 Open Access
Open Access
ARTICLE
Combination of 6-Benzylaminopurine and Thidiazuron Promotes Highly Efficient Shoot Regeneration from Cotyledonary Node of Mature Peanut (Arachis hypogaea L.) Cultivars
1 Department of Crop Genetics and Breeding, College of Agronomy, Jilin Agricultural University, Changchun, 130118, China
2 Department of Biochemistry and Molecular Biology, College of Life Science, Jilin Agricultural University, Changchun, 130118, China
* Corresponding Authors: Abraham Lamboro. Email: ; Jun Zhang. Email:
(This article belongs to the Special Issue: Integrating Agronomy and Plant Physiology for Improving Crop Production)
Phyton-International Journal of Experimental Botany 2022, 91(12), 2619-2631. https://doi.org/10.32604/phyton.2022.021404
Received 12 January 2022; Accepted 02 April 2022; Issue published 29 August 2022
Abstract
Efficient in vitro plantlet regeneration is an important step to successfully transform genes for the improvement of agronomic traits. A combination of 6-benzylaminopurine (BAP) and thidiazuron (TDZ) plant growth regulators was applied to evaluate shoot regeneration capacity whereas α-naphthalene acetic acid (NAA) combination with 6-benzylaminopurine (BAP), and 2, 4-dichlorophenoxyacetic acid (2, 4-D) with 6-benzylaminopurine were tested to optimize root induction for two peanut cultivars. The result showed combination (BAP with TDZ) was found to be effective in promoting shoot. The highest shoot regeneration frequency (93%) was obtained on a medium supplemented with 4 mg/L BAP and 0.5 mg/L TDZ while an average regeneration frequency (87%) was achieved in a medium containing combinations of 2 mg/L BAP with 1 mg/L TDZ. The shooting rate increased for both cultivars as the concentrations of BAP increased and TDZ decreased. The highest rooting rate (93%) was obtained on a medium supplemented with 3.5 mg/L NAA with 2.5 mg/L BAP for both cultivars. The rooting rate increased as the concentration of auxin to cytokinin ratio increased. The maximum rooting rate (83%) was obtained on MS medium supplemented with 0.3 mg/L 2, 4-D with 0.2 mg/L BAP for the cultivar N3. The result indicated that BAP with NAA was much better than BAP with 2, 4-D in rooting rate. Thus, the protocol developed was genotype independent and effective for peanut tissue culture.Keywords
Peanut (Arachis hypogaea L.) is an oil-bearing legume crop. It is an allotetraploid derived between two diploid peanut Arachis duranensis (AA) and Arachis ipaensis (BB) [1]. The total production of 17,519, 600 tonnes volume per year peanut is produced in China. Therefore, China is the first in the world in production of peanuts [2]. Next to soybean, the peanut is an important oil-bearing legume and is widely grown in Africa and Asia. Peanut seeds are sources of carbohydrates, protein, and oil as major constituents [3]. It has an antioxidant capacity [4].
An efficient in vitro regeneration system is a basic and important step to transform genes and it is highly advantageous to apply gene-editing techniques in order to produce transgenic plants with the desired trait. In response to growth regulators like 2, 4-D, and naphthalene acetic acid (NAA) explant sources such as embryo axes produce roots [5]. To produce regenerant plantlets of peanut cotyledonary node used continuously [5,6]. In order to produce independently transformed plants in legumes or any other crops using a cotyledonary node as a source of explant is considered preferable to other sources since it is time-efficient and responsive to genetic transformation [5–10].
Earlier in vitro regeneration reports have shown varied shoot regeneration rates using different explant sources [5,6,11]. The main factors for variation of regeneration rate or frequency were the source of explant, the type and concentration of plant growth regulators used. For instance, regeneration rates of 80% to 81% [6], 86% and 98% [5], 81.5% [5] were few among several reports for the peanut plant. There were some in vitro regeneration reports in peanut and other crops using various explant sources including a cotyledonary node in mungbean [7,8], a cotyledonary node in black gram [9,12], deembryonated cotyledons in black gram [13], plumular apices in chickpea [14], immature embryonic axes and cotyledonary nodes in soybean [15], the cotyledonary node in soybean [10], cotyledon in oilseed crop [16], embryo in wheat [17], hypocotyl and cotyledon in cabbage [18], leaf base segment in millet [19], cotyledon and hypocotyl in Brassica oleracea [20], the nodal segment in Cinnamomum camphora [21], cotyledon and cotyledonary node in Toona ciliate [22].
In peanut epicotyl [23], hypocotyl [24], somatic embryos [25], and cotyledonary node [5,6,26,27] were reported effective in in vitro peanut regeneration. However, the plant variety, culture media, culture condition, and source of explant had been reported as the factors that influence tissue culture system or condition [28]. Some reports indicated that cultivated peanut is considered to be relatively recalcitrant to tissue culture [5,27,29]. Tissue culture is used widely throughout the development of plants in general and peanut in particular. Peanut culture is very important to produce plantlets from cells or tissue, which are then used for cloning, somaclonal variations and to develop transgenic plants with important agronomic traits. Hence, the development of an effective and reproducible protocol for peanuts is very important in order to genetically engineer peanut crop plants for further improvement of economically important agronomic traits. To our knowledge, so far there is no previously developed regeneration system for the Chinese peanut cultivar Yu-hua-14 and N3 using cotyledonary node as a source of explant. Therefore, the aim of this study was to evaluate the effect of different combinations of BAP and TDZ on shoot regeneration capacity in peanut cultivars.
2.1 Plant Material and Cultivar Selection
Mature, dry, and healthy seeds of peanut cultivars Yu-hua-14 and N3 were used in this study. The seeds were obtained from the department of crop genetics and breeding, Jilin Agricultural University, China.
2.2 Explant Preparation and in Vitro Culture Condition
The embryo axis was soaked for about 14 h in sterilized double-distilled water. The seeds were sterilized in 10% (w/v) sodium hypochlorite (NaClO) solution for 7 min and 1 min in 70% (w/v) ethanol to reduce microbial contaminants. Then, washed for 6–7 min in sterilized distilled water. Murashige and Skoog (MS) salts with vitamin and sucrose were purchased from Coolaber Science and Technology Co., Ltd., Beijing, China. Both cytokinin and auxin hormones and agar were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., China. 3% (w/v) sucrose and 0.8% (w/v) agar were used and pH was adjusted 5.7 before autoclave. Both epicotyls and hypocotyls from 3-week-old plantlets were removed and transferred to shoot elongation medium (SEM) for about 21 days. Then elongated shoots were removed and transferred on to root induction medium (RIM) for about one month. The plantlet’s growth conditions were 25/25°C day/night, 16 h photoperiod, and 130–150 μmol m−2 s−1 fluorescent light.
2.3 Optimization of Shoot Induction and Elongation Media
BAP with TDZ combinations was applied in the shoot induction medium (SIM) and shoot elongation medium (SEM). To test effect of BAP with TDZ on shoot induction, we used BAP + TDZ (1 + 1, 2 + 1, 3 + 0.5, 4 + 0.5, 5 + 0.5 mg/L). Shoots grown in a combination of 3 mg/L BAP with 0.5 mg/L TDZ were transferred to SEM with BAP + TDZ (1 + 1, 2 + 1, 3 + 0.5, 4 + 0.5, 5 + 0.5 mg/L). The experimental design was a complete randomized design with three replication. In a glass jar 10 CNs were placed and from these half were used after 30 days to record phenotypic data such as shoot number, shooting rate, shoot length and fresh shoot weight were recorded while the remaining were used for rooting.
2.4 Optimization of Root Induction Media
Two different auxins with cytokinin combination (2, 4-D with BAP and NAA with BAP) were used distinctly in RIM. Shoots grown in 4 mg/L BAP with 0.5 mg/L TDZ SIM and SEM and shoots grown in 2 mg/L BAP with 1 mg/L TDZ SEM were transferred to 2, 4-D with BAP combination (0.15 + 0.1, 0.2 + 0.15, 0.3 + 0.2, 0.4 + 0.3 mg/L) and NAA with BAP combination (4 + 4, 3 + 2, 3.5 + 2.5, 4 + 1.5 mg/L). After 30 days morphological data such as rooting rate, root number, root fresh weight, and length were recorded and analyzed. The rooted plants were transferred into plastic pots containing soil mixtures such as sandy soil, vermiculite, and manure at the ratio of 1:1:1. The plantlets were covered under transparent plastic bags for retaining humidity and hardened under 25/25°C day/night, 16 h photoperiod, and 130–150 μmol m−2 s−1 fluorescent light for two weeks. The plastic bags were gradually withdrawn for 2 h for 4 days. The acclimatized plantlets were transferred into pots containing sand and soil and moved to the greenhouse.
Shoot number, shooting rate, shoot length, fresh shoot weight, rooting rate, root number, root fresh weight, and root length were used for Analysis of Variance (ANOVA). Each experiment had a completely randomized design and was replicated three times. Appropriate standard deviation and means separations were calculated according to Tukey’s Multiple Range Test at a confidence interval of 95%. Minitab 17 software (Minitab Inc., State College, P.A., USA) was used for analysis. Different letters on the graphs showed that the means were statistically different at the P < 0.05 level.
3.1 Shoot Induction and Elongation Using BAP and TDZ
BAP with TDZ combinations promoted shoot initiation in peanut cultivars. However, the shoot regeneration rate and shoot bud formation varied with the hormone combinations. In this study, BAP with TDZ combination was found to be advantageous in stimulating shoot. A maximum shoot regeneration rate (93%) was achieved on a MS medium containing 4 mg/L BAP with 0.5 mg/L TDZ (Fig. 2B) and an average shooting rate (87%) was obtained in a medium containing a combination of 2 mg/L BAP with 1 mg/L TDZ for N3 cultivar. However, the maximum shooting rate (92%) was achieved for the cultivar Yu-hua-14 in a medium containing 4 mg/L BAP with 0.5 mg/L TDZ (Fig. 2B). The shooting rate increased for both cultivars as the level of BAP concentration increased and TDZ decreased. Moreover, the shooting rate decreased on a medium containing 5 mg/L BAP with 0.5 mg/L TDZ (Fig. 2B). There was no significance difference between cultivars at 4 mg/L BAP with 0.5 mg/L TDZ, 3 mg/L BAP with 0.5 mg/L TDZ and 5 mg/L BAP with 0.5 mg/L TDZ (Fig. 2B). Indicating that combination of BAP with TDZ hormones in shoot induction and shoot elongation medium was found to be worthy in finding better quality shoots in peanut (Figs. 1, 2A).
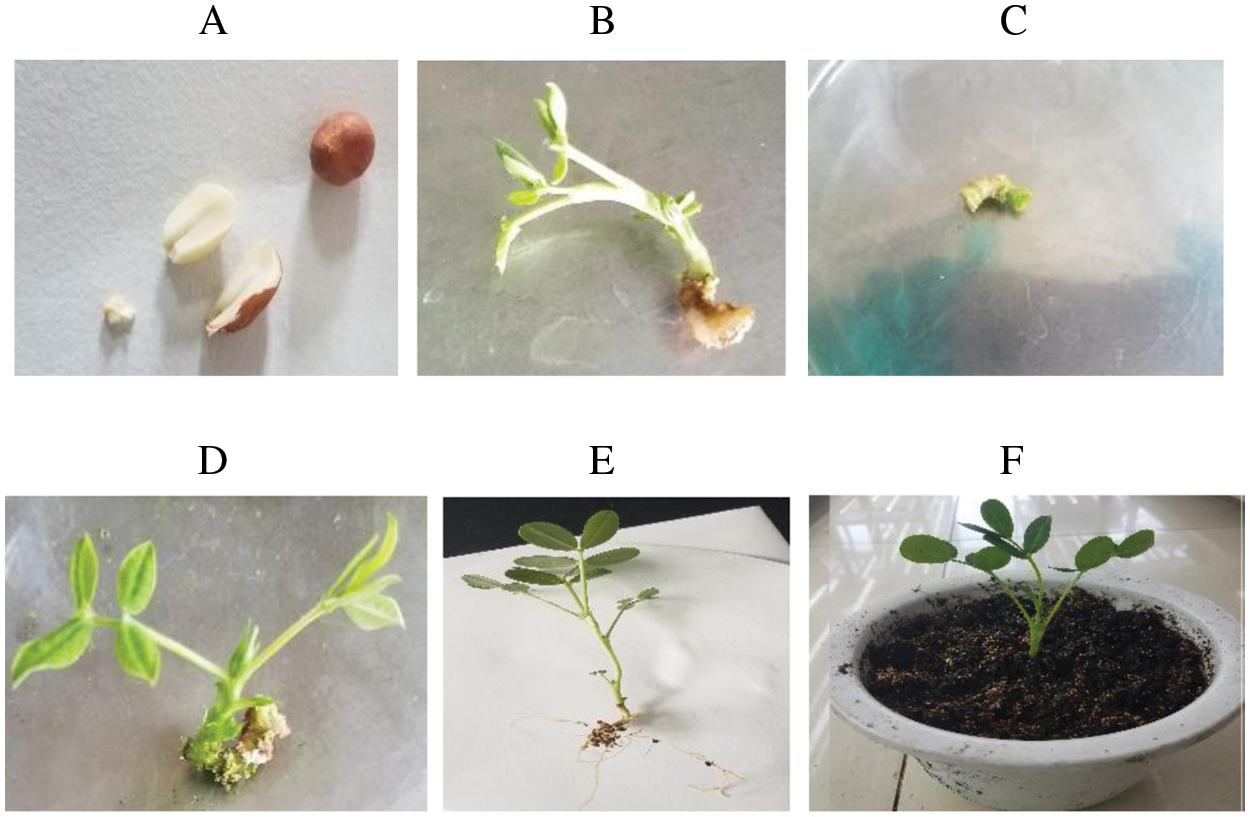
Figure 1: Cotyledonary node regeneration system of peanut variety Yu-hua-14. A healthy mature seeds and embryo axes, B three-week-old regenerated peanut plantlets from shoot initiation medium, C cotyledonary node, D one-month-old shoots regeneration from the cotyledonary node, E regeneration of roots, F regenerated plants transferred to soil
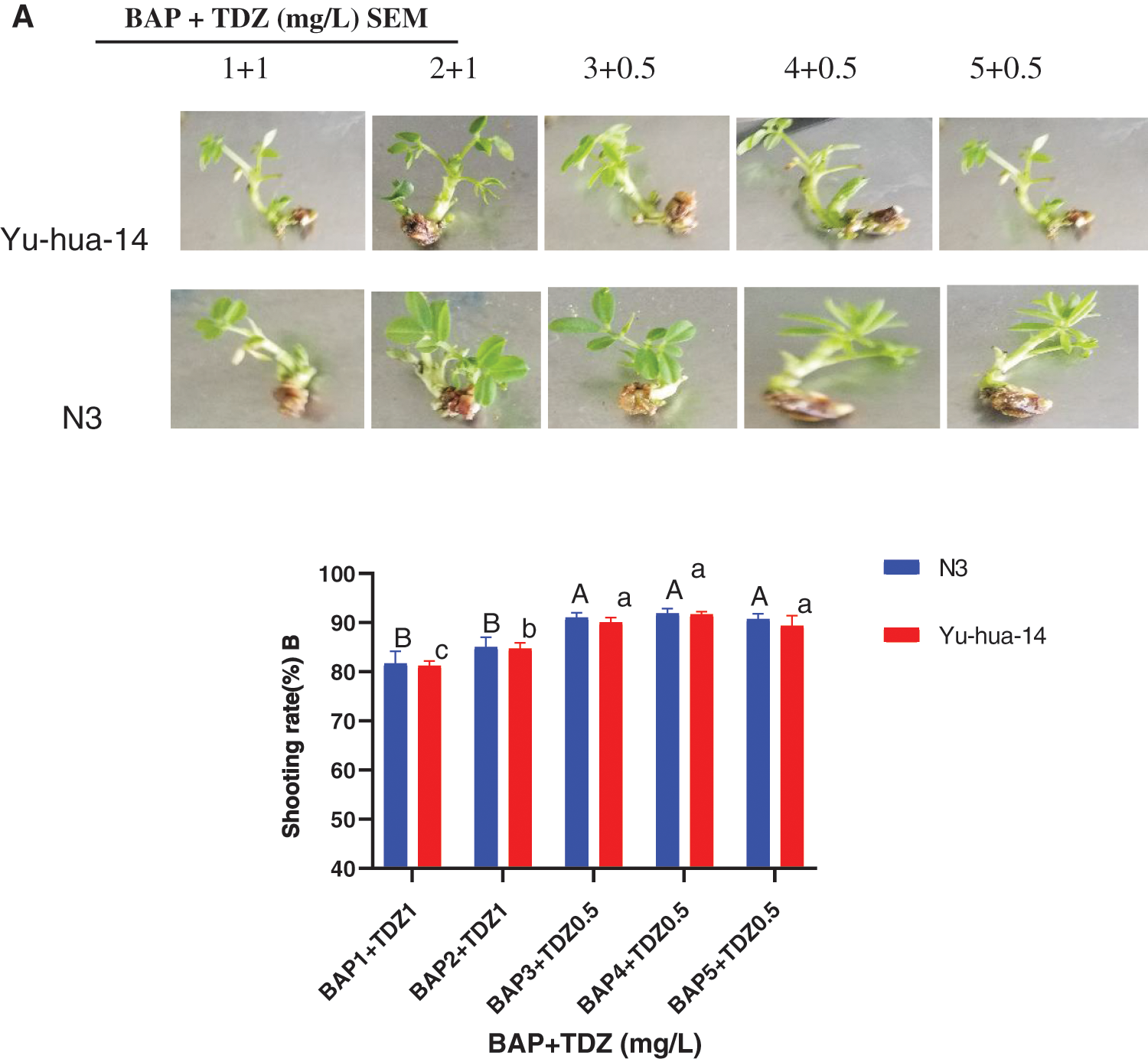
Figure 2: 6-benzylaminopurine and thidiazuron effect on shoot elongation response in peanut cultivars Yu-hua-14 and N3 at different concentrations of BAP with TDZ. A three-week-old Yu-hua-14 and N3 peanut cultivars under different concentrations of BAP with TDZ combination, B shooting rate
The maximum shoot number (5 ± 0.577) and average shoot number (4 ± 0.577) were obtained in a medium containing 5 mg/L BAP with 0.5 mg/L TDZ and 4 mg/L BAP with 0.5 mg/L TDZ, respectively (Fig. 3A). Shoot number increased when BAP with TDZ concentration increased. There was no significant difference found between cultivars in shoot number containing 3 mg/L BAP with 0.5 mg/L TDZ and 4 mg/L BAP with 0.5 mg/L TDZ (Fig. 3A).
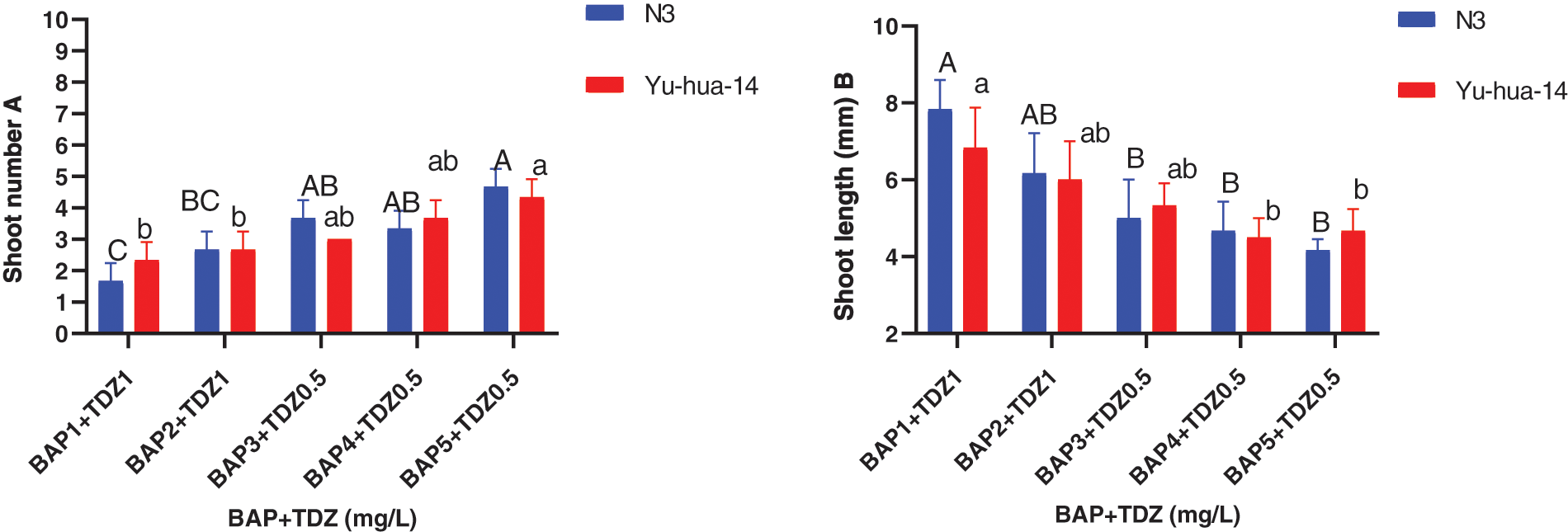
Figure 3: 6-benzylaminopurine and thidiazuron effect on shoot elongation response in peanut cultivars Yu-hua-14 and N3 at different concentrations of BAP with TDZ. A shoot number, B shoot length
Shoot length ranged between (4 and 8 mm). The maximum shoot length (8 mm) was found on a medium containing 1 mg/L BAP with a 1 mg/L TDZ combination. Shoot length decreased as the concentration of BAP with TDZ increased (Fig. 3B). The average shoot weight (0.48 g) for Yu-hua-14 and (0.55 g) for N3 were obtained on a medium containing 4 mg/L BAP with 1 mg/L TDZ. No significant difference was observed for shoot weight (Fig. 4).
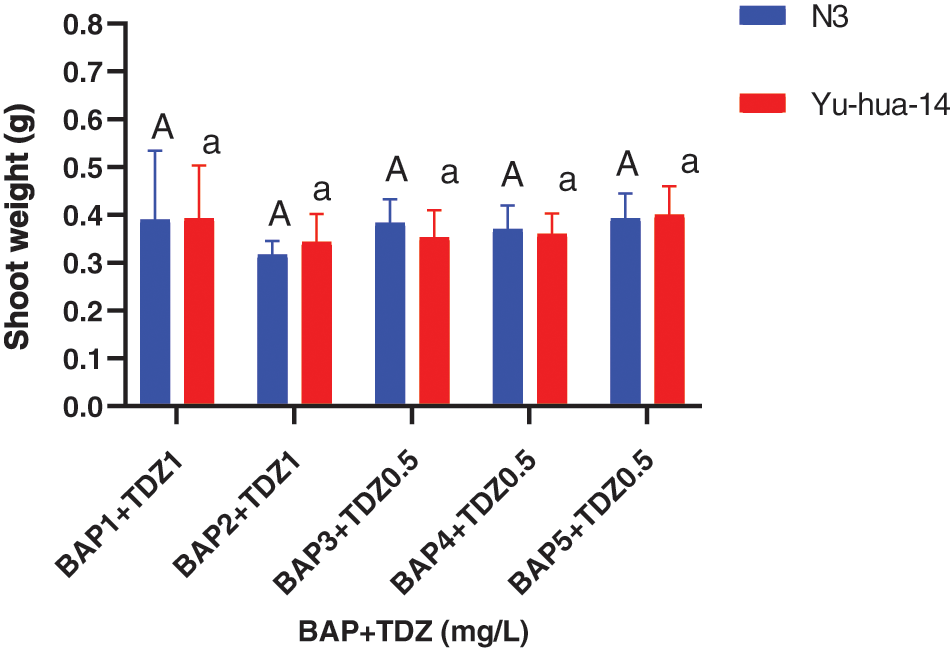
Figure 4: 6-benzylaminopurine and thidiazuron effect on shoot weight in peanut cultivars Yu-hua-14 and N3 at different concentrations of BAP with TDZ on shoot weight
In in vitro root culture condition, 21 day-old plantlets were placed on MS medium containing NAA with BAP and 2, 4-D with BAP combinations. The Difference in NAA with BAP and 2, 4-D with BAP medium were tested to check the improvement in peanut cultivars. Healthy and well-developed shoots developed roots initiation within 7 days after placed on the rooting medium (Figs. 5 and 8A).

Figure 5: Effect of NAA with BAP combination on the in vitro root induction response of peanut cultivars Yu-hua-14 and N3 at different concentrations of auxin/cytokinin ratio treatment initially grown on BAP with TDZ combination medium. One-month-old Yu-hua-14 and N3 peanut cultivars under different concentrations of NAA with BAP
3.2 Optimization of Root Using NAA with BAP
Rooting rates ranged from 50% to 93% and the maximum rooting rate (93%) was obtained on MS medium containing 3 mg/L NAA with 2 mg/L BAP, 3 mg/L NAA with 2.5 mg/L BAP, and 4 mg/L NAA with 1.5 mg/L BAP for N3 cultivar, respectively (Fig. 6A). However, a 93% rooting rate was found on a medium containing 3.5 mg/L NAA with 2.5 mg/L BAP for both cultivars. The rooting rate increased as the concentration of NAA to BAP ratio increased. Nevertheless, at 4 mg/L NAA with 1.5 mg/L BAP rooting rate decreased (Fig. 6A). There was no significant difference found in all combinations except 4 mg/L NAA with 4 mg/L BAP. Morphologically healthy roots developed in all media 3 mg/L NAA with 2 mg/L BAP and 3.5 mg/L NAA with 2.5 mg/L BAP were found better for root initiation.
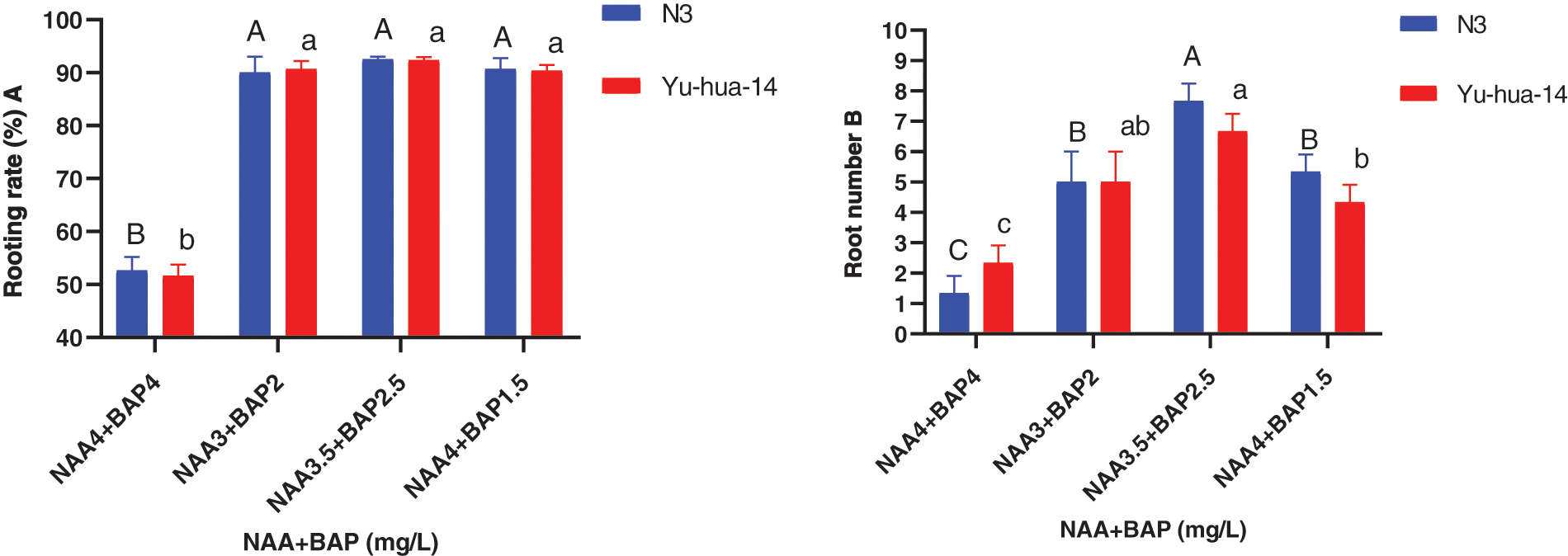
Figure 6: Effect of NAA with BAP combination on the in vitro root induction response of peanut cultivars Yu-hua-14 and N3 at different concentrations of auxin treatment initially grown on BAP with TDZ combination medium. A rooting rate, B root number
The highest root number (8) and the average root number (6) were obtained for the cultivar N3 on a medium containing 3.5 mg/L NAA with 2.5 mg/L BAP and 3 mg/L NAA with 2 mg/L BAP, respectively. Whereas the maximum root number (7) was found for Yu-hua-14 on a medium containing 3.5 mg/L NAA with 2.5 mg/L BAP (Fig. 6B). The highest root length (18 mm) for Yu-hua-14 and (21 mm) for N3 were found on MS medium containing 4 mg/L NAA with 4 mg/L BAP and 3 mg/L NAA with 2 mg/L BAP, respectively (Fig. 7A). Root fresh weight ranged from 0.15 to 0.55 g for N3. The maximum root fresh weight (0.55 g) was obtained on a medium containing 4 mg/L NAA with 4 mg/L BAP for N3. Average root fresh weight (0.45 g) for N3 and 0.42 g for Yu-hua-14 were recorded on hormone combination 3.5 mg/L NAA with 2.5 mg/L BAP (Fig. 7B). There was no significant difference found for root fresh weight at all hormone combinations except 4 mg/L NAA with 4 mg/L BAP.
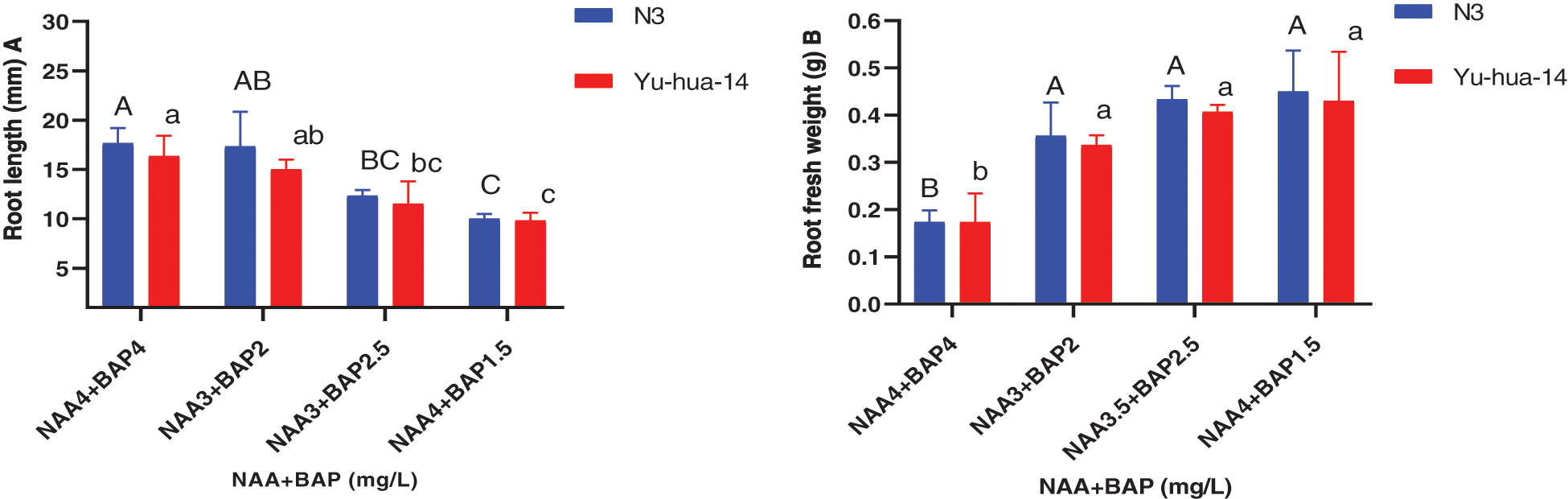
Figure 7: Effect of NAA with BAP combination on the in vitro root induction response of peanut cultivars Yu-hua-14 and N3 at different concentrations of auxin/cytokinin ratio treatment initially grown on BAP with TDZ combination medium. A root length, B root fresh weight
3.3 Optimization of Root Using 2, 4-D with BAP
The effect of 2, 4-D with BAP combination on root development was highly significant (P < 0.005). The rooting rate ranged from 51–83% for N3 and 52–83% for Yu-hua-14 (Fig. 8B). The maximum rooting rate (83%) was obtained on MS medium containing 0.3 mg/L 2, 4-D with 0.2 mg/L BAP for the cultivar N3 and 0.4 mg/L 2, 4-D with 0.3 mg/L BAP for Yu-hua-14. Average rooting rate (80%) was obtained on a medium containing 0.3 mg/L 2, 4-D with 0.2 mg/L BAP for Yu-hua-14 (Fig. 8B). The rooting rate decreased as the concentration exceeds 0.3 mg/L 2, 4-D with 0.2 mg/L BAP combination for the peanut cultivar N3.
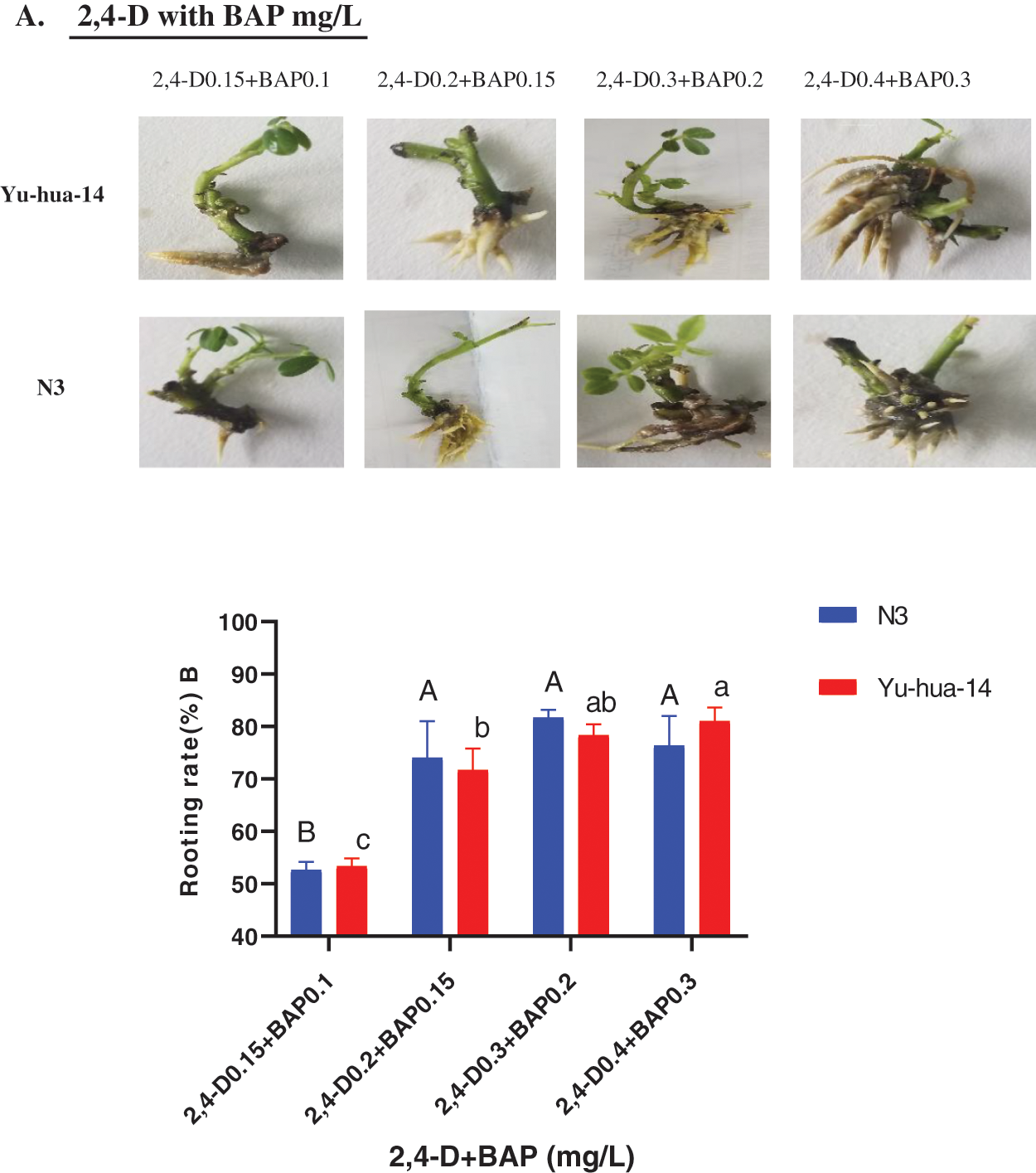
Figure 8: Effect of 2, 4-D and BAP combination on the in vitro root induction response of peanut cultivars Yu-hua-14 and N3 at different concentrations of auxin/cytokinin ratio treatment initially grown on BAP and TDZ combination medium. A one-month-old Yu-hua-14 and N3 peanut cultivars under different concentration of 2, 4-D and BAP, B rooting rate
Root number varied from 1–6 for N3 and 1–7 for Yu-hua-14. The maximum root number 7 ± 2 for Yu-hua-14 and 6 ± 0.577 for N3 were obtained on MS medium containing a combination of 0.4 mg/L 2, 4-D with 0.3 mg/L BAP, and 0.3 mg/L 2, 4-D with 0.2 mg/L BAP, respectively (Fig. 9A). The average root number 4 ± 0.577, was obtained on a medium containing 0.2 mg/L 2, 4-D with 0.15 mg/L BAP for Yu-hua-14. The average root number 5 ± 1.155 was obtained on a medium containing 0.2 mg/L 2, 4-D with 0.15 mg/L BAP for N3 (Fig. 9A). Root number increased as the concentration of hormone combination increased.
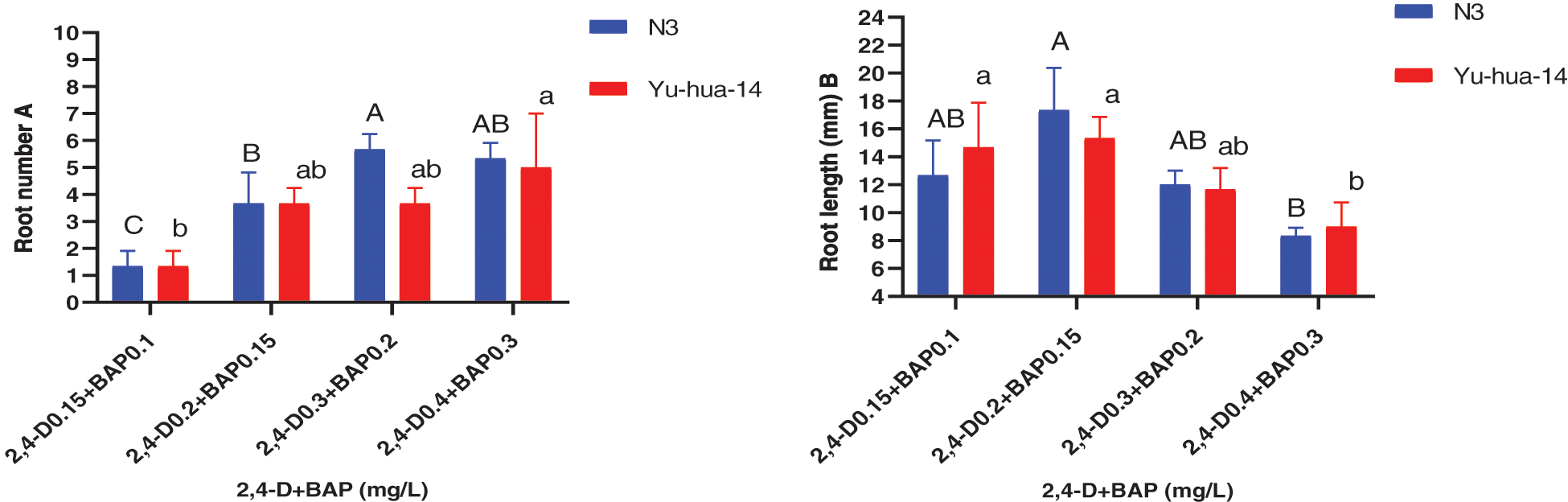
Figure 9: Effect of 2, 4-D and BAP combination on the in vitro root induction response of peanut cultivars Yu-hua-14 and N3 at different concentrations of auxin/cytokinin ratio treatment initially grown on BAP and TDZ combination medium. A root number, B root length
The MS medium containing 0.2 mg/L 2, 4-D with 0.15 mg/L BAP gave highest root length (20 mm) for N3 cultivar while highest root length (17 mm) was obtained for Yu-hua-14 on MS medium containing 0.15 mg/L 2, 4-D with 0.1 mg/L BAP (Fig. 9B). Root fresh weight ranged from 0.1–0.4 g. The maximum root fresh weight was obtained on MS medium containing 0.3 mg/L 2, 4-D with 0.2 mg/L BAP for both peanut cultivars (Fig. 10).
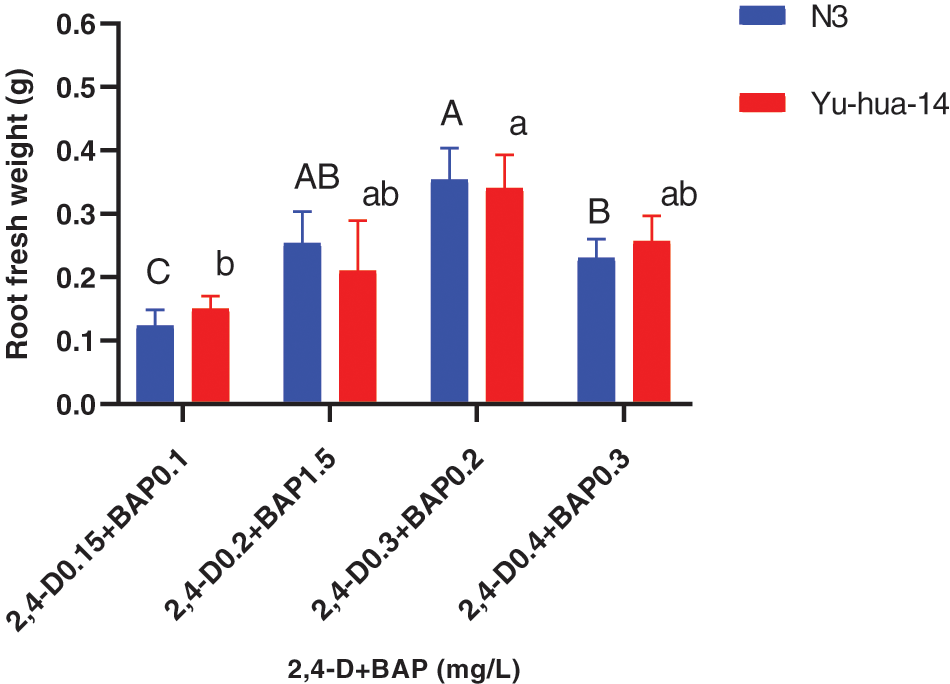
Figure 10: Effect of 2, 4-D and BAP combination on the in vitro root induction response of peanut cultivars Yu-hua-14 and N3 initially grown on BAP and TDZ combination medium on root fresh weight
4.1 Shoot Induction and Elongation
In this study, we optimized in vitro plantlet regeneration and protocol development for peanut cultivars. In in vitro culture conditions, surface sterilization of the embryo axis is very important and effective to minimize pollutants.
The effect of BAP with TDZ combination was evaluated for shoot initiation and elongation. In the present study, morphologically more healthy peanut plantlets were obtained in a shoot induction medium containing 2 mg/L BAP with 1 mg/L TDZ and 3 mg/L BAP with 0.5 mg/L TDZ (Fig. 2A). Indicating 2 mg/L BAP with 1 mg/L TDZ and 3 mg/L BAP with 0.5 mg/L TDZ combinations were found good for shoot induction. In shoot elongation medium, we used shoots initially grown on MS medium containing 3 mg/L BAP with 0.5 mg/L TDZ. Thus, the medium containing 4 mg/L BAP with 0.5 mg/L TDZ combination resulted maximum regeneration rate (93%) for the cultivar N3 and (92%) for Yu-hua-14 (Fig. 2B). There was no significant difference between cultivars. The rate of shoot regeneration was reduced with an increase in BAP and TDZ concentration (more than 5 mg/L BAP with 0.5 mg/L TDZ) in the culture medium (Fig. 2B). Indicating lower cytokinin combinations are better for shoot regeneration.
The induction of shoot in peanuts has been previously reported using different hormone concentrations. Limbua et al. [6] reported maximum shoot regeneration rate (98%) on medium containing 5 mg/L with 1 mg/L TDZ combination whereas highest 81.5% shoot bud formation were reported by [11,30] on medium containing 3 mg/L BAP with 0.92 mg/L NAA. The maximum shooting rate (68%) was reported [31] on a medium containing 8.88 μМ BAP with 1.15 μМ NAA. In a soybean, using spermidine at 137.69 μМ, the highest shooting rate (96.94%) was reported by [32]. A high shoot regeneration frequency (93.86%) was reported in sunflower [33] on MS medium supplemented with 9.84 μМ 2-isopentenyladenine (2-iP), 2.85 μМ indole-3-acetic acid (IAA) and 0.45 μМ thidiazuron (TDZ) and highest shooting rate (97.7%) was reported in peanut [34] medium containing N6-benzyladenine (BA) with 2, 4-D combinations. The shoot regeneration capacity difference might be because of the variation of the concentrations of hormones and the genotype.
The highest shoot number (5 ± 0.577), maximum shoot length (8 mm) was obtained on a medium containing 5 mg/L BAP with 0.5 mg/L TDZ and 1 mg/L BAP with 1 mg/L TDZ) (Figs. 3A and 3B). The medium containing 4 mg/L BAP with 0.5 mg/l TDZ and 4 mg/L BAP with 0.5 mg/L TDZ were found to be preferable hormone combination in terms of good morphology of the peanut cultivars (Fig. 2A). Cytokinins plant growth regulators are a very important factor in the induction of shoots from cultured tissues. In this study, BAP at higher concentrations and TDZ at lower concentrations resulted in the production of multiple shoots. We obtained 0.48 g shoot weight for Yu-hua-14 and 0.55 g for N3 (Fig. 4). And no significant difference was observed between the cultivars. Indicating, the protocol developed in the present study was genotype independent. Plantlets regenerated from cotyledonary node culture were smaller in size than normal seedlings and this might be because of the nutrients supplied via the culture medium may not be the same as the nutrient present in the seed.
The effect of NAA with BAP was observed for root induction in peanut. Different concentrations of NAA with BAP were tested (Figs. 5, 6A, 6B, 7A and 7B). A mixture of NAA with BAP increased the regeneration rate and the number of roots for both peanut cultivars. Between NAA and BAP combination tested, the maximum rooting rate (93%) was found for the N3 cultivar. A maximum root number (8) was achieved on a medium containing 3.5 mg/L NAA with 2.5 mg/L BAP (Figs. 6A, 6B). But highest root length (21 mm) was achieved on the medium containing 3 mg/L NAA with 2 mg/L BAP (Fig. 7A). The maximum root fresh weight (0.55 g) was achieved on a medium containing 4 mg/L NAA with 1.5 mg/L BAP for N3 (Fig. 7B).
The maximum root regeneration rate (93%) and root number (7) were obtained for the cultivar Yu-hua-14 on a medium containing 3.5 mg/L NAA with 2.5 mg/L BAP. In this study highest root regeneration rate and maximum root number were obtained on a medium containing 3.5 mg/L NAA with 2.5 mg/L BAP for both cultivars (Figs. 6A, 6B). Indicating 3.5 mg/L NAA with 2.5 mg/L BAP combination was a better hormone combination to generate maximum rooting rate and root number. The highest root length (18 mm) was obtained in the combination of 4 mg/L NAA with 4 mg/L BAP while maximum root fresh weight (0.55 g) was found on a medium containing 4 mg/L NAA with 1.5 mg/L BAP (Figs. 7A, 7B).
Varied root regeneration percent had been previously reported on different hormone combinations [6]. High regeneration frequency (70%) was reported by [6]. Whereas (68.3%) root regeneration reported [9] on MS medium containing 2 mg/L BAP with 1.5 mg/L NAA in black gram. In peanut [27] reported 96.33% regeneration rate on medium containing 1 mg/L NAA. However, Limbua et al. [6] reported 100% rooting rate on a medium containing 5.7 μM NAA. Comparatively a high ratio of cytokinin to auxin stimulates shoot formation while increased auxin to cytokinin ratio stimulates root formation [35].
In this study, 2, 4-D with BAP induced root regeneration of peanut cultivars (Figs. 8A, 8B). We evaluated the effectiveness of the combination of the two hormones for the root formation. Medium containing 0.3 mg/L 2, 4-D with 0.2 mg/L BAP was found relatively more suitable for rooting than other concentration combinations. Maximum rooting rate (83%), root number (6 ± 0.577), root length (20 mm), and root fresh weight (0.4 g) were observed in the N3 peanut cultivar (Figs. 8B, 9A, 9B and 10). In Yu-hua-14, the highest rooting rate was (83%) observed on a medium containing 0.4 mg/L 2, 4-D with 0.3 mg/L BAP combinations (Fig. 8B). Medium containing 0.3 mg/L 2, 4-D with 0.2 mg/L BAP and 0.4 mg/L 2, 4-D with 0.3 mg/L BAP gave better regeneration rate for both cultivars as compared to other hormone combinations. Similar to our result, Limbua et al. [6] reported that the rooting rate was 83%. The highest root number, root length, and root fresh weight were 7 ± 2, 17 mm, and 0.4 g, respectively (Figs. 9A, 9B and 10).
In general, a maximum rooting rate (93%) was achieved on a medium containing 3.5 mg/L NAA with 2.5 mg/L BAP for both cultivars. The rooting rate increased as the concentration of auxin to cytokinin ratio increased. The maximum rooting rate (83%) was achieved on MS medium supplemented with 0.3 mg/L 2, 4-D with 0.2 mg/L BAP for the cultivar N3. The result showed that BAP with NAA was much better than BAP with 2, 4-D in rooting rate. The plants with a more number of roots at the time of transfer to the pots become well established. The combination of NAA with BAP and 2, 4-D with BAP helped in the development of roots, but a reduced rate of root forming as compared to cytokinins alone.
A combination of 2 mg/L BAP with 1 mg/L TDZ and 3 mg/L BAP with 0.5 mg/L TDZ were found to be favorable for shoot initiation. Using BAP with TDZ combination was effective for shoot initiation and elongation media. The application of 4 mg/L BAP with 0.5 mg/L TDZ was more effective for shoot elongation. BAP in combination with NAA was found to be effective and much better than BAP with 2, 4-D in rooting rate, root number, and root length. Plantlets regenerated from cotyledonary node culture were smaller in size than normal seedlings and this might be because of the nutrients supplied via the culture medium may not be the same as the nutrient present in the seed.
Acknowledgement: We would like to thank Professor Piwu Wang for allowing Plant Biotechnology Center to do the experiment.
Authorship: AL, conducted the experiment, wrote and revised the manuscript. JZ, XH, and SY supervised the whole process and revised and edited the manuscript. AL, BS, DY, XL analyzed data. All authors read and approved the final manuscript.
Funding Statement: Also we would like to thank Jilin Province’s Key Research and Development Project (20180201070NY) for financial support.
Conflicts of Interest: The authors declare there is no conflict of interest
References
1. Bertioli, D. J., Cannon, S. B., Froenicke, L., Huang, G., Farmer, A. D. et al. (2016). The genome sequence of Arachis duranensis and Arachis ipaensis, the diploid ancestor of cultivated peanut. Nature Genetics, 48, 438–446. DOI 10.1038/ng.3517. [Google Scholar] [CrossRef]
2. UNFAO (2019). The state of food agriculture: moving forward on food loss and waste reduction. The State of the World. http://www.fao.org/faostat. [Google Scholar]
3. Davis, J. P., Price, K. M., Dean, L. L., Sweigart, D. S., Cottonaro, J. M. et al. (2016). Peanut oil stability and physical properties across a range of industrially relevant oleic acid/linoleic acid ratios. Peanut Science, 43(1), 1–11. DOI 10.3146/PS14-17.1. [Google Scholar] [CrossRef]
4. Tedesco, M. P., Monaco-Lourenco, C. A., Carvalho, R. A. (2017). Characterization of oral disintegrating film of peanut skin extract-potential route for buccal delivery of phenolic compounds. International Journal of Biological Macromolecules, 97, 418–425. DOI 10.1016/j.ijbiomac.2017.01.044. [Google Scholar] [CrossRef]
5. Hsieh, Y. F., Jain, M., Wang, J., Gallo, M. (2017). Direct organogenesis from cotyledonary node explants suitable for agrobacterium-mediated transformation in peanut (Arachis hypogaea L.). Plant Cell, Tissue and Organ Culture, 128, 161–175. DOI 10.1007/s11240-016-1095-1. [Google Scholar] [CrossRef]
6. Limbua, P. G., Ngugi, M. P., Oduor, R. O. (2019). In vitro regeneration protocol of Kenyan adapted groundnut (Arachis hypogaeaL.) genotypes using cotyledonary node explants. Journal of Plant Biochemistry and Physiology, 7, 1. DOI 10.4172/2329-9029.1000233. [Google Scholar] [CrossRef]
7. Mojumder, S., Hossain, M. D., Haque, M. S., Nasiruddin, K. M. (2015). In vitro regeneration of bina mungbean varieties. Journal of Environmental Science and Natural Resources, 7(2), 47–52. DOI 10.3329/jesnr.v7i2.22203. [Google Scholar] [CrossRef]
8. Sagare, D. B., Mohanty, I. C. (2015). In vitro regeneration system in green gram (Vigna radiate (L) wilczek, cv. SujataA recalcitrant legume crop. Research Journal of Agricultural Science, 6(1), 64–67. DOI 2196-1409-2014-014. [Google Scholar]
9. Adlinge, P. M., Samal, K. C., Kumara Swamy, R. V., Rout, G. R. (2014). Rapid in vitro plant regeneration of black gram (Vigna mungoL. Hepper) var. Sarala, an important legume crop. Proceeding of the National Academy of Science, India Section B: Biological Sciences, 84, 823–827. DOI 10.1007/s40011-013-0281-8. [Google Scholar] [CrossRef]
10. Cheng, T. Y., Saka, H., Voqui-Dinh, T. H. (1980). lant regeneration from soybean cotyledonary note segments in culture. Plant Science Letters, 19(2), 91–99. DOI 10.1016/0304-4211(80)90084-X. [Google Scholar] [CrossRef]
11. Tiwari, S., Tuli, R. (2009). Multiple shoot regeneration in seed-derived immature leaflet explants of peanut (Arachis hypogaea L.). Scientia Horticulturae, 121, 223–227. DOI 10.1016/j.scienta.2009.01.029. [Google Scholar] [CrossRef]
12. Muruganantham, M., Amutha, S., Selvaraj, N., Vengadesan, G., Ganapath, A. (2007). Efficient agrobacterium-mediated transformation of Vigna mungo using immature cotyledonary-node explants and phosphinothricin as the selection agent. In Vitro Cellular and Developmental Bioligy-Plant, 43(6), 550–557. DOI 10.1007/s11627-007-9060-7. [Google Scholar] [CrossRef]
13. Anandan, R., Deenathayalan, T., Bhuvaneshwari, R., Monisha, M. M., Prakash, M. (2019). An efficient protocol for rapid plant regeneration from deembryonated cotyledons of black gram [Vigna mungo (L.) Hepper]. Indian Journal Agricultural Research, 53, 589–593. DOI 10.18805/IJARe.A-5169. [Google Scholar] [CrossRef]
14. Aasim, M., Sibel, D., Fereshteh, R., Mortaza, H. (2013). Multiple shoot regeneration of plumular apices of chickpea. Turkish Journal Agriculture Forestry, 37, 33–39. DOI 10.3906/tar-1204-38. [Google Scholar] [CrossRef]
15. Pathak, N., Tiwari, S., Mishra, M. K. (2017). Regeneration of plantlets from immature explants culture in Glycine max (L.) Merrill. Legume Research: An International Journal, 40(1), 69–73. DOI 10.18805/lr.v0i0.7020. [Google Scholar] [CrossRef]
16. Zimik, M., Arumugam, N. (2017). Induction of shoot regeneration in cotyledon explants of the oilseed crop (Sesamum indicum L.). Journal of Genetic Engineering Biotechnology, 15, 303–308. DOI 10.1016/j.jgeb.2017.07.006. [Google Scholar] [CrossRef]
17. Agil, F., Orgec, M., Karakas, F. P., Verma, S. K., Zencirci, N. (2022). In vitro mature embryo culture protocol of einkom (Triticum monococoum ssp. monococoum) and bread (Triticum aestivum L.) wheat under boron stress. Plant Cell, Tissue and Organ Culture (PCTOC), 148(2), 293–304. DOI 10.1007/s11240-021-02186-007-9060-7. [Google Scholar] [CrossRef]
18. Sivanandhan, G., Choi, S. B., Jiae, M., Choi, S. R., Kim, S. G. et al. (2019). High frequency in vitro regeneration of Chinese cabbage (cv. Kenshin) from hypocotyl and cotyledon explants. Horticultural Science and Technology, 37, 640–650. DOI 10.7235/HORT.20190064. [Google Scholar] [CrossRef]
19. Rathinapriya, P., Satish, L., Rameshkumar, R., Pandiana, S., Ramesh, M. (2019). Role of activated charcoal and amino acids in developing an efficient regeneration system for foxtail millet (Setaria italica (L.) Beauv.) using leaf base segments. Physiology and Molecular Biology of Plants, 25, 533–548. DOI 10.1007/s12298-018-0619-z. [Google Scholar] [CrossRef]
20. Gambhir, G., Kumar, P., Srivastava, D. K. (2017). High frequency regeneration of plants from cotyledon and hypocotyl cultures in Brassica oleracea cv. pride of India. Biotechnology Reports, 15, 107–113. DOI 10.1016/j.btre.2017.02.005. [Google Scholar] [CrossRef]
21. Du, L., Li, Y. P., Yao, Y., Hang, L. W. (2015). An efficient protocol for plantlet regeneration via direct organogenesis by using nodal segments from embryo-cultured seedlings of Cinnamomum camphora L. PLoS One, 10, e0127215. DOI 10.1371/journal.pone.0127215. [Google Scholar] [CrossRef]
22. Song, H., Mao, W., Shang, Y., Zhou, W., Li, P. et al. (2021). A regeneration system using cotyledons and cotyledonary node explants of Toona ciliate. Journal of Forestry Research, 32, 967–974. DOI 10.1007/s11676-020-01189-5. [Google Scholar] [CrossRef]
23. Shan, L., Tang, G., Xu, P., Liu, Z., Bi, Y. (2009). High efficiency in vitro plant regeneration from epicotyl explants of Chinese peanut cultivars. In Vitro Cell and Developmental Biology-Plant, 45, 525–531. DOI 10.1007/s11627-009-9255-1. [Google Scholar] [CrossRef]
24. Matand, K., Prakash, C. S. (2007). Evaluation of peanut genotypes for in vitro plant regeneration using thidiazuron. Journal of Biotechnology, 130, 202–207. DOI 10.1016/j.jbiotec.2007.02.014. [Google Scholar] [CrossRef]
25. Joshi, M. V., Sahasrabudhe, N. A., Hazra, S. (2003). Responses of peanut somatic embryos to thidiazuron. Biology Plant, 46(2), 187–192. DOI 10.1023/A:1022886107591. [Google Scholar] [CrossRef]
26. Limua, P. G., Ngugi, M. P., Oduor, R. O. (2022). Genatic transformability of selected kenyan groundnut (Arachis hypogaea L.) genotypes with IPT gene using cotyledonary node explants. Advences in Agriculture, 2022, 2516843. DOI 10.1155/2022/2516843. [Google Scholar] [CrossRef]
27. Lamboro, A., Han, X., Yang, S., Li, X., Yao, D. et al. (2022). High-frequency direct organogenesis from cotyledonary node explants and plantlet regeneration of peanut (Arachis hypogaea) cultivars. International Journal of Agriculture and Biology, 27, 105–114. DOI 10.17957/IJAB/15.1906. [Google Scholar] [CrossRef]
28. Manjulatha, M., Sreevathsa, R., Kumar, A. M., Sudhakar, C., Prasad, T. et al. (2014). Overexpression of a pea DNA helicase (PDH45) in peanut (Arachis hypogaea L.) confers improvement of cellular level tolerance and productivity under drought stress. Molecular Biology, 56, 111–125. DOI 10.1007/s12033-013-9687-z. [Google Scholar] [CrossRef]
29. Akasaka, Y., Daimon, H., Mii, M. (2000). Improved plant regeneration from cultured leaf segments in peanut (Arachis hypogaea L.) by limited exposure to thidiazuron. Plant Science, 156, 169–175. DOI 10.1016/S0168-9452(00)00251-X. [Google Scholar] [CrossRef]
30. Palanivel, S., Parvathi, S., Jayabalan, N. (2002). Callus induction and plantlet regeneration from mature cotyledonary segments of groundnut (Arachis hypogaea L.). Journal of Plant Biology, 45(1), 22–27. DOI 10.1007/BF03030428. [Google Scholar] [CrossRef]
31. Das, A., Kumar, S., Nandeesha, P., Yadav, I. S., Saini, J. et al. (2014). An efficient in vitro regeneration system of field pea (Pisum sativum L.) via shoot organogenesis. Journal of Plant Biochemistry and Biotechnology, 23, 184–189. DOI 10.1007/s13562-013-0200-3. [Google Scholar] [CrossRef]
32. Arun, M., Subramanyam, K., Theboral, J., Ganapathi, A., Manickavasgam, M. (2014). Optimized shoot regeneration for Indian soybean: The influence of exogenous polyamine. Plant Cell, Tissue and Organ Culture, 117, 305–309. DOI 10.1007/s11240-014-0431-6. [Google Scholar] [CrossRef]
33. Sujatha, M., Vijay, S., Vasavi, S., Sivaraj, N., Rao, S. C. (2012). Combination of thidiazuron and 2-isopentenyladenine promotes highly efficient adventitious shoot regeneration from cotyledons of mature sunflower (Helianthus annuus L.) seeds. Plant Cell, Tissue and Organ Culture, 111(3), 359–372. DOI 10.1007/s11240-012-0202-1. [Google Scholar] [CrossRef]
34. Sharma, K. K., Anjaiah, V. V. (2000). An efficient method for production of transgenic plants of peanut (Arachis hypogaea L.) through Agrobacterium tumefaciens-mediated genetic transformation. Plant Science, 159(1), 7–19. DOI 10.1016/s0168-9452(00)00294-6. [Google Scholar] [CrossRef]
35. Öpik, H., Rolfe, S. A. (2005). The physiology of flowering plants. New York, NY: Cambridge University Press. DOI 10.1017/CBO9781139164450. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools