
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015852
ARTICLE
Expression and Interaction Analysis of FAZ1 Protein in Brassica oleracea
1College of Agronomy and Biotechnology, Southwest University, Chongqing, 400715, China
2State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, School of Agriculture, Yunnan University, Kunming, 650091, China
*Corresponding Author: Liquan Zhu. Email: zhuliquan@swu.edu.cn
#Contributed equally to this work and are co-first authors
Received: 18 January 2021; Accepted: 25 March 2021
Abstract: To identify and characterize genes involved in reproductive tissue abscission in Brassica oleracea, the transcript data of pollinated pistil was analyzed. A differentially expressed gene, named BoFAZ1(FLOWER ABSCISSION ZONE1) was identified, which contains one exon and encompass a 139aa. Furthermore, a T-DNA insertion mutant (SALK_302_G01) (faz1 mutant) was obtained from Arabidopsis thaliana mutant library. Floral organ shedding from mutants was delayed and a V-shaped structure in the boundary region between the stalk and torus of the sepal abscission zone was obtained in faz1 mutant. The cell density of this structure was lower than that of the corresponding region in the wild-type control. In the transgenic plants, the normal development of the stalk zone of faz1 was recovered completely by transforming a 1919-bp DNA fragment of BoFAZ1 into the faz1 mutant. In Addition, our data showed that BoFAZ1 was expressed in mature pollen grains, but not in the bracts, roots, stems, leaves, and sepals. Its expression in the filaments, stigma, and pistil exfoliation layer gradually increased after pollination. Subcellular localization experiments showed that BoFAZ1 was located in the cell membrane. A myristoylation site was found at the N-terminus of BoFAZ1. Removal of this site resulted in protein dislocation in the cytoplasm, cell membrane and nucleus. Finally, a yeast two-hybrid test indicated that BoH3.2 (histone H3.2), a protein involved in abscission zone development, interacted with BoFAZ1. This interaction was verified by a GST pull-down assay. In summary, our data indicated that BoFAZ1 was involved in the formation of the pistil abscission zone in B. oleracea.
Keywords: Abscission; AZ; B. oleracea; flower development organ boundary
Abscission in plants is a physiological process of shedding unwanted organs, such as leaves, floral organs, fruits, and seeds at specific points in their life cycle or in response to environmental cues [1]. This process occurs in a developmentally defined region of cells called the abscission zone (AZ), which is established at the base of the organs to be shed. The abscission zones (AZs) consisting of 1–50 small, undifferentiated, or meristematic cell layers are critical mediators to make the organs of various plant parts detached properly from the main body [2–4]. Most studies on abscission have focused on the roles of plant hormones on the development and activation of AZs. Most of the classical plant hormones have been shown to affect the timing of abscission. In general, the accepted concept is that ethylene promotes the establishment of AZs, whereas auxin has the opposite effect [5].
Besides plant hormones, multiple transcription factors, small signaling peptides, and membrane traffic regulators have been found to play essential roles in the development of AZs and the shedding of plant organs in many species. For example, the transcriptional activators BLADE-ON-PETIOLE1 (BOP1) and BLADE-ON-PETIOLE2 (BOP2) redundantly controlled the patterning of the proximal regions of developing leaves and floral organs. In Arabidopsis thaliana leaves, BOP1 and BOP2 were involved in AZ formation. It was revealed that the double mutants of bop1/bop2 failed to produce normal AZ in leaves [6,7]. The tobacco BOP2 homologue was associated with regulating the corolla (petal) AZ [8]. In rice, the SUPERNUMERARY BRACT (SNB) promoted the expression of two abscission-related genes, qSH1 and SH5, which participated in seed abscission via regulation of lignin deposition and organ detachment in specific areas [9]. In addition, mutations in the REPLUMLESS gene, which is a homolog of BELL1(BEL1) in A. thaliana, resulted in a lack of AZs in temperate indica rice cultivars [10,11]. In wheat, the transcription factor APETALA2 (AP2) had impacts on flowering, glume shape, and seed shedding [12,13]. The development of floral AZs was disrupted when BREVIPEDICELLUS (BP, also known as KNAT1) was knocked out in A. thaliana [14]. The A. thaliana ZINC FINGER PROTEIN2 (AtZFP2) gene expressed in the AZ of stamens, petals, and sepals affected the structure of AZs in the Arabidopsis flower stalk [15]. ARABIDOPSIS THALIANA HOMEOBOX GENE1 (ATH1) was required for the formation of the stamen AZ through inhibiting the growth of floral organ receptacle boundaries [16]. The transcription factor, ASYMMETRIC LEAVES1 (AS1), played an important role in the morphology and structure of the AZ boundary in flowers [17]. Additionally, JOINTLESS, MACROCALYX (MC), and SLMBP21, two members of the MADS-box gene family, were required in the development of the tomato flower AZ [18]. 1-aminocyclopropane-1-carboxylate oxidase (ACO) family and LeLX genes were expressed in the tomato petiole. All these three genes had implications in AZ development [19]. The Tomato Hybrid Proline-rich Protein (THyPRP) gene was specifically expressed in the flower AZ substantially inhibited tomato pedicel abscission following flower removal [20]. HAWAIIAN SKIRT (HWS), a F-box protein, prevented the fusion of adjacent sepals and affected the timing of their abscission [21]. However, the molecules and mechanism regulating floral organ abscission in Brassica is still largely unknown.
To explore potential regulators of floral abscission in Brassica, a differentially expressed gene, designated as FLOWER ABSCISSION ZONE1 (BoFAZ1) was identified from the pollinated pistil of Brassica oleracea for the first time by analyzing the transcriptome data from databases. A T-DNA insertion mutant of BoFAZ1 in Arabidopsis thaliana exhibited the procrastination of floral organ shedding from the stalk. The expression of BoFAZ1 in the filaments, stigma, and pistil exfoliation layer was gradually increased after pollination. Subcellular localization data indicated that BoFAZ1 is a cell membrane protein. Finally, a yeast two-hybrid test revealed that BoFAZ1 interacted with BoH3.2 (histone H3.2), a protein involved in abscission zone development. In summary, our data suggested that BoFAZ1 is involved in the formation of pistil abscission zone in Brassica. The data presented herein may be useful for future studies on the development of the pistil AZ and the shedding of flower organs from B. oleracea plants.
2.1 Growth Conditions of Plant Materials
Brassica oleracea var. capitata L. seeds, provided by the Chongqing Key Laboratory of Olericulture, were planted and grown in a glasshouse in the Xiema Cruciferous Research Institute, Southwest University, Chongqing, China. To investigate the potential regulator during Brassica floral organ abscission, the pistils were collected at 0, 15, 30, 60 min after self- and cross-pollination and stored at −80°C freezer until total RNA extraction.
The FAZ1 mutants SALK_302_G01 were from the mutant library and ProFAZ1: GUS was in the A. thaliana Columbia ecotype background. The FAZ1/Pro35S:BoFAZ1 plants were generated with natural crosses. The T-DNA insertions of FAZ1 homozygous lines were identified by polymerase chain reaction (PCR) genotyping with the primers listed in Tab. S1. Seeds were germinated and grown on half-strength Murashige and Skoog (MS) medium containing 1% sucrose and 0.7% agar. All plants were grown at 23°C under long-day conditions (16-h light/8-h dark).
2.2 Bioinformatics Analysis and Data Analysis
The ClutsalW program was used for multiple protein sequence alignments and MEGA X was used to construct the Bar chart.
2.3 RNA Extraction and qRT-PCR Analysis
Total RNA was extracted from floral tissues at different time intervals after pollination with the RNAprep Pure Plant Kit, Tiangen, Beijing, China. First-strand cDNA was synthesized from 1 μg total RNA with the PrimeScript RT Reagent Kit with gDNA Eraser. A quantitative real-time (qRT)-PCR assay was performed with the SYBR Premix Ex Taq Kit using the 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) with the following PCR program: 95°C for 30 s; 40 cycles of 95°C for 10 s, 56°C for 10 s, and 72°C for 10 s. Gene expression analyses were conducted with three replicates, and expression levels were normalized against that of Actin2 according to the 2−ΔΔCt method [22]. Details regarding the qRT-PCR primers are provided in Tab. S1.
2.4 Plasmid Construction and Transformation
A 1.5-kb promoter region upstream of the FAZ1 start codon was amplified by PCR with the primers listed in Tab. S1. The amplified fragments were inserted into the SalI/EcoRI sites of the pCAMBIA1391 vector to generate the pBoFAZ1: GUS recombinant plasmid. For subcellular localization analysis, FAZ1 cDNA was cloned into the p35S: GFP vector. GST pull-down assay was according to [23]. The primer sequences used are listed in Supplementary Tab. S1.
2.5 Scanning Electron Microscopy (SEM)
Mutant and wild type flower organs were examined with a stereo microscope and photographed. The sepals and petals were removed with tweezers, and the stamens and pistils were placed on conductive gels before they were analyzed with a scanning electron microscope. Experimental flowers were fixed in formaldehyde-acetic acid (45% ethanol, 2.5% acetic acid, and 2.5% formalin) at room temperature overnight, dehydrated in an ethanol series, and critical-point dried (DCP-1). Samples were mounted on aluminum stubs, silver painted, sputter-coated with Au/Pd, and imagined under a SEM microscope.
2.6 Genetic Complementation Test
The cDNA fragment of BoFAZ1, the homologous gene of AtFAZ1 in Arabidopsis, was amplified from Brassica oleracea. A recombinant plasmid with FAZ1 coding sequence fused to the CaMV 35S promoter, and was generated and transformed into the mutant faz1 of A. thaliana according to a previously described protocol.
Plant materials were stained with a solution comprising 1 mM 5-bromo-4-chloro-3-indolyl β-D-glucuronide, 50 mM NaPO4 (pH 7.0), 0.4 mM K3Fe(CN)6, and 0.1% (v/v) Triton X-100 at 37°C for 6 h. After the GUS staining, chlorophyll was removed with a 4-h treatment with 70% ethanol. Samples were then analyzed, and images were captured with an MVX10 stereomicroscope (Olympus, Tokyo, Japan).
2.8 Subcellular Localization of BoFAZ1 Protein
A recombinant plasmid with FAZ1 coding sequence fused to a GFP-encoding gene driven by the CaMV 35S promoter was generated and transfected into A. thaliana according to a previously described protocol [24]. An empty vector (i.e., GFP-encoding gene only) was used as a control. Confocal fluorescent images of the transformed A. thaliana cells were acquired with the LSM 510 Meta inverted laser scanning confocal microscope (Carl Zeiss, Jena, Germany).
2.9 Yeast Two-Hybrid Screening
The Matchmaker Gold Yeast Two-Hybrid System (Clontech, Tokyo, Japan) was used to detect proteins interacting with BoFAZ1. To identify interacting proteins, we used BoFAZ1d as the bait to screen a B. oleracea cDNA library. Competent yeast cells were prepared with LiAc following the manufacturer’s instructions. The pGBKT7-BoFAZ1d recombinant plasmid was transfected into Y2HGold yeast cells, whereas the pGADT7-Library recombinant plasmid was inserted into Y187 yeast cells. Bait toxicity and recombinant plasmid activation tests were performed at 30°C on synthetic defined (SD/−Leu and SD/−Trp) media in Petri plates. The Y187 [pGADT7-T] × Y2Hgold [pGBKT7-Lam] transformation was used as a negative control, whereas the Y187 [pGADT7-T] × Y2H Gold [pGBKT7-53] transformation served as a positive control. The screening of protein interactions was performed for each pGBKT7-PUB7 derivative and pGADT7-Library on DDO (SD/−Leu/−Trp) and QDO/A (SD/−Ade/−His/−Leu/−Trp/AbA) media in Petri plates following a 3-day incubation period.
GST pull down assay was conducted to study the interaction between BoFAZ1 and BoH3.2. BoFAZ1d was cloned into pGEX-4T-1vector, while BoH3.2 was cloned into pET-15b vector. The pET- BoH3.2 and pGEX- BoFAZ1 fused proteins were isolated and purified from E. coli (Transetta DE3). The pGEX4T-1 vector and pGEX-BoFAZ1d fusion proteins were incubated with glutathione-agarose beads, which were then used to pull down 6×His tagged pET-BoH3.2. The protein solutions after pull down were analyzed by western blot analysis.
3.1 BoFAZ1 was Differentially Expressed in the Pistils after Self-Pollinated
According to GO term classifications, one of the differentially expressed genes (DEG) was selected, which was significantly up-regulated expressed after self-pollination [25]. qRT-PCR assays further confirmed that the expression level of BoFAZ1 displayed an 11, 20 and 65-fold increase at 15, 30, and 60 min after self-pollination (SP), respectively, and BoFAZ1 expression was slowly increased after cross-pollination (CP) (Fig. 1).

Figure 1: Expression analysis and gene amplification of BoFAZ1. mRNA expression of BoFAZ1 at 0, 15, 30, and 60 min after pollinations obtained from transcriptomic data analysis. SP: Self-pollination; CP: Cross pollination
To obtain the genomic and encoding full-length sequence of BoFAZ1 from B. oleracea, polymerase chain reactions (PCR) with individual gDNA and cDNA as amplification templates were carried out by using a specific primer pair. This was designed based on the transcriptome sequence data. The cDNA and gDNA sequencing results revealed that BoFAZ1 consisted of 420 bp, with only one exon. The deduced amino acid sequence analysis suggested that BoFAZ1 encoded a 139 aa peptide, with a molecular weight of approximately 17 kDa. An analysis from the NCBI database indicated that BoFAZ1 was located on chromosome C3. Additionally, the TMHMM server showed that a signal peptide was missing on BoFAZ1, whereas the ScanProsite online program revealed a myristoylation site at the amino acid positions 2–7 of BoFAZ1 (i.e., N-terminus).
3.2 Homologous Analysis of the BoFAZ1 Gene in Brassicaceae
To characterize this novel gene in Brassicaceae, a multiple protein sequence alignment was performed to reveal the amino acid similarity among BoFAZ1, BnFAZ1, BrFAZ1 and AtFAZ1 from B. oleracea, B. napus, B. rapa and A. thaliana, respectively (Fig. 2A). The alignment showed that the full length of the BoFAZ1 protein shared substantial identity within these proteins. For example, the BoFAZ1 and BnFAZ1 sequences had only one amino acid difference, whereas the BoFAZ1 and BrFAZ1 sequence had three amino acid variation. To further analyze the phylogenetic relationships between FAZ1 in B. oleracea, a phylogenetic tree was constructed using the Gramene online tool (http://www.gramene.org/) (Fig. 2B). The tree indicated that the highest similarity and collinearity were observed between BoFAZ1 (accession number: Bo3g109530) and BnFAZ1 (accession number: BnaCnng41410D). The similarity between Bo3g109530 and Bo8g077850 was very low. Furthermore, a synteny comparison was conducted to analyze the conservation of genes surrounding FAZ1. As shown in Fig. 2C, genes belonging to similar gene families were located at both sides of the FAZ1 gene in B. oleracea. In summary, the FAZ1 gene was highly conserved in Brassicaceae.

Figure 2: FAZ1 sequence alignment and phylogenetic relationships. A, Alignment of protein sequences of multiple species. At: Arabidopsis, Bo: Brassica oleracea, Bn: Brassica napus, Br: Brassica rape. B, Phylogenetic analysis of FAZ1 of B. oleracea. C, Gene conservation analysis surrounding FAZ1. The 10 genes flanking either side of FAZ1 are color-coded based on the gene family
3.3 Phenotypic Analysis of the at FAZ1 Mutant
To investigate the potential biological function of BoFAZ1 in B. oleracea, T-DNA insertion mutants were generated in A thaliana given the fact that A thaliana is one of the classical model plants and a single-copy homolog of BoFAZ1 in A. thaliana. A thaliana T-DNA insertion mutant (SALK_302_G01) (i.e., faz1 mutant) was analyzed to characterize the potential effects of FAZ1 on the flower AZ. The sequencing results revealed that the T-DNA was inserted in the second exon of the FAZ1 locus in this mutant (Fig. 3A). To our surprise, a phenotypic comparison suggested that the abscission of the petals and sepals in the faz1 mutants was delayed around 4–5 days in comparison to that in the wild-type controls (Fig. 3B). To take a closer look, scanning electron microscopy (SEM) images displayed that the boundary between the sepal and torus was smooth in the wild-type controls, whereas it formed a V-shaped structure in the mutants (Figs. 3E and 3F). Additionally, the cell density of this V-shaped structure was around 30% lower than that of the corresponding region in the wild-type controls (Figs. 3C, 3D and 3I). To confirm whether the mutation of (SALK_302_G01) resulted in the mutant phenotype, we performed a complementation experiment by transforming a 1919-bp DNA fragment into the Atfaz1 mutant that contained the cDNA of BoFAZ1. BoFAZ1, the homologous gene of AtFAZ1, after transformation to faz1 showed that the mutant faz1 restored the characteristics of the wild type (Figs. 3C, 3D and 3G−3I).

Figure 3: FAZ1 regulated floral organ abscission in Arabidopsis thaliana. A, Diagram of the genomic region flanking the T-DNA insertion site (left) and genotype faz1 mutants by PCR (right). LP: left primer for FAZ1; RP: right primer for FAZ1; LB1.3: T-DNA left border primer; LP + RP: detection of wild-type (WT); LB1.3+RP: detection of the homozygous faz1 mutant. Actin: Actin of Genome sequence. B, Representative siliques from wild type (WT), faz1 T-DNA insertion mutant (faz1) and complementary experimental plant by Com-bofaz1. Flowers of the WT had abscised, but the mutant flowers delayed abscission. The phenotype was recovered by Com-bofaz1. C-E, Scanning electron microscope (SEM) of floral AZ of WT. (C), faz1 mutants (E) and Com-bofaz1 (G). D, F, H, SEM higher magnification of floral AZ of WT (D), faz1 mutant (F) and Com-bofaz1(H). I, Cell numbers in the AZ of WT control, faz1 mutant plants, and Com-bofaz1 plants. Scale bar: 500 μm
The novel FAZ1 gene may associate with floral organ abscission. This triggered us to study its expression pattern during the flowering of B. oleracea. To this end, the promoter region approximately 1.5 kb upstream of the start codon from B. oleracea was amplified by PCR and fused to the β-glucuronidase (GUS) reporter vector. This construct was inserted into A. thaliana plants. Three independent transgenic lines were obtained and examined to confirm that the GUS expression activity in these lines were consistent with each other. The GUS staining results disclosed a lack of positive signals in the cotyledons, hypocotyls, and roots, indicating that BoFAZ1 was not expressed in these tissues. During the reproduction stage, GUS signals were detected in the mature pollen grains as well as in the filament, stigma, and exfoliation layer (Figs. 4A−4H). Thus, BoFAZ1 was apparently expressed in immature pollen grains, but the expression level gradually decreased as the flower buds developed. In contrast, BoFAZ1 expression levels gradually increased in the filament, stigma, and exfoliation layer.

Figure 4: BoFAZ1 promoter-driven GUS gene expression patterns in transgenic Arabidopsis thaliana. A–B, no GUS activity in seedlings. C, GUS staining in pollen grains. D–F, GUS staining in flowers at developmental stages. G, GUS staining in style and flower AZ. H, Strong GUS staining in flower AZ. Scale bar: (A−F) 500 μm, (G) 100 μm, (H) 1 mm
To further elucidate the subcellular localization of the BoFAZ1 protein, a BoFAZ1: green fluorescent protein (GFP) fusion protein as well as GFP alone (as a control) were transiently expressed in separate A. thaliana protoplasts. The green fluorescence coming from the BoFAZ1:GFP fusion protein was detected in the cell membrane (Fig. 5). Another construct (BoFAZ1d:GFP) was also constructed and transiently transfected into A. thaliana protoplasts for the production of a GFP fusion protein without the seven amino acids, a possible myristoylation site at the N-terminus of BoFAZ1. An examination of A. thaliana protoplasts under fluorescent microscope indicated BoFAZ1d was localized in the cytoplasm, nucleus and cell membrane which was as same as the cellular location of the control GFP protein (Fig. 4). Accordingly, the myristoylation site is likely required for regulating the cellular localization of BoFAZ1.
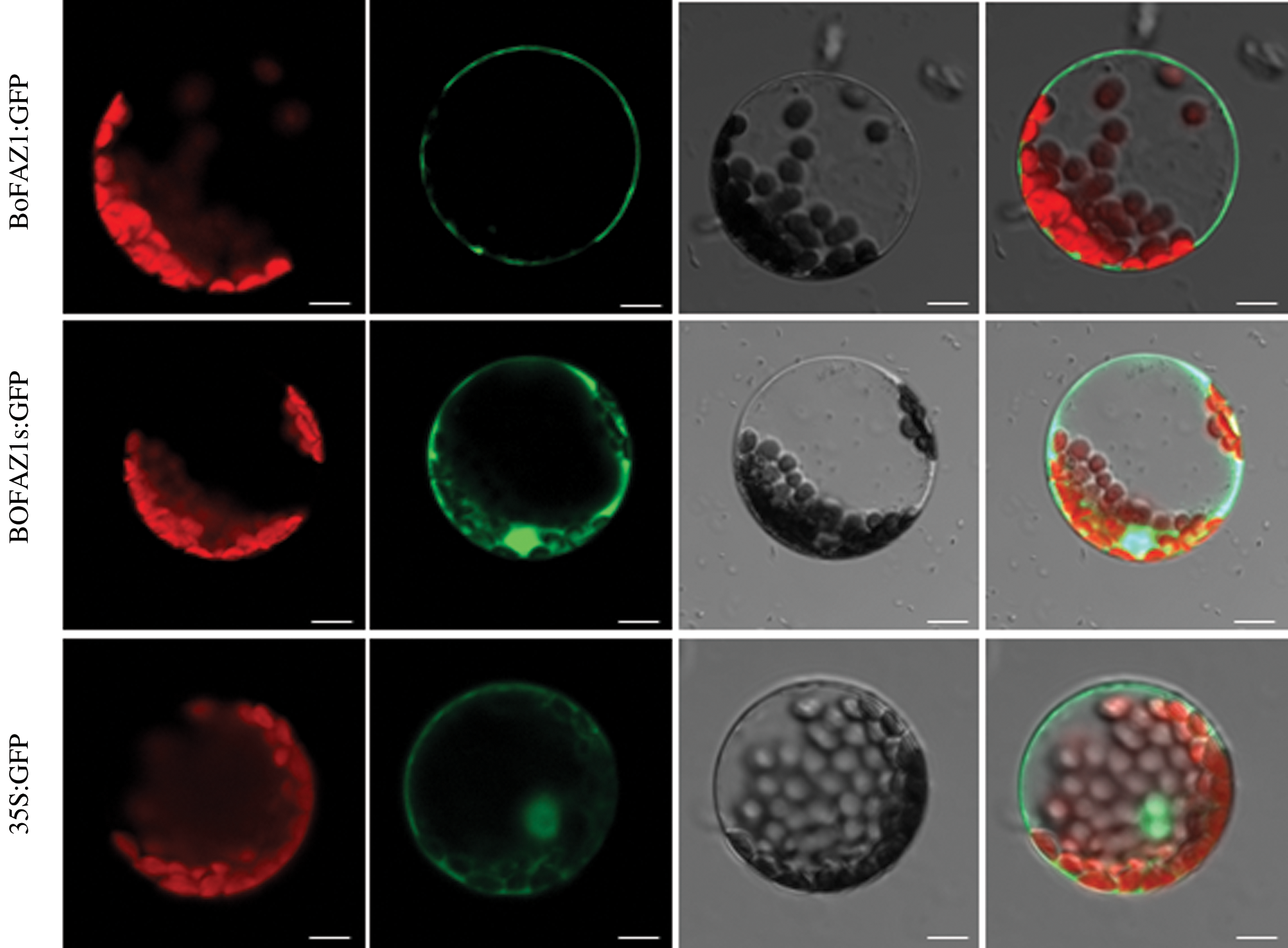
Figure 5: Subcellular localization of BoFAZ1. The BoFAZ1:GFP fusion protein was detected in the cell membrane, whereas the BoFAZ1d:GFP fusion protein was localized in the cytoplasm, nucleus, and cell membrane. The GFP alone (control) was primarily localized in the cytoplasm, nucleus and cell membrane. Chloroplast, chlorophyll auto-fluorescence; GFP:GFP fluorescence; Light: bright light field; Merge: combination of GFP fluorescence, bright light, and chlorophyll auto-fluorescence images. Scale bar, 10 μm
3.5 FAZ1 Interacts with Histone H3.2
To investigate the mechanism by which BoFAZ1 affects the development of the flower AZ, one feasible approach was to examine the possible interacting proteins which may be associated with the flower AZ development or abscission process. To this purpose, a yeast two-hybrid system was applied in this study. FAZ1d sequence was fused to the BD domain sequence of the pGBK-T7 vector to generate the bait plasmid, while floral cDNA was inserted into pGADT7 vector to construct the prey plasmids. The yeast two-hybrid clones screening and DNA sequencing results showed that a clone harboring FAZ1d and BoH3.2 (histone H3.2) can grow on the selection QDO/A medium, whereas no growth was observed in the negative control (Fig. 6). These data suggested that BoFAZ1d can directly interact with the histone protein BoH3.2. To further confirm the interaction between FAZ1and BoH3.2, an in-vitro GST pull-down assay was performed. The BoFAZ1d sequence was fused to expressing vector pGEX4T-1 to generate the GST-BoFAZ1d fusion protein. The BoH3.2 fragment was cloned into another expressing vector pET43.1a to produce the His6-tagged BoH3.2 protein. GST pull-down assay did verify the interaction of BoFAZ1 and BoH3.2 (Fig. 6B). It is known that Histone H3 is one of the key factors responsible for the nucleosome formation. Moreover, the genes encoding histone H3 (H3.1 and H3.2) were highly expressed in the meristematic tissues and abscission region. Therefore, BoFAZ1 may play an important role in floral abscission possibly via interaction with BoH3.2 in the B. oleracea floral organ.
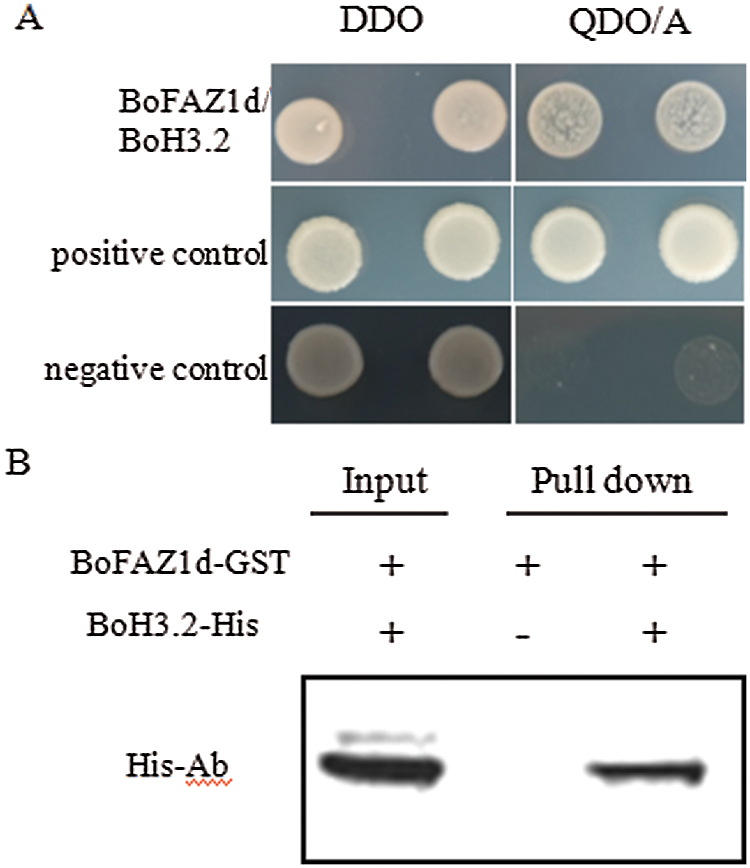
Figure 6: Interaction between BoFAZ1d and BoH3.2. A, Yeast two-hybrid assay for the interaction between BoFAZ1d and BoH3.2. Yeast cells were grown on agar-solidified DDO (SD/-Leu/-Trp) and QDO/A (SD/-Ade/-His/-Leu/-Trp/AbA) media, respectively. Yeast clones were formed on the positive control and cells transfected with BoFAZ1d and BoH3.2, but not the negative control. B, In vitro GST pull-down assay for the interaction of BoFAZ1 and BoH3.2. Purified BoH3.2-His tagged protein was pulled down by BoFAZ1d-GST fusion protein and was detected by western blot analysis using an His- antibody
In this study, we first discovered that the BoFAZ1 may be involved in the floral abscission in B. oleracea likely through the interaction with the histone BoH3.2 protein. As a single copy in A. thaliana and B. oleracea genomes, the membrane protein BoFAZ1 was specifically expressed in reproductive organs including flower AZ accompanied with flowering and subsequent abscission of unwanted organs after pollination and fertilization. This discovery provides a new perspective in the molecular basis of the floral abscission in B. oleracea.
Gubert reported that instead of developing at the sepal base, AZs are usually formed as an inverted V-shape within the proximal regions of the medial and lateral sepals in the A. thaliana as1 mutant [17]. In rice, the APETALA2-like transcription factor SUPERNUMERARY BRACT induces seed shattering by upregulating the qSH1 and SH5 expression levels, while altering the seed size by modulating the longitudinal cell elongation [9]. The delayed abscission of petals and sepals in the A. thaliana faz1 mutants (Fig. 3) as well as our scanning electron microscopy results are consistent with the results of the Gubert et al. study [17]. In the transgenic plants, the phenotype of faz1 was recovered completely by Com-bofaz1, indicating that BoFAZ1 has the same function as AtFAZ1 in Arabidopsis. These results implied that the loss-of-function mutation of FAZ1 delayed the abscission of the flower organ due to changes in the structure of the flower AZ. Consequently, we believe that FAZ1 influences the abscission of flower organs and eventually affects the shedding of fruits, seeds, and stems.
A previous study reported that N-terminal myristoylated proteins have diverse biological effects that influence protein subcellular localization, protein–protein interactions, and signal transduction [26]. Proteins with their myristoylation site modification are generally localized in the plasma membrane. The demyristylation via a myristoylation inhibitor or a mutation to the Gly2 of a myristoylation site prevented the resulting protein from localizing in the cell membrane [27,28]. Recent studies concluded that myristoylation modifications regulate protein stability by modulating the ubiquitination pathway through a changing activity of the c-Src kinase [29]. In the current study, BoFAZ1 was found to localize in the cell membrane, whereas BoFAZ1d, which lack a myristoylation site, was detected in the cytoplasm and nucleus. These results indicated that the supposing myristoylation site did control the cellular localization of BoFAZ1, which is consistent with the known effects of N-myristoylation sites in many proteins.
We observed the interaction between BoFAZ1 and BoH3.2 in yeast two-hybrid and GST pull-down assays. The histone genes H1, H2A, H2B, H3, and H4 are often expressed in eukaryotes, wherein they have important effects on the eukaryotic chromatin and are responsible for the formation of nucleosomes [30]. Additionally, various post-translational modifications of histone H3, such as methylation, acetylation, and phosphorylation, are important for regulating gene transcription. The histone H3 genes (H3.1 and H3.2) are highly expressed in plant meristematic tissues [31]. The H3.2 protein is highly acetylated in meristematic tissues [32]. An earlier investigation of rice proved that H3.2 expression was responsive to abscisic acid (ABA) treatment [33]. Another study revealed that ABA accumulated in the AZ after the floral organ was manually removed in Lupinus luteus [34]. Therefore, H3.2 may affect the development of the AZ through the ABA signaling pathway. The interaction between FAZ1 and H3.2 suggests that FAZ1 may affect the development of the pistil AZ by modulating the ABA signaling pathway via its interaction with H3.2.
BoFAZ1 was first identified in Brassica oleracea. The loss-of-function mutation of FAZ1 delayed the abscission of the flower organ and changes the structure of the flower AZ. In the transgenic plants, the phenotype of faz1 was recovered completely by Com-bofaz1. The possible mechanism may be that BoFAZ1 participates in the formation of the pistil abscission zone through the BoH3.2 (histone H3.2), a protein involved in the abscission zone development. Further studies are needed to find the possible mechanism of the pistil abscission zone in Brassica as noted in the present study.
Author Contributions: ZHC, LXP and ZLQ designed the experiments. ZHC, LXP, ZTH and LQY performed the experiments. ZHC, LXP and ZYZ analyzed the data. HDK and XQQ contributed analysis tools. ZHC and LXP wrote the manuscript. ZHC and ZLQ revised the manuscript. All authors have read and agreed to the submitted version of the manuscript.
Funding Statement: This work was supported by the National Natural Science Foundation of China (31572127), A Special Foundation of Central Institution Basic Research (XDJK2017C032).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Roberts, J. A., Elliott, K. A., Gonzalez, C. Z. H. (2002). Abscission, dehiscence, and other cell separation processes. Annual Review of Plant Biology, 3, 131–158. DOI 10.1146/annurev.arplant.53.092701.180236. [Google Scholar] [CrossRef]
2. Van, N. S. (2009). Development of the abscission zone. Stewart Postharvest Review, 1, 6. DOI 10.2212/spr.2009.1.5. [Google Scholar] [CrossRef]
3. Liljegren, S. J. (2012). Organ abscission: Exit strategies require signals and moving traffic. Current Opinion in Plant Biology, 15, 670–676. DOI 10.1016/j.pbi.2012.09.012. [Google Scholar] [CrossRef]
4. Niederhuth, C. E., Cho, S. K., Seitz, K., Walker, J. C. (2013). Letting go is never easy: Abscission and receptor-like protein kinases. Journal of Integrative Plant Biology, 55, 1251–1263. DOI 10.1111/jipb.12116. [Google Scholar] [CrossRef]
5. Meir, S., Philosoph, H. S., Sundaresan, K. S., Selvaraj, S., Burd, R. et al. (2010). Microarray analysis of the abscission related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiology, 154, 1929–1956. DOI 10.1104/pp.110.160697. [Google Scholar] [CrossRef]
6. Hepworth, S. R., Zhang, Y., McKim, S. X., Haughn, G. W. (2005). BLADE-ON-PETIOLE dependent signaling controls leaf and floral patterning in arabidopsis. Plant Cell, 17, 1434–1448. DOI 10.1105/tpc.104.030536. [Google Scholar] [CrossRef]
7. Jun, J. H., Ma, C. M., Fletcher, J. C. (2010). BLADE-On-pETIOLE1 coordinates organ determinacy and axial polarity in arabidopsis by directly activating ASYMMETRIC LEAVES2. Plant Cell, 22, 62–76. DOI 10.1105/tpc.109.070763. [Google Scholar] [CrossRef]
8. Pinyopich, A., Ditts, G. S., Savidge, B., Liljegren, S. J., Baumann, E. et al. (2003). Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature, 424(6944), 85–88. [Google Scholar]
9. Jiang, L. Y., Ma, X., Zhao, S. S., Tang,Y. Y., Liu, F. X. et al. (2019). The APETALA2-like transcription factor SUPERNUMERARY BRACT3 controls rice seed shattering and seed size. The Plant Cell, 31, 17–36. DOI 10.1105/tpc.18.00304. [Google Scholar] [CrossRef]
10. Adrienne, H. K., Roeder, C. F., Martin, F. (2003). The role of the REPLUMLESS homeodomain protein in patterning the arabidopsis fruit. Current Biology, 9(13),1630–1635. DOI 10.1016/j.cub.2003.08.027. [Google Scholar] [CrossRef]
11. Ciro, N. H., Mora, N. P. (2020). Anatomical and genetic bases underlying convergent evolution of fleshy and dry dehiscent fruits in cestrum and brugmansia (Solanaceae). International Journal of Developmental Biology. DOI 10.1387/ijdb.200080np. [Google Scholar] [CrossRef]
12. Simons, K. J., Fellers, J. P., Trick, H. N., Zhang, Z., Tai, Y. S. et al. (2006). Molecular characterization of the major wheat domestication gene Q. Genetics, 172, 547–555. DOI 10.1534/genetics.105.044727. [Google Scholar] [CrossRef]
13. Debernaridi, J. M., Lin, H. Q., Chuck, G., Faris, J. D., Dubcovsky, J. (2017). microRNA172 plays a crucial role in wheat spike morphogenesis and grain threshablility. Development, 144, 1966–1975. DOI 10.1242/dev.146399. [Google Scholar] [CrossRef]
14. Wang, X. Q., Xu, W. H., Ma, L. G., Fu, Z. M., Deng, X. W. et al. (2006). Requirement of KNAT1/BP for the development of abscission zones in arabidopsis thaliana. Journal of Integrative Plant Biology, 48 (1), 15–26. DOI 10.1111/j.1744-7909.2005.00085.x-i1. [Google Scholar] [CrossRef]
15. Cai, S., Lashbrook, C. C. (2008). Stamen abscission zone transcriptome profiling reveals New candidates for abscission control: Enhanced retention of floral organs in transgenic plants overexpressing arabidopsis ZINC FINGER PROTEIN2. Physiologia Plantarum, 146(3), 1305–1321. DOI 10.1104/pp.107.110908. [Google Scholar] [CrossRef]
16. Li.Y., Pi, L., Huang, H., Xu, L. (2012). ATH1 and KNAT2 proteins act together in regulation of plant inflorescence architecture. Journal of Experimental Botany, 63(3), 1423–1433. DOI 10.1093/jxb/err376. [Google Scholar] [CrossRef]
17. Gubert, M. C., Christy, M. E., Ward, D. L., Groner, D. W., Liljegren, S. J. (2014). ASYMMETRIC LEAVES1 regulates abscission zone placement in arabidopsis flowers. BMC Plant Biology, 14, 195. DOI 10.1186/s12870-014-0195-5. [Google Scholar] [CrossRef]
18. Mao, L., Begum, D., Chuang, H. (2000). JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature, 406(6789), 910–913. DOI 10.1038/35022611. [Google Scholar] [CrossRef]
19. Shi, Z. H., Jiang, Y., Han, X. Q., Liu, X., Cao, R. S. et al. (2017). SlPIN1 regulates auxin efflux to affect flower abscission process. Scientific Reports, 7(1), 1–13. DOI 10.1038/s41598-017-15072-7. [Google Scholar] [CrossRef]
20. Sundaresan, S., Hadas, S. P., Ma, C., Jiang, C. Z., Riov, J. et al. (2018). The tomato hybrid proline-rich protein regulates the abscission zone competence to respond to ethylene signals. Horticulture Research, 5, 28. DOI 10.1038/s41438-018-0033-2. [Google Scholar] [CrossRef]
21. González, Z. H., Rompa, U., Peters, J. L., Bhatt, A. M., Wagstaff, C. et al. (2007). HAWAIIAN SKIRT: An F-box gene that regulates organ fusion and growth in arabidopsis. Plant Physiology, 144, 1370–1382. DOI 10.1104/pp.106.092288. [Google Scholar] [CrossRef]
22. Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25(4), 402–408. DOI 10.1006/meth.2001.1262. [Google Scholar] [CrossRef]
23. Lian, X. P., Zhang, H. C., Zeng, J., Wang, Y. K., Bai, X. J. et al. (2020). C2h2-like zinc finger protein 1 causes pollen and pistil malformation through the auxin pathway. Plant Growth Regulation, 90, 505–518. DOI 10.1007/s10725-019-00568-1. [Google Scholar] [CrossRef]
24. Yoo, S. D., Cho, Y. H., Sheen, J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nature Protocols, 2(7), 1565. DOI 10.1038/nprot.2007.199. [Google Scholar] [CrossRef]
25. Pu, M., Lian, X. P., Luo, S. L., Zhang, H. C., Wang, Y. K. et al. (2018). Transcriptome analysis of stigma after pollination in brassica oleracea var. capitata. Acta Horticulturae Sinica, 45(1), 71–84 (in Chinese). [Google Scholar]
26. Sorek, N., Bloch, D., Yalovsky, S. (2009). Protein lipid modifications in signaling and subcellular targeting. Current Opinion in Plant Biology, 12, 714–720. DOI 10.1016/j.pbi.2009.09.003. [Google Scholar] [CrossRef]
27. Perez, M., Greenwald, D. L., De, L. A., Torre, J. C. (2004). Myristoylation of the RING finger Z protein is essential for arenavirus budding. Journal of Virology, 78, 11443–11448. DOI 10.1128/JVI.78.20.11443-11448.2004. [Google Scholar] [CrossRef]
28. Strecker, T., Maisa, A., Daffis, S. (2006). The role of myristoylation in the membrane association of the lassa virus matrix protein Z. Virology Journal, 3, 93. DOI 10.1186/1743-422X-3-93. [Google Scholar] [CrossRef]
29. Patwardhan, P., Resh, M. D. (2010). Myristoylation and membrane binding regulate c-src stability and kinase activity. Molecular and Cellular Biology, 30, 4094–4107. DOI 10.1128/MCB.00246-10. [Google Scholar] [CrossRef]
30. Takahashi, S., Osabe, K., Fukushima, N., Takuno, S., Miyaji, N. et al. (2018). Genome-wide characterization of DNA methylation, small RNA expression, and histone H3 lysine nine di-methylation in brassica rapa L. DNA Research, 25(5), 511–520. DOI 10.1093/dnares/dsy021. [Google Scholar] [CrossRef]
31. Kaludov, K. N., Seavy, M., Robinson, G., Hurt, M. M. (1997). A mouse histone H1 variant, H1b, binds preferentially to a regulatory sequence within a mouse H3.2 replication-dependent histone gene. The Journal of Biological Chemistry, 272(24), 15120–15127. DOI 10.1074/jbc.272.24.15120. [Google Scholar] [CrossRef]
32. Martina, H., Konrad, B. (2012). Transcription in the absence of histone H3.2 and H3k4 methylation. Current Biology, 22, 2253–2257. DOI 10.1016/j.cub.2012.10.008. [Google Scholar] [CrossRef]
33. Qiu, S. P., Huang, J., Pan, L. J., Wang, M. M., Zhang, H. S. (2006). Salt induces expression of RH3.2A, encoding an H3.2-type histone H3 protein in rice (Oryza sativa L.). Acta Genetica Sinica, 33(9), 833–840. DOI 10.1016/S0379-4172(06)60117-0. [Google Scholar] [CrossRef]
34. Wilmowicz, E., Frankowski, F., Kuckoa, A., Nowakowska, A., Kopcewicz, J. (2016). The influence of abscisic acid on the ethylene biosynthesis pathway in the functioning of the flower abscission zone in lupinus luteus. Journal of Plant Physiology, 206, 49–58. DOI 10.1016/j.jplph.2016.08.018. [Google Scholar] [CrossRef]
Appendix

 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |