

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020295
ARTICLE
Metabolomics Analysis of Metabolites in Forsythia suspense Fruit Using UPLC/ESI-Q TRAP-MS/MS
Institute of Cash Crops, Hebei Academy of Agricultural and Forestry Sciences, Shijiazhuang, 050051, China
*Corresponding Author: Tao Jiang. Email: jttaojiang@163.com
Received: 16 November 2021; Accepted: 19 January 2022
Abstract: Forsythiae Fructus, the fruit of Forsythia suspense is a traditional Chinese hebal medicine that has the antiviral and antioxidant effects in China. Modern analytical chemistry studies showed that the extracts of Forsythiae Fructus contain many bioactive components, such as flavonoids, lignans, phenolic acids, and terpenoids, which can be used to anti-inflammatory and treat toxicity, tonsillitis, ulcers, pharyngitis and acute nephritis. In order to study the types and quantities of metabolites in Forsythiae Fructus, we isolated, identified and analysed metabolites between two varieties of Forsythiae Fructus using UPLC/ESI-Q TRAP-MS/MS. The results showed that a total of 407 metabolites were identified in Forsythiae Fructus using UPLC/ESI-Q TRAP-MS/MS, including 21 terpenoids, 68 phenolic acids, 63 flavonoids, 43 amino acids and derivatives, 22 alkaloids, 55 lipids, 24 lignans and coumarins, 31 nucleotides and derivatives, 29 organic acids, and 51 other metabolites. Among, lignans and coumarins, terpenoids, organic acids, lipids, and phenolic acids were rich in Forsythiae Fructus, which accounted for more than 60% of the total metabolite content. Differential metabolite analysis revealed that 80 metabolites differed significantly between the two types of Forsythiae Fructus. Our results greatly enrich the Forsythiae Fructus phytochemical composition database and provide valuable information for further study of the metabolites of Forsythiae Fructus.
Keywords: Forsythiae Fructus; traditional Chinese medicine; UPLC/ESI-Q TRAP-MS/MS; component; metabolite
Forsythia suspensa (Thunb.) Vahl is a perennial shrub plant which is widely distributed in Japan, Korea, and China. The fruit of Forsythia suspense named Forsythiae Fructus is a traditional Chinese herbal medicine that has antiviral and antioxidant effects [1,2]. Modern analytical chemistry studies showed that the extracts of Forsythiae Fructus contain many bioactive components, such as terpenoids, lignans, phenolic acids, and flavonoids [3–6]. Among them, the flavonoids of Forsythiae Fructus have been proven to be responsible for their neuroprotective effects [7]. Lignans of Forsythiae Fructus have been shown to be a cytoprotective effect against cell damage [8]. The total phenolic of Forsythiae Fructus is considered to have anti-microbial activity [9]. Xue et al. [10] reported that eleven terpenoid compounds are obtained from Forsythiae Fructus and some of them show cytotoxity against gastric cancer cells. Forsythoside A is a phenylethanoid glycoside from Forsythiae Fructus that has an antiviral effect against influenza virus [2]. Therefore, systematic analysis of the metabolites of Forsythiae Fructus has great significance for the exploitation and utilization of Forsythia suspensa.
UPLC/ESI-Q TRAP-MS/MS has been used for the identification and analysis of plant metabolites, which has the advantages of wide coverage, fast separation, and high throughput and sensitivity [11,12]. Zhu et al. [13] showed that 820 chemicals were identified based on ESI–QqTOF–MS/MS metabolic methods in tomato fruit. Zou et al. [14] detected 518 metabolies from soybean growth between drought stress and non-stress by UPLC-MS/MS, and the contents of sugars, amino acids, organic acid, and nucleotides were reduced under drought stress. Fayek et al. [15] reported that 16 different Vicia species were subjected to a chemotaxonomic study using UPLC-MS metabolome, and a total of 89 metabolites were observed.
UPLC technology was also used to identify metabolites in Forsythiae Fructus. Zhou et al. [16] identified 45 kinds of chemical components of Forsythia suspensa by UPLC-Q-TOF-MS, and 19 compounds were identified for the first time. Wei et al. [17] detected twenty-one bioactive constituents using UFLC-Q TRAP-MS/MS technology in seed, stalk and pericarp of Forsythiae Fructus, and phenylethanoid glycosides, lignans, flavonoids and phenolic acids were main compounds. Although more than 100 bioactive compounds have been identified in Forsythiae Fructus, the number and type of metabolites in Forsythiae Fructus are still unclear. In our study, two varieties of Forsythiae Fructus were chosen for this study, one with surface nodules, the other smooth. They are the main types of Forsythiae Fructus in Hebei province, and will be major sowing varieties. We identified and analyzed the metabolites of these Forsythiae Fructuses using UPLC/ESI-Q TRAP-MS/M, and found the differential metabolites between two varieties of Forsythiae Fructus. Our results contribute valuable information for metabolite studies on Forsythiae Fructus.
Forsythiae Fructus were collected from healthy Forsythia suspensa on 15 August at Shexian Forsythia suspensa base, Hebei Province, China. We collected two types of Forsythiae Fructus (Fig. 1): one type of fruit displayed surface nodules (LG), the other smooth (GG). All Forsythiae Fructus samples were frozen using liquid nitrogen and stored in −80°C ultra-low temperature refrigerator.

Figure 1: Phenotypes of two varieties of Forsythiae Fructus
Freeze-dried Forsythiae Fructus samples were crushed using a mixer mill (MM 400, VERDER RETSCH, Shanghai, China) for 2.0 min at a frequency of 50 Hz. Then, 200 mg powder was extracted for 12 h with 70% methanol aqueous solution (V/V = 70%). After centrifugation at 12,000 g for 10 min, the extracts of Forsythiae Fructus were adsorbed by a CNWBOND Carbon-GCB SPE cartridge (250 mg, 3 mL; ANPEL, Shanghai, China) and filtered through a 0.22-μm microfiltration membrane (SCAA-104; ANPEL, Shanghai, China).
2.3 Ultra Performance Liquid Chromatography (UPLC) Conditions
Forsythiae Fructus extracts were analyzed by UPLC-ESI-MS/MS system (UPLC, Shim-pack UFLC SHIMADZU CBM30A system, Shanghai, China). The UPLC analysis method was in accordance with the method of Wang et al. [18]. Analytical conditions of UPLC: column, Waters (Shanghai, China) ACQUITY UPLC HSS T3 C18 (1.8 µm, 2.1 mm × 100 mm); solvent system, water (0.04% acetic acid): acetonitrile (0.04% acetic acid); gradient program, 95:5 V/V at 0 min, 5:95 V/V at 11.0 min, 5:95 V/V at 12.0 min, 95:5 V/V at 12.1 min, 95:5 V/V at 15.0 min; flow rate, 0.40 mL/min; temperature, 40°C; injection volume: 2 μL. After UPLC separation, the eluent was analysed by MS in Linear Ion Trap (LIT) and Triple Quadrupole (TQ) modes.
Linear ion trap (LIT) and triple quadrupole (QQQ) scans were acquired on a triple Q TRAP, API 6500 Q TRAP LC/MS/MS system (Applied Biosystems, Shanghai, China) equipped with an ESI turbo ion-spray interface, which was operated in positive and negative ion mode. Operation parameters of ESI source were set with the following: ion source, turbo spray; source temperature 400°C; ion spray voltage (IS) 5000 V. The ion source gas I (GSI), gas II (GSII), and curtain gas (CUR) were set at 25.0, 55.0, and 60.0 psi, respectively. Mass calibration and instrument tuning were performed with 10 and 100 μmol/L polypropylene glycol solutions in LIT and QQQ modes, respectively. Declustering potential and collision energy measurements were done for individual MRM transitions. QQQ scans were acquired as multiple reaction monitoring (MRM) experiments with collision gas (nitrogen) set to 5 psi. A specific set of MRM transitions was monitored for each period on the basis of the metabolites eluted within the period [19].
2.5 Qualitative and Quantitative Analysis of Metabolites
Qualitative analysis of the metabolites was in according to the method of Jiang et al. [12], which were performed based on the Metware Database (Metware Biotechnology Co., Ltd., Wuhan, China). Home-made software with reference to MetDNA of Zhu’s laboratory and Masterview from AB Sciex was used to provide the matching score of metabolites from the Metware Database. Most metabolite compounds were standards in the database, and no standards metabolites were available in the MS2T library, and mainly the peaks were used to query the MS 2 spectral data from the public metabolite databases, such as METLIN, MoToDB, KNAPSAcK, HMDB, and MassBank [20]. The metabolites Forsythiae Fructus were quantitatively analyzed using multiple reaction monitoring [21]. After the induced ionization of the collision chamber, the precursor ions were fragmented to form many fragmented ions. Then, a characteristic fragment ion was selected to eliminate the interference of non-target ions by triple quadrupole filtering. After the metabolite mass spectrometry data of different Forsythiae Fructus samples were obtained, all substance mass peaks were integrated into the peak area, and the same metabolite in different samples was integrated and corrected.
Unsupervised principal component analysis (PCA), hierarchical cluster analysis (HCA), and partial least squares-discriminant analysis (OPLS-DA) were performed with R [22]. The unsupervised PCA was performed by the statistics function prcomp in R, and the data were unit variance scaled before the unsupervised PCA. The HCA results of Forsythiae Fructus samples and metabolites were presented as heatmaps with dendrograms, while pearson correlation coefficients (PCC) between samples were calculated and presented as heatmaps using the cor function in R. Both HCA and PCC were carried out by the R package pheatmap. For HCA, normalized signal intensities of metabolites were visualized to a color spectrum. Differential metabolites were determined by an absolute Log2FC (fold change) ≥ 1 and variable importance in projection (VIP) ≥ 1.
Each experiment was repeated in three biological replicates. Statistical analysis was performed with Excel 2013 software (Microsoft Office, USA). Data were presented as means ± standard deviations (SD), and the statistical significance levels were analyzed using the least significant difference (P < 0.05).
3.1 Metabolites in Forsythiae Fructus
In order to isolate, identify and analyse the variation of differential metabolites between GG and LG, we detected the metabolites in Forsythiae Fructus using UPLC/ESI-Q TRAP-MS/MS technology. The total ion current (TIC) chromatogram of mixed quality control (QC) samples showed the intensity of all ions in the mass spectrum at each time point (Fig. 2A). The multi-peak diagram of multiple reaction monitoring (MRM) metabolite detection showed the ion flow spectrogram of multi-substance extraction (Fig. 2B). The superposition diagram of the TIC of metabolites showed that the TIC curves had high overlap, which indicated that the data were repeatable and reliable (Fig. 2C). A total of 407 metabolites were isolated and identified in Forsythiae Fructus. R software program was used to draw the heatmap of metabolites after unit variance scaling (UV), and the accumulation pattern of metabolites was described using hierarchical cluster analysis (HCA) (Fig. 3A). The 407 metabolites were classified into 10 categories, including 21 terpenoids, 68 phenolic acids, 63 flavonoids, 43 amino acids and derivatives, 22 alkaloids, 55 lipids, 24 lignans and coumarins, 31 nucleotides and derivatives, 29 organic acids, and 51 other metabolites (Fig. 3B and Table S1).

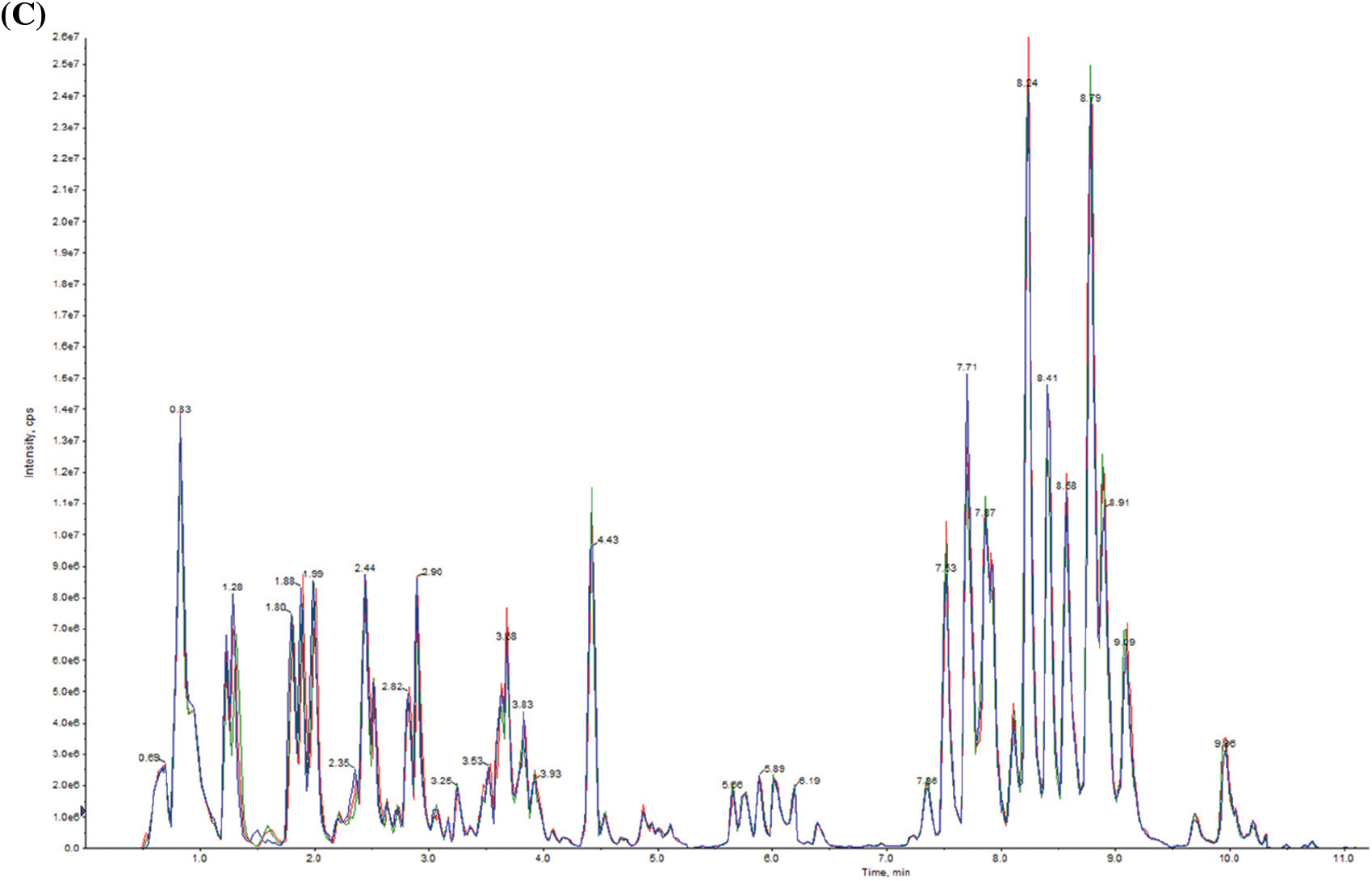
Figure 2: Quantitative and qualitative analyses of metabolites in Forsythiae Fructus. (A) Total ion current of samples based on mass spectrometry detection. (B) Multi-peak detection plot of metabolites in the multiplereaction monitoring (MRM) mode. (C) The overlapped total ion chromatogram of Forsythiae Fructus
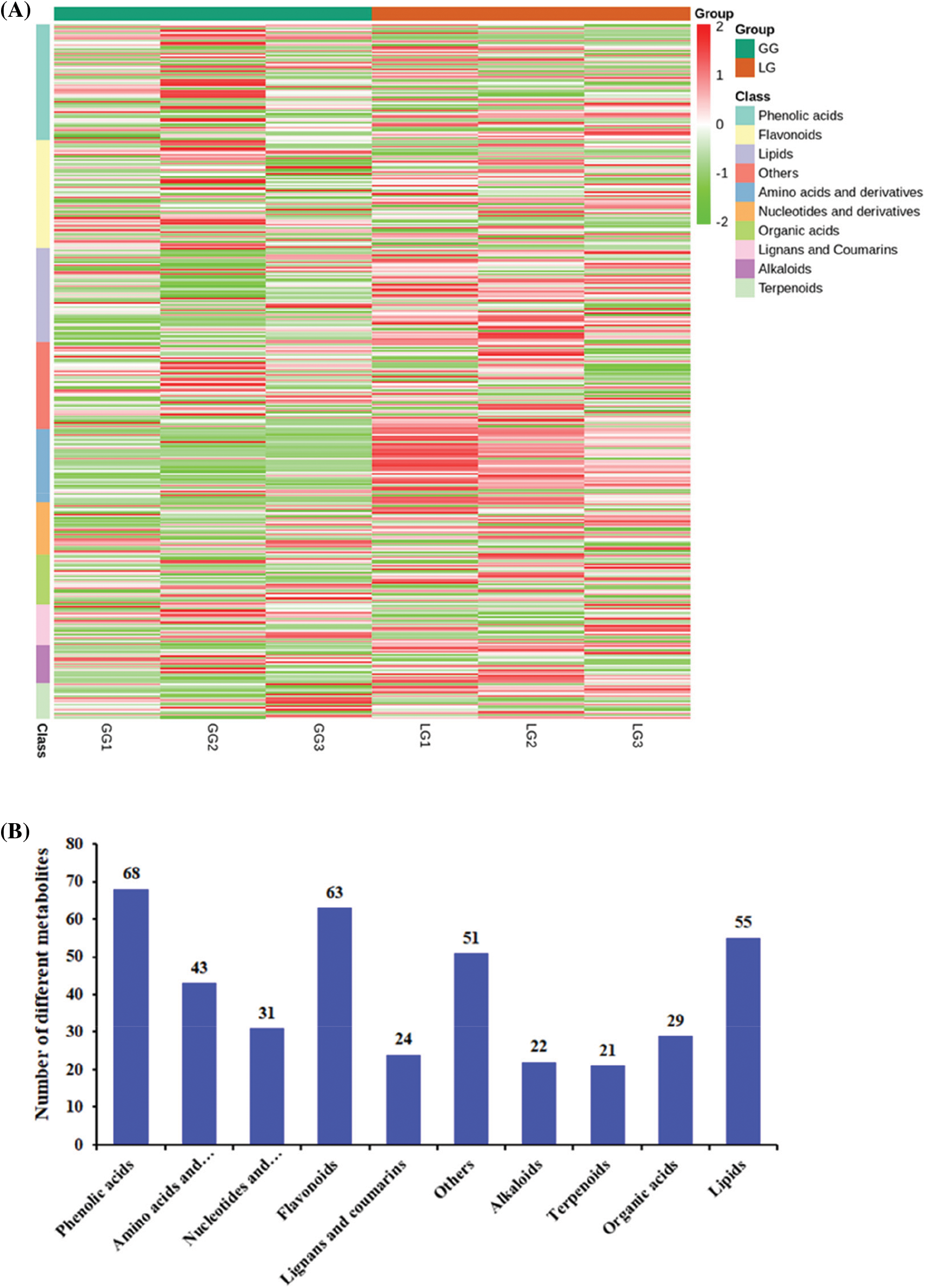
Figure 3: Analysis of metabolites in Forsythiae Fructus. (A) Cluster heat map of metabolites between GG and LG. The abundance of each metabolite is represented with a different color. Red indicates highly abundant metabolites, whereas metabolites with low abundance are shown in green. (B) The number of metabolites in different categories
3.2 Analysis of Main Metabolites in Forsythiae Fructus
Among 407 metabolites, lignans and coumarins, terpenoids, organic acids, lipids, and phenolic acids were abundant in Forsythiae Fructus, which accounted for more than 60% of the total metabolite content. Lipids are important active ingredients in plants, but the compositions of lipids were still unclear in Forsythiae Fructus. In our metabolite data, the contents of lipids were highest in all identified metabolites. A total of 55 lipids were detected, among phosphatidylcholines, lysophosphatidylcholines, myristic acid, and 15s-hydroperoxyeicosatetraenoic acid were major lipid compounds in Forsythiae Fructus (Fig. 4A). Terpenoids play an important role during the development of plants, and over 40,000 terpenoids are found in natural products [23]. In our study, we identified 21 terpenoids, including 3 monoterpenoids, 2 sesquiterpenoids, and 16 triterpenes (Fig. 4B). Forsythide, monoterpenoids with vasorelaxant effects [24], were also found in our study. Organic acids have been shown to be biologically active natural compounds. Some studies showed organic acids had anti-inflammatory, antioxidant, and antimicrobial activity [25]. However, the research of organic acids is still limited in Forsythiae Fructus. In this study, 29 organic acids were detected, with malic acid and citric acid accounting for over 60% of the organic acids detected (Fig. 4C).
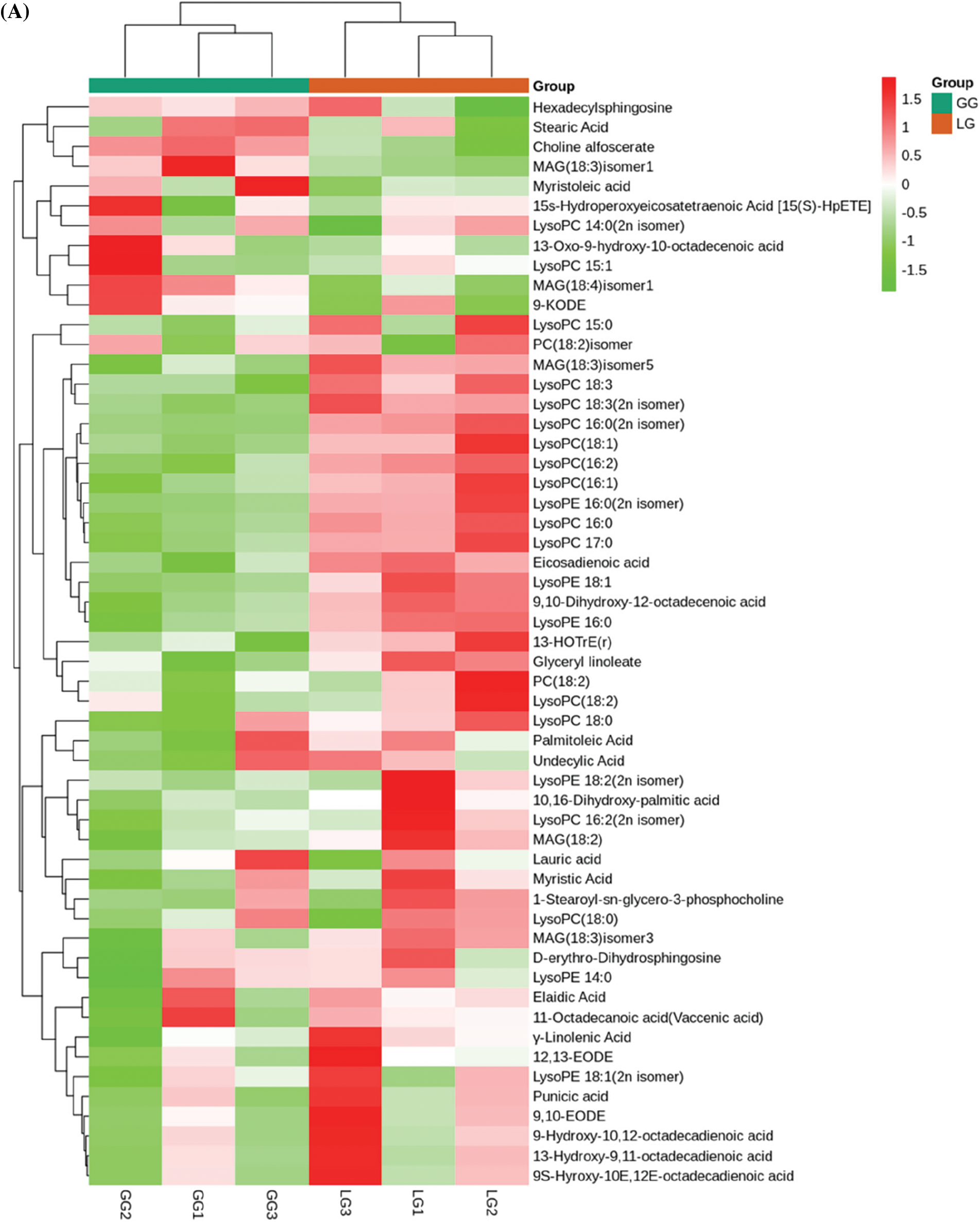
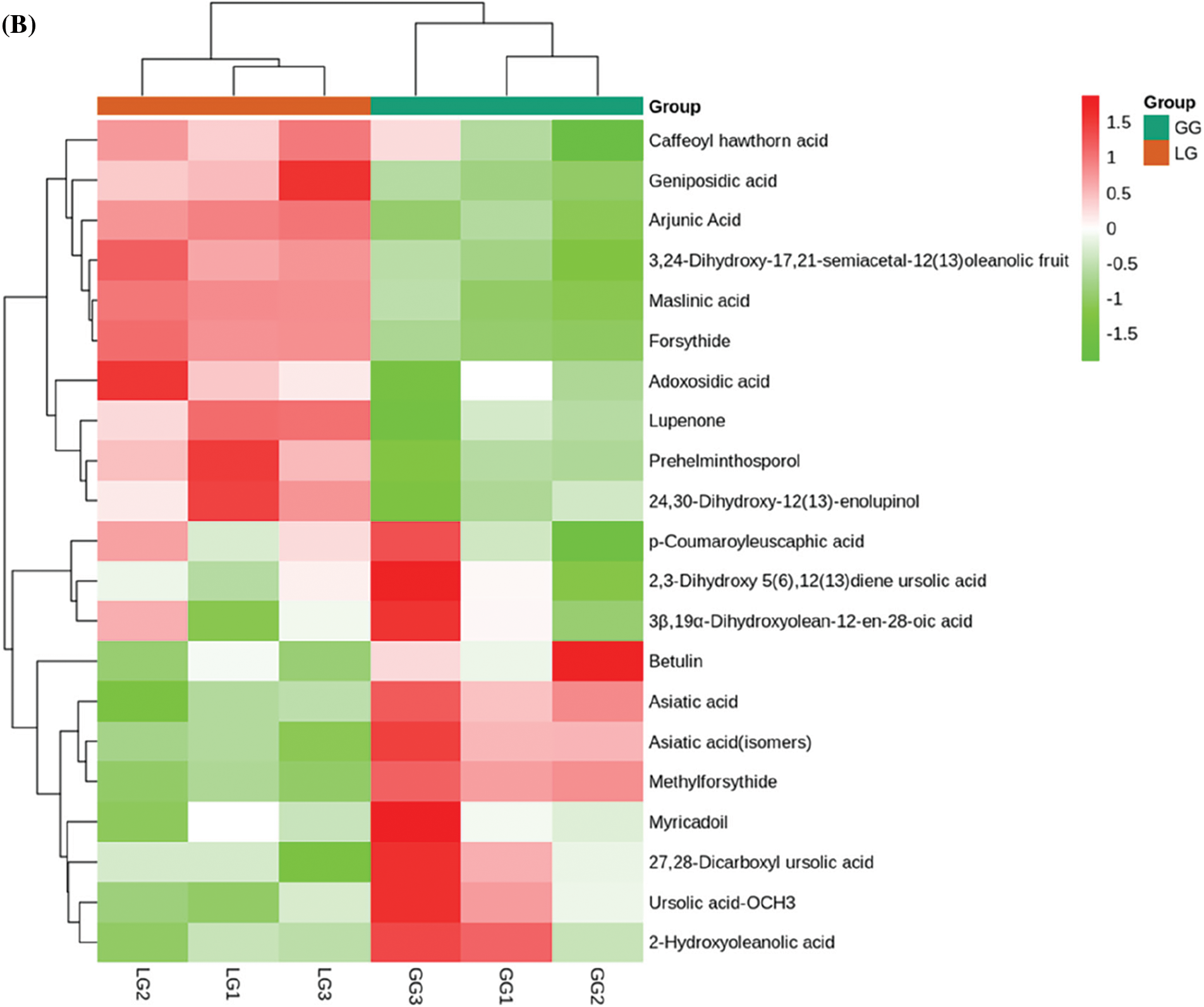
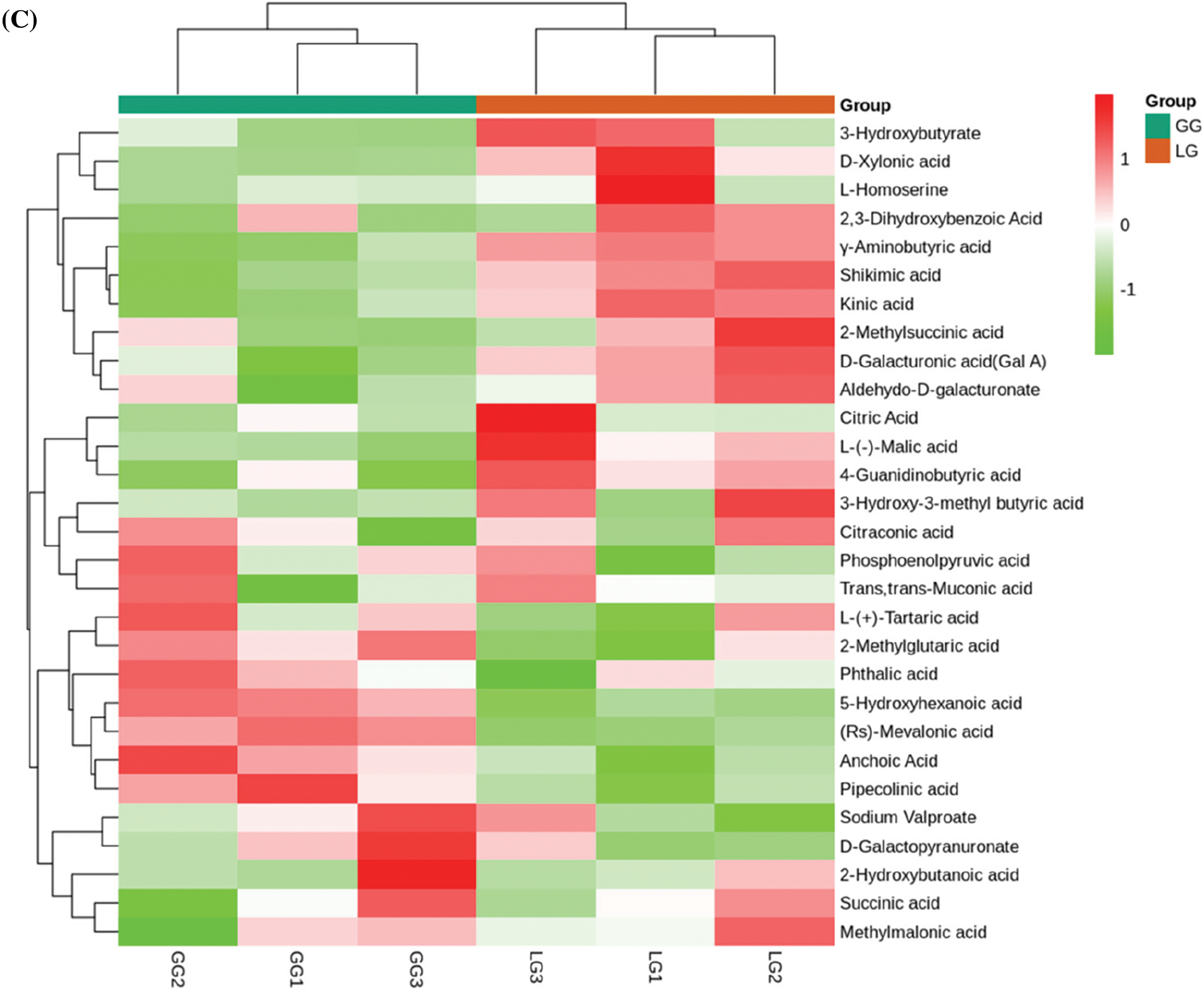
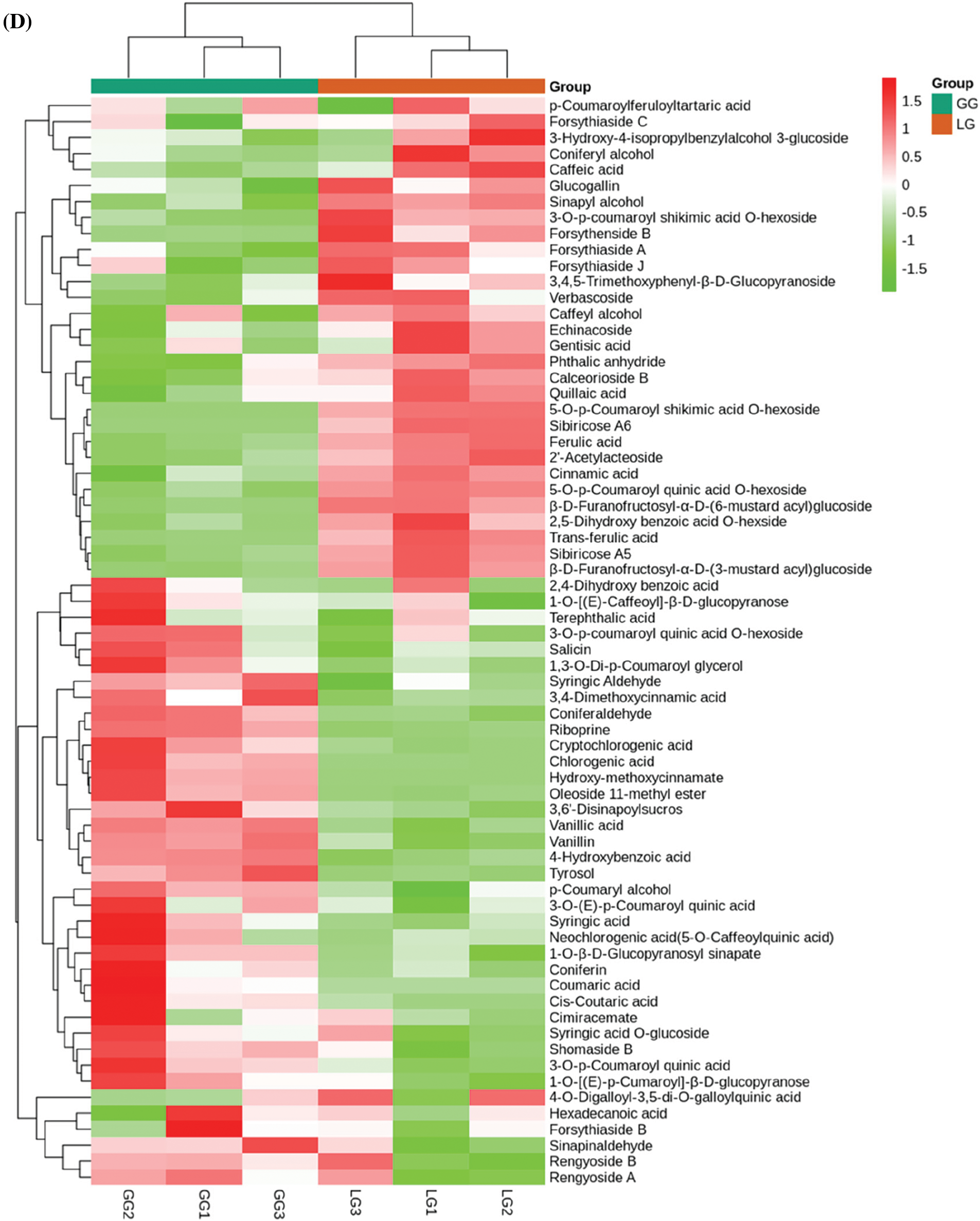
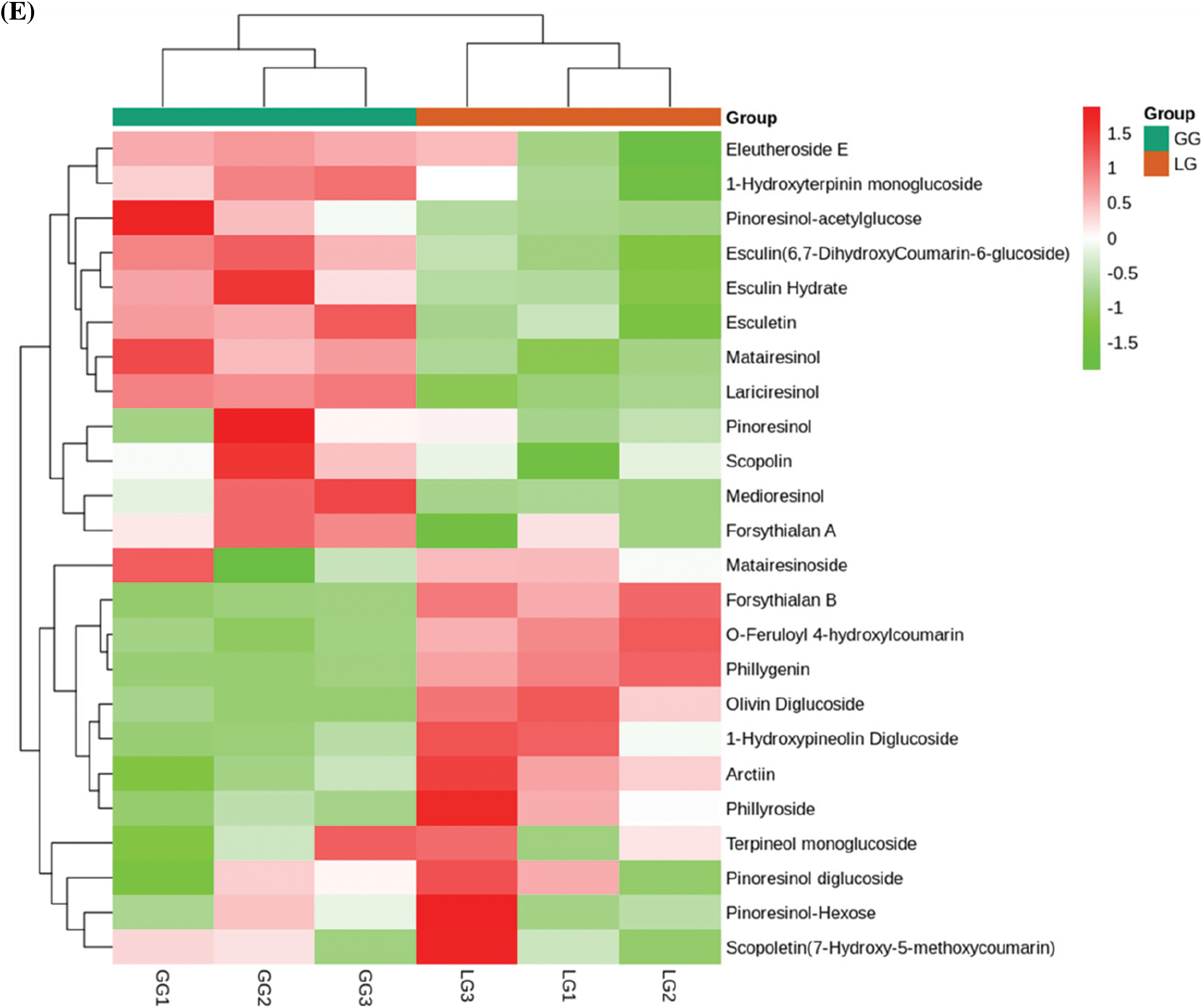
Figure 4: Analysis of secondary metabolites between GG and LG. (A) Lipids, (B) Terpenoids, (C) Organic acids, (D) Phenolic acids, (E) Lignans and coumarins. Each sample is represented by a column, and each metabolite is represented by a row. The abundance of each metabolite is represented by a different color. Ratios of fold changes are shown as red or green shading. Data represent the mean values of three biological replicates for each sample
Phenolic acids are abundant in vegetables, fruit, and herbs [26]. However, less attention is focused on the bioactivity of phenolic acids in Forsythiae Fructus. Sixty-eight phenolic acids were identified in Forsythiae Fructus, and chlorogenic acid, forsythenside, rengyoside, and forsythiaside were major phenolic acids extracted from Forsythiae Fructus (Fig. 4D). Among, forsythiaside A, forsythiaside B, forsythiaside C, forsythiaside J, rengyoside A, rengyoside B, and forsythenside B are unique bioactive compounds in Forsythiae Fructus that were more and more attention in clearing heat and detoxifying effect [27]. Lignans and coumarins are plant secondary metabolites derived from phenylpropanoid biosynthesis pathway. They are considered to have an important anticancer role [28]. We found 24 lignans and coumarins compounds in Forsythiae Fructus, including 18 lignans and 6 coumarins (Fig. 4E). Matairesinoside, terpineol monoglucoside, and pinoresinol-Hexose were found to make up about 80% of total lignans and coumarins detected. We also had identified forsythialan A, forsythialan B, phillyroside, and phillygenin in lignans and coumarins compounds.
3.3 Differential Metabolite Analysis between GG and LG
Principle component analysis (PCA) was used to reveal the differential metabolites between the groups and the intra-group samples. In our study, two principal components were extracted and had values of 55.87% and 13.59%, respectively; the cumulative contribution rate reached 69.46% (Fig. 5A). Analysis of the PCA score plot indicated that GG and LG were clearly separated from one another. We further analyzed the metabolite data using orthogonal partial least squares discriminant analysis (OPLS-DA). Q2 is an important index to evaluate the models in OPLS-DA. A Q2 value greater than 0.5 shows an effective model, and Q2 value greater than 0.9 shows an excellent model. In our study, the Q2 value was 0.95 in GG vs. LG (Fig. 5B). Thus, the OPLS-DA models were reliable and effective method to screen the differential metabolites between groups.
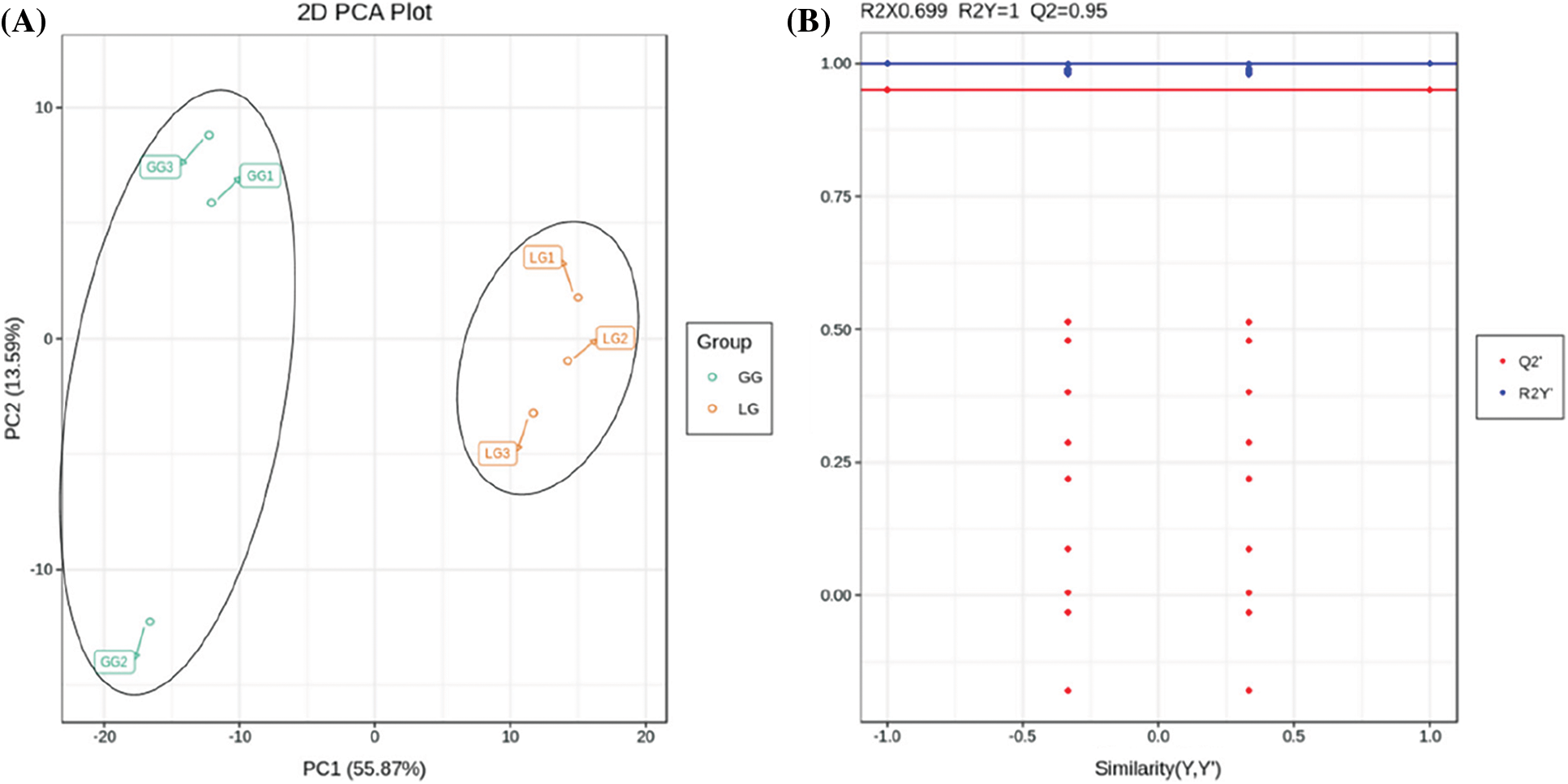
Figure 5: Analysis of differential metabolites based on principle component analysis (PCA) and orthogonal partial least squares discriminant (OPLS-DA). (A) PCA score plots of mass spectrum data. (B) OPLS-DA models of GG vs. LG. The horizontal axis represents the similarity of Y and Y′, and the vertical axis is the value of R2Y and Q2
Differential metabolites were screened by the absolute Log2FC (fold change) ≥ 1 and VIP value ≥ 1; 80 metabolites among the 407 metabolites were significantly different between GG and LG, including 56 upregulated metabolites and 24 downregulated metabolites (Fig. 6A). Amino acids are important compounds that can increase the yield of plants [29]. In this study, we found that the contents of amino acids and derivatives were significantly different between GG and LG, and amino acids and derivatives content was 2.58-fold in LG than in GG. Among, DL-norvaline, L-leucine, L-proline, L-valine, L-isoleucine, α-aminocaproic acid, and tryptophan were major amino acids. Further analysis of metabolites, we found that the contents of flavonoids, lignans and coumarins, other metabolites, alkaloids, terpenoids, organic acid, and lipids were higher in LG than in GG, whereas the contents of phenolic acid and nucleotides and derivatives were low in LG than in GG (Fig. 6B).
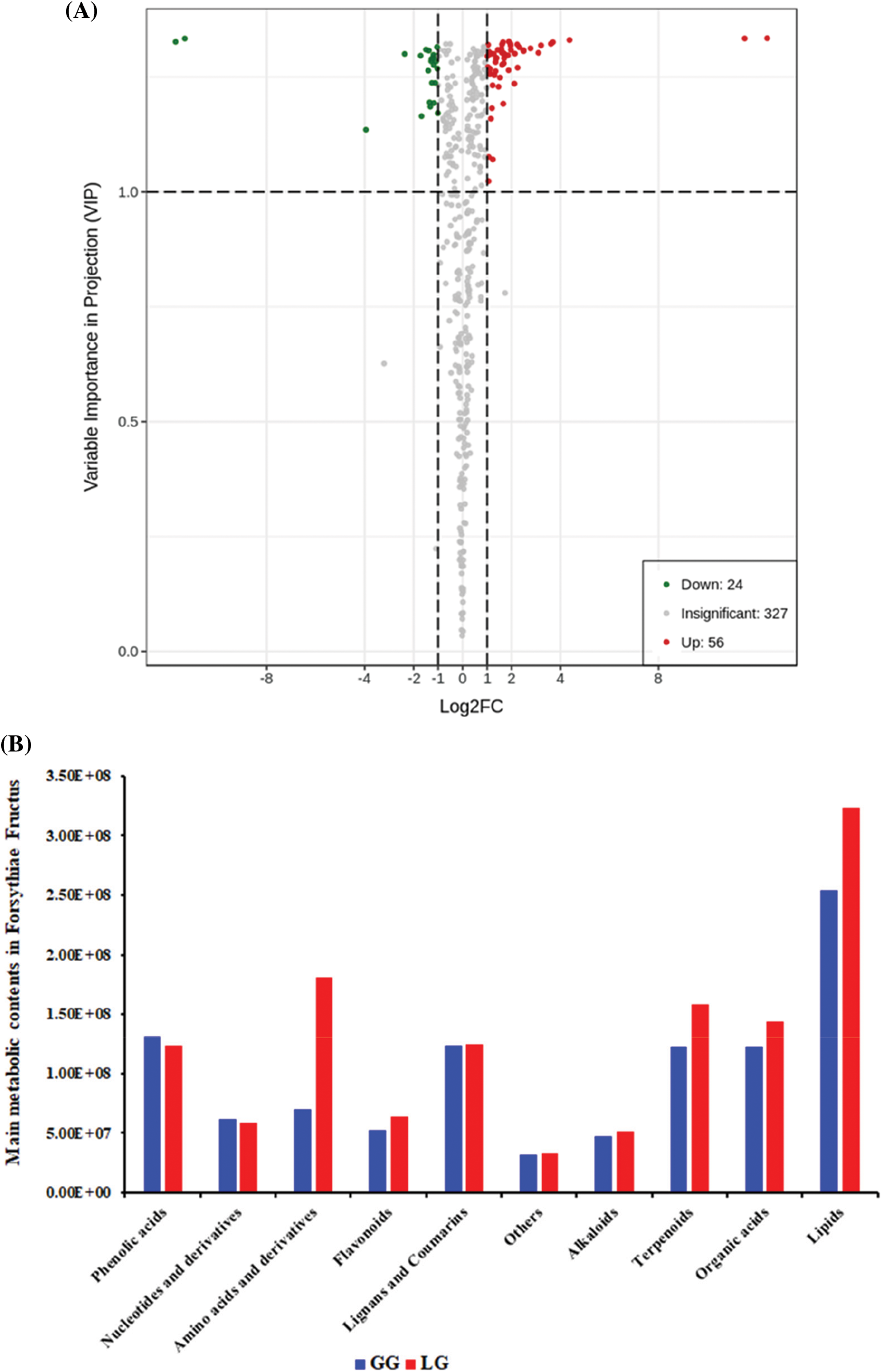
Figure 6: Analysis of differential metabolites in Forsythiae Fructus. (A) Volcano plot of the differential metabolites between GG and LG. Up- and down-regulated metabolites are shown in red and green, respectively. (B) Main metabolic contents in Forsythiae Fructus
Forsythiae Fructus is a traditional medicinal herb in China for centuries, and it can be used to anti-inflammatory and treat toxicity, tonsillitis, ulcers, pharyngitis and acute nephritis [30,31]. According to the Chinese Pharmacopoeia, more than 40 Chinese medicinal preparations contain Forsythiae Fructus [32]. Forsythiae Fructus has abundant secondary metabolites, including flavonoids, lignans, phenylethanoid glycosides, and terpenoids, and some of the above metabolites have been reported to have different biological activities [33]. Qu et al. [34] reported that forsythin and forsythiaside had antioxidant and antibacterial activities. Phillyrin is an important chemical compound of Forsythia suspensa (Thunb.), which can attenuate LPS-induced pulmonary inflammation [35]. Zhang et al. [7] identified four new compounds (forsythoneoside E and F, forsythobiflavone A and B) from the fruit of Forsythia suspense, and each compound exhibited neuroprotective effect.
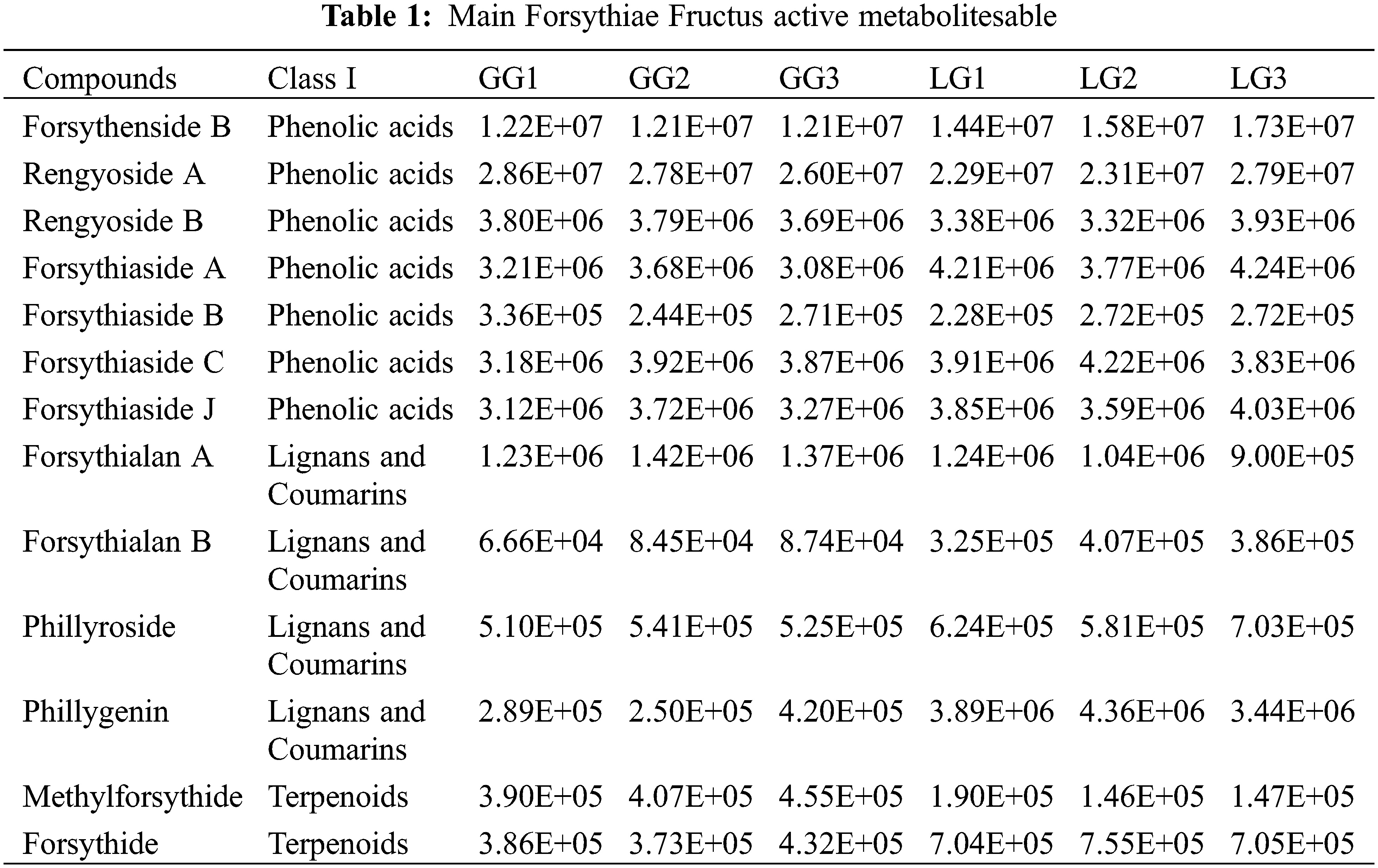
In recent years, metabolomics has been utilized to gain insight into the chemical compounds in plants [36–38]. For example, 45 kinds of chemical components were identified in Forsythiae Fructus by UPLC-Q-TOF-MS, which included 7 phenylethanol glycosides, 5 lignans, 5 terpenes, 12 flavonoids, 7 organic acids, 2 phenols, 2 quinones, 2 glycosides and 3 other components [16]. In this study, in order to elucidate the variation of metabolites between GG and LG, metabolomics analysis was performed using UPLC/ESI-Q TRAP-MS/MS. We identified 407 differential metabolites in Forsythiae Fructus, among which 80 metabolites were significantly different between LG and GG (Fig. 3 and Fig. 6A). Further analysis of the metabolites indicated that the main metabolic components were lignans and coumarins, terpenoids, organic acids, lipids, and phenolic acids in Forsythiae Fructus, which accounted for more than 60% of the total metabolite content (Fig. 4). Differential metabolite analysis between GG and LG showed that LG contained high levels of secondary metabolites, such as amino acids, flavonoids, lignans and coumarins, alkaloids, terpenoids, and lipids, whereas GG contained a high level of phenolic acid and nucleotides and derivatives (Fig. 5B). This suggested that the LG type of Forsythiae Fructus will be an important germplasm resource in the future Forsythia suspensa breeding. Phillyrin, forsythoside A, and forsythoside C which are bioactive compounds in Forsythiae Fructus exhibit antioxidant, antibacterial, and antiviral activity [39]. In this study, a total of 13 main Forsythiae Fructus active metabolites were found in GG and LG (Table 1). Of these, forsythiaside A, forsythiaside B, forsythiaside C, forsythiaside J, forsythenside B, rengyoside A, and rengyoside B belonged to the phenolic acids. Forsythialan A, forsythialan B, phillyroside, and phillygenin were belong to the lignans and coumarins. Forsythide and methylforsythide were belong to the terpenoids. The above metabolites were the main medicinal ingredients in Forsythiae Fructus.
We identified 407 metabolites in Forsythiae Fructus using UPLC/ESI-Q TRAP-MS/MS. The main metabolic components were lignans and coumarins, terpenoids, organic acids, lipids, and phenolic acids in Forsythiae Fructus, which accounted for more than 60% of the total metabolite content. Differential metabolite analysis revealed that 80 metabolites differed significantly between the two types of Forsythiae Fructus. Our results greatly enrich the Forsythiae Fructus phytochemical composition database and provide valuable information for further study of the metabolites of Forsythiae Fructus.
Acknowledgement: We thank LetPub (https://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author Contributions: C. X., W. T., and X. L. performed the experiments. T. J. and S. Q. analyzed the data. L. D. and T. J. wrote the paper. All authors read and approved the final version of the paper.
Funding Statement: This work was supported by Evaluation and Application of Germplasm Resources in Technical System of Traditional Chinese Medicine Industry in Hebei Province (HBCT201806201), Hebei Modern Seed Industry Science and Technology Special Project (19226345D), and China Agriculture Research System (CARS-21).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Zhang, H. Y., Piao, X. S., Zhang, Q., Li, P., Yi, J. Q. et al. (2013). The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poultry Science, 92(8), 1981–1988. DOI 10.3382/ps.2013-03081. [Google Scholar] [CrossRef]
2. Law, A. H. Y., Yang, C. L. H., Lau, A. S. Y., Chan, G. C. F. (2017). Antiviral effect of forsythoside A from Forsythia suspensa (Thunb.) Vahl fruit against influenza A virus through reduction of viral M1 protein. Journal of Ethnopharmacology, 209(1), 236–247. DOI 10.1016/j.jep.2017.07.015. [Google Scholar] [CrossRef]
3. Nikaido, T., Ohmoto, T., Kinoshita, T., Sankawa, U., Nishibe, S. et al. (1981). Inhibition of cyclic AMP phosphodiesterase by lignans. Chemical Pharmaceutical Bulletin, 29(12), 3586–3592. DOI 10.1248/cpb.29.3586. [Google Scholar] [CrossRef]
4. Kitagawa, S., Hisada, S., Nishibe, S. (1984). Phenolic compounds from Forsythia leaves. Phytochemistry, 23(8), 1635–1636. DOI 10.1016/S0031-9422(00)83456-1. [Google Scholar] [CrossRef]
5. Shamsur-Rouf, A. S., Ozaki, Y., Rashid, M. A., Rui, J. (2001). Dammarane derivatives from the dried fruits of Forsythia suspensa. Phytochemistry, 56(8), 815–818. DOI 10.1016/S0031-9422(01)00028-0. [Google Scholar] [CrossRef]
6. Qu, H. H., Zhang, Y. M., Wang, Y., Li, B. X., Sun, W. J. (2008). Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated from Forsythia suspense. Journal of Pharmacy and Pharmacology, 60(2), 261–266. DOI 10.1211/jpp.60.2.0016. [Google Scholar] [CrossRef]
7. Zhang, F., Yang, Y. N., Feng, Z. M., Jiang, J. S., Zhang, P. C. (2017). Four new phenylethanoid and flavonoid glycoside dimers from the fruits of Forsythia suspensa and their neuroprotective activities. RSC Advances, 7(40), 24963–24969. DOI 10.1039/C7RA04229A. [Google Scholar] [CrossRef]
8. Piao, X. L., Cho, E. J., Jang, M. H., Cui, J. (2009). Cytoprotective effect of lignans from Forsythia suspensa against peroxynitrite-induced LLC-PK1 cell damage. Phytotherapy Research, 23(7), 938–942. DOI 10.1002/ptr.2834. [Google Scholar] [CrossRef]
9. Qu, J. L., Yan, X. J., Li, C. Y., Wen, J., Lu, C. N. et al. (2017). Comparative evaluation of raw and ripe fruits of Forsythia suspensa by HPLC-ESI-MS/MS analysis and anti-microbial assay. Journal of Chromatographic Science, 55(4), 451–458. DOI 10.1093/chromsci/bmw203. [Google Scholar] [CrossRef]
10. Xue, J., Xie, L., Liu, B. R., Yu, L. X. (2010). Triterpenoids from the fruits of Forsythia suspense. Chinese Journal of Natural Medicines, 8(6), 414–418. DOI 10.1016/S1875-5364(11)60004-6. [Google Scholar] [CrossRef]
11. Li, J., Yang, P., Yang, Q. H., Gong, X. W., Ma, H. C. et al. (2019). Analysis of flavonoid metabolites in buckwheat leaves using UPLC-ESI-MS/MS. Molecules, 24(7), 1310. DOI 10.3390/molecules24071310. [Google Scholar] [CrossRef]
12. Jiang, T., Zhang, M. D., Wen, C. X., Xie, X. L., Tian, W. et al. (2020). Integrated metabolomic and transcriptomic analysis of the anthocyanin regulatory networks in Salvia miltiorrhiza Bge. flowers. BMC Plant Biology, 20(1), 349. DOI 10.1186/s12870-020-02553-7. [Google Scholar] [CrossRef]
13. Zhu, G. T., Wang, S. C., Huang, Z. J., Zhang, S. B., Liao, Q. G. et al. (2018). Rewiring of the fruit metabolome in tomato breeding. Cell, 172(1–2), 249–261. DOI 10.1016/j.cell.2017.12.019. [Google Scholar] [CrossRef]
14. Zou, J. N., Yu, H., Yu, Q., Jin, X. J., Cao, L. et al. (2021). Physiological and UPLC-MS/MS widely targeted metabolites mechanisms of alleviation of drought stress-induced soybean growth inhibition by melatonin. Industrial Crops and Products, 163, 113323. DOI 10.1016/j.indcrop.2021.113323. [Google Scholar] [CrossRef]
15. Fayek, N. M., Mekky, R. H., Dias, C. N., Kropf, M., Heiss, A. G. et al. (2021). UPLC-MS metabolome-based seed classification of 16 Vicia species: A prospect for phyto-equivalency and chemotaxonomy of different accessions. Journal of Agricultural and Food Chemistry, 69(17), 5252–5266. DOI 10.1021/acs.jafc.0c06054. [Google Scholar] [CrossRef]
16. Zhou, M. Y., Hou, J. H., Sun, G. D., Wang, W. M. (2019). Identification of 45 kinds of chemical components of Forsythia suspensa by UPLC-Q-TOF-MS. China Pharmacy, 30(22), 3067–3073. DOI 10.6039/j.issn.1001-0408.2019.22.09. [Google Scholar] [CrossRef]
17. Wei, L. F., Mei, Y. Q., Zou, L. S., Chen, J. L., Tan, M. X. et al. (2020). Distribution patterns for bioactive constituents in pericarp, stalk and seed of Forsythiae Fructus. Molecules, 25(2), 340. DOI 10.3390/molecules25020340. [Google Scholar] [CrossRef]
18. Wang, A., Li, R., Ren, L., Gao, X. L., Zhang, Y. G. et al. (2018). A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chemistry, 260(125), 124–134. DOI 10.1016/j.foodchem.2018.03.125. [Google Scholar] [CrossRef]
19. Chen, W., Gong, L., Guo, Z. L., Wang, W. S., Zhang, H. Y. et al. (2013). A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites, application in the study of rice metabolomics. Molecular Plant, 6(6), 1769–1780. DOI 10.1093/mp/sst080. [Google Scholar] [CrossRef]
20. Yang, F. Y., Yang, T. R., Liu, K., Yang, Q., Wan, Y. Q. et al. (2019). Analysis of metabolite accumulation related to pod color variation of Caragana intermedia. Molecules, 24(4), 717. DOI 10.3390/molecules24040717. [Google Scholar] [CrossRef]
21. Wang, Z. R., Cui, Y. Y., Vainstein, A., Chen, S. W., Ma, H. et al. (2017). Regulation of Fig (Ficus carica L.) fruit color, metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway. Front Plant Sciences, 8, 1990. DOI 10.3389/fpls.2017.01990. [Google Scholar] [CrossRef]
22. Wang, S. C., Tu, H., Wang, J., Chen, W., Liu, X. Q. et al. (2016). Spatio-temporal distribution and natural variation of metabolites in citrus fruits. Food Chemistry, 199, 8–17. DOI 10.1016/j.foodchem.2015.11.113. [Google Scholar] [CrossRef]
23. Bonfill, M., Malik, S., Mirjalili, M. H., Goleniowski, M., Cusido, R. et al. (2013). Production and genetic engineering of terpenoids production in plant cell and organ cultures. Natural Products, 60(6), 2761–2796. DOI 10.1007/978-3-642-22144-6_123. [Google Scholar] [CrossRef]
24. Lizuka, T., Sakai, H., Moriyama, H., Suto, N., Nagai, M. et al. (2009). Vasorelaxant effects of forsythide isolated from the leaves of Forsythia viridissima on NE-induced aortal contraction. Phytomedicine, 16(4), 386–390. DOI 10.1016/j.phymed.2008.09.011. [Google Scholar] [CrossRef]
25. Duffy, C. F., Power, R. F. (2001). Antioxidant and antimicrobial properties of some Chinese plant extracts. International Journal of Antimicrobial Agents, 17(6), 527–529. DOI 10.1016/S0924-8579(01)00326-0. [Google Scholar] [CrossRef]
26. Russell, W., Duthie, G. (2011). Plant secondary metabolites and gut health: The case for phenolic acids. Proceedings of the Nutrition Society, 70(3), 389–396. DOI 10.1017/S0029665111000152. [Google Scholar] [CrossRef]
27. Tong, C., Chen, T., Chen, Z. W., Wang, H., Wang, X. F. et al. (2021). Forsythiaside a plays an anti-inflammatory role in LPS-induced mastitis in a mouse model by modulating the MAPK and NF-κB signaling pathways. Research in Veterinary Science, 136, 390–395. DOI 10.1016/j.rvsc.2021.03.020. [Google Scholar] [CrossRef]
28. Teodor, E. D., Moroeanu, V., Radu, G. L. (2020). Lignans from medicinal plants and their anticancer effect. Mini-Reviews in Medicinal Chemistry, 20(12), 1083–1090. DOI 10.2174/1389557520666200212110513. [Google Scholar] [CrossRef]
29. Tzin, V., Galili, G. (2010). New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Molecular Plant, 3(6), 956–972. DOI 10.1093/mp/ssq048. [Google Scholar] [CrossRef]
30. Ozaki, Y., Rui, J., Tang, Y., Satake, M. (1997). Antiinflammatory effect of Forsythia suspensa Vahl and its active fraction. Biological Pharmaceutical Bulletin, 20(8), 861–864. DOI 10.1248/bpb.20.861. [Google Scholar] [CrossRef]
31. Nishibe, S., Okabe, K., Tsukamoto, H., Sakushima, A., Hisada, S. et al. (1982). Studies on the Chinese crude drug Forsythiae Fructus VI. The structure and antibacterial activity of suspersaside isolated from Forsythia suspensa. Chemical Pharmaceutical Bulletin, 30(12), 4548–4553. DOI 10.1248/cpb.30.4548. [Google Scholar] [CrossRef]
32. Chinese Pharmacopoeia Commission (2015). The pharmacopoeia of the People’s Republic of China. Beijing, China: Chinese Medical Science Press. [Google Scholar]
33. Jia, J. P., Zhang, F. S., Li, Z. Y., Qin, X. M., Zhang, L. W. (2015). Comparison of fruits of Forsythia suspensa at two different maturation stages by NMR-based metabolomics. Molecules, 20(6), 10065–10081. DOI 10.3390/molecules200610065. [Google Scholar] [CrossRef]
34. Qu, H. H., Li, B. X., Li, X., Tu, G. Z., Lü, J. et al. (2008). Qualitative and quantitative analyses of three bioactive compounds in different parts of Forsythia suspensa by high-performance liquid chromatography-electrospray ionization-mass spectrometry. Microchemical Journal, 89(2), 159–164. DOI 10.1016/j.microc.2008.02.002. [Google Scholar] [CrossRef]
35. Zhong, W. T., Wu, Y. C., Xie, X. X., Zhou, X., Wei, M. M. et al. (2013). Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-κB activation in acute lung injury mice. Fitoterapia, 90(18), 132–139. DOI 10.1016/j.fitote.2013.06.003. [Google Scholar] [CrossRef]
36. Jiang, T., Guo, K. Y., Liu, L. D., Tian, W., Xie, X. L. et al. (2020). Integrated transcriptomic and metabolomic data reveal the flavonoid biosynthesis metabolic pathway in Perilla frutescens (L.) leaves. Scientific Reports, 10(1), 16207. DOI 10.1038/s41598-020-73274-y. [Google Scholar] [CrossRef]
37. Yang, G., Liang, K. N., Zhou, Z. Z., Wang, X. Y., Huang, G. H. (2020). UPLC-ESI-MS/MS-Based widely targeted metabolomics analysis of wood metabolites in teak (Tectona grandis). Molecules, 25(9), 2189. DOI 10.3390/molecules25092189. [Google Scholar] [CrossRef]
38. Wang, F. Y., Huang, Y. J., Wu, W., Zhu, C. Y., Zhang, R. M. et al. (2020). Metabolomics analysis of the peels of different colored citrus fruits (Citrus reticulata cv. ‘Shatangju’) during the maturation period based on UHPLC-QQQ-MS. Molecules, 25(2), 396. DOI 10.3390/molecules25020396. [Google Scholar] [CrossRef]
39. Xia, Y. G., Yang, B. Y., Wang, Q. H., Liang, J., Wei, Y. H. et al. (2009). Quantitative analysis and chromatographic fingerprinting for the quality evaluation of Forsythia suspensa extract by HPLC coupled with photodiode array detector. Journal of Pharmaceutical and Biomedical Analysis, 32(23–24), 4113–4125. DOI 10.1002/jssc.200900488. [Google Scholar] [CrossRef]
Appendix
Supplementary Table S1: The metabolites in Forsythiae Fructus
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |