
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.016290
ARTICLE
Potential Antidiabetic and Anti-Genotoxic Activities of Silver Nanoparticles of Alkaloid Extract of Rhazya stricta in Rat Animal Model
Biology Department, Faculty of Science, University of Tabuk, Tabuk, 47512, Saudi Arabia
*Corresponding Author: Mohammed Ali Ashehri. Email: ma.alshehri@ut.edu.sa
Received: 23 February 2021; Accepted: 29 April 2021
Abstract: In most of Arabian courtiers Rhazya stricta is extensively utilized in public medicine for several diseases treatment. In this study, crude alkaloid extract of R. stricta (CAERS) coated with silver nanoparticles (CAERS-AgNPs) as potential treatment against diabetes in DM animal model was evaluated. Swiss albino male rats (n = 80) were injected with STZ to induce Diabetes Mellitus type-2 (DM). DM-rats were injected different doses of CAERS or CAERS-nanoparticles (CAERS-NPs) for 2 months. The results exhibited that mRNA expression of insulin and insulin receptor was down-regulated, activity levels of antioxidant enzymes were decreased, generation of ROS mediated DNA adducts and apoptosis in DM-rats was increased significantly than those in negative control rats. In contrast, the expression of insulin and insulin receptor genes was up-regulated, activity levels of antioxidant enzymes, ROS generation, DNA adducts and apoptosis incidence in DM-rats supplemented with high dose of CAERS and all doses of CAERS-NPs were improved. In conclusion: R. stricta nanoparticles improved the anti-diabetic effect of the plant much more than the powder form of the extract. This action could be attributed to modification of the chemical and physical properties of the plant materials. The properties modification might be improved the ability of plant compounds to penetrate the cell membrane which facilitating release of plant materials into the target cells.
Keywords: Diabetes; Rhazya stricta; nanotechnology; gene expression; DNA damage; apoptosis
Saudi Arabia ranked as the second highest country in the Middle East in Diabetes mellitus (DM) rate as declared by the WHO. It has been reported that more than seven million are suffering from diabetes in Saudi Arabia and three million are considered as pre-diabetes [1]. Diabetes is defined as a disorder in the metabolism of many compounds leading to decrease insulin secretion, high blood sugar levels, and disturbances in the metabolism of carbohydrates, proteins and fats. DM has been found to be highly correlated with vascular complications, morbidity and mortality accompanied by lower quality of life [2].
The medicinal plant, namely, Rhazya stricta (Apocynaceae) is widely distributed in Arabian countries and Indian sub-continent. The R. stricta leaf extract contains mainly glycosides, alkaloids, tannins and triterpenes [3]. R. stricta leaf extract has been used for treatment several ailments such as diabetes [4], cancer [5] and inflammation [3].
Saudi Arabia is one of the most Arabian countries having widely R. stricta. The leaves of R. stricta collected from different locations of Saudi Arabia contained high levels of flavonoid compounds [6–8]. Alkaloid diversity and genome data of R. stricta has been also documented [9]. Several terpenoid indole alkaloids (TIAs) are isolated from R. stricta leaves which could be utilized in several biotechnology applications [10]. Additionally, the extract of R. stricta is considered as an important source of d-tocopherols which possess anti-cancer, antioxidant and anti-inflammatory activities [11,12].
The nanotechnology promising field involves the use of nano-materials to achieve preferred goals in different fields including medicine (diagnosis of diseases), agriculture (pesticide management) [13,14]. Use of such field is attributed to the specific physio-chemical characteristics of metal nanoparticles enhancing biochemical properties, elevated reactivity and surface area. One of the important metal nanoparticles is silver which is extensively used for diagnostic purposes, drug delivery and disease treatment [15–19].
The new field, namely, nanomedicine is a combination between nanotechnology and medicine which is created to promote human health care. Diabetic treatment is one of the several applications of nanomedicine in treating diseases [20]. They utilized biodegradable polymeric carriers “nanospheres”. This nano-material act as artificial pancreas as an alternative to pancreas transplantation. So, as many of patients with diabetes in the Saudi Arabia are using medicinal plants in combination with medicines to treat diabetes, in this study we have investigated the use of crude alkaloid extract of R. stricta (CAERS) coated with silver nanoparticles (Ag-NPs) as potential treatment against diabetes in DM animal model.
Streptozotocin (STZ) drug used for induction of diabetes was obtained from Sigma–Aldrich, St. Louis Missouri, USA. Chemicals used for molecular biology analyses were obtained from Invitrogen, Carlsbad, CA, USA (such as Trizol reagent), Fermentas, Glen Burnie, MD, USA (such as reverse transcription and PCR kits) and Stratagene, California, USA (such as SYBR Green Mix). Moreover, remaining used chemicals were purchased from ordinary commercial suppliers.
Swiss albino male rats (130–145 g) were used in this experiment. The rats were supplemented with regular diet and water ad libitum. Following one week adaptation time, several animal groups (n = 9) were designed (10 rats each) in which the animals of each group were housed in separate plastic cages. The rats were housed in animal care room free from any contamination and controlled light periods and temperature. The utilized rats were received human care according to the Tabuk University Ethical guidelines and the experimental design was approved from the Ethical committee of Tabuk University.
2.2.1 Induction of Diabetic Animals
Fasted rats were injected intraperitonealy (i.p.) with one dose of STZ solution (50 mg/kg b.wt.) [21] to induce diabetes. Three days later of injection, blood samples were aspirated from STZ-exposed animals and levels of glucose were estimated. When, the glucose levels exceeded 250 mg/dl [22], the rats were considered as diabetic animals and were included in this study. Healthy control animals were injected with citrate buffer solution.
The R. stricta leaves are collected in 2020 from Tabuk, KSA. The plant samples were dried using solar energy once transfer the samples into the laboratory. The R. stricta leaves were authenticated by the Biology Department, University of Tabuk, Saudi Arabia.
2.2.3 Alkaloid Extract Preparation
Leaves of R. stricta were used to prepare a crude alkaloid extract according to Elkady [23]. In brief, dried powder of R. stricta leaves (350 g) were soaked in methyl alcohol (1 L, 80%) for one week at 24°C. Afterwards, the R. stricta methanolic extract was evaporated and the rest residue was suspended in H2O and filtered. The water extract was mixed with glacial acetic acid (10%) and then extracted with chloroform which had weakly neutral and basic alkaloids compounds. The residual of aqueous solution was mixed and alkalinized with NaOH (pH = 11). Using chloroform the alkaline water layer was extracted to a good yield of chloroform fraction having strongly alkaloid compound termed as crude alkaloid extract of R. stricta (CAERS).
2.2.4 Biosynthesis of Silver Nanoparticles
Preparation of silver nitrate nanoparticles (1 mM) was carried out in aqueous solution in which the fresh CAERS was added at a ratio of 9:1 (v:v). The solution mixture of silver nanoparticles (Ag-NPs) and CAERS was placed with constant rotation in a shaker at 27 ± 2°C for six h [24].
2.2.5 Nanoparticles Characterization of CAERS-AgNPs
The prepared CAERS-AgNPs was characterized using UV-visible spectrophotometry. Therefore, to prove the biosynthesize of CAERS-AgNPs, a UV-visible spectrophotometry (UVD 3200) had been used. Additionally, to perform the X-ray diffraction assessment of CAERS-AgNPs, X-ray Diffractometer (Equinox 3000) was used. Furthermore, a Hitachi (S-4160) scanning electron microscopy was utilized to analyze the size and shape of the biosynthesized CAERS-AgNPs. Also, to measure particle and molecule sizes of the prepared CAERS-AgNPs a Nno-z 590 Malvern-Zetasizer was implemented. The characterization measurement of DVLE-AgNPs was determined to classify the functional groups responsible in reduction of silver ion existed in CAERS. Consequently, potassium bromide with the pellets of CAERS combined with Ag-NPs (1:100) for FTIR spectrum analysis by a Bruker Tensor 27 spectrophotometer was used.
The protocol of the in vivo experiments was approved by the Ethical committee of Faculty of Science, University of Tabuk, Tabuk, Saudi Arabian. All protocols and procedures concerning animal handling and care of animals (NIH guidelines) followed to the ARRIVE guidelines [25] were taken into consideration.
Animals used in this study were allocated in eight groups (10 rats per a group) as follows: Group 1: Rats injected orally with citrate buffer solution and served as control. Group 2: Animals were exposed (i.p.) to single dose of STZ (50 mg/kg b.wt.) to induce Diabetes Mellitus type-2 (DM) as described above [26]. Groups 3–5: diabetic-rats were injected orally with 0.11, 0.22, and 0.33 g/kg body weight, respectively, of crude alkaloid extract of R. stricta (CAERS) for 2 months [27]. Groups 6–8: diabetic-rats were injected orally with 0.11, 0.22, and 0.33 g/kg body weight, respectively, of CAERS-nanoparticles (CAERS-NPs) for 60 days. At termination of the experiment, samples of the blood were taken to measure levels of glucose. Afterwards, using ether animals from all treated groups were anesthetized and then sacrificed speedily. Consequently, pancreas and liver tissues of the animals were collected for the bio analyses.
2.3.1 Isolation of Total RNA and Reverse Transcription Reaction
The total RNA of collected pancreas samples of all treated animals was extracted using TRIzol® extraction reagent (Invitrogen, Carlsbad, CA, USA). Subsequent to achievement of the RNA extraction, the pellets of the isolated RNA were kept in water containing DEPC. The isolated RNA was exposed to RNAse-free DNAse kit (Invitrogen, Germany) to break down the potential residues of the DNA [28]. Afterwards, the extracted RNA was divided in aliquots and kept under −80°C until use for reverse transcription.
To synthesize the cDNA copies of the pancreas samples of treated animals synthesis kit of First Strand cDNA of Fermentas company (RevertAidTM, Fermentas, Glen Burnie, MD, USA) was utilized to reverse isolated RNA to cDNA through the reaction of reverse transcription (RT). The PCR program of the RT to obtain the cDNA copy was adjusted as follows: (a) 10 min at 25°C; (b) 60 min at 42°C; and (c) 5 min at 95°C. After termination of the RT reaction, the PCR tubes containing cDNA copies were kept at −20°C up to use for qRT-PCR [29].
2.3.2 Quantitative Real Time-PCR
The synthesized cDNA copies of the pancreas samples were used for the qRT-PCR reaction using SYBR green kit (Stratagene, California, USA). Melting curve was performed for each reaction and specific studied gene. The Ct values of the qRT-PCR of investigated genes were normalized on those of the β-actin housekeeping gene (Tab. 1). The quantitative values of the tested genes under investigation to the housekeeping gene were assessed using 2−ΔΔCT method.

2.3.3 Assessment of ROS Generation
To determine the intracellular ROS generation in pancreatic samples of treated animals was carried out using fluorescent probe of the oxidation-sensitive DCFH-DA through a flow cytometer. Through the reaction the pancreatic cells were freely absorbed the non-fluorescent compound DCFH-DA. The fluorescence activity was determined at excitation 488 nm and emission 525 nm by the flow cytometer. The counted cells for each measurement were approximately equal to 1 × 105 cells in which the test was carried out in triplicate [31].
2.3.4 Assessment of DNA Adducts (8-OHdG and 2-dG)
The 8-OHdG/2-dG ratio generated in pancreatic tissues of all treated rats was carried out. Total DNA was isolated from rat samples by homogenizing its tissues in lysis buffer (pH 7.4) and incubating the samples over night (~ for 16 h) in presence proteinase K at 50–60°C. The incubated animal samples were then treated with RNase enzyme at 50°C for 10 min according to Abdu et al. [32]. Chloroform/isoamyl alcohol method was used to extract the genomic DNA from the experimental groups. The isolated DNA pellet was dissolved in Tris/EDTA solution up to use. Using HPLC (CoulArray system, Model 5600) with several electrochemical sensors the formation ratio of 8-OHdG/2-dG was determined [33].
2.3.5 Apoptosis Detection by Annexin V-Fitc Assay
To assess the apoptosis in pancreatic tissues of all treated rats a protocol of the Annexin V-FITC assay kit (Sigma-Aldrich, Germany) was used. Pancreatic samples collected from all experimental groups were homogenized in PBS, washed in distilled water and left at 4°C. After homogenization process Annexin V-FITC was mixed with the cell suspension binding buffer and the mixture was kept in the dark for 10 min. Afterwards, the incubated cells were washed and then re-suspended in the binding buffer followed by adding Propidium Iodide (PI) solution. The cells were kept on ice and consequently analyzed by flow cytometry (Becton Dickinson, San Jose, CA, USA). After flow cytometry measurement CellQuest software (Becton Dickinson, San Jose, CA, USA) was used to analyze the obtained data. Finally, apoptosis was calculated as summation of early apoptotic cells and late apoptotic cells according to Dabour et al. [34].
2.3.6 Antioxidant Enzymes Analysis
According to the protocols of commercially available kits, liver samples collected from treated groups were homogenized in lysis buffer using clean mortar. At the reaction termination the samples were centrifuged and the supernatants were used for the measurement of catalase (CAT) and glutathione peroxidase (GPx) enzymes following the instructions of utilized kits [35].
All data obtained from the previous analyses were expressed as mean ± SEM and analyzed using General Liner Models (GLM) of Statistical Analysis System (SAS). Afterwards, to assess the significant differences between investigated groups a Scheffé-test was utilized. All significance statements were based on probability of P < 0.05.
3.1 Levels of Glucose in CAERS and CAERS-NPs Treated DM-Rats
Glucose levels in serum of diabetic are presented in Tab. 2. The measurement exhibited that CAERS and CAERS-NPs reduced the levels of glucose in diabetic rats after 2 months treatment with clearly significant differences compared to DM-rats without treatment. The reduction impact on the glucose levels was observed with all doses of CAERS and CAERS-NPs. Moreover, the reduction in the glucose levels at the treatment with the high dose of CAERS-NPs reached point very close to levels of glucose in control rats.

3.2 Gene Expression Profile in CAERS and CAERS-NPs Treated DM-Rats
The expression profile of the studied genes related to diabetes, namely, insulin and insulin receptor in pancreatic samples collected from all treated groups is summarized in Figs. 1 and 2. The results found that expression levels of the studied genes were decreased significantly (P < 0.001) in DM-rats in comparison to those in healthy control rats.
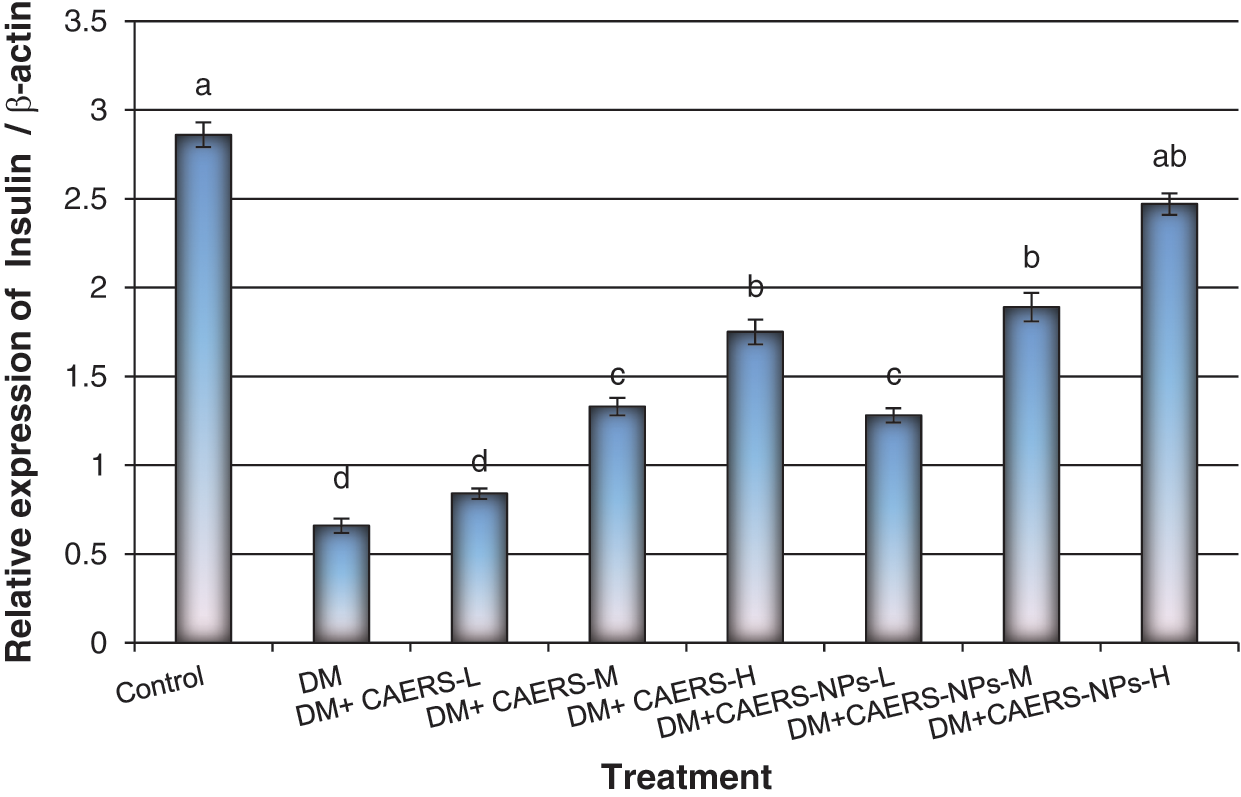
Figure 1: Expression levels of insulin gene in pancreatic tissues of DM rats treated with CAERS and CAERS-nanoparticles. Data are presented as mean ± SEM. a,b,c,d followed by different superscripts are significantly different (P ≤ 0.05)
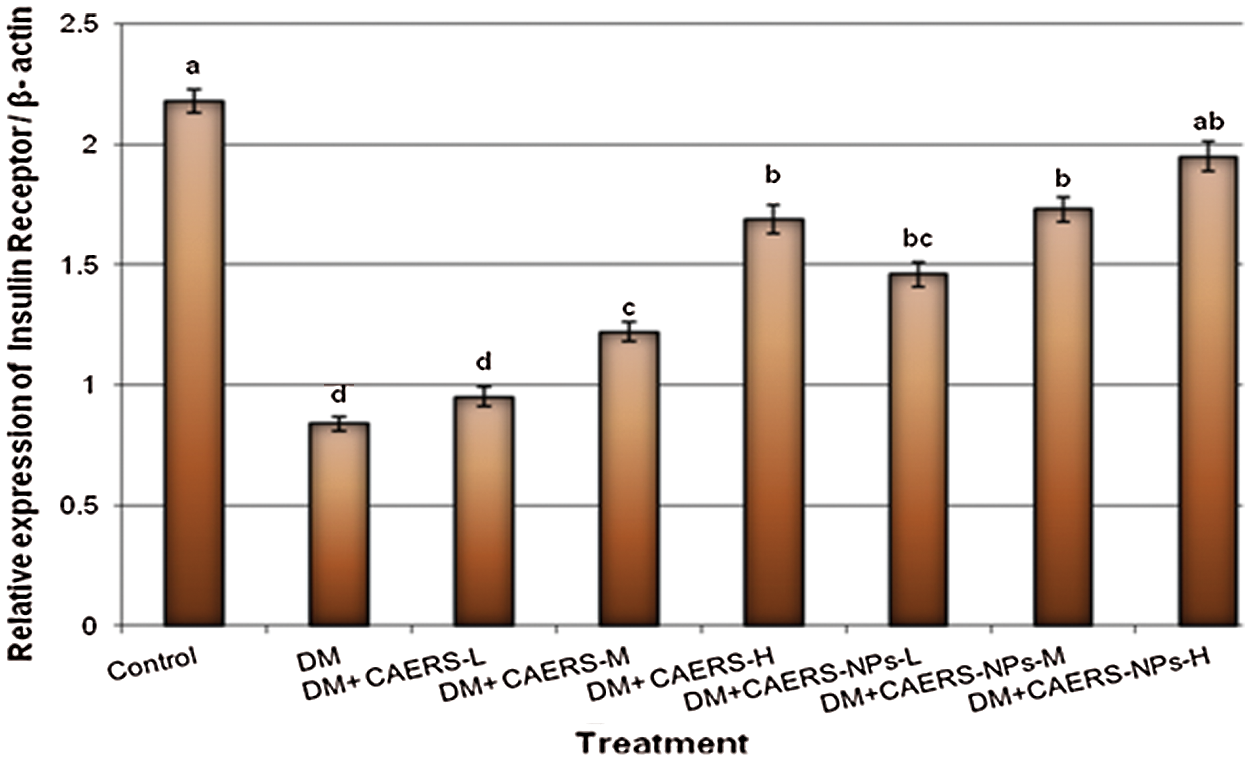
Figure 2: Expression levels of insulin receptor gene in pancreatic tissues of DM rats treated with CAERS and CAERS-nanoparticles. Data are presented as mean ± SEM. a,b,c,d followed by different superscripts are significantly different (P ≤ 0.05)
In contrast, insulin and insulin receptor genes were over expressed with significant (P < 0.05 and P < 0.01, respectively) high values in diabetic rats treated with medium and high doses of CAERS in comparison to those in DM-rats without treatment. Moreover, the genes under study were (P < 0.01 and P < 0.001, respectively) over expressed in diabetic rats treated with all three doses of CAERS-NPs in regard to those in DM-rats without treatment. Furthermore, insulin and insulin receptor genes were highly over expressed in DM-induced rats treated with CAERS-NPs at the highest dose as their expression reached levels very close to that in healthy control rats.
3.3 ROS Generation in CAERS and CAERS-NPs Treated DM-Rats
The effect of CAERS and CAERS-NPs on the formation of the intracellular ROS ratio in pancreas samples of DM-animals is presented in Fig. 3. The current findings indicated that the ROS generation induced by STZ in DM-rats was increased significantly compared with those in control animals. In contrast, the ROS generation ratio in DM-rats treated with CAERS at the medium and high doses decreased significantly (P < 0.05) in comparison to that in DM-rats without treatment. Additionally, treatment of diabetic animals with CAERS-NPs at the all tested doses decreased significantly (P < 0.01 and P < 0.001, respectively) the ROS generation in comparison to that in DM-rats without treatment.
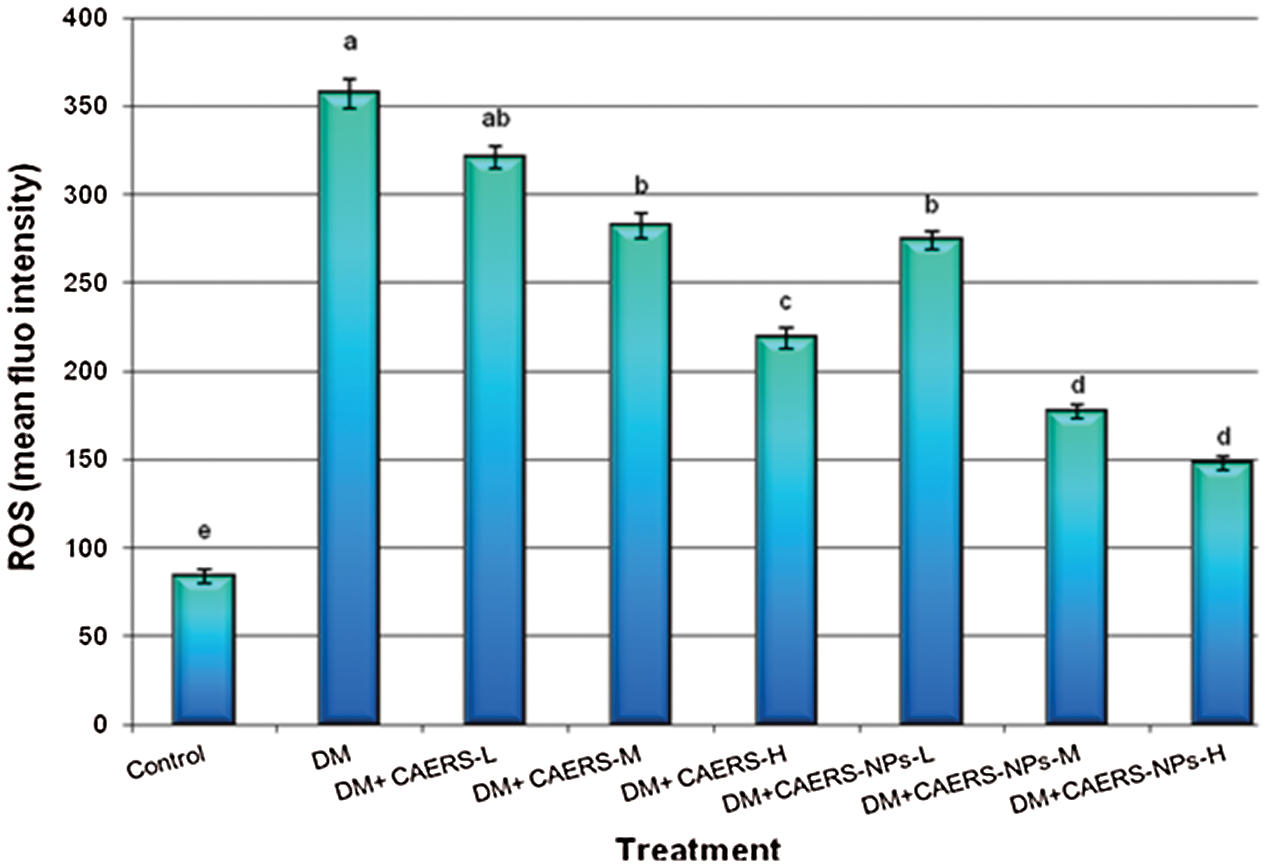
Figure 3: The changes of intracellular ROS levels in pancreatic tissues of DM-rats treated with CAERS and CAERS-nanoparticles. Data are presented as mean ± SEM. a,b,c,d,e followed by different superscripts are significantly different (P ≤ 0.05)
3.4 Formation of DNA Adducts in CAERS and CAERS-NPs Treated DM-Rats
The formation of 8-OHdG/2-dG ratio in pancreatic samples of DM-animals treated with CAERS and CAERS-NPs is illustrated in Fig. 4. The obtained results exhibited that the ratio of 8-OHdG/2-dG formation induced by STZ in DM-animals was significantly increased compared with that in control animals. However, the formation ratio of the DNA adducts in DM-animals treated with CAERS at medium and high doses decreased significantly (P < 0.05) in comparison to that in untreated DM-animals. Also, treatment of diabetic animals CAERS-NPs at the all three tested doses decreased significantly (P < 0.01 and P < 0.001, respectively) the DNA adducts formation compared with that in untreated DM-rats.
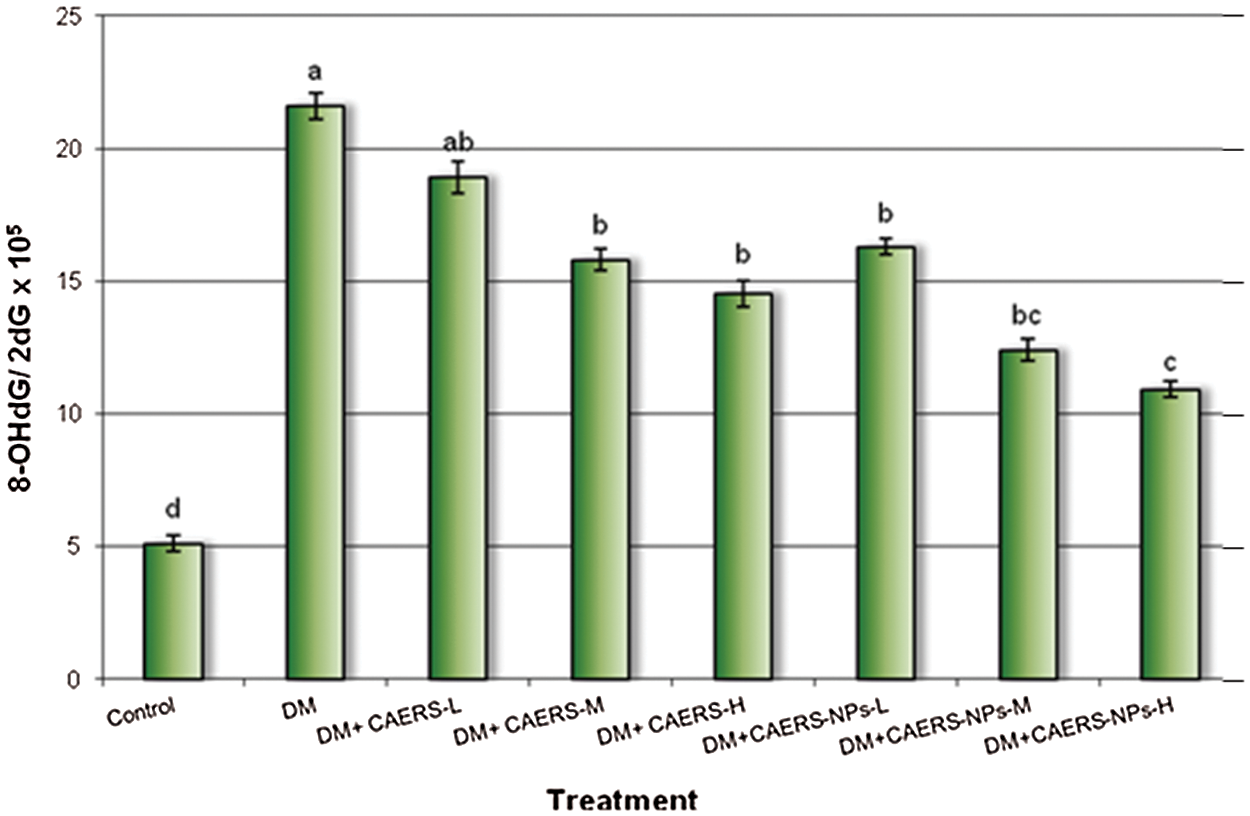
Figure 4: Generation of 8-OHdG in pancreatic tissues of DM-rats treated with CAERS and CAERS-nanoparticles. DNA damage was expressed as the ratio of oxidized DNA base (8-OHdG) to nonoxidized base (2-dG) in pancreatic tissue DNA. a,b,c,d followed by different superscripts are significantly different (P ≤ 0.05)
3.5 Apoptosis in CAERS and CAERS-NPs Treated DM-Rats
The incidence of apoptosis using flow cytometry analysis combined with FITC-Annexin V/PI staining in pancreatic samples of DM-animals treated with CAERS and CAERS-NPs is summarized in Figs. 5a and 5b. The results found that the incidence of apoptosis (%) induced by STZ in DM-animals increased significantly in comparison to that in control rats (Fig. 5a).


Figure 5: (a) Flow cytometry analysis of annexin-V and propidium iodide staining in pancreatic tissues of untreated control and diabetic rats (DM). The upper left (UL) quadrant represents necrotic cells, the left lower (LL) quadrant represents healthy cells, the upper right (UR) quadrant represents early apoptotic cells and the lower right (LR) quadrant represents late apoptotic cells. Apoptosis was calculated as summation of UR + LR. Values represent average percentage (±SEM) of at least five samples. (b) Flow cytometry analysis of annexin-V and propidium iodide staining in pancreatic tissues of diabetic rats (DM) treated with high dose of CAERS (DM + CAERS-H) and diabetic rats (DM) treated with high dose of CAERS-nanoparticles (DM + CAERS-NPs-H). The upper left (UL) quadrant represents necrotic cells, the left lower (LL) quadrant represents healthy cells, the upper right (UR) quadrant represents early apoptotic cells and the lower right (LR) quadrant represents late apoptotic cells. Apoptosis was calculated as summation of UR + LR. Values represent average percentage (±SEM) of at least five samples
The apoptosis rates in DM-rats treated with CAERS and CAERS-NPs at low and medium doses were decreased without significant differences (P > 0.05) compared with untreated DM-animals. However, treatment of DM-animals with CAERS and CAERS-NPs at the high dose suppressed significantly (P > 0.05) the incidence of apoptosis (Fig. 5b).
3.6 Antioxidant Enzyme Activity in CAERS and CAERS-NPs Treated DM-Rats
The activity of the antioxidant enzymes (GPx and CAT) in hepatic samples of DM-animals treated with CAERS and CAERS-NPs is presented in Tab. 3. The obtained activity values indicated that GPx and CAT activities declined significantly in DM-animals in comparison to those in control rats. Nevertheless, the GPx activity levels in DM-animals treated with CAERS (at the highest dose) and CAERS-NPs (at the medium and high doses) were increased with significant differences in comparison to that in untreated DM-animals. In addition, activity levels of CAT were increased significantly in DM-animals treated with CAERS (at medium and high doses) and CAERS-NPs (at all doses) compared with those in untreated DM-animals.

R. stricta is extensively utilized in public medicine for treatment wide range of diseases especially in Arabian courtiers. Several studies have been used the extract of R. stricta to assess the toxicological and pharmacological actions [36,37].
The present study revealed that CAERS and CAERS-NPs reduced significantly the glucose levels in diabetic rats after 2 months treatment compared to untreated DM-rats. Moreover, CAERS-NPs at the high dose modulated the glucose levels in DM-rats nearly to the glucose levels in healthy control animals. In same line, several studies indicated that R. stricta extracts decreased the levels of glucose levels in Balb-C mice [38]. Moreover, Baeshin et al. [27], oral supplementation of alkaloids extracts of R. stricta reduced the levels of glucose and improved significantly the levels pf insulin in DM rats. The current findings proved that the active compounds of R. stricta extracts have the ability to improve the beta cells function existing in the pancreatic islets that synthesize insulin.
Our previous studies revealed that several alkaloids and flavonoid and compounds are present and isolated from R. stricta collected from different areas of Saudi Arabia [7,8]. These compounds could be associated with beta cells function improvement in diabetic rats. This explanation is correlated with our findings based on the expression analysis of insulin and insulin receptor mRNAs. The gene expression analysis in the present study exhibited that DM-rats treated with CAERS (at medium and high doses) and CAERS-NPs (at all three doses) increased the expression levels of the diabetes related genes in pancreatic samples of DM-rats in comparison to those in untreated DM-animals. Furthermore, the expression levels of diabetes related genes in pancreatic samples of DM-animals treated with CAERS-NPs (at the highest dose) reached levels near to those in healthy control rats. In the same line, Baeshen et al. [39] reported that oral supplementation of R. stricta leaves extracts increased the secretion levels of insulin and up-regulated the expression of adiponectin related after 2 and 4 weeks of treatments. Our results also found that the expression of insulin and insulin receptor is improved after 2 moth treatment of CAERS and CAERS-NPs.
The present work found that generation of ROS mediated DNA adducts and apoptosis in DM-rats was significantly higher than those in control rats. However, the generation of ROS rate, DNA adducts and apoptosis rates in DM-rats treated with medium and high doses of CAERS and all doses of CAERS-NPs were decreased compared with DM-rats without treatment.
There are reported data exhibited a link between ROS generation and diabetes progression as well as elevation of oxidative stress. This link is considered as one of the common reasons inducing of beta-cells disorder in the pancreas and consequently causing diabetes [40]. Moreover, Niedowicz et al. [41] reported that the oxidative stress plays an important role in the elevation of complication and pathogenesis of diabetes [42]. Therefore, using of antioxidants in the pathogenesis of diabetes is much recommended to prohibit or at least minimize diabetes complications [43,44]. For that reason several plant extracts have been widely investigated to discover its potential antioxidants to avoid or decreased the negative process caused by exposure to oxidative stressors [45]. So, various natural compounds are proved to have antioxidants activities such as trans-13-Octadecenoic acid [46], thioureas derivatives [47], 9-Octadecenoic acid (Z)-, methyl ester [46], squalene [48], acetamide derivatives [49] and uleine [50] which are mitigating the toxicity effects of the oxidative stress. Hence, R. stricta extracts occurred its possible alleviative impact against DM through lowering the glucose levels in which this action might be attributed to several phytoconstituents exist in the extract.
In the present study the R. stricta extract exhibited positive effects against diabetes in which it revealed preventive impact against ROS generation, DNA adducts and apoptosis as well as improvement for antioxidant enzymes. The activity levels of GPx and CAT increased significantly in DM-rats treated CAERS and CAERS-NPs in a dose-dependent manner. In the same line, Ali [51] reported that R. stricta extract exhibited protective impact against hepatotoxicity induced by paracetamol exposure in rats. They suggested that the improvement impact of R. stricta against paracetamol was attributed to increase the activity of antioxidants enzymes such as superoxide dismutase (SOD) and glutathione activity.
The present study showed that nanoparticles form of R. stricta improved its effect against diabetes much more than the powder form of the extract. The mechanism of naoparticles in improving the effect of R. stricta was explained by Castillo-Henríquez et al. [52], who reported that nanoparticles of the plant improved the ability of plant compounds to enter the cell membrane due to modification of the chemical and physical properties of the plant materials. So, plant nano-extract act to accelerate the diffusion and dissolution of the extract and facilitating the plant compounds release.
This study found that CAERS and CAERS-NPs exhibited positive effects against diabetes in which it revealed preventive impact against ROS generation, DNA adducts and apoptosis as well as improvement for antioxidant enzymes. Moreover, R. stricta nanoparticles improved the anti-diabetic effect of the plant much more than the powder form of the extract. The nanoparticles of the plant might be improved the ability of plant compounds to penetrate the cell membrane due to modification of the chemical and physical properties of the plant materials which facilitating the plant compounds release into the target cells.
Acknowledgement: The author is grateful to the University of Tabuk, Saudi Arabian, for unlimited help and support to carry out these experiments.
Funding Statement: The author received no specific funding for this study.
Conflicts of Interest: The author declares that he has no conflicts of interest to report regarding the present study.
1. Al Dawish, M. A., Robert, A. A., Braham, R., Al Hayek, A. A., Al Saeed, A. et al. (2016). Diabetes mellitus in Saudi Arabia: A review of the recent literature. Current Diabetes Reviews, 12(4), 359–368. DOI 10.2174/1573399811666150724095130. [Google Scholar] [CrossRef]
2. Alqurashi, K. A., Aljabri, K. S., Bokhari, S. A. (2011). Prevalence of diabetes mellitus in a Saudi community. Annals of Saudi Medicine, 31(1), 19–23. DOI 10.4103/0256-4947.75773. [Google Scholar] [CrossRef]
3. Baeshin, N., Sabir, J., Qari, S. (2009). Cytogenetic and molecular evaluations of genetic effects of leaf extract of Rhazya stricta (Decne) on allium cepa root tip meristems. Egyptian Journal of Genetics and Cytology, 38, 73–83. [Google Scholar]
4. Ahmed, A., Asad, M. J., Ahmad, M. S., Qureshi, R., Shah, S. I. et al. (2015). Antidiabetic and hypolipidemic potential of Rhazya stricta decne extract and its fraction. International Current Pharmaceutical Biotechnology, 4(2), 353–361. DOI 10.3329/icpj.v4i2.21484. [Google Scholar] [CrossRef]
5. Alagrafi, F. S., Alawad, A. O., Abutaha, N. M., Nasr, F. A., Alhazzaa, O. A. et al. (2017). In vitro induction of human embryonal carcinoma differentiation by a crude extract of Rhazya stricta. BMC Complementray Medicine and Therapies, 17-342. DOI 10.1186/s12906-017-1852-7. [Google Scholar] [CrossRef]
6. Bukhari, N. A., Al-Otaibi, R. A., Ibrahim, M. M. (2017). Phytochemical and taxonomic evaluation of Rhazya stricta in Saudi Arabia. Saudi Journal of Biological Sciences, 24, 1513–1521. DOI 10.1016/j.sjbs.2015.10.017. [Google Scholar] [CrossRef]
7. Aziz, A. T., Alshehri, M. A., Alanazi, N. A., Panneerselvam, C., Trivedi, S. et al. (2020). Phytochemical analysis of Rhazya stricta extract and its use in fabrication of silver nanoparticles effective against mosquito vectors and microbial pathogens. Science of the Total Environment, 700, 134443. DOI 10.1016/j.scitotenv.2019.134443. [Google Scholar] [CrossRef]
8. Aziz, A. T., Alshehri, M. A., Panneerselvam, C., Murugan, K., Trivedi, S. et al. (2018). The desert wormwood (Artemisia herba-alba)–from Arabian folk medicine to a source of green and effective nanoinsecticides against mosquito vectors. Journal of Photochemistry & Photobiology, B: Biology, 180, 225–234. DOI 10.1016/j.jphotobiol.2018.02.012. [Google Scholar] [CrossRef]
9. Sabir, J. S. M., Jansen, R. K., Arasappan, D., Calderon, V., Noutahi, E. et al. (2016). The nuclear genome of Rhazya stricta and the evolution of alkaloid diversity in a medically relevant clade of apocynaceae. Scientific Reports, 6, 33782. DOI 10.1038/srep33782. [Google Scholar] [CrossRef]
10. Akhgari, A., Oksman-Caldentey, K. M., Rischer, H. (2017). Biotechnology of the medicinal plant Rhazya stricta: A little investigated member of the apocynaceae family. Biotechnology Letters, 39, 829–840. DOI 10.1007/s10529-017-2320-7. [Google Scholar] [CrossRef]
11. Ahsan, H., Ahad, A., Iqbal, J., Siddiqui, W. A. (2014). Pharmacological potential of tocotrienols: A review. Nutrition & Metabolism, 11(1), 52. DOI 10.1186/1743-7075-11-52. [Google Scholar] [CrossRef]
12. Nehdi, I. A., Sbihi, H. M., Tan, C. P., Al-Resayes, S. I. (2016). Seed oil from harmal (Rhazya stricta decne) grown in Riyadh (Saudi ArabiaA potential source of δ-tocopherol. Journal of Saudi Chemical Society, 20, 107–113. DOI 10.1016/j.jscs.2014.09.005. [Google Scholar] [CrossRef]
13. Jain, P. K., Lee, K. S., El-Sayed, I. H., El-Sayed, M. A. (2006). Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biologicals imaging and biomedicine. The Journal of Physical Chemistry, B, 110, 7238–7248. DOI 10.1021/jp057170o. [Google Scholar] [CrossRef]
14. Panneerselvam, C., Murugan, K., Roni, M., Aziz, A. T., Suresh, U. et al. (2016). Fernsynthesized nanoparticles in the fight against malaria: LC/MS analysis of pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitology Research, 115, 997–1013. DOI 10.1007/s00436-015-4828-x. [Google Scholar] [CrossRef]
15. Benelli, G., Caselli, A., Canale, A. (2017). Nanoparticles for mosquito control: Challenges and constraints. Journal of King Saud University, 29, 424–435. DOI 10.1016/j.jksus.2016.08.006. [Google Scholar] [CrossRef]
16. Benelli, G., Kadaikunnan, S., Alharbi, N. S., Govindarajan, M. (2018). Biophysical characterization of acacia caesia-fabricated silver nanoparticles: Effectiveness on mosquito vectors of public health relevance and impact on non-target aquatic biocontrol agents. Environmental Science and Pollution Research, 25(11), 10228–10242. DOI 10.1007/s11356-017-8482-y. [Google Scholar] [CrossRef]
17. Benelli, G., Maggi, F., Pavela, R., Murugan, K., Govindarajan, M. et al. (2017). Mosquito control with green nanopesticides: Towards the one health approach? A review of non-target effects. Environmental Science and Pollution Research, 25(11), 10184–10206. DOI 10.1007/s11356-017-9752-4. [Google Scholar] [CrossRef]
18. Benelli, G., Maggi, F., Romano, D., Stefanini, C., Vaseeharan, B. et al. (2017). Nanoparticles as effective acaricides against ticks–A review. Ticks and Tick-Borne Disease, 8(6), 821–826. DOI 10.1016/j.ttbdis.2017.08.004. [Google Scholar] [CrossRef]
19. Benelli, G., Pavela, R., Maggi, F., Petrelli, R., Nicoletti, M. (2017). Commentary: Making green pesticides greener? The potential of plant products for nanosynthesis and pest control. Journal of Cluster Science, 28, 3–10. DOI 10.1007/s10876-016-1131-7. [Google Scholar] [CrossRef]
20. Gupta, R. (2017). Diabetes treatment by nanotechnology. Journal of Biotechnology & Biomaterials, 7, 268. DOI 10.4172/2155-952X. [Google Scholar] [CrossRef]
21. Hounsom, L., Horrobin, D. F., Tritschler, H., Corder, R., Tomlinson, D. R. (1998). A lipioc acid-gamma linolenic acid conjugate is effective against multiple indices of experimental diabetic neuropathy. Diabetologia, 41, 839–843. DOI 10.1007/s001250050996. [Google Scholar] [CrossRef]
22. Cam, M., Yavuz, O., Guven, A., Ercan, F., Bukan, N. et al. (2003). Protective effects of chronic melatonin treatment against renal injury in streptozotocin-induced diabetic rats. Journal of Pineal Research, 35, 212–220. DOI 10.1034/j.1600-079X.2003.00082.x. [Google Scholar] [CrossRef]
23. Elkady, A. I. (2013). Crude alkaloid extract of Rhazya stricta inhibits cell growth and sensitizes human lung cancer cells to cisplatin through induction of apoptosis. Genetics and Molecular Biology, 36(1), 12–21. DOI 10.1590/S1415-47572013005000009. [Google Scholar] [CrossRef]
24. Prasannaraj, G., Venkatachalam, P. (2017). Hepatoprotective effect of engineered silver nanoparticles coated bioactive compounds against diethylnitrosamine induced hepatocarcinogenesis in experimental mice. Journal of Photochemistry and Photobiology B, 167, 309–320. DOI 10.1016/j.jphotobiol.2017.01.009. [Google Scholar] [CrossRef]
25. Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., Altman, D. G. (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology, 8(6), DOI 10.1371/journal.pbio.1000412. [Google Scholar] [CrossRef]
26. Hwang, S. H., Kang, I. J., Lim, S. S. (2017). Antidiabetic effect of fresh nopal (Opuntia ficus-indica) in Low-dose streptozotocin-induced diabetic rats fed a high-fat diet. Evidence-Based Complementary and Alternative Medicine, 4380721, 8. DOI 10.1155/2017/4380721. [Google Scholar] [CrossRef]
27. Baeshin, N. A., Yaghmoor, S. S., Al Ashmaoui, H. M., Kumosani, T. A., Saini, K. S. (2014). The indole-alkaloid fraction of Rhazya stricta improves key biochemical parameters associated with metabolic syndrome in rats. Bothalia Journal, 44(5), 358–371. [Google Scholar]
28. Salem, N. A., Wahba, M. A., Eisa, W. H., El-Shamarka, M., Khalil, W. (2018). Silver oxide nanoparticles alleviate indomethacin-induced gastric injury: A novel antiulcer agent. Inflammopharmacology, 26, 1025–1035. DOI 10.1007/s10787-017-0424-2. [Google Scholar] [CrossRef]
29. Khalil, W. K. B., Booles, H. F., Hafiz, N. A., El-Bassyouni, G. E. (2018). Ameliorative effects of brachidontes variabilis calcium carbonate against bone loss in ovariectomized rats. International Journal of Pharmacology, 14, 477–487. DOI 10.3923/ijp.2018.477.487. [Google Scholar] [CrossRef]
30. Hegazy, E. M., Hafiz, N. A., Rozik, N. N., Khalil, W. K. B. (2018). Comparative study between powder and nanoparticles of dried cactus (Opuntia ficus-indica L.) fruit peels in streptozotocin-induced diabetic rats: Anti-microbial and anti-genotoxic capacity. Annual Research & Review in Biology, 26(6), 1–14. DOI 10.9734/ARRB. [Google Scholar] [CrossRef]
31. Khalil, W. K. B., Abdu, F. (2013). Effects of salvia officinalis extract and its nano-encapsulated form on methylmercury induced neurotoxic-stress in male rats. World Applied Sciences Journal, 24(7), 826–837. [Google Scholar]
32. Abdu, S. B., Abdu, F., Khalil, W. K. B. (2017). Ginger nanoparticles modulate the apoptotic activity in male rats exposed to dioxin-induced cancer initiation. International Journal of Pharmacology, 13, 946–957. [Google Scholar]
33. Girgis, E., Khalil, W. K. B., Emam, A. N., Mohamed, M. B., Rao, K. V. (2012). Nanotoxicity of gold and gold-cobalt nanoalloy. Chemical Research in Toxicology, 25(5), 1086–1098. [Google Scholar]
34. Dabour, K., Al Naggar, Y., Masry, S., Naiem, E., Giesy, J. P. (2019). Cellular alterations in midgut cells of honey bee workers (Apismillefera L.) exposed to sublethal concentrations of CdO or PbO nanoparticles or their binary mixture. Science of the Total Environment, 651, 1356–1367. [Google Scholar]
35. Elmegeed, G., Khalil, W. K. B., Abdel Raouf, A., Abdelhalim, M. M. (2008). Synthesis and in vivo anti-mutagenic activity of novel melatonin derivatives. European Journal of Medicinal Chemistry, 43, 763–770. [Google Scholar]
36. Tanira, M. O. M., Ali, B. H., Bashir, A. K., Chandranath, I. (1996). Some pharmacologic and toxicologic studies on Rhazya stricta decne in rats, mice and rabbits. General Pharmacology, 27, 1261–1267. [Google Scholar]
37. Adam, S. E. I. (1998). Toxicity of Rhazya stricta to sheep. Veterinary and Human Toxicology, 40, 68–69. [Google Scholar]
38. Ahmed, A., Gulfraz, M., Asad, M. J., Qureshi, R., Bibi, S. et al. (2016). Hypoglycemic and hypocholesterolemic activity of leave of few medicinal plants against steptozotocin induced hyperglycemia. Pakistan Journal of Pharmaceutical Sciences, 29(6), 2065–2070. [Google Scholar]
39. Baeshen, N. A., Lari, S. A., Al Doghaither, H. A. R., Ramadan, H. A. (2010). Effect of Rhazya stricta extract on rat adiponectin gene and insulin resistance. Journal of American Science, 6(12), 1237–1245. [Google Scholar]
40. Robertson, R. P. (2004). Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. Journal of Biological Chemistry, 279, 42351–42354. DOI 10.1074/jbc.R400019200. [Google Scholar] [CrossRef]
41. Niedowicz, D., Daleke, D. (2005). The role of oxidative stress in diabetic complications. Cell Biochemistry and Biophysics, 43, 289–330. DOI 10.1385/CBB:43:2:289. [Google Scholar] [CrossRef]
42. DCCT (1993). The diabetes control and complications trial research group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine, 329, 977–986. DOI 10.1056/NEJM199309303291401. [Google Scholar] [CrossRef]
43. Coleman, M. D., Fernandes, S., Khanderia, L. A. (2003). Preliminary evaluation of a novel method to monitor a triple antioxidant combination (vitamins E, C and [alpha]-lipoic acid) in diabetic volunteers using in vitro methaemoglobin formation. Environmental Toxicology and Pharmacology, 14, 69–75. DOI 10.1016/S1382-6689(03)00027-9. [Google Scholar] [CrossRef]
44. Ozkan, Y., Yilmaz, O. K., Ihsan, A. O., Yasemin, E. (2005). Effects of triple antioxidant combination (vitamin E, vitamin C and a-lipoic acid) with insulin on lipid and cholesterol levels and fatty acid composition of brain tissue in experimental diabetic and non-diabetic rats. Cell Biology International, 29, 754–760. DOI 10.1016/j.cellbi.2005.04.011. [Google Scholar] [CrossRef]
45. Kaur, C., Kapoor, H. C. (2001). Antioxidants in fruits and vegetables ± the millennium’s health. International Journal of Food Science & Technology, 36, 703–725. DOI 10.1046/j.1365-2621.2001.00513.x. [Google Scholar] [CrossRef]
46. Asghar, S. F., Choudahry, M. I., Habib-Ur, R., Atta-Ur, R. (2011). Gas chromatography-mass spectrometry (GC-mS) analysis of petroleum ether extract (oil) and bioassays of crude extract of iris germanica. International Journal of Genetics and Molecular Biology, 3(7), 95–100. DOI 10.5897/IJGMB.9000024. [Google Scholar] [CrossRef]
47. Naz, S., Zahoor, M., Umar, M. N., Ali, B., Ullah, R. et al. (2019). Enzyme inhibitory, antioxidant and antibacterial potentials of synthetic symmetrical and unsymmetrical thioureas. Drug Design, Development and Therapy, 13, 3485–3495. DOI 10.2147/DDDT. [Google Scholar] [CrossRef]
48. Amarowicz, R. (2009). Squalene: a natural antioxidant? European Journal of Lipid Science and Technology, 111, 411–412. DOI 10.1002/ejlt.200900102. [Google Scholar] [CrossRef]
49. Autore, G., Caruso, A., Marzocco, S., Nicolaus, B., Palladino, C. et al. (2010). Acetamide derivatives with antioxidant activity and potential anti-inflammatory activity. Molecules, 15, 2028–2038. DOI 10.3390/molecules15032028. [Google Scholar] [CrossRef]
50. Pereira Rocha, M., Rodrigues Valadares Campana, P., de Oliveira Scoaris, D., de Almeida, V. L., Dias Lopes, J. C. et al. (2018). Biological activities of extracts from aspidosperma subincanum mart. and in-silico prediction for inhibition of acetylcholinesterase. Phytotherapy Research, 32(10), 2021–2033. DOI 10.1002/ptr.6133. [Google Scholar] [CrossRef]
51. Ali, B. H. (2002). The effect of treatment with the medicinal plant Rhazya stricta decne on gentamicin nephrotoxicity in rats. Phytomedicine, 9(5), 385–389. DOI 10.1078/09447110260571607. [Google Scholar] [CrossRef]
52. Castillo-Henríquez, L., Alfaro-Aguilar, K., Ugalde-Álvarez, J., Vega-Fernández, L., de Oca-Vásquez, G. M. (2020). Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials, 10, 1763. DOI 10.3390/nano10091763. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |