
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015617
ARTICLE
Comparison of Morphological and Anatomical Characteristics of Taxus chinensis var. mairei Seedlings Root under Waterlogging Stress in Different Substrates
College of Horticulture and Gardening, Yangtze University, Jingzhou, 434025, China
*Corresponding Author: Die Hu. Email: hudie.16@163.com
Received: 31 December 2020; Accepted: 01 March 2021
Abstract: Four different ratios of river sand, ceramic pellets, vermiculite and perlite (1:1), and field soil were selected as the substrates in this experiment, and four gradient levels of root waterlogging, half waterlogging, full waterlogging and normal were set to investigate the effects of different gradients of waterlogging stress on the root morphology of Taxus chinensis var. mairei seedlings under different substrates. In this study, the root anatomical structure of Taxus chinensis var. mairei under different waterlogging stress was observed by the paraffin section method. The roots of T. chinensis var. mairei were diarch, with no pith and resin canals. There was a large number of tannins in the pericycle of the aerial adventitious roots of seedlings adapted to waterlogging. Also, the endodermis has obvious casparian strip thickening, and there were 4-5 layers of large parenchymatous cells in the close to the inner side of the pericycle in the vascular cylinder, which could increase the storage capacity, and transport capacity of the root. Under the treatment of root waterlogging stress, the development of plant roots in the mixed substrate of vermiculite and, perlite was the earliest. Under half waterlogging stress, T. chinensis var. mairei seedlings treated with various substrates all could better adapt to the environment of waterlogging stress. Under the stress of fully waterlogging, the roots of seedlings planted in river sand substrate developed secondary growth.
Keywords: Waterlogging stress; Taxus chinensis var. mairei; root morphological; anatomy
In recent years, floods have occurred frequently in southern China and the Yangtze River basin, especially in summer, increased rainfall leads to higher underground water level, which further makes Taxus chinensis var. mairei vulnerable to water stress. The root system is an important organ for absorbing water and mineral nutrients, and it is also the first place where plants experience soil stress. The anatomical structure of the root system is a direct reflection of root system development level [1].
T. chinensis var. mairei is an evergreen tree of the Taxus genus in the Taxus family, a relict tree species of the Tertiary period, and one of the main source plants of the new anti-cancer natural drug of paclitaxel [2,3]. T. chinensis var. mairei can also uptake harmful substances from the air, and at the same time secrete alkaloids into the air to exert a role of disinfection and sterilization. At present, there have been some studies on the morphological anatomy of Taxus plants. For instance, Bobrov studied Seed morphology and anatomy of austrotaxus spicata (taxaceae) and its systematic position [4], and Vessella discussed Morphological and molecular data from Madeira support the persistence of an ancient lineage of Taxus baccata L. in macaronesia and call for immediate conservation actions [5].
T. chinensis var. mairei belong to slowly growing, shallow-rooted plant sand affected by waterlogging. The taproot of T. chinensis var. mairei is not obvious, and its lateral roots developed but not stout. The aim of the study was to describe the morphological and anatomical structure changes within the root system of T. chinensis var. mairei in response to waterlogging stress. Thus, three levels of water stress treatments of root waterlogging (half, full and control) in different substrates were designed to study. Our study can provide atheoretical basis for T. chinensis var. mairei cultivation, especially in southern regions and low altitude areas where waterlogging conditions occur.
The experimental materials for this study were two-year-old T. chinensis var. mairei seedlings in the greenhouse of the College of Horticulture and Gardening, Yangtze University, Jingzhou City (30°21′N, 112°8′E) in Hubei Province, China. The environment has subtropical monsoon climate with mean rainfall of 1100 mm–1300 mm. Annual mean temperature is 15.9°C–16.6°C, and annual sunshine duration is 1800 h–2000 h. Annual frost period is 242–263 days. The day/night light cycle was 16 h/8 h, temperature was 25°C/20°C, and relative humidity was 70% in the greenhouse.
Four substrates were used in this experiment. The comparison of the four substrates is shown in Tab. 1. The substrates were selected from the pastoral soil of the Jianghan Plain as a control, and compared with several substrates with different moisture retention properties and relatively stable structure, to study the morphological performance of T. chinensis var. mairei seedlings in response to water stress in different substrates.

2.3 Experimental Design and Methods
The T. chinensis var. mairei seedlings were planted in glass containers (8 cm in caliber and 18 cm in height), respectively. After one month of normal moisture management under greenhouse conditions, with robust growth, disease-free, and consistent in growth were waterlogged, with 30 plants for each treatment. Repeated three times for each treatment.
A two-factor randomized block design was adopted. Sixteen treatments were applied. Factor 1 was different substrate treatments: (1) river sand; (2) ceramsite; (3) vermiculite and perlite (in a ratio of 1:1); (4) pastoral soil. Factor 2 was different waterlogging treatments, according to the method of plant water gradient division, as follows: root waterlogging (saturated soil water content, the water level was flush with the substrate, simulating water damage hazard in the natural state, W1); half waterlogging (the water surface was about 2 cm higher than the substrate, simulating a higher water level, which was belonging to mild waterlogging stress, W2); full waterlogging (water content of the substrate is too saturated, the water surface is about 4 cm higher than the substrate, simulating waterlogging, W3), normal (control, grown normally in pastoral soil without water stress treatment, W4). The day/night temperature was 25°C/20°C during experiment. Replenish water daily with a measuring cylinder to the specified water level. At 90 days of waterlogging, ten plants were taken from each treatment to observe root morphology.
Slices were prepared by paraffin sectioning, and then the anatomical structure of the root system under an optical microscope were observed. Briefly, the same aerial adventitious roots in each treatment group and normal roots without water stress were collected, and then the root tissues were cut into 0.5–1 mm long sections and fixed in FAA solution. Next, the conventional paraffin section method used to make slices. The fixed sections were further cut into 10 µm thick slices, then stained with saffron-fast green double staining, followed by alcohol graded dehydration, transparent with xylene, and sealed with Canadian gum [6]. Finally, the slices were observed, and, photographed by OlympusDp70 bio-research level microscope [7,8].
3.1 Effects of Root Waterlogging on the Structure of Adventitious Roots of T. chinensis var. mairei Seedlings in Different Substrates
We dissected and observed the adventitious root cross-section of the seedlings of T. chinensis var. mairei in the root waterlogging treatment group. The cross-section of the roots under each substrate treatment was roughly circular or nearly elliptical, and the structure was the same as that of soil-grown roots. The root systems of the seedlings in each substrate treatment group were all diarch, with two primary xylem bundles, and various ergastic substance were stored in the cells. In the treatment group without water stress, the cross-section of soil-grown roots was irregular in shape. The root system began to develop an incomplete secondary structure, with a thick epidermis layer, irregular and alternate arrangement of cortical parenchyma cells, large cell volume, and orderly and tight arrangement of endodermis cells in strips. In the vascular cylinder, there were two layers of parenchyma cells immediately inside the pericycle, and the approximately square primary xylem remains in the center of the root. At the same time, it was accompanied by the occurrence of lateral roots, which originate from the pericycle (Figs. 1(J)–(L), Figs. 2(J)–(L)).
In the river sand substrate group, the primary structure of adventitious roots was clearly visible. The epidermis consisted of a layer of approximately square parenchyma cells, which were arranged neatly and tightly, and the stratum cuticle was thin. The cortex occupied most of the cross-section of aerial adventitious roots. The exodermis was composed of two layers of neatly arranged and compacted cells, and the endodermis was thickened with casparian strip. The pericycle was a layer of parenchyma cells, and in the vascular cylinder next to pericycle inside, there were observed 4–5 layers of large parenchyma cells, which were approximately round or irregular and obviously larger than other cells. In the structure of cortex and vascular cylinder, most cells contained ergastic substance (Figs. 1(A)–(C), Figs. 2(A)–(C)).In the ceramsite substrate group, the root system was exhibited primary structure, and the proportion of cortical parenchyma cells was roughly equal to that of the river sand substrate group, but the arrangement of parenchyma cells was more orderly and regular, and the parenchyma cells volume and intercellular space were respectively smaller and slightly larger than that of the river sand substrate group. The endodermis also had a thickening of casparian strip, and the vascular cylinder was composed of 3–4 layers of parenchyma cells immediately adjacent to the pericycle (Figs. 1(D)–(F), Figs. 2(D)–(F)). In the mixed substrate group of vermiculite and perlite, the root system had appeared secondary growth and could develop into vascular cambium. The cortical parenchyma cells were large in size and incomplete in shape, and all the parenchyma cells were connected (Figs. 1(G)–(I), Figs. 2(G)–(I)).
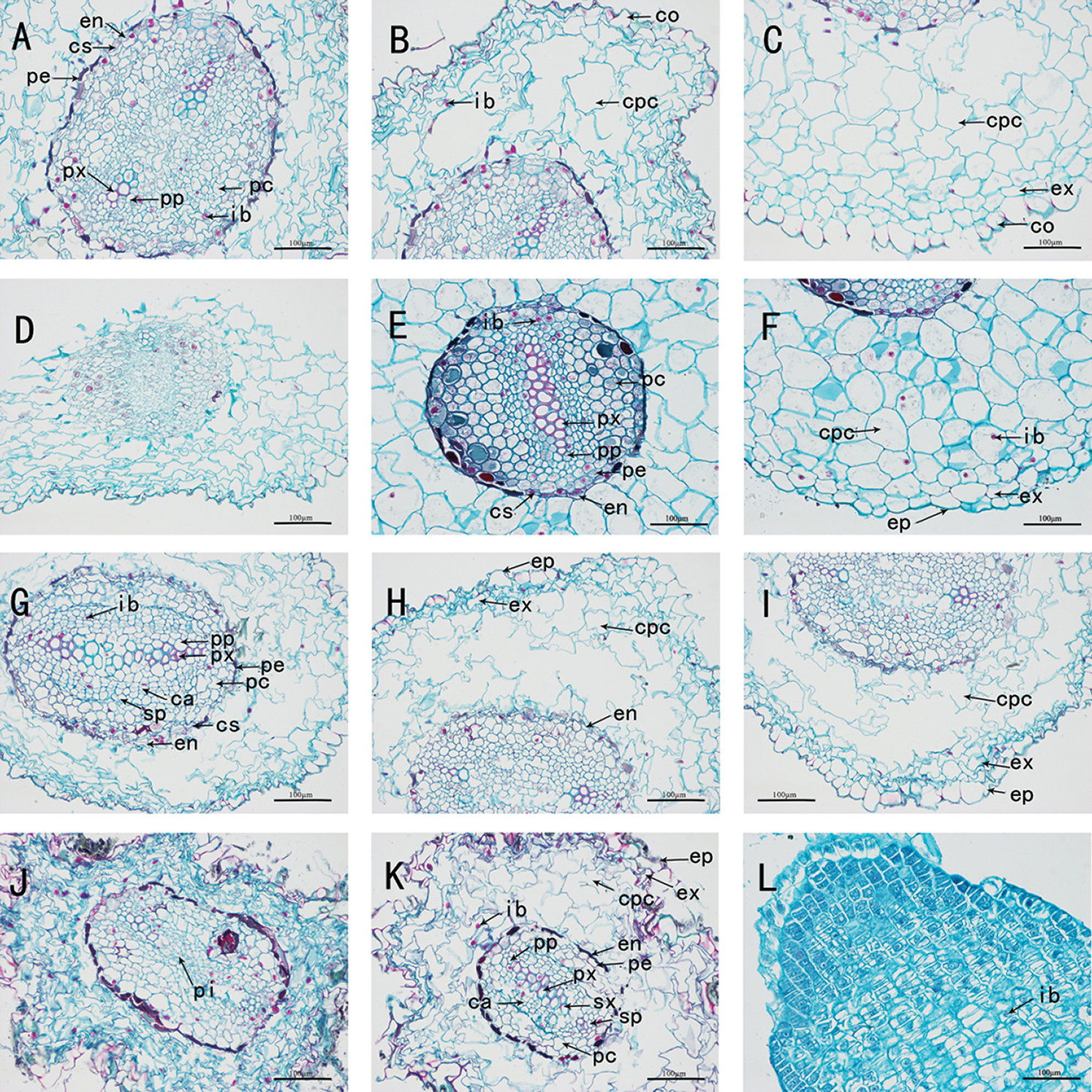
Figure 1: The anatomy of adventitious root of Taxus chinensis var. mairei seedlings grown in different substrates (the river sand substrate (A–B–C), ceramsite (D–E–F), vermiculite and perlite mixed matrix (G–H–I)) within root waterlogging and control conditions (J–K–L) (200 x)). En: endodermis, Cs: Casparian strip, Pe: pericycle, Px: primary xylem, Pp: primary phloem, Ib: inclusion, Pc: parenchyma cell, Co: cortex, Cpc: cortical parenchyma cell, Ex: exodermis, Ep: epidermis, Ca: cambium, Sp: secondary phloem, Pi: pith, Sx: secondary xylem
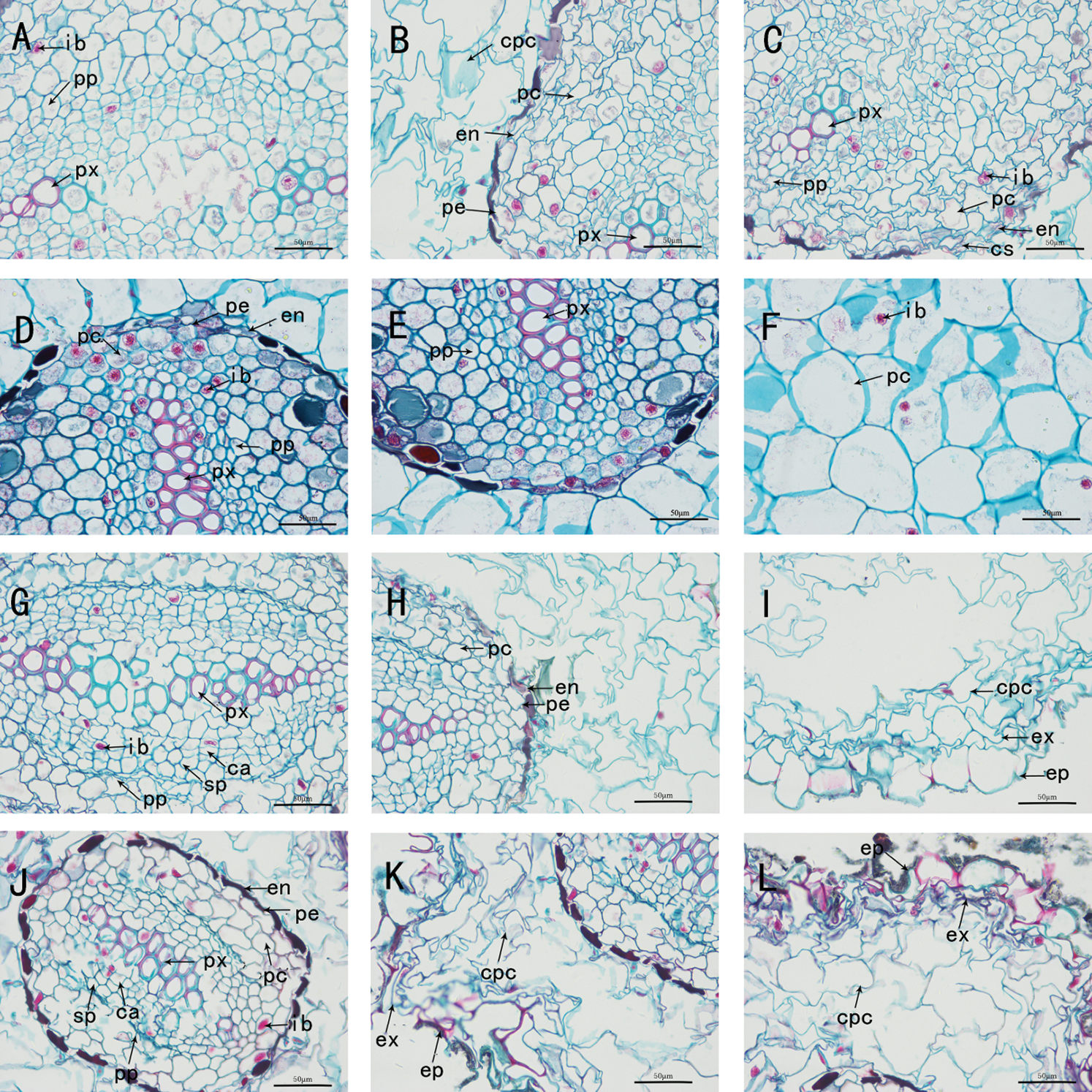
Figure 2: The anatomy of adventitious root of Taxus chinensis var. mairei seedlings grown in different substrates (the river sand substrate (A–B–C), ceramsite (D–E–F), vermiculite and perlite mixed matrix (G–H–I)) within root waterlogging and control conditions (J–K–L) (400 x)). Ib: inclusion, Pp: primary phloem, Px: primary xylem, Cpc: cortical parenchyma cell, Pc: parenchyma cell, En: endodermis, Pe: pericycle, Cs: Casparian strip, Ca: cambium, Sp: secondary phloem, Ex: exodermis, Ep: epidermis
3.2 Effects of Half Waterlogging on the Structure of Adventitious Roots of T. chinensis var. mairei Seedlings in Different Substrates
We dissected and observed the adventitious root cross-section of the seedlings of Taxus chinensis var. mairei in the half waterlogging treatment group. In the group of river sand substrate, vermiculite and perlite mixed substrate, the adventitious roots of T. chinensis var. mairei seedlings all began to occur secondary growth, but the growth and development were still in the early stage. The roots had two primary xylem bundles, both of which were diarch, and various ergastic substance were observed in the cells of each group (Figs. 3(A)–3(C), 3G, 3H, 3L). The anatomical structure of the roots in the ceramsite substrate group showed the characteristics of primary structure, which was roughly the same as the root structure under root waterlogging stress (Figs. 3(D)–3(F)).
In the river sand substrate group treated with half waterlogging, the roots began to grow secondary structure. Vascular cambium not only observed secondary xylem, cambium, and secondary phloem, but also observed the phloem ray in secondary phloem, and xylem ray in the secondary xylem. These structures can play a role in lateral transport and storage functions when plants are subjected to waterlogging stress. In addition, the proportion of parenchyma cells adjacent to the pericycle in the vascular cylinder was reduced, compared with root waterlogging stress. The circumferential diameter was expanded when they undergone the cambium cells carry out the periclinal division and the anticlinal division. The activity of the vascular cambium caused the roots constantly thickened and their positions constantly shifted outward, produced into secondary phloem outward and inwardly differentiated into secondary xylem (Figs. 3(A)–3(C), Figs. 4(A)–4(C)). Under half-flood stress, the root structure in the mixed substrate group of vermiculite and perlite was the same as that under the root waterlogging stress. The root system began to secondary growth, while it was slightly earlier than that under the root waterlogging stress. The fact was that the division speed was not identical in each part of cambium ring, the concave cells of the wavy cambium were produced forward with the fast division speed, and that the speed of inward differentiation into secondary xylem cells was greater than that of outward differentiated into secondary phloem cells. Notably, the number of cell divisions in each place was the same. Thus, the concave part of the wavy ring was slowly pushed to the outside, so that the entire cambium was in the shape of a ring (Figs. 3G, 3H, 3L and Figs. 4G, 4H, 4L). In the ceramsite substrate group, the cross-section of the root showed an irregular shape, which was the same as the root structure of in the root waterlogging treatment group, and the adventitious roots also exhibited a primary structure. (Figs. 3(D)–3(F), Figs. 4(D)–4(F)).
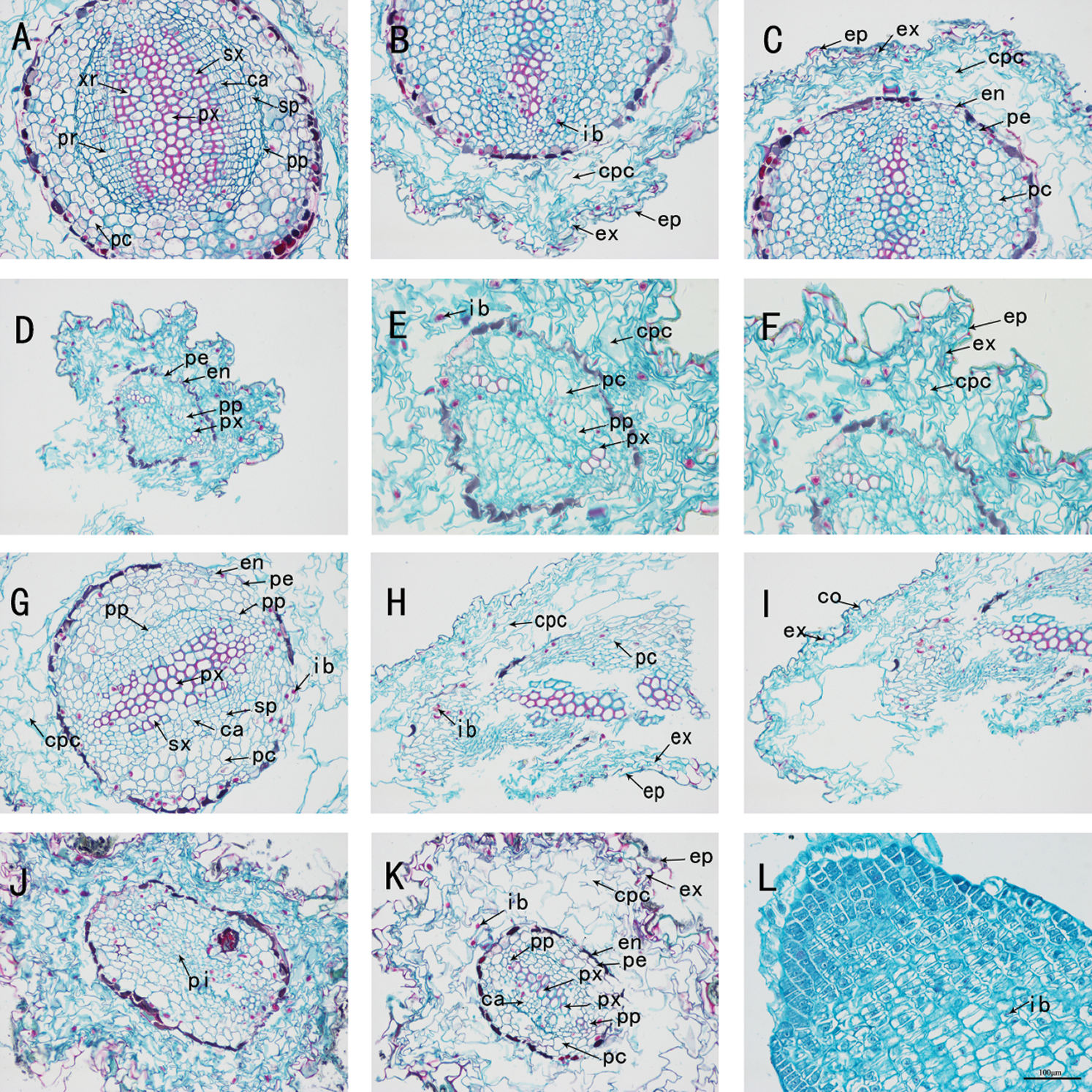
Figure 3: The anatomy of adventitious root of Taxus chinensis var. mairei seedlings grown in different substrates (the river sand substrate (A–B–C), ceramsite (D–E–F), vermiculite and perlite mixed matrix (G–H–I)) within half waterlogging and control conditions (J–K–L) (200 x)). Xr: xylem ray, Pr: phloem ray, Sx: secondary xylem, Px: primary xylem, Pc: parenchyma cell, Ca: cambium, Sp: secondary phloem, Pp: primary phloem, Ib: inclusion, Cpc: cortical parenchyma cell, Ex: exodermis, Ep: epidermis, En: endodermis, Pe: pericycle, Co: cortex, Pi: pith
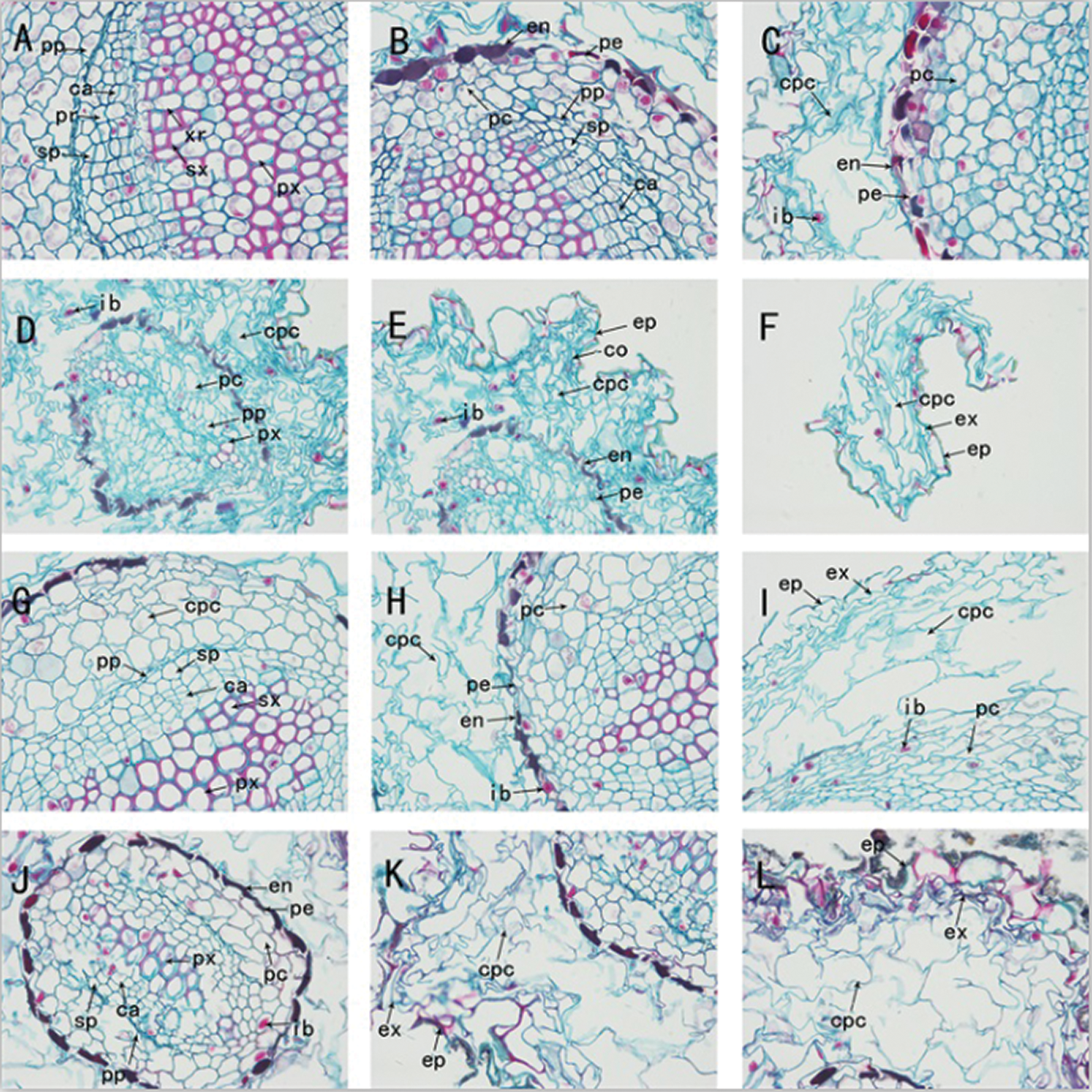
Figure 4: The anatomy of adventitious root of Taxus chinensis var. mairei seedlings grown in different substrates (the river sand substrate (A–B–C), ceramsite (D–E–F), vermiculite and perlite mixed matrix (G–H–I)) within half waterlogging and control conditions (J–K–L) (400 x)). Pp: primary phloem, Ca: cambium, Pr: phloem ray, Sp: secondary phloem, Xr: xylem ray, Sx: secondary xylem, Px: primary xylem, En: endodermis, Pe: pericycle, Pc: parenchyma cell, Cpc: cortical parenchyma cell, Ib: inclusion, Co: cortex, Ex: exodermis, Ep: epidermis
3.3 Effects of Full Waterlogging on the Structure of Adventitious Roots of T. chinensis var. mairei Seedlings in Different Substrates
We dissected and observed the adventitious root cross-section of the seedlings of T. chinensis var. mairei in the full waterlogging treatment group. Under the treatment of full waterlogging stress, the root structure of the adventitious roots of T. chinensis var. mairei seedlings in the river sand substrate group began to occurrence secondary growth, while the secondary growth of root in the vermiculite and perlite mixed substrate group was not obvious, but in the ceramsite substrate group, the root structure still characterized by the primary structure. Compared with the half-flooded treatment, the primary structure of adventitious roots in the ceramsite substrate group under the full waterlogging treatment was clearer and more complete (Figs. 5(D)–5(F)). The root cross-sections of each group were roughly round or nearly ellipse, and the roots were all diarch, and the cell structure was also roughly the same, but the degree of development was different.
In the anatomical structure of the root tissue of the river sand substrate group, the epidermis, cortex, vascular cylinder and vascular cambium can be observed. The proportion of parenchyma cells in the cortex was larger in the full waterlogging treatment which was consisting of 7–8 layers of cortical parenchyma cells, compared with half-flooded stress. Also, there were 5–6 layers of parenchyma cells in the vascular cylinder adjacent to pericycle, thereby the proportion was also greater than the half-flooded stress treatment group. In addition, although the roots have shown the characteristics of forming secondary structures, the development of secondary growth of the roots was slightly later than that of the half-flooded stress treatment group (Figs. 5(A)–5(C), Figs. 6(A)–6(C)). In the ceramsite substrate group, compared to half-flooded stress treatment, the cortical parenchyma cells were arranged more neatly and tightly, with fewer gaps, and a greater amount of ergastic substances were contained in the pericycle close to the endodermis (Figs. 5(D)–5(F), Figs. 6(D)–6(F)).In the mixed substrate group of vermiculite and perlite, the parenchyma cells of the cortex were arranged orderly and regularly. Different from the root structure under half-flooded stress, the root structure was in the primary growth stage under full-flooded stress. The tannins stained blue could be clearly observed in the pericycle, and 2-3 layers of parenchymal cells have existed in the vascular cylinder (Figs. 3(G)–3(I)–6(G)–6(I)).
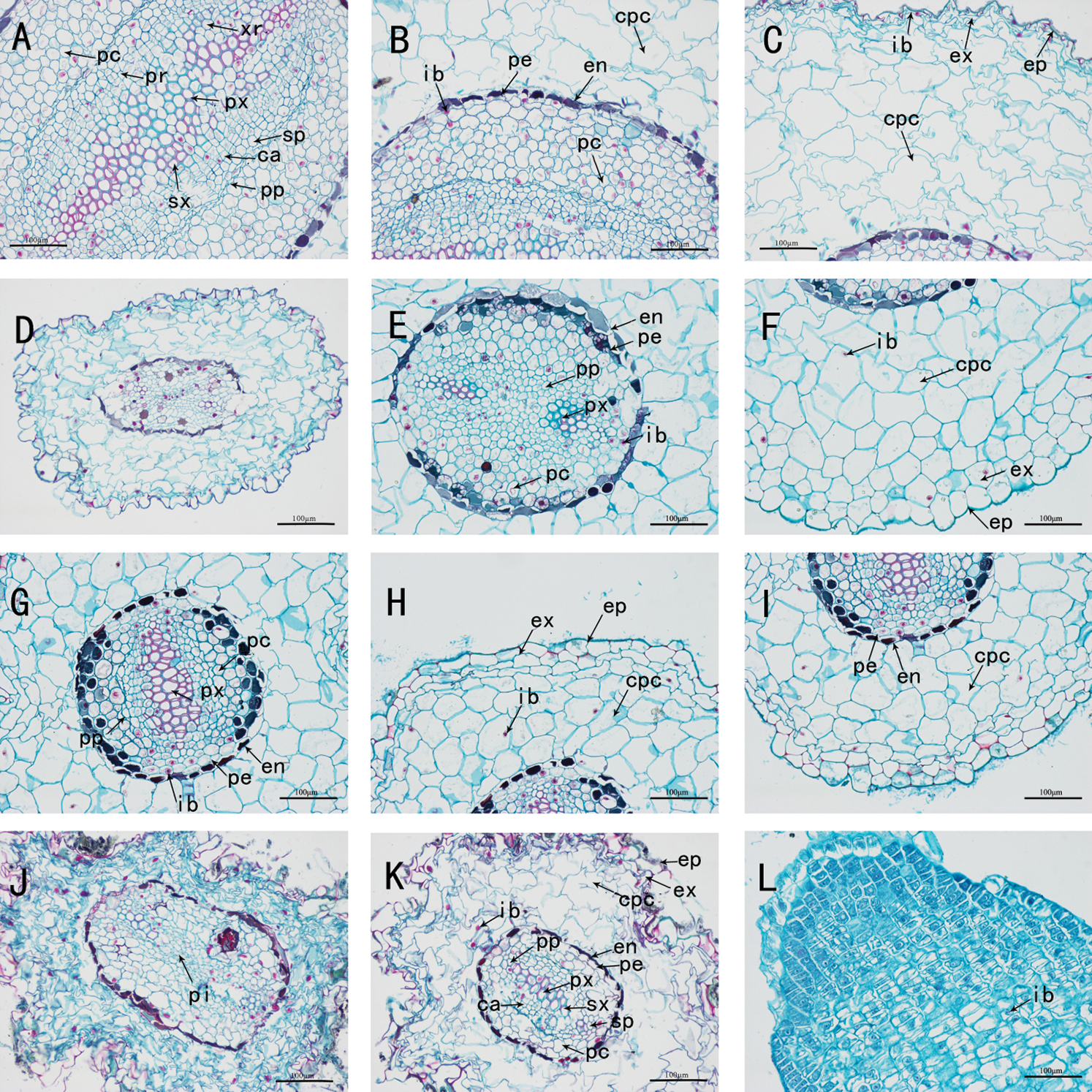
Figure 5: The anatomy of adventitious root of Taxus chinensis var. mairei seedlings grown in different substrates (the river sand substrate (A–B–C), ceramsite (D–E–F), vermiculite and perlite mixed matrix (G–H–I)) within full waterlogging and control conditions (J–K–L) (200 x)). Xr: xylem ray, Pc: parenchyma cell, Pr: phloem ray, Px: primary xylem, Sp: secondary phloem, Ca: cambium, Pp: primary phloem, Sx: secondary xylem, Ib: inclusion, Pe: pericycle, En: endodermis, Cpc: cortical parenchyma cell, Ex: exodermis, Ep: epidermis, Pi: pith
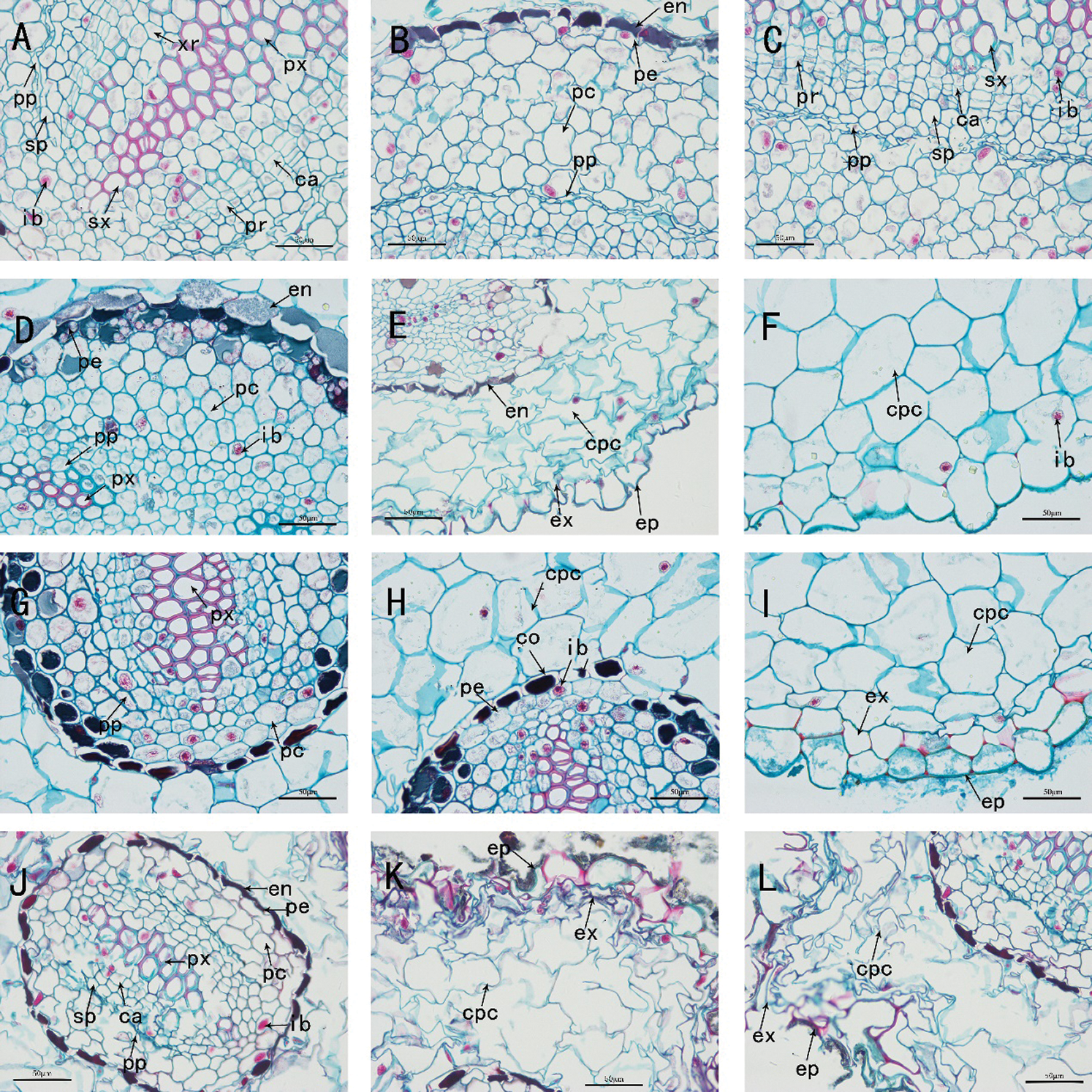
Figure 6: The anatomy of adventitious root of Taxus chinensis var. mairei seedlings grown in different substrates (the river sand substrate (A–B–C), ceramsite (D–E–F), vermiculite and perlite mixed matrix (G–H–I)) within full waterlogging and control conditions (J–K–L) (200 x)). Pp: primary phloem, Sp: secondary phloem, Ib: inclusion, Xr: xylem ray, Sx: secondary xylem, Px: primary xylem, Ca: cambium, Pr: phloem ray, En: endodermis, Pe: pericycle, Pc: parenchyma cell, Cpc: cortical parenchyma cell, Ex: exodermis, Ep: epidermis, Co: cortex
The primary structure of aerial adventitious roots of T. chinensis var. mairei consists of epidermis, cortex and vascular cylinder, which is the same as the root structures of other seed plants [9,10]. The difference is that there is almost no pith in the primary structure of aerial adventitious roots of T. chinensis var. mairei, and exists 4–5 layers of large parenchyma cells in the inner side of the pericycle. The anatomical structure of the aerial adventitious roots of T. chinensis var. mairei reported by Li, showed that there were 2–4 layers of large parenchyma cells in the vascular cylinder immediately inside the pericycle [11]. These indicating that the large parenchyma cells inside the pericycle were conducive to enhancing the water storage capacity of aerial adventitious roots under water-logging stress. Compared with the species of genus Cathaya in the Pinaceae which is also a gymnosperm, the biggest difference is that the aerial adventitious roots of T. chinensis var. mairei belong to diarch and no resin canals [12]. The roots of Cathaya are diarch-triarch with resin canals distribution, which are the same as the results of Xu et al. [13]. Aerenchyma transports and stores oxygen for the hypoxic part of the plant in a water-wet environment. Under waterlogging stress, some plant roots will produce aerenchyma, the primary root of two species of poplars that developed aerenchyma under the stress of waterlogging, indicating that poplar was a waterlogging tolerant tree [14]. In this study, the T. chinensis var. mairei did not form a structure similar to the aerenchyma, indicating that the T. chinensis var. mairei was not resistant to waterlogging, which was the same as the conclusion of Baek [15].
Waterlogging stress seriously affects the metabolism of plants [16]. Many plants are inhibited in growth after being flooded and die in severe cases. However, many plants also adapt to the environment of waterlogging by altering their morphological, physiological and biochemical indicators [17–19]. In this study, under the treatment of waterlogging stress, to adapt to the water flooded environment, the seedlings of T.chinensis var. mairei grew adventitious roots that contained a lot of tannins in the pericycle, which could partly avoid the water shortage inside the roots. Li et al. [11] also observed abundant tannins in the epidermal cells of the stems and leaves of T. chinensis var. mairei in Yuanbaoshan Nature Reserve in northwest Guangxi. Studies have found that tannins could largely resist waterlogging stress [20,21]. Moreover, the endodermis of the adventitious roots of T. chinensis var. mairei seedlings has an obvious thickening of the casparian strip, which prevents the horizontal transport of water and solutes directly through the cell wall and intercellular space into the vascular cylinder. Thus, water and solutes must pass through the selective permeability membrane of endodermis cells to reach into the protoplast, and then into the vascular cylinder, thereby protecting the vascular cylinder [22,23].
When plants encounter a changeable climate or soil environment, they will autonomously adjust their behavior, activate different genes, and produce different physiological and biochemical metabolic systems to adapt to environmental changes and ensure the survival and reproduction of their species, which is the ecological adaptability of plants [24,25]. Nutrient elements and water supply in the substrate soil play an important role in plant productivity [26,27]. In this study, under three different waterlogging stress conditions, the development degree of plant roots in each substrate soil was different. Compared with the underground roots treated without waterlogging stress: Under root waterlogging stress, the root structure of the vermiculite and perlite mixed substrate group was roughly the same as the underground root structure, and the degree of cell differentiation was slightly earlier than that of the river sand and ceramsite substrate group; Under half-flooded treatment, except for the ceramsite group, the root systems of the other groups showed a certain tolerance and were able to adapt to the environment of half-waterlogging stress; Under the full-flooded stress, the secondary growth and secondary structure of root system in the river sand substrate group were weaker than the normal underground roots. In general, under half-flooded stress, the T. chinensis var. mairei seedlings in each substrate treatment group could better adapt to the flooded stress environment and tolerate waterlogging adversity to a certain extent. It was well established that appropriate spaces in plant organs retain oxygen under hypoxia and anoxia stresses [28,29].
In summary, the cell structure and development characteristics of the adventitious roots of T. chinensis var. mairei seedlings are rough as follows: (1) The roots of T. chinensis var. mairei were diarch, with no pith and resin canals. (2) Abundant tannins in pericycle of aerial adventitious roots adapted to waterlogging. (3) Waterlogging stress caused thickening of casparian bands of T. chinensis var. mairei. (4) Large parenchymatous cells formed in the vascular cylinder under waterlogging stress. (5) Mix of vermiculite and perlite promoted roots development under waterlogging stress.
Funding Statement: This work was supported by the National Natural Science Foundation of China [Grant No. 31270740].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Kummerova, M., Zezulka, S., Babula, P., Vanova, L. (2013). Root response in pisum sativum and zea mays under fluoranthene stress: Morphological and anatomical traits. Chemosphere, 90(2), 665–673. DOI 10.1016/j.chemosphere.2012.09.047. [Google Scholar] [CrossRef]
2. Zhang, Q., Liu, H., Sun, G., Wilson, I. W., Wu, J. et al. (2015). Baseline survey of root-associated microbes of Taxus chinensis (Pilger) Rehd. PLoS One, 10(3), e0123026. DOI 10.1371/journal.pone.0123026. [Google Scholar] [CrossRef]
3. Fei, Y. J., Tang, W. (2018). Effects of endogenous indole-3-acetic acid and acetic acid and polyamines on adventitious root formation of Taxus chinensis Lemee et levl. var. mairei. Propagation of Ornamental Plants, 18(2), 64–74. [Google Scholar]
4. Bobrov, A. V. F. C., Melikian, A. P., Romanov, M. S., Sorokin, A. N. (2004). Seed morphology and anatomy of Austrotaxus spicata (Taxaceae) and its systematic position. Botanical Journal of the Linnean Society, 145(4), 437–443. DOI 10.1111/j.1095-8339.2004.00285.x. [Google Scholar] [CrossRef]
5. Vessella, F., Simeone, M. C., Fernandes, F. M., Schirone, A., Gomes, M. P. et al. (2013). Morphological and molecular data from madeira support the persistence of an ancient lineage of taxus baccata L. in macaronesia and call for immediate conservation actions. Caryologia: International Journal of Cytology, Cytosystematics and Cytogenetics, 66(2), 162–177. [Google Scholar]
6. Yang, J. P. (2006). Improvement of traditional paraffin section preparation methods. Journal of Biology, (1), 45–46. [Google Scholar]
7. Zhang, X., Yang, C. D., Seago, J. L. (2018). Anatomical and histochemical traits of roots and stems of Artemisia lavandulaefolia and A. selengensis (Asteraceae) in the Jianghan plain, China. Flora, 239(3), 87–97. DOI 10.1016/j.flora.2017.11.009. [Google Scholar] [CrossRef]
8. Yang, C. D., Yang, X. L., Zhang, X., Zhou, C. Y., Zhang, F. et al. (2019). Anatomical structures of alligator weed (Alternanthera philoxeroides) suggest it is well adapted to the aquatic-terrestrial transition zone. Flora, 253, 27–34. DOI 10.1016/j.flora.2019.02.013. [Google Scholar] [CrossRef]
9. Yang, C. D., Zhang, X., Zhou, C. Y., Seago, Jr, J. L. (2011). Root and stem anatomy and histochemistry of four grasses from the Jianghan Floodplain along the Yangtze River China. Flora, 206(7), 653–661. DOI 10.1016/j.flora.2010.11.011. [Google Scholar] [CrossRef]
10. Uwe, D., Philipp, F., Hajirezaei, M. R. (2016). Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Frontiers in Plant Science, 7(133), 381. [Google Scholar]
11. Li, F. Y., Liang, S. C. (2013). Anatomical structure and environmental adaptability of Taxus chinensis var. mairei in Yuanbaoshan. Plants of Guangxi, (2), 80–85. [Google Scholar]
12. Liu, M. (2004). Introduction to morphological anatomy of seed plants, pp. 140–142. Beijing: Science and Technology Press. [Google Scholar]
13. Xu, B., Zhu, T., Peng, Z. H. (2012). Research on the distribution of resin canals of cedrus deodara based on wood anatomy. Applied Mechanics and Materials, 246–247, 1079–1084. DOI 10.4028/www.scientific.net/AMM.246-247.1079. [Google Scholar] [CrossRef]
14. Chen, Y. L., Du, K. B., Jiang, F. X., Peng, Y. J., Tu, B. K. et al. (2015). Influences of waterlogging stress on cell structure of primary roots of two poplar species. Scientia Silvae Sinicae, 51(3), 163–169. [Google Scholar]
15. Baek, J. S., Chung, N. J. (2012). Seed wintering and deterioration characteristics between weedy and cultivated rice. Rice, 5(1), 97. DOI 10.1186/1939-8433-5-21. [Google Scholar] [CrossRef]
16. Bashar, K. K., Tareq, M. Z., Islam, M. S. (2020). Unlocking the mystery of plants’ survival capability under waterlogging stress. Plant Science Today, 7(2), 142–153. DOI 10.14719/pst.2020.7.2.663. [Google Scholar] [CrossRef]
17. Lin, K. H., Kuo, W. S., Chiang, C. M. (2013). Study of sponge gourd ascorbate peroxidase and winter squash superoxide dismutase under respective flooding and chilling stresses. Scientia Horticulturae, 162(3), 333–340. DOI 10.1016/j.scienta.2013.08.016. [Google Scholar] [CrossRef]
18. Yin, X., Komatsu, S. (2015). Quantitative proteomics of nuclear phosphoproteins in the root tip of soybean during the initial stages of flooding stress. Journal of Proteomics, 119(4), 183–195. DOI 10.1016/j.jprot.2015.02.004. [Google Scholar] [CrossRef]
19. Yu, B., Zhu, J., Zhou, G. L., Li, Z. Y., Peng, Y. Q. (2018). Effects of waterlogging stress on growth and adventitious roots anatomical structure in cucumber seedling. Northern Horticulture, 42(3), 61–64. [Google Scholar]
20. Xiao, B., Jespersen, D. (2019). Morphological and physiological responses of seashore paspalum and bermudagrass to waterlogging stress. Journal of the American Society for Horticultural Science, 144(5), 305–313. DOI 10.21273/JASHS04737-19. [Google Scholar] [CrossRef]
21. Phukan, U. J., Mishra, S., Shukla, R. K. (2016). Waterlogging and submergence stress: Affects and acclimation. Critical Reviews in Biotechnology, 36(5), 956–966. DOI 10.3109/07388551.2015.1064856. [Google Scholar] [CrossRef]
22. Naseer, S., Lee, Y., Lapierre, C., Franke, R., Nawrath, C. et al. (2012). Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proceedings of the National Academy of Sciences of the United States of America, 109(25), 10101–10106. DOI 10.1073/pnas.1205726109. [Google Scholar] [CrossRef]
23. Peng, Y. Q., Zhu, J., Li, W. J., Huang, Y., Li, Y. Y. et al. (2019). Effects of waterlogging stress on growth, physiological characteristics and anatomical structure of adventitious roots of grafted Momordica charantia seedlings. Journal of plant physiology, 55(6), 756–766. [Google Scholar]
24. Xu, W. Z., Zhu, L. X., Zhao, G. (2006). Application of plant ecological adaptability in hydroponics. Subtropical Plant Science, 35, 28–32. [Google Scholar]
25. Barrios-Masias, F. H., Hernandez-Espinoza, L. H. (2020). Physiological and anatomical changes in tomato roots in response to low water stress. Scientia Horticulturae, 265(19), 109208–109216. DOI 10.1016/j.scienta.2020.109208. [Google Scholar] [CrossRef]
26. Wu, D., Xu, X., Chen, Y., Shao, H., Sokolowski, E. et al. (2019). Effect of different drip fertigation methods on maize yield, nutrient and water productivity in two-soils in Northeast China. Agricultural Water Management, 213, 200–211. DOI 10.1016/j.agwat.2018.10.018. [Google Scholar] [CrossRef]
27. Mandre, M., Lukjanova, A. (2011). Biochemical and structural characteristics of scots pine (pinus sylvestris L.) in an alkaline environment. Estonian Journal of Ecology, 60(4), 264–283. DOI 10.3176/eco.2011.4.02. [Google Scholar] [CrossRef]
28. Sachs, M. (2008). Plant anaerobic stress ii. strategy of avoidance of anaerobiosis and other aspects of plant life under hypoxia and anoxia. Plant Stress, 2(1), 1–19. [Google Scholar]
29. Colmer, T. D. (2003). Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment, 26(1), 17–36. DOI 10.1046/j.1365-3040.2003.00846.x. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |