

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.022473
ARTICLE
Role of Organic Amendments to Mitigate Cd Toxicity and Its Assimilation in Triticum aestivum L.
1College of Agriculture, Bahauddin Zakariya University, Bahadur Sub-Campus Layyah, 31200, Pakistan
2Cholistan Institute of Desert Studies, The Islamia University of Bahawalpur, Bahawalpur, 63100, Pakistan
3Directorate General Soil Survey of Punjab, Agriculture Department, Lahore, 54780, Pakistan
4National Agricultural Research Centre, Islamabad, 44000, Pakistan
5Plant Production Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, 11451, Saudi Arabia
6Department of Agricultural Engineering, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, 64200, Pakistan
7School of Biological Sciences, The University of Western Australia, Perth, WA 6009, Australia
8Department of Environmental Sciences, Shaheed Benazir Bhutto University Sheringal, Dir (U), Khyber Pakhtunkhwa, 18000, Pakistan
9Academy of Biology and Biotechnology, Southern Federal University, Rostov-on-Don, 344006, Russia
10Department of Agronomy, Faculty of Agriculture, Kafrelsheikh University, Kafr El Sheikh, 33511, Egypt
*Corresponding Authors: Allah Wasaya. Email: wasayauaf@gmail.com; Ayman EL Sabagh. Email: ayman.elsabagh@agr.kfs.edu.eg
Received: 11 March 2022; Accepted: 28 April 2022
Abstract: In soil biota, higher and enduring concentration of heavy metals like cadmium (Cd) is hazardous and associated with great loss in growth, yield, and quality parameters of most of the crop plants. Recently, in-situ applications of eco-friendly stabilizing agents in the form of organic modifications have been utilized to mitigate the adverse effects of Cd-toxicity. This controlled experiment was laid down to appraise the imprints of various applied organic amendments namely poultry manure (PM), farmyard manure (FYM), and sugarcane press mud (PS) to immobilize Cd in polluted soil. Moreover, phytoavailability of Cd in wheat was also accessed under an alkaline environment. Results revealed that the addition of FYM (5–10 ton ha−1) in Cd-contaminated soil significantly increased germination rate, leaf chlorophyll content, plant height, spike length, biological and grain yield amongst all applied organic amendments. Moreover, the addition of FYM (5–10 ton ha−1) also reduced the phytoavailability of Cd by 73–85% in the roots, 57–83% in the shoots, and 81–90% in grains of wheat crop. Thus, it is affirmed that incorporation of FYM (5–10 ton ha−1) performed better to enhance wheat growth and yield by remediating Cd. Thus, the application of FYM (5–10 ton ha−1) reduced the toxicity induced by Cd to plants by declining its uptake and translocation as compared to all other applied organic amendments to immobilize Cd under sandy alkaline polluted soil.
Keywords: Cadmium toxicity; mitigation; chlorophyll attributes; morphometry; yield attributes; cereal
Heavy metal stress has become a global issue and has diverted the vast attention of researchers. The increasing prevalence of toxicant like heavy metals creates critical environmental concern that has simultaneously posed a real hazard to cultivated soil, human and atmospheric deposition [1,2]. The use of contaminated sewage sludge, industrial effluents, cadmium (Cd) containing fertilizers, insecticides and pesticides are the origin point of heavy metals (HMs), causing chronic and acute health problems when these pollutants transfer from soil to the food chain [3]. Moreover, at high concentrations, several heavy metals have been reported to inhibit plant growth and disrupt many physiological processes of crop plants [4]. The uptake of heavy metals depends on several factors including their form, concentration, soil pH, soil organic matter contents, and plant species [5].
Anthromorphic proceedings including the dumping of municipal disposal, mining work, smelting, metal production, and using of industrial phosphatic fertilizers resulted in an escalated amount of Cd in the environment, which is carcinogenic to human health [6,7]. A higher accumulation of Cd in plants restricts intake and movement of water and essential nutrients, as well as causes oxidative impairment, interrupts plant cellular metabolic activities, and impedes plant morphological and physiological development [8].
Previous studies have shown that Cd-toxicity in polluted soil may decrease plant growth [9,10] by interruption of physiological activities such as water balance, transpiration rate, photosynthetic rate, and decreased chlorophyll contents [1,11,12]. Due to the inhibiting and disturbing nature of Cd, it is not used as a favourable element for various plant species [13]. Its uptake effect the chemical composition of root at the time of seed germination [14]. In Cd stressful conditions, the plant development (both morphological and physiological) gets disturbed and becomes inefficient to build up and detoxicate Cd ions, [15]. Accumulation of Cd in plants also hinders the efficiency of nutrient uptake and affects its availability in soil [16]. For instance, Cd exposure caused the deficiency of iron in sugar beet roots [17] and inhibited the P, K, S, Ca, Zn, and Mn uptake in plants [18].
For remediation of heavy metal from polluted soils, various conventional soil remedial techniques are in use such as excavation, landfilling, soil washing, etc. So, the in-situ immobilization technique has been anticipated because it is more effective and protective for structural damage, and ecologically viable to remediate heavy metals pollution from contaminated soils [19]. Various additives and amendments have been applied as in situ treatments to reduce phytoavailability and exposure of toxic elements to living organisms [20]. Various approaches are deployed for the indemnification of Cd-toxicity from soil such as organic and inorganic amendments and agricultural practices [21]. The use of organic amendments can decrease the bio-availability of metal pollutants in the soil [22]. The uptake and translocation of metal in plants and mobility in soils can be decreased by their application [23]. Determining the chemical composition of animal manure is hard to determine, because of the huge quantity and changes in the concentration of nutrients. A lot of factors could alter the chemical and nutrient status of animal manure [24].
Poultry manure not only reduce the bio-mobility of heavy metals but also reduces its environmental risks [25]. The farmyard manure (FYM) application at sowing temporarily enhancement in soil pH, which reduces heavy metals and enhances phosphorus availability [26]. Press mud also helps to suppress heavy metal toxicity in plants [27]. Amongst cereals, wheat is a staple crop and, is being cultivated on an acreage of 8.80 m.ha in Pakistan with an average yield of 2.85 tons per hectares [28]. Bread wheat was grown on an estimated area of 9052 thousand hectares with an annual production of 25.6 million tons [29]. Numerous studies have reported that Cd uptake in wheat is governed by roots and then translocates toward shoots and finally to grains [30,31]. The Cd-toxicity diminishes the performance of the wheat crop by affecting its growth stages, photosynthetic activity, and nutritional utilization [32].
HMs occurrence is a huge hurdle for the successful cultivation of wheat and continuous poisonous and accumulation towards the soil to food chain [33] in many areas of Pakistan. Therefore, it is important to find out an alternative to reduce the heavy metals toxicity for the successful cultivation of wheat worldwide on a sustainable basis with minimum expenditure. Consequently, the present experiment was designed with the specific objective to envisage the alleviation effects of manures as soil immobilizing agents to reduce the bioavailability and Cd-accumulation in wheat under a sandy alkaline environment.
A pot trial was initiated at the Agronomical research area of Agriculture College, at Sub-Campus Layyah of Bahauddin Zakariya University Multan, Pakistan. The soil was collected from a nearby research area from a depth of 0 to 15 cm. The composite soil sample was processed further for physico-chemical characterization after air drying, grinding, and sieving through a 2 mm sieve. The texture of the soil was determined by the hydrometer process of particle size analysis, whereas soil organic matter content was estimated by Walky and Black technique. pH-meter and EC-meter were used to measure the pH value and electrical conductivity (EC) of the sampled soil, respectively by making soil and water slurry at 1:2.5 and 1:5 (w/v) ratios, respectively. Similarly, soil elements (N, K, P) concentrations were measured by the ammonium-acetate method and Olsen’s method, respectively [34,35]. The measured characteristics of the sampled soil are mentioned in Fig. 1.
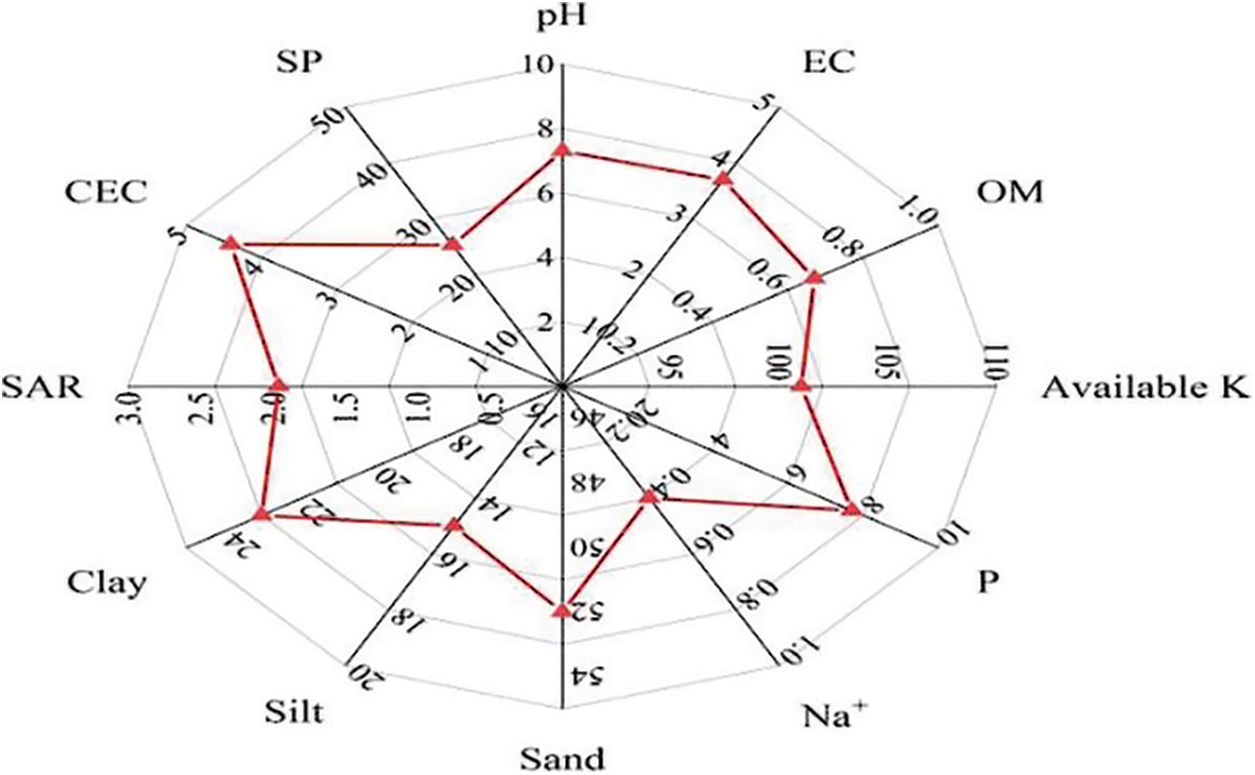
Figure 1: OM: soil organic matter (%), EC: electrical conductivity of the soil (ds m−1), Available K: available potassium (mg kg−1), P: phosphorus (ppm), Na+: sodium (mmole L−1), Sand: (%), Silt: (%), Clay: (%), SAR: sodium absorption ratio (mmole L−1)1/2, CEC: cation exchange capacity of the soil (cmolc kg−1), SP: saturation percentage (%)
For Cd treatment, the soil was contaminated with CdNO3.4H2O (5 mg kg−1 of soil) and incubated for two months at 25°C and 60% moisture content. Afterward, various organic manures like poultry manure (PM), farmyard manure (FYM), and sugar cane pressmud (PS) were added to the Cd-spiked soil. The mixture was further incubated for two months at room temperature by maintaining moisture level at 60%. After incubation, the soil sample was dried, crushed, and sieved (2 mm sieve). Soil samples, from depth of 0–15 and 15–30 cm, were collected. 1 g of soil sample was mixed with 20 ml of acetic acid solution, shaked and kept for 18 h. After that, digestion of these samples was done with di-acids H2SO4–H2O2 by keeping the samples under fumehood for 24 h. The samples were digested on hotplate until cleared liquour was obtained, then filtered and made final volume by adding distilled water. The samples were directly injected to atomic absorption spectrophotometer (AAS, PerkinElmer, PE400, California, USA) for determination of Cd concentration. Before using AAS, different samples of known elemental concentration were used for the calibration. The calibration curve was used to detect the unknown concentration of the known element in a certain solution. The AAS was calibrated by using several known standard concentration of Cd (0, 1, 2, 4, 6, 8 mg L−1). The absorbance of every Cd standard solution was determined by AAS and then calibration curve of concentration and absorbance was drawn.
Then, 5 kg of this soil mixture was stuffed in each pot. The treatments consisted of control, no amendment; PM-5, poultry manure 5 t ha−1; PM-10, poultry manure 10 t ha−1; FYM-5, farmyard manure 5 t ha−1; FYM-10, farmyard manure 10 tons ha−1, PS-5, press mud 5 tons ha−1 and PS-10, press mud 10 tons ha−1. Four replications per treatment were assigned under a completely randomized design.
After that, ten seeds of bread wheat cultivar glaxy-2013 were uniformaly dibbled in every individual pot. The recommended doses of fertilizers were applied in each pot. After having satisfactory germination, each pot was maintained by having five healthier plants. To maintain moisture, all pots were irrigated with the same quantity of water till maturity.
Germination percentage (GP) was calculated for determining the viability of seeds. It is the percentage of emerging seedlings in relation to the total wheat seeds and calculated by the method of [36], given as
Whereas, average plant height (cm), number of productive tillers on a plant basis, leaf area, and chlorophyll content were measured for the identification of plant growth. After tillering, stage total production tillers on each plant were counted and recorded. Average plant height was taken from tip to the basal portion of each plant. The greenness of leaves was observed by taking in account the average of five leaves per pot by using SPAD-502 meter. After that, wheat leaves were detached from the stem at the collar region and measured for leaf area by using a leaf area meter. At maturity, three plants from each pot were taken to estimate biological yield and then threshed manually to get grain yield.
In order to determine Cd concentration in different plant parts including roots, shoots, and grains, the samples were taken at harvesting maturity. Before processing further, the samples were treated with 1 mM solution of CaSO4 to eliminate any type of contamination. Samples were further cleaned with distilled water and then dried by placing them in a hot air oven at 80°C for 48 h. Oven dried plant roots, shoots, and grains samples were crushed to pass a 30-mesh screen. One gram sample, separately from the roots, shoots, and grains from each treatment, was collected in a conical flask, further added with 10 ml solution of HNO3:HClO4 (v/v, 3:1 ratio) and retained overnight followed by heating on a hot plate until a clear transparent solution was attained. The digested samples for roots, shoots, and grains were then used to determine Cd concentration by Atomic Absorption Spectrometer.
Collected data were evaluated statistically by using software SAS, version 9.1. Analysis of variance was done according to [37] and Tukey’s HSD-test was applied to match mean values of the treatments using 5% level of probability. Further, radar graph for soil physico-chemical properties and bar graphs for mean comparison was made by using the software Origin Pro. Whereas, correlation heat-map was generated to find out the relationship among Cd in different plant parts and soil N, P, and K by using the correlation-matrix function of the software R studio.
Different treatments of organic amendments used to mitigate Cd stress, significantly affected the soil pH (Fig. 2). Results demonstrated that the soil pH reached a maximum in that treatment which was amended with poultry manure (PM) @ 10-ton ha−1and it was about 6.31% higher than the control treatment which was treated with Cd only (Fig. 2), while amending the soil with (PM) @ 5-ton ha−1, FYM @ 5 and 10-ton ha−1, PS @ 5 and 10-ton ha−1 increase the soil pH to about 4.25%, 2.74%, 3.16%, 3.57% and 4.66%, respectively, as compared to the unamended soil.
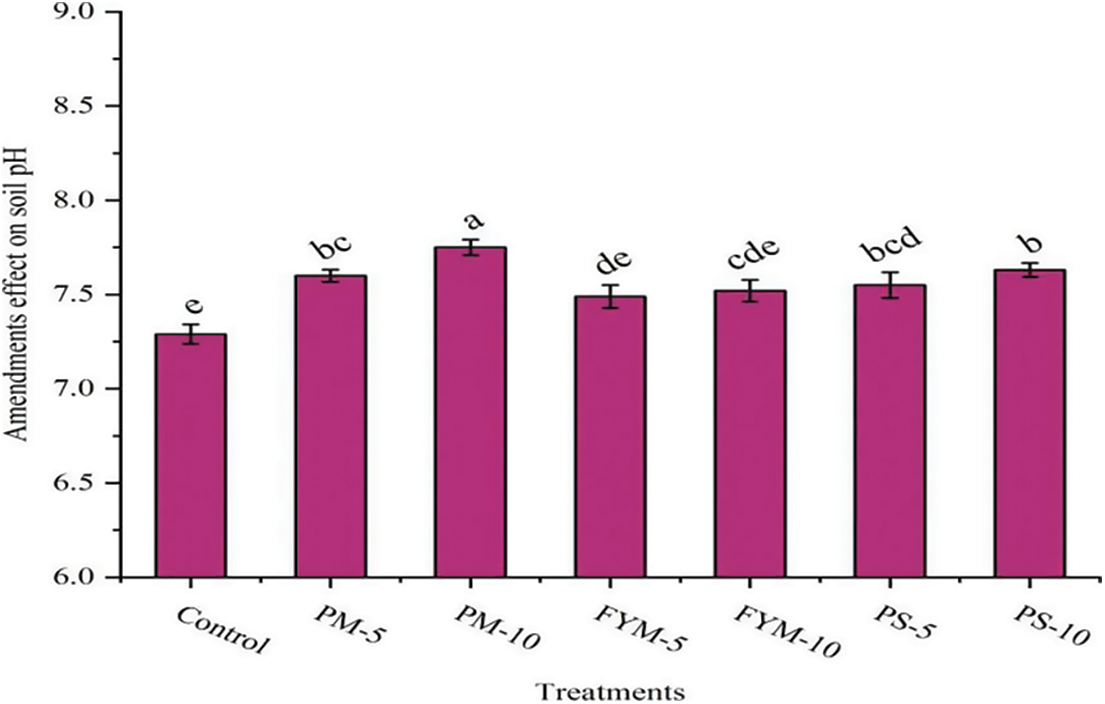
Figure 2: Influence of organic amendments application on soil pH ± S.E
The value showing different letters are significant at 5% probability, whereas values with similar letters are not statistically different at 5% probability.
PM-5, poultry manure @ 5-ton ha−1; PM-10, poultry manure @ 10-ton ha−1; FYM-5, Farmyard manure @ 5-ton ha−1; FYM-10, Farmyard manure @ 10-ton ha−1; PS-5, press-mud @ 5-ton ha−1; PS-10, press-mud @ 10-ton ha−1.
Similarly, organic amendments affected significantly and decreased (P < 0.05) the plant available Cd in the soil compared with non-treated soil (Fig. 3). Rapid reduction in soil Cd was observed with the addition of different levels of each organic source. Farmyard manure (FYM) proved a better treatment against cd-toxicity compared with other organic sources. Further, a maximum decrease (88%) in plant-available Cd compared with control treatment was recorded in soil received FYM-10 (Fig. 3). while the addition of other organic amendments as (PM) @ 5 and 10-ton ha−1, FYM @ 5-ton ha−1, PS @ 5 and 10-ton ha−1 decrease the Cd concentration in soil to about 49%, 75%, 69%, 60% and 71%, respectively, in relation with control condition.
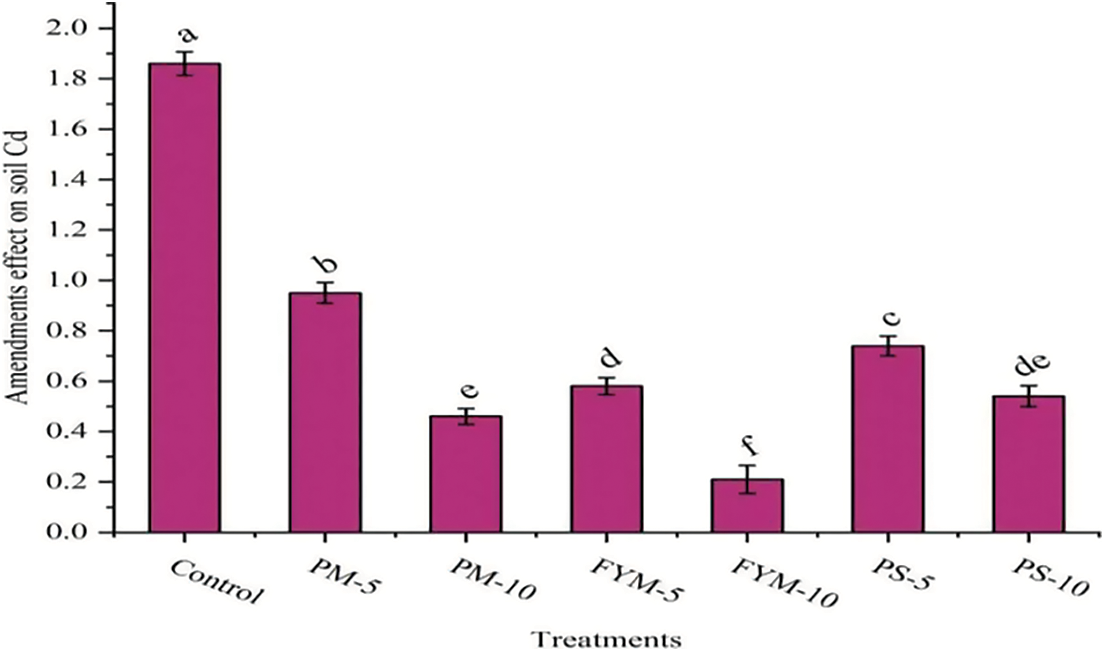
Figure 3: Influence of organic amendments application on the concentration of Cd in the soil ± S.E
The value showing different letters are significant at 5% probability, whereas values with similar letters are not statistically different at 5% probability.
PM-5, poultry manure @ 5-ton ha−1; PM-10, poultry manure @ 10-ton ha−1; FYM-5, Farmyard manure @ 5-ton ha−1; FYM-10, Farmyard manure @ 10-ton ha−1; PS-5, press-mud @ 5-ton ha−1; PS-10, press-mud @ 10-ton ha−1.
The addition of different organic stuff into the Cd contaminated soil significantly ameliorated the chlorophyll content of wheat plants (Fig. 4). It is evident from Fig. 4 that FYM produced higher chlorophyll contents, which further depicts that, in comparison to control treatment, (3.86 times) higher chlorophyll contents were noted in the treatment which was amended with FYM-10 and then subsequently observed in PM-10 (2.98 times), PS-10 (2.60 times), FYM-5 (2.40 times), PM-5 (1.81 times) and PS-5 (1.63 times).
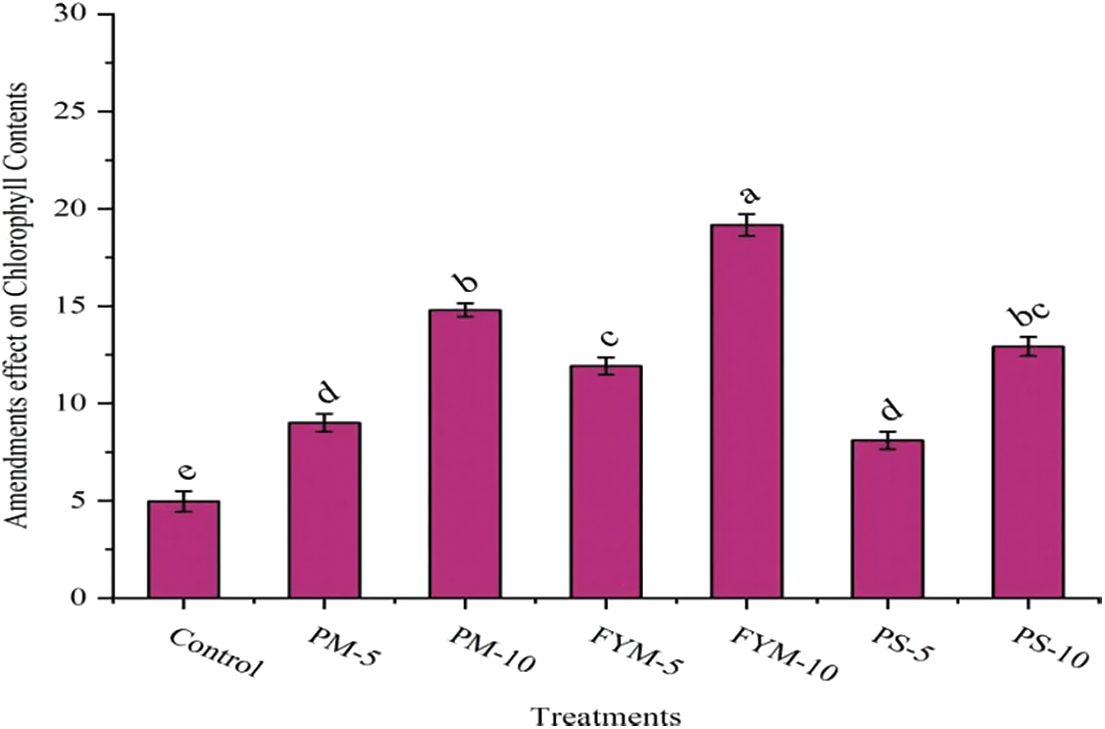
Figure 4: Influence of organic amendments application on chlorophyll contents of wheat ± S.E
The value showing different letters are significant at 5% probability, whereas values with similar letters are not statistically different at 5% probability.
PM-5, poultry manure @ 5-ton ha−1; PM-10, poultry manure @ 10-ton ha−1; FYM-5, Farmyard manure @ 5-ton ha−1; FYM-10, Farmyard manure @ 10-ton ha−1; PS-5, press-mud @ 5-ton ha−1; PS-10, press-mud @ 10-ton ha−1.
Cadmium toxicity showed adverse effects on seed germination, but its inhibitory effect was reduced with the addition of organic amendments. The addition of FYM at all rates significantly improved seed germination rate about 62–186%; while, poultry manure, and press mud gave better results when applied in higher quantities of 10 tons ha−1. A maximum rate of germination was noticed where FYM was applied in a higher quantity of 10 tons ha−1 (Table 1). Likewise, the maximum number of tillers was recorded in the treatment where FYM was applied at the rate of 10 tons ha−1 as compared to the control (Table 1).
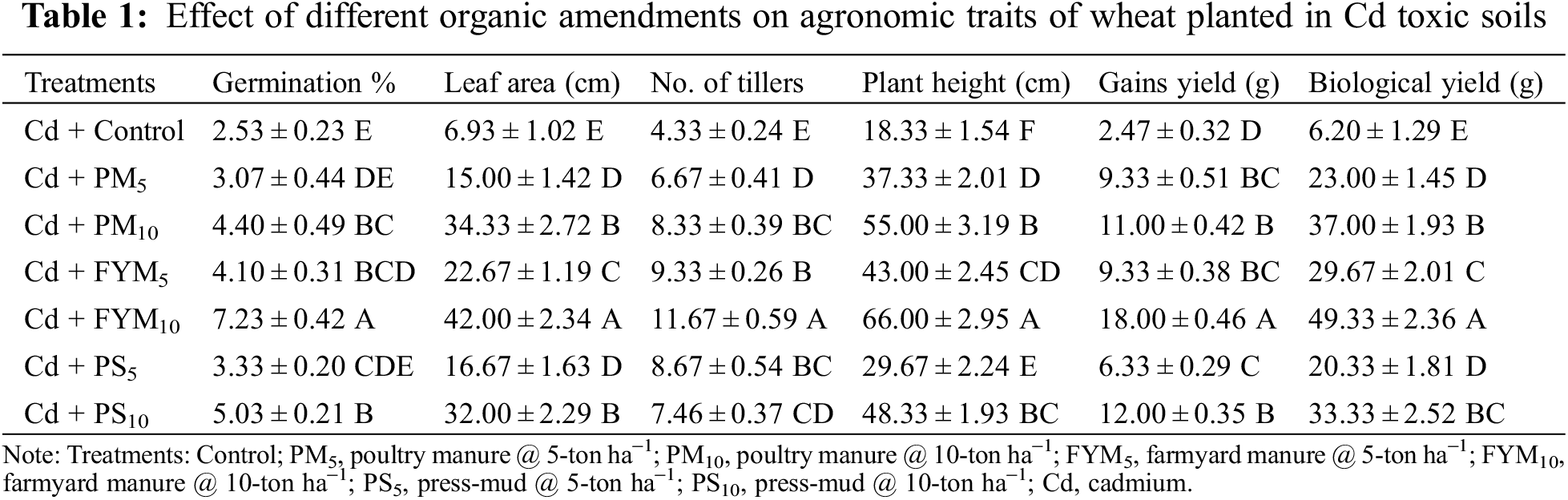
A significant variation in plant height was observed after the application of different organic amendments (Table 1). Among the different application sources of organic amendments, the maximum height of wheat plants was observed by using 10 tons ha−1 and it was 260% higher as compared to the control treatment followed by PM, PS @ 10 tons ha−1, FYM, PM and PS @ 5 tons ha−1 respectively. The lowest plant height was measured in the control treatment (Table 1).
Various treatments of organic amendments significantly affected the leaf area of wheat during the growing season of the crop under Cd stress. Among the different amendments sources, the application of FYM with 5–10 tons ha−1 concentration showed higher leaf area as compared to other amendments (Table 1) which were 231–506% higher than the control treatment. Whereas, the lowest leaf area was measured in the control treatment. The total dry matter produced by crop plants was determined by biological yield. Applications of various organic amendments significantly affect biological yield. All amendment sources enhanced the biological yield of wheat; however, the highest biological yield was received from the treatment where FYM was added at the rate of 10 tons ha−1 which was almost seven times higher than the control treatment (Table 1). Similarly, other amendments enhance the biological yield by the following ratio; PM @ 5–10 tons ha−1 (3–7 times), FYM @ 5 tons ha−1 (5 times), PS @ 5–10 tons ha−1 (3–5 times), respectively.
Addition of organic amendments substantially enhanced the grain yield of wheat. In this scenario, the addition of FYM at the rate of 10 tons ha−1 showed the highest yield of wheat grains, which was noted 630% higher than the control treatment (Table 1). With the addition of various organic stuff, the analyzed levels of Cd in plant parts were found significantly decreased (Table 2). Furthermore, among plant parts, the maximum Cd was analyzed in roots than in shoots and grains, respectively. Amongst all organic additives, the lowest Cd concentration was observed in FYM10 treatment, where it was decreased by 85% in roots, 83% in shoots, and 90% in grains when checked with the control (Table 2).
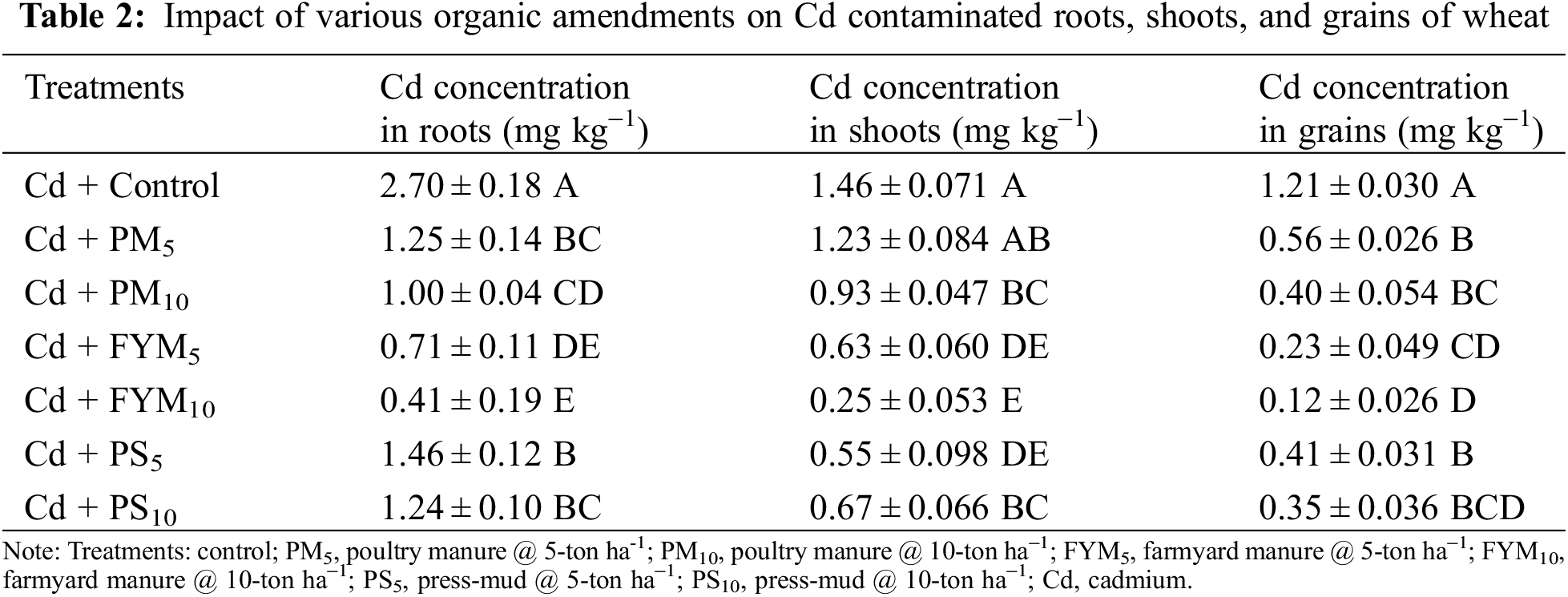
The soil nutrient status regarding N, P, and K was upgraded considerably by using various amendments (Table 3). Under diverse applied organic amendments, the highest levels of N, P, and K (484%, 365%, and 389%, higher than the control, respectively) figured out with the modification of Cd toxicity was done with FYM10 treatment (Table 3).
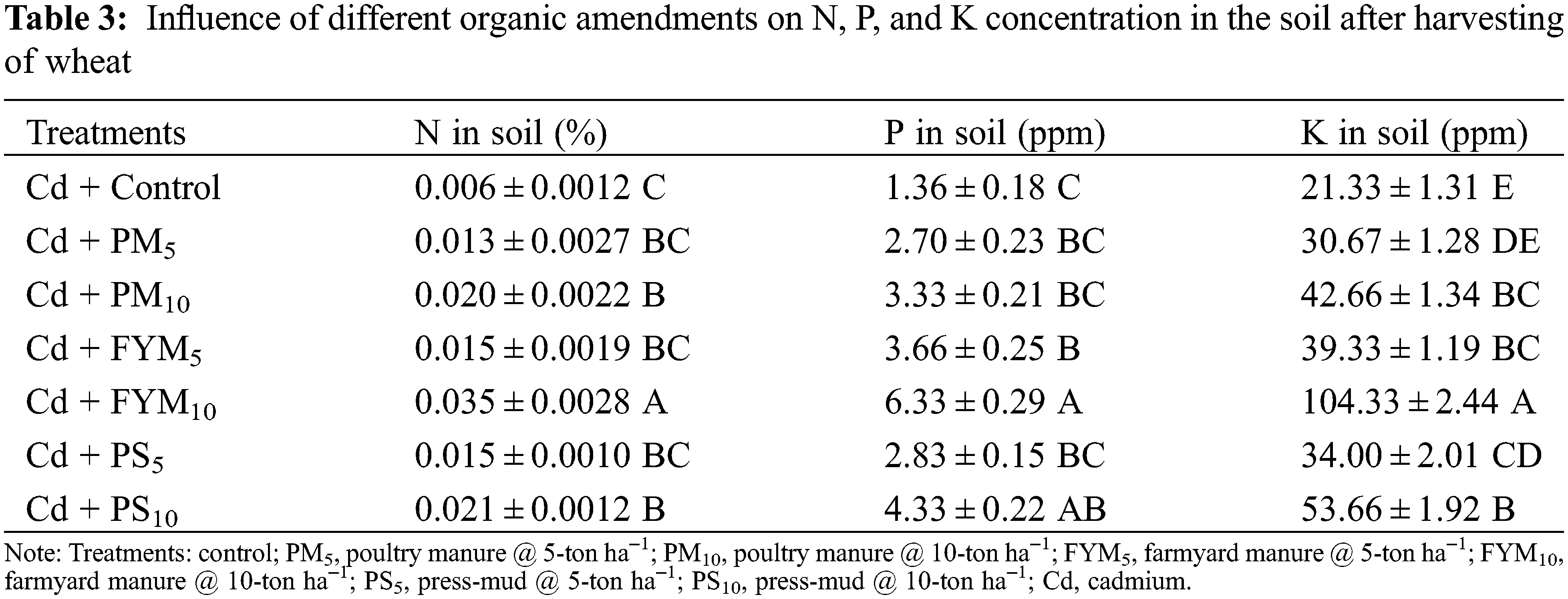
After considering the correlation ratio of the chosen parameters (soil N, P, K, soil pH, Cd in soil, roots, shoots, and grains of wheat plant), it was it was found that there were 31% strong positive correlation and 28% strong negative correlation; while, the remaining were moderately correlated either as positive and negative. The presence of N, P, and K in soil showed a strong positive correlation with each other, while the negative correlation with Cd in soil, plant roots, aboveground shoots, and in grains samples. All the components were correlated significantly to each other, except soil pH and K in the soil. Soil pH showed a non-significant correlation with all the remaining components except itself; while K in soil showed non-significant association with pH and in soil samples as well as roots and grains (Fig. 5).

Figure 5: Correlation of (A: Soil N, P, K; B: Cd in soil, roots, shoots, grains and soil pH) N = nitrogen, P = phosphorus, K = potassium, Cd = cadmium
Cd enriched soils also known as the polluted soils, disturbed the soil environment and have negative effect on growth and development of plant when it accumulates in plant body parts [38]. The current study elaborates how organic amendments alleviate Cd-induced changes in wheat plant growth, heavy-metal uptake by roots, and its distribution in different plant parts and physiological attributes. The toxicity of Cd in the control treatment resulted in a very low germination percentage. Due to Cd toxicity breakdown of stored food material in seed [39] results in a lower germination rate [40]. In the current study, the application of different organic materials resulted in a high germination rate compared with control which could be due to the efficacy of organic material for immobilizing Cd; thus, decreasing availability to germinating seeds. However, results have shown this effect widely varied with the type of organic material which could be related to the degree of decomposition of that material and relative persistency in soil. The highest seed germination in FYM-10 could be associated with ease of decomposition of FYM to become the part of soil more efficiently than other organic sources which might have caused higher confinement of Cd in the soil. Previously, the same was reported by [41] that application of FMY increased seed germination rate under Cd stress. The addition of FYM improved the nutrient status and physical characteristics of soil and effectively decreased Cd uptake in plants [42]. Cd-induced reduction in growth and biological yield in wheat was observed in this study which has already been reported by previous studies [43,44]. Similarly, plant leaf area and the total number of leaves have also been reported to be decreased by Cd [45]. The presence of Cd creates irregularities in plant growth such as browning and decomposing of roots, reduction in the elongation of root and shoots, variation in the ultrastructure of chloroplast, which produced chlorosis and reduced the photosynthesis activity [46,47]. The current study revealed that the applied quantities of organic materials especially FYM improved the leaf area in wheat plants. Similarly, a previous study showed that FYM application in Cd and Zinc-contaminated soils significantly modified growth parameters by reducing their uptake in maize crop [48].
The addition of FYM in higher quantity improved total plant tillers as well as the height of individual plant height on an average basis in Cd contaminated soil (Table 2). The application of 30 mg FYM ha−1 significantly increased the plant height, number of fertile tillers m−2, yield attributes, and wheat harvest index [49]. The response of plant growth to FYM was increased with an increasing application rate. By applying the FYM, the improvement in plant response in terms of growth parameters, possibly attributed to the proliferation of mineralization and absorption of nutrients which later translated into better plant height than control plants. The biological and grain yield of wheat was being reduced under Cd toxicity [50] stated that manure application under Cd stress boosted plant growth and biomass of wheat crop and reduced the uptake of Cd concentration in plants. The difference in biological yield might be due to decreasing organic amendments level. Similar results were also reported by [51] who observed that increasing organic manure amendment quantities bring a significant increment in yield parameters of the potato crop. The increase in wheat yield was perhaps caused by the effects of organic manures on growth-related characteristics which resulted in improved biomass production, leaf growth, chlorophyll density resulting in more photo-assimilate production and ultimately translated into higher grain yield [52].
It has been found that Cd-reduced the plant height [53] and nutrient uptake such as calcium, zinc, and iron level in Zea mays L. [54]. In this study, Cd stress also reduced the plant development, absorption of nutrients and their translocation in the plant parts. It has been reported that, in different growing condition, the status of different nutrients in wheat plant tissues were decreased by Cd-toxicity [33]. It is evident that the concentration of major nutrients like N, P, and K found to be reduced after harvesting of wheat in cadmium toxic soils. The organic amendments have ability to retain nutrients in soils and improved the soil structure and fertility [55]. In the present study, the use of organic amendments remarkably upscaled the concentrations of NPK within the soil as compared to the control. However, a significant increase in NPK contents compared with other treatments recorded with FYM-10 treatment. As it was previously reported that the application of FYM soil not only improves characteristics of soil in all aspects but also supplies the crops with all kinds of required nutrients [56]. The increased levels of high-demanding nutrients like N, P, and K are possible because of the addition of organic amendments that significantly enhanced the nutrient mobility in soil [55].
In the present study, the addition of organic amendments reduced Cd concentration to a great extent in various samples of wheat. The reduction of Cd in plant tissue might be due to various mechanisms of organic amendments. The use of organic amendment in minimizing the Cd concentration would involve the immobilization of Cd by organic fraction [57]. Similarly, nutrients supplying the ability of organic amendments might have improved wheat production and Cd uptake. Because in the present study, nutrients (NPK) concentrations increased significantly in organically amended soil in relation to the check treatment, whereas Cd distribution among different samples of wheat was negatively correlated with nutrient contents in soil (Fig. 5). Dilution effect due to increase in plant biomass minimizes the hazardous effect on plants grown in contaminated soil [58]. Moreover, different nutrients have different effects in reducing the harmful effects of Cd. Nitrogen supply improves Cd binding protein in plants which helps in detoxification and reduces its accumulation in seed [59]. Similarly, the formation of insoluble Cd phosphate in soil due to P declines its accumulation in seed [60]. In this study, although different organic sources reduced Cd accumulation in wheat, however, minimal Cd accumulation was recorded with FYM-10 treatment. These results suggested that Cd mobility and phytoavaibility vary with the kind of organic matter as well as the rate of its application.
The amendment of soils with FYM produced wheat plants with comparatively lesser Cd concentration in different tissues of plant than the soils amended by other types of organic matters (Table 2). The quantification of Cd, in the soils which have hazardous quantities of heavy metals importantly Cd, by lowering its amount in useable parts of plant materials is highly demanded for producing safe food [61]. Factors including soil pH, fertilizer type, plant species, and soil texture influence the effect of N on the intake of Cd by plants [62]. Adaptation of various agricultural approaches including organically and inorganically produced amendments, and other practices have a negative relationship with cadmium mobilization and its absorption in crop plants [63]. The use of organic fertilizers has also restricted the ions of heavy metals to be transported from soil to plants through [64]. Nutrients present in organic amendments decrease Cd concentration in shoots, grains [65], and roots [66].
The present study concludes that the application of various organic amendments was found useful in making the soil environment conducive for plant growth by lowering the concentration of Cd in soil and by optimizing soil properties to a suitable limit as well as improving the nutrient status of the soil. Moreover, the application of organic amendments has the potential to ameliorate the quantity of Cd not only in the soil but also in different plant parts (root, shoot and grain). Organic amendments also enhanced the agronomic performance of wheat crop by increasing nutrient accessibility in the form of N,P and K in the soil environment. It is therefore recommended to use FYM for phytostabilization of soils and to optimize the yield potential of wheat crop in soils which have higher levels of cadmium.
Acknowledgement: The authors extend their appreciation to the Researchers Supporting Project No. (RSP-2021/390), King Saud University, Riyadh, Saudi Arabia. The authors thank Dr. Vishnu D. Rajput and Dr. Tatiana Minkins for their contributions to improve the paper.
Funding Statement: This research was funded by the Researchers Supporting Project No. (RSP-2021/390), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ali, B., Qian, P., Jin, R., Khan, M., Aziz, R. et al. (2014). Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biologia Plantarum, 58, 131–138. DOI 10.1007/s10535-013-0358-5. [Google Scholar] [CrossRef]
2. Ali, B., Mwamba, T. M., Yang, C., Daud, M. K., Wu, Y. et al. (2014). Improvement of element uptake and antioxidative defense in Brassica napus under lead stress by application of hydrogen sulfide. Plant Growth Regulation, 74, 261–273. DOI 10.1007/s10725-014-9917-9. [Google Scholar] [CrossRef]
3. Alengebawy, A., Abdelkhalek, S. T., Qureshi, S. R., Wang, M. Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics, 9, 42. DOI 10.3390/toxics9030042. [Google Scholar] [CrossRef]
4. Shahkolaie, S. S., Baranimotlagh, M., Dordipour, E., Khormali, F. (2020). Effects of inorganic and organic amendments on physiological parameters and antioxidant enzymes activities in Zea mays L. from a cadmium-contaminated calcareous soil. South African Journal of Botany, 128, 132–140. DOI 10.1016/j.sajb.2019.10.007. [Google Scholar] [CrossRef]
5. Arshad, M., Ali, M., Noman, M. S., Ali, S. A., Rizwan, Q. et al. (2015). Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Archives of Agronomy and Soil Science, 62, 533–546. DOI 10.1080/03650340.2015.1064903. [Google Scholar] [CrossRef]
6. Karadas, C., Kara, D. (2011). In vitro gastro-intestinal method for the assessment of heavy metal bioavailability in contaminated soils. Environ Science and Pollution Research, 18, 620–628. DOI 10.1007/s11356-010-0404-1. [Google Scholar] [CrossRef]
7. Qadir, M., Ghafoor, A., Murtaza, G. (2000). Cadmium concentration in vegetables grown on urban soils irrigated with untreated municipal sewage. Environment, Development and Sustainability, 2, 11–19 DOI 10.1023/A:1010061711331. [Google Scholar] [CrossRef]
8. Haider, F. U., Liqun, C., Coulter, J. A., Cheema, S. A., Wu, J. et al. (2021). Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicology and Environmental Safety, 211, 111887. DOI 10.1016/j.ecoenv.2020.111887. [Google Scholar] [CrossRef]
9. Gozubenli, H. (2010). Seed vigor of maize grown on the contaminated soils by cadmium. Asian Journal of Plant Sciences, 9, 168–171. DOI 10.3923/ajps.2010.168.171. [Google Scholar] [CrossRef]
10. Gupta, D., Abdullah. (2011). Toxicity of copper and cadmium on germination and seedling growth of maize (Zea mays L.) seeds. Indian Journal of Scientific Research, 2, 67–70. [Google Scholar]
11. Zhou, W. B., Qiu, B. S. (2005). Effects of cadmium hyperaccumulation on physiological characteristics of Sedum alfredii Hance (Crassulaceae). Plant Science, 169, 737–745. DOI 10.1016/j.plantsci.2005.05.030. [Google Scholar] [CrossRef]
12. Benavides, M. P., Gallego, S. M., Tomaro, M. L. (2005). Cadmium toxicity in plants. Brazilian Journal of Plant Physiology, 17, 21–34. DOI 10.1590/S1677-04202005000100003. [Google Scholar] [CrossRef]
13. Popa, C., Petrus, M., Bratu, A. M. (2022). Alfalfa (Medicago sativa) sprouts respiratory responses to cadmium stress using IR LPAS. Molecules, 27(6), 1891. DOI 10.3390/molecules27061891. [Google Scholar] [CrossRef]
14. Popa, C., Bratu, A. M., Bacalum, M., Prepelita, P. (2020). Application of the laser technology on the effect of Cd phytotoxicity in the detection of NH3, C2H4, C2H5OH and CO2 biomolecules at Triticum aestivum plantlets. Sustainable Chemistry and Pharmacy, 15, 100208. DOI 10.1016/j.scp.2019.100208. [Google Scholar] [CrossRef]
15. Małecka, A., Konkolewska, A., Hanć, A., Barałkiewicz, D., Ciszewska, L. et al. (2019). Insight into the phytoremediation capability of Brassica juncea (v. malopolskaMetal accumulation and antioxidant enzyme activity. International Journal of Molecular Sciences, 20, 4355. DOI 10.3390/ijms20184355. [Google Scholar] [CrossRef]
16. Dobrikova, A. G., Apostolova, E. L., Hanć, A., Yotsova, E., Borisova, P. (2021). Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicology and Environmental Safety, 209, 111851. DOI 10.1016/j.ecoenv.2020.111851. [Google Scholar] [CrossRef]
17. Chang, Y. C., Zouari, M., Gogorcena, Y., Lucena, J. J., Abadía, J. (2003). Effects of cadmium and lead on ferric chelate reductase activities in sugar beet roots. Plant Physiology and Biochemistry, 41, 999–1005. DOI 10.1016/j.plaphy.2003.07.007. [Google Scholar] [CrossRef]
18. Metwally, A., Safronova, V. I., Belimov, A. A., Dietz, K. J. (2005). Genotypic variation of the response to cadmium toxicity in Pisum sativum L. Journal of Experimental Botany, 56, 167–178. [Google Scholar]
19. Liang, Y., Cao, X., Zhao, L., Arellano, E. (2014). Biochar and phosphate-induced immobilization of heavy metals in contaminated soil and water: Implication on simultaneous remediation of contaminated soil and groundwater. Environmental Science and Pollution Research, 22, 4665–4674. DOI 10.1007/s11356-013-2423-1. [Google Scholar] [CrossRef]
20. Vangronsveld, J., Herzig, R., Weyens, N., Boulet, J., Adriaensen, K. et al. (2009). Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environmental Science and Pollution Research International, 16, 765–794. DOI 10.1007/s11356-009-0213-6. [Google Scholar] [CrossRef]
21. Qayyum, M. F., Rehman, M. Z. U., Ali, S., Rizwan, M., Naeem, A. et al. (2017). Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere, 174, 515–523. DOI 10.1016/j.chemosphere.2017.02.006. [Google Scholar] [CrossRef]
22. Zhang, M., Gao, B., Varnoosfaderani, S., Hebard, A., Yao, Y. et al. (2013). Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresource Technology, 130, 457–462. DOI 10.1016/j.biortech.2012.11.132. [Google Scholar] [CrossRef]
23. Pichtel, J., Bradway, D. (2008). Conventional crops and organic amendments for Pb, Cd and Zn treatment at a severely contaminated site. Bioresource. Technology, 99, 1242–1251. DOI 10.1016/j.biortech.2007.02.042. [Google Scholar] [CrossRef]
24. Celi, P., Cowieson, A. J., Fru-Nji, F., Steinert, R. E., Kluenter, A. M. et al. (2017). Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Animal Feed Science and Technology, 234, 88–100. DOI 10.1016/j.anifeedsci.2017.09.012. [Google Scholar] [CrossRef]
25. Aljerf, L., Aljurf, M. (2020). Improvements in the ecological and nutritional aspects of down’s syndrome. Preprints. DOI 10.21203/rs.3.rs-30313/v1. [Google Scholar] [CrossRef]
26. De Bauw, P., Shimamura, E., Rakotoson, T., Andriamananjara, A., Verbeeck, M. et al. (2021). Farm yard manure application mitigates aluminium toxicity and phosphorus deficiency for different upland rice genotypes. Journal of Agronomy and Crop Science, 207, 148–162. DOI 10.1111/jac.12436. [Google Scholar] [CrossRef]
27. Mushtaq, Z., Asghar, H. N., Zahir, Z. A. (2021). Comparative growth analysis of okra (Abelmoschus esculentus) in the presence of PGPR and press mud in chromium contaminated soil. Chemosphere, 262, 127865. DOI 10.1016/j.chemosphere.2020.127865. [Google Scholar] [CrossRef]
28. FAO. FAOSTAT Data (2018). http://www.fao.org/faostat. [Google Scholar]
29. Jaitley, A. (2017). Economic Survey 2016-17 (No. id: 11601). [Google Scholar]
30. Naeem, A., Ghafoor, A., Farooq, M. (2015). Suppression of cadmium concentration in wheat grains by silicon is related to its application rate and cadmium accumulating abilities of cultivars. Journal of the Science of Food and Agriculture, 95, 2467–2472. DOI 10.1002/jsfa.6976. [Google Scholar] [CrossRef]
31. Rehman, M. Z. U., Rizwan, M., Ghafoor, A., Naeem, A., Ali, S. et al. (2015). Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environmental Science and Pollution Research, 22, 16897–16906. DOI 10.1007/s11356-015-4883-y. [Google Scholar] [CrossRef]
32. Rizwan, M., Ali, S., Abbas, T., Rehman, M. Z. U., Hannan, F. et al. (2016). Cadmium minimization in wheat: A critical review. Ecotoxicology and Environmental Safety, 130, 43–53. DOI 10.1016/j.ecoenv.2016.04.001. [Google Scholar] [CrossRef]
33. Murtaza, G., Javed, W., Hussain, A., Qadir, M., Aslam, M. (2017). Soil-applied zinc and copper suppress cadmium uptake and improve the performance of cereals and legumes. International Journal of Phytoremediation, 19, 199–206. DOI 10.1080/15226514.2016.1207605. [Google Scholar] [CrossRef]
34. Lu, R. K. (1999). Analytical methods of soil agrochemistry, pp. 85–96. China Agricultural Science and Technology Press, Beijing. [Google Scholar]
35. Parkinson, M., Allen, N. (1975). A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Communications in Soil Science and Plant Analysis, 6, 1–11. DOI 10.1080/00103627509366539. [Google Scholar] [CrossRef]
36. Yue, S., Zhou, Y., Zhang, Y., Xu, S., Gu, R. et al. (2019). Effects of salinity and temperature on seed germination and seedling establishment in the endangered seagrass Zostera japonica Asch. & Graebn. in northern China. Marine Pollution Bulletin, 146, 848–856. DOI 10.1016/j.marpolbul.2019.07.037. [Google Scholar] [CrossRef]
37. Steel, R. G. D., Torrie, J. H., Dicky, D. A. (1997). Principles and procedures of statistics, a biometrical approach, 3rd Edition, pp. 352–358. McGraw Hill, Inc. Book Co., New York. [Google Scholar]
38. Paul, A. L., Chaney, R. L. (2017). Effect of soil amendments on Cd accumulation by spinach from a Cd-mineralized soil. Journal of Environmental Quality, 46(4), 707–713. DOI 10.2134/jeq2016.07.0251. [Google Scholar] [CrossRef]
39. Raziuddin, Hassan, G., Akmal, M., Shah, S., Mohammad, F. et al. (2011). Effects of cadmium and salinity on growth and photosynthesis parameters of brassica species. Pakistan Journal of Botany, 43, 333–340. [Google Scholar]
40. Ahmad, I., Akhtar, M. J., Zahir, Z. A., Jamil, A. (2012). Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pakistan Journal of Botany, 44, 1569–1574. [Google Scholar]
41. Rajendra, P., Singh, R. K., Archna, R., Singh, D. K. (2000). Partial factor productivity of nitrogen and its use efficiency in rice and wheat. Fertiliser News, 45, 63–65. [Google Scholar]
42. Asagba, S. O., Ezedom, T., Kadiri, H. (2017). Influence of farmyard manure on some morphological and biochemical parameters of cowpea (Vigna unguiculata) seedling grown in cadmium-treated soil. Environmental Science and Pollution Research, 24, 23735–23743. DOI 10.1007/s11356-017-9988-z. [Google Scholar] [CrossRef]
43. Naeem, A., Rehman, M. Z. U., Akhtar, T., Zia, M. H., Aslam, M. (2018). Silicon nutrition lowers cadmium content of wheat cultivars by regulating transpiration rate and activity of antioxidant enzymes. Environmental Pollution, 242, 126–135. DOI 10.1016/j.envpol.2018.06.069. [Google Scholar] [CrossRef]
44. Azhar, M., Rehman, M. Z. U., Ali, S., Qayyum, M. F., Naeem, A. et al. (2019). Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere, 227, 72–81. DOI 10.1016/j.chemosphere.2019.04.041. [Google Scholar] [CrossRef]
45. Khan, N. A., Samiullah, Singh, S., Nazar, R. (2007). Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress. Journal of Agronomy and Crop Science, 193, 435–444. [Google Scholar]
46. Liu, J., Cao, C., Wong, M., Zhang, Z., Chai, Y. (2010). Variations between rice cultivars in iron and manganese plaque on roots and the relation with plant cadmium uptake. Journal of Environmental Sciences, 22, 1067–1072. DOI 10.1016/S1001-0742(09)60218-7. [Google Scholar] [CrossRef]
47. Miyadate, H., Adachi, S., Hiraizumi, A., Tezuka, K., Nakazawa, N. et al. (2011). OsHMA3, a P18-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytologist, 189, 190–199. DOI 10.1111/j.1469-8137.2010.03459.x. [Google Scholar] [CrossRef]
48. Putwattana, N., Kruatrachue, M., Kumsopa, A., Pokethitiyook, P. (2015). Evaluation of organic and inorganic amendments on maize growth and uptake of Cd and Zn from contaminated paddy soils. International Journal of Phytoremediation, 17, 165–174. DOI 10.1080/15226514.2013.876962. [Google Scholar] [CrossRef]
49. Jan, A., Amanullah, Noor, M. (2011). Wheat response to farm yard manure and nitrogen fertilization under moisture stress conditions. Journal of Plant Nutrition, 34, 732–742. DOI 10.1080/01904167.2011.540688. [Google Scholar] [CrossRef]
50. Ahmad, A., Hadi, F., Ali, N. (2015). Effective phytoextraction of cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L) plant under various treatments of fertilizers, plant growth regulators and sodium salt. International Journal of Phytoremediation, 17, 56–65. DOI 10.1080/15226514.2013.828018. [Google Scholar] [CrossRef]
51. Angelova, V., Ivanova, R., Pevicharova, G., Ivanov, K. (2010). Effect of organic amendments on heavy metals uptake by potato plants. Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, pp. 84–87. Brisbane, Australia. [Google Scholar]
52. Gopinath, K. A., Saha, S., Mina, B. L., Pande, H., Kundu, S. et al. (2008). Influence of organic amendments on growth, yield and quality of wheat and on soil properties during transition to organic production. Nutrient Cycling in Agroecosystems, 82, 51–60. DOI 10.1007/s10705-008-9168-0. [Google Scholar] [CrossRef]
53. Anjum, S. A., Tanveer, M., Hussain, S., Bao, M., Wang, L. et al. (2015). Cadmium toxicity in maize (Zea mays L.Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environmental Science and Pollution Research, 22, 17022–17030. DOI 10.1007/s11356-015-4882-z. [Google Scholar] [CrossRef]
54. Castillo-Michel, H. A., Hernandez, N., Martinez-Martinez, A., Parsons, J. G., Peralta-Videa, J. R. et al. (2009). Coordination and speciation of cadmium in corn seedlings and its effects on macro-and micronutrients uptake. Plant Physiology and Biochemistry, 47, 608–614. DOI 10.1016/j.plaphy.2009.02.005. [Google Scholar] [CrossRef]
55. Maman, N., Mason, S. (2013). Poultry manure and inorganic fertilizer to improve pearl millet yield in Niger. African Journal of Plant Science, 7, 162–169. [Google Scholar]
56. Ndukwe, O. O., Muoneke, C. O., Baiyeri, K. P., Tenkouano, A. (2011). Growth and yield responses of plantain genotypes as influenced by organic and inorganic fertilizers. Journal of Plant Nutrition, 34, 700–716. DOI 10.1080/01904167.2011.540686. [Google Scholar] [CrossRef]
57. Rehman, M. Z. U., Zafar, M., Waris, A. A., Rizwan, M., Ali, S. et al. (2020). Residual effects of frequently available organic amendments on cadmium bioavailability and accumulation in wheat. Chemosphere, 244, 125548. DOI 10.1016/j.chemosphere.2019.125548. [Google Scholar] [CrossRef]
58. Chattha, M. U., Arif, W., Khan, I., Soufan, W., Chattha, M. B. et al. (2021). Mitigation of cadmium induced oxidative stress by using organic amendments to improve the growth and yield of mash beans [Vigna mungo (L.)]. Agronomy, 11, 2152. DOI 10.3390/agronomy11112152. [Google Scholar] [CrossRef]
59. Zhang, F., Wan, X., Zhong, Y. (2014). Nitrogen as an important detoxification factor to cadmium stress in poplar plants. Journal of Plant Interactions, 9, 249–258. DOI 10.1080/17429145.2013.819944. [Google Scholar] [CrossRef]
60. Gupta, D. K., Chatterjee, S., Datta, S., Veer, V., Walther, C. (2014). Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere, 108, 134–144. DOI 10.1016/j.chemosphere.2014.01.030. [Google Scholar] [CrossRef]
61. Rizwan, M., Ali, S., Adrees, M., Rizvi, H., Rehman, M. Z. U. et al. (2016). Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environmental Science and Pollution Research, 23, 17859–17879. DOI 10.1007/s11356-016-6436-4. [Google Scholar] [CrossRef]
62. Huang, X., Duan, S., Wu, Q., Yu, M., Shabala, S. (2020). Reducing cadmium accumulation in plants: Structure–function relations and tissue-specific operation of transporters in the spotlight. Plants, 9, 223. DOI 10.3390/plants9020223. [Google Scholar] [CrossRef]
63. Abbas, T., Rizwan, M., Ali, S., Adrees, M., Mahmood, A. et al. (2018). Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicology and Environmental Safety, 148, 825–833. DOI 10.1016/j.ecoenv.2017.11.063. [Google Scholar] [CrossRef]
64. Lwin, C. S., Seo, B. H., Kim, H. U., Owens, G., Kim, K. R. (2018). Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil Science and Plant Nutrition, 64, 156–167. DOI 10.1080/00380768.2018.1440938. [Google Scholar] [CrossRef]
65. Li, M., Mohamed, I., Raleve, D., Chen, W., Huang, Q. (2016). Field evaluation of intensive compost application on Cd fractionation and phytoavailability in a mining-contaminated soil. Environmental. Geochemistry and Health, 38, 1193–1201. DOI 10.1007/s10653-015-9784-y. [Google Scholar] [CrossRef]
66. Abedi, T., Mojiri, A. (2020). Cadmium uptake by wheat (Triticum aestivum L.An overview. Plants, 9, 500. DOI 10.3390/plants9040500. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |