
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.016004
ARTICLE
Encapsulation of Immature Somatic Embryos of Coffea arabica L. for in Vitro Preservation
1Laboratorio de Biotecnología Vegetal, Instituto Tecnológico de Tuxtla Gutiérrez, Tecnológico Nacional de México, Tuxtla Gutiérrez, Chiapas, 29050, México
2CONACYT-Tecnológico Nacional de México/Instituto Tecnológico de Tuxtla Gutiérrez, Tuxtla Gutiérrez, Chiapas, 29050, México
*Corresponding Author: Nancy Ruiz-Lau. Email: nruizla@conacyt.mx
Received: 30 January 2021; Accepted: 29 April 2021
Abstract: The present study aimed to develop a protocol for somatic embryogenesis and encapsulation of coffee embryos (Coffea arabica L.), for the conservation of genotypes with characteristics of commercial interest. Somatic embryos were induced from leaf explants in Murashige and Skoog medium (MS) supplemented with 1 mg · L−1 of 2,4-dichlorophenoxiacetic acid (2,4-D) combined with 2 mg · L−1 of benzyladenine (BA). Somatic embryos (SE) at the globular stage were encapsulated in a sodium alginate matrix; two treatments were tested: MS + 5 mg · L−1 BA + 1 mg · L−1 NAA + 3% (w/v) alginate, and MS + 7 mg · L−1 BA + 5.7 mg · L−1 indoleacetic acid (IAA) + 3% (w/v) alginate. Alginate was complexed with 100 mM calcium chloride (CaCl2). Viability of the encapsulated SE was determined by staining with 0.01% fluorescein diacetate (FDA) after 0, 15, 30, and 45 days of storage at 4°C. Embryo viability was 100% in both treatments.
Keywords: Coffee; explants; embryo; artificial seeds
Coffee is the most important agricultural product, with an estimated value of 70 thousand million dollars. It is crucial for the economy of more than 60 countries, and it is the main income for over 100 million people [1,2]. Therefore, conservation of vegetal material for breeding programs is essential. The genus Coffea currently has more than 100 species described and, among this, C. arabica L. and C. canephora Pierre are the main species in the market worldwide [3]. C. arabica is the most cultivated species, due to its organoleptic characteristics; however, it is highly susceptible to coffee leaf rust (CLR), a disease caused by the fungus Hemileia vastatrix, an obligate parasite.
CLR cause losses of one to two thousand million dollars annually [2], and it is one of the main limiting factors of the worldwide Arabica coffee production [2,4].
Breeding programs to generate resistant varieties to CLR require germplasm conservation protocols for CLR-resistant phenotypes. Naturally CLR-resistant individual C. arabica plants have been identified and described within plantations. Conservation of this germplasm is possible through in vitro cultivation, which unlike traditional propagation techniques, allows clonal micropropagation of a specific plant genotype in a relatively short time.
The current propagation method for C. arabica is based mainly on seeds, which does not allow uniformity in the plantations; therefore, the technology of artificial seeds associated with the somatic embryos production is an alternative for more efficient coffee plant propagation [5]. Artificial seeds are defined as artificially encapsulated somatic embryos, shoot buds, cell aggregates, or any other tissue that can be used for sowing as a seed, with the ability to convert into a plant under in vitro or ex vitro conditions, and retaining this potential after storage. The synthetic seed production technique is a method for mass propagation of promising plant genotypes, and it is an alternative propagation technology in many commercially essential crops [5,6].
Cryopreservation methods can be used for the short- and long-term conservation of selected coffee varieties, allowing extensive scaling for the commercial production of resistant phenotypes or elite clones. C. arabica L. seeds are partially tolerant to desiccation and storage [3]; thus, their intermediate behavior make them suitable candidates for the development of artificial seeds. However, despite the existence of successful protocols for somatic embryogenesis in coffee, studies about encapsulation or artificial seeds for germplasm conservation are limited. Therefore, the present study aimed to develop a protocol for the induction of somatic embryogenesis of C. arabica L., and the further encapsulation and cold storage of embryos, as a first step in the conservation of coffee genotypes with characteristics of commercial interest.
The collection of leaves from the apical (AL) or middle (ML) parts of the mother plants, as explant source, was carried out in eight-month-old coffee plants (C. arabica) that showed phenotypic resistance to CLR in plots located in the town of Sajonia, municipality of La Concordia, Chiapas, Mexico (latitude of 15.834722’N, longitude of −92.940833’W and an altitude of 740 m above sea level). One week before the collection, mother plants were sprinkled daily with a Captan (5 g · L−1), Agrimycin (5 g · L−1) solutions, or a Captan-Agrimycin mixture (5 g · L−1, each), as a previous disinfection step to reduce contamination of explants. Once the leaves were collected from mother plants, they were disinfected with a modification of the protocol of [7], as follows: leaves were washed with liquid soap (trichloro® Axion, 10 min) and then placed in an antioxidant/fungicide solution (30 g · L−1 sucrose, 150 mg · L−1 citric acid, 100 mg · L−1 ascorbic acid, 2.5 g · L−1 Captan® and 1 g · L−1 Agrimycin®) to taken them to plant biotechnology laboratory of the Intituto Tencnologico de Tuxtla Gutiérrez, Chiapas (approximately 3–4 h).
2.2 Induction of Somatic Embryogenesis
The collected leaves were disinfected with 30% commercial chlorine (10 min) and 70% ethanol (5 min). Leaf explants of approximately 1.5 cm2 were cut eliminating the midrib of the AL and ML leaves and then placed in semi-solid culture medium MS + 1 mg · L−1 2,4-D and 2 mg · L−1 of BA, 30 g · L−1 sucrose, [8] pH of 5.7 ± 1, with 2.5 g · L−1 phytagel and incubated at 26 ± 2°C in the dark. The influence of the explant position (abaxial o adaxial) on the embryogenic response was determined by placing three explants with the bundle and three with the underside in direct contact with the culture medium in the same bottle, performing ten repetitions of each position of the explant.
After 45 days in darkness, the explants with developing calluses were moved to a photoperiod of 16/8 light/dark hours. The parameters evaluated were percentage of contamination at eight days after sowing (das), the oxidation and formation of embryogenic calluses, the embryogenic response of AL and ML leaves, and the position of the leaf explants (abaxial o adaxial) over the semi-solid medium.
2.3 Encapsulation of Somatic Embryos
For encapsulation, embryos in the globular stage were isolated from embryogenic callus with 45 days of induction, under a stereoscope-microscope (Luxed 4D, LABOMED). The encapsulation process was carried out by suspending somatic embryos in a sodium alginate solution, consisting in 3% sodium alginate (Sigma, St. Louis, MO) in MS medium, with different PGR (Treatment 1: 5 mg · L−1 BA and 1 mg · L−1 IAA; Treatment 2: 7 mg · L−1 BA and 5.7 mg · L−1 IAA). Subsequently, drops with individual alginate-embedded embryos were taken with a micropipette and added to a 100 mM CaCl2 solution, as a complexing agent [9].
After 30 min of stirring, encapsulated embryos were decanted and rinsed with sterile distilled water.
For viability studies, encapsulated embryos (artificial seeds) were stored either in empty Petri dishes at 4.0 ± 1°C [10] or in vitro in the germination medium (MS with 3 mg · L−1 of GA3).
The viability determination of artificial seeds was performed with 0.01% FDA in acetone (w/v) [11]. For that, embryos were extracted from the alginate capsule under a stereomicroscope (Luxed 4D, LABOMED). Extracted embryos were placed on a slide with 20 μL of FDA solution and observed under an epifluorescence microscope (Motic BA410). Viability of encapsulated embryos was evaluated after different time of storage (0, 15, 30, and 45 days), using ten embryos per treatment. Embryos generated from oxidized callus were used as the negative control.
The mean and standard deviation were estimated for the percentage of callus formation, oxidation, and contamination. The explant position and treatment effect on embryogenic callus formation was analyzed by one-way ANOVA followed by a post hoc Tukey test for the inference of significant statistical difference with an α = 0.05 (Statgraphics Centurion XVII software).
3.1 Induction of Somatic Embryogenesis
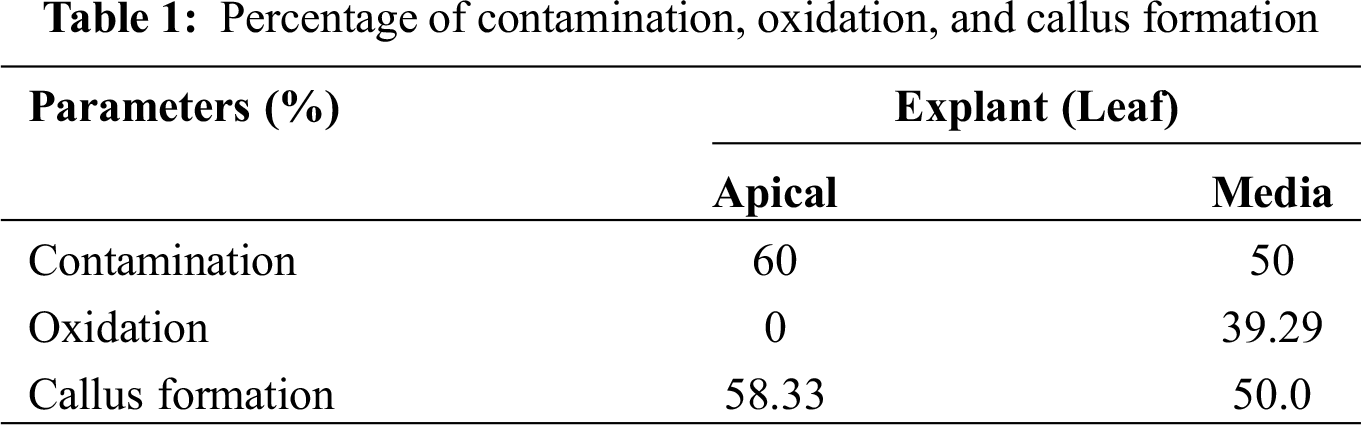
Explants from AL developed 40% of aseptic explants, with no oxidation, and 50% were obtained from ML, with 39.29% oxidation (Tab. 1). The contamination was mainly by fungi, which were visualized after eight das. Regarding the percentage of callus formation, AL had a small but significant better response than ML (Tab. 1). With respect to the explant position, both AL and ML have a 100% callus formation response when the adaxial side of the leaf explant was in contact with the medium, but only AL developed callus (16.67%) when the abaxial side was in contact (Fig. 1). All callus generated somatic embryos (Fig. 2). These results suggest a dependence on the position of the explant for the callus formation.
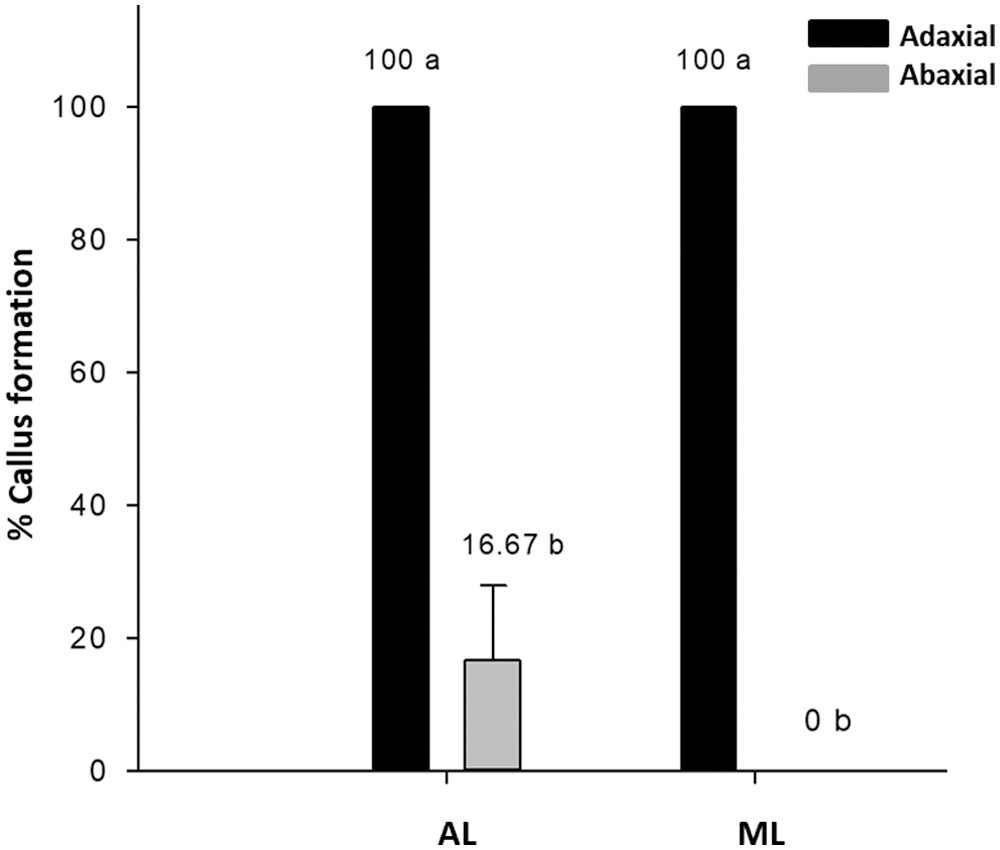
Figure 1: Influence of the explant position (adaxial and abaxial sides of the leaf), on the embryogenic callus formation. AL: apical leaf; ML: middle leaf. Tukey HSD 95%
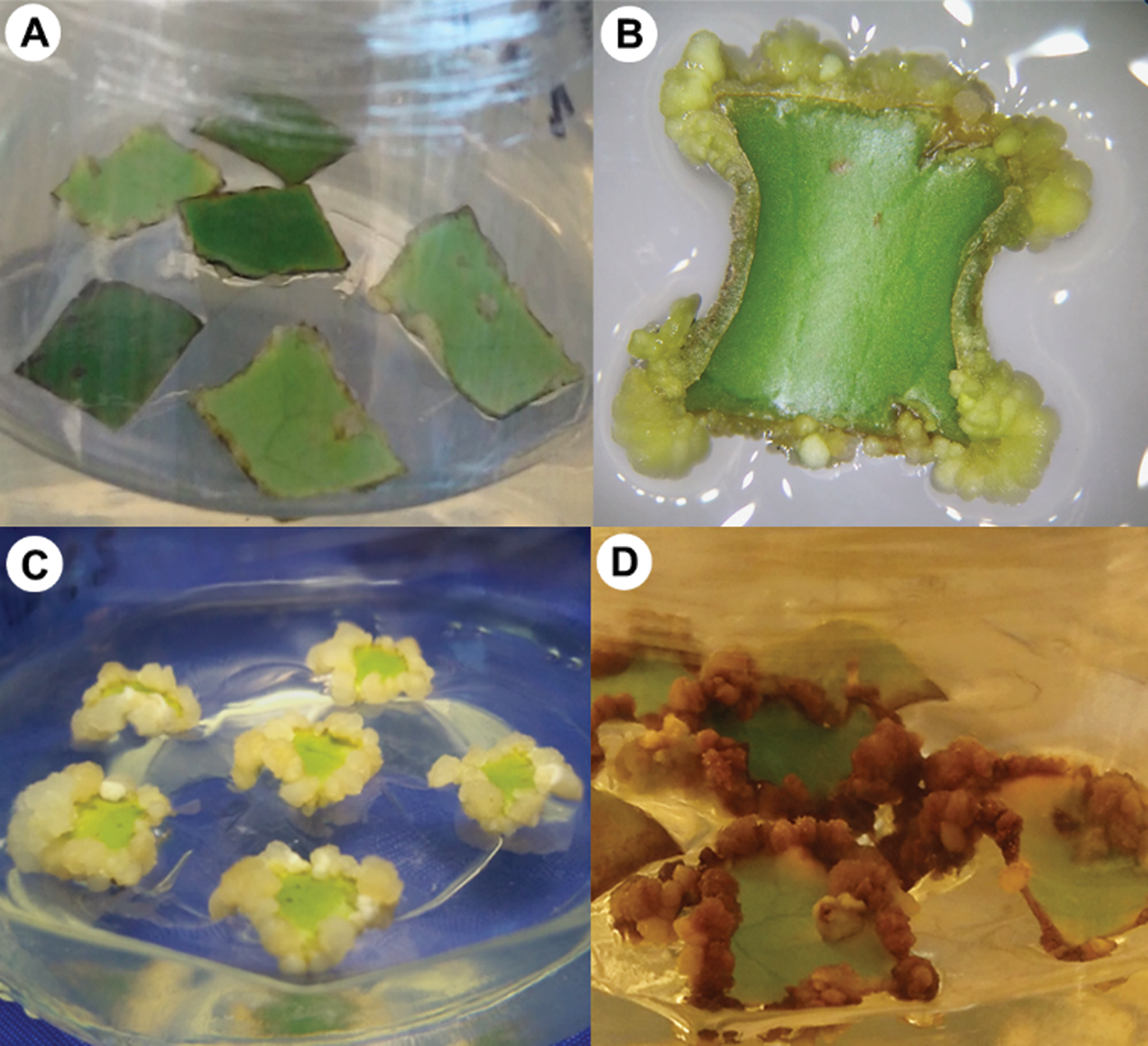
Figure 2: Induction of somatic embryogenesis in Coffea arabica L. A. Leaf explants in MS medium with 1 mg · L−1 2,4-D + 2 mg · L−1 BA. Callus developing at 15 (B), 30 (C), and 55 (D) days after sowing.
3.2 Viability of Encapsulated Embryos
The artificial seed were obtained by submerging the somatic embryos in the globular stage in a sodium alginate solution and CaCl2 as complexing agent (Fig. 3). With this technique, an artificial seed has been generated consisting of a somatic embryo with a seminal cover and an artificial endosperm [5,6]. Fifty-two embryos were encapsulated. Twelve were placed in a semi-solid culture medium for germination for 45 days, and 40 embryos were stored at 4°C during 15, 30, and 45 days.
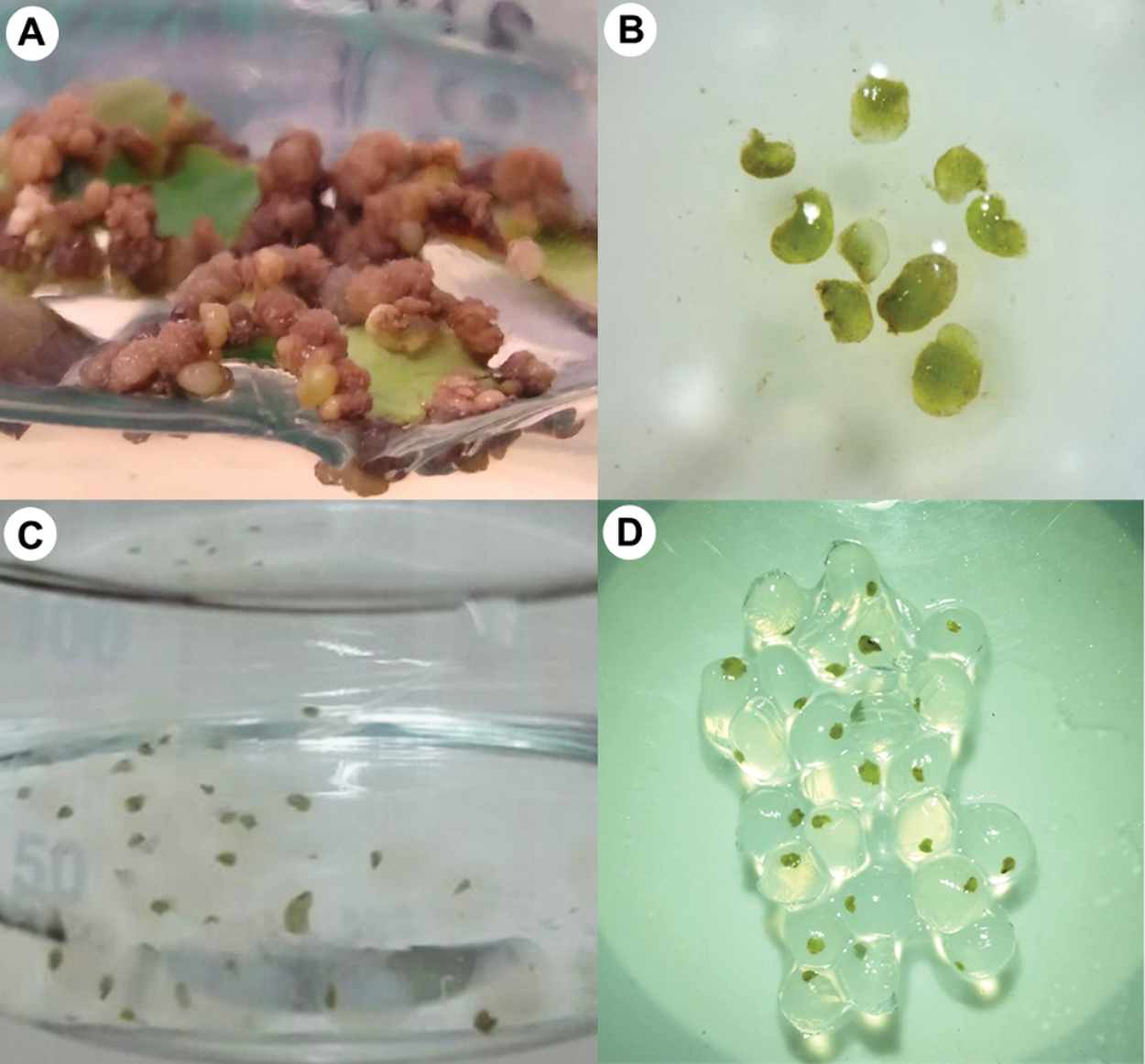
Figure 3: Encapsulation of Coffea arabica L. somatic embryos. A. Somatic embryos developing on embryogenic callus; B. Isolated somatic embryos; C and D. Encapsulation of somatic embryos.
After 45 days, neither of the two treatments allowed germination of the encapsulated embryos. Therefore, viability of encapsulated embryos was determined by the FDA test. Vital staining with FDA showed 100% viability in all embryos evaluated from both treatments. This result suggests that the embryo developmental stage restrain germination. Further studies may be necessary to evaluate the germination capacity of encapsulated embryos in dependence of their developmental stage.
The encapsulation efficiency and the viability of stored embryos were verified by the FDA test (Fig. 4). The results showed that all embryos remained viable after 15, 30, and 45 days of storage. Therefore, the encapsulation provided not only physical protection to embryos but also provided the nutrients and carbon source during storage, preventing their drying and loss of viability.
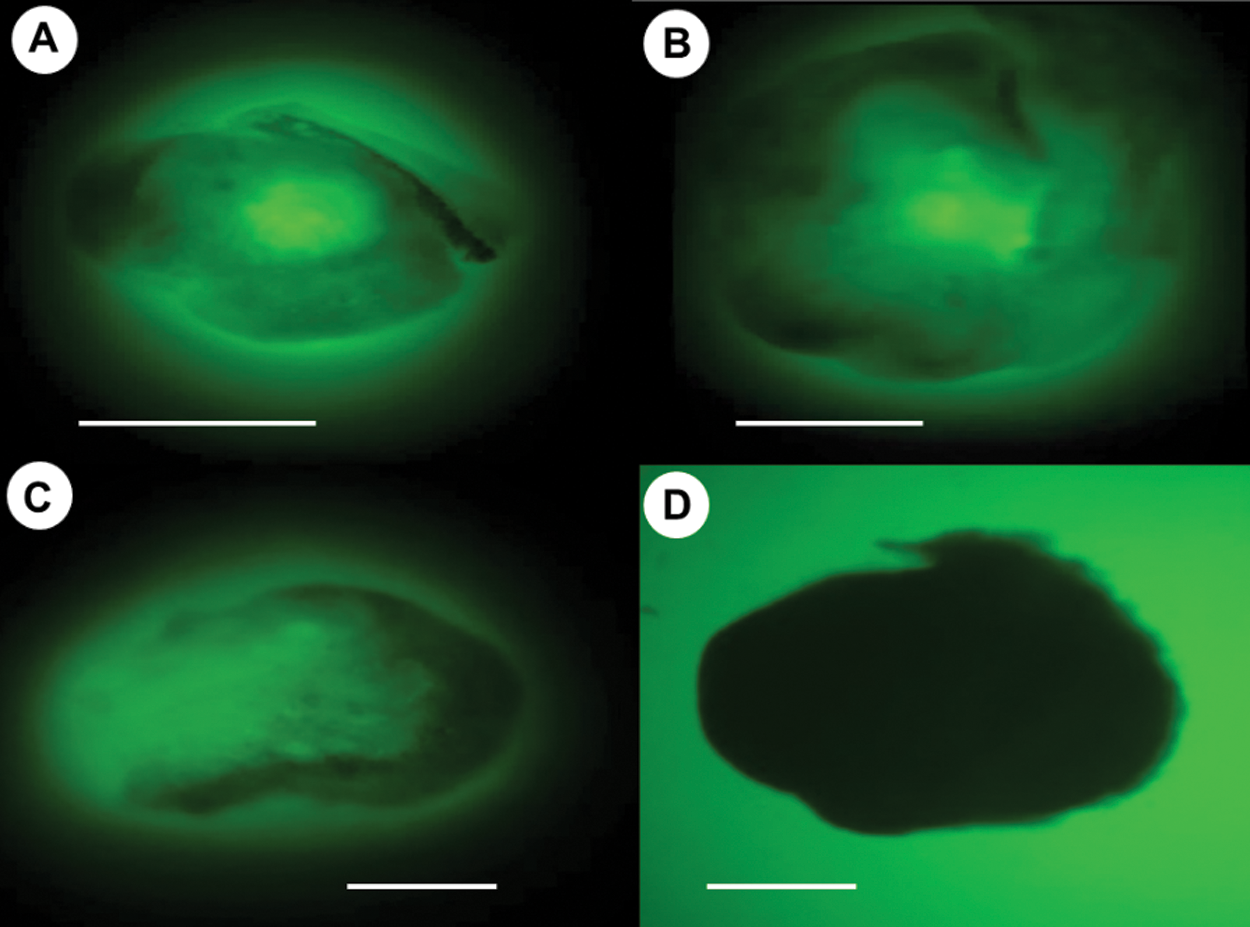
Figure 4: Viability of encapsulated somatic embryos of C. arabica L. Encapsulated globular-stage somatic embryos at 15 (A) 30 (B) and 45 (C) days in storage at 4°C were stained with 0.01% FDA and observed under the Motic BA410 10x epifluorescence microscope. As negative control non-viable embryos were also stained (D).
4.1 Somatic Coffee Embryogenesis of Coffea arabica
With the protocol followed in this work, the onset of callus formation was observed after the second induction week. It has been reported that many factors influence the success of somatic embryogenesis, including the type and age of explant, the physiological state of the mother plant, the explant interaction with disinfectant agents, and the presence of nutrients and PGR (endogenous and added), as well as its oxidation when applying a disinfection method. However, genotype remains the most determining factor for effective somatic embryogenesis in coffee [12].
Our results showed 100% formation of callus, being greater than previously reported by [8]. Evaluating the effect of explant position in the culture medium, on callus formation, revealed that putting the adaxial side of the leaves in contact with the culture medium gave a better response (100% in both AL and ML) than putting the abaxial side (~17% in AL and 0% in ML). This behavior can be explained by the leaf’s ability to transfer external nutriments and PGR from exposed to internal tissues. In this organ, the nutrient absorption is achieved by diffusion of nutrients between epidermal and mesophyll tissues [13]. The fact that coffee leaves are hypostomatic (stomata are distributed in the underside surface), suggests that gas exchange in explants caused a better response in the formation of embryogenic callus.
With respect to the culture medium, several authors have mentioned that the generation of somatic embryos is less efficient when semi-solid medium is used. In semi-solid medium, it is possible that nutriments and PGR are poorly diffused, embryos and their concentration is less homogeneous than in liquid medium [12]. Pinto et al. [5] mentioned that more somatic embryos are produced in liquid than in semi-solid medium. This may have occurred because 1) the calli inoculated on a solid medium has less contact area for nutrient absorption than that in a liquid medium, or 2) the secondary somatic embryogenesis was observed in liquid medium. In our work, encapsulated embryos did not culminate in gemination, possibly because they were in an immature stage. Nonetheless, our protocol for embryo encapsulation did not affect the viability of embryos and could be used as an alternative for the conservation of elite genotypes.
4.2 Artificial Seeds and Their Viability
Rihan et al. [10] mentioned that the viability of artificial seeds and their storage capacity may be affected by the matrix material or the simulated endosperm. The nutriment supplement, the concentration of PGR and the physical environment can determine the quality of the artificial seeds while stored at 4°C. The matrix provides not only physical protection to embryos but also a source of carbon source and PGR to control and maintain growth. The fluorescein diacetate (FDA) test was an effective method that allowed the visualization of viable somatic embryos (Fig. 4), indicating that the two methods used to prepare the encapsulation matrix (Treatment 1 and Treatment 2) were suitable to maintain the viability of the embryos. [11] mentioned that FDA produces fluorescence after being activated by intracellular esterases. These enzymes are widely found in mammalian and plant cells and microorganisms, and their activity is commonly used as an indicator of cell viability [14].
The storage conditions of artificial seeds are essential to increase their viability. In this study, encapsulated embryos were stored at 4°C, according to the data reported by [15]; they encapsulated Rauvolfia serpentina sprouts and stored them for 14 weeks at different temperatures, founding that sprouts stored at 4°C developed the highest percentage of seedlings or shoots (68.5–100%). In the present work, a 100% of embryo viability was obtained after 45 days of storage at 4°C. Other jobs also reported a 4°C storage temperature for other species. Winkelmann et al. [16] encapsulated embryos in the globular stage of Cyclamen persicum at 4°C for 30 days obtaining a 68% viability. In a different report, Cartes et al. [17] encapsulated somatic embryos in the torpedo-cotyledonary stages of Nothofagus alpina, in which the best treatment (97% survival) consisted in MS medium supplemented with 0.5 mg · L−1 IAA, 0.5 mg · L−1 NAA, 2 mg · L−1 BA, 2 mg · L−1 Fe-EDTA, and 30 g · L−1 sucrose plus 3% alginate followed by 30 min immersion in 5.5 g · L−1 calcium chloride.
On the other hand, Pinto et al. [5] showed that the encapsulation of somatic embryos of C. arabica in early stages of development decreased their germination capability at the end of the encapsulation treatment. These results are consistent with results found in the present work, where embryos in the globular stage were encapsulated but no germination was observed, even when encapsulation did not affect their viability. Therefore, it would be important to further evaluate whether the encapsulation of embryos in more advanced stages of development improves their germination capacity as artificial seeds. In this way, this methodology could be used as an alternative for the preservation of C. arabica elite germplasm.
It was found that somatic embryos of Coffea arabica L., encapsulated in the globular stage in sodium alginate remained 100% viable after a month and a half of cold storage at 4°C. However, it is necessary to further evaluate if encapsulation of embryos at more advanced stages could improve their germination capacity. Similarly, it was determined that the position of the leaf in the plant (apical and middle leaves) and the position of the explant in contact with the culture medium (adaxial or abaxial) are factors that influence the somatic embryogenesis in Coffea arabica L.
Acknowledgement: The authors acknowledge Consejo Nacional de Ciencia y Tecnologia for the EPA Master’s scholarship (CONACYT 597370).
Funding Statement: Project financed by the Tecnologico Nacional de Mexico in the call Support for Scientific and Applied Research Projects, Technological Development and Innovation 2015 (CI-02-2015).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. ICO (2016). World Coffee Production. International Coffee Organization. http://www.ico.org/prices/po-production.pdf. [Google Scholar]
2. Talhinhas, P., Batista, D., Diniz, I., Vieira, A., Silva, D. N. et al. (2017). The coffee leaf rust pathogen hemileia vastatrix: One and a half centuries around the tropics. Molecular Plant Pathology, 18(8), 1039–1051. DOI 10.1111/mpp.12512. [Google Scholar] [CrossRef]
3. Boas Coelho, S. V., Dellyzete, V. S., Da Rosa, F., Botelho, F. T., Lima, B. J. et al. (2018). Cryopreservation in coffea canephora pierre seeds: Slow and fast cooling. Ciência e Agrotecnologia, 42(6), 588–597. DOI 10.1590/1413-70542018426017718. [Google Scholar] [CrossRef]
4. Avelino, J., Cristancho, M., Georgiou, S., Imbach, P., Aguilar, L. et al. (2015). The coffee rust crises in Colombia and Central America (2008–2013Impacts, plausible causes and proposed solutions. Food Security, 7(2), 303–321. DOI 10.1007/s12571-015-0446-9. [Google Scholar] [CrossRef]
5. Pinto, R. T., Ribeiro, L. O., Torres, L. F., Freitas, N. C., Barbosa, S. et al. (2020). Coffee synthetic seed production is influenced by somatic embryo development stage and nutritional factors related to the encapsulation matrix. Acta Horticulture, 1285, 31–38. DOI 10.17660/ActaHortic.2020.1285.5. [Google Scholar] [CrossRef]
6. Chandra, K., Pandey, A., Kumar, P. (2018). Synthetic seed-future prospects in crop improvement. International Journal of Agriculture Innovations and Research, 6(4), 2319–1473. https://ijair.org/index.php/issues?view=publication&task=show&id=1119. [Google Scholar]
7. López-Gómez, P., Iracheta-Donjuan, L., Castellanos-Juárez, M., Méndez-López, I., Aguirre-Medina, J. F. et al. (2011). Variación en la tolerancia a desinfectantes de genotipos élite de coffea spp. cultivados in vitro. Revista Mexicana de Ciencias Agrícolas, 2(5), 645–657. DOI 10.29312/remexca.v2i5.1615. [Google Scholar] [CrossRef]
8. Ahmed, W., Feyissa, T., Disasa, T. (2013). Somatic embryogenesis of coffee (Coffea arabica L.) hybrid using leaf explants.the Journal of Horticultural Science and Biotechnology, 88(4), 469–475. DOI 10.1080/14620316.2013.11512993. [Google Scholar] [CrossRef]
9. Benelli, C. (2016). Encapsulation of shoot tips and nodal segments for in vitro storage of “Kober 5BB” grapevine rootstock. Horticulturae, 2, 10. DOI 10.3390/horticulturae2030010. [Google Scholar] [CrossRef]
10. Rihan, H. Z., Kareem, F., El-Mahrouka, M., Fuller, M. P. (2017). Artificial seeds (principle, aspects and applications). Agronomy, 7(71), 1–15. DOI 10.3390/agronomy7040071. [Google Scholar] [CrossRef]
11. Panjaitan, S. B., Abdullah, S. N. A., Aziz, M. A., Meon, S., Omar, O. (2009). Somatic embryogenesis from scutellar embryo of oryza sativa L. var. MR219. Pertanika Journal of Tropical Agricultural Science, 32(2), 185–194. https://core.ac.uk/download/pdf/42992827.pdf. [Google Scholar]
12. Campos, N. A., Panis, B., Carpentier, S. C. (2017). Somatic embryogenesis in coffee: The evolution of biotechnology and the integration of omics technologies offer great opportunities. Frontiers in Plant Science, 8(1460), 1–12. DOI 10.3389/fpls.2017.01460. [Google Scholar] [CrossRef]
13. Murillo, C. R., Piedra, G. M. G., León, R. G. (2013). Absorción de nutrientes a través de la hoja. Uniciencia, 27(1), 232–244. https://dialnet.unirioja.es/servlet/articulo?codigo=4945327. [Google Scholar]
14. Card, S. D., Tapper, B. A., Lloyd-West, C., Wright, K. M. (2013). Assessment of fluorescein-based fluorescent dyes for tracing neotyphodium endophytes in planta. Mycologia, 105(1), 221–229. DOI 10.3852/12-062. [Google Scholar] [CrossRef]
15. Ray, A., Bhattacharya, S. (2008). Storage and plant regeneration from encapsulated shoot tips of rauvolfia serpentine–An effective way of conservation and mass propagation. South African Journal of Botany, 74, 776–779. DOI 10.1016/j.sajb.2008.06.002. [Google Scholar] [CrossRef]
16. Winkelmann, T., Meyer, L., Serek, M. (2004). Germination of encapsulated somatic embryos of cyclamen persicum. HortSciense, 39(5), 1093–1097. DOI 10.21273/HORTSCI.39.5.1093. [Google Scholar] [CrossRef]
17. Cartes, R. P., Castellanos, B. H., Ríos, L. D., Sáez, C. K., Spierccolli, H. S. et al. (2009). Encapsulated somatic embryos and zygotic embryos for obtaining artificial seeds of rauli-beech (Nothofagus alpina (Poepp. & endl.) oerst.). Chilean Journal of Agricultural Research, 69(1), 112–118. DOI 10.4067/S0718-58392009000100014. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |