

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.017929
ARTICLE
Diversity of Saxicolous Lichens along an Aridity Gradient in Central México
1Facultad de Ciencias Naturales, Universidad Autónoma de Querétaro, Querétaro, 73230, México
2Departamento de Biología y Geología, Física y Química Inorgánica Tulipán s/n, Universidad Rey Juan Carlos, Móstoles, Madrid, 28933, España
3Cátedra CONACYT–Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo,Morelia, Michoacán, 58030, México
*Corresponding Author: Mariela Gómez-Romero. Email: mgomezr@conacyt.mx
Received: 17 June 2021; Accepted: 23 September 2021
Abstract: Lichens are symbiotic organisms that comprise a fungus and a photosynthetic partner wich are recognized as a good indicator of climate change. However, our understanding of how aridity affects the diversity of saxicolous lichens in drylands is still limited. To evaluate the relationship between saxicolous lichen diversity and aridity in a central México dryland, a geographical transect was established of 100 km to build an aridity gradient in the semiarid zone of the State of Querétaro, Mexico, comprising ten sampling sites with a 10 km separation. Species richness, abundance and diversity of soil lichen species were recorded using two sampling methods: the quadrat-intercept and the line-intercept method, to compare their performance in assessing soil lichen diversity in drylands. The number of species and Shannon diversity of saxicolous lichens were higher at intermediate values of the aridity index (AI = 0.10–0.34). Quadrat intercept and point intercept methods gave quite similar results, which means that the selected method does not influence the results in a significant way. This study confirms the role of saxicolous lichens as climate change indicators and reveals the importance of the sampling method selection in the evaluation of different parameters of soil lichen diversity in drylands.
Keywords: Climate change; drylands; ecological indicators; environmental stress; precipitation; temperature
Lichens are symbiotic organisms comprising a fungal partner (mycobiont) and a photosynthetic partner (photobiont they may be green alga and/or cyanobacteria). One major feature of lichens is their poikilohydric and poikilothermic nature, which means that they cannot actively regulate their water status and internal temperature Nash et al. [1]. Lichens, even though they can tolerate relatively well harsh environmental conditions, like a high exposure to light, low water availability and elevated temperatures, some of them are species that show a wide range of sensitivity to changes in the surrounding environment. Therefore, lichen richness and diversity are considered good indicators of climate changes Matos et al. [2].
Previous research on lichens as indicators of environmental changes showed that their abundance and diversity respond to climate type and variability from the subtropics to drylands [3–6], but also in cold regions [7]. An important climate variable is aridity, which is an essential driver of lichen taxonomic and functional diversity along wide environmental gradients. This support the hypothesis that lichen distribution is markedly determined by climatic gradients [7–9].
The distribution and diversity of lichens in the boreal region, and vascular plants, are also affected by changes in climate such as precipitation and temperature [10–14] as well as organisms in general. However, lichens are a particular interesting model organisms because of their high sensitivity to changes in water availability and temperature, due to their poikilohydric nature. Rainfall and non-rainfall water inputs are important sources of hydration [15].
For the next decades, the Intergovernmental Panel on Climate Changes has predicted an increase in drought frequency and intensity, combined with extreme temperatures IPCC [16,17], which are expected to affect lichen distribution, abundance and diversity [18]. Although evidence supports lichens as a good indicator of climate changes [9,19–21], only few studies had evaluated how lichen diversity responds to aridity [22,23]. This is a fact for Mexican drylands, which comprise almost 65% of the country surface area [24].
Drylands are characterized by low water availability and elevated temperatures, two variables that define the aridity of an ecosystem [25]. Because of a global trend towards warmer and drier air, will affect lichen-dominated dryland biocrust communities and their role in regulating ecosystem functions worldwide [26,27]. Aridity can be defined as the lack of available water and multiple indices can be used to calculate it. This index has been used to evaluate plant species distribution, diversity, and development [28,29]. As in plants, the lack of water has direct effects on lichen physiology via lichen water relations [15], e.g., thallus hydration and desiccation [30], determining their establishment and success. However, lichen resistance to water availability is a specific characteristic, thus, lichen diversity is expected to show marked shifts along aridity gradients.
When assessing lichen diversity, several sampling methods are available, for example, the line-intercept method is based on recording species presence and abundance along a longitudinal transect [31,32], and it has the advantage of being quite easy to apply [33]. The line-intercept method has another variant; the quadrat-intercept method consists of placing sampling quadrats along the transect at a systematic distance spacing [34]. Both methods have been widely used in plant research because they are able to reflect plant diversity patterns. The line-intercept method records plant species abundance and diversity by recording the plant lineal spatial configuration of multiple landscape components [35]. On the other hand, the quadrat-intercept method allows a simultaneous assessment of species presence and abundance in a defined area at a given spatial scale [36], although it requires a major sampling effort [37]. Surprisingly, studies comparing the effectiveness of each method in revealing lichen diversity patterns are uncommon in the literature.
Mexico needs more studies on vegetation distribution and diversity, since they are relatively scarce and limited compared to other regions [38,39]. Moreover, there is a major knowledge difference regarding biocrust distribution and diversity in Mexican and South American ecosystems [40]. However, there are good examples of the high diversity and the important role that biocrust lichens play in Mexican drylands [18,41–43]. Therefore, is crucial to have a better knowledge of how lichen diversity responds to environmental conditions in Mexican open drylands, where lichens are a common ecosystem component due to the harsh environmental conditions given by the semi-arid climate and the soil rockiness [44]. These ecosystems already cover around 45% of the Earth’s land mass [45] and are expected to increase by up to 23% by the end of the 21st century due to predicted increases in aridity under climate change [46]. Also, there is a need to validate the suitability of the multiple sampling methods to assess soil lichen diversity in drylands.
In this study, it was evaluated lichen abundance and diversity inhabiting rock outcrops along an aridity gradient using two different sampling methods: quadrat-intercept and line-intercept method. These methods had been using to evaluate changes in the vascular plant covers in drylands [47]; however, their differential suitability to detect changes in lichen abundance and diversity hasn´t been well study and evaluate. By evaluating how different lichen species respond to environmental gradients driven by climate, and specifically aridity, we will better understand the potential implications of the forecasted climate change effects on Mexican drylands and in worldwide dryland biodiversity. The hypothesis was that an increased in aridity leads to lower lichen diversity, supporting the theory that lichens are good bio-indicators of climate change in drylands [2].
This study was conducted in the State of Queretaro, central Mexico, in the physiographic province of the central Neo-Volcanic axis in the border of the Sierra Madre Oriental province, where the climate is dry and semi-arid, with mean annual precipitation and temperature ranging from 435 to 709 mm and from 18°C to 22°C. Topography is characterized by gentle rolling hills to flat, surrounded by mountain ranges, plateaus and hillocks that retain the humidity coming with the winds from the Mexican Gulf and the Central Plateau, which run from north to south INEGI [48]. Altitude values are below 2000 m asl and for this study, the geographic transect was 100 km long, crossing the central semi-arid region in the State of Queretaro, from San Juan del Río Community to Peñamiller (Fig. 1), with a daily temperature range of 7°C during the day. Ten sampling sites were in the aridity transect, each separated 10 km apart (Fig. 1). At each site, we registered the geographic coordinates, altitude, and lichen species presence and abundance.
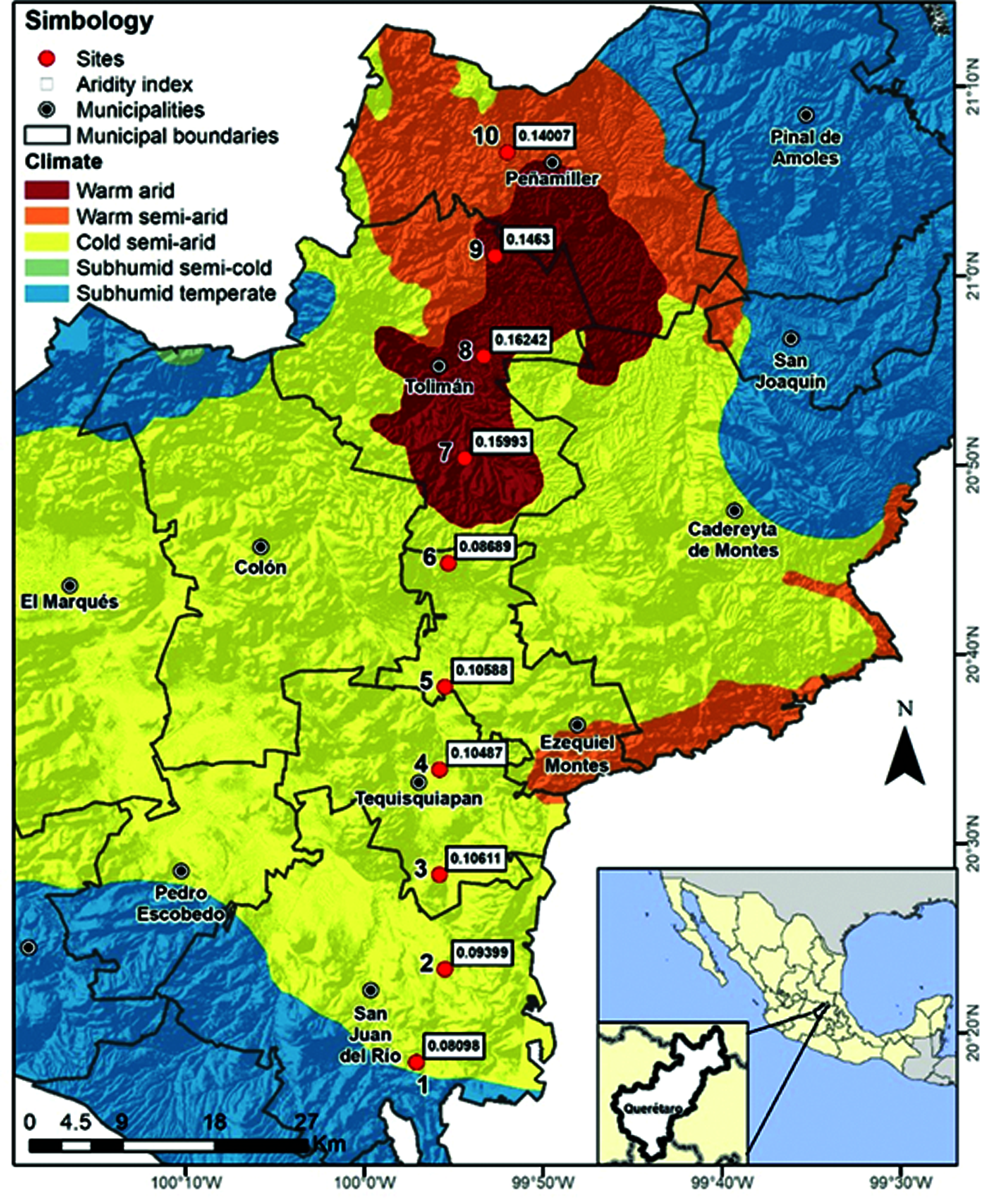
Figure 1: Study sites along the aridity gradient in central Mexico drylands
2.2 Characterization of Climatic Variables
Was used the geographic coordinates and altitude values from each site to calculate climatic variables and build our aridity gradient. Many studies have calculated aridity as the relationship between temperature and precipitation using the Annual Aridity Index, defined as AAI = (DD50.5)/MAP where DD5 is the temperature in decimal degrees of each day with temperatures >5°C and MAP is the annual mean precipitation [28,49,50]. Climate variables were obtained using thin plate splines, a climatic model developed for Mexico [51]. This model was based on data surface interpolation using the software ANUSPLINE [52] and fed on normalized monthly temperature (maximum, mean and minimum) and precipitation data collected from ∼4000 Mexican climate stations. Then, were used the data obtained with the model to calculate the Annual Arity Index (AAI), defined as AAI = (DD50.5)/MAP; where DD5 is the temperature in decimal degrees of each day with temperature >5°C and MAP is the annual mean precipitation over 29 years (1961–1990) [28] (Fig. 1). The Annual Aridity Index is known to be tightly linked to vascular plant species and group distribution [49].
Was used two different methods to assess changes in lichen abundance and diversity along the aridity gradient. The study site was characterized by the presence of rocky outcrops. The minimum exclusion of an area with human activity was at least 100 m, in most cases the distance were kilometers. First, was used the quadrat-intercept method, which consists of regularly locating multiple sampling quadrats of a particular size along a transect. Were established four transects (20 m), S-W oriented at each site. Along each transect, 12 sampling quadrats (30 × 30 cm) were in open microsites (i.e., plant interspaces), 50 cm apart from each other (Fig. 2a).
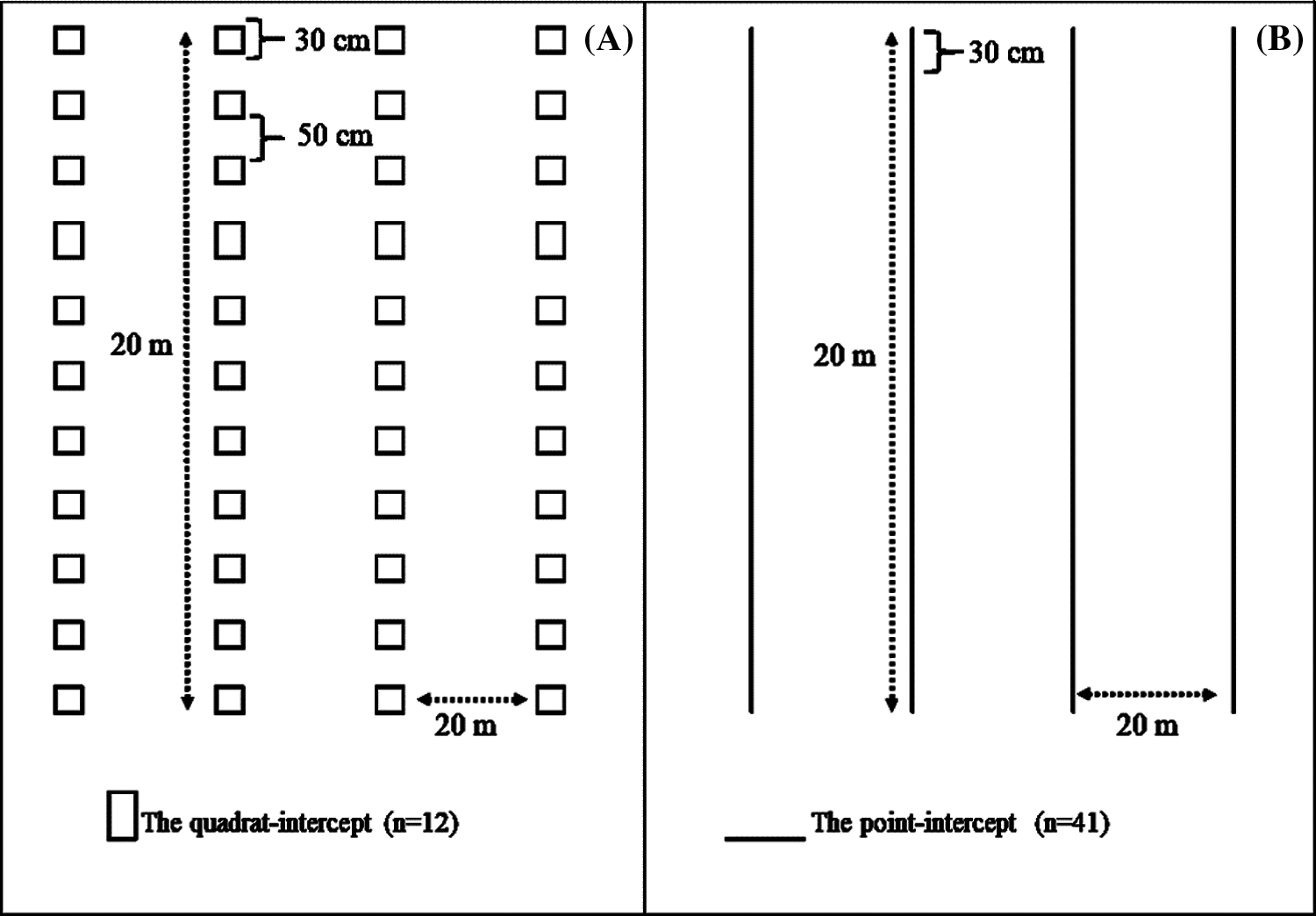
Figure 2: Sampling methods used to evaluate lichen diversity metrics. (a) Quadrat intercept method, (b) Point intercept method in central Mexico drylands
Second, were employed the point-intercept method Elzinga et al. [53], based on four transects of 20 m located 20 m apart from each other, each containing 41 sampling points 30 cm from each other. Lichen species were registered at each sampling quadrat and point along transects (Fig. 2b), and the species cover was calculated as the proportion of quadrats or intercepted points where the species was present.
Lichen species were identified following [1,54] based on lichen morphological and chemical attributes (i.e., presence of lichen secondary compounds such as organic acids). To identify lichen secondary compounds, we used the following chemical tests: sodium hypochlorite (NaOCl) [55], potassium hydroxide KOH (10%), calcium hypochlorite (Ca(Cl)2) and paraphenildiamine (C6H8N2, 95%) [56] and potassium iodide (KI) [57].
To characterize lichen diversity along the aridity gradient, we used several indices. Firstly, we calculated the number of species on the sites. Secondly, we calculated the Shannon-Wiener index (H) to estimate the heterogeneity of the community based on two parameters: the number of species present in the sample and their relative abundance [58]. The Shannon-Wiener index is defined as
To evaluate the relationship between altitude and the aridity index, was carried a lineal regression analysis using the PROC REG procedure in the SAS software (Version 9.3) SAS [60]. Our model followed the equation Yij = β0 + β1Xi + eij, where Yij = mean annual aridity index, β0 = intercept, β1 = slope, Xi = altitude (m asl) in the i-th sampling point, and eij = error. To evaluate the relationship between lichen diversity variables (number of species and Shannon-Wiener index) and the aridity index, we followed the same procedure but, in this case, we used a quadratic regression following the equation Yij = β0 + β1Xi + β1Xi2 + eij.
Along the studied geographic gradient, the aridest sites were in the north (aridity index = 0.14–0.16), where the altitude is around 16,000 m asl Then, aridity decreased towards the southern part of the gradient, where altitude reaches 2,200 m asl INEGI [48]. Within the gradient, sites that are close to each other show contrasting values for the aridity index due to pronounced changes in topography at small spatial scales. The aridity index was significantly and negatively related with altitude, at lower altitude, higher annual aridity index (r2 = 0.84, P < 0.001). In addition, sites were separated into two groups: sites 1 to 6 (aridity index < 0.12) and sites 7 to 10 (aridity index > 0.12; Fig. 3), suggesting there were marked differences in local environmental conditions.
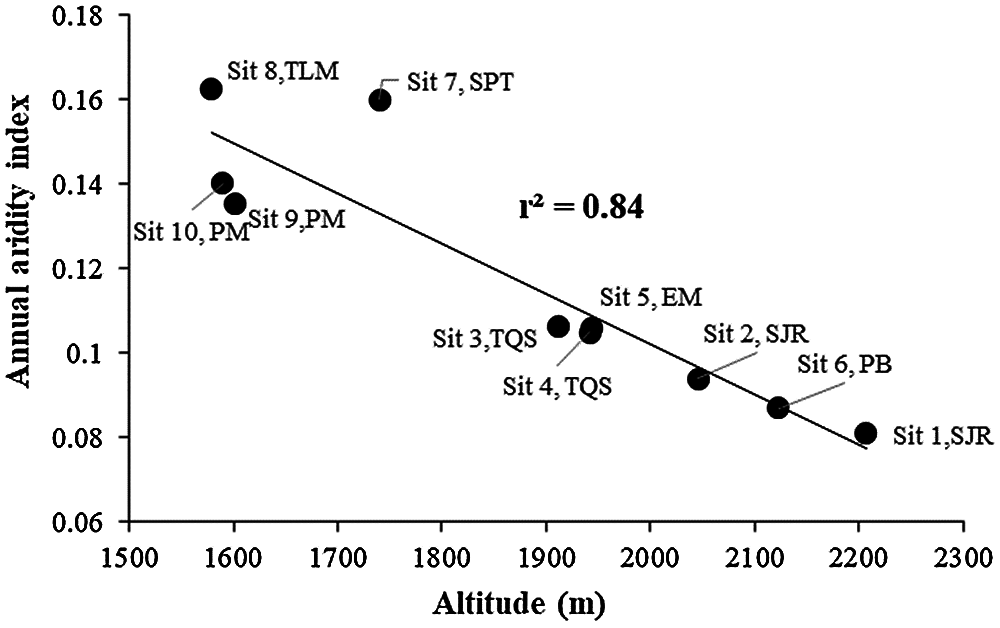
Figure 3: Relationship between the annual aridity index (AAI) and altitude along the aridity gradient in central Mexico drylands
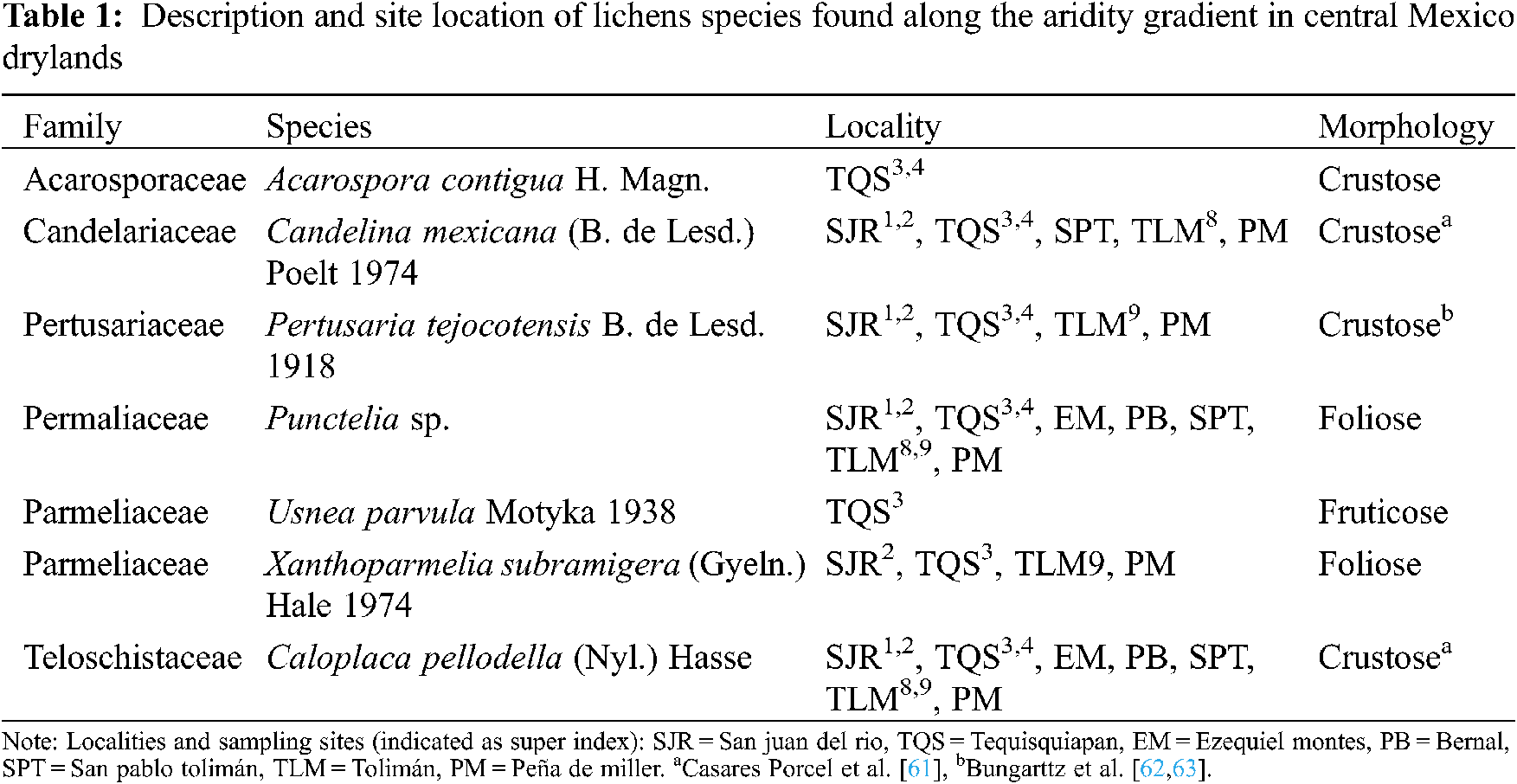
In the study were found seven lichen species belonging to five different families. Five species presented crustose growth form, one species was foliose, and another species was fruticose (Table 1). It is worth to stand out that by registring the presence of Usnea parvula, a rare species in drylands, although this species was found in one site only, showing an intermediate value of the aridity index (Table 1, Fig. 3). The most abundant species were Caloplaca pellodella, followed by Punctelia sp., with a cover ranging from 50% to 65% and from 10% to 20%, respectively, as indicated by both sampling methods (quadrat-intercept and point intercept; Fig. 4). Additionally found that the quadrat-intercept method registered higher cover values for all the species, compared to the point intercept method.
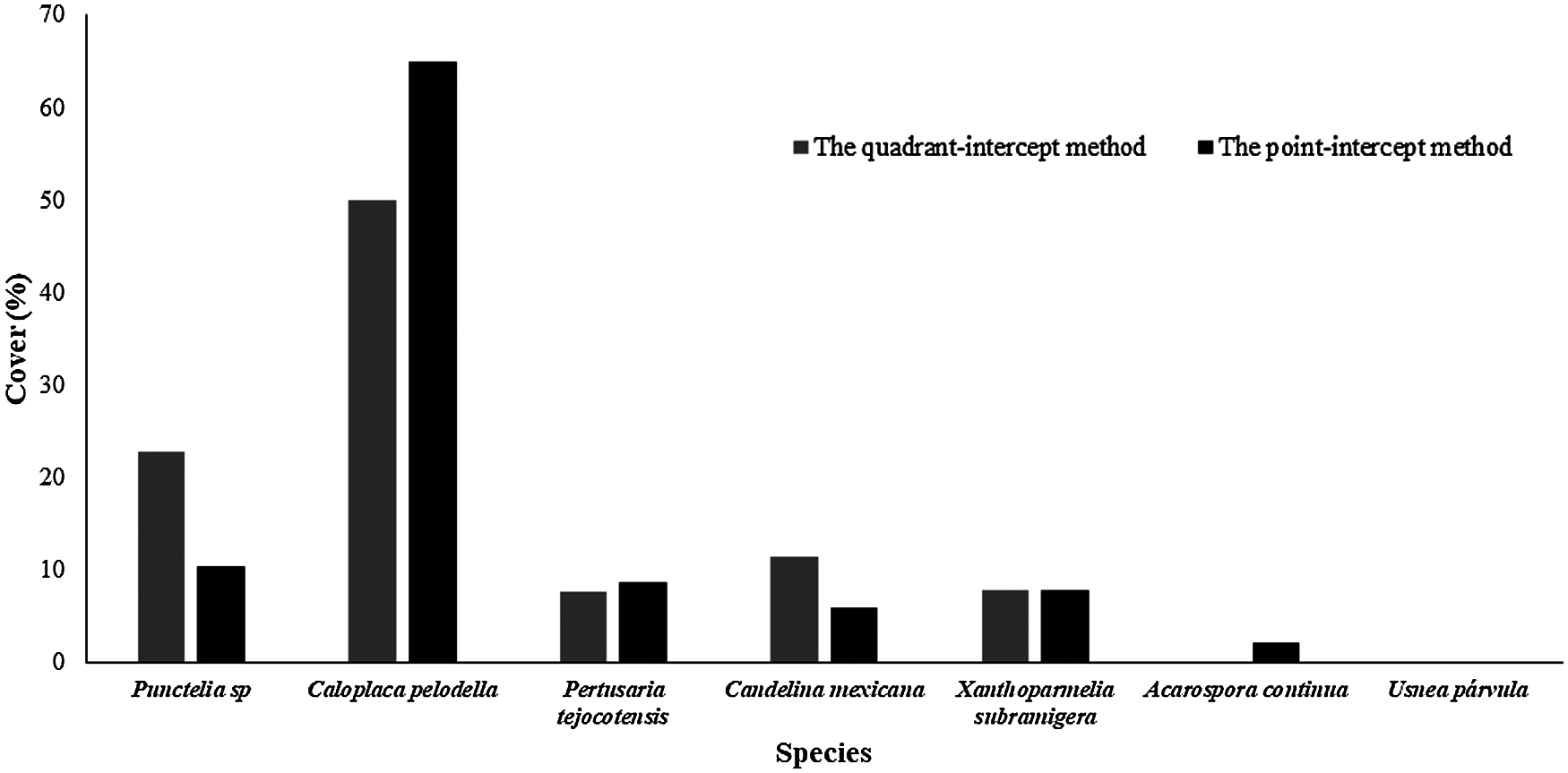
Figure 4: Cover of lichen species registered by the quadrat and point-intercept method along the aridity gradient in central Mexico drylands
The number of lichen species and the aridity index showed a non-linear relationship regardless of the sampling method. However, this relationship was moderated but not significant with the quadrat-intercept method (r2 = 0.43, P = 0.080), but strong and significant in the point-intercept method (r2 = 0.66, P = 0.017, Fig. 5a). In both cases, the number of lichen species was higher for intermediate values of the aridity index (0.10–0.14) and lower in the extremes of the gradient (aridity index <0.10 and >0.14). The relationship between the Shannon-Wiener index and the aridity index followed a similar pattern than the number of lichen species for both sampling methods; however, in this case, none of the relationships were significant (quadrat intercept: r2 = 0.39, P = 0.116, and point intercept: r2 = 0.52, P = 0.086; Fig. 5b).

Figure 5: Lichen diversity metrics along the aridity gradient in central Mexico drylands. (a) Number of lichen species, (b) shannon index
The results suggested that that aridity influenced the diversity of saxicolous lichens along the studied gradient. Rock outcrops characterize the semi-arid region of the State of Queretaro where lichens are a common feature (Table 1) [64]. Similar lichen communities were found in surrounding areas like Guanajuato [64,65] and Hidalgo States [63]. Some lichen species typically found in such harsh environmental conditions are infrequent like U. parvula is particularly uncommon in semi-arid drylands. The most abundant species in the studied region showed a crustose growth, which has been identified as a resistant growth form due to morphological and physiological attributes [66].
The number of lichen species showed a quadratic relationship with aridity, higher at intermediate levels of aridity (AAI between 0.10 and 0.14, Fig. 5a), supporting the hypothesis that climate acts as an important environmental filter on lichen species richness [2,4,67]. In particular, the aridest environments may have limited the number of species able to tolerate the low water availability while in the less arid extreme of the gradient, lichens may compete for space and resources with other organisms such as vascular plants [4,23]. The biodiversity loss of plants and soil microorganisms may have especially strong consequences under low and high aridity conditions [68]. Most lichen species found in this study were pioneers in hostile environments where vascular plant cover is scarce [44], showing a saxicolous habit in Mexican drylands [64]. Indeed, the less arid sites of our study gradient were less rocky than the more arid sites, providing less stressful habitats for vascular plant communities. 34% of lichens in Mexico have rocks as their substrate. The most common substrate where they grow is the bark of trees (46%) [69]. There is variability in the answer you can show the lichens at specific level in similar environmental factors [70,71]. The Shannon-Wiener index showed similarities to the number of lichens species, i.e., higher at intermediate levels of aridity but lower in the gradient extremes. These results agree with previous research showing that the most stressful habitats lead to less diverse lichen communities [4,72], because the increase in a few species that can cope better when water availability is reduced [73]. However, the strong relationship between the Shannon-Wiener index and the AAI was marginally significant (0.05 < P< 0.12, Fig. 5b), suggesting moderate data variability and that more sites should be included in the gradient or that lichen diversity should be assessed along a wider range of aridity.
The quadrat-intercept method allows to collect a higher amount of information in the field, e.g., the number and abundance of individuals for each species at the local scale, making this method useful to evaluate changes in lichen abundance and diversity. However, this method requires a greater effort to collect the data [36], compared to the point-intercept method. The point-intercept method has found to be useful to assess the spatial configuration of multiple ecosystem components Álvarez et al. [35]. The result suggested that, by comparing the two sampling methods, they gave slightly different results. To represent the number of species and diversity of saxicolous lichens along the studied aridity gradient. Since choosing the right sampling method is critical to obtain valid data to evaluate diversity patterns [74] and in a cost-effective way [43], our findings contribute to improve the methodological approach to be used when assessing changes of saxicolous lichens inhabiting drylands. The climate changes, relationships between plant traits and soil functions are likely to change [75,76]. The response of lichen diversity to aridity found in our study confirms the role of lichens as ecological indicators of environmental changes [22,77]. Specifically, lichens can be considered reliable indicators of climate change in drylands [2,4,78], where forecasted changes in temperature and precipitation regimes are predicted to have critical impacts on ecosystem structure and functioning [79]. Abundances is of paramount importance to understanding how these compositional changes will affect communities and ecosystem functioning in response to ongoing climate change [80]. Expected increases in aridity will affect lichen abundance and diversity [81,82], and lichen species such as C. pellodella and Punctelia sp. are expected to become dominant due to their morphological and physiological traits, which may confer a higher resistance to an aridest environment. Could contribute to primary production and the regulation of greenhouse emissions [83].
The amount number and diversity of saxicolous lichen species respond to changes in aridity in semi-arid drylands of central México. Diversity decreases at both ends of the gradient. Lichen richness was measured with the point intercept method correlated more strongly with aridity. Quadrat intercept and point intercept methods gave quite similar results, which means that the selected method doesn´t influence the results in a significant way. To provide better evidence in the changes of climate and anticipate their impacts on the structure and function of drylands, further studies should be conducted using lichens as early and reliable ecological indicators.
Acknowledgement: The authors thank Ph.D. Teresa García Gasca, (rector of the Autonomous University of Querétaro), Postgraduate Office, Sara Mercado and Irma Avilés Carrillo for their assistance. We also thank Ph.D. Cristina Branquinho for her observations.
Author Contributions: JCSC and LCZ planned and designed the research, JCSC and ASV conducted fieldwork, JCSC and VHCS processed and analyzed the data, JCSC, MGR, LCZ wrote the manuscript and all authors contributed to the final review.
Funding Statement: This research was supported by the Funding Internships and the Postgraduate Office of the Autonomous University of Querétaro. LCZ MSCA-IF-2017 (Grant Agreement 795380 [INDECRUST]) under the European Community’s H2020-EU.1.3.2 Programme.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Nash III, T. H., Ryan, B. D., Diederich, P., Gries, C., Bungarts, F. (2007). Lichen flora of the greater sonoran desert region, vol. III, pp. 567. Tempe, Arizona, USA: Lichens Unlimited, Arizona State University. [Google Scholar]
2. Matos, P., Pinho, P., Aragón, G., Martínez, I., Nunes, A. et al. (2015). Lichen traits responding to aridity. Journal of Ecology, 103(2), 451–458. DOI 10.1111/1365-2745.12364. [Google Scholar] [CrossRef]
3. Watson, R. T. (2004). Climate change 2001: Synthesis report. IPCC, Geneva: Cambridge University Press. [Google Scholar]
4. Concostrina-Zubiri, L., Martínez, I., Rabasa, S. G., Escudero, A. (2014). The influence of environmental factors on biological soil crust: From a community perspective to a species level approach. Journal of Vegetation Science, 25, 503–513. DOI 10.1111/jvs.12084. [Google Scholar] [CrossRef]
5. Aptroot, A., van Herk, C. (2006). Further evidence of the effects of global warming on lichens, particularly those with trentepohlia phycobionts. Environmental Pollution, 146, 293–298. DOI 10.1016/j.envpol.2006.03.018. [Google Scholar] [CrossRef]
6. Koch, M. N., Branquinho, C., Matos, P., Pinho, P., Lucheta, F. et al. (2016). The application of lichens as ecological surrogates of air pollution in the sub tropics: A case study in south Brazil. Enviromental Science and Pollution Research, 23, 20819–20834. DOI 10.1007/s11356-016-7256-2. [Google Scholar] [CrossRef]
7. Green, T. A., Sancho, L. G., Pintado, A., Schroeter, B. (2011). Functional and spatial pressures on terrestrial vegetation in Antarctica forced by global warming. Polar Biology, 34, 1643–1656. DOI 10.1007/s00300-011-1058-2. [Google Scholar] [CrossRef]
8. Giordani, P., Incerti, G. (2008). The influence of climate on the distribution of lichens: A case study in a borderline área (Liguria, NW Italy). Plant Ecology, 195, 257–272. DOI 10.1007/s11258-007-9324-7. [Google Scholar] [CrossRef]
9. Nascimbene, B., Casazza, G., Benesperi, R., Catalano, I., Cataldo, D. et al. (2016). Climate change forest the decline of epiphytic lobaria species in Italy. Biological Conservation, 201, 377–384. DOI 10.1016/j.biocon.2016.08.003. [Google Scholar] [CrossRef]
10. Fitzpatrick, C. M., Gove, D. A., Sanders, J. N., Dunn, R. R. (2008). Climate change, plant migration, and range collapse in a global biodiversity hotspot: The banksia (Proteaceae) of Western Australia. Global Change Biology, 14, 1337–1352. DOI 10.1111/j.1365-2486.2008.01559.x. [Google Scholar] [CrossRef]
11. Johansson, P. (2008). Consequences of disturbance on epiphytic lichens in boreal and near boreal forests. Biological Conservatin, 141, 1933–1944. DOI 10.1016/j.biocon.2008.05.013. [Google Scholar] [CrossRef]
12. Rehfeldt, G. E., Ferguson, D. E., Crookston, N. L. (2009). Aspen, climate and sudden decline in Western USA. Forest Ecology Management, 258, 2353–2364. DOI 10.1016/j.foreco.2009.06.005. [Google Scholar] [CrossRef]
13. Vitasse, Y., Porté, A. J., Kremer, A., Michalet, R., Delzon, S. (2009). Responses of canopy duration to temperature changes in four temperate tree species: Relative contributions of spring and autumn leaf phenology. Oecology, 161, 187–198. DOI 10.1007/s00442-009-1363-4. [Google Scholar] [CrossRef]
14. Boch, S. D., Schöning, I., Fischer, M. (2016). Acer pseudoplatanus, fagus sylvatica, fraxinus excelsior and quercus petraea. Biodiversity and Conservation, 25(2), 225–238. DOI 10.1007/s10531-015-1037-y. [Google Scholar] [CrossRef]
15. Raggio, J., Green, T., Sancho, L., Pintado, A., Colesie, C. et al. (2017). Metabolic activity duration can be effectively predicted from macroclimatic data for biological soil crust habitats across Europe. Geoderma, 306, 10–17. DOI 10.1016/j.geoderma.2017.07.001. [Google Scholar] [CrossRef]
16. IPCC (2013). Climate change 2013: The physical science basis. In: Stocker, T. F., Qin, D., Plattner, G. K., Tignor, M., Allen, S. K. et al. (Eds.Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change, pp. 1535. Cambridge, UK and New York, NY, USA: Cambridge University Press. [Google Scholar]
17. IPCC (2014). Summary for policymakers. In: Field, C. B., Barros, V. R., Dokken, D. J., Mach, K. J., Mastrandrea, M. D. et al. (Eds.Climate change 2014: Impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change, pp. 1132. Cambridge, UK and New York, NY, USA: Cambridge University Press. [Google Scholar]
18. Gutiérrez Gutiérrez, M., Pando-Moreno, M., González Rodríguez, H., Mendoza Aguilar, D. (2017). Efecto del micrositio en la composición de costras biológicas del suelo en un área de matorral micrófilo del desierto chihuahuense, México. Interciencia, 42(4), 212–214. [Google Scholar]
19. Pinho, P., Máguas, C., Branquinho, C. (2010). Modeling ecology of lichens communities based on photobiont type in relation to potential solar radiation and neighborhood land-use. In: Nash III, T., Geiser, L., McCune, B., Triebel, D., Tomescu, A. M., Sanders, W. (Eds.Bibliotheca lichenologica. Biology of Lichens-Symbiosis, Ecology, Environmental Monitoring Cystematics and Cyber Applications. vol. 105, pp. 149–160. California: J. Cramer. [Google Scholar]
20. Marini, L., Nascimbene, J., Nimis, P. L. (2011). Large-scale patterns of epiphytic lichen species richness: Photobiont-dependent response to climate and forest struture. Science of the Total Environment, 409, 4381–4386. DOI 10.1016/j.scitotenv.2011.07.010. [Google Scholar] [CrossRef]
21. Colesie, C., Green, A., Haferkamp, I., Büdel, B. (2014). Habitat stress initiates changes in composition, CO2 gas exchange and C-allocation as life traits in biological soil crusts. The ISME Journal, 8, 2115–2115. DOI 10.1038/ismej.2014.47. [Google Scholar] [CrossRef]
22. Branquinho, C., Matos, P. (2015). Lichens as ecological indicators to tack atmospheric changes: Future challenges. In: Lindenmayer, D., Barton, P., Pierson J. (Eds.Indicators and surrogates of biodiversity and environmental change, pp. 216. Melburne, London: Csiro Publishing. [Google Scholar]
23. Concostrina-Zubiri, L., Matos, P., Giodani, P., Branquinho, C. (2018). Biocrust tissue traits as potential indicators of global change in the Mediterranean. Plant Soil, 429, 159–174. DOI 10.1007/s11104-017-3483-7. [Google Scholar] [CrossRef]
24. SEMARNAT. Secretaría de Medio Ambiente y Recursos Naturales (2016). Executive summary. Mexico state of the environment report compendium of environment statistics, key environmental indicators, environmental performance indicators and green growth indicators. Version 2015. SEMARNAT. [Google Scholar]
25. Maestre, F. T., Bowker, M. A. Y., Cantón, Y., Castillo-Monroy, A. P., Cortina, J. et al. (2011). Ecology and functional roles of biological soil crusts in semi-arid ecosystems of Spain. Journal of Arid Environments, 75, 1282–1291. DOI 10.1016/j.jaridenv.2010.12.008. [Google Scholar] [CrossRef]
26. Reed, S. C., Delgado-Baquerizo, M., Ferrenberg, S. (2019). Biocrust science and global change. New Phytologist, 223(3), 1047–1051. DOI 10.1111/nph.15992. [Google Scholar] [CrossRef]
27. Baldauf, S., Porada, P., Raggio, J., Maestre, F. T., Tietjen, B. (2021). Relative humidity predominantly determines long-term biocrust-forming lichen cover in drylands under climate change. Journal of Ecology, 109, 1370–1385. DOI 10.1111/1365-2745.13563. [Google Scholar] [CrossRef]
28. Sáenz-Romero, C., Rehfeldt, G. E., Crookston, N. L., Duval, P., St-Amant, R. et al. (2010). Spline models of contemporary, 2030, 2060 and 2090 climates for Mexico and their use in understanding climate-change impacts on the vegetation. Climatic Change, 102, 595–623. DOI 10.1007/s10584-009-9753-5. [Google Scholar] [CrossRef]
29. Soto-Correa, J. C., Sáenz-Romero, C., Lindig-Cisneros, R., de-la-Barrera, E. (2014). Lupinus elegans kunth assisted migration on common garden field tests. Revista Fitotecnia Mexicana, 37(2), 107–116. DOI 10.35196/rfm.2014.2.107. [Google Scholar] [CrossRef]
30. Gauslaa, Y., Coxson, D. S., Solhaug, K. A. (2012). The paradox of higher light tolerance during desiccation in rare old forest cyanolichens than in more widespread co-occurring chloro- and cephalolichens. New Phytologist, 195(4), 812–822. DOI 10.1111/j.1469-8137.2012.04221.x. [Google Scholar] [CrossRef]
31. Bowker, M. A., Belnap, J., Davidson, D. W., Goldstein, H. (2006). Correlates of biological soil crust abundance across a continuum of spatial scales: Support for a hierarchical conceptual model. Journal of Applied Ecology, 43(1), 152–163. DOI 10.1111/j.1365-2664.2006.01122.x. [Google Scholar] [CrossRef]
32. Bowker, M. A., Belnap, J. (2008). A simple classification of soil types as habitats of biological soil crusts on the Colorado plateau, USA. Journal of Vegetation Science, 19(6), 831–840. DOI 10.3170/2008-8-18454. [Google Scholar] [CrossRef]
33. Canfield, H. R. (1941). Application of the line interception method in sampling range vegetation. Journal of Forestry, 39, 388–394. DOI 10.1093/jof/39.4.388. [Google Scholar] [CrossRef]
34. Nelson, P. R., McCune, B., Roland, C., Stehn, S. (2015). Non-parametric methods reveal non-linear functional trait variation of lichens along environmental and fire age gradients. Journal of Vegetation Science, 26(5), 848–865. DOI 10.1111/jvs.12286. [Google Scholar] [CrossRef]
35. Álvarez, M., Córdoba, S., Escobar, F., Fagua, G., Gast, F. et al. (2006). Manual de métodos para el desarrollo de inventarios de biodiversidad. Programa de Inventarios de Biodiversidad, 2nd edition, pp. 236. Bogotá, Colombia: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. [Google Scholar]
36. Ramirez-Gonzalez, A. (2006). Ecología, métodos de muestreo y análisis de poblaciones y comunidades, pp. 273. Bogotá, Colombia: Pontificia Universidad Javeriana. [Google Scholar]
37. Flores, J., Álvarez-Sánchez, J. (2011). Flora y vegetación. In: Bautista, F., Palacio, J. y Delfín-González, H. (Eds.Técnicas de muestreo para manejadores de recursos naturales, 2da edition, pp. 389–413, 770. México: Universidad Nacional Autónoma de México. [Google Scholar]
38. Sánchez-Velázquez, L. R., Galindo-González, J., Díaz-Fleischer, F. (Eds.) (2008). Ecología, manejo y conservación de los ecosistemas de montaña en méxico, pp. 393. México: Mundi Prensa México, S. A. de C. V., CONABIO, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Universidad Veracruzana. [Google Scholar]
39. Dirzo, R., Aguirre, A., López, J. C. (2009). Diversidad florística de las selvas húmedas en paisajes antropizados. Investigación Ambiental, 1, 17–22. [Google Scholar]
40. Weber, B., Bowker, M., Zhang, Y. B., Belnap, J. (2016). Natural recovery of biological soil crusts after disturbance. Chapter 23. In: Weber, B., Büdel, B., Belnap J. (Eds.Biological soil crusts as an organizing principle in drylands, pp. 479–498. Switzerland: Springer, International Publishing AG, Cham. [Google Scholar]
41. Rivera-Aguilar, V., Montejano, G., Rodríguez-Zaragoza, S., Durán-Díaz, A. (2006). Distribution and composition of cyanobacteria, mosses and lichens of the biological soil crusts of the tehuacán valley, puebla, México. Journal of Arid Environments, 67(2), 208–225. DOI 10.1016/j.jaridenv.2006.02.013. [Google Scholar] [CrossRef]
42. Rivera-Aguilar, V., Godínez-Alvarez, H., Moreno-Torres, R., Rodríguez-Zaragoza, S. (2009). Soil physico-chemical properties affecting the distribution of biological soil crusts along an environmental transect at zapotitlán drylands, Mexico. Journal of Arid Environments, 73(11), 1023–1028. DOI 10.1016/j.jaridenv.2009.05.003. [Google Scholar] [CrossRef]
43. Concostrina-Zubiri, L., Huber-Sannwald, E., Martínez, I., Flores, J. F., Escudero, A. (2013). Biological soil crusts greatly contribute to small-scale soil heterogeneity along a grazing gradient. Soil Biology and Biochemistry, 64, 28–36. DOI 10.1016/j.soilbio.2013.03.029. [Google Scholar] [CrossRef]
44. Herrera, T., Ulloa, M. (1998). El reino de los hongos, micología básica y aplicada, 2da Edition. Universidad Autónoma de México/Fondo de cultura económica. México, pp. 552. [Google Scholar]
45. Prăvălie, R. (2016). Dry lands extent and environmental issues a global approach. Earth-Science Reviews, 161, 259. DOI 10.1016/j.earscirev.2016.08.003. [Google Scholar] [CrossRef]
46. Huang, J., Yu, H., Guan, X., Wang, G., Guo, R. (2016). Accelerated dryland expansion under climate change. Nature Climate Change, 6, 166–171. DOI 10.1038/nclimate2837. [Google Scholar] [CrossRef]
47. Nunes, A., Tapia, S., Pinho, P., Correia, O., Braquino, C. (2014). Advantages of the point-intercept method for assessing functional diversity in semi-arid areas. iForest Biogeosciences and Forestry, 8, 471–479. DOI 10.3832/ifor1261-007. [Google Scholar] [CrossRef]
48. INEGI (1998). Anuario estadístico del estado de querétaro, pp. 13. México: Instituto Nacional de Estadística, Geografía e Informática. [Google Scholar]
49. Rehfeldt, G. E. (2006). A spline model of climate for the western United States. USDA Forest Service Gen. Tech. Rep. RMRS–GTR–165. Fort Collins, Clorado, U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, pp. 21. [Google Scholar]
50. Soto-Correa, J. C., Sáenz-Romero, C., Lindig-Cisneros, R., Sánchez-Vargas, N., Cruz-de-León, J. (2012). Genetic variation between Lupinus elegans kunth provenances, altitudinal seed zoning and assisted migration. Agrociencia, 46, 593–608. [Google Scholar]
51. Crookston, N. L. (2010). Research on forest climate change: Potential effects of global warming on forests and plant climate relationships in western North America and Mexico. http://forest.moscowfsl.wsu.edu/climate/. [Google Scholar]
52. Hutchinson, M. F. (2004). Anuspline version 4.3. Canberra, Australia: Centre for resource and environmental studies. The Australian Nacional University. [Google Scholar]
53. Elzinga, C. L., Salzer, D. W., Willoughby, J. W. (2001). Measuring and monitoring plant populations, pp. 477. Denver, CO, USA: Bureau of Land Management National Business Center. [Google Scholar]
54. Brodo, I. M., Duran-Sharnoff, S., Sharnoff, S. (2001). Lichens of North America, pp. 795. New Haven and London: Yale University Press. [Google Scholar]
55. Chaparro de, V. M., Aguirre, L. J. (2002). In: Hongos liquenizados. vol. 1. pp 220. Bogotá Colombia: El Malpensante, S. A. (Ed.). Colombia: Universidad Nacional de Colombia. [Google Scholar]
56. Santesson, R. (1973). In: Ahmadjian, V., Hale, M. (Eds.pp. 697. Identification and isolation of lichen substances. New York and London: Academic Press. [Google Scholar]
57. Marcano, V. (1994). Introducción al estudio de los líquenes y su clasificación, pp. 14–97. Merida: Museo de la Ciencia. [Google Scholar]
58. Shannon, C. E., Weaver, W. (1949). The mathematical theory of communication, pp. 144. USA, Urbana, IL, EEUU: University of Illinois Press. [Google Scholar]
59. Magurran, A. E. (1988). Ecological diverssity and its measurement, pp. 179. Londres: Croom Helm. [Google Scholar]
60. SAS Institute Inc. (2004). SAS/STAT 9.3 User’s Guide, pp. 4975. Cary, North Carolina, USA: SAS Institute Inc. [Google Scholar]
61. Casares Porcel, M., Gutiérrez, L., García Rowe, J., Carballal, R. (Eds.) (2006). Sistemática de los líquenes. In: Capítulo 8. Proyecto andalucía enciclopedia de la naturaleza, Tomo 21, pp. 191–228. España: Giralda. [Google Scholar]
62. Bungarttz, F., Elix, J. A., Yánez-Ayabaca, A., Archer, A. W. (2015). Endemism in the pertusaria (Pertusariales, lichenized ascomycota) from de galapagos islands. Telopea Journal of Plant Systematics, 18, 325–369. DOI 10.7751/telopea8895. [Google Scholar] [CrossRef]
63. Cortés-Hernández, V., Gómez-Peralta, M., Hernández-Soberano, C., Ruíz-Contreras, M. (2012). Los líquenes de acueducto de san josé altlán, municipio de huichapan, hidalgo, México. Biológicas, 14(1), 13–17. [Google Scholar]
64. Cardona-Ponce, S. M., Puyy-Alquiza, M. J. (2016). Catálogo de líquenes saxícolas del distrito minero de guanajuato, localidad Cerro de la Bufa. Jóvenes en la Ciencia, 4(1), 541–545. [Google Scholar]
65. Puy-Alquiza, M. J., Gómez-Peralta, M., Reyes-Zamudio, V., Gregorio-Cripiano, M. R., Miranda-Avilés, R. et al. (2018). Diversidad de macroliquenes saxícolas en méxico: Caso de estudio del distrito minero de guanajuato. Acta Botánica Mexicana, 123, 37–50. DOI 10.21829/abm123.2018.1246. [Google Scholar] [CrossRef]
66. Barreno, E., Pérez-Ortega, S. (2003). Líquenes de la reserve natural de integral de muniellos. Cuadernos de Medio Ambiente. Asturias: Consejería de Medio Ambiente, vol. 5, pp. 522. [Google Scholar]
67. Laguna-Defior, C., Pintado, A., Green, T. G. A. J., Blanquer, J. M., Sancho, L. G. (2016). Distrubutional and ecophysiological study on the artarctic lichens species pair Usnea antarctical/Usnea aurantiaco-atra. Polar Biology, 39, 1183–1195. DOI 10.1007/s00300-015-1832-7. [Google Scholar] [CrossRef]
68. Hu, W., Ran, J., Dong, L., Du, Q., Ji, M. et al. (2021). Aridity-driven shift in biodiversity–soil multifunctionality relationships. Nature Communications, 12, 5350. DOI 10.1038/s41467-021-25641-0. [Google Scholar] [CrossRef]
69. Herrera-Campos, M. A., Lücking, R., Pérez-Pérez, R. E., Miranda-González, R., Sánchez, N. et al. (2014). Biodiversidad de líquenes en méxico. Revista Mexicana de Biodiversidad, 85, 82–99. DOI 10.7550/rmb.37003. [Google Scholar] [CrossRef]
70. Concostrina-Zubiri, L. (2012). Composiciòn, estructura y dinámica de la costra biológica del suelo en ambientes áridos (Ph.D. Thesis). Universidad Rey Juan Carlos, España. [Google Scholar]
71. Concostrina-Zubiri, L., Martínez, I., Huber-Sannwald, E., Escudero, A. (2013). Efectos y respuestas de la costra biológica del suelo en ecosistemas áridos: Avances recientes a nivel de especie. Ecosistemas, 22(3), 95–100. DOI 10.7818/ECOS.2013.22-3.13. [Google Scholar] [CrossRef]
72. Pinho, P., Augusto, S., Martins-Loução, M. A., Pereira, M. J., Soares, A. et al. (2008). Causes of change in nitrophytic and oligotrophic lichen species in a Mediterranean climate: Impact of land cover and atmospheric pollutants. Environtal Pollution, 154, 380–389. DOI 10.1016/j.envpol.2007.11.028. [Google Scholar] [CrossRef]
73. Grime, J. P. (2006). Trait convergence and trait divergence in herbaceous plant communities: Mechanisms and consequences. Journal of Vegetation Science, 17, 255–260. [Google Scholar]
74. Moreno, C. E., Barragán, F., Pineda, E., Pavón, N. P. (2011). Reanálisis de la diversidad alpha: Alternativas para interpretar y comparar información sobre comunidades ecológicas. Revista Mexicana de Biodiversidad, 82, 1249–1261. DOI 10.22201/ib.20078706e.2011.4.745. [Google Scholar] [CrossRef]
75. Sayer, E. J., Oliver, A. E., Fridley, J. D., Askew, A. P., Mills, R. T. E. et al. (2017). Links between soil microbial communities and plant traits in a species-rich grassland under long-term climate change. Ecology and Evolution, 7, 855–862. DOI 10.1002/ece3.2700. [Google Scholar] [CrossRef]
76. Manning, P., van der Plas, F., Soliveres, S., Allan, E., Maestre, F. T. et al. (2018). Redefining ecosystem multifunctionality. Nature Ecology and Evolution, 2, 427–436. DOI 10.1038/s41559-017-0461-7. [Google Scholar] [CrossRef]
77. Nimis, P. L., Scheidegger, C., Wolseley, P. A. (2002). Monitoring with lichens-monitoring lichens: An introduction. In: Nimis P. L., Scheidegger, C. and Wolseley, P. A. (Eds.Monitoring with lichens-monitoring lichens. NATO Science Series (Series IV: Earth and Environmental Sciences). Netherlands, Dordrecht: Springer, pp. 1–4. DOI 10.1007/978-94-010-0423-7. [Google Scholar] [CrossRef]
78. Giordani, P., Brunialti, G., Bacaro, G., Nascimbene, J. (2012). Functional traits of epiphytic lichens as potential indicators of environmental conditions in forest ecosystems. Ecological Indicators, 18, 413–420. DOI 10.1016/j.ecolind.2011.12.006. [Google Scholar] [CrossRef]
79. Maestre, F. T., Salguero-Gomez, R., Quero, J. L. (2012). It is getting hotter inhere: Determining and projecting the impacts of global environmental change on drylands. Philosophical Transactions of the Royal Society of London, 367, 3062–3075. DOI 10.1098/rstb.2011.0323. [Google Scholar] [CrossRef]
80. Berdugo, M., Maestre, F. T., Kéfi, S., Gross, N., Le Bagousse-Pinguet, Y. et al. (2019). Aridity preferences alter the relative importance of abiotic and biotic drivers on plant species abundance in global drylands. Journal Ecology, 107, 190–202. DOI 10.1111/1365-2745.13006. [Google Scholar] [CrossRef]
81. Nascimbene, J., Marini, L. (2015). Epiphytic lichen diversity along elevational gradients: Biological traits reveal a complex response to water and energy. Journal of Biogeography, 42, 1222–1232. DOI 10.1111/jbi.12493. [Google Scholar] [CrossRef]
82. Bässler, C., Cadotte, M. W., Beudert, B., Heibl, C., Blaschke, M. et al. (2016). Contrasting patterns of lichen functional diversity and species richness across an elevation gradient. Ecography, 39, 689–698. DOI 10.1111/ecog.01789. [Google Scholar] [CrossRef]
83. Delgado-Baquerizo, M., Maestre, F. T., Reich, P. B., Jeffries, T. C., Gaitan, J. J. et al. (2016). Microbial diversity drives multifunctionality in terrestrial ecosystems. Nature Communications, 28, 10541. DOI 10.1038/ncomms10541. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |