
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015871
ARTICLE
Effects of Piriformospora indica on the Respiration of Taxus chinensis var. mairei under Water Stress
College of Horticulture and Gardening, Yangtze University, Jingzhou, 434025, China
*Corresponding Author: Die Hu. Email: hudie.16@163.com
Received: 20 January 2021; Accepted: 31 March 2021
Abstract: Seedlings of Taxus chinensis var. mairei were used as experimental materials to study the adaptation of Piriformospora indica to this plant under water stress. The materials were divided into two groups, namely, with or without inoculation with P. indica. Each group was subjected to four different levels of water stress. Vitality and physiological and biochemical indexes of the roots of T. chinensis var. mairei were regularly measured. Under water stress, T. chinensis var. mairei had significantly decreased root vitality; root vitality was higher in inoculated roots than in uninoculated roots. Under intense water stress, the inoculated roots had a higher soluble sugar content than the uninoculated roots. Under water stress, T. chinensis var. mairei experienced decreased activity of aerobic respiratory metabolic enzymes. The activity of anaerobic respiratory metabolic enzymes and alcohol dehydrogenase initially increased and then decreased, whereas that of lactate dehydrogenase increased. The inoculated roots had a higher activity of respiratory metabolic enzymes than the uninoculated roots. As water stress was further intensified, the roots had significantly decreased activity of aerobic respiratory metabolic enzymes and significantly increased activity of anaerobic respiratory metabolic enzymes. The activity of respiratory metabolic enzymes decreased faster in the uninoculated roots than in the inoculated roots. This study demonstrated that Piriformospora indica plays a positive role in enhancing the antihypoxic ability of T. chinensis var. mairei, thereby alleviating plant damage due to water stress.
Keywords: Taxus chinensis var. mairei; Piriformospora indica; water stress; respiration
As a relict tree species of the Tertiary Period, Taxus chinensis var. mairei is listed as a “the state-protected one-grade rare and endangered plants” [1,2]. This tree is an important novel natural anticancer drug with a special anticancer mechanism and good anticancer effects [3]. Taxol is mainly derived from plant resources of Taxus Linn [4]. Owing to its rarity and medicinal value, wild resources of Taxus plants have been severely exploited. In addition, T. chinensis var. mairei can purify the air by absorbing harmful substances. Thus, this species plays a role in the sterilization, disinfection, and protection of the ecological environment [5]. However, T. chinensis var. mairei is difficult to sow because it has a low seedling survival rate [6,7], and it is intolerant to flooding. Thus, its survival situation is relatively grim.
Colonization of plants by Piriformospora indica can make them resistant to biotic and abiotic stresses [8], increase plant biomass [9], and increase the formation of lateral roots and root hairs [10]. Colonized plant roots are more developed than uncolonized roots, and the survival rate is high [11,12]. Water stress is an important factor affecting plant growth [13]. It has a direct impact on roots. Thus, plant roots can act as an important sign of response to waterlogging stress [14–17]. When a tree species with a weak water tolerance is subjected to water stress, its photosynthesis weakens [18], and its growth and development are inhibited [19], causing root death and plant wilting [20,21].
In this study, P. indica was inoculated into the roots of T. chinensis var. mairei under potted conditions to study the adaptation of this tree species to water stress. This study aimed to provide a theoretical basis and scientific methods for cultivating T. chinensis var. mairei against stress. This work also aimed to increase the stress resistance of T. chinensis var. mairei in southern areas with a high water content [1].
This experiment was conducted in Yangtze University. Jingzhou City (111°15′–114° 05′E, N29° 26′–31° 37′) is located in the central and southern parts of Hubei Province. Areas with a subtropical monsoon climate such as Jingzhou City has four distinct seasons, sufficient sunshine, and abundant precipitation. This city has a total solar radiation of about 104–110 kcal/cm2, an average annual sunshine hours of 1800–2000 h, an annual average temperature of 15.9°C–16.6°C, an annual rainfall of 1100–1300 mm, and an annual frost-free period of 250–267 d. The experimental seedlings were placed outdoors. In a previous study [22], we established that T. chinensis var. mairei can be grown in Jingzhou City.
In March 2019, seedlings of T. chinensis var. mairei with good growth and consistent growth vigor were transplanted in pots and placed in a garden to allow natural growth. The substrate consisted of a mixture of nutrient soil, river sand, and perlite at a ratio of 2:1:1. After 3 months of routine management, the treatment was commenced in June. First, 100 mL of P. indica diluent was cultured in potato dextrose agar medium (200 g of potato, 20 g of glucose, 15–20 g of agar, and 1000 mL distilled water) for 5 d and then in potato dextrose liquid medium (11 g of potato infusion, 20 g of dextrose, 15 g of agar, and 1000 mL distilled water) for 1 week. This culture was added to each pot in the T. chinensis var. mairei treatment group. The same amount of deionized water was added into each pot in the control group. Forty days after the inoculation, the root tips of T. chinensis var. mairei were cleaned and cut into sections of about 1 cm. The root tips were soaked in 10% KOH for almost 12 h. Afterward, the root tips were rinsed with deionized water for 6–9 times, placed on glass slides, and then dropped with an appropriate amount of trypan blue for about 12 min to observe the plant tissues under a stereomicroscope.
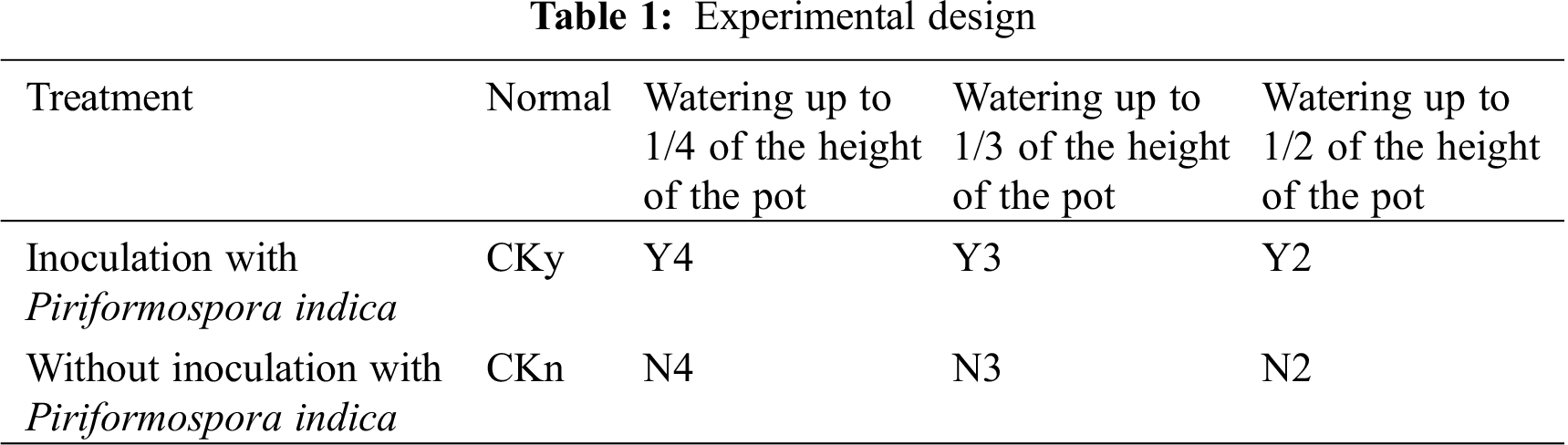
The materials were divided into two groups. One group was inoculated with P. indica, whereas the other group was not inoculated. Each group of test materials was subjected to four different degrees of water stress: each group was watered up to 0, 1/2, 1/3, and 1/4 of the height of the pot, and the water level was maintained at these levels; these groups were labelled as Ckn, N2, N3, and N4 for the uninoculated seedlings, respectively, and Cky, Y2, Y3, and Y4 in the inoculated seedlings, respectively (Tab. 1). All pots were 10.6 cm in height, with an outer diameter of 11.9 cm and an inner diameter of 11.4 cm. Two groups of different water stresses were repeated for each treatment with 20 plants, for a total of 160 plants. After 40 d of inoculation, samples were collected every 4 days for a total of five times. The physiological and biochemical indexes of the underground roots of T. chinensis var. mairei were measured each time.
2.3 Determination of Test Indexes
Root vitality was measured via the triphenyl tetrazolium chloride method [23]. Soluble sugar content was determined by anthrone colorimetry [24]. Malic acid content was quantified by NaOH titration [25]. Malate dehydrogenase (MDH) activity was assessed following a previously described method [26]. Activities of succinate dehydrogenase (SDH), lactate dehydrogenase (LDH), and alcohol dehydrogenase (ADH) were evaluated using the lactic acid kit of Nanjing Jiancheng Bioengineering Institute. Lactic acid content was measured using the same kit.
Data were analyzed by one-way ANOVA with Duncan’s multiple range tests to separate means by using the program SAS 10.0 (SAS Institute, Inc., Cary, NC). Correlation analysis was conducted using SPSS 18.0. Different letters in the graphs or the tables indicate significant differences at P < 0.05.
3.1 P. indica Colonization in the Roots of T. chinensis var. mairei and Comparison of Plant Growth under Different Water Stresses
The roots of uninoculated T. chinensis var. mairei had no chlamydospores within the roots’ cells (Fig. 1b). By contrast, 40 d after the inoculation, chlamydospores were observed within the roots’ cells of inoculated plants (Fig. 1a). The chlamydospores of P. indica were arranged in clusters in the roots.

Figure 1: Comparison of colonization of Piriformospora indica in the roots of Taxus chinensis var. mairei
On the 16th day, the growth of uninoculated T. chinensis var. mairei seedlings and their roots gradually deteriorated as water stress intensified Fig. 2. The growth of CKN seedlings was the best; they had luxuriant leaves, well-developed root systems, and more new roots. N4 seedlings vigorously grew, with better growth and new roots in their root systems. N3 seedlings were curved and thin, with sparse roots and few new roots. N2 seedlings had the worst growth; they were thin and weak, with yellow leaves, wilted roots, and no new roots.

Figure 2: Growth of Taxus chinensis var. mairei seedlings and their roots under different water stresses on the 16th day
3.2 Effects of P. indica on the Root Vitality of T. chinensis var. mairei under Water Stress
On the same day, the root vitality of Y4, Y3, Y2, N4, N3, and N2 seedlings decreased as water stress intensified (Fig. 3). On the 4th day, the difference in root vitality between CKn, Y4, Y3, and N4 seedlings was not significant (P > 0.05). However, on the 8th day, the difference in root vitality between CKn, N4, N4, and N2 seedlings was significant (P < 0.05). However, on the 12th day, the difference in root vitality between CKy, Y4, Y3, and Y2 seedlings was significant (P < 0.05). Moreover, root vigor index was higher in the inoculated roots than in the uninoculated roots. Results demonstrated that T. chinensis var. mairei experienced low root vitality as water stress intensified. Inoculated T. chinensis var. mairei had a higher root vitality than the uninoculated seedlings. Moreover, the root vitality of the uninoculated T. chinensis var. mairei decreased much faster and was substantially lower than that of inoculated seedlings.

Figure 3: Root activity in the inoculated (CKy, Y4, Y3, and Y2) and uninoculated (Ckn, N4, N3, and N2) roots of T. chinensis var. mairei under water stress after 4, 8, 12, and 16 day of watering
3.3 Effects of P. indica on the Soluble Sugar Content in the Roots of T. chinensis var. mairei under Water Stress
As water stress intensified, the content of soluble sugars in the roots of T. chinensis var. mairei increased. The content initially increased and then decreased (Fig. 4). On the 4th day of inoculation, the differences between CKy, Y4, Y3, and Y2 were significant (P < 0.05). Similarly, on the 16th day of inoculation, the differences between CKy and Y3, Y3, and Y4 were significant (P < 0.05); however, the differences between Y2, Y3, and Y4 were not significant (P > 0.05). Results showed that soluble sugar content was higher in the inoculated group than in the uninoculated group. On the 4th day, the differences between N4, N3, and CKn were significant (P < 0.05). On the 16th day, the differences between CKn and N2 were significant (P < 0.05), but those between N3, N2, and N4 were not significant (P > 0.05). The soluble sugar content of each treatment group was lower than that of the control group. Inoculated T. chinensis var. mairei seedlings had a higher soluble sugar content in the roots than the uninoculated seedlings; the reduction in soluble sugar content was slower in the former than that in the latter.

Figure 4: Soluble sugar content in inoculated (CKy, Y4, Y3, and Y2) and uninoculated (Ckn, N4, N3, and N2) roots of T. chinensis var. mairei under water stress after 4, 8, 12, and 16 days of watering
3.4 Effect of P. indica on MDH Activity in the Roots of T. chinensis var. mairei under Water Stress
As water stress intensified over time, MDH activity in the two groups gradually decreased (Fig. 5). On the 4th day of inoculation, the differences between Y4, Y3, Y2, and CKy were significant (P < 0.05). On the 16th day of inoculation, the difference between Y3 and Y4 was not significant (P > 0.05), whereas that between Y2, Y3, and CKy was significant (P < 0.05). As water stress further intensified, MDH activity in the roots decreased. On the 4th day, the differences between CKn, N4, N2, and N3 were significant (P < 0.05). On the 16th day, the differences between the treatment groups significant (P < 0.05). Uninoculated T. chinensis var. mairei had a faster and a greater reduction in MDH activity than the inoculated seedlings. The inoculated T. chinensis var. mairei had a higher MDH activity in the roots than the uninoculated seedlings.

Figure 5: Malate dehydrogenase activity in inoculated (CKy, Y4, Y3, and Y2) and uninoculated (Ckn, N4, N3, and N2) roots of T. chinensis var. mairei under water stress after 4, 8, 12, and 16 days of watering
3.5 Effects of P. indica on SDH Activity in the Roots of T. chinensis var. mairei under Water Stress
As water stress intensified over time, the activity of MDH in the two groups gradually decreased, but the decrease was slower in the inoculated group than in the uninoculated group (Fig. 6). On the 4th day of inoculation, the differences between CKy, Y3, and Y2 were significant (P < 0.05), but the difference between CKy and Y4 was not significant (P > 0.05). On the 16th day of inoculation, the differences between the treatments were significant (P < 0.05). On the 4th day, the differences between CKn and N2 and N3 were significant (P < 0.05) but not with N4 (P > 0.05). On the 16th day, the differences between CKn and N4, N2, and N3 were significant (P < 0.05). As water stress further intensified, SDH activity in the roots decreased. Inoculated T. chinensis var. mairei had a higher SDH activity in the roots than the uninoculated seedlings. The uninoculated T. chinensis var. mairei had a faster and greater reduction in SDH activity in the roots than the inoculated seedlings.

Figure 6: Succinate dehydrogenase activity in inoculated (CKy, Y4, Y3, and Y2) and uninoculated (Ckn, N4, N3, and N2) roots of T. chinensis var. mairei under water stress after 4, 8, 12, and 16 days of watering
3.6 Effects of P. indica on the Malic Acid Content in the Roots of T. chinensis var. mairei under Water Stress
As water stress intensified over time, the malate content in the two groups gradually decreased (Fig. 7). On the 4th day of inoculation, the differences between CKy and Y4, Y3, and Y2 were not significant (P > 0.05). On the 12th day, the differences between CKy and Y4, Y3, and Y2 (P < 0.05) and between Y3 and Y4 were significant (P < 0.05). As water stress further intensified, the malic acid content in the roots decreased. On the 16th day, the differences between CKy and Y4, Y3, and Y2 were significant (P < 0.05). On the 4th day, the differences between CKn and N2 and N3 were significant (P < 0.05) but not significant between Ckn and N4 (P > 0.05). On the 8th and 16th days, the differences between the treatments were significant (P < 0.05). The uninoculated T. chinensis var. mairei seedlings had a faster and greater reduction in malic acid content in the roots than the inoculated seedlings. The inoculated T. chinensis var. mairei seedlings had a higher malic acid content in the roots than the uninoculated seedlings.

Figure 7: Malate content in inoculated (CKy, Y4, Y3, and Y2) and uninoculated (Ckn, N4, N3, and N2) roots of T. chinensis var. mairei under water stress after 4, 8, 12, and 16 days of watering
3.7 Effect of P. indica on LDH Activity in the Roots of T. chinensis var. mairei under Water Stress
As water stress intensified over time, the activity of LDH in the uninoculated seedlings initially increased and then stabilized; by contrast, in the inoculated group, LDH activity initially increased and then decreased (Fig. 8). On the 4th day of inoculation, the differences between CKy and Y4, Y3, and Y2 were not significant (P > 0.05). On the 16th day, the differences between CKy and Y4, Y3, and Y2 and between CKy and Y3 and Y2 were significant (P < 0.05). On the 4th day, the differences between CKn and N4 and N3 were not significant (P > 0.05), but the difference between CKn and N2 was significant (P < 0.05). On the 16th day, the differences between CKn and N4, N3, and N2 were significant (P < 0.05), but the difference between N2 and N3 was not significant (P > 0.05), with increased LDH activity in the roots. As water stress further intensified over time, the T. chinensis var. mairei seedlings had increased LDH activity in the roots. The inoculated T. chinensis var. mairei seedlings had a higher and faster increase in LDH activity in the roots than the uninoculated seedlings. Hence, inoculation with P. indica increased the LDH activity in the roots of the T. chinensis var. mairei seedlings.
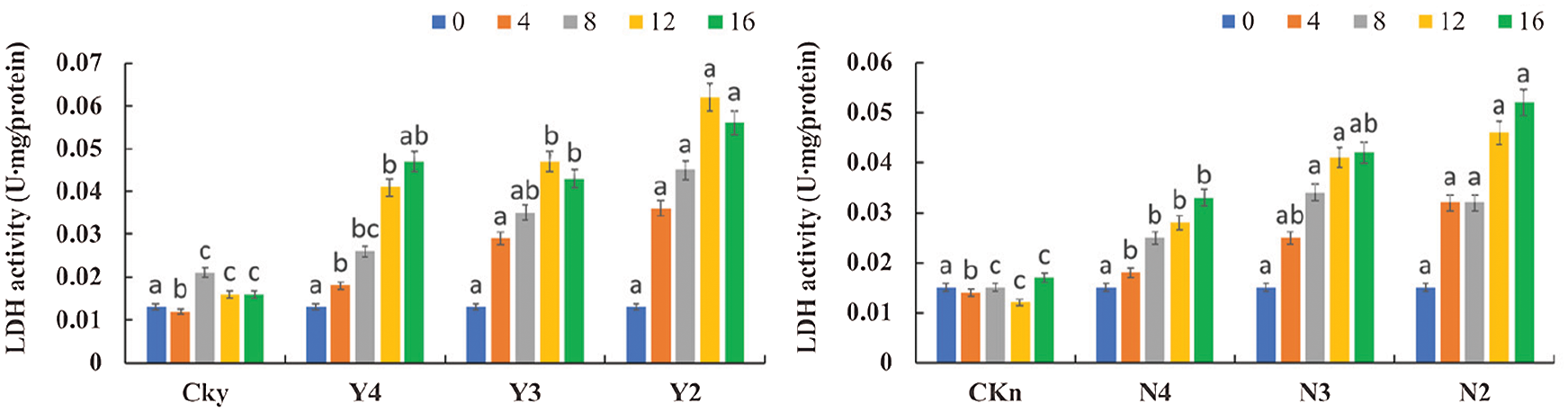
Figure 8: Lactate dehydrogenase activity in inoculated (CKy, Y4, Y3, and Y2) and uninoculated (Ckn, N4, N3, and N2) roots of T. chinensis var. mairei under water stress after 4, 8, 12, and 16 days of watering
3.8 Effects of P. indica on ADH Activity in the Roots of T. chinensis var. mairei under Water Stress
Over time, ADH activity in the two treatment groups initially increased and then decreased (Fig. 9). On the 8th day of inoculation, the differences between CKy and Y4, Y3, and Y2 were significant (P < 0.05). On the 16th day, the differences between CKy and Y3 and Y2 were significant (P < 0.05), but the difference between Y3 and Y2 was not significant (P > 0.05). On the 8th day, the differences between CKn and N4, N3, and N2 were significant (P < 0.05). On the 16th day, the differences between CKn and N3 and N2 were significant (P < 0.05), but the difference between N4 and N3 was not significant (P > 0.05). Thus, as water stress intensified, ADH activity in the roots of T. chinensis var. mairei increased. ADH activity in the roots initially increased and then decreased. The inoculated T. chinensis var. mairei seedlings has a higher and a faster increase in ADH activity in the roots than the uninoculated seedlings. Therefore, inoculation with P. indica increased the ADH activity in the roots of T. chinensis var. mairei.

Figure 9: Alcohol dehydrogenase activity in inoculated (CKy, Y4, Y3, and Y2) and uninoculated (Ckn, N4, N3, and N2) roots of T. chinensis var. mairei under water stress after 4, 8, 12, and 16 days of watering
3.9 Effects of P. indica on Lactic Acid Content in the Roots of T. chinensis var. mairei under Water Stress
As water stress intensified over time, the lactic acid content in the two treatment groups initially increased and then decreased (Fig. 10). On the 8th and 12th days of inoculation, the differences between CKy and Y4, Y3, and Y2 were significant (P < 0.05). On the 16th day, the differences between CKy and Y4, Y3, and Y2 were significant (P < 0.05), but the difference between Y4 and Y3 was not significant (P > 0.05). On the 8th day, the differences between CKn and N4, N3, and N2 were significant (P < 0.05). On the 16th day, the difference between N4 and N3 was not significant (P > 0.05), but the differences between CKn and N4 and N2 were significant (P < 0.05). As water stress further intensified over time, lactic acid content initially increased and then decreased. The inoculated T. chinensis var. mairei seedlings had a higher and faster increase in lactic acid content in the roots than the uninoculated seedlings. The uninoculated T. chinensis var. mairei seedlings has a greater reduction in lactic acid content than the inoculated seedlings. Hence, inoculation with P. indica increased lactic acid content in the roots of T. chinensis var. mairei seedlings.

Figure 10: Lactic acid content in inoculated (CKy, Y4, Y3, and Y2) and uninoculated (Ckn, N4, N3, and N2) roots of T. chinensis var. mairei under water stress after 4, 8, 12, and 16 days of watering
4.1 P. indica Colonization in the Roots of T. chinensis var. mairei and Comparison of Plant Growth under Different Degrees of Water Stress
The T. chinensis var. mairei seedlings inoculated with P. indica did not have chlamydospores on the 15th day after inoculation. After 40 d, more chlamydospores were observed in the roots, and the root hairs and the root system substantially increased. This result was consistent with that of Liang et al. [27], who reported that inoculation of P. indica on Medicago truncatula notably promotes root growth at 15–30 d. It also showed that gymnosperms have a longer infection of the test mycorrhiza after inoculation with P. indica.
As water stress intensified, the growth of the T. chinensis var. mairei seedlings and their roots gradually deteriorated, indicating that this species is not tolerant to flooding and water stress is not conducive to its growth. This finding was consistent with that of Ivanov et al. [28], who reported that pine and spruce seedlings grow poorly and even die under water stress.
4.2 Effects of P. indica on Root Vitality and Respiratory Substrates in the Roots of T. chinensis var. mairei under Water Stress
Under different water stress treatments, the T. chinensis var. mairei seedlings had substantially decreased root vitality. The inoculated T. chinensis var. mairei seedlings had higher root vitality and slower reduction rate in this index than the uninoculated seedlings, indicating that P. indica can increase the root vitality of T. chinensis var. mairei and reduce the damage to its roots caused by water stress. This result was consistent with that of Ghodrat et al. [29], who noted that the root vitality of cotton declines under water stress.
The T. chinensis var. mairei seedlings inoculated with P. indica had a higher soluble sugar content in the roots than the uninoculated seedlings. Moreover, its soluble sugar content increased as water stress intensified. This observation was consistent with that of Khosravi et al. [30], who found that the soluble sugar content of wheat increases under flooding stress. Soluble sugars can adjust the osmotic pressure in plants, indicating that inoculation with P. indica helps retain the photosynthetic metabolites of seedlings, thereby increasing the osmotic adjustment capacity of the root system.
4.3 Effects of P. indica on the Activity of Aerobic Respiratory Metabolic Enzymes and Products in the Roots of T. chinensis var. mairei under Water Stress
The T. chinensis var. mairei seedlings inoculated with P. indica had a higher MDH and SDH activity and higher malic acid content in the roots than the uninoculated seedlings, indicating that P. indica can increase the aerobic respiration rate of T. chinensis var. mairei. As water stress further intensified over time, the MDH and SDH activity and the content of malic acid of the T. chinensis var. mairei seedlings gradually decreased, suggesting that the aerobic respiration in the roots of this species gradually decreased under water stress. This finding was consistent with that of Guibin et al. [31], who reported that the MDH activity and aerobic respiration intensity in the roots of Eucalyptus decreases under flooding stress, as well as with that of Pereira et al. [32], who observed that the malic acid content in the roots of Spinach gradually decreases under flooding stress.
4.4 Effects of P. indica on the Activity of Anaerobic Respiratory Metabolic Enzymes and Products in the Roots of T. chinensis var. mairei under Water Stress
Under water stress, the ADH activity and the lactic acid content in the roots initially substantially increased and then decreased. Moreover, LDH activity increased as was stress intensified. This result was similar to that of Du et al. [33], who reported that the ADH activity of wheat seedling roots initially increases and then stabilizes under waterlogging stress. These findings indicated that T. chinensis var. mairei has a limited aerobic respiration under water stress and instead mainly performs anaerobic respiration. In addition, the inoculated T. chinensis var. mairei seedlings had a higher LDH and ADH activity and a higher lactic acid content in the roots than the uninoculated seedlings. This trend was similar to that observed by Li et al. [34], who found that white clover has a high lactic acid content in the roots under hypoxia, indicating that P. indica plays a positive role in enhancing the antihypoxic ability of T. chinensis var. mairei.
To sum up, this study demonstrated that P. indica can enhance the root vitality and waterlogging resistance of T. chinensis var. mairei. This work provides a theoretical basis and scientific method for promoting and planting T. chinensis var. mairei in humid areas in southern China. However, this study has several limitations. Only T. chinensis var. mairei was selected in studying respiration under water stress. Follow-up studies should include other species of Taxus or other precious tree species. Moreover, the mechanism of the effect of P. indica on the respiration of T. chinensis var. mairei under water stress must be investigated further. Given that the regulation of respiratory metabolism under hypoxic environment involves a more complicated mechanism, future studies should focus on the molecular level and resolve related problems via biochemical or molecular technologies.
Funding Statement: This work was supported by the National Natural Science Foundation of China (No. 31270740).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Gao, R. M., Shi, X. D., Fan, L. Y., Sun, Y. Y., Guo, X. H. (2016). Natural distribution and community ecological characteristics of taxus chinensis var. mairei in Shanxi Province, China. The Journal of Applied Ecology, 27(6), 1820. DOI 10.13287/j.1001-9332.201606.029. [Google Scholar] [CrossRef]
2. Zhang, D. Q., Zhou, N. (2013). Genetic diversity and population structure of the endangered conifer taxus wallichiana var. mairei (Taxaceae) revealed by simple sequence repeat (SSR) markers. Biochemical Systematics and Ecology, 49, 107–114. DOI 10.1016/j.bse.2013.03.030. [Google Scholar] [CrossRef]
3. Qiao, W., Ling, F., Yu, L., Huang, Y., Wang, T. (2017). Enhancing taxol production in a novel endophytic fungus. Aspergillus Aculeatinus Tax-6, Isolated from Taxus Chinensis var. Mairei. Fungal Biology, 121(12), 1037–1044. DOI 10.1016/j.funbio.2017.08.011. [Google Scholar] [CrossRef]
4. Nimasow, G., Nimasow, O. D., Rawat, J. S., Tsering, G., Litin, T. (2016). Remote sensing and GIS-based suitability modeling of medicinal plant (Taxus baccata linn.) in tawang district, arunachal pradesh, India. Current Science, 110(2), 219–227. DOI 10.18520/cs/v110/i2/219-227. [Google Scholar] [CrossRef]
5. Wei, Q., Yin, C. W. (2019). Chemical composition of essential oils from the stems of taxus chinensis var. mairei. Journal of Essential Oil Bearing Plants, 22(4), 1144–1149. DOI 10.1080/0972060X.2019.1668864. [Google Scholar] [CrossRef]
6. Song, L. L., Zhang, H. N., Zhao, H. Q., Jiang, Y. L., Hou, M. F. (2014). In vitro germination and seedling development of taxus chinensis var. mairei by embryo culture. Journal of Agricultural Science & Technology, 16, 1355–1363. https://www.researchgate.net/publication/288536196. [Google Scholar]
7. Jian, Z. Y., Meng, L., Wang, N., Xu, G. F., Shi, Y. (2016). Characteristic and protection of rare and endangered taxus chinensis var. mairei in the taihang mountains. Nutrición Hospitalaria, 33(3), 698–702. https://www.redalyc.org/articulo.oa?id=309246400029. [Google Scholar]
8. Lin, H. F., Xiong, J., Zhou, H. M., Chen, C. M., Lin, F. Z. et al. (2019). Growth promotion and disease resistance induced in anthurium colonized by the beneficial root endophyte piriformospora indica. BMC Plant Biology, 19(1), 40. DOI 10.1186/s12870-019-1649-6. [Google Scholar] [CrossRef]
9. Gosal, S. K., Sharma, M., Gosal, S. S., Chhibba, I. M., Bhatnagar, K., Varma, A. (2011). Biohardening with piriformospora indica improves survival rate, growth, iron uptake and cane yield of micropropagated sugarcane. International Sugar Journal, 113(1349), 382–388. https://www.redalyc.org/articulo.oa?id=309246400029. [Google Scholar]
10. Li, Q., Kuo, Y. W., Lin, K. H., Huang, W., Deng, C. et al. (2021). Piriformospora indica colonization increases the growth, development, and herbivory resistance of sweet potato (Ipomoea batatas L.). Plant Cell Reports, 40(2), 339–350. DOI 10.1007/s00299-020-02636-7. [Google Scholar] [CrossRef]
11. Das, A., Kamal, S., Shakil, N. A., Sherameti, I., Oelmüller, R. et al. (2012). The root endophyte fungus piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, coleus forskohlii. Plant Signaling & Behavior, 7(1), 103–112. DOI 10.4161/psb.7.1.18472. [Google Scholar] [CrossRef]
12. Kumar, M., Yadav, V., Kumar, H., Sharma, R., Singh, A. et al. (2011). Piriformospora indica enhances plant growth by transferring phosphate. Plant Signaling & Behavior, 6(5), 723–725. DOI 10.4161/psb.6.5.15106. [Google Scholar] [CrossRef]
13. Candan, N., Tarhan, L. (2012). Tolerance or sensitivity responses of mentha pulegium to osmotic and waterlogging stress in terms of antioxidant defense systems and membrane lipid peroxidation. Environmental and Experimental Botany, 75, 83–88. DOI 10.1016/j.envexpbot.2011.08.014. [Google Scholar] [CrossRef]
14. Knapp, A. K., Hoover, D. L., Wilcox, K. R., Avolio, M. L., Koerner, S. E. et al. (2015). Characterizing differences in precipitation regimes of extreme wet and dry years: Implications for climate change experiments. Global Change Biology, 21(7), 2624–2633. DOI 10.1111/gcb.12888. [Google Scholar] [CrossRef]
15. Knapp, A. K., Avolio, M. L., Beier, C., Carroll, C. J., Collins, S. L. et al. (2017). Pushing precipitation to the extremes in distributed experiments: Recommendations for simulating wet and dry years. Global Change Biology, 23(5), 1774–1782. DOI 10.1111/gcb.13504. [Google Scholar] [CrossRef]
16. Bárzana, G., Carvajal, M. (2020). Genetic regulation of water and nutrient transport in water stress tolerance in roots. Journal of Biotechnology, 324, 134–142. DOI 10.1016/j.jbiotec.2020.10.003. [Google Scholar] [CrossRef]
17. Niu, X., Hu, T., Liu, T., Wu, X., Feng, P. et al. (2014). Appropriate partial water stress improving maize root absorbing capacity. Transactions of the Chinese Society of Agricultural Engineering, 30(22), 80–86. DOI 10.3969/j.issn.1002-6819.2014.22.010. [Google Scholar] [CrossRef]
18. Zhang, Y. J., Xie, Z. K., Wang, Y. J., Su, P. X., An, L. P. et al. (2011). Effect of water stress on leaf photosynthesis, chlorophyll content, and growth of oriental lily. Russian Journal of Plant Physiology, 58(5), 844. DOI 10.1134/S1021443711050268. [Google Scholar] [CrossRef]
19. Ekmekci, Y., Bohms, A., Thomson, J. A., Mundree, S. G. (2005). Photochemical and antioxidant responses in the leaves of xerophyta viscosa baker and digitaria sanguinalis L. under water deficit. Zeitschrift für Naturforschung, 60(5–6), 435–443. DOI 10.1515/znc-2005-5-612. [Google Scholar] [CrossRef]
20. Seo, C., Lee, S., Kang, S., Park, Y., Kim, A. et al. (2017). Selection of suitable plant growth regulators for augmenting resistance to waterlogging stress in soybean plants (Glycine max L.). Korean Journal of Crop Science, 62(4), 325–332. https://www.dbpia.co.kr/journal/articleDetail?nodeId=NODE07470438. [Google Scholar]
21. Pereira, A. (2016). Plant abiotic stress challenges from the changing environment. Frontiers in Plant Science, 7, 1123. DOI 10.3389/fpls.2016.01123. [Google Scholar] [CrossRef]
22. Cao, J., Liu, C., Wu, Y., Li, H., Li, M. (2017). Geographic distribution and ecological zoning of taxus chinensis var. mairei in China. Acta Horticulturae, 1185, 265–276. DOI 10.17660/ActaHortic.2017.1185.34. [Google Scholar] [CrossRef]
23. Hawrylak-Nowak, B., Matraszek, R., Pogorzelec, M. (2015). The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiologiae Plantarum, 37(2), 41. DOI 10.1007/s11738-015-1788-9. [Google Scholar] [CrossRef]
24. Wei, F., Zheng, Q. K., Luo, S. Q., Qiu, J., Yang, W. F. (2014). A method for measuring soluble sugars and starch in bark and xylem of rubber tree. Chinese Journal of Tropical Agriculture, 4, 12–16. DOI CNKI:SUN:RDNK.0.2014-04-002. [Google Scholar]
25. Ma, B., Chen, J., Zheng, H., Fang, T., Ogutu, C. et al. (2015). Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chemistry, 172, 86–91. DOI 10.1016/j.foodchem.2014.09.032. [Google Scholar] [CrossRef]
26. Sales, C. R., da Silva, A. B., Carmo-Silva, E. (2020). Measuring rubisco activity: Challenges and opportunities of NADH-linked microtiter plate-based and 14C-based assays. Journal of Experimental Botany, 71(18), 5302–5312. DOI 10.1093/jxb/eraa289. [Google Scholar] [CrossRef]
27. Li, L., Li, L., Wang, X., Zhu, P., Wu, H. et al. (2017). Plant growth-promoting endophyte piriformospora indica alleviates salinity stress in medicago truncatula. Plant Physiology and Biochemistry, 119, 211–223. DOI 10.1016/j.plaphy.2017.08.029. [Google Scholar] [CrossRef]
28. Ivanov, Y., Kartashov, V., Zlobin, E., Kuznetsov, V. (2018). Water deficit-dependent changes in non-structural carbohydrate profiles, growth and mortality of pine and spruce seedlings in hydroculture. Environmental and Experimental Botany, 157, 151–160. DOI 10.1016/j.envexpbot.2018.10.016. [Google Scholar] [CrossRef]
29. Hamidi, R., Sharafzadeh, S., Ghodrat, V., Bazrafshan, F., Alizadeh, O. (2016). Effect of water stress at flowering stage on morphophysiological characteristics of cotton (Gossypium hirsutum L.) cultivars in southern Iran. ecology. Environment and Conservation, 22, 1763–1773. https://www.researchgate.net/publication/317204820. [Google Scholar]
30. Khosravi, M. S., Heidari, R., Jamei, R., Kouhi, S. M. M., Moudi, M. (2018). Comparative growth and physiological responses of tetraploid and hexaploid species of wheat to flooding stress. Acta Agriculturae Slovenica, 111(2), 285–292. DOI 10.14720/aas.2018.111.2.04. [Google Scholar] [CrossRef]
31. de Sá Martins, R., Faria, J. M. R., Rossini, B. C., Marino, C. L., Dos Santos, L. D. et al. (2020). Proteomic analyses unraveling water stress response in two eucalyptus species originating from contrasting environments for aridity. Molecular Biology Reports, 47(7), 5191–5205. DOI 10.1007/s11033-020-05594-1. [Google Scholar] [CrossRef]
32. Pereira, C., Dias, M. I., Petropoulos, S. A., Plexida, S., Chrysargyris, A. et al. (2019). The effects of biostimulants, biofertilizers and water-stress on nutritional value and chemical composition of Two spinach genotypes (Spinacia oleracea L.). Molecules, 24(24), 4494. DOI 10.3390/molecules24244494. [Google Scholar] [CrossRef]
33. Du, H. Y., Liu, D. X., Liu, G. T., Liu, H. P., Kurtenbach, R. (2018). Relationship between polyamines and anaerobic respiration of wheat seedling root under water-logging stress. Russian Journal of Plant Physiology, 65(6), 874–881. DOI 10.1134/S1021443718060055. [Google Scholar] [CrossRef]
34. Li, Z., Cheng, B., Yong, B., Liu, T., Peng, Y. et al. (2019). Metabolomics and physiological analyses reveal β-sitosterol as an important plant growth regulator inducing tolerance to water stress in white clover. Planta, 250(6), 2033–2046. DOI 10.1007/s00425-019-03277-1. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |