
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015591
ARTICLE
Screening and Assessment of Selected Chilli (Capsicum annuum L.) Genotypes for Drought Tolerance at Seedling Stage
1Plant Genetic Resources Centre, Bangladesh Agricultural Research Institute, Gazipur, 1701, Bangladesh
2Molecular Breeding Laboratory, Plant Breeding Division, Bangladesh Agricultural Research Institute, Gazipur, 1701, Bangladesh
3Department of Agronomy, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Dhaka, 1207, Bangladesh
4Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
*Corresponding Authors: Md. Motiar Rohman. Email: motiar_1@yahoo.com; Mirza Hasanuzzaman. Email: mhzsauag@yahoo.com
Received: 30 December 2020; Accepted: 23 February 2021
Abstract: This study was undertaken to investigate oxidative stress tolerant mechanisms in chilli (Capsicum annuum L.) under drought genotypes through evaluating morphological, physiological, biochemical and stomatal parameters. Twenty genotypes were evaluated for their genetic potential to drought stress tolerant at seedling stage. Thirty days old seedlings were exposed to drought stress induced by stop watering for the following 10 days and re-watering for the following one week as recovery. Based on their survival performance, two tolerant genotypes viz. BD-10906 and BD-109012 and two susceptible genotypes viz. BD-10902 and RT-20 were selected for studying the oxidative stress tolerance mechanism. Drought reduced root and shoot length, dry weight, ratio, petiole weight and leaf area in both tolerant and susceptible genotypes, and a higher reduction was observed in susceptible genotypes. Lower reduction of leaf area and photosynthetic pigments were also found in tolerant genotypes. Moreover, tolerant genotypes showed higher recovery than susceptible genotypes after the removal of stress. A higher reduction of relative water content (RWC) may cause an imbalance between absorbed and transpirated water in susceptible genotypes. Higher accumulation of proline in tolerant genotypes might be helpful to for better osmotic maintenance than that in susceptible genotypes. Tolerant genotypes showed higher antioxidant activity as they showed DPPH radical scavenging percentage than the susceptible genotypes. Moreover, closer stomata in tolerant genotypes than susceptible ones helped to avoid dehydration in tolerant genotypes. Thus, the above morphological, physiological, biochemical and stomatal parameters helped to show better tolerance in chilli under drought stress.
Keywords: Chilli; drought; stress tolerance; morphology; physiology; biochemical altern
Drought is of increasing concern worldwide due to increased evapotranspiration and evaporation losses from plants and canopy free soils, respectively. Water is a major factor influencing plant productivity, so when water is insufficient in the soil, and atmospheric conditions cause a continuous loss of water, drought stress occurs. Drought accounts as one of the leading causes for a decrease in the average yield of more than 50% of many crops in the world [1,2]. Chilli (Capsicum annuum L.) belongs to the family of solanaceae is very sensitive to drought which may be due to shallowly distributed root systems, and water stress may cause a dramatic reduction in their desired production. Chilli is a valuable spice and also one of the most important cash crops grown in Bangladesh. The area and production of chilli in Kharif (summer, April to September) are 19,000 hectares (ha) and 36,000 metric ton (mt), respectively, while 83,000 ha and 105,000 mt were recorded in Rabi (winter, October to March), respectively. The yield was around 1.42 t ha-1 [3]. Several studies reported that chilli yield was drastically reduced due to drought stress [4]. The severity of such stress becomes quite high when the crop becomes severely damaged either due to very low soil water availability resulting in low or no yield. Plants can be affected by drought at any time of their life, but the critical stages are at germination and seedling growth [5]. Drought stress can induce morphological, physiological, biochemical and stomatal changes in plants because of the reduction in plant tissue water content and water potential [6]. In Bangladesh, drought is being also considered as one of the main causes to reduce the crop growth and yield over the last few decades. In the Rabi season, about 1.2 million ha of agricultural land suffers droughts of different magnitudes due to shortage of rainfall and caused significant yield loss of different rabi crops including chilli [7]. Several studies reported that the seedling growth parameters such as root and shoot length, ratio, and dry weight used as useful traits in the selection of tolerant genotypes to water stress such as wheat [8–10], soybean [11], safflower [12] and tomato [13,14]. However, there has been limited information on drought stress in chilli. With this view, the present study has been undertaken to investigate the morphological, physiological, stomatal and biochemical responses in tolerant and susceptible chilli genotypes under drought stress that might help in selecting tolerant and susceptible chilli genotypes as well as understanding oxidative stress under drought at seedling stage.
2.1 Plant Materials and Stress Application
To compare the drought tolerance in the seedling stage, 20 chilli genotypes were selected based on their drought tolerance performance found in the germination stage in previous study [15]. From there, ten best performed and ten least performed genotypes were selected and tested for drought tolerance (Tab. 1). The germinated seeds in the pot were properly watered for 30 days. At this stage, watering was stopped for the following 10 days, and the dead and alive seedlings were counted for ten days. After that, they were subsequently rewatered. After one week of rewatering, only the alive seedlings of the genotypes survived were counted. Therefore, the first ten days [31–40 days after emergence (DAE)] were considered as drought stress period and onward re-watering (41–47 DAE) was considered as the recovery period. The moisture content of the pot soil was determined by a portable soil moisture meter (PMS-714, LUTRON, Taiwan). This experiment was repeated three times under the same condition. Based on the results of survival capacity, the most two tolerant and susceptible genotypes were used to study the morphological, physiological, stomatal and biochemical responses. Ten seedlings with three replications were evaluated under control, drought stress and recovery period in this study.
Table 1: List of genotypes used in this study for screening of drought tolerant
2.2 Determination of Growth Parameters
Root and shoot length were measured from three randomly selected fresh seedlings from each treatment and expressed in cm. Similarly, three fresh seedlings from each treatment were dried at 80°C for 48 h, then weighed and considered as dry weight (DW), expressed in g. The root-shoot weight ratio was calculated from the dry weights. Leaf area (LA) was determined through an area meter (LI-3100, LI-COR Environmental, USA) and expressed in cm2. The average weight of three-leaf petioles was recorded on precision balance and expressed in gram (g).
2.3 Measurement of Leaf Relative Water Content
RWC of the leaves of plants in different treatments of different genotypes was determined following the procedure of Savita [16]. Similar size leaf samples were weighed immediately to record the fresh weight of the sample. Then, the samples were hydrated to full turgidity by floating on de-ionized water in a closed petri-dish for 4 hours under normal room light and temperature. After 4 hours, the samples were taken out of water and surface moisture was removed quickly and lightly with filter paper and immediately weighed to obtain fully turgid weight. The samples were dried in an oven at 70°C for 48 h and weighed (after being cooled down in a desiccator) to determine dry weight of the sample. The RWC was calculated by using the following formula and expressed as a percent.
2.4 Measurement of Chlorophyll and Carotenoids
Leaves were extracted with 80% (v/v) acetone (centrifuging at 5000 × g), absorbance of the supernatant of the plant samples was measured at 663, 645 and 470 nm, and chlorophyll (Chl) content was calculated according to Arnon [17] and expressed in mg g-1 FW.
Carotenoids (Car) content was estimated using the formula of Kirk et al. [18] and expressed in mg g−1 FW.
where,
D = Optical density; V = Final volume of 80% acetone (ml) and W = Fresh weight of leaf sample taken
Proline colorimetric determination preceded according to Bates et al. [19] based on proline’s reaction with ninhydrin. Fresh leaf tissue (0.5 g) was homogenized in 10 ml of 3% sulfosalicylic acid in ice. The homogenate was centrifuged at 11,500 × g for 15 min. Two mL of the filtrate was mixed with 2 ml of acid ninhydrin and 2 ml of glacial acetic acid. After incubation at 100°C for 1 h, it was cooled and 4 ml of toluene was added. The optical density of the chromophore containing toluene was read spectrophotometrically at 520 nm using toluene as a blank. The amount of proline was determined by comparing with a standard curve.
2.6 Estimation of Total Antioxidant Capacity (TAC)
Diphenyl-picrylhydrazyl (DPPH) radical degradation method [20,21] was used to estimate the antioxidant activity. Absorbance was taken against methanol at 734 nm by using UV-visible spectrophotometer (UV-1800, Shimadzu, Japan). The percent of DPPH inhibition relative to the control were used to determine antioxidant activity using the following equation:
where, Abs. blank is the absorbance of the control reaction [10 µl methanol for TAC (DPPH) instead of leaf extract] and Abs. sample is the absorbance of the test compound. Trolox was used as the reference standard, and the results were expressed as μgTrolox equivalent g−1 DW.
2.7 Observation of Stomatal Behavior
White color transparent nail polish was coated on the abbatial epidermis of the youngest fully expanded leaf and kept in the sunlight for at least 15 min for proper drying. It was then carefully removed by the scotch tape and put on the sterilized slide. Then all slides were transferred under a fluorescence microscope for data measurement.
The experiment was laid out in two factor completely randomized design. Each experiment was repeated twice independently with three replications.
Data of all parameters were reported as the mean ± SE. The data were statistically analyzed by analysis of variance (ANOVA) using Statistix 10 software. Least significance difference (LSD) at 5% level of probability was used to compare the mean values. Comparisons with P ≤ 0.05 were considered significantly different.
3.1 Estimation of Survival Performance Based on Death and Recovery Percentage
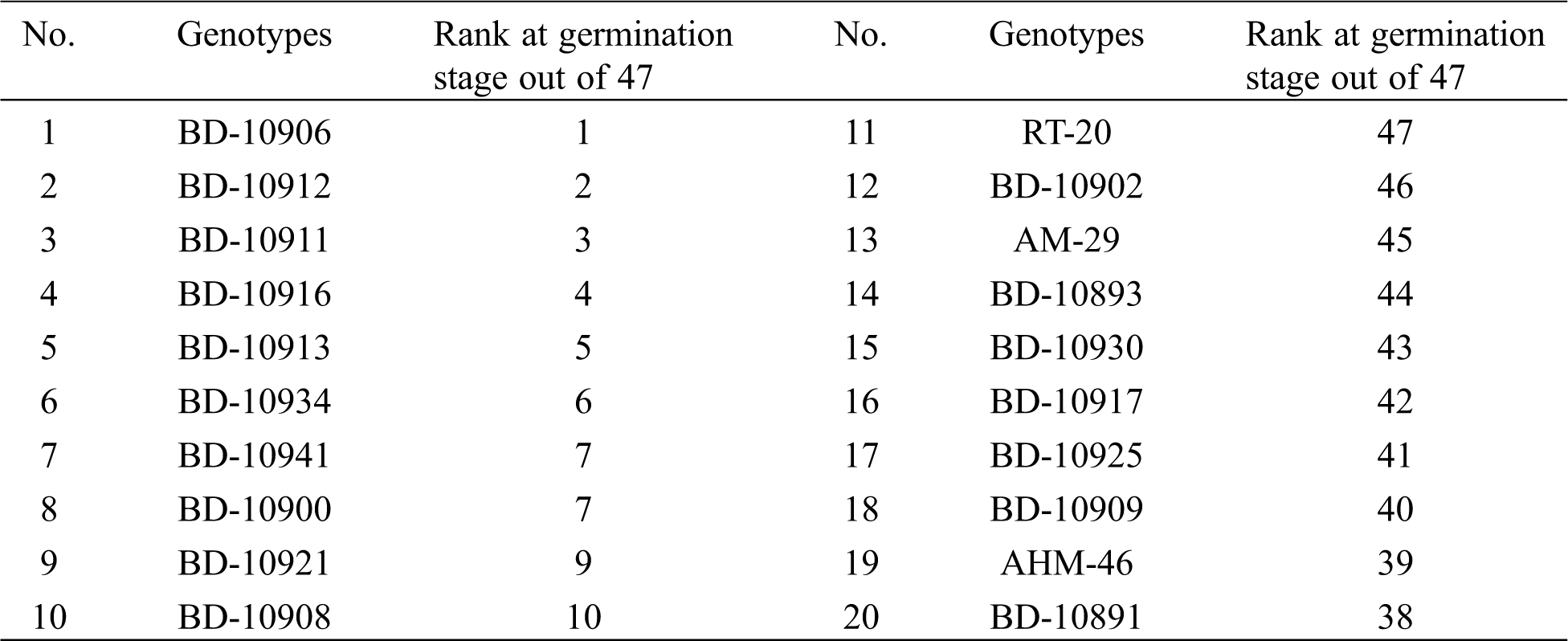
Ten seedlings of each genotype (10 from drought tolerant and 10 from sensitive) in each replication were grown and subjected to screening at the seedling stage. The lowest percentage of seedling death was registered in the genotypes BD-10906 followed by BD-109012 with their value of 11.46% and 11.56%, respectively. On the other hand, the highest percentage of seedling death was recorded in the genotypes BD-10902 and RT-20 with their respective values of 67% and 68% (Fig. 1a). Genotype BD-10906 and BD-10912 showed maximum recovery (76% and 77%), whereas the minimum recovery was found in the genotypes RT-20 and BD-10902 with recovery percentage of 3 and 4, respectively (Fig. 1b). Based on the results, the most drought tolerant genotypes were BD-10906 and BD-10912; contrarily, BD-10902 and RT-20 were the most susceptible. A comparison can be seen on death and survival percentages of the genotypes under drought stress, where BD-10906 and BD-10902 were found as the most tolerant and susceptible one, respectively (Fig. 2).
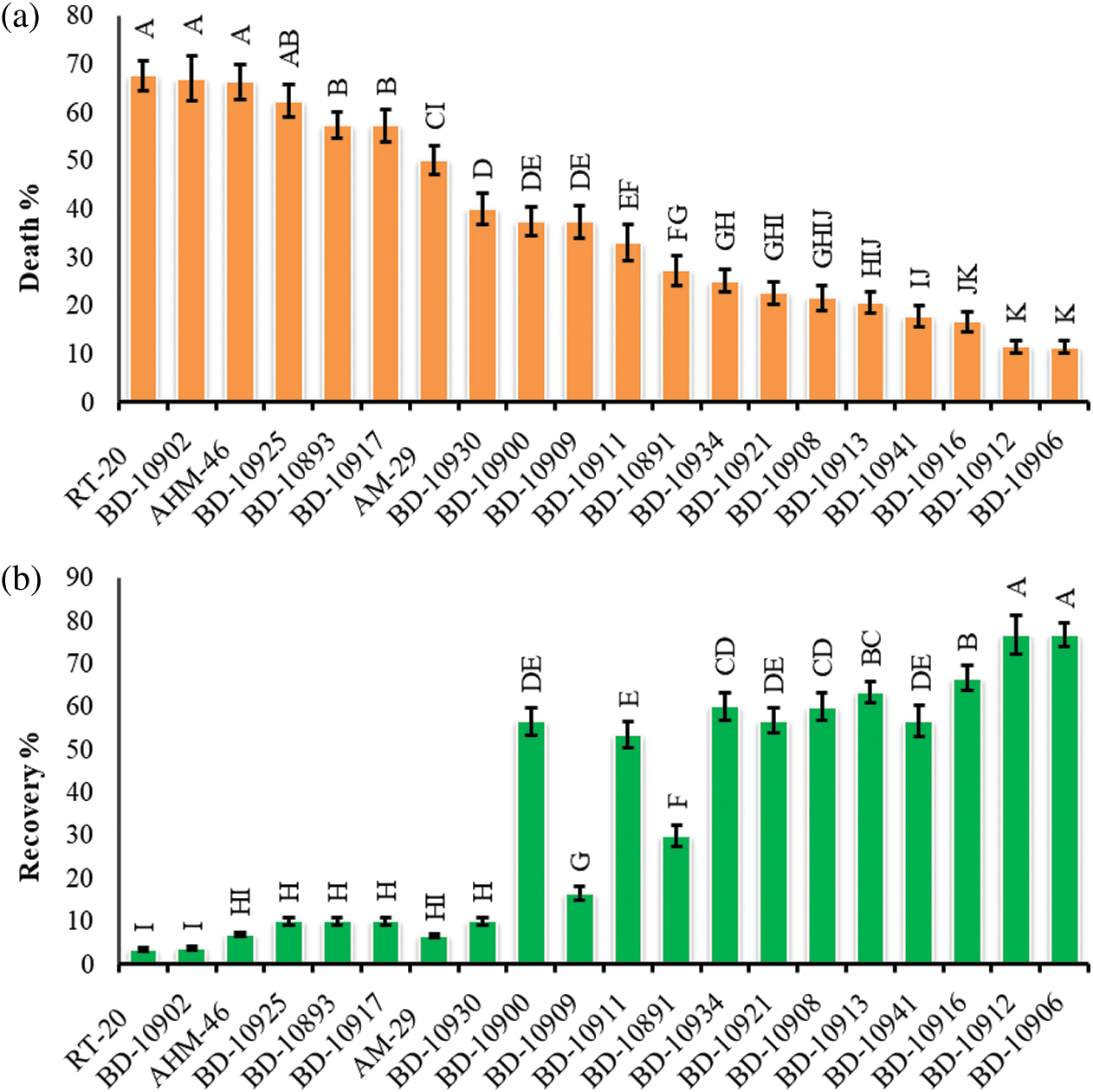
Figure 1: Death (a) and recovery percentage (b) of chilli seedlings under drought stress. Values in bar are mean ± SE of three replications. Different letters on bars are significantly different at P ≤ 0.05
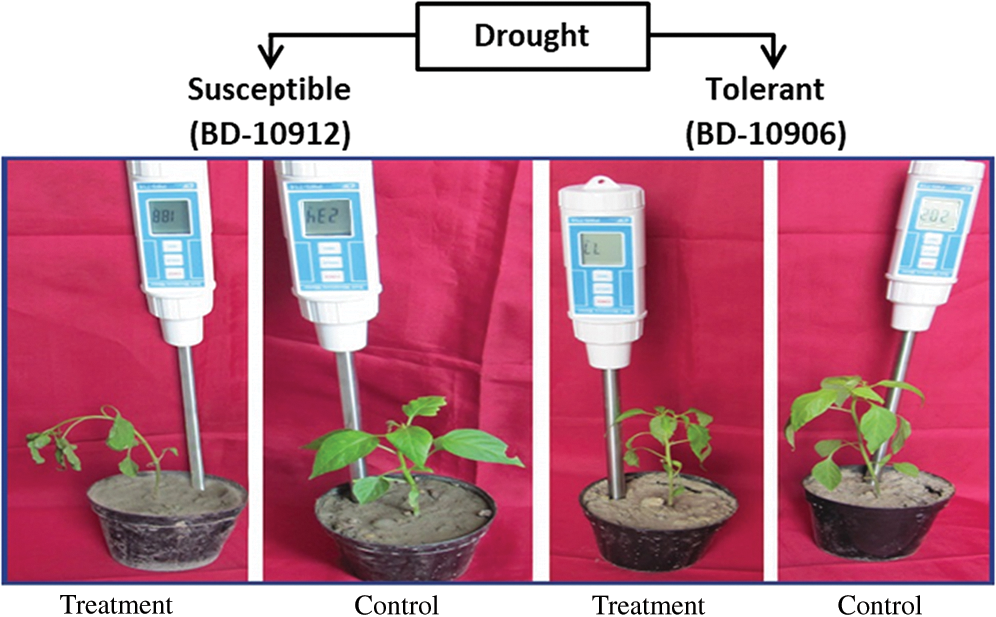
Figure 2: Phenotypeic comparison of the most drought tolerant (BD-10906) and most susceptible (BD-10902) chilli genotypes. Thirty days old seedlings were exposed to drought for 10 days
3.2 Evaluation of Morphological Parameters in Selected Genotypes
Comparatively longer root was observed in drought-tolerant genotypes, BD-10906 and BD-10912 (Fig. 3a). Significant variations were observed among genotypes, treatments and interactions.
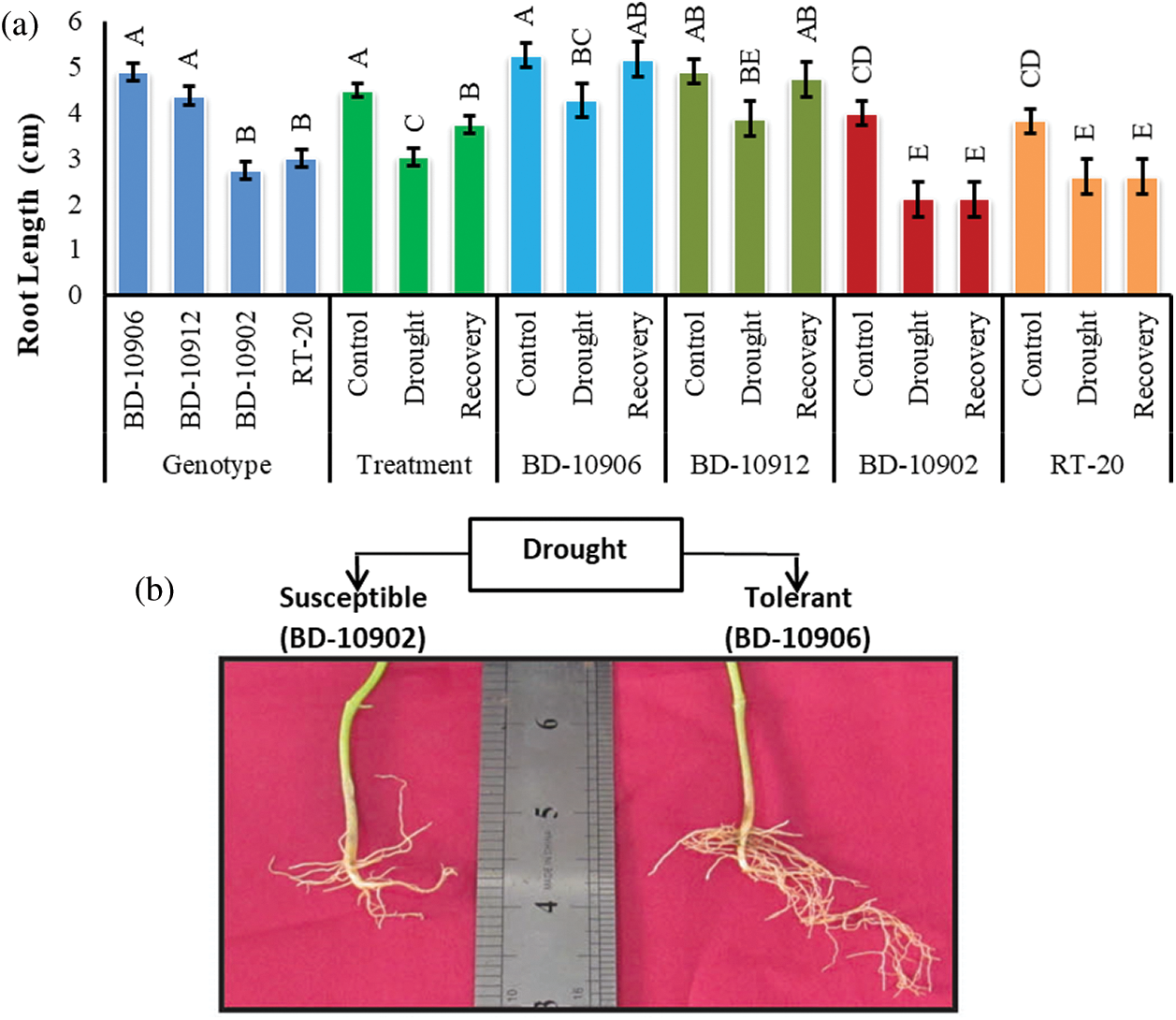
Figure 3: Effect of drought stress on root length at seedling stage in drought tolerant (BD-10906 and BD-10912) and susceptible (BD-10902 and RT-20) genotypes (a) and comparative plate root length is showing between tolerant (BD-10906) and susceptible (BD-10902) genotypes in drought (b) Values in bar are mean ± SE of three replications and different letters on bars are significantly different at P ≤ 0.05
Reduction of the root length in susceptible genotypes was remarkably higher than the tolerant genotypes (Fig. 3a). Moreover, tolerant genotypes also showed higher recovery in root length than that of susceptible genotypes. Root density in tolerant genotypes was also higher in tolerant genotypes than susceptible genotypes (Fig. 3b showd only in one genotype from each group).
Shoot length (SL) showed significant variation in genotypes, treatments and interactions (Fig. 4). In interaction effect, it was clear that drought stresse decreased SL in both tolerant and susceptible genotypes, and SL of susceptible genotypes was more affected by drought. At the same time, no recovery was observed in susceptible genotypes (Fig. 4).
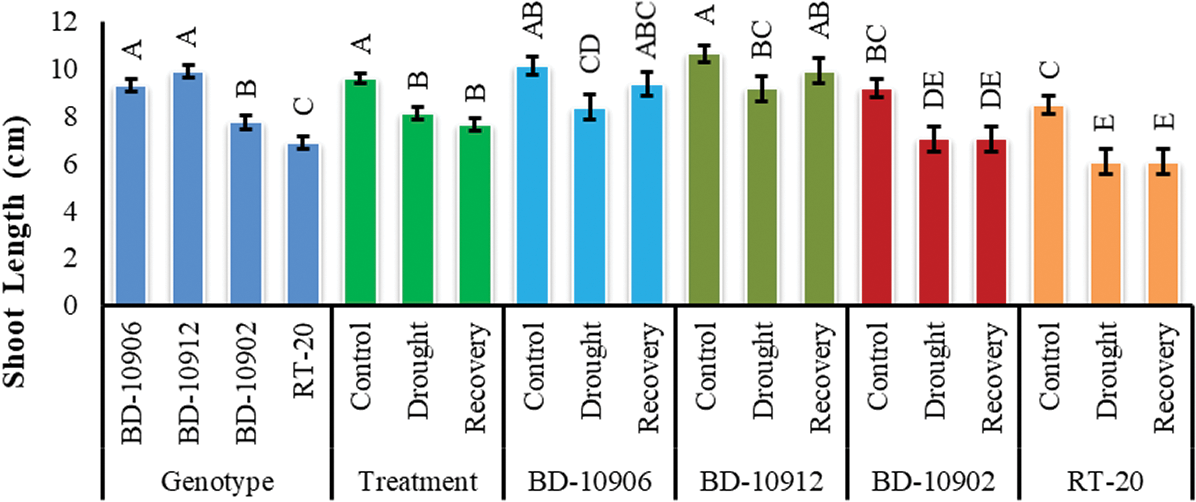
Figure 4: Effect of drought stress on shoot length at seedling stage. Values in bar are mean ± SE of three replications and different letters on bars are significantly different at P ≤ 0.05
The root dry weight (RDW) of both tolerant and susceptible chilli genotypes was significantly affected by drought stress and variation was found in RDW among genotypes, treatments and interactions (Fig. 5a). Data of interaction effects suggested that RDW decreased significantly in both tolerant genotypes BD-10906 and BD-10912 as compared to their respective control. In recovery, RDW increased by almost 47% in both genotypes over drought. In contrary, drought reduced root dry weight in susceptible genotypes BD-10902 and RT-20 by 44% and 40%, respectively. It was noted that the reduction was found to continue in the susceptible genotypes even in recovery (Fig. 5a).
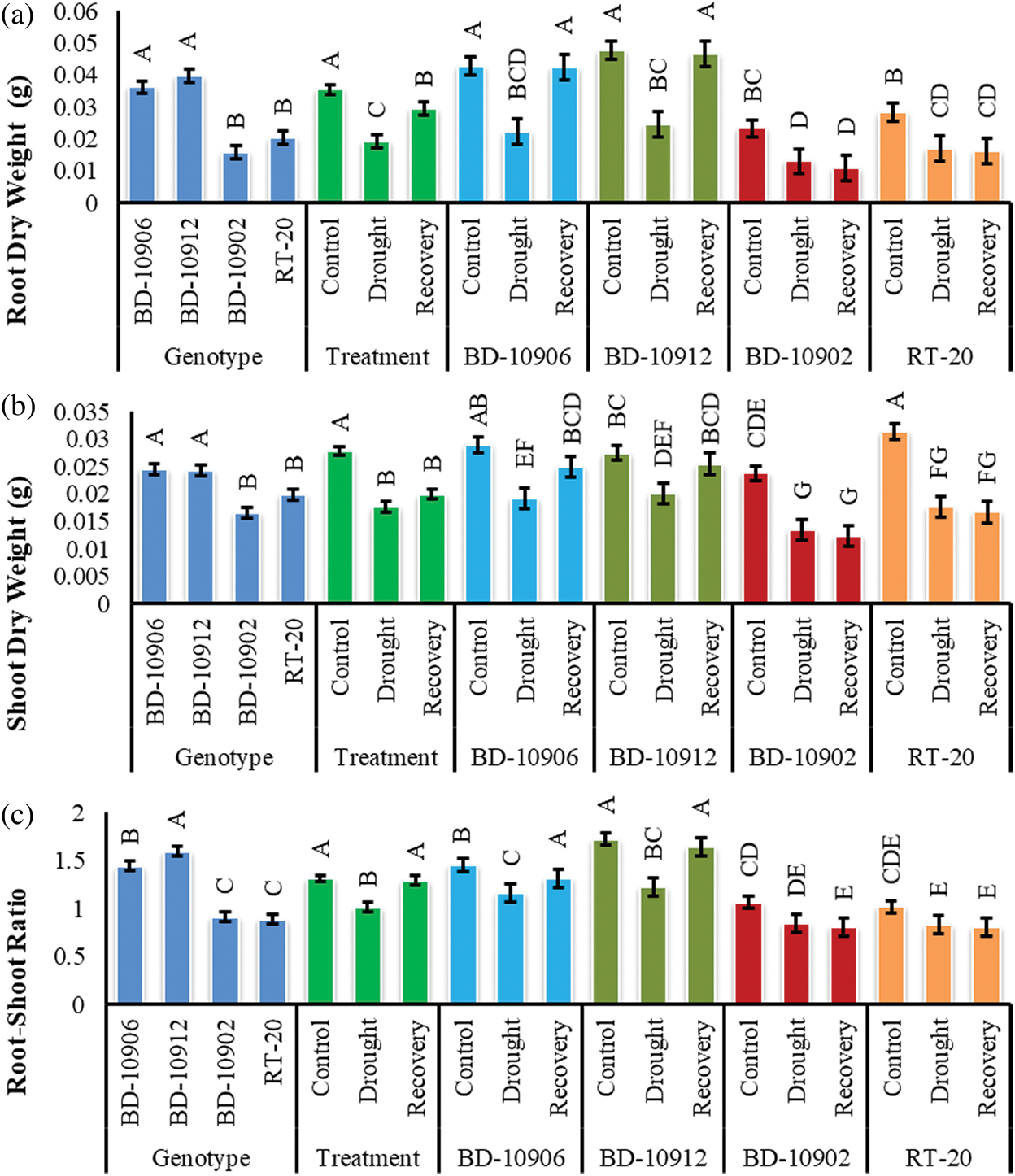
Figure 5: Effect of drought stress on root dry weight (a), shoot dry weight (b) and root-shoot ratio (c) at seedling stage. Values in bar are mean ± SE of three replications and different letters on bars are significantly different at P ≤ 0.05
Drought caused a significant reduction in shoot dry weight (SDW) in all the genotypes, and significant variations were also found in the interactions (Fig. 5). Remarkably, reduction in susceptible genotypes was higher than that in the tolerant genotypes (Fig. 5b). Data showed that s compared to control, drought significantly reduced SDW by 34% and 27% in tolerant genotypes BD-10906 and BD-10912, respectively. On the other hand, 43% and 44% reductions were obtained in susceptible genotypes BD-10902 and RT-20, respectively. Although tolerant genotypes showed an increment in recovery, a further reduction was noticed in susceptible genotypes (Fig. 5b).
As compared to control, drought decreased root-shoot ratio (RSR) in tolerant genotypes BD-10906 and BD-10912 by 21% and 29%, respectively. On the other hand, RSR was recovered by 12% and 25% in BD-10906 and BD-10912, respectively, relative to drought (Fig. 5c). On the other hand, drought reduced RSR in susceptible genotypes BD-10902 and RT-20 by 23% and 28%, respectively. However, no improvement was observed in recovery in susceptible genotypes.
Drought significantly reduced leaf area (LA) in all tested genotypes under stress as compared to respective control. Significant variations were also found in the interactions (Fig. 6a). Remarkably, the reduction in susceptible genotypes was higher than the tolerant genotypes. As compared to the control, drought significantly reduced LA by 15% and 16% in tolerance genotypes BD-10906 and BD-10912, respectively. On the other hand, 26% and 19% reduction were obtained in susceptible genotypes BD-10902 and RT-20, respectively. However, the data of interaction effects showed that only tolerant genotypes recovered LA during recovery period by 9% and 7%, respectively (Fig. 6a).
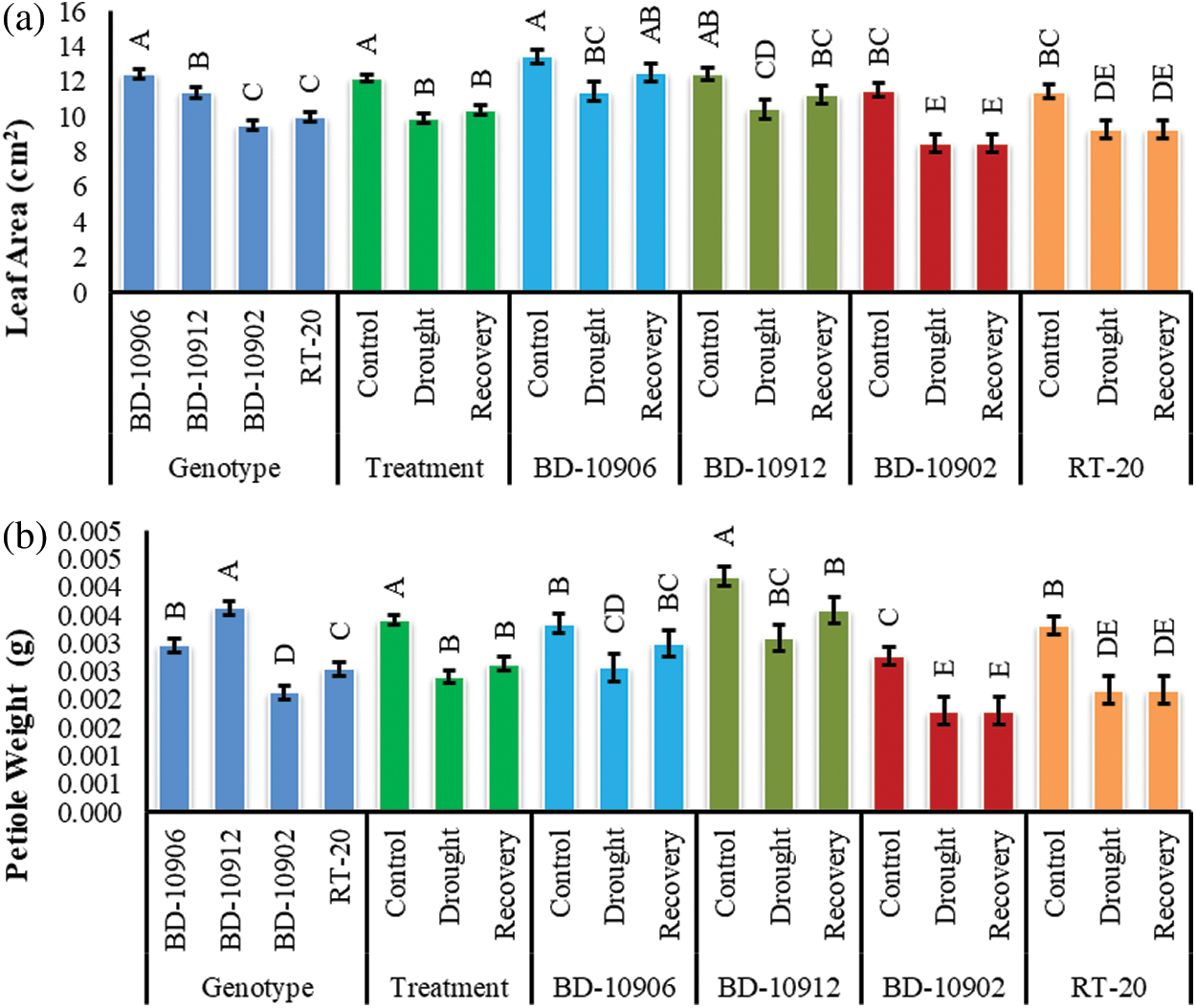
Figure 6: Effect of drought stress on leaf area (a) and petiole weight (b) at seedling stage. Values in bar are mean ± SE of three replications and different letters on bars are significantly different at P ≤ 0.05
Drought decreased petiole weight (PW) by 23% and 26% in tolerant genotypes BD-10906 and BD-10912, respectively, while 35% and 34% reductions were observed in susceptible genotypes BD-10902, respectively (Fig. 6b). However, in recovery period no increment was observed in susceptible genotypes.
3.3 Evaluation of Physiological and Biochemical Parameters
3.3.1 Relative Water Content in Leaf
Relative water content (RWC) of chilli leaf was decreased in drought condition (Fig. 7). Significant variation was also found among the genotypes. Interaction effects suggested that drought mediated decreased RWC was improved in tolerant genotypes in recovery period (Fig. 7).
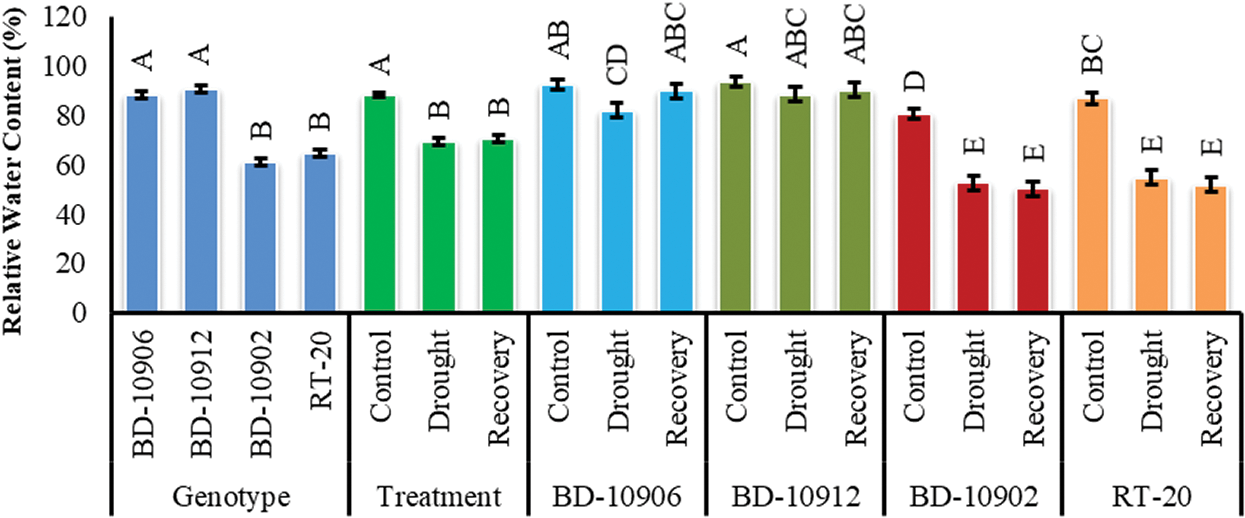
Figure 7: Effect of drought stress on RWC. Values in bar are mean ± SE of three replications and different letters on bars are significantly different at P ≤ 0.05
Although loss of chlorophyll a (Chl a) content was noticed by drought in both tolerant and susceptible genotypes, the degradation was highly significant in susceptible genotypes (Fig. 8). Importantly, the tolerant genotypes maintained higher Chl a in stress and recovery period. It was also noticed that the content further improved in tolerant genotypes in recovery period (Fig. 8a).
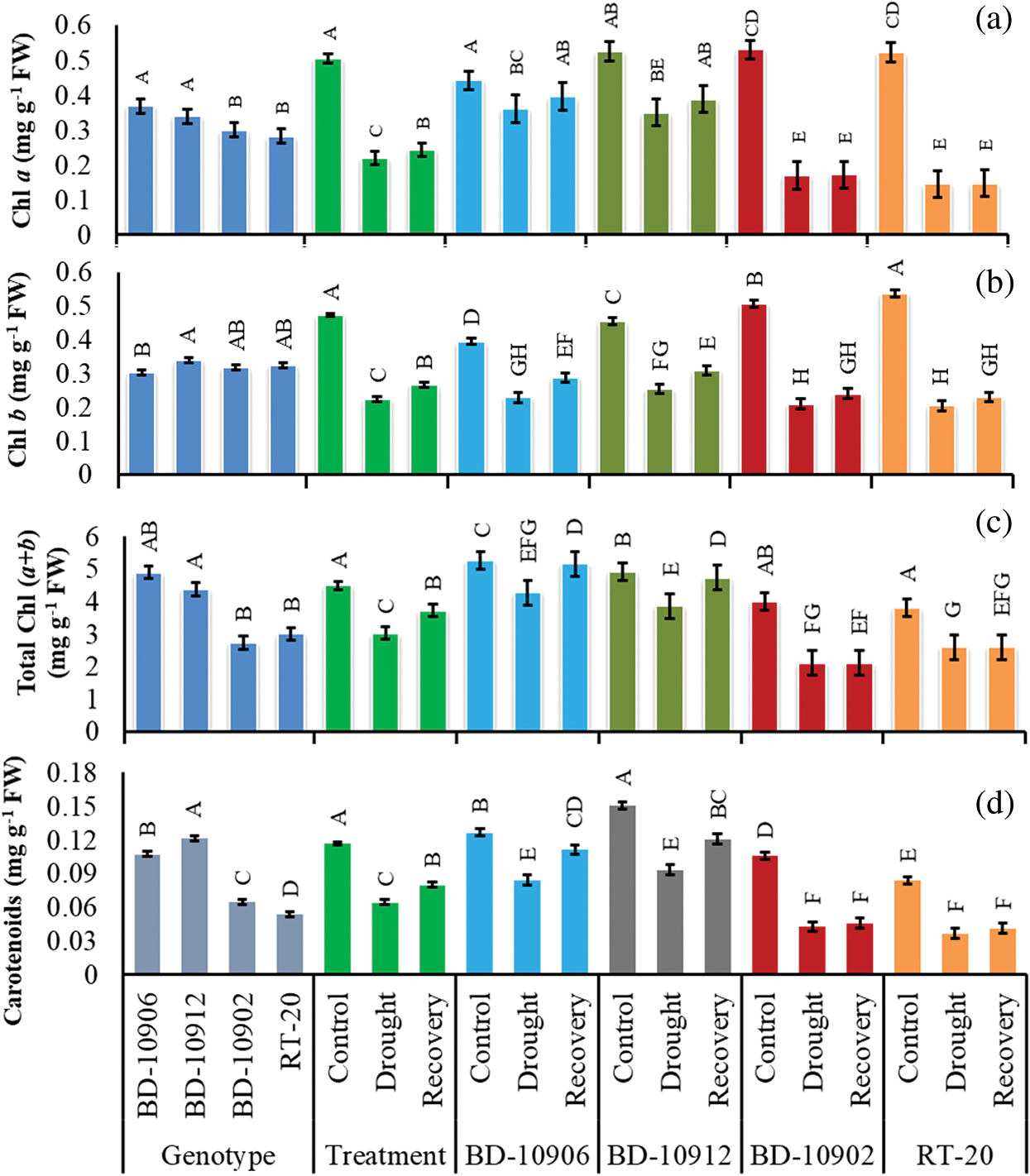
Figure 8: Effect of drought stress on Chl a, (a), Chl b (b), total Chl (c) and carotenoids (d) at seedling stage. Values in bar are mean ± SE of three replications and different letters on bars are significantly different at P ≤ 0.05
In interaction effects, it showed that drought stress decreased Chl b in tolerant and susceptible genotypes, and the degradation was higher in susceptible genotypes (Fig. 8b). As compare to control, drought decreased Chl b by 42% and 44% in tolerant genotypes BD-10906 and BD-10912, respectively. In recovery, relative to drought, 20% and 18% increases in Chl b were observed in BD-10906 and BD-10912, respectively (Fig. 8b). On the other hand, drought reduced Chl b in susceptible genotypes BD-10902 and RT-20 by 59% and 62%, respectively, and in recovery, the recovered by 12% and 11%, respectively, over drought.
3.3.4 Total Chlorophyll Content
Total Chlorophyll Chl (a + b) content of tolerant and susceptible chilli genotypes was significantly affected by drought stress (Fig. 8c). Drought stress remarkably decreased total chlorophyll content in tolerant and susceptible genotypes, but the reduction was higher in susceptible genotypes. In recovery, the content increased in tolerant genotypes only (Fig. 8c).
Results depicted that the carotenoid (Car) content in susceptible genotypes was more affected by drought stress, and the content was significantly higher in tolerant genotypes than susceptible one under drought stress. Data also suggested that compared to control, drought decreased Car content by 33% and 38% in tolerant genotypes BD-10906 and BD-10912, respectively. In recovery, the content further increased in the tolerant genotypes by 12% and 20%, respectively (Fig. 8d). On the other hand, drought reduced Car by 60% and 56% in susceptible genotypes BD-10902 and RT-20, respectively, and in recovery, the content increased by 7% and 10%, respectively.
The data on the effect of drought on proline (Pro) accumulation was presented in Fig. 9. Significant variation was found in Pro content in genotypes, treatments and interactions. Interaction effects showed that both tolerant and susceptible genotypes accumulated more Pro under drought stress as compared to control. However, the accumulation was significantly higher in tolerant genotypes. However, in recovery, the content decreased in all genotypes (Fig. 9).
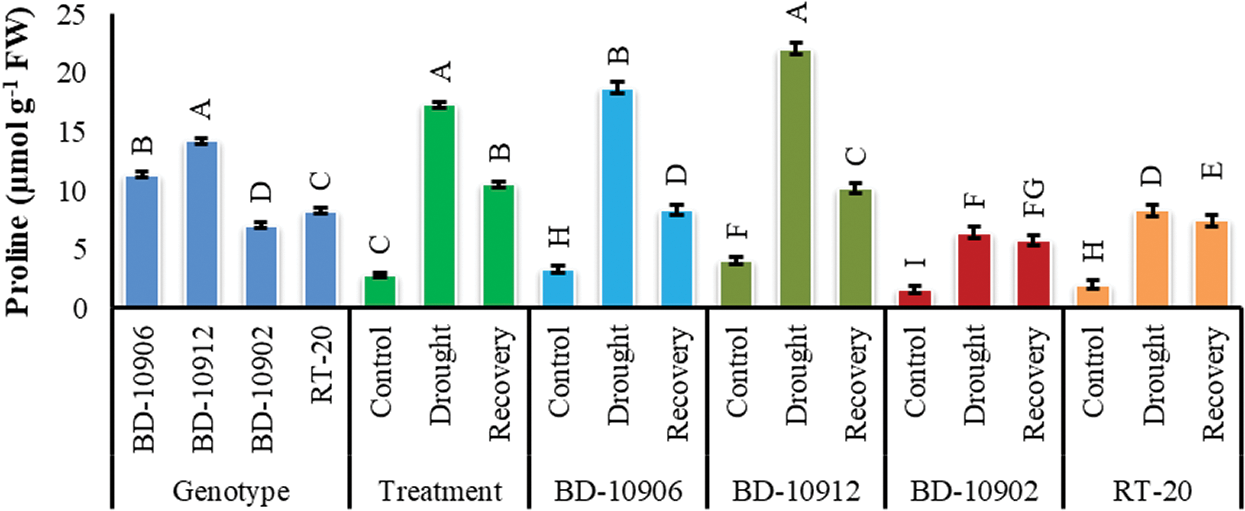
Figure 9: Effect of drought stress on Proline content at seedling stage. Values in bar are mean ± SE of three replications and different letters on bars are significantly different at P ≤ 0.05
3.4 Estimation of Total Antioxidant Capacity (TAC) by DPPH Radical Scavenging
Although the loss of TAC was noticed by drought in both tolerant and susceptible genotypes, the degradation was highly significant in susceptible genotypes (Fig. 10). Importantly, the tolerant genotypes maintained higher TAC in the stress and recovery period. Importantly, the reduced TAC in tolerant genotypes by drought was further increased significantly in the recovery period.
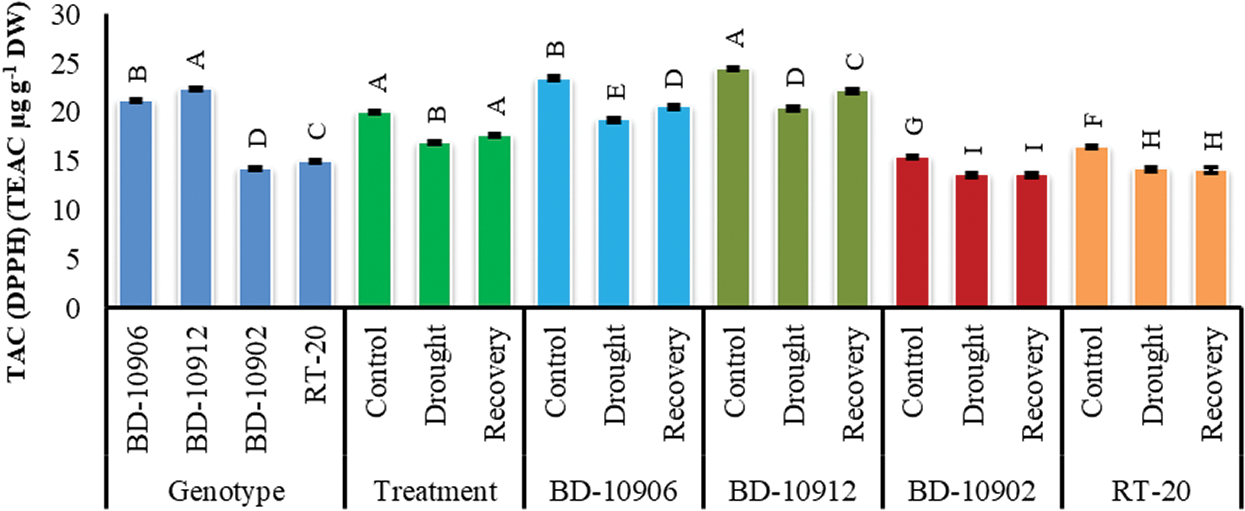
Figure 10: Effect of drought stress on total antioxidant capacity (TAC) at seedling stage. Values in bar are mean ± SE of three replications and different letters on bars are significantly different at P ≤ 0.05
3.5 Evaluation of Anatomical Features
In general, tolerant cultivars efficiently decreased their water loss by means of the reduction in stomata density, dimensions and area and consequently avoid dehydration effects. In this study, numbers of stomata were investigated and compared them between tolerant and susceptible chilli genotype in drought condition. Interestingly, drought tolerant genotypes had more number of stomata than susceptible genotypes [Fig. 11 showed only for tolerant genotypes BD-10912 (a) and susceptible genotypes RT-20 (b)]. It was also noticeable that the stomata were closer in tolerant genotypes than those in susceptible genotypes.
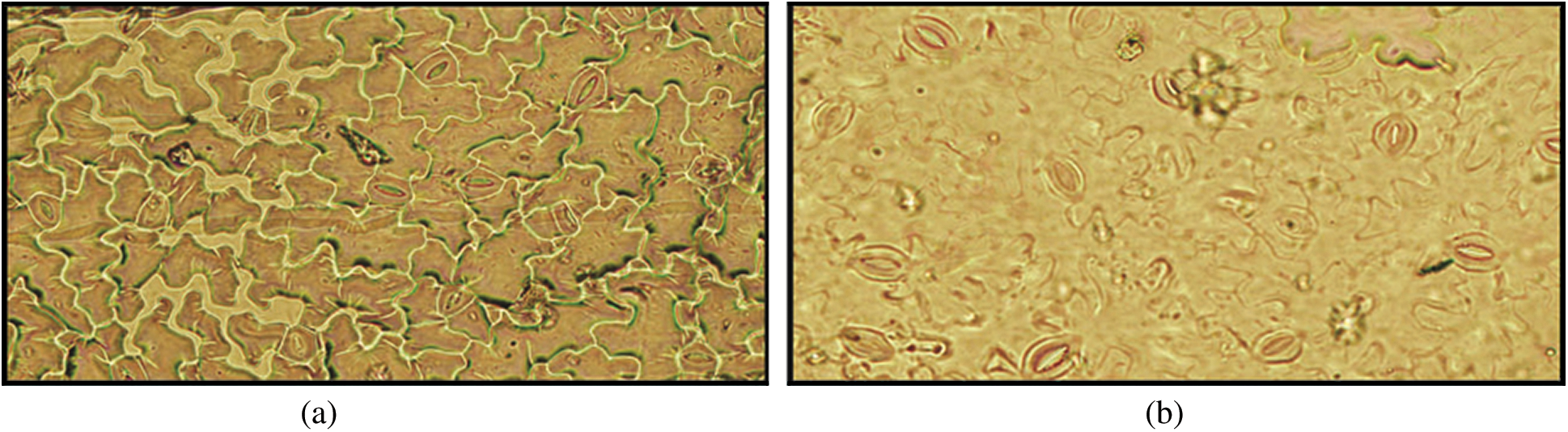
Figure 11: Effect of drought stress on stomatal behavior in tolerant genotype (BD-10912) (a) and susceptible genotype (RT-20) (b)
4.1 Estimation of Survival Performance
Usually, drought tolerant genotypes possess lower dead percentage and higher recovery percentage at their seedling stage. In this study, the genotypes which are sensitive to drought stress at germination stage had higher death percentage and lower recovery percentage at their seedling stage (Figs. 1a, 1b). It should be mentioned that in our previous study, BD-10906 and BD-10912 were the most drought tolerant genotypes in germinating stage, and had low death and high recovery percentage in seedling stage [15]. In contrast, the water stress sensitive genotypes BD-10902 and RT-20 had higher death and lower recovery percentage at seedling stage. From our previous and current studies, its indicated that genotypes that are tolerant to drought stress at germination stage are also tolerant to drought stress at seedling stage, and those genotypes which are sensitive to water stress at germination stage are sensitive to drought stress at their seedling stage as well. It was also reflected that genotypes with higher death percentage had lower survival rate in recovery (Tab. 1 and Figs. 1a, 1b). These findings are supported by Ghebremariam et al. [14] in tomato where tolerant and susceptible tomato genotypes had similar tolerability and susceptibility, respectively to drought stress during both germination and seedling stages.
4.2 Evaluation of Morphological Parameters
A well-developed root system in drought stress is an criterion to select drought tolerant genotypes. Because, such type root system can uptake soil moisture from lower layers of soil, and thus, it is the most important trait for selecting genotypes at seedling stage [22–24]. Therefore, the genotypes with higher root length and volume show resistance to drought stress [12,25,26]. In this investigation, drought treated plants had lower length and dry weight of root than the controls, and drought susceptible genotypes BD-10902 and RT-20 showed higher reduction than the tolerant genotypes during drought and recovery period (Figs. 3a, 3b and 5a). Similar results were noticed in shoot length and dry weight (Figs. 4 and 5b). The reduction of root-shoot length may be due to an impediment of cell division and elongation [27,28] resulting in decrease of root-shoot length and finally reduced dry weight [10]. This is consistent with previous studies of Farooq et al. [12] and Mahmoud et al. [29] who have shown that drought stress reduces the root-shoot growth in safflower and wheat, respectively. Pace et al. [30] suggested that increased tap root length, at the expense of thickening, in response to drought was a common response in cotton. This response may permit cotton plants to survive drought by accessing water from deeper in the soil profile than the soil horizons tapped during periods of recovery. Ahmad et al. [31] reported reduced shoot dry weight under water stress conditions for various wheat genotypes, which also supported our results. This reduction may be due to suppression of cell expansion and cell growth resulting from low turgor pressure [32,33]. Accordingly, the root-shoot ratio (RSR) was reduced in the drought stress treated seedling of both tolerant and susceptible genotypes than their respective control, whereas tolerant plants contained more root-shoot ratio compared to susceptible one (Fig. 5c). Besides, tolerant genotypes were able to revive again with higher RSR in recovery (Fig. 5c). The results implicated that tolerant genotypes with a higher proportion of roots could compete more effectively for soil nutrients, while those with a higher proportion of shoots could collect more light energy. However, several studies reported the seedling growth parameters such as root and shoot length, ratio and dry weight as useful traits in selection of tolerant genotypes to drought stress in tomato [14,34], wheat [10,29], safflower [12] and cotton [30].
The leaf area of a plant is the best measure of photosynthetic size. In this experiment, detrimental effects of drought stress on leaf area and petiole weight were determined in all tested genotypes. The effect of the stress were higher in sensitive genotypes, and failed to increase leaf area and petiole weight in recovery period (Figs. 6a and 6b). The mechanism, by which plant leaf area was reduced under drought stress, is through reduction of cell division leading to the reduction of cell size [35]. Previous studies conducted by Boutraa et al. [36] and Foulkes et al. [37] reported more reduction in leaf area of susceptible wheat genotypes in drought. Pace et al. [30] reported that photosynthate partitioning in the drought-treated tolerant plants of cotton was apparently preferentially directed to leaf area expansion during the recovery period.
4.3 Evaluation of Physiological and Biochemical Parameters
Since relative water content (RWC) relates well with cell volume, it can accurately indicate the balance between absorbed water by plant and that lost through transpiration [38,39] hence, it is considered as an important marker for selecting tolerant plants to drought [40–43]. In the present investigation, a decrease in RWC was observed with drought treatment both in tolerant and susceptible genotypes where more detrimental effects were observed in susceptible plants which still declined in recovery period. However, an increasing trend in RWC in tolerant plants during recovery period (Fig. 7). Similar results in RWC due to drought stress were reported earlier. While screening for water stress tolerance in long storage tomato genotypes, it was observed that leaf RWC significantly decreased as drought stress progressed [44]. In another water stress tolerance study in wheat by Arjenaki et al. [45], it was shown that wheat cultivars having higher RWC are more tolerant to drought stress. Thus, the higher RWC obtained in stress tolerance plants is a consequence of better water regulation during transpiration and photosynthesis processes [46,47].
Photosynthetic pigments remain major drivers of plants’ photosynthetic capacity due to their crucial role in both absorptions (chlorophyll) and dissipation (carotenoids) of light energy [48]. The loss of photosynthetic pigment levels in abiotic stress including drought is one of the main causes of inactivation of photosynthesis. Thus, a reduction in photosynthetic capacity in plants exposed to water stress is expected. Numerous studies reported that inhibition of photosynthesis was attributed to destroying the photosynthetic pigments or chlorophyll due to oxidation of pigments or simply preventing its synthesis [49–53] and this process might be derived with ROS, mainly H2O2 that is accumulated under water stress because it provokes the inactivation/oxidation of the pigments pre-existing in chloroplasts [54]. The decrease in Chl a, Chl b, total Chl and Car is commonly observed phenomenon under drought stress. In a study by Pirzad et al. [55], it was observed that water stress; both excess and deficit water significantly decreases leaf chlorophyll [Chl a, b and total Chl] concentrations. In this study, drought stress caused significant reduction in the contents of Chl and Car in both tolerant (BD-10906 and BD-10912) and susceptible (BD-10902 and RT-20) chilli genotypes (Figs. 8a–8d). However, the pigment reduction was higher in sensitive seedlings of chilli as compared to tolerant seedlings. This reduction of Chl and Car under water stress could be due to the increased activity of chlorophyllase enzyme or due to the disruption of fine structure of chloroplast and instability of pigment protein complexes by ions [56]. Higher Chl and Car in drought tolerant genotypes suggested better photosynthetic capacity under drought stress [10] as well as a better tolerant mechanism which prevented Chl degradation in tolerant genotypes than susceptible ones. Similar results were observed by other others who reported that Chl contents of drought resistant genotypes of barley, wheat, and maize were higher compared to sensitive genotypes in drought stress conditions [45,57,58]. In recovery, removal of drought stress led to increase concentration of chlorophyll (Chl a, Chl b, total Chl and Car) in tolerant genotypes (Figs. 8a–8d). These increases suggested their role in photosynthesis and protective role from active stomatal regulation along with a balance of relative water content consequently, actively functioning to scavenge ROS [59–61] and thereby reducing the Chl degradation under stress condition. Menconi et al. [62] opined that in well water condition, gas exchange parameters retrieved and this could be due to an increase in leaf expansion and photosynthetic pigments for functioning photosynthetic machinery. In the present study, both leaf area and photosynthetic pigments increased in tolerant genotypes in recovery and this increment suggested their positive correlation to each other.
Proline, an essential amino acid, is known to participate in the biosynthesis of primary metabolism during growth and development [63,64]. Proline also accumulates in response to the imposition of a wide range of stress responses in plants.
It has been well documented as an osmotic regulator helping in the reduction of osmotic damage [65,66]. It is further hypothesized that the accumulation of Pro in leaves could play a protective role aside from osmoregulation during water stress [67]. Dien et al. [68] suggested that osmotic adjustment is a biochemical mechanism that helps plants to acclimate to water deficit. One mechanism for osmotic adjustment is the accumulation of compatible solutes, such as the amino acid; Pro. The results of the present study expressed that Prol was highly accumulated in leaves of tolerant chilli seedling under drought stress compared to control, and that removal of water stress resulted in more Pro accumulation in susceptible genotypes (Fig. 9). These results agreed with other previous studies, which mentioned that Pro is highly accumulated by the tolerant plant under drought stress. In another study by Dien et al. [68] proline content was significantly higher in tolerant rice cv. DA8 and Thierno Bande variety under drought stress conditions. Similar results were also obtained by Ranganayakulu et al. [69] who reported that accumulation of Pro content was increased in both drought treated tolerant and susceptible cultivars, but the percent of increase was higher in tolerant cv. K134 than the susceptible cv. JL-24.
At the end of the recovery period, the Pro content of the tolerant genotypes decreased rapidly, reaching values similar to those under the control condition (Fig. 9). Several studies have indicated that Pro accumulated during episodes of water stress is lost rapidly when the water deficit or abundance is eliminated [68,69]. Once water stress is removed, Pro is oxidized into 1-pyrroline-5-carboxylate (P5C) by proline dehydrogenase, also known as proline oxidase, the first enzyme in the Pro degradation pathway. Then, P5C is converted back into glutamate by the enzyme P5C dehydrogenase [70].
4.3.4 DPPH Radical Scavenging Activity
Antioxidant constituents of plant origin are very important substances that have the ability to defend the body from injuries caused by free radical induced oxidative stress [71]. BD-10906 and BD-10912 are potential candidates of antioxidant activity as their scavenging percentage was found to be higher than the susceptible genotypes BD-10902 and RT-20. Moreover, BD-10906 and BD-10912 showed the obvious tolerance as their scavenging percentage was further increased with the removal of drought stress (Fig. 10). High level of DPPH activity has been correlated with tolerance to different stress conditions [72].
4.4 Stomatal Behavior and Density Observation
The ability of plants to be able to regulate the size of the stomatal opening is a very important mechanism to control water loss and survive. This ability is especially important during water stress when the loss of water can have serious consequences for the plants. Water stress can cause reduced growth and in severe cases caused plant death.
Since stomatal closure has negative effects on CO2 uptake, photosynthesis, transpirational cooling as well as water and nutrient uptake, it is important to close the stomata only when the benefit of water retention outweighs the negative effects [73]. The efficiency of stomatal openings is not only determined by the size of the opening, but also by the number of stomata [74]. In this investigation, results exhibited that the genotypes BD-10906 and BD-10912 showing drought tolerancehad more number of stomata under stress condition, and most of them are visually closed (Figs. 11a and 11b). However, an opposite trend was observed in respect of susceptible genotypes. These findings are in agreement with the outcomes of Kusvuran et al. [75] who found more closure stomata in drought tolerant that those in susceptible genotypes melon. Previously, Mehri et al. [76] showed higher number of stomata in drought tolerant wheat genotypes than sensitive genotypes. Nonetheless, most of the stomata were opened among the observation in susceptible genotypes. Stomatal closure occurs when the two guard cells surrounding the stomata opening lose turgor pressure and close the opening [77]. Many signals induce stomatal closure, among these the best-known signal is probably abscisic acid (ABA) [73]. In this study, although stomatal limitation might be occurred in tolerance genotypes, escaping non-stomatal limitation to photosynthesis which includes reducing leaf water potential and relative water content which may reduce efficiency and activity of RuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) enzyme thereby limiting carbon dioxide fixation. Yuan et al. [61] reported that under moderate to severe water stress, non-stomatal limitation is the primary cause of decline in photosynthesis. Moreover, these tolerant genotypes might maintain active stomatal regulation and able to reduce stomatal conductance under drought stress.
In the present experiment, the role of the morphological, physiological, biochemical and anatomical parameters were studied and compared between tolerant and susceptible genotypes in their adaptation to drought stress conditions in seedling stage. Our findings reveal that a comparatively longer and dense root system in tolerant genotypes than those in susceptible genotypes might help to improve water uptake and maintain turgor and osmotic adjustment in drought stress. Moreover, expanded leaf contained a higher amount of photosynthetic pigments maintained photosynthetic activity. Higher accumulation of stress metabolites like Pro could prevent tolerant genotypes from osmotic damages. Thus, tolerant genotypes showed higher antioxidant activity compared to susceptible genotypes. Additionally, tolerant genotypes rapidly closed stomata which may positively contribute to the level of tolerance under drought stress. Distinct changes among the tolerant and susceptible genotypes in respect of morphological, physiological, biochemical, anatomical and total antioxidative parameters have been identified in this investigation, and these findings create an opportunity to confirm ROS, enzymatic and non-enzymatic antioxidant, glyoxalases and their related metabolites as these are directly related with tolerant and susceptible mechanism of the plants.
Acknowledgement: The authors are thankful to Md. Robyul Islam for their nice cooperation during study period and critical criticism in preparation of this manuscript.
Funding Statement: The cost of reagents of the study was supported by the R&D projects of Ministry of Science and Technology, Government of the Bangladesh.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, W., Vinocur, B., Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta, 218(1), 1–14. DOI 10.1007/s00425-003-1105-5. [Google Scholar] [CrossRef]
2. Bayoumi, T. Y., Manal, H. E., Metwali, E. M. (2008). Application of physiological and biochemical indices as a screening technique for drought tolerant in wheat genotypes. African Journal of Biotechnology, 7, 2341–2352. [Google Scholar]
3. Bangladesh Bureau of Statistics (BBS) (2019). Year book of agricultural statistics 2018, bangladesh bureau of statistics. Dhaka: Ministry of Planning, Government of the Bangladesh. [Google Scholar]
4. Sezen, S. M., Yazar, A., Eker, S. (2006). Effect of drip irrigation regimes on yield and quality of field grown bell pepper. Agricultural Water Management, 81(1–2), 115–131. DOI 10.1016/j.agwat.2005.04.002. [Google Scholar] [CrossRef]
5. Pessarakli, M. (1999). Hand book of plant and crop stress. 2nd Edition. New York, USA: Marcel Dekker Inc. [Google Scholar]
6. Misra, A. N., Misra, N., Singh, R. (2011). Nictric oxide ameliorates stress responses in plants. Plant, Soil and Environment, 57(3), 95–100. DOI 10.17221/202/2010-PSE. [Google Scholar] [CrossRef]
7. Dey, N. C., Alam, M. S., Sajjan, A. K., Bhuiyan, M. A., Ghose, L. et al. (2012). Assessing environmental and health impact of drought in the northwest Bangladesh. Journal of Environmental Science and Natural Resources, 4(2), 89–97. DOI 10.3329/jesnr.v4i2.10141. [Google Scholar] [CrossRef]
8. Awan, S. I., Ahmed, M. S., Farooq, J., Ahmad, S. D., Ilyas, M. et al. (2011). Genetic model analysis on seedling and maturity traits in wheat under rainfed conditions. Frontiers of Agriculture in China, 5(4), 486–496. DOI 10.1007/s11703-011-1113-3. [Google Scholar] [CrossRef]
9. Baloch, M. J., Dunwell, J., Khakwani, A. A., Dennett, M., Jatoi, W. A. et al. (2012). Assessment of wheat cultivars for drought tolerant via osmotic stress imposed at early seedling growth stages. Journal of Agricultural Research, 50(3), 299–310. [Google Scholar]
10. Ahsan, A. F. M. S. (2018). Physiological and biochemical mechanisms of drought tolerant in wheat, (Ph.D. Dissertation). Mymensingh, Bangladesh: Department of Agronomy, Bangladesh Agricultural University. [Google Scholar]
11. Kim, Y. H., Hwang, S. J., Waqas, M., Khan, A. L., Lee, J. H. et al. (2015). Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Frontiers in Plant Science, 6(63), 963525. DOI 10.3389/fpls.2015.00714. [Google Scholar] [CrossRef]
12. Farooq, A., Bukhari, S. A., Akram, N. A., Ashraf, M., Wijaya, L. et al. (2020). Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants, 9(1), 104. DOI 10.3390/plants9010104. [Google Scholar] [CrossRef]
13. Gotame, T. P. (2006). Morphological and physiological responses of tomato cv. NCL 1 subjected to excess water stress. In: Karmacharya, S. B., Dhakal, M. R., Jha, S. N., Mandal, T. N., Chettri, M. K., Subba, B. R., Koirala, U., Niroula, B., Limbu, K. P. (eds.Natural resource management, pp. 445–450. P. G. Campus, Biratnagar, Nepal Biological Society, Biratnagar & Ecological Society, Kathmandu, Nepal. [Google Scholar]
14. Ghebremariam, K. M., Liang, Y., Li, C., Li, Y., Qin, L. (2013). Screening of tomato inbred-lines for drought tolerance at germination and seedling stage. Journal of Agricultural Science, 5(11), 1916–9760. DOI 10.5539/jas.v5n11p93. [Google Scholar] [CrossRef]
15. Molla, M. R., Ahmed, I., Ara, R., Hassan, L., Rohman, M. M. (2019). Screening of chilli (Capsicum annum L.) genotypes for drought tolerant at seedling emergence stage. Journal of Plant Sciences, 7(4), 76–85. DOI 0.11648/j.jps.20190704.12. [Google Scholar]
16. Savita (2016). Effect of waterlogging, salinity and their interaction on growth, oxidative and carbohydrate metabolism in pigeon pea (Cajanus cajan L. Millsp.) genotypes (Ph.D. Thesis). Hisar, India: Department of Botany and Plant Physiology, Chaudhary Charan Singh Haryana Agricultural University. [Google Scholar]
17. Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiology, 24(1), 1–15. DOI 10.1104/pp.24.1.1. [Google Scholar] [CrossRef]
18. Kirk, J. T. O., Allen, R. L. (1965). Dependence of chloroplast pigment synthesis on protein sunthesis: Effect of actidione. Biochemical and Biophysical Research Communications, 21(6), 523–530. DOI 10.1016/0006-291X(65)90516-4. [Google Scholar] [CrossRef]
19. Bates, L. S., Waldren, R. P., Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
20. Sarker, U., Oba, S. (2019). Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Scientific Reports, 9(1), 168. DOI 10.1038/s41598-019-52033-8. [Google Scholar] [CrossRef]
21. Sarker, U., Oba, S. (2019b). Nutrients, minerals, antioxidant pigments and phytochemicals, and antioxidant capacity of the leaves of stem amaranth. Scientific Reports, 9(1), 20413. DOI 10.1038/s41598-019-50977-5. [Google Scholar] [CrossRef]
22. Morgan, J. M., Hare, R. A., Fletcher, R. J. (1986). Genetic variation in osmo-regulation in bread and durum wheat and its relationship to grain yield in a range of field environments. Australian Journal of Agricultural Research, 37(5), 449–457. DOI 10.1071/AR9860449. [Google Scholar] [CrossRef]
23. Dhanda, S. S., Behl, R. K., Elbassam, L. (1995). Breeding wheat genotypes for water deficit environments. Landbanforschung Volkenrode, 45, 159–167. [Google Scholar]
24. Baloch, M. J., Dunwell, J., Khakwani, A. A., Dennett, M., Jatoi, W. A. (2012). Assessment of wheat cultivars for drought tolerant via osmotic stress imposed at early seedling growth stages. Journal of Agricultural Research, 50(3), 299–310. [Google Scholar]
25. Leishman, M. R., Westoby, M. (1994). The role of seed size in seedling establishment in dry soil conditions-experimental evidence from semi-arid species. Journal of Ecology, 82(2), 249–258. DOI 10.2307/2261293. [Google Scholar] [CrossRef]
26. Cardoso, J. A., Jiménez, J. C., Rao, I. M. (2014). Waterlogging-induced changes in root architecture of germplasm accessions of the tropical forage grass Brachiaria humidicola. AoB Plants, 6(Suppl. A), 91. DOI 10.1093/aobpla/plu017. [Google Scholar] [CrossRef]
27. Raziuddin, Swati, Z. A., Bakht, J., Farhatullah, Khan, N. U. et al. (2010). In situ assessment of morpho-physiological responses of wheat (Triticum aestivum L.) genotypes to drought. Pakistan Journal of Botany, 42, 3183–3195. [Google Scholar]
28. Khakwani, A. A., Dennett, M. D., Munir, M. (2011). Early growth response of six wheat varieties under artificial osmotic stress condition. Pakistan Journal of Agricultural Sciences, 48, 118–123. [Google Scholar]
29. Mahmoud, A. E., Mohamed, I. H. (2014). A diallel analysis of drought tolerance indices at seedling stage in bread wheat (Triticum aestivum L.). Plant Breeding and Biotechnology, 2(3), 276–288. DOI 10.9787/PBB.2014.2.3.276. [Google Scholar] [CrossRef]
30. Pace, P. F., Cralle, H. T., El-Halawany, S. H. M., Cothren, J. T., Senseman, S. A. (1999). Drought-induced changes in shoot and root growth of young cotton plants. Journal of Cotton Science, 3(4), 183–187. [Google Scholar]
31. Ahmad, F., Rahmatullah, Aziz, T., Maqsood, M. A., Tahir, M. A., Kanwal, S. (2007). Effect of silicon application on wheat (Triticum aestivum L.) growth under water deficiency stress. Emirates Journal of Food and Agriculture, 19(2), 1–7. DOI 10.9755/ejfa.v12i1.5170. [Google Scholar] [CrossRef]
32. Rouhi, V., Samson, R., Lemeur, R., Damme, P. V. (2007). Photosynthetic gas exchange characteristics in three different almond species during drought stress and subsequent recovery. Environmental and Experimental Botany, 59(2), 117–129. DOI 10.1016/j.envexpbot.2005.10.001. [Google Scholar] [CrossRef]
33. Jaleel, C. A., Gopi, R., Sankar, B., Gomathinayagam, M., Panneerselvam, R. (2008). Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. Comptes Rendus Biologies, 331(1), 42–47. DOI 10.1016/j.crvi.2007.11.003. [Google Scholar] [CrossRef]
34. Rana, M. K., Kalloo (1989). Morphological attributes associated with the adaptation under water deficit condition in tomato. Journal of Vegetation Science, 16(1), 32–38. [Google Scholar]
35. Schuppler, U., He, P. H., Peter, C. L. J., Munns, J. R. (1998). Effect of water stress on cell division and cell-division-cycle 2-like cell-cycle kinase activity in wheat leaves. Plant Physiology, 117(2), 667–678. DOI 10.1104/pp.117.2.667. [Google Scholar] [CrossRef]
36. Boutraa, T., Akhkha, A., Al-Shoaibi, A. A., Alhejeli, A. M. (2018). Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. Journal of Taibah University for Science, 3(1), 39–48. DOI 10.1016/S1658-3655(12)60019-3. [Google Scholar] [CrossRef]
37. Foulkes, M. J., Sylvester-Bradley, R., Weightman, R., Snape, J. W. (2007). Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Research, 103(1), 11–24. DOI 10.1016/j.fcr.2007.04.007. [Google Scholar] [CrossRef]
38. Hassanzadeh, M., Ebadi, A., Panahyan-e-Kivi, M., Eshghi, A. G., Jamaati-e- Somarin, S. et al. (2009). Evaluation of drought stress on relative water content and chlorophyll content of sesame (Sesamum indicum L.) genotypes at early flowering stage. Research Journal of Environmental Sciences, 3(3), 345–350. DOI 10.3923/rjes.2009.345.350. [Google Scholar] [CrossRef]
39. Lugojan, C., Ciulca, S. (2011). Evaluation of relative water content in winter wheat. Journal of Horticulture, Forestry and Biotechnology, 15, 173–177. [Google Scholar]
40. Kumutha, D., Sairam, R. K., Meena, R. C. (2008). Role of root carbohydrate reserves and their mobilization in imparting waterlogging tolerance in greengram [(Vigna radiata (L.) Wilczek)] genotypes. Indian Journal of Plant Physiology, 33, 735–744. [Google Scholar]
41. Sairam, R. K., Kumutha, D., Ezhilmathi, K., Chinnusamy, V., Meena, R. C. (2009). Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeonpea. Biologia Plantarum, 53(3), 493–504. DOI 10.1007/s10535-009-0090-3. [Google Scholar] [CrossRef]
42. Prasanna, Y. L., Ramarao, G. (2014). Effect of waterlogging on physiological and biochemical parameters and seed yield in greengram genotypes. International Journal of Food, Agriculture and Veterinary Sciences, 4(2), 176–183. [Google Scholar]
43. Soltys-Kalina, D., Plich, J., Strzelczyk-Żyta, D., Śliwka, J., Marczewski, W. (2016). The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breeding Science, 66(2), 328–331. DOI 10.1270/jsbbs.66.328. [Google Scholar] [CrossRef]
44. Patane, C., Scordia, D., Testa, G., Cosentino, S. L. (2016). Physiological screening for drought tolerance in Mediterranean long-storage tomato. Plant Science, 249, 25–34. DOI 10.1016/j.plantsci.2016.05.006. [Google Scholar] [CrossRef]
45. Arjenaki, F. G., Jabbari, R., Morshedi, A. (2012). Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. International Journal of Agriculture and Crop Sciences, 4(11), 726–729. DOI cabdirect.org/cabdirect/abstract/20123364516. [Google Scholar]
46. Jones, H. G. (1998). Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany, 49, 387–398. DOI 10.1093/jxb/49.Special_Issue.387. [Google Scholar] [CrossRef]
47. Lobato, A. K. S., Costa, R. C. L., Neto, M. A. M., Oliveira Neto, C. F., Santos Filho, B. G. et al. (2009). Consequences of the water deficit on nitrogen compounds in pepper (cv. Vermelho gigante) plants. Research Journal of Biological Sciences, 4, 760–764. [Google Scholar]
48. Kamanga, M. R., Mbega, E., Ndakidem, P. (2018). Drought tolerance mechanisms in plants: Physiological responses associated with water deficit stress in Solanum lycopersicum. Advances in Crop Science and Technology, 6(3), 362. DOI 10.4172/2329-8863.1000362. [Google Scholar] [CrossRef]
49. Montagu, K. D., Woo, K. C. (1999). Recovery of tree photosynthetic capacity from seasonal drought in the wet-dry tropics: the role of phyllode and canopy processes in Acacia auriculiformis. Functional Plant Biology, 26(2), 135–145. DOI 10.1071/PP98034. [Google Scholar] [CrossRef]
50. Anjum, S. A., Xie, X., Farooq, M., Wang, L., Xue, L. et al. (2011). Effect of exogenous methyl jasmonate on growth, gas exchange and chlorophyll contents of soybean subjected to drought. African Journal of Biotechnology, 10(47), 9640–9646. DOI 10.5897/AJB10.2641. [Google Scholar] [CrossRef]
51. Saraswathi, S. G., Paliwal, K. (2011). Drought induced changes in growth, leaf gas exchange and biomass production in Albizia lebbeck and Cassia siamea seedlings. Journal of Environmental Biology, 32, 173–178. [Google Scholar]
52. Chakraborty, U., Pradhan, B. (2012). Oxidative stress in five wheat varieties (Triticum aestivum L.) exposed to water stress and study on their antioxidant enzyme defense system, water stress responsive metabolites and H2O2 accumulation. Brazil Journal of Plant Physiology, 24(2), 117–130. DOI 10.1590/S1677-04202012000200005. [Google Scholar] [CrossRef]
53. Rohman, M. M., Talukder, M. Z. A., Hossain, M. G., Uddin, M. S., Amiruzzaman et al. (2016). Saline sensitivity leads to oxidative stress and increases the antioxidants in presence of proline and betaine in maize (Zea mays L.) inbred. Plant Omics, 9(1), 35–47. DOI 10.21475/poj.16.09.04.pne31. [Google Scholar] [CrossRef]
54. Almási, A., Apatini, D., Bóka, K., Böddi, B., Gáborjányi, R. (2000). BSMV infection inhibits chlorophyll biosynthesis in barley plants. Physiological and Molecular Plant Pathology, 56(6), 227–233. DOI 10.1006/pmpp.2000.0266. [Google Scholar] [CrossRef]
55. Pirzad, A., Shakiba, M. R., Zehtab-Salmasi, S., Mohammadi, S. A., Darvishzadeh, R. et al. (2011). Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. Journal of Medicinal Plants Research, 5, 2483–2488. DOI 10.5897/JMPR.9000503. [Google Scholar] [CrossRef]
56. Sevengor, S., Yasar, F., Kusvuran, S., Ellialtioglu, S. (2011). The effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidative enzymes of pumpkin seedling. African Journal of Agricultural Research, 6(21), 4920–4924. DOI 10.5897/AJAR11.668. [Google Scholar] [CrossRef]
57. Pastori, G. M., Trippi, V. S. (1992). Oxidative stress induces high rate of glutathione reductase synthesis in a drought resistant maize strain. Plant and Cell Physiology, 33, 957–961. DOI 10.1093/oxfordjournals.pcp.a078347. [Google Scholar] [CrossRef]
58. Rong-Hua, L. I., Guo, G. U. O. P., Baum, M., Grando, S., Ceccarelli, S. (2006). Evaluation of chlorophyll content and florescence parameters in indicators of drought tolerance in barley. Agriculture Science (China), 5(10), 751–757. DOI 10.1016/S1671-2927(06)60120-X. [Google Scholar] [CrossRef]
59. Alam, M. M., Nahar, K., Hasanuzzaman, M., Fujita, M. (2014). Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics, 7(4), 271. DOI 10.1016/S1671-2927(06)60120-X. [Google Scholar] [CrossRef]
60. Rohman, M. M., Begum, S., Talukder, M. Z. A., Akhi, A. H., Amiruzzaman, M. (2016b). Drought sensitive maize inbred shows more oxidative damage and higher ROS scavenging enzymes, but not glyoxalases than a tolerant one at seedling stage. Plant Omics, 9(4), 220–232. DOI 10.21475/poj.16.09.04.pne31. [Google Scholar] [CrossRef]
61. Yuan, X. K., Yang, Z. Q., Li, Y. X., Liu, Q., Han, W. (2016). Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica, 54(1), 28–39. DOI 10.1007/s11099-015-0122-5. [Google Scholar] [CrossRef]
62. Menconi, M., Sgherri, C. L. M., Pinzino, C., Navari-Izzo, F. (1995). Activated oxygen production and detoxification in wheat plants subjected to a water deficit programme. Journal of Experimental Botany, 46(9), 1123–1130. DOI 10.1093/jxb/46.9.1123. [Google Scholar] [CrossRef]
63. Hare, P. D., Cress, W. A., van Staden, J. (1999). Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. Journal of Experimental Botany, 50(333), 413–434. DOI 10.1093/jxb/50.333.413. [Google Scholar] [CrossRef]
64. Funck, D., Winter, G., Baumgarten, L., Forlani, G. (2012). Requirement of proline synthesis during Arabidopsis reproductive development. BMC Plant Biology, 12(1), 191–203. DOI 10.1186/1471-2229-12-191. [Google Scholar] [CrossRef]
65. Slama, I., Ghnaya, T., Savouré, A., Abdelly, C. (2008). Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. Comptes Rendus Biologies, 331(6), 442–451. DOI 10.1016/j.crvi.2008.03.006. [Google Scholar] [CrossRef]
66. Reddy, P. S., Jogeswar, G., Rasineni, G. K., Maheswari, M., Reddy, A. R. et al. (2015). Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiology and Biochemistry, 94, 104–113. DOI 10.1016/j.plaphy.2015.05.014. [Google Scholar] [CrossRef]
67. Jungklang, J., Saengnil, K., Uthaibutra, J. (2017). Efects of water-defcit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi Journal of Biological Sciences, 24(7), 1505–1512. DOI 10.1016/j.sjbs.2015.09.017. [Google Scholar] [CrossRef]
68. Dien, D. C., Mochizuki, T., Yamakawa, T. (2019). Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Production Science, 22(4), 530–545. DOI 10.1080/1343943X.2019.1647787. [Google Scholar] [CrossRef]
69. Ranganayakulu, G. S., Sudhakar, C., Sivakumar, R. P. (2015). Effect of water stress on proline metabolism and leaf relative water content in two high yielding genotypes of groundnut (Arachis hypogaea L.) with contrasting drought tolerance. Journal of Experimental Biology and Agricultural Sciences, 3(1), 97–103. [Google Scholar]
70. Hare, P. D., Cress, W. A., Staden, J. V. (1998). Dissecting the roles of osmolyte accumulation during stress. Plant, Cell and Environment, 21(6), 535–553. DOI 10.1046/j.1365-3040.1998.00309.x. [Google Scholar] [CrossRef]
71. Ahmad, N., Fazal, H., Abbasi, B. H., Rashid, M., Mahmood, T. et al. (2010). Efficient regeneration and antioxidant potential in regenerated-tissues of Piper nigrum L. Plant Cell, Tissue and Organ Culture (PCTOC), 102(1), 129–134. DOI 10.1007/s11240-010-9712-x. [Google Scholar] [CrossRef]
72. Kang, H. M., Salveit, M. E. (2002). Reduced chilling tolerance in elongating cucumber seedling. Radicals is related to their reduced antioxidant enzyme and DPPH radical scavenging activity. Physiologia Plantarum, 115(2), 244–250. DOI 10.1034/j.1399-3054.2002.1150210.x. [Google Scholar] [CrossRef]
73. Arve, L. E., Torre, S., Olsen, J. E., Tanino, K. K., Shanker, A. et al. (2011). Stomatal responses to drought stress and air humidity. In: Shanker, A., Venkateswarlu, B. (eds.Abiotic stress in plants- mechanisms and adaptations. Intech Open. [Google Scholar]
74. Metwally, A. W., Beck, G., Struckme, B. E. (1971). Density and behavior of stomata of Pelargonium hortorum-Grown under 3 soil moisture regimes. Journal of the American Society for Horticultural Science, 96, 31. [Google Scholar]
75. Kusvuran, S., Dasgan, H. Y., Kuçukkomurcu, S., Abak, K. (2010). Relationship between drought tolerance and stomata density in melon. Acta Horticulturae, 871, 291–300. DOI 10.17660/ActaHortic.2010.871.39. [Google Scholar] [CrossRef]
76. Mehri, N., Fotovat, R., Sba, J., Jabbari, F. (2009). Variation of stomata dimensions and densities in tolerant and susceptible wheat cultivars under drought stress. Journal of Food Agriculture and Environment, 7(1), 167–170. [Google Scholar]
77. Outlaw, W. H. (2003). Integration of cellular and physiological functions of guard cells. Critical Reviews in Plant Sciences, 22(6), 503–529. DOI 10.1080/713608316. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |