
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015822
ARTICLE
Potentiality of Different Seed Priming Agents to Mitigate Cold Stress of Winter Rice Seedling
1Agro Innovation Laboratory, Department of Agronomy, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
2International Rice Research Institute, Bangladesh Office, Dhaka, 1213, Bangladesh
3Rice Research and Training Center, Field Crops Research Institute, Agricultural Research Center, Sakha, 33717, Egypt
4Department of Biology, College of Science, Taif University, Taif, 21944, Saudi Arabia
5Bangladesh Wheat and Maize Research Institute, Dinajpur, 5200, Bangladesh
6Department of Agronomy, Faculty of Agriculture, Kafrelsheikh University, Kafr Elsheikh, 33516, Egypt
*Corresponding Authors: Md. Parvez Anwar. Email: parvezanwar@bau.edu.bd; Ayman EL Sabagh. Email: ayman.elsabagh@agr.kfs.edu.e.g
Received: 16 January 2021; Accepted: 29 March 2021
Abstract: Seed priming has proved to be an effective pre-germination seed invigoration technique for different crops to improve seed and seedling performance under different abiotic stresses. In Bangladesh, winter rice is very often exposed to cold waves just after sowing in the nursery bed resulting in poor seed germination and seedling emergence, yellowish and thin seedlings production, and a very low survival rate. Seed priming may mitigate the cold stress during seed germination and seedling emergence and helps in the quality seedling production of winter rice. To evaluate the efficacy of different seed priming techniques in increasing seedling emergence, growth, vigor and survivability of winter rice cultivars under cold stress, a pot experiment was conducted at the Department of Agronomy, Bangladesh Agricultural University during December 2018 to January 2019. The experiment comprised two factors, (A) Winter rice variety namely, i) BRRI dhan29 and ii) BRRI dhan36; (B) Seed priming agent namely i) Control (no priming), ii) 20000 ppm NaCl, iii) 30000 ppm NaCl, iv) 20000 ppm KCl, v) 30000 ppm KCl, vi) 20000 ppm CaCl2, vii) 30000 ppm CaCl2, viii) 50 ppm CuSO4, ix) 75 ppm CuSO4, x) 10000 ppm ZnSO4, xi) 15000 ppm ZnSO4, xii) 2 ppm Na2MoO4, xiii) 3 ppm Na2MoO4, xiv) 100 ppm PEG (Polyethylene glycol 4000) and xv) 150 ppm PEG. Seeds were sown on two different dates viz., 1st December and 1st January so that seedlings are exposed to cold stress at different stages. The experiment was laid out in a completely randomized design (CRD) with three replications. Results indicated that (in most of the cases) seed priming has a positive impact on seedling emergence rate (%), root length, shoot length, root shoot ratio, root dry weight, shoot dry weight, seedling dry weight and survival rate (%). Among the priming agents, KCl and CaCl2 performed best; while priming with NaCl and PEG showed no advantages over no priming for both the sowing dates. In general, BRRI dhan36 performed better than BRRI dhan29 in terms of seedling growth because of its higher tolerance to cold stress. But, both the varieties performed similarly in terms of emergence rate and survival rate. Thus, priming is an effective tool to increase seed germination, better seedling growth, and higher seedling survivability of winter rice under cold stress, and KCl (20000 ppm) or CaCl2 (20000 ppm) can be considered as a viable priming agent.
Keywords: Seed invigoration; seedling emergence; seedling mortality; seedling growth; low temperature stress
Rice is grown in tropical and sub-tropical areas of the world, and is often threatened by low temperature stress. Rice plant is sensitive to chilling stress especially at the seedling and reproductive stages. Low temperature at an early stage may result in reduced germination, poor seedling establishment, stunted seedlings, yellowing or withering, and reduced tillering [1]. Unforeseeable cold stress (<10°C) at the reproductive stage delays heading and causes pollen sterility resulting in grain yield reduction [2]. This stress arrests the expression of full genetic potential of a rice variety through direct inhibition of metabolic reactions and, indirectly through cold-induced osmotic, oxidative and other stresses [3]. Under the sub-tropical condition of Bangladesh, winter rice is greatly affected by low temperature stress during crop establishment and reproductive stages [1]. Due to extreme low temperatures in December and January, the seedling mortality in nursery beds, as well as main field, often goes up to 90%, especially in the northern part of the country [4]. Therefore, farmers often cover the nursery beds with plastic sheet on cold nights to protect the young seedlings from low temperature and cold injury. Because of low temperature, seedling growth is often slow and farmers usually transplant relatively old seedlings (>45 days) to protect from cold injury [1]. Recently, more than 2.0 million hectares of rice crop in northern and north-eastern parts of Bangladesh have been affected by acute cold spell causing partial to total crop damage [4]. In the haor (wet-land ecosystem) areas of Bangladesh, farmers need to early transplanting of rice to protect the crop from the flash floods during the maturity stage and early-planted winter rice has often face low temperature at the reproductive stages (panicle initiation to flowering) [5]. As stated by Smillie et al. [6], photosynthesis is damaged by low temperature which limits growth and yield as there is less carbohydrate available for seed production.
Seed priming has proved to be an effective seed invigoration technique that might be resulted in increase seed performance by increasing the rate, percentage, and uniformity of seed germination and seedling emergence under stress conditions (e.g., salinity, temperature and drought stress). Usually, seed priming is used to curtail the duration between seed sowing and seedling emergence and to synchronize emergence [7,8]. Priming allows some of the metabolic processes essential for germination to occur. In this process seeds are soaked in different solutions with high osmotic potential that inhibits the seeds from absorbing sufficient moisture for radicle protrusion, thus suspending the seeds in the lag phase [9]. Beneficial effects of seed priming include enhanced, rapid and uniform emergence of seedlings [10–14], which leads to crops growing faster, flowering earlier and yielding higher [15,16]. Besides, seed priming enhances the uptake of nutrients and controls seed-borne pathogens [8]. A number of researches on vegetables, floriculture and some field crops demonstrated that seed priming has effectively improved germination and early seedling growth especially at suboptimal conditions [13,17]. Generally, various seed priming techniques, which include hydropriming, osmopriming, chemical priming, hormonal priming, biological priming, redox priming, and solid-matrix priming, are used to induce pre-germination changes [13,18,19]. Hydro priming increased the yield as a result of better germination and vigorous growth in mung bean [20], lentils [21] and in rice [22] under flooding stress, whereas, improved germination was also observed in polyethylene glycol-6000 (PEG-6000) primed rapeseeds [14].
Although, seed priming has been extensively studied as a tool for boosting up seed germination and seedling growth of different crop species including rice, but this issue has not been properly addressed so far especially with winter rice in sub-tropical conditions. Therefore, research is needed on this particular issue to increase the yield of rice in a sustainable way. The present study was therefore undertaken to evaluate the efficacy of different seed priming agents in increasing seed emergence, seedling growth, vigor and survivability of winter rice under cold stress and to assess the rice varietal responses to different seed priming agents under cold stress.
2.1 Experimental Site and Soil
The pot experiment was conducted at the Net House and Agro Innovation Laboratory, Department of Agronomy, Bangladesh Agricultural University during December 2018 to January 2019. The experimental site was situated at 23°77’ N latitude and 90°33’ E longitude at an altitude of 18.6 meter above sea level. The experimental soil was slightly acidic in nature (pH 6.8) with silty loam texture and low organic matter content (1.59%). The general fertility level of the soil was moderate (0.13% N, 13.9 ppm available phosphorus, 16.3 ppm available potassium and 0.28 ppm exchangeable potassium). The climate of the experimental site is characterized by scanty rainfall associated with moderately low temperatures during the experimental period. Average relative humidity in December 2018 and January 2019 were recorded 80.2 and 75.3%, respectively. Monthly total rainfall and cumulative sunshine hours during December and January were recorded as 17.7 and 2.13 mm and 201.7 and 228.4 hrs, respectively. The weekly mean temperature during the experimental period has been presented in Fig. 1.

Figure 1: Weekly average maximum, minimum and mean temperature in December 2018 and January 2019
2.2 Experimental Treatments and Design
The experiment comprised two factors. Factor A includes two high yielding winter rice varieties, namely i) BRRI dhan29 and ii) BRRI dhan36; factor B includes 15 seed priming agents such as i) Control (no priming), ii) 20000 ppm NaCl, iii) 30000 ppm NaCl, iv) 20000 ppm KCl, v) 30000 ppm KCl, vi) 20000 ppm CaCl2, vii) 30000 ppm CaCl2, viii) 50 ppm CuSO4, ix) 75 ppm CuSO4, x) 10000 ppm ZnSO4, xi) 15000 ppm ZnSO4, xii) 2 ppm Na2MoO4, xiii) 3 ppm Na2MoO4, xiv) 100 ppm PEG (Polyethylene glycol) and xv) 150 ppm PEG. The experiment was laid out in a completely randomized design (CRD) with three replications. Altogether there were 90 pots in the experiment. Plastic pot of size of 25 cm height and 22 cm diameter was used as the experimental unit.
2.3 Description of the Rice Varieties
BRRI dhan29: This variety was developed by the Bangladesh Rice Research Institute (BRRI) in 1980 from crossing between BG 90-2 and BR15-46-5 lines. It is a long duration and late harvest rice variety. It is characterized with weakly photoperiod sensitivity suitable for late transplanting among modern varieties in Bangladesh. It is a non-lodging type of rice variety and it takes about 160–165 days to complete its life cycle after transplanting with the average yield range of 7–8 t ha−1 [23].
BRRI dhan36: BRRI dhan36 was also developed by BRRI from the line IR 54791-19-2-3 (IRRI) and was released in 1998. Generally, the plant height is about 90 cm, clean rice, long slender. Planting time is mid to late November. Harvesting time is early to mid-April. General yield is 5–6 t ha−1. It is moderately resistant to tungro, tolerant to cold at the seedling stage [23].
All the priming agents used in the experiment were of laboratory grade. Details of the priming agents are presented in Tab. 1
Table 1: Description of the priming agents

2.5 Conduction of the Experiment
Seeds of both the rice varieties were soaked in different priming agents solution (previously prepared using distilled water) as per treatments for 24 h at room temperature, (25 ± 2°C). The ratio of seed weight to solution volume was 1:5 (g mL−1). Then, seeds were removed from the priming agent solution followed by washing several times with distilled water to remove the traces of chemicals. Then, seeds were dried back to the original moisture content by forced air. Dried seeds were put in polythene bags and stored in a refrigerator at 5 ± 1°C until used. While control treatment received no prior seed priming.
Both the primed (stored in refrigerator) and non-primed seeds were soaked in water for 24 h and then taken out of water and kept thickly in cloth bags. The seeds started sprouting after 48 h and were sown in the pot after 72 h.
2.5.3 Pot Preparation, Seed Sowing and Management
Each pot was filled up to 3/4th with 4.6 kg soil. Cowdung @ 100 g pot−1 was added and thoroughly mixed with the soil. Water was added and puddled manually to make a favorable condition for sowing seed. No chemical fertilizer was added to the pot soil. Sprouted seeds were sown in the pot @ 300 seeds pot−1. Seeds were sown on two different dates viz., 1 December and 1 January so that seedlings are exposed to cold stress at different stages. The seeds were protected from the birds after sowing. Weeds were manually uprooted when observed. Watering was done by a watering can as and when necessary to ensure the normal growth of the seedlings. Every pot was regularly monitored to record any insect pest or disease infestation. But no occurrence of insects or diseases was recorded.
Data were collected on emergence rate (%), survival rate (%), root and shoot length, root and shoot dry weight and seedling dry weight. For recording data on seedling growth, 20 seedlings were randomly uprooted from each pot at 10, 20 and 30 days after sowing (DAS). The emergence percentage was calculated at 15 DAS using the following formula [22]:
Root length was measured from the base of the plant up to the end of the longest root and expressed in mm. Shoot length was measured from the base of the plant up to the tip of the longest leaf and expressed in mm. For root and shoot dry weight, 20 randomly selected seedlings were measured after separately drying the root and shoot in an oven at 60°C for 72 h. Finally root and shoot dry weight of each seedling was calculated and expressed in mg. The dry weight of the seedling was calculated as the sum of root and shoot dry weight and expressed in mg. The survival percentage is the ratio of the number of survived seedlings (at 30 DAS) to the number of seedlings that emerged (at 15 DAS) and expressed as a percent. It was calculated by the following formula:
The recorded data were undergone to homogeneity and normality checked prior to analysis of variance (ANOVA). The results of the homogeneity and normality test revealed that the data were normally distributed. ANOVA was done to test for statistical significance among treatments with the help of the computer package MSTAT-C. The mean differences among the treatments were adjudged using Duncan’s Multiple Range Test (DMRT) at a 5% level of significance.
Variety had no significant effect on the emergence percentage of winter rice at both the sowing dates (Tab. 2). But numerically, BRRI dhan36 showed a higher emergence percentage compared to BRRI dhan29. Tab. 2 also shows that for both the varieties, the emergence percentage was higher in December sowing compared to January sowing. The priming agent exerted a significant effect on the emergence percentage of winter rice at both sowing dates (Tab. 3). In general, at both the sowing dates seed priming showed a positive effect on the emergence percentage. Priming with NaCl at any concentration reduced the emergence percentage. On the other hand, priming with 3 ppm Na2MoO4 resulted in a similar emergence percentage as shown in control. While all other priming agents increased the emergence percentage of winter rice compared to control (no priming). Irrespective of priming agents, December sowing showed a higher emergence percentage than January sowing.
Table 2: Effect of variety on seedling emergence (%) and survival (%) of winter rice at different sowing dates

Table 3: Effect of seed priming agent on emergence (%) and survival (%) of winter rice at different sowing dates

3.2 Seedling Survival Percentage
Variety showed no significant effect on the survival percentage of winter rice seedlings at both the sowing dates (Tab. 2). Numerically, BRRI dhan36 showed a higher survival percentage compared to BRRI dhan29. Like the emergence percentage, the survival percentage also was higher in December sowing than January sowing. A significant effect of priming agent was found for the survival percentage of winter rice seedlings at both the sowing dates (Tab. 3). A clear advantage of seed priming in terms of seedling survival percentage over no priming was evident only for priming with KCl, CaCl2, and CuSO4 at December sowing and KCl, CaCl2, CuSO4 and ZnSO4 at January sowing. It may be noted that December sowing showed a higher survival percentage than January sowing.
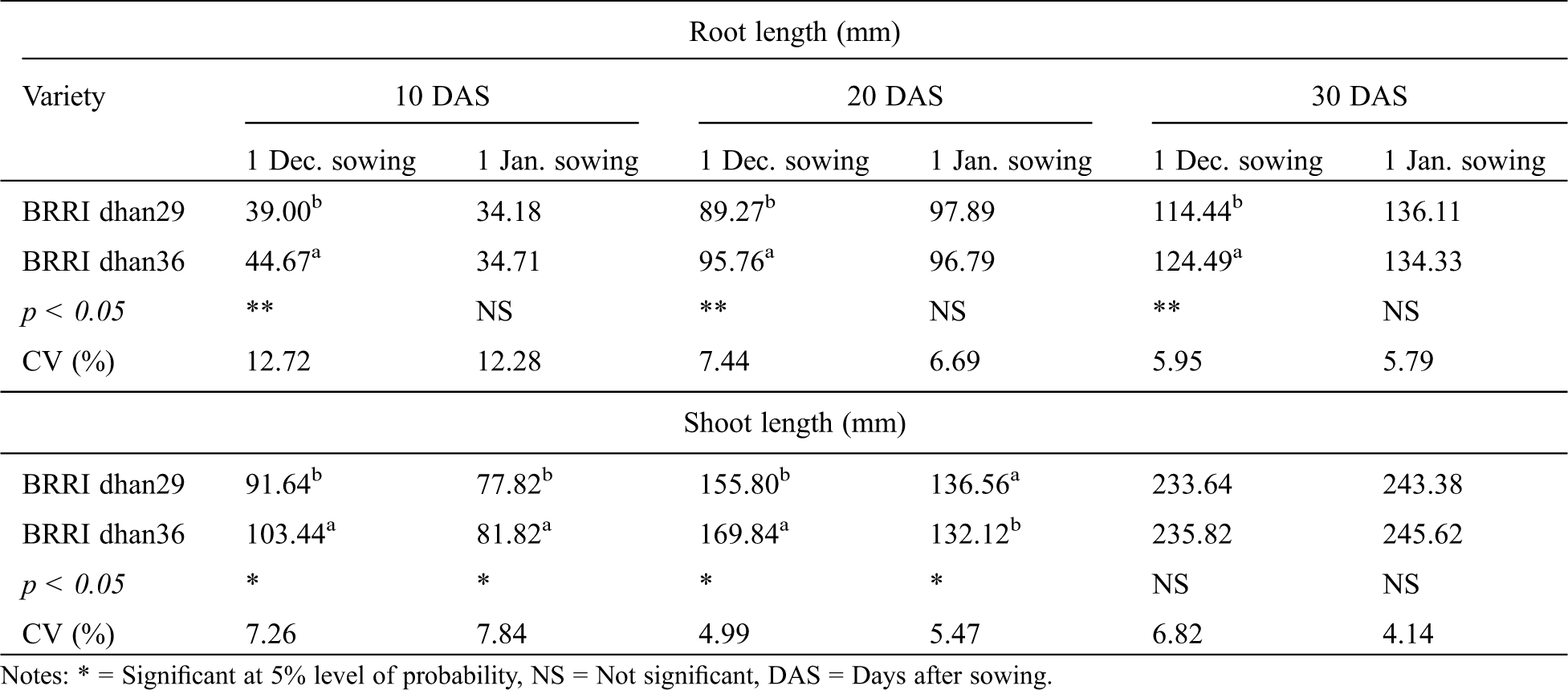
Varietal effect on seedling root length was found significant in December sowing but non-significant in January sowing at all the 3 observations (Tab. 4). In December sowing, seedling root length of BRRI dhan36 was respectively 5, 6 and 10 mm higher than those of BRRI dhan29 at 10, 20 and 30 days after sowing (Tab. 4).
Table 4: Effect of variety on root length and shoot length of winter rice seedlings at different sowing dates
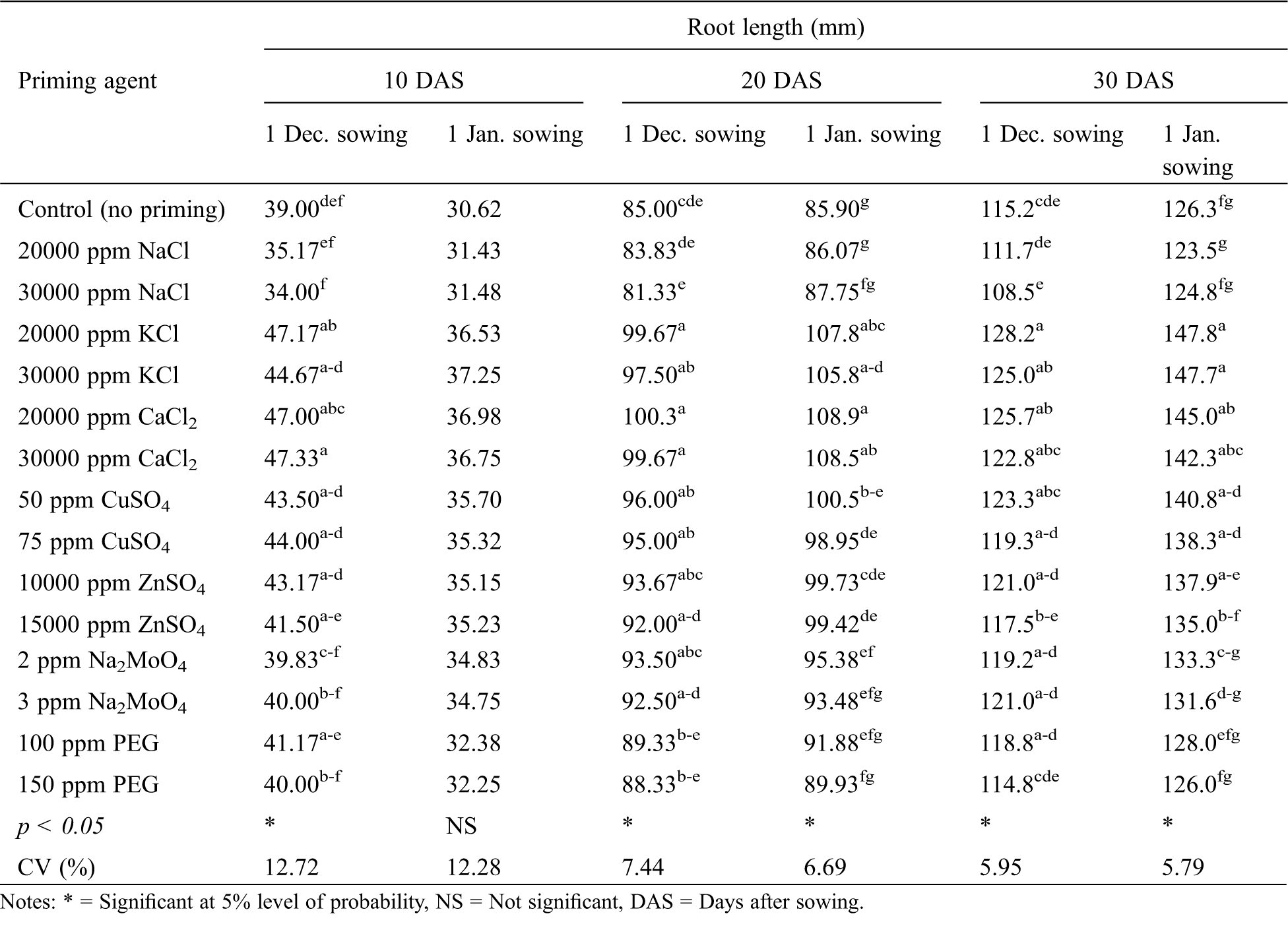
The priming agent exerted a significant effect on the root length of winter rice seedlings at all the observations for both the sowing dates except at 10 DAS for January sowing (Tab. 5). Seed priming with KCl or CaCl2 at either concentration resulted in the longest root at all the growth stages for both the sowing dates. In general, seed priming with NaCl or PEG produced no advantages in terms of root length, over no priming treatment. On the other hand, seed priming with CuSO4 or ZnSO4 produced a higher root length compared to no priming (Tab. 5).
Table 5: Effect of seed priming agent on root length of winter rice seedlings at different sowing dates
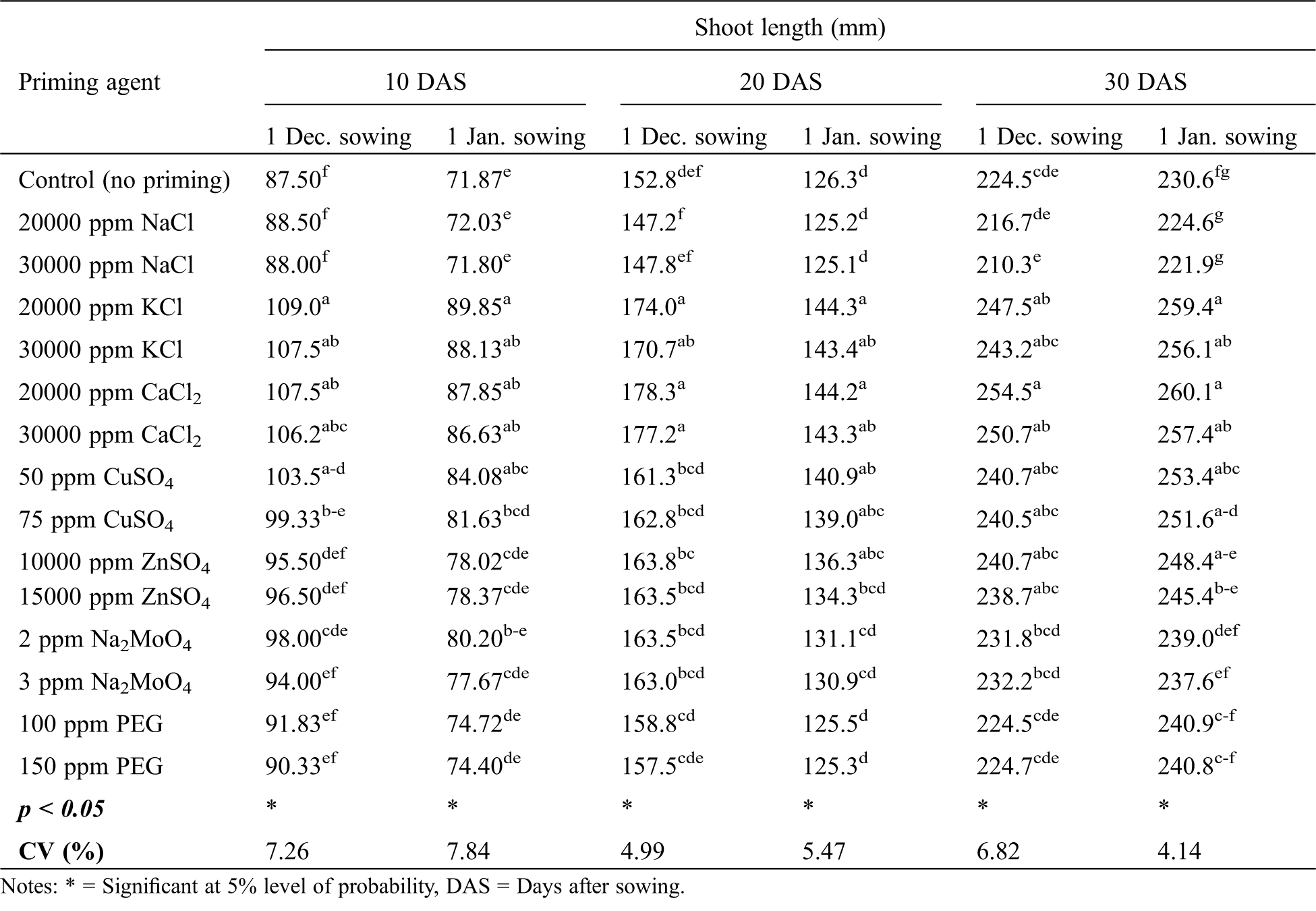
A significant effect of variety on seedling shoot length of winter rice was observed only at early stages (10 and 20 DAS), but not at later growth stage i.e., 30 DAS (Tab. 4). In case of December sowing, BRRI dhan36 performed better than BRRI dhan29 at 10 and 20 DAS. But for January sowing, BRRI dhan36 produced a longer shoot than BRRI dhan29 at 10 DAS; while at 20 DAS, BRRI dhan29 produced a longer shoot than BRRI dhan36 (Tab. 4). Seedling shoot length of winter rice was significantly affected by different seed priming agents at both the sowing dates (Tab. 6). In general, seed priming with KCl or CaCl2 performed the best in terms of seedling shoot length at all the growth stages for both the sowing dates. Seed priming with CuSO4 or ZnSO4, on the other hand, produced the longest shoot only at 20 and 30 DAS compared to control. No advantages of seed priming with NaCl, Na2MoO4 and PEG were evident as all those priming agents performed similarly to control in terms of seedling shoot length of winter rice (Tab. 6).
Table 6: Effect of seed priming agent on shoot length of winter rice seedlings at different sowing dates
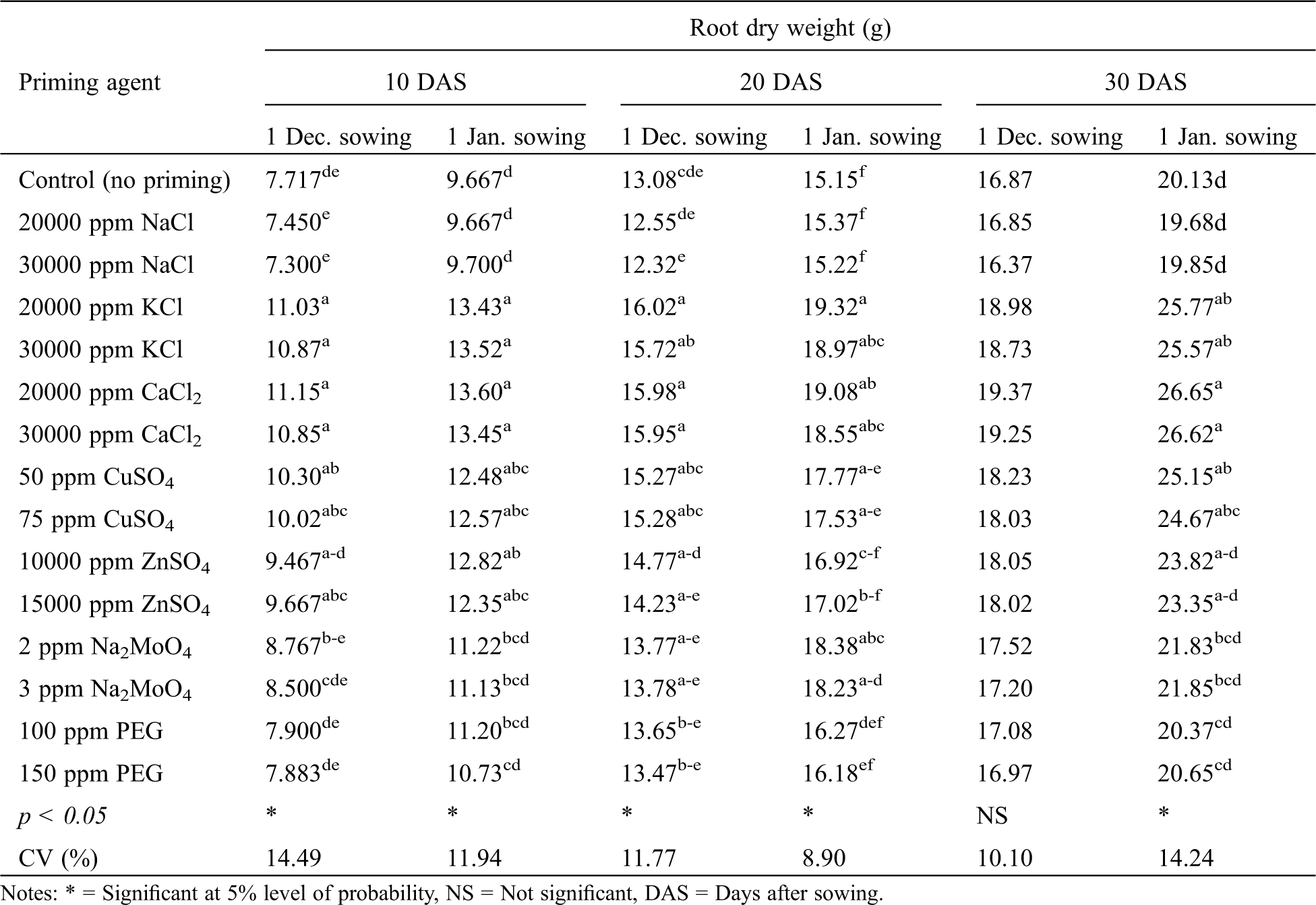
Variety significantly affected seedling root dry weight of winter rice at 20 and 30 DAS for December sowing and 10, 20 and 30 DAS for January sowing (Tab. 7). For both the sowing dates, higher seedling root dry weight was recorded with BRRI dhan36 at all the growth stages compared to BRRI dhan29. Seed priming agent significantly affected seedling root dry weight of winter rice at all the observations for January sowing; while for December sowing, a significant effect was found at 10 and 20 DAS only (Tab. 8). Seed priming with KCl, CaCl2, CuSO4 and ZnSO4 produced the highest and statistically similar seedling root dry weight at all the observations. While, no advantage of NaCl, Na2MoO4 and PEG priming were evident since they produced statistically similar seedling root dry weight as observed in case of no priming (Tab. 8).
Table 7: Effect of variety on root dry weight, shoot dry weight and seedlings dry weight of winter rice at different sowing dates
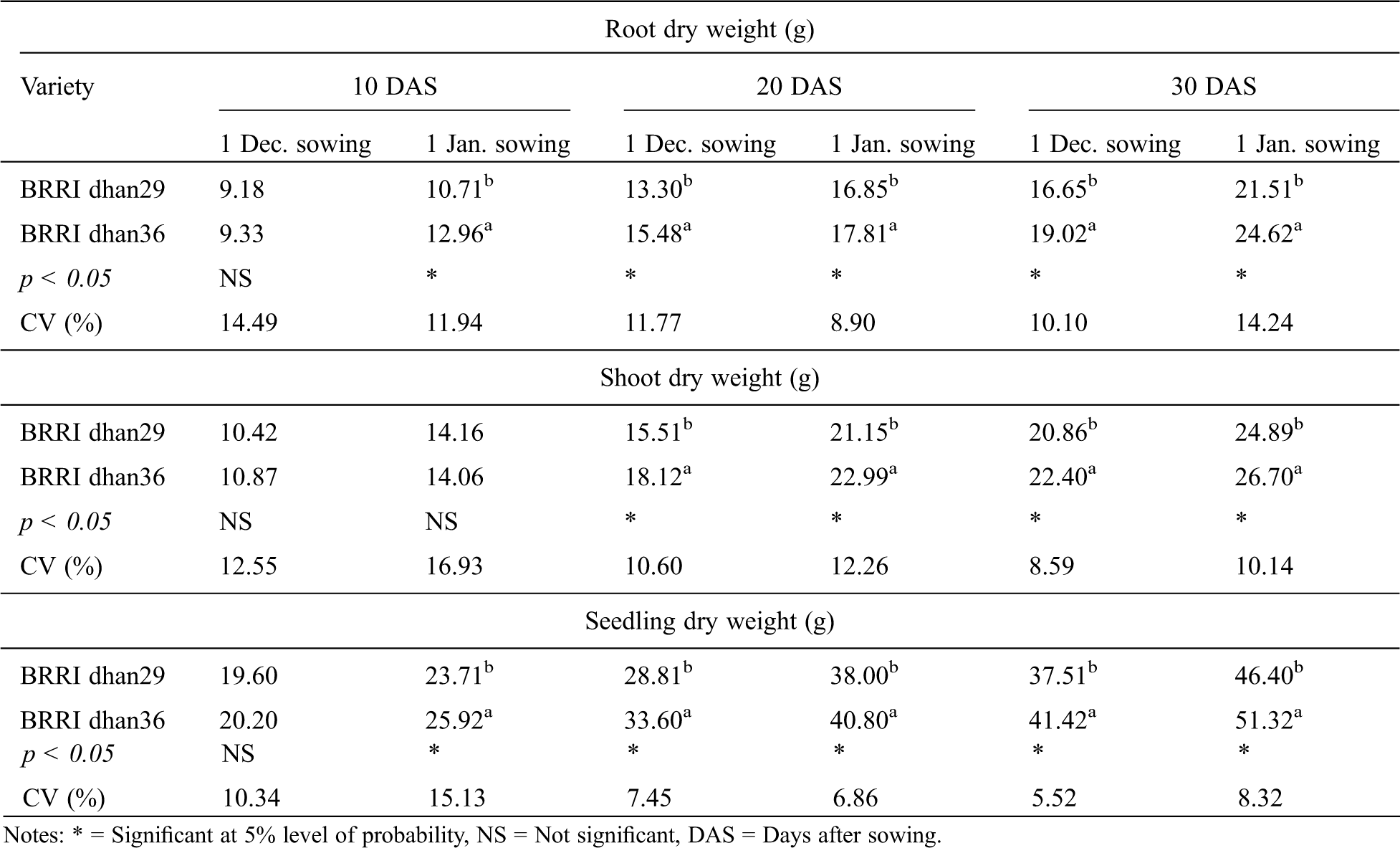
Table 8: Effect of seed priming agent on root dry weight of winter rice seedlings at different sowing dates
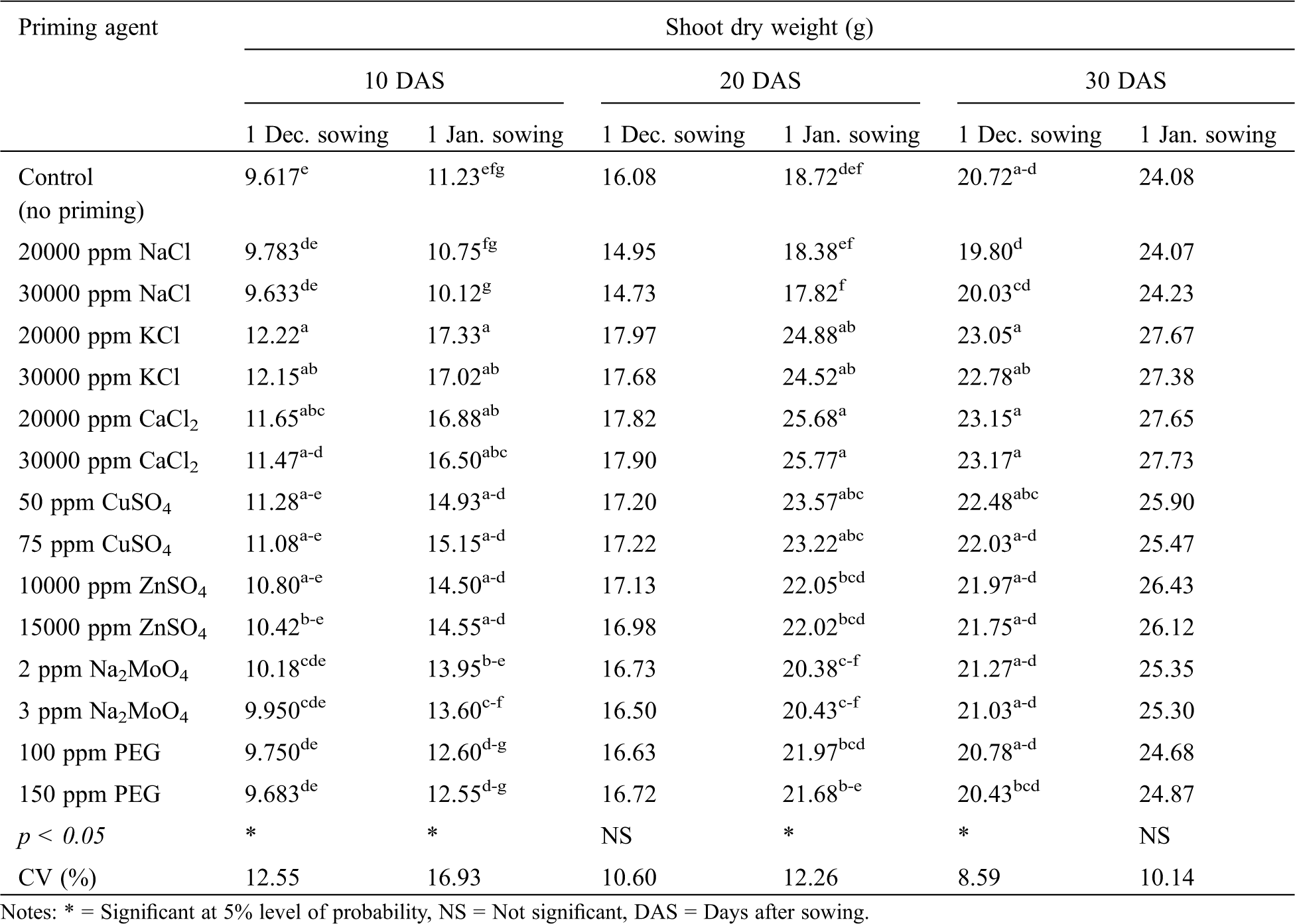
Seedling shoot dry weight of winter rice was significantly affected by variety at 20 and 30 DAS for both the December and January sowing, but not at 10 DAS at either sowing (Tab. 7). For, both the sowing dates, BRRI dhan36 produced a higher seedling shoot dry weight than BRRI dhan29 at both 20 and 30 DAS. Priming agent produced a significant effect on seedling shoot dry weight of winter rice at 10 DAS for January sowing and only for 30 DAS at December sowing (Tab. 9). Like the other parameters studied, seed priming with KCl and CaCl2 resulted in the highest seedling shoot dry weight at all the sampling dates for both December and January sowing. Although, seed priming with CuSO4, ZnSO4 and Na2MoO4 also performed the best in terms of seedling shoot dry weight in some cases (Tab. 9).
Table 9: Effect of seed priming agent on shoot dry weight of winter rice seedlings at different sowing dates
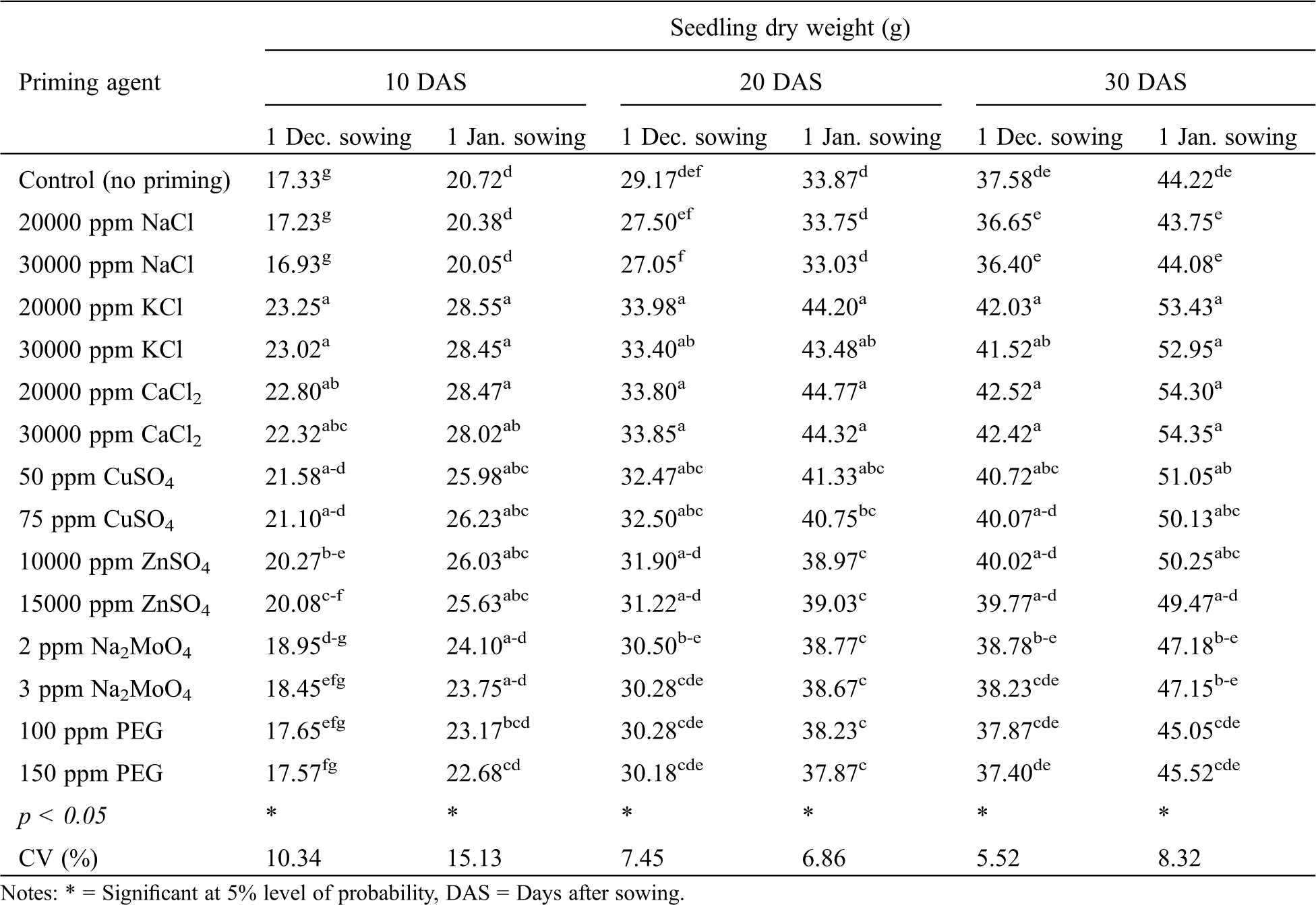
The seedling dry weight of winter rice was significantly affected by variety at all the observations for both December and January sowing, except at 10 DAS for December sowing (Tab. 7). Like other parameters, higher seedling dry weight was recorded with BRRI dhan36 compared to BRRI dhan29. A significant effect of seed priming agent on the seedling dry weight of winter rice was observed at all the growth stages for both December and January sowing (Tab. 10). In general, seed priming with KCl, CaCl2, CuSO4 and ZnSO4 resulted in the highest and statistically similar seedling dry weight. While other priming agents showed no advantages in terms of seedling dry weight production compared to no priming.
Table 10: Effect of priming agent on seedling dry weight of winter rice seedlings at different sowing dates
Seedling emergence, survival and establishment are very crucial for plant growth and productivity under suboptimal/adverse conditions like moisture stress, salinity stress, temperature stress etc. As evident by earlier reports [24,25], seed priming, a prior encounter with a particular stress, is known to bestow plants with greater tolerance when exposed to subsequent stress of same or other type. It was therefore hypothesized that pre-sowing seed treatment could be helpful for faster and higher seed emergence, better survival, increased growth and more vigor of winter rice seedlings which is very often exposed to cold stress at nursery bed.
Seed priming enhanced seed germination and seedling performance compared with the non-primed seed in the current study confirms the potentiality of seed priming to mitigate cold stress during seed germination and seedling establishment in rice. Except NaCl and PEG, other priming agents in this study had a positive impact on seed germination, seedling emergence and survival percentage, and seedling growth in cold stress, and seed priming with KCl or CaCl2 performed the best. It was reported from the many previous studies that the delayed and non-uniform germination of rice under cold stress was mainly due to reducing water uptake, cell membrane injury, less cellular respiration, and elevated reactive oxygen species (ROS) levels [26–29]. In low temperatures, hindered the water uptake and root growth, resulting in a decreased seed germination was reported by Li et al. [27]. Oliver et al. [30] reported cold stress may create a metabolic imbalance in plant tissues and reduce the growth of the seedlings by suppressing cell elongation and division. Rahman et al. [31] reported the respiration is one of the most important metabolic events during seed germination which provides the energy for coleoptile elongation, and the respiration rate during seed germination is closely related to temperature. Low temperature reduces the respiration of seeds, and the longer the duration of low temperature, the greater the reduction of respiration rate [32].
Higher seedling growth under cold stress in the current study from the primed seed treatments was might be the consequence of rapid emergence due to earlier production of emergence metabolites. Khan et al. [33] opined that rapid imbibitions during priming disrupt the cell membrane, cause localized cells in cotyledons resulting in more vigorous seedlings. Starch metabolism, total soluble sugar contents and α-amylase activity are strongly linked to the seed germination and seedling growth of rice [32,34]. Du et al. [34] reported seed priming increase the antioxidants superoxide dismutase (SOD), peroxidase, catalase, and glutathione peroxidase contents in seedlings and decreases the stress-related malondialdehyde (MDA) content compared to non-primed seed and in this way, these enzymes protect the plants by alleviating low-temperature chilling effects. These findings are in line with those who also confirmed increased germination rate along with faster and synchronized emergence as a consequence of pre-sowing seed priming in different crop species including rice [35–37]. Arif et al. [38] reported genetic and structural repair along with rapid development of the immature embryo during seed priming are plausible justifications for increased germination rate.
Although NaCl mediated seed priming did not enhance seed emergence percentage in this study, conflicting findings have also been reported by Mamun et al. [35] in rice and Yadav et al. [39] in capsicum, where Osmo-hardening with NaCl enhanced germination rate. This was might be due to the variation in crop species used and NaCl concentration tested in different studies.
In this study, rice varietal effect was not significant for seed emergence and seedling survival percentage at either sowing date. But, most of the seedling growth parameters at different growth stages were significantly influenced by rice variety for both the sowing dates. It was evident that BRRI dhan36 performed better in terms of seedling growth than BRRI dhan29. This was might be due to the fact that BRRI dhan36 is considered as a moderately cold-tolerant rice variety especially at the seedling stage [3,26]. Varietal differences in seed germination and seedling growth of rice has also been reported by many researchers [35,36] which is mostly due to the genetic make-up of the variety.
Both seedling emergence percentage and seedling survival percentage were found higher in 1st December sowing compared to 1st January sowing. This might be the consequence of lower minimum and the average temperature in the 1st week of January (10.1°C and 18.2°C, respectively) than those of 1st week of December (15.0°C and 21.4°C, respectively). Xu et al. [37] reported that both the speed and rate of germination of rice seeds are reduced at the temperature below 18°C. Cruz et al. [40] also confirmed the reduction in germination percentage of rice seeds at low temperatures. Cruz and Milach [41] revealed that poor germination rate under chilling stress is due to damage to the plasma membrane resulting in the leakage of solutes especially amino acids and carbohydrates from the germinating rice seeds.
Root and shoot length and dry matter accumulation of rice seedlings at different stages were found significantly higher with BRRI dhan36 than with BRRI dahn29 at both the sowing dates. This was due to higher cold tolerance of BRRI dhan36 than BRRI dhan29 as reported by Rashid and Yasmeen [3]. Similar to the emergence percentage and seedling survival percentage, seedling growth and dry matter accumulation were recorded higher when sown on 1st December than 1st January sowing. This was due to comparatively low temperatures in January than in December. As recorded during the experimental period, the average temperature in December and January were respectively 20.3 and 18.1°C, respectively. Reduction in seedling growth due to cold stress has also been reported by many others [42–44]. Rice seedling growth, evaluated in terms of root and shoot length and dry weight, was positively influenced by seed priming for both the sowing. In most of the cases, all the priming agents performed better than unprimed control, but in some cases, performances of NaCl or PEG was not satisfactory, which may be due to their phytotoxic effects on rice seedlings.
The present study confirms the potentiality of seed priming as a tool for increasing seedling emergence and survival percentage, and enhancing seedling growth of rice even under cold stress. Seed priming with KCl and CaCl2 emerged as the best priming agents. Conclusively, these findings will provide new avenues for advancing seed priming to mitigate cold stress in winter rice particularly at the seedling stage; however, needs further validation this results in larger field trials in the natural environment. However, the present study did not examine the physiological and biochemical changes in rice seedlings under the influence of seed priming and cold stress to obtain a better understanding of priming-induced mechanisms and to provide a basis for further analyses.
Acknowledgement: The authors thankfully acknowledge the laboratory facilities provided by the Department of Agronomy, Bangladesh Agricultural University to conduct this research activity. The authors extend their appreciation to Taif University for funding current work by Taif University Researchers Supporting Project number (TURSP-2020/120), Taif University, Taif, Saudi Arabia.
Funding Statement: The current work was funded by Bangladesh Agricultural University Research System (BAURES, Bangladesh Agricultural University, Mymensingh through the research project number: 2018/597/BAU. Taif University Researchers Supporting Project number (TURSP-2020/120), Taif University, Taif, Saudi Arabia provided the APC of this publication.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ahmed, S., Humphreys, E., Chauhan, B. S. (2016). Optimum sowing date and cultivar duration of dry-seeded boro on the high Ganges River floodplain of Bangladesh. Field Crops Research, 190, 91–102. DOI 10.1016/j.fcr.2015.12.004. [Google Scholar] [CrossRef]
2. Lee, M. H. (2001). Low Temperature Tolerance in Rice: The Korean Experience. In: Fukai, S., Basnayake, J. (Eds.ACIAR proceedings 101; increased lowland rice production in the mekong region, pp. 138–146. Australian Centre for International Agricultural Research (ACIARCanberra. [Google Scholar]
3. Rahman, M. M., Aziz, M. A., Musa, M. A., Kumar, J. (1995). Prospects of Pulse Crops in the Rice-Based Cropping System in Bangladesh. Fragile Lives in Fragile Ecosystems, Proceedings of the International Rice Research Conference, pp. 439–449. International Rice Research Institute, Manila, Philippines. [Google Scholar]
4. Rashid, M. M., Yasmeen, R. (2017). Cold injury and flash flood damage in boro rice cultivation in Bangladesh: A review. Bangladesh Rice Journal, 21(1), 13–25. DOI 10.3329/brj.v21i1.37360. [Google Scholar] [CrossRef]
5. Zhang, Q., Chen, Q., Wang, S., Hong, Y., Wang, Z. (2014). Rice and cold stress: Methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice Journal, 7, 24. DOI 10.1186/s12284-014-0024-3. [Google Scholar] [CrossRef]
6. Hetherington, S. E., He, J., Smillie, R. M. (1998). Photoinhibition at low temperature in chilling-sensitive and -resistant plants. Plant Physiology, 90(4), 1609–1615. DOI 10.1104/pp.90.4.1609. [Google Scholar] [CrossRef]
7. Hussain, S., Khan, F., Hussain, H. A., Nie, L. (2016). Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Frontiers in Plant Science, 7, 116. DOI 10.3389/fpls.2016.00116. [Google Scholar] [CrossRef]
8. Wang, W., Chen, Q., Hussain, S., Mei, J., Dong, H. et al. (2016). Seedling growth and associated metabolic events under chilling stress. Scientific Reports, 6, 19637. DOI 10.1038/srep19637. [Google Scholar] [CrossRef]
9. Taylor, A. G., Allen, P. S., Bennett, M. A., Bradford, K. J. (1998). Seed enhancements. Seed Science Research, 8(2), 245–256. DOI 10.1017/S0960258500004141. [Google Scholar] [CrossRef]
10. Kaya, M. D., Okcub, G., Ataka, M., Cikilic, Y., Kolsaricia, O. (2006). Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). European Journal of Agronomy, 24, 291–295. DOI 10.1016/j.eja.2005.08.001. [Google Scholar] [CrossRef]
11. Farooq, M., Basra, S. M. A., Ahmad, N. (2007). Improving the performance of transplanted rice by seed priming. Plant Growth Regulation, 51, 129–137. DOI 10.1007/s10725-006-9155-x. [Google Scholar] [CrossRef]
12. Juraimi, A. S., Anwar, M. P., Selamat, A., Puteh, A., Man, A. (2012). The influence of seed priming on weed suppression in aerobic rice. Pakistan Journal of Weed Science Research, 18, 257–264. [Google Scholar]
13. Jisha, K. C., Vijayakumari, K., Puthur, J. T. (2013). Seed priming for abiotic stress tolerance: An overview. Acta Physiology Plantarum, 35, 1381–1396. DOI 10.1007/s11738-012-1186-5. [Google Scholar] [CrossRef]
14. Kubala, S., Garnczarska, M., Wojtyla, L., Clippe, A., Kosmala, A. et al. (2015). Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Science, 231, 94–113. DOI 10.1016/j.plantsci.2014.11.008. [Google Scholar] [CrossRef]
15. Ibrahim, E. A. (2016). Seed priming to alleviate salinity stress in germinating seeds. Journal of Plant Physiology, 192, 38–46. DOI 10.1016/j.jplph.2015.12.011. [Google Scholar] [CrossRef]
16. Kaur, S., Gupta, A. K., Kaur, N. (2005). Seed priming increases crop yield possibly by modulating enzymes of sucrose metabolism in chickpea. Journal of Agronomy and Crop Science, 19, 81–87. DOI 10.1111/j.1439-037X.2004.00140.x. [Google Scholar] [CrossRef]
17. Farooq, M., Basra, S. M. A., Hafeez, K., Ahmad, N. (2005). Thermal hardening: A new seed vigor enhancement tool in rice. Journal of Integrative Plant Biology, 47, 187–193. DOI 10.1111/j.1744-7909.2005.00031.x. [Google Scholar] [CrossRef]
18. Hossain, M. A., Lee, Y., Cho, J. I., Ahn, C. H., Lee, S. K. et al. (2010). The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice. Journal of Plant Molecular Biology, 72(4–5), 557–566. DOI 10.1007/s11103-009-9592-9. [Google Scholar] [CrossRef]
19. Paparella, S., Araujo, S. S., Rossi, G., Wijayasinghe, M., Carbonera, D. et al. (2015). Seed priming: State of the art and new perspectives. Plant Cell Reports, 34, 1281–1293. DOI 10.1007/s00299-015-1784-y. [Google Scholar] [CrossRef]
20. Rashid, A., Harris, D., Hollington, P., Ali, S. (2004). On-farm seed priming reduces yield losses of mungbean (Vigna radiata) associated with mungbean yellow mosaic virus in the north west frontier province of Pakistan. Crop Protection, 23(11), 1119–1124. DOI 10.1016/j.cropro.2004.04.002. [Google Scholar] [CrossRef]
21. Ghassemi-Golezani, K., Aliloo, A. A., Valizadeh, M., Moghaddam, M. (2008). Effects of hydro and osmo-priming on seed germination and field emergence of lentil (Lens culinaris medik.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 36(1), 29–33. DOI 10.15835/nbha36186. [Google Scholar] [CrossRef]
22. Mondal, S., Khan, M. I. R., Entila, F., Dixit, S., Cruz, P. C. S. et al. (2020). Responses of AG1 and AG2 QTL introgression lines and seed pre-treatment on growth and physiological processes during anaerobic germination of rice under flooding. Scientific Reports, 10, 1021. DOI 10.1038/s41598-020-67240-x. [Google Scholar] [CrossRef]
23. BRRI. (2019). Modern Rice Cultivation, pp. 96. 22nd Special Edition, Bangladesh Rice Research Institute, Gazipur 1701, Bangladesh. [Google Scholar]
24. Wahid, A., Noreen, A., Basra, S. M. A., Gelani, M., Farooq, M. (2008). Priming induced metabolic changes in sunflower achenes improve germination and seedling growth. Botanical Studies, 49, 343–350. [Google Scholar]
25. Patanea, C., Cavallaro, V., Cosentino, S. L. (2009). Germination and radicle growth in unprimed and primed seed of sweet sorghum as affected by reduced water potential in NaCl at different temperatures. Industrial Crops and Products, 30, 1–8. DOI 10.1016/j.indcrop.2008.12.005. [Google Scholar] [CrossRef]
26. Nayyar, H., Chander, K., Kumar, S., Bains,T. (2005). Glycinebetaine mitigates cold stress damage in chickpea. Agronomy for Sustainable Development, 25, 381–388. DOI 10.1051/agro:2005033. [Google Scholar] [CrossRef]
27. Li, X., Jiang, H., Liu, F., Cai, J., Dai,T. et al. (2013). Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitricoxide and gibberellin. Plant Growth Regulation, 71, 31–40. DOI 10.1007/s10725-013-9805-8. [Google Scholar] [CrossRef]
28. Ye, C., Fukai, S., Godwin, I., Reinke, R., Snell, P. et al. (2009). Cold tolerance in rice varieties at different growth stages. Crop and Pasture Science, 60, 328–338. DOI 10.1071/CP09006. [Google Scholar] [CrossRef]
29. Cheng, C., Yun, K. Y., Ressom, H. W., Mohanty, B., Bajic,V. B. et al. (2007). An early response regulatory cluster induced by low temperature and hydrogen peroxide in seedlings of chilling-tolerant japonica rice. BMC Genomics, 8, 175. DOI 10.1186/1471-2164-8-175. [Google Scholar] [CrossRef]
30. Oliver, S. N., Dennis, E. S., Dolferus, R. (2007). ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiology, 48, 1319–1330. DOI 10.1093/pcp/pcm100. [Google Scholar] [CrossRef]
31. Rahman, M., Ijaz, M., Qamar, S., Bukhari, S. A. (2019). Abiotic stress signaling in rice crop. In: Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J. K., (Eds.Advance in rice research for abiotic stress tolerance, vol. 27, pp. 551–569. Abiotic Stress Signaling in Rice Crop. India Woodhead Publishing. DOI 10.1016/B978-0-12-814332-2.00027-7. [Google Scholar] [CrossRef]
32. Ray, B. P., Chanda, M. C., Sayem, M. A., Roy, A. K. (2016). Genetic analysis and marker assisted selection (MAS) of cold tolerant rice. International Journal of Biotechnology and Allied Fields, 4(9), 339–345. [Google Scholar]
33. Khan, A., Khalil, S. K., Khan, A. Z., Marwat, K. B., Afzal, A. (2008). The role of seed priming in semi-arid area for mungbean phenology and yield. Pakistan Journal of Botany, 40, 2471–2480. [Google Scholar]
34. Du, B., Luo, H., He, L., Zhang, L., Liu, Y. et al. (2019). Rice seed priming with sodium selenate: Effects on germination, seedling growth, and biochemical attributes. Scientific Reports, 9, 4311. DOI 10.1038/s41598-019-40849-3. [Google Scholar] [CrossRef]
35. Mamun, A. A., Naher, U. A., Ali, M. Y. (2018). Effect of seed priming on seed germination and seedling growth of modern rice varieties. The Agriculturist, 16(1), 34–43. DOI 10.3329/agric.v16i1.37532. [Google Scholar] [CrossRef]
36. Basra, S. M. A., Afzal, I., Rashid, A. R., Farooq, M. (2005). Pre-sowing seed treatment to improve germination and seedling growth in wheat (Triticum aestivum L.) seeds. Pakistan Journal of Arid Agriculture, 5, 11–16. [Google Scholar]
37. Xu, L. M., Zhou, L., Zeng, Y. W., Wang, F. M., Zhang, H. L. et al. (2008). Identification and mapping of quantitative trait loci for cold tolerance at the booting stage in a japonica rice near isogenic line. Plant Science, 174, 340–347. DOI 10.1016/j.plantsci.2007.12.003. [Google Scholar] [CrossRef]
38. Arif, M., Jan, M. T., Marwat, K. B., Khan, M. A. (2008). Seed priming improves emergence and yield of soybean. Pakistan Journal of Botany, 40, 1169–1177. [Google Scholar]
39. Yadav, P. V., Kumara, M., Ahmed, Z. (2011). Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Research Journal of Seed Science, 4(3), 125–136. DOI 10.3923/rjss.2011.125.136. [Google Scholar] [CrossRef]
40. Cruz, R. P., Sperotto, R. A., Cargnelutti, D., Adamski, J. M., Terra, T. F. et al. (2013). Avoiding damage and achieving cold tolerance in rice plants. Food and Energy Security, 2, 96–119. DOI 10.1002/fes3.25. [Google Scholar] [CrossRef]
41. Cruz, R. P. D., Milach, S. C. K. (2004). Cold tolerance at the germination stage of rice: Methods of evaluation and characterization of genotypes. Scientia Agricola, 6, 11–8. DOI 10.1590/S0103-90162004000100001. [Google Scholar] [CrossRef]
42. Wang, W., Peng, S., Chen, Q., Mei, J., Dong, H. et al. (2016). Effects of pre-sowing seed treatments on establishment of dry direct seeded early rice under chilling stress. AoB Plants, 8, 1–11. DOI 10.1093/aobpla/plw074. [Google Scholar] [CrossRef]
43. Sadeghi, H., Khazaei, F., Yari, L., Sheidaei, S. (2011). Effect of seed osmopriming on seed germination behavior and vigor of soybean (Glycine max L.). ARPN Journal of Agriculture and Biological Science, 6, 39–43. [Google Scholar]
44. Rowse, H. R. (1995). Drum priming-a non-osmotic method of priming seeds. Seed Science and Technology, 24, 281–294. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |