

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.018223
ARTICLE
Effect of Putrescine on Low-Temperature Acclimation in Chlamydomonas reinhardtii
1Molecular Biology and Genetics Department, Faculty of Science and Letter, Bilecik Şeyh Edebali University, Bilecik, 11230, Turkey
2Biotechnology Application and Research Center, Bilecik Şeyh Edebali University, Bilecik, 11230, Turkey
3Biotechnology Department, Art and Sciences Faculty, Niğde Ömer Halisdemir University, Niğde, 51240, Turkey
4Botany Department, Centre for Environmental Studies, Ege University, Izmir, 35100, Turkey
*Corresponding Authors: Dilek Unal. Email: dilek.unal@bilecik.edu.tr; Bengu Turkyilmaz Unal. Email: bturkyilmaz@ohu.edu.tr
Received: 08 July 2021; Accepted: 18 September 2021
Abstract: Putrescine is reported to be necessary for cold acclimation under low-temperature stress. In this study, the effect of low-temperature on some physiological and biochemical parameters has been investigated using the green algae Chlamydomonas reinhardtii. The lipid peroxidation rate, amount of Rubisco protein, activities of antioxidant enzymes and gene expression of polyamine biosynthesis (odc2, and spd1), heat shock proteins (hsp70c, hsp90a, and hsp90c), and PSII repair mechanisms (psba, rep27, and tba1) were determined to understand the low-temperature response. Exogenous putrescine application significantly increased Rubisco protein concentration and catalase enzyme activities under low-temperature stress. Moreover, real-time RT-PCR results and gene expression analysis showed that polyamine metabolism induced gene expression at low-temperatures in the first 24 h. In the same way, the gene expression of heat shock proteins (hsp70c, hsp90a, and hsp90c) decreased under low-temperature treatment for 72 h; however, application of putrescine enhanced the gene expression in the first 24 h. The results obtained indicated that molecular response in the first 24 h could be important for cold acclimation. The psba and tba1 expressions were reduced under low-temperatures depending on the exposure time. In contrast, the exogenous putrescine enhanced the expression level of the psba response to low-temperature at 24 and 72 h. The results obtained in this study indicate that putrescine could play a role in the PS II repair mechanisms under low-temperature stress.
Keywords: Chlamydomonas reinhardtii; cold acclimation; heat shock protein; low-temperature stress; PS II repair; putrescine
| Nomenclature | |
| ADC: | Arginine decarboxylase |
| APX: | Ascorbate peroxidase |
| CAT: | Catalase |
| GR: | Glutathione reductase |
| HSP: | Heat shock protein |
| MDA: | Malondialdehyde |
| ODC: | Ornithine decarboxylase |
| PSII: | Photosystem II |
| ROS: | Reactive oxygen species |
| RuBisCO: | Ribulose-1, 5-bisphosphate carboxylase/oxygenase |
| UV: | Ultra violet |
The polyamines like putrescine, spermidine, and spermine are biogenic amines with multiple in vivo effects at the cellular level in different organisms [1,2]. The key enzyme in polyamine metabolism is ornithine decarboxylase (ODC) which catalyzes putrescine biosynthesis via ornithine’s decarboxylation. In higher plants, putrescine is mainly synthesized via arginine decarboxylation by catalyzing the arginine decarboxylase enzyme (ADC), encoded by the adc1 and adc2 genes [3,4]. However, in C. reinhardtii, putrescine formation is generally controlled by the ODC activity, encoded by the odc2 gene [5,6]. Moreover, the putrescine is converted into spermidine by spermidine synthase (EC 2.5.1.16) encoded by the spd1 gene in C. reinhardtii [5]. The polyamines and their genes are significant in protecting photosynthetic organisms from abiotic stresses, such as low temperature, salinity, drought, osmotic shock, and heavy metals [7–9]. Earlier studies have also indicated that putrescine primarily responds to low-temperature acclimation in higher plants [3].
PS II includes two main core proteins: D1 and D2. The D1 protein of thylakoid membranes is the most sensitive and regulates the synthesis/degradation of the D1 protein and psbA, encoding of the D1 protein, gene expression during the dark to light shifts, and under varied stress conditions [10–12]. Previous studies have demonstrated that D1 protein synthesis is regulated at the translational level RNA binding protein complex. It consists of four major proteins: a chloroplast poly(A) binding (cPAB1), a protein disulfide isomerase (cPDI), a novel 38 kDa RNA binding protein, and a 55 kDa protein [13–15]. The chloroplastic redox potential controls the psbA mRNA translation initiation via reducing the cPAB1 protein by cPDI. Somanchi et al. [16] demonstrated that tba1 is a soluble chloroplastic oxidoreductase-like protein, and its expression is required for cPAB1 RNA binding activity and D1 translation. In addition, a nucleus-encoded protein, REP27, is necessary for protein turnover of the D1 reaction center, thus allowing finishing of the translation process, maturation, and activation of D1 into a functional PSII reaction center holo complex in C. reinhardtii [17,18].
In photosynthetic organisms, stress tolerance is also related to an imbalance in the Photosystem II (PSII) repair process. The production of reactive oxygen species (ROS) can directly disrupt the chloroplast structure and has been an important limiting factor in repairing photodamaged PSII under environmental stress conditions [7]. The balance between photodamaged PSII and its repaired form is related to ROS accumulation [19,20]. Earlier studies have shown that low temperature [21,22], metal [9], salt [23], and oxidative stresses [24] prevent photodamaged PSII repair by hindering psbA gene transcription and translation. However, there is little data on the effect of environmental stress on tba1 and rep27 expression.
Polyamine metabolisms are also important in photosynthetic regulation under different environmental factors. Putrescine is the key element for higher plants as a cold acclimation factor [3,25,26]. Its action in cold acclimation is not completely known. In the present study, we have tried to understand the role of putrescine on the transcription of photosynthetic genes involved in PSII repair mechanisms using the model organism C. reinhardtii.
The objectives of our investigations were as follows: (i) to define the effects of exogenously treated putrescine transcription level of polyamine biosynthesis genes, some of the PSII repair mechanisms genes (psbA, rep27, and tba1), and heat shock protein (HSP) genes using Quantitative RT-PCR under low-temperature stress; (ii) to define physiological responses to low-temperature in C. reinhardtii by analyzing the growth rate, lipid peroxidation, rubisco protein amount, and antioxidant enzymes activity in presence and absence of putrescine.
2.1 Materials and Culture Conditions
C. reinhardtii (CC1010) was obtained from the Chlamydomonas Research Center culture collection. Five Erlenmeyer flasks (250 mL) of C. reinhardtii were used during the trials. The culture was grown in TAP medium at 24°C, under 120 µmol m−2 s−1 light intensity, and continuous light.
All experiments were carried out in 3 replicates. The chemicals used in the trials were obtained from Sigma-Aldrich and Merck.
The C. reinhardtii culture was pre-incubated at 24°C in the presence of 100 μM putrescine for 3 h before starting the low-temperature experiment. Untreated and putrescine-treated cells were incubated in a growth chamber at 10°C under continuous 120 µmol m−2s−1 light intensity for 24, 48, and 72 h. The cells not treated with low temperature and putrescine were used as control. The absorbance of cell growth was measured on a UV-spectrophotometer at 750 nm, and cell number was counted using a Neubauer hemocytometer.
2.2.2 Lipid Peroxidation Analysis
The lipid peroxidation rate was determined following the malondialdehyde (MDA) content (TBARS) method [27]. The amount of MDA formed was calculated following the absorption difference between 532 and 600 nm.
2.2.3 Rubisco Protein Concentration Analysis
The Plant RuBisCO ELISA Kit was used to determine the amount of ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO) protein (Cat No. CK-E91378). Approximately 30 mg of cells were rinsed with 0.05 M PBS (containing 2% PVPP and 1 mM EDTA), homogenized with 1 mL of 0.05 MPBS and stored in a freezer (−20°C) overnight. After two freeze-thaw cycles, homogenates were centrifuged at 5000× g for 10 min to break the cell membranes. 50 μL Standard or Sample was added to the other wells. After 60 min of incubation at 37°C, each well was washed by aspirating. The procedure was repeated three times. The wells were filled with Wash Buffer (100 μL) and left for 2 min, and the liquid was completely removed. After this, 50 μL of HRP-conjugate working solution was added and mixed well with a pipette. Incubation was done at 37°C for 60 min. The plate was again washed 5 times, and 50 μL of chromogen solution A and B were added to each well. Incubation was carried out for 20 min at 37°C. When the last four wells with the lowest standard concentrations turned into a distinct blue color, 50 μL Stop Solution was added to each well. The optical density of each well was determined for 10 min at 450 nm using a microplate reader.
2.2.4 Antioxidant Enzymes Activity Analysis
C. reinhardtii cultures of each experimental group were centrifuged to 20 mg and then ground using liquid nitrogen. 50 mM sodium phosphate buffer (pH 7.8) was added to each experimental group’s samples and homogenized with 12.000 g for 15 min, followed by centrifuging at 4°C for 15 min. The enzyme activity and total protein content were determined in the obtained supernatants [28]. Specific enzyme activities were determined and recorded as unit mg−1 protein.
The catalase activity (E.C.1.11.1.6) was determined in the mixture of the extract using 100 mM phosphate buffer and 6 mM H2O2. The decomposition of H2O2 was monitored at 240 nm. 1 unit CAT activity was defined as 1 µmol H2O2 destroyed per minute [29]. Each treatment comprised three replicates.
The activity of APX (E.C. 1.11.1.11) was determined by observing the reduction in A 290 using an extinction factor of 2.8 mM cm−1 [30].
Glutathione Reductase (GR) activity was detected using the GR Assay Kit protocol (Cayman Chemical Company, USA, Cat No. 703202).
RNA isolation was done using TRIZOL reagent. 50 mg samples were triturated with liquid nitrogen. 1 mL of TRIZOL reagent was added to the ground samples, transferred to the Eppendorf tube, and homogenized. 0.2 mL of chloroform was added to each Eppendorf tube and vortexed vigorously for 15 seconds. Incubated samples were centrifuged at +4°C at 12.000× g for 15 min at room temperature for 3 min. After washing the RNA pellet once with 75% ethanol, 1 mL of 75% ethanol was added per 1 mL of TRIZOL used for initial homogenization. Samples were centrifuged at 7.500× g for 5 min at 8°C after vortexing. The washing procedure was repeated once. The A260/A280 ratio of partially dissolved RNA samples was <1.8. Reverse Transcriptase-PCR and a High Capacity cDNA Reverse Transcription Kit were used as Invitrogen, Cat No. 4398814.
Primer sequences for each gene under real-time PCR are shown in Table 1. Real-Time RT-PCR was performed using a PowerUpTM SYBR® Green Master Mix. The 18S rRNA was used as a housekeeping gene to normalize expression changes. All real-time PCRs (Applied Biosystems USA) were performed under the following conditions: 2 min at 50°C, 2 min at 95°C and 15 s at 95°C for 35 cycles, and 60 in 96-well MicroAmp® Fast Optical reaction plates. In this analysis, three technical replicates were analyzed using two biological replicates for each sample. The amplification efficiency of Real-Time PCR was analyzed according to Livak and Schmittgen [31]. The gene expression relative quantification among the groups was analyzed by 2−ΔΔCt method [31], where Ct is the cycle number at which the fluorescent signal rised statistically above the background.

Tukey test [32] (SPSS for Windows version 11.0) was used for statistical analysis. The critical value for significance was p < 0.05 or p < 0.01.
The psbA gene expression levels increased 5.24 and 2.13-fold under low temperature during 24 and 48 h, respectively (Fig. 1A). However, the psbA expression decreased by 2.23-fold after 72 h treatment. The putrescine application showed a positive effect on the psbA gene expression by an increase of 2.65-and 1.5-fold after 24 and 72 h, respectively.
The tba1, which plays a role in regulating the RNA binding activity of cPAB1, was also induced in response to low-temperature stress. Fig. 1B depicts the gene expression profiles of tba1 in non-putrescine and putrescine-treatment cells under low-temperature stress. Compared with the control, tba1 gene expression increased by 2.0-and 2.09-fold after 24 and 48 h, respectively. These findings confirm the psbA gene expression results. The putrescine application showed a simulative effect on the tba1 gene expression and increased 2.71-fold within 24 h.
The rep27 gene expression level, a nucleus-encoded protein gene, which is the likely orthologue of LPA1 in the green alga C. reinhardtii, did not enhance significantly following the low-temperature application neither after 24 nor 48 h (Fig. 1C). In contrast, the gene expression level of rep27 significantly decreased after 72 h. In the putrescine-treatment culture under low-temperature stress, gene expression of rep27 increased 2.44-and 2.21-fold after 24 and 48 h, respectively, in comparison to the control (Fig. 1C).

Figure 1: The effects of exogenous putrescine on some genes of the PSII repair mechanisms: (A) psbA gene expression, (B) tba1 gene expression, and (C) rep27 gene expression in Chlamydomonas reinhardtii cultures under low-temperature stress compared with the control. a: control, b: 24 h, c: 48 h, d: 72 h, e: put-24 h, f: put-48 h, g: put-72 h
Under normal conditions, growth rate and the increase in cell numbers during 48 and 72 h were lower than previously (Fig. 2). Growth rates and maximum cell densities of algae showed a significant decrease at low temperature (p < 0.05) compared to the control groups after 72 h. Exogenously applied putrescine significantly enhanced cell growth under low temperature (Fig. 2).

Figure 2: The effects of putrescine on the growth rate of C. reinhardtii under low temperature compared with normal conditions
The low temperature significantly increased the MDA content of C. reinhardtii after 24, 48 and 72 h (P < 0.05) (Table 2). An increase in MDA content indicates cell membrane injury during low-temperature stress. However, the difference in MDA production levels between the untreated and putrescine treated samples was significant (p < 0.01). The sample treated exogenously with putrescine had a lower MDA concentration than the other groups. Our findings indicated that the exogenous putrescine addition avoided higher lipid peroxidation under low temperature.

The odc2 was up-regulated in the treatment with low temperature after 24 (0.91-fold) and 48 h (0.73-fold) (Fig. 3A), analyzed by real-time RT-PCR. Treatment with low temperature for 72 h resulted in a lower odc2 gene expression level (2.76-fold). In the putrescine added groups, the odc2 gene expression level increased 3.15-fold within the first 24 h; however, odc2 gene expression decreased 1.14-and 0.3-fold after 48 and 72 h, respectively. The spd1 gene expression decreased 4.2-fold at low temperature after 72 h (Fig. 3B); however, treatment with exogenous putrescine at low temperature enhanced the spd1 gene expression after 24 h (Fig. 3B).

Figure 3: The effects of exogenous putrescine on two main polyamine biosynthesis genes in C. reinhardtii culture under low-temperature stress, compared with the control. (A) odc2 gene expression. (B) spd1 gene expression. a: control, b: 24 h, c: 48 h, d: 72 h, e: put-24 h, f: put-48 h, g: put-72 h
The concentration of rubisco protein reduced gradually in the samples treated for different times (24, 48, and 72 h) at low temperatures (Table 3). The concentration of rubisco protein was approximately 2 times lower than the control in C. reinhardtii under low temperature after 72 h. Exogenous putrescine application significantly improved the Rubisco protein concentration under low-temperature stress (p < 0.05). Additionally, in samples treated with exogenous putrescine, the amount of rubisco protein was approximately 1.5 times higher than the control after 72 h.

The GR activity gradually reduced under low-temperature stress (Fig. 4A). However, the application of putrescine only significantly enhanced the GR activity under low temperature after 48 h. The CAT activity significantly increased under low temperature in C. reinhardtii culture within 24 h (p < 0.05). However, this activity decreased by 1.65-fold after 72 h compared with the control (Fig. 4B). Putrescine application enhanced CAT activity by 2.04-, 1.59-and 1.56-fold at low temperature after 24, 48, and 72 h respectively. The APX activity did not change significantly compared with the control after 24 h (Fig. 4C). This activity significantly increased by 1.35-fold after 48 h but decreased by 1.25-fold after 72 h, compared with the control. Application of putrescine enhanced APX activity under low temperature only after 24 h.

Figure 4: Effects of exogenous putrescine on antioxidant enzyme activity [(A) GR activity, (B) CAT activity, (C) APX activity] in C. reinhardtii culture under low-temperature stress. *Represents a statistically significant difference at p < 0.05 or ** p < 0.01 when compared with the control
The hsp70c gene expression level increased 2.12 fold after 24 h and decreased 2.76 fold after 72 h, respectively, compared to control (Fig. 5A). An application of putrescine in the C. reinhardtii culture under low temperature lead to an enhancement of 2.88-fold in the first 24 h. The hsp90a gene expression level increased 2-and 2.09-fold after 24 and 48 h (Fig. 5B), while the hsp90c gene expression did not increase significantly. In the putrescine application, the hsp90c gene expression level was enhanced by 3.27 fold in the first 24 h (Fig. 5C) but did not significantly increase the hsp90a gene expression level (Fig. 5B).

Figure 5: The effects of exogenous putrescine on some heat shock protein genes. (A) hsp70c gene expression, (B) hsp90a gene expression, and (C) hsp90c gene expression in C. reinhardtii culture under low-temperature stress, compared with the control. a: control, b: 24 h, c: 48 h, d: 72 h, e: put-24 h, f: put-48 h, g: put-72 h
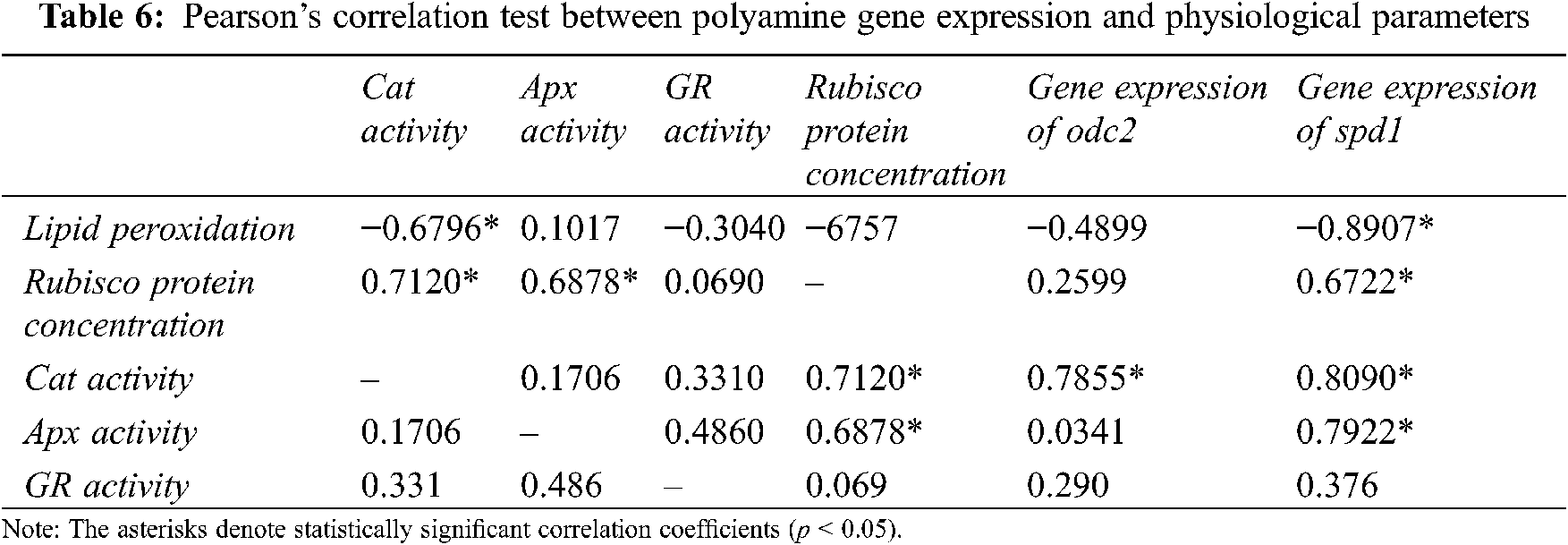
Pearson’s correlation analysis showed that polyamine gene expression was related to the psbA and tba1 gene expression levels. The odc2 gene expression level was strongly positively correlated with psbA and tba1 genes (Table 4). In contrast, the spd1 gene expression level showed a weak correlation with the gene expressions of psbA and tba1. The rep27 gene expression was strongly correlated with the odc2 and spd1 gene expression levels (Table 4). The hsp70c, hsp90a, and hsp90c have revealed a strong positive correlation with psbA, rep27, and tba1 gene expression (Table 4). The lipid peroxidation rate had a strong negative correlation with the rep27 gene expression (Table 5). The PSII repair mechanism gene expression was strongly correlated with CAT activity (Table 5). The lipid peroxidation level was negatively correlated with the Rubisco protein concentration, the spd1 gene expression, and catalase activity (Table 6). The gene expression of heat shock proteins also showed a strong positive correlation with CAT activity (Table 7).



The rate of replacement of the damaged D1 protein, which is involved in the turnover-repair cycle, could play an important role in the tolerance of photodamage under low temperature. psbA probably plays an important role in sustaining stable photosynthesis capability by maintaining PSII from oxidative damage under stress conditions. Sippola et al. [33] reported that psbA transcript accumulation shows a dramatic increase under lower temperature stress in Synechococcus sp. PCC7942. It has also been stated that in tomato WHIRLY1 (SlWHY1) up-regulates psbA transcription under cold stress [34,35]. In the present study, the psbA mRNA transcript increased during the first 24 and 48 h; however,the gene expression level sharply decreased after 72 h (Fig. 1A). Reactive oxygen species are an important limiting factor for the PSII repair process. Previous studies demonstrated that oxidative stress inhibits the repair of photodamaged PSII by limiting the transcription and translation of psbA genes [24]. Tba1 expression is necessary for translational initiation of psbA mRNA [16]. Some of the earlier studies also showed that the REP27 protein plays a critical role in psbA mRNA translation, the insertion of newly synthesized D1 protein into PSII template in place of the damaged D1 protein, and an activation of the newly assembled reaction center complex [17,18,36]. Although the role of tba1 and rep27 have been demonstrated in PSII repair mechanisms, the alteration of their transcript level under environmental stress conditions is not completely understood. In the present study, low-temperature stress led to an increase in the expression level of the tba1 gene after 24 and 48 h (Fig. 1B), while rep27 gene expression was not induced by low temperature (Fig. 1C). However, the tba1 and rep27 expressions decreased sharply after 72 h. Our lipid peroxidation and growth results indicate that low temperature stress has negative effects after 72 h and decreases the tolerance capacity in C. reinhardtii cultures (Table 2 and Fig. 2). Thus, the tba1 and rep27 expression could be reduced by increasing oxidative stress, by preventing the PSII repair mechanism under low temperature stress.
Many studies showed that exogenous treatment of polyamines, especially spd and spm, retard the loss of D1 and D2 under various environmental stresses and attenuate the reduction in PSII major protein transcripts [7,9,37,38]. Hamdani et al. [37] suggested that polyamines play a role in maintaining D1 and D2 proteins of PSII under stress conditions by delaying their apparent degradation, which may occur. Our earlier studies also confirmed that the psbA expression was reduced under aluminum stress but it was raised by spermidine treatment [9]. Hu et al. [7] also showed that spermidine enhances psbA gene expression under salinity-alkalinity stress. Similarly, exogenously applied putrescine enhanced the expression level of psbA and tba1 in response to low temperature within 24 h (Figs. 1A and 1B). Exogenous putrescine also enhanced odc2 and spd1 gene expression within 24 h under low-temperature conditions (Figs. 3A and 3B).
The odc2 gene expression was strongly correlated with psbA and tba1 gene expression, although spd1 gene expression showed a weak correlation (Table 4). On the other hand, the rep27 gene expression was strongly correlated with the spd1 and odc2 gene expression, indicating that ODC pathways have some partial role in the PSII repair mechanisms. These compounds could (1) behave as direct radical scavengers, (2) be conjugated to antioxidant molecules or (3) bind to antioxidant enzymes, and then permeate to the oxidative stress site [39]. Moreover, Byrd et al. [40] reported that putrescine might play a role in reducing oxidative stress under cold stress. Also, exogenous putrescine administration improved oxidative stress in cold-stored okra by eliminating ROS [41]. In the present study, we also found that putrescine can ameliorate lipid peroxidation rate under low-temperature stress (Table 2). Although the role of putrescine in the PSII repair mechanism is still unclear, our results indicate that exogenously applied putrescine might help in the psbA translation via reducing oxidative stress.
Another important mechanism for low-temperature acclimation is the HSP action in plant cells [42,43]. According to Krishna et al. [42], the accumulation of hsp90 mRNA increases in response to cold temperature. Zhang et al. [43] found that Hsp70 has a role in plants during low-temperature acclimation. Zou et al. [44] suggested that only some HSP family members respond to cold stress. In the present study, transcription of hsp70c and hsp90a was induced under low-temperature conditions within 24 h (Figs. 5A and 5B). However, the transcript level of hsp90c genes was little affected by low temperature, although hsp90c was significantly reduced after 72 h (Fig. 5C). This indicates that hsp70c and hsp90a could play a role in stress response to low temperature; extending the low-temperature period can reduce the transcript level of other HSP proteins in C. reinhardtii cultures. Previous studies showed that HSPs have a role in protection against photoinhibition and PSII repair mechanisms [45–47]. Schroda et al. [45] demonstrated that a chloroplast-targeted Hsp70b might contribute to the process of PSII repair during photoinhibition. Our results also indicated that the HSP gene transcription level was correlated with translation factors of PSII repair mechanism genes (Table 4). In this investigation, we found that there was also the transcriptional level of HSP proteins due to the putrescine effects during cold stress in C. reinhardtii cultures. Königshofer et al. [48] reported that HSP synthesis under heat stress could be affected by the cell polyamine metabolic status in cell suspension cultures of tobacco and alfalfa. Sagor et al. [49] also reported that spermine enhanced gene expression of four heat shock proteins (hsp101, hsp90, hsp70, and hsp16.7). Our results indicated that external putrescine increased the level of expression of HSP-encoding genes.
Low-temperature stress affects the photosynthetic process involving the carbon reduction cycle [40,50]. According to Zhou et al. [51], low temperature significantly decreases the rubisco content in higher plants. In our studies on C. reinhardtii (Table 2), reaction to low temperature related to changes in the amount of Rubisco protein. A similar result was reported by Zhou et al. [51]. Earlier studies proved that exogenously applied polyamines prevent Rubisco reduction under osmotic stress in higher plants [50,52,53]. Sagor et al. [49] showed that inhibition of s-adenosyl methionine decarboxylase activity promotes a decreased Rubisco activation state under chilling stress. Our data strongly indicated that exogenous putrescine enhanced the amount of Rubisco protein in C. reinhardtii cells under low temperature (Table 3).
The cellular antioxidant mechanisms of enzymatic and non-enzymatic nature play important roles in cold acclimation [54]. Antioxidant enzymes give protection against oxidative damage caused by cold stress. The catalase plays an important role in chilling tolerance in maize [55] and is particularly significant for hydrogen peroxide (H2O2) removal in C3 plants [56]. Gechev et al. [57] indicated that chilling reduces CAT activity in Nicotiana tabacum. In our study, low-temperature conditions led to important changes in CAT activity (Fig. 4B). Earlier findings have shown a good correlation between reducing catalase activity and H2O2 accumulation [55,58]. Similarly, increased lipid peroxidation rate led to an increase in oxidative stress and a decrease in the CAT enzyme activity in C. reinhardtii under low temperature (Fig. 4B). Some previous studies also show that polyamines could moderately reduce ROS formation by inhibiting the NADPH oxidase activity (EC 1.6.3.1) with spermine, spermidine, and putrescine [59–61]. According to Shu et al. [62], the exogenous spermine significantly increases the GR, superoxide dismutase, ascorbate peroxidase, and peroxidase activities. Verma et al. [63] noticed that putrescine affects various antioxidant enzyme activities, including CAT. In the present investigation, exogenous putrescine enhanced CAT and GR activities, especially CAT activity, which was strongly correlated with the odc2 gene expression (Fig. 3A and Table 6).
In conclusion, our results demonstrated that exogenous putrescine provided cold acclimation by decreasing lipid peroxidation and increasing catalase and Rubisco protein concentration in C. reinhardtii cultures. Our observations also suggest that putrescine may provide stability to the PS II repair processes, and act as a signal molecule possibly interacting with heat shock proteins. In addition, our results revealed that putrescine and PSII repair mechanisms interact with the low-temperature stress response. Elucidation of how the effect on PS II repair mechanisms is achieved will certainly require future studies.
Acknowledgement: The corresponding author would like to thank her university authorities for support, and collaborating authors from other university.
Funding Statement: This study was partially supported by the Bilecik Seyh Edebali University Research Foundation (2014-02-BIL-04-03).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Bouchereau, A., Aziz, A., Larher, F., Martin-Tanguy, J. (1999). Polyamines and environmental challenges: Recent development. Plant Science, 140(2), 103–125. DOI 10.1016/S0168-9452(98)00218-0. [Google Scholar] [CrossRef]
2. Handa, A. K., Fatima, T., Mattoo, A. K. (2018). Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Frontiers in Chemistry, 6, 10. DOI 10.3389/fchem.2018.00010. [Google Scholar] [CrossRef]
3. Alcázar, R., Cuevas, J. C., Planas, J., Zarza, X., Bortolotti, C. et al. (2011). Integration of polyamines in the cold acclimation response. Plant Science, 180(1), 31–38. DOI 10.1016/j.plantsci.2010.07.022. [Google Scholar] [CrossRef]
4. Jiao, C., Lan, G., Sun, Y., Wang, G., Sun, Y. (2020). Dopamine alleviates chilling stress in watermelon seedlings via modulation of proline content, antioxidant enzyme activity, and polyamine metabolism. Journal of Plant Growth Regulation, 40(1), 277–292. DOI 10.1007/s00344-020-10096-2. [Google Scholar] [CrossRef]
5. González-Ballester, D., Casero, D., Cokus, S., Pellegrini, M., Merchant, S. S. et al. (2010). RNA-seq analysis of sulfur-deprived Chlamydomonas cells reveals aspects of acclimation critical for cell survival. The Plant Cell, 22(6), 2058–2084. DOI 10.1105/tpc.109.071167. [Google Scholar] [CrossRef]
6. Tassoni, A., Awad, N., Griffiths, G. (2018). Effect of ornithine decarboxylase and norspermidine in modulating cell division in the green alga Chlamydomonas reinhardtii. Plant Physiology and Biochemistry, 123, 125–131. DOI 10.1016/j.plaphy.2017.12.014. [Google Scholar] [CrossRef]
7. Hu, L., Xiang, L., Li, S., Zou, Z., Hu, X. H. (2016). Beneficial role of spermidine in chlorophyll metabolism and D1 protein content in tomato seedlings under salinity-alkalinity stress. Physiologia Plantarum, 156(4), 468–477. DOI 10.1111/ppl.12398. [Google Scholar] [CrossRef]
8. Masson, P. H., Takahashi, T., Angelini, R. (2017). Molecular mechanisms underlying polyamine functions in plants. Frontiers in Plant Science, 8, 14. DOI 10.3389/fpls.2017.00014. [Google Scholar] [CrossRef]
9. Sen, G., Eryilmaz, I. E., Ozakca, D. (2014). The effect of aluminium-stress and exogenous spermidine on chlorophyll degradation, glutathione reductase activity and the photosystem II D1 protein gene (psbA) transcript level in lichen Xanthoria parietina. Phytochemistry, 98(12), 54–59. DOI 10.1016/j.phytochem.2013.11.021. [Google Scholar] [CrossRef]
10. Giardi, M. T., Masojídek, J., Godde, D. (1997). Effects of abiotic stresses on the turnover of the D1 reaction centre II protein. Physiologia Plantarum, 101(3), 635–642. DOI 10.1111/j.1399-3054.1997.tb01048.x. [Google Scholar] [CrossRef]
11. Marín-Navarro, J., Manuell, A. L., Wu, J., Mayfield, S. P. (2007). Chloroplast translation regulation. Photosynthesis Research, 94(2), 359–374. DOI 10.1007/s11120-007-9183-z. [Google Scholar] [CrossRef]
12. Kizilkaya, T. I., Akcaalan, S., Unal, D. (2021). Determination of photosynthesis-related and ascorbate peroxidase gene expression in the green algae (Chlorella vulgaris) under high-temperature conditions. International Journal of Secondary Metabolite, 8(1), 59–69. DOI 10.21448/ijsm.794617. [Google Scholar] [CrossRef]
13. Barnes, D., Cohen, A., Bruick, R. K., Kantardjieff, K., Fowler, S. et al. (2004). Identification and characterization of a novel RNA binding protein That associates with the 5’-untranslated region of the chloroplast psbA mRNA. Biochemistry, 43(26), 8541–8550. DOI 10.1021/bi035909j. [Google Scholar] [CrossRef]
14. Kim, J., Mayfield, S. P. (1997). Protein disulfide isomerase as a regulator of chloroplast translational activation. Science, 278(5345), 1954–1957. DOI 10.1126/science.278.5345.1954. [Google Scholar] [CrossRef]
15. Yohn, C. B., Cohen, A., Danon, A., Mayfield, S. P. (1998). A poly (A) binding protein functions in the chloroplast as a message-specific translation factor. Proceedings of the National Academy of Sciences of the United States of America, 95(5), 2238–2243. DOI 10.1073/pnas.95.5.2238. [Google Scholar] [CrossRef]
16. Somanchi, A., Barnes, D., Mayfield, S. P. (2005). A nuclear gene of Chlamydomonas reinhardtii, Tba1, encodes a putative oxidoreductase required for translation of the chloroplast psbA mRNA. The Plant Journal, 42(3), 341–352. DOI 10.1111/j.1365-313X.2005.02378.x. [Google Scholar] [CrossRef]
17. Dewez, D., Park, S., García-Cerdán, J. G., Lindberg, P., Melis, A. (2009). Mechanism of REP27 protein action in the D1 protein turnover and photosystem II repair from photodamage. Plant Physiology, 151(1), 88–99. DOI 10.1104/pp.109.140798. [Google Scholar] [CrossRef]
18. Park, S., Khamai, P., Garcia-Cerdan, J. G., Melis, A. (2007). REP27, a tetratricopeptide repeat nuclear-encoded and chloroplast-localized protein, functions in D1/32-kD reaction center protein turnover and photosystem II repair from photodamage. Plant Physiology, 143(4), 1547–1560. DOI 10.1104/pp.107.096396. [Google Scholar] [CrossRef]
19. Nishiyama, Y., Allakhverdiev, S. I., Murata, N. (2011). Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiologia Plantarum, 142(1), 35–46. DOI 10.1111/j.1399-3054.2011.01457.x. [Google Scholar] [CrossRef]
20. Fagerlund, R. D., Forsman, J. A., Biswas, S., Vass, I., Davies, F. K. et al. (2020). Stabilization of photosystem II by the PsbT protein impacts photodamage, repair and biogenesis. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1861(10), 148234. DOI 10.1016/j.bbabio.2020.148234. [Google Scholar] [CrossRef]
21. Murata, N., Takahashi, S., Nishiyama, Y., Allakhverdiev, S. I. (2007). Photoinhibition of photosystem II under environmental stress. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1767(6), 414–421. DOI 10.1016/j.bbabio.2006.11.019. [Google Scholar] [CrossRef]
22. Chi, Y. X., Yang, L., Zhao, C. J., Muhammad, I., Zhou, X. B. et al. (2021). Effects of soaking seeds in exogenous vitamins on active oxygen metabolism and seedling growth under low-temperature stress. Saudi Journal of Biological Sciences, 28(6), 3254–3261. DOI 10.1016/j.sjbs.2021.02.065. [Google Scholar] [CrossRef]
23. Allakhverdiev, S. I., Nishiyama, Y., Miyairi, S., Yamamoto, H., Inagaki, N. et al. (2002). Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbAGenes in Synechocystis. Plant Physiology, 130(3), 1443–1453. DOI 10.1104/pp.011114. [Google Scholar] [CrossRef]
24. Nishiyama, Y., Allakhverdiev, S. I., Yamamoto, H., Hayashi, H., Murata, N. (2004). Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry, 43(35), 11321–11330. DOI 10.1021/bi036178q. [Google Scholar] [CrossRef]
25. Cuevas, J. C., López-Cobollo, R., Alcázar, R., Zarza, X., Koncz, C. et al. (2008). Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiology, 148(2), 1094–1105. DOI 10.1104/pp.108.122945. [Google Scholar] [CrossRef]
26. Yin, X., Yang, Y., Lv, Y., Li, Y., Yang, D. et al. (2020). BrrICE1. 1 is associated with putrescine synthesis through regulation of the arginine decarboxylase gene in freezing tolerance of turnip (Brassica rapa var. rapa). BMC Plant Biology, 20(1), 504. DOI 10.1186/s12870-020-02697-6. [Google Scholar] [CrossRef]
27. Heath, R. L., Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198. DOI 10.1016/0003-9861(68)90654-1. [Google Scholar] [CrossRef]
28. Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
29. Bergmeyer, N. (1970). Methoden der Enzymatischen Analyse, vol. 1, pp. 636–647. Berlin: Akademie Verlag. [Google Scholar]
30. Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22(5), 867–880. DOI 10.1093/oxfordjournals.pcp.a076232. [Google Scholar] [CrossRef]
31. Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4), 402–408. DOI 10.1006/meth.2001.1262. [Google Scholar] [CrossRef]
32. Tukey, J. W. (1954). Some selected quick and easy methods of statistical analysis. Transactions of the New York Academy of Sciences, 16, 88–97. [Google Scholar]
33. Sippola, K., Kanervo, E., Murata, N., Aro, E. M. (1998). A genetically engineered increase in fatty acid unsaturation in Synechococcus sp. PCC, 7942 allows exchange of D1 protein forms and sustenance of photosystem II activity at low temperature. European Journal of Biochemistry, 251(3), 641–648. DOI 10.1046/j.1432-1327.1998.2510641.x. [Google Scholar] [CrossRef]
34. Zhuang, K., Kong, F., Zhang, S., Meng, C., Yang, M. et al. (2019). Whirly1 enhances tolerance to chilling stress in tomato via protection of photosystem II and regulation of starch degradation. New Phytologist, 221(4), 1998–2012. DOI 10.1111/nph.15532. [Google Scholar] [CrossRef]
35. Zhuang, K., Wang, J., Jiao, B., Chen, C., Zhang, J. et al. (2020). WHIRLY1 maintains leaf photosynthetic capacity in tomato by regulating the expression of RbcS1 under chilling stress. Journal of Experimental Botany, 71(12), 3653–3663. DOI 10.1093/jxb/eraa145. [Google Scholar] [CrossRef]
36. Metis, A. (2019). Dunaliella salina. The Alga Dunaliella Biodiversity, Physiology. In: Genomics and Biotechnology, pp. 273. USA: CRC Press. [Google Scholar]
37. Hamdani, S., Gauthier, A., Msilini, N., Carpentier, R. (2011). Positive charges of polyamines protect PSII in isolated thylakoid membranes during photoinhibitory conditions. Plant and Cell Physiology, 52(5), 866–873. DOI 10.1093/pcp/pcr040. [Google Scholar] [CrossRef]
38. Li, L., Gu, W., Li, J., Li, C., Xie, T. et al. (2018). Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiology and Biochemistry, 129(196), 35–55. DOI 10.1016/j.plaphy.2018.05.017. [Google Scholar] [CrossRef]
39. Drolet, G., Dumbroff, E., Legge, R., Thompson, J. (1986). Radical scavenging properties of polyamines. Phytochemistry, 25(2), 367–371. DOI 10.1016/S0031-9422(00)85482-5. [Google Scholar] [CrossRef]
40. Byrd, G. T., Ort, D. R., Ogren, W. L. (1995). The effects of chilling in the light on ribulose-1, 5-bisphosphate carboxylase/oxygenase activation in tomato (Lycopersicon esculentum Mill.). Plant Physiology, 107(2), 585–591. DOI 10.1104/pp.107.2.585. [Google Scholar] [CrossRef]
41. Gupta, K., Dey, A., Gupta, B. (2013). Plant polyamines in abiotic stress responses. Acta Physiologiae Plantarum, 35(7), 2015–2036. DOI 10.1007/s11738-013-1239-4. [Google Scholar] [CrossRef]
42. Krishna, P., Sacco, M., Cherutti, J. F., Hill, S. (1995). Cold-induced accumulation of hsp90 transcripts in Brassica napus. Plant Physiology, 107(3), 915–923. DOI 10.1104/pp.107.3.915. [Google Scholar] [CrossRef]
43. Zhang, C., Guy, C. (2006). In vitro evidence of Hsc70 functioning as a molecular chaperone during cold stress. Plant Physiology and Biochemistry, 44(11–12), 844–850. DOI 10.1016/j.plaphy.2006.09.012. [Google Scholar] [CrossRef]
44. Zou, J., Liu, A., Chen, X., Zhou, X., Gao, G. et al. (2009). Expression analysis of nine rice heat shock protein genes under abiotic stresses and ABA treatment. Journal of Plant Physiology, 166(8), 851–861. DOI 10.1016/j.jplph.2008.11.007. [Google Scholar] [CrossRef]
45. Schroda, M., Vallon, O., Wollman, F. A., Beck, C. F. (1999). A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. The Plant Cell, 11(6), 1165–1178. DOI 10.1105/tpc.11.6.1165. [Google Scholar] [CrossRef]
46. Schuster, G., Even, D., Kloppstech, K., Ohad, I. (1988). Evidence for protection by heat-shock proteins against photoinhibition during heat-shock. The EMBO Journal, 7(1), 1–6. DOI 10.1002/j.1460-2075.1988.tb02776.x. [Google Scholar] [CrossRef]
47. Hu, S., Ding, Y., Zhu, C. (2020). Sensitivity and responses of chloroplasts to heat stress in plants. Frontiers in Plant Science, 11, 375. DOI 10.3389/fpls.2020.00375. [Google Scholar] [CrossRef]
48. Königshofer, H., Lechner, S. (2002). Are polyamines involved in the synthesis of heat-shock proteins in cell suspension cultures of tobacco and alfalfa in response to high-temperature stress? Plant Physiology and Biochemistry, 40(1), 51–59. DOI 10.1016/S0981-9428(01)01347-X. [Google Scholar] [CrossRef]
49. Sagor, G., Berberich, T., Takahashi, Y., Niitsu, M., Kusano, T. (2013). The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic research, 22(3), 595–605. DOI 10.1007/s11248-012-9666-3. [Google Scholar] [CrossRef]
50. He, L., Nada, K., Kasukabe, Y., Tachibana, S. (2002). Enhanced susceptibility of photosynthesis to low-temperature photoinhibition due to interruption of chill-induced increase of S-adenosylmethionine decarboxylase activity in leaves of spinach (Spinacia oleracea L.). Plant and Cell Physiology, 43(2), 196–206. DOI 10.1093/pcp/pcf021. [Google Scholar] [CrossRef]
51. Zhou, Y. H., Yu, J. Q., Mao, W. H., Huang, L. F., Song, X. S. et al. (2006). Genotypic variation of rubisco expression, photosynthetic electron flow and antioxidant metabolism in the chloroplasts of chill-exposed cucumber plants. Plant and Cell Physiology, 47(2), 192–199. DOI 10.1093/pcp/pci234. [Google Scholar] [CrossRef]
52. Besford, R., Richardson, C., Campos, J., Tiburcio, A. (1993). Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta, 189(2), 201–206. DOI 10.1007/BF00195077. [Google Scholar] [CrossRef]
53. Hassan, N., Ebeed, H., Aljaarany, A. (2020). Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiology and Molecular Biology of Plants, 26(2), 233–245. DOI 10.1007/s12298-019-00744-7. [Google Scholar] [CrossRef]
54. Saadati, S., Baninasab, B., Mobli, M., Gholami, M. (2020). Cold tolerance in olive leaves of three cultivars related to some physiological parameters during cold acclimation and de-acclimation stages. Journal of Agricultural Science and Technology, 22(5), 1313–1326. [Google Scholar]
55. Prasad, T. K. (1997). Role of catalase in inducing chilling tolerance in pre-emergent maize seedlings. Plant Physiology, 114(4), 1369–1376. DOI 10.1104/pp.114.4.1369. [Google Scholar] [CrossRef]
56. Willekens, H., Chamnongpol, S., Davey, M., Schraudner, M., Langebartels, C. et al. (1997). Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. The EMBO Journal, 16(16), 4806–4816. DOI 10.1093/emboj/16.16.4806. [Google Scholar] [CrossRef]
57. Gechev, T., Willekens, H., van Montagu, M., Inzé, D., van Camp, W. et al. (2003). Different responses of tobacco antioxidant enzymes to light and chilling stress. Journal of Plant Physiology, 160(5), 509–515. DOI 10.1078/0176-1617-00753. [Google Scholar] [CrossRef]
58. Chamnongpol, S., Willekens, H., Moeder, W., Langebartels, C., Sandermann, H. et al. (1998). Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proceedings of the National Academy of Sciences of the United States of America, 95(10), 5818–5823. DOI 10.1073/pnas.95.10.5818. [Google Scholar] [CrossRef]
59. Kubiś, J. (2008). Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. Journal of Plant Physiology, 165(4), 397–406. DOI 10.1016/j.jplph.2007.02.005. [Google Scholar] [CrossRef]
60. Shen, W., Nada, K., Tachibana, S. (2000). Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiology, 124(1), 431–440. DOI 10.1104/pp.124.1.431. [Google Scholar] [CrossRef]
61. Andronis, E. A., Moschou, P. N., Toumi, I., Roubelakis-Angelakis, K. A. (2014). Peroxisomal polyamine oxidase and NADPH-oxidase cross-talk for ROS homeostasis which affects respiration rate in Arabidopsis thaliana. Frontiers in Plant Science, 5(109), 132. DOI 10.3389/fpls.2014.00132. [Google Scholar] [CrossRef]
62. Shu, S., Yuan, L. Y., Guo, S. R., Sun, J., Yuan, Y. H. (2013). Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiology and Biochemistry, 63, 209–216. DOI 10.1016/j.plaphy.2012.11.028. [Google Scholar] [CrossRef]
63. Verma, S., Mishra, S. N. (2005). Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. Journal of Plant Physiology, 162(6), 669–677. DOI 10.1016/j.jplph.2004.08.008. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |