

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.021644
ARTICLE
Termites Improve the Horizontal Movement of Carbonized Particles: A Step towards Sustainable Utilization of Biochar
1Department of Environmental Sciences, COMSATS University Islamabad, Vehari Campus, Vehari, 61100, Pakistan
2Department of Plant Production, College of Food and Agriculture, King Saud University, Riyadh, 11451, Saudi Arabia
3Graduate School of Integrated Sciences for Life, Hiroshima University, Hiroshima, 739-8528, Japan
4Crop Sciences Institute, National Agricultural Research Centre, Islamabad, 44000, Pakistan
5VIT School of Agricultural Innovations and Advanced Learning (VAIAL), Vellore Institute of Technology (VIT), Vellore, 632014, India
6Department of Agronomy, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Shaikh, 33516, Egypt
*Corresponding Author: Ayman EL Sabagh. Email: ayman.elsabagh@agr.kfs.edu.eg
Received: 25 January 2022; Accepted: 16 March 2022
Abstract: Soil amendments containing carbonized materials increase the soil carbon reservoir, influence plant productivity, and, ultimately, help to clean the environment. There is data on the effect of such additions on soil physicochemical properties or plant growth, but few studies have focused on how these carbonized materials are distributed by termite species in the soil ecosystem. It is the first comprehensive study of the transportation of biochar (BC) by termite species under tropical environmental conditions in Pakistan. The present study was carried out to test the hypothesis that if termite species I) were involved in the distribution of biochar particles II) if yes, then how far these particles were transported during the study period (10 days) and III) check their preference between the enriched BC (EBC) and non-enriched BC. BC was enriched with the cattle slurry after its pyrolysis in the study. The results showed that EBC particles were significantly more widely distributed than non-enriched BC particles, but both types of BC were transported more than 4 cm (ring 4) within 10 days (at the end of the experiment). The current study also revealed that EBC was easily attached to the setae, cuticle, and legs of termites, implying that it could potentially be transported over a greater distance. Furthermore, transportation of EBC over larger distances indicated a potential preference of termite species between the EBC and BC particles. During the study, however, the preference among the termite species was also observed. Under the prevailing study conditions, the Coptotermes heimi and Heteroterme indicola species transported the EBC further than Microtermes obesi and Odontotermes obesus. These findings revealed that transportation preferences were observed among the four termite species. In conclusion, the current study found that termites were involved in the distribution of BC particles, with a preference for EBC and that these have the potential to transport BC particles more than 4 cm within 10 days. Furthermore, two species Coptotermes heimi and Heteroterme indicola may be more suitable candidates for EBC transpiration in Pakistani soils. It was necessary to conduct additional research into the effect of temperature on the transportation process.
Keywords: Enriched biochar; termite species; distribution; micro-particles; feeding stations; soil productivity
The incorporation of biochar (BC) into agricultural soils resulted in improved soil fertility as well as a reduction in greenhouse gas emissions [1–5]. Some BC feedstuffs are naturally alkaline, and adding more of this BC to the soil may raise its pH [6]. Furthermore, BC improves nutrient mobility, particularly in acidic soils, though it may also improve mineral immobilization in alkaline soils [1]. In recent years, researchers have focused more on maximizing the benefits of BC and encouraging soil microbial activity [7]. The activity and population of various microorganisms in the soil ecosystem are affected by BC [8–10]. However, soil microbial activity may be affected by any change in soil pH [11–13]. A significant positive relationship was found between inoculum population density, BC feed stuff and BC pH [14,15]. Some studies have found that increasing soil acidity (pH) increases microbial biomass (for example, from pH 3.75 to 7.1). but later reduces it [16]. Furthermore, fungal and some bacterial populations respond differentially to pH changes in soil. Bacterial population increase as soil pH rises up to 7 [17–19], whereas, fungal populations show no change in total biomass or a dramatic decrease in fungal growth at higher pH [20–22].
BC can alter and stimulate the soil biochemical cycle because it can survive in soil for centuries [20,23,24]. BC has a high concentration of recalcitrant C but a low concentration of leachable C and other components [6]. BC differs from soil organic carbon (SOC) structurally and chemically because it contains far more aromatic C with a stable structure than other carbonous materials such as lignin [25,26]. Soil microbiomes benefit from a high concentration of BC in the form of C, N, K, P and other elements [14–27]. Furthermore, when compared to other carbon sources, BC is readily and easily available to soil microbes, allowing them to begin their activities more quickly [28,29]. Soil enzymatic activities are an important indicator of a healthy soil ecosystem because they respond quickly to changes in soil properties [30]. As a result, SOC can boost soil enzymatic activities [28]. Enriched BC has a higher biological activity than BC or fresh BC [31–34]. The significant effect of microbial biomass and its respiration is directly related to the addition of BC contents and concentration [20–35]. It also increases nutrients availability and microbial respiration [36]. Several studies discovered that applying biochar at a rate of 10–100 g kg−1 soil improved its physicochemical and biological properties [37–41]. Furthermore, co-application of biochar (@ 5 tha−1) and synthetic fertilizer can ensure long-term plant nutrient availability [42]. Previous research has shown that BC amendments can increase agricultural productivity and microbial biomass in arid and poor agricultural soils [43].
Termites play an important role in the soil food web and fungal association. Termites are well-known soil engineers because they improve the structure and functions of the soil through their feeding, nesting, and burrowing habits [44]. They also form the channels in the soil, such as fungal energy channels, which enhance the interactive role of other soil microbial systems [45]. Furthermore, termites ingest soil organic matter by producing different enzymes that decompose the soil necromass [46] and distribute it in different soil layers via feces or excretions [47]. Soil aggregation and porosity were also improved and the bulk density of the soil was reduced [48]. Termites also provide shelter for soil microbes and promote the growth of fungal colonies [49]. Although much research has been done on the effects of soil BC on soil microbial biomass, earthworms, and their interactions, little attention has been paid to the effect of collembola, termites, and mites on the BC degradation and movement in the soil. According to our best knowledge this is the first laboratory-scale study of termites and their effects on biochar movement in Pakistan. Previously, no study had been conducted with a specific focus on Pakistan, neither on a laboratory scale nor in field trials. This study will lay the groundwork for future studies on the role of biological individuals in soil BC distribution. Furthermore, no research has been conducted to investigate the role of mesofauna in the distribution and transportation of BC amendments. Biochar distribution is most likely caused by i) the attachment on their body, i.e., cuticle and setae, ii) animal movement over feeding stuff and thus, movement pushing, and iii) feeding and defecation. Additionally, the characteristics of the BC feeding stock influence biochar transportation. The current study was conducted based on the above hypothesis to determine the role of termite species in biochar movement; if yes, then how far the biochar particles would be distributed and transported over the course of 10 days study. Furthermore, how do termite species differ between EBC and non-enriched BC? To answer to these questions, the current study used four termite species with EBC and BC amendments. The primary goal of this research was to gain a better understanding of the effects of various EBC and BC amendments. The main objective of this study was to better understand the effects of BC types and their distribution in the soil by different termite species.
2.1 Collection of Termite Species
The four most promising termite species were extracted from soil collected at the COMSATS University Islamabad, Vehari Campus, and characterized under a light microscope based on their unique characteristics. Coptotermes heimi (Wasmann), Heteroterme indicola (Wasmann), Microtermes obesi (Holmgren) and Odontotermes obesus (Rambur) were the termite species used in this study. During manuscript, the name of these species is abbreviated as Coptotermes heimi (C. heimi), Heteroterme indicola (H. indicola), Microtermes obesi (M. obesi) and Odontotermes obesus (O. obesus). At the time of sample collection, the body sizes of all species were the same and constant (12 mm). Prior to the start of starvation, all of the species were placed on plaster of paris and fed with yeast. The termite species were starved 14 days before the experiment began [45].
The poplar (Liriodendron tulipifera L.) wood chips (2–3 cm diameter) were collected from a local furniture market in Vehari, Punjab-Pakistan and used as feedstock for BC production. Before the carbonization process, the feedstock was air-dried in natural sunlight. The BC was created through slow pyrolysis (300°C) of poplar wood chips in an anaerobic environment with electricity as the energy source. The low temperature was used during the pyrolysis to conserve the volatile compounds, carbon, and nitrogen contents that would have been lost at high temperatures, and soil microbial activity is enhanced by increasing the bio-available carbon contents in the biochar [45]. There were no preservatives (chemicals) used before or after production. During pyrolysis, a small amount of steam was produced and exhausted externally. After cooling, the BC material was manually crushed with a mortar and pestle and sieved (100–200 μm). The specific particle fraction (100–200 μm) was used in the study because previous research demonstrated that it was an optimal size for F. candida mediated potential transport [32] and was also easy to count on photos for quantitative analysis. The BC’s water holding capacity, pH, and nutrient concentrations were measured and are shown in Table S1.
Dairy manure effluent (DME; slurry of cattle) was collected in a plastic drum from a local cattle farm located 2 km from the campus. The DME is taken to the postgraduate laboratory of the Department of Environmental Sciences, COMSATS University Islamabad, Vehari Campus. The enrichment began on the same day. The slurry was centrifuged at 20000 rpm and the supernatant was thoroughly filtered (0.5 μm filter papers). The EBC was made by adding the BC (1% w/w; 1 g 100 g−1 cattle slurry) and shaking it for 24 h. Gao et al. [33] found that a longer shaking time resulted in better enrichment. After shaking, the material was centrifuged, filtered, and oven-dried until a constant weight was achieved. So, this nutrient-enriched biochar was termed EBC, while the other biochar was labelled non-enriched biochar (BC). The BC was kept at room temperature in a glass bottle for later use in the study.
2.4 Experimental Design and Layout
The experimental units were small plastic cups (10 cm diameter), which were then filled with a one cm layer of plaster of paris that had been wetted until saturated. The treatments consisted of 2 mg of BC or EBC sprinkled into the center of the cup in a 0.5 cm circle called a “feeding station”. The cups were covered with transparent lids to prevent any airflow interruption to the BC material inside. Throughout the study, the cups containing EBC and BC treatments were kept at room temperature (26 ± 2°C). Each cup held 15 termites from each species. The experiment was repeated eight times, totaling 40 experimental units. Ring 1, ring 2, ring 3, and ring 4 are four concentric circles of 1, 2, 3, and 4 cm in diameter that are represented inside the cup as rings 1, 2, 3 and 4. The particles carried out by the termite species in each ring were counted. Two controls were also included in the experiment (positive and negative controls). The experiment was carried out for ten days. Every sample was photographed once a day at a distance of 25 cm. A stereomicroscope (10× magnification) with a cold light source was used for all particle measurements.
The current study evaluated data using the R software (v.3.3.1), and the “nlme” package for general least square models (LSM). The function “varIdent” was used to examine the heterogeneity of the data. The package “lsmeans” was used to perform pair-wise comparisons of LSMs of factors. The graphs were created using Origin Pro v.2018.
The findings revealed that particle distribution was significantly affected by all four rings, i.e., BC types and four termite species (Figs. 1–4).
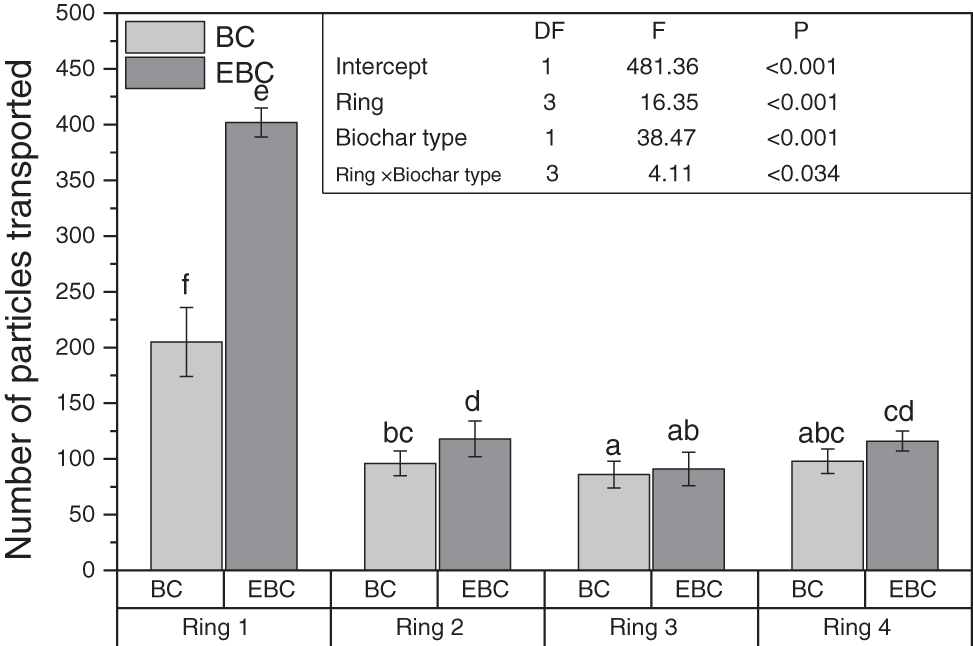
Figure 1: The number of particles distributed horizontally C. heimi species in the four rings diameter (ring 1 = 1 cm, ring 2 = 2 cm, ring 3 = 3 cm, ring 4 = 4 cm) around the feeding station after ten days of the experiment. The shading of bars indicated the different biochar types (BC, non-enrich biochar; EBC, enriched biochar). The line over each bar indicated the Mean ± SE (n = 8). The same letters over each bar do not differ significantly from each other at a 5% probability level according to pairwise comparisons using least square means. The control value is zero, so it was not shown in the graph

Figure 2: The number of particles distributed horizontally by H. indicola species in the four rings diameter (ring 1 = 1 cm, ring 2 = 2 cm, ring 3 = 3 cm, ring 4 = 4 cm) around the feeding station after ten days of the experiment. The shading of bars indicated the different biochar types (BC, non-enrich biochar; EBC, enriched biochar). The line over each bar indicated the Mean ± SE (n = 8). The same letters over each bar do not significantly differ from each other at a 5% probability level according to pairwise comparisons using least square means. The control value is zero, so it was not shown in the graph

Figure 3: The number of particles distributed horizontally by M. obesi species in the four rings diameter (ring 1 = 1 cm, ring 2 = 2 cm, ring 3 = 3 cm, ring 4 = 4 cm) around the feeding station after ten days of the experiment. The shading of bars indicated the different biochar types (BC, non-enrich biochar; EBC, enriched biochar). The line over each bar indicated the Mean ± SE (n = 8). The same letters over each bar do not significantly differ from each other at a 5% probability level according to pairwise comparisons using least square means. The control value is zero, so it was not shown in the graph

Figure 4: The number of particles distributed horizontally by O. obesus species in the four rings diameter (ring 1 = 1 cm, ring 2 = 2 cm, ring 3 = 3 cm, ring 4 = 4 cm) around the feeding station after ten days of the experiment. The shading of bars indicated the different biochar types (BC, non-enrich biochar; EBC, enriched biochar). The line over each bar indicated the Mean ± SE (n = 8). The same letters over each bar do not significantly differ from each other at a 5% probability level according to pairwise comparisons using least square means. The control treatment value is zero, so did not show in the figure graph
All four species are common in Punjab-Pakistan soil, and thus play an important role in the soil biological system. C. heimi was the most active of the four species and had a dominant role in the distribution of BC particles in the current study. The data in Fig. 1 showed that the C. heimi termite species transported more EBC particles (402) than BC particles (205) in ring 1 compared to the other three rings. However, ring 4 contained more EBC particles than ring 3 (Fig. 1; Tables S1, S3 and S4; Fig. S1). The BC distribution pattern for H. indicola species was almost identical, with maximum EBC particles (372) over BC particles (191) (Fig. 2), but it differed significantly from the C. heimi species. The data also showed that these two species distributed the vast majority of the particles (C. heimi and H. indicola).
In terms of particles distribution, M. obesi and O. obesus had the highest EBC particle counts (292 vs. 212; 251 vs. 166) than BC particles, respectively.
In general, BC particles enriched with cattle slurry were more widely distributed than biochar particles without enrichment (Figs. 1–4; Fig. S2). In terms of particle distribution among the rings, ring 1 had the most particle distribution, followed by ring 2, ring 3, and ring 4 (Figs. 1–4; Fig. S1; Table S1, S3, and S4).
Even after the termites had left the biochar piles, EBC particles attracted more termite species than biochar without enrichment. There are also block spots in the intestines and on body parts that the termite species has browsed and consumed. As a result, the plastic cups containing these particles were defecated elsewhere in the plastic glasses. Furthermore, a higher proportion of EBC particles than BC particles were found to be grazed.
The principal component analysis (PCA) of the various rings revealed that rings 1, ring 2, and ring 3 are all in the same box, indicating that they are correlated, whereas ring 4 is in the opposite box, indicating that it is not correlated with the other three rings (Fig. 5; Fig. S1). All of the data related to the rings falls into the F1 over to the F2, F3, and F4 (Table S1), while the variability and cumulative data were both 87% in each (Table S2). The squared cosines of the variables were found in ring 1 (88.6%), ring 2 (93.4%), ring 3 (94.4), and ring 4 (75.1%) (Table S4). When each variable (rings) to the factor with the greatest squared cosine, the values in F1 are equal.

Figure 5: Principle component analysis of the different termite species and rings
The distribution of objects within the soil was determined by its biological ecosystems, which included earthworms, mites and springtails. Previous research has shown that soil mesofauna play a role in the distribution of small particles such as BC microparticles, hydrochar, fungal spores, and microplastics [50]. Despite this, it has been discovered that the springtail distributed the BC within the soil [50]. Using four termite species, Coptotermes heimi (Wasmann), Heteroterme indicola (Wasmann), Microtermes obesi (Holmgren), and Odontotermes obesus (Rambur) abbreviated as C. heimi, H. indicola, M. obesi and O. obesus, respectively, we studied their ability to transfer different types of BC at different feeding stations. It was also investigated whether various termite species reacted differently to different BC types in terms of distribution. It was discovered that all four species distributed both types of BC greater than 4 cm (ring 4) at the various feeding stations during the specific period (ten days). However, at the end of the experiment, there was a clear difference between all four species based on how far and how frequently biochar distribution was done. EBC was more widely available than BC.
Termite species (C. heimi and H. indicola, M. obesi) moved the BC particles by attaching to their setae or body and thus transported them relatively farther, but the distribution was only limited to smaller particles size. Furthermore, the termite species (C. heimi and H. indicola) produced micro-particles more efficiently than the other two species by grazing larger chunks of biochar and converting them into micro-particles (M. obesi and O. obesus). Maaß et al. [50] and Salem et al. [51] reported similar results regarding the ingesting of larger particles into micro-particles. More feeding, on the other hand, is likely to have, resulted in more defecation into the feeding station. Surprisingly, ingestion or defecation would be important only for smaller particles (diameter ≤ 100 μm) or those transformed into micro-particles by grazing activities. The abundance of mesofauna in the soil micro-climate suggested that distribution could be significant and should be investigated further.
When the distribution frequencies of both BC types in the four species were examined, EBC was found to be preferable to BC (Figs. 1–4). This could be due to a fungal hyphae grazer with a strong preference for the specific species [52]. The current study results are most likely to change the abiotic features [53] of the soil microclimate imposed by the BC types, which ultimately, supported some specific type of microbial colonies on their surface [54,55]. Furthermore, the species (C. heimi and H. indicola) responded to the soil micro-climate better than the others (M. obesi and O. obesus). The possibility is that the former two species grazed more than the latter, resulting in a more dispersed distribution of BC particles. More research is needed to fully understand the potential ecotoxicology of the micro-climate on the mesofauna.
Previously, the EBC was shown to increase microbial diversity [55], but bacteria and fungi may react differently to changing soil microclimates, such as pH [56,57]. Besides that, the surface/shape characteristics (how quickly and easily particles were carried out by termite species), the micro-arthropods like EBC as a source of food [54], which could be due to its greater nutritional benefits than the EBC used in the experiment. Furthermore, the presence of bacteria in the termite gut may actively work on EBC and decompose easily through grazing activities, resulting in greater distribution elsewhere in the feeding station than other types of BC. These gut bacteria may be unable to survive outside the gut/abdomen on the feces [58] of micro-arthropods, altering the microbial biomass slightly [59].
The current study found that all termite species were involved in the horizontal distribution of both types of BC. Although, the frequencies of BC distributions were different in all four termite species, this could be due to species reactions to soil abiotic characteristics and BC shape. This type of distribution is critical in the soil ecosystem for transporting particles from one location (from where they applied) to another. As a result, horizontal and slightly vertical transport tends to explain the interaction of soil biota with BC on a local scale [60]. Furthermore, the interaction with soil minerals is thought to have facilitated the transport of BC particles to deeper and deeper soil layers, increasing soil productivity [61].
The results of the present study indicated that the termite species are involved in the horizontal distribution of BC in a variety of ways. However, mineral distribution and transportation were found in EBC particles as they travelled from EBC to BC. Moreover, the Coptotermes heimi and Heteroterme indicola species move more BC particles than Microtermes obesi and Odontotermes obesus species, which are closely related. Additionally, this study found the potential termite species for the transportation of BC in the soil ecosystem. Our findings suggested that the soil mesofauna could serve as a vector for the distribution of BC particles and that the mesofauna had a distinct preference for certain BC types, which should be investigated in greater depth in future experiments. It also should focus on the effect of the different particle sizes and soil temperatures in order to have a better understanding of the interaction with other soil organisms as well as the potential for ecotoxicity over time.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: M.A., N.M., H.M.R.J.; data collection: M.A., N. M., H.M.R.J., M.A.S., analysis and interpretation of results: M.A., N.M., H.M.R.J., M.A.S., L.L., A.E.L.S.; draft manuscript preparation: M.A., N.M., M.A.S., A.E.L.S., I.A., K.F.A., K.R. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgement: The authors extend their appreciation to the Researchers Supporting Project No. (RSP-2021/298), King Saud University, Riyadh, Saudi Arabia.
Funding Statement: This work was supported by the Researchers Supporting Project No. (RSP-2021/298), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Abbasi, M. K., Anwar, A. A. (2015). Ameliorating effects of biochar derived from poultry manure and white clover residues on soil nutrient status and plant growth promotion-greenhouse experiments. PLoS One, 10(6), e0131592. DOI 10.1371/journal.pone.0131592. [Google Scholar] [CrossRef]
2. Collier, S. M., Ruark, M. D., Oates, L. G., Jokela, W. E., Dell, C. J. (2014). Measurement of greenhouse gas flux from agricultural soils using static chambers. Journal of Visualized Experiments, (9052110. DOI 10.3791/52110. [Google Scholar] [CrossRef]
3. Pokharel, P., Chang, S. X. (2019). Manure pellet, woodchip and their biochars differently affect wheat yield and carbon dioxide emission from bulk and rhizosphere soils. Science of the Total Environment, 659, 463–472. DOI 10.1016/j.scitotenv.2018.12.380. [Google Scholar] [CrossRef]
4. Thangarajan, R., Bolan, N. S., Tian, G., Naidu, R., Kunhikrishnan, A. (2013). Role of organic amendment application on greenhouse gas emission from soil. Science of the Total Environment, 465, 72–96. DOI 10.1016/j.scitotenv.2013.01.031. [Google Scholar] [CrossRef]
5. Wu, D., Senbayram, M., Zang, H., Ugurlar, F., Aydemir, S. et al. (2018). Effect of biochar origin and soil pH on greenhouse gas emissions from sandy and clay soils. Applied Soil Ecology, 129, 121–127. DOI 10.1016/j.apsoil.2018.05.009. [Google Scholar] [CrossRef]
6. Baiamonte, G., Crescimanno, G., Parrino, F., De-Pasquale, C. (2019). Effect of biochar on the physical and structural properties of a desert sandy soil. Catena, 175, 294–303. DOI 10.1016/j.catena.2018.12.019. [Google Scholar] [CrossRef]
7. Han, G., Lan, J., Chen, Q., Yu, C., Bie, S. (2017). Response of soil microbial community to application of biochar in cotton soils with different continuous cropping years. Scientific Reports, 7, 10184. DOI 10.1038/s41598-017-10427-6. [Google Scholar] [CrossRef]
8. Su, P., Lou, J., Brookes, P. C., Luo, Y., He, Y. et al. (2017). Taxon-specific responses of soil microbial communities to different soil priming effects induced by addition of plant residues and their biochars. Journal of Soils and Sediments, 17, 674–684. DOI 10.1007/s11368-015-1238-8. [Google Scholar] [CrossRef]
9. Zhou, H., Zhang, D., Wang, P., Liu, X., Cheng, K. et al. (2017). Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: A meta-analysis. Agriculture, Ecosystems & Environment, 239, 80–89. DOI 10.1016/j.agee.2017.01.006. [Google Scholar] [CrossRef]
10. Gul, S., Whalen, J. K., Thomas, B. W., Sachdeva, V. H. D. (2015). Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agriculture, Ecosystems & Environment, 206, 46–59. DOI 10.1016/j.agee.2015.03.015. [Google Scholar] [CrossRef]
11. Biederman, L. A., Harpole, W. S. (2013). Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy, 5, 202–214. DOI 10.1111/gcbb.12037. [Google Scholar] [CrossRef]
12. Jenkins, J. R., Viger, M., Arnold, E. C., Harris, Z. M., Ventura, M. et al. (2017). Biochar alters the soil microbiome and soil function: Results of next-generation amplicon sequencing across Europe. GCB Bioenergy, 9, 591–612. DOI 10.1111/gcbb.12371. [Google Scholar] [CrossRef]
13. Sheng, Y., Zhu, L. (2018). Biochar alters microbial community and carbon sequestration potential across different soil pH. Science of Total Environment, 622, 1391–1399. DOI 10.1016/j.scitotenv.2017.11.337. [Google Scholar] [CrossRef]
14. Chen, Q., Qin, J., Sun, P., Cheng, Z., Shen, G. (2018). Cow dung-derived engineered biochar for reclaiming phosphate from aqueous solution and its validation as slow-release fertilizer in soil-crop system. Journal of Cleaner Production, 172, 2009–2018. DOI 10.1016/j.jclepro.2017.11.224. [Google Scholar] [CrossRef]
15. Dahlawi, S., Naeem, A., Rengel, Z., Naidu, R. (2018). Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Science of the Total Environment, 625, 320–335. DOI 10.1016/j.scitotenv.2017.12.257. [Google Scholar] [CrossRef]
16. Glaser, B., Lehr, V. I. (2019). Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Scientific Reports, 9, 9338. DOI 10.1038/s41598-019-45693-z. [Google Scholar] [CrossRef]
17. Enagbonma, B. J., Ajilogba, C. F., Babalola, O. O. (2020). Metagenomic profiling of bacterial diversity and community structure in termite mounds and surrounding soils. Archives of Microbiology, 202, 2697–2709. DOI 10.1007/s00203-020-01994-w. [Google Scholar] [CrossRef]
18. Zaheer, A., Malik, A., Sher, A., Qaisrani, M. M., Mehmood, A. et al. (2019). Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi Journal of Biological Sciences, 26(5), 1061–1067. DOI 10.1016/j.sjbs.2019.04.004. [Google Scholar] [CrossRef]
19. Enagbonma, B. J., Babalola, O. O. (2019). Environmental sustainability: A review of termite mound soil material and its bacteria. Sustainability, 11, 3847. DOI 10.3390/su11143847. [Google Scholar] [CrossRef]
20. Hardy, B., Sleutel, S., Dufey, J. E., Cornelis, J. T. (2019). The long-term effect of biochar on soil microbial abundance, activity and community structure is overwritten by land management. Frontiers in Environmental Science, 7, 110. DOI 10.3389/fenvs.2019.00110. [Google Scholar] [CrossRef]
21. House, G. L., Bever, J. D. J. R. E. (2019). Biochar soil amendments in prairie restorations do not interfere with benefits from inoculation with native arbuscular mycorrhizal fungi. Restoration Ecology, 28(4), 785–795. DOI 10.1111/rec.12924. [Google Scholar] [CrossRef]
22. Zimmerman, A. R., Ouyang, L. (2019). Priming of pyrogenic C (biochar) mineralization by dissolved organic matter and vice versa. Soil Biology & Biochemistry, 130, 105–112. DOI 10.1016/j.soilbio.2018.12.011. [Google Scholar] [CrossRef]
23. Sagrilo, E., Jeffery, S., Hoffland, E., Kuyper, T. W. (2015). Emission of CO2 from biochar-amended soils and implications for soil organic carbon. GCB Bioenergy, 7, 1294–1304. DOI 10.1111/gcbb.12234. [Google Scholar] [CrossRef]
24. Maestrini, B., Nannipieri, P., Abiven, S. (2015). A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy, 7, 577–590. DOI 10.1111/gcbb.12194. [Google Scholar] [CrossRef]
25. Ameur, D., Zehetner, F., Johnen, S., Jöchlinger, L., Pardeller, G. et al. (2018). Activated biochar alters activities of carbon and nitrogen acquiring soil enzymes. Pedobiologia, 69, 1–10. DOI 10.1016/j.pedobi.2018.06.001. [Google Scholar] [CrossRef]
26. El-Naggar, A., Lee, S. S., Rinklebe, J., Farooq, M., Song, H. et al. (2019). Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma, 337, 536–554. DOI 10.1016/j.geoderma.2018.09.034. [Google Scholar] [CrossRef]
27. Aller, D., Rathke, S., Laird, D., Cruse, R., Hatfield, J. (2017). Impacts of fresh and aged biochars on plant available water and water use efficiency. Geoderma, 307, 114–121. DOI 10.1016/j.geoderma.2017.08.007. [Google Scholar] [CrossRef]
28. Javeed, H. M. R., Ali, M., Qamar, R., Shehzad, M., Rehman, H. et al. (2021). Effect of date biochar pyrolyzed at different temperature on physiochemical properties of sandy soil and wheat crop response. Communications in Soil Science and Plant Analysis, 52(18), 2110–2124. DOI 10.1080/00103624.2021.1919698. [Google Scholar] [CrossRef]
29. Hailegnaw, N. S., Mercl, F., Pračke, K., Száková, J., Tlustoš, P. (2019). High temperature-produced biochar can be efficient in nitrate loss prevention and carbon sequestration. Geoderma, 338, 48–55. DOI 10.1016/j.geoderma.2018.11.006. [Google Scholar] [CrossRef]
30. Jain, S., Mishra, D., Khare, P., Yadav, V., Deshmukh, Y. et al. (2016). Impact of biochar amendment on enzymatic resilience properties of mine spoils. Science of the Total Environment, 544, 410–421. DOI 10.1016/j.scitotenv.2015.11.011. [Google Scholar] [CrossRef]
31. Jaafar, N. M., Clode, P. L., Abbott, L. K. (2015). Biochar-soil interactions in four agricultural soils. Pedosphere, 25, 729–736. DOI 10.1016/S1002-0160(15)30054-0. [Google Scholar] [CrossRef]
32. Agegnehu, G., Srivastava, A. K., Bird, M. I. (2017). The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Applied Soil Ecology, 119, 156–170. DOI 10.1016/j.apsoil.2017.06.008. [Google Scholar] [CrossRef]
33. Gao, S., DeLuca, T. (2016). Influence of biochar on soil nutrient transformations, nutrient leaching, and crop yield. Advances in Plants Agriculture Research, 4(5), 348–362. DOI 10.15406/apar.2016.04.00150. [Google Scholar] [CrossRef]
34. Li, Z., Schneider, R. L., Morreale, S. J., Xie, Y., Li, C. et al. (2018). Woody organic amendments for raining soil water, improving soil properties and enhancing plant growth in desertified soils of Ningxia, China. Geoderma, 310, 143–152. DOI 10.1016/j.geoderma.2017.09.009. [Google Scholar] [CrossRef]
35. Munera-Echeverri, J. L., Martinsen, V., Strand, L. T., Cornelissen, G., Mulder, J. (2020). Effect of conservation farming and biochar addition on soil organic carbon quality, nitrogen mineralization, and crop productivity in a light textured acrisol in the sub-humid tropics. PLoS One, 15(2), e0228717. DOI 10.1371/journal.pone.0228717. [Google Scholar] [CrossRef]
36. Servin, A. D., de la Torre-Roche, R., Castillo-Michel, H., Pagano, L., Hawthorne, J. et al. (2017). Exposure of agricultural crops to nanoparticle CeO2 in biochar-amended soil. Plant Physiology & Biochemistry, 110, 147–157. DOI 10.1016/j.plaphy.2016.06.003. [Google Scholar] [CrossRef]
37. Wu, Y., Xu, G., Shao, H. B. (2014). Furfural and its biochar improve the general properties of a saline soil. Solid Earth, 5(2), 665–671. DOI 10.5194/se-5-665-2014. [Google Scholar] [CrossRef]
38. Mukherjee, A., Zimmerman, A. R. (2013). Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma, 193, 122–130. DOI 10.1016/j.geoderma.2012.10.002. [Google Scholar] [CrossRef]
39. Irfan, M., Chen, Q., Yue, Y., Pang, R., Lin, Q. et al. (2016). Co-production of biochar, bio-oil and syngas from halophyte grass (Achnatherum splendens L.) under three different pyrolysis temperatures. Bioresource Technology, 211, 457–463. DOI 10.1016/j.biortech.2016.03.077. [Google Scholar] [CrossRef]
40. Prayogo, C., Jones, J. E., Baeyens, J., Bending, G. D. (2014). Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biology and Fertility of Soils, 50, 695–702. DOI 10.1007/s00374-013-0884-5. [Google Scholar] [CrossRef]
41. Al Marzooqi, F., Yousef, L. F. (2017). Biological response of a sandy soil treated with biochar derived from a halophyte (Salicornia bigelovii). Applied Soil Ecology, 114, 9–15. DOI 10.1016/j.apsoil.2017.02.012. [Google Scholar] [CrossRef]
42. Saha, A., Basak, B. B., Gajbhiye, N. A., Kalariya, K. A., Manivel, P. (2019). Sustainable fertilization through co-application of biochar and chemical fertilizers improves yield, quality of andrographis paniculata and soil health. Industrial Crops and Products, 140, 111607. DOI 10.1016/j.indcrop.2019.111607. [Google Scholar] [CrossRef]
43. Singh, C., Tiwari, S., Gupta, V. K., Singh, J. S. (2018). The effect of rice husk biochar on soil nutrient status, microbial biomass and paddy productivity of nutrient poor agriculture soils. Catena, 171, 485–493. DOI 10.1016/j.catena.2018.07.042. [Google Scholar] [CrossRef]
44. Silver, W., Perez, T., Mayer, A., Jones, A. (2021). The role of soil in the contribution of food and feed. Philosophical Transactions of the Royal Society B, 376, 20200181. DOI 10.1098/rstb.2020.0181. [Google Scholar] [CrossRef]
45. Deenik, J. L., McClellan, A., Uehara, G. (2009). Biochar volatile matter content effects on plant growth and nitrogen transformations in a tropical soil. Western Nutrient Management Conference, vol. 8, pp. 26. Salt Lake City, Utha, USA. [Google Scholar]
46. Lavelle, P., Spain, A., Fonte, S., Bedano, J. C., Blanchart, E. et al. (2020). Soil aggregation, ecosystem engineers and the C cycle. Acta Oecologica, 105, 103561. DOI 10.1016/j.actao.2020.103561. [Google Scholar] [CrossRef]
47. Mbau, S. K., Karanja, N., Ayuke, F. (2015). Short-term influence of compost application on maize yield, soil macrofauna diversity and abundance in nutrient deficient soils of Kakamega County, Kenya. Plant and Soil, 387, 379–394. DOI 10.1007/s11104-014-2305-4. [Google Scholar] [CrossRef]
48. Fonte, S. J., Kong, A. Y., van Kessel, C., Hendrix, P. F., Six, J. (2007). Influence of earthworm activity on aggregate-associated carbon and nitrogen dynamics differs with agroecosystem management. Soil Biology and Biochemistry, 39(5), 1014–1022. DOI 10.1016/j.soilbio.2006.11.011. [Google Scholar] [CrossRef]
49. Galindo-Castañeda, T., Brown, K. M., Kuldau, G. A., Roth, G. W., Wenner, N. G. et al. (2019). Root cortical anatomy is associated with differential pathogenic and symbiotic fungal colonization in maize. Plant, Cell & Environment, 42(11), 2999–3014. DOI 10.1111/pce.13615. [Google Scholar] [CrossRef]
50. Maaß, S., Daphi, D., Lehmann, A., Rillig, M. C. (2017). Transport of microplastics by two collembolan species. Environmental Pollution, 225, 456–459. DOI 10.1016/j.envpol.2017.03.009. [Google Scholar] [CrossRef]
51. Salem, M., Kohler, J., Rillig, M. C. (2013). Palatability of carbonized materials to Collembola. Applied Soil Ecology, 64, 63–69. DOI 10.1016/j.apsoil.2012.10.009. [Google Scholar] [CrossRef]
52. Fountain, M. T., Hopkin, S. P. (2005). Folsomia candida (CollembolaA “Standard” soil arthropod. Annual Review of Entomology, 50, 201–222. DOI 10.1146/annurev.ento.50.071803.130331. [Google Scholar] [CrossRef]
53. George, C., Kohler, J., Rillig, M. C. (2016). Biochars reduce infection rates of the root-lesion nematode Pratylenchus penetrans and associated biomass loss in carrot. Soil Biology and Biochemistry, 95, 11–18. DOI 10.1016/j.soilbio.2015.12.003. [Google Scholar] [CrossRef]
54. Ding, Y., Liu, Y., Liu, S., Li, Z., Tan, X. et al. (2016). Biochar to improve soil fertility. A review. Agronomy for Sustainable Development, 36(2), 1–18. DOI 10.1007/s13593-016-0372-z. [Google Scholar] [CrossRef]
55. Domene, X., Hanley, K., Enders, A., Lehmann, J. (2015). Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Applied Soil Ecology, 89, 10–17. DOI 10.1016/j.apsoil.2014.12.005. [Google Scholar] [CrossRef]
56. Rousk, J., Bååth, E., Brookes, P. C., Lauber, C. L., Lozupone, C. et al. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME Journal, 4(10), 1340–1351. DOI 10.1038/ismej.2010.58. [Google Scholar] [CrossRef]
57. Ding, J., Yin, Y., Sun, A. Q., Lassen, S. B., Li, G. et al. (2019). Effects of biochar amendments on antibiotic resistome of the soil and collembolan gut. Journal of Hazardous Materials, 377, 186–194. DOI 10.1016/j.jhazmat.2019.05.089. [Google Scholar] [CrossRef]
58. Hoffmann, A., Thimm, T., Dröge, M., Moore, E. R. B., Munch, J. C. et al. (1998). Intergeneric transfer of conjugative and mobilizable plasmids harbored by Escherichia coli in the gut of the soil microarthropod Folsomia candida (Collembola). Applied and Environmental Microbiology, 64, 2652. DOI 10.1128/AEM.64.7.2652-2659.1998. [Google Scholar] [CrossRef]
59. Thimm, T., Hoffmann, A., Borkott, H., Charles Munch, J., Tebbe, C. C. (1998). The Gut of the soil microarthropod Folsomia candida (Collembola) is a frequently changeable but selective habitat and a vector for microorganisms. Applied and Environmental Microbiology, 64, 2660. DOI 10.1128/AEM.64.7.2660-2669.1998. [Google Scholar] [CrossRef]
60. Peng, X., Wang, W. (2016). Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of Northern China. Soil Biology and Biochemistry, 98, 74–84. DOI 10.1016/j.soilbio.2016.04.008. [Google Scholar] [CrossRef]
61. Reibe, K., Götz, K. P., Roß, C. L., Döring, T. F., Ellmer, F. et al. (2015). Impact of quality and quantity of biochar and hydrochar on soil collembola and growth of spring wheat. Soil Biology and Biochemistry, 83, 84–87. DOI 10.1016/j.soilbio.2015.01.014. [Google Scholar] [CrossRef]



Figure S1: Eigenvalues and Cumulative variability among the factors

Figure S2: Principal component analysis among different rings; A plot of percentage abundance


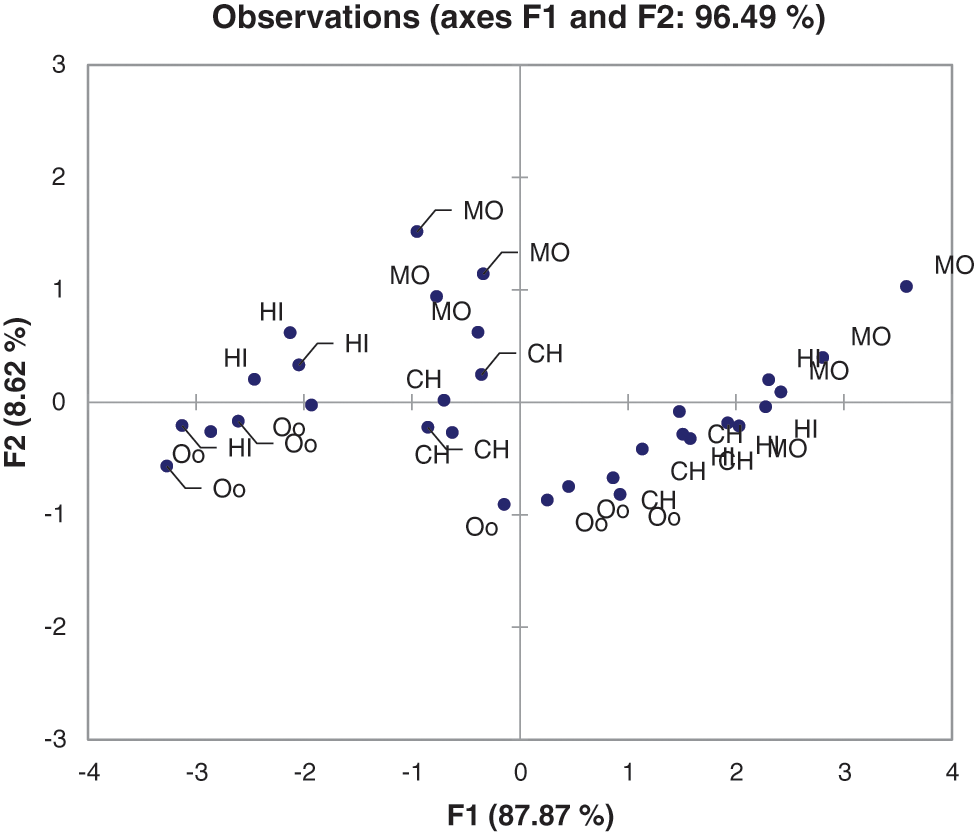
Figure S3: Principal component analysis of among different termite species
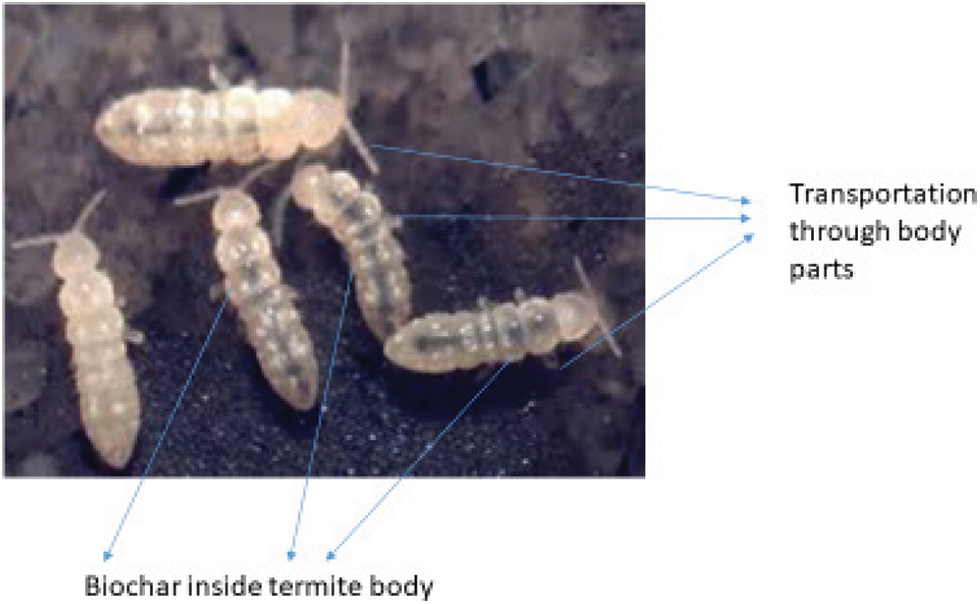
Figure S4: Termites covered with biochar micro-particles on the body parts and inside the gut
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |