
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014826
REVIEW
Morpho-Physiological, Biochemical and Molecular Adaptation of Millets to Abiotic Stresses: A Review
1Department of Bioresources, School of Biological Sciences, University of Kashmir, Srinagar, 190006, India
2Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
*Corresponding Authors: Khalid Rehman Hakeem. Email: kur.hakeem@gmail.com; Reiaz Ul Rehman. Email: rreaizbiores@gmail.com
Received: 02 November 2020; Accepted: 12 February 2021
Abstract: Abiotic stresses such as drought, heat, cold, nutrient deficiency, excess salt and hazardous metals can hamper plantgrowth and development. Intensive agriculture of only a few major staple food crops that are sensitive and intolerant to environmental stresses has led to an agrarian crisis. On the other hand, nutritionally rich, gluten free and stress tolerant plants like millets are neglected and underutilized. Millets sustain about one-third of the world’s population and show exceptional tolerance to various abiotic and biotic stresses. Millets are C4 plants that are adapted to marginal and dry lands of arid and semi-arid regions, and survive low rainfall and poor soils. Abiotic stresses significantly affect plant growth which ultimately results in reduced crop yields. However, various adaptation mechanisms have evolved in millets to withstand different stresses. This review aims at exploring various of these morphophysiological, biochemical and molecular aspects of mechanisms in millets. Morphological adaptations include short life span, smallplant height and leaf area, dense root system, adjusted flowering time, increased root and decreased shoot lengths, high tillering, and leaf folding. A high accumulation of various osmoprotectants (proline, soluble sugars, proteins) improves hyperosmolarity and enhances the activity of antioxidant enzymes (e.g., Ascorbate peroxidase, Superoxide dismutase, Catalase, Peroxidase) providing defense against oxidative damage. Physiologically, plants show low photosynthetic and stomatal conductance rates, and root respiration which help them to escape from water stress. Molecular adaptations include the upregulation of stress-related transcriptional factors, signalling genes, ion transporters, secondary metabolite pathways, receptor kinases, phytohormone biosynthesis and antioxidative enzymes. Lack of genetic resources hampers improvement of millets. However, several identified and characterized genes for stress tolerance can be exploited for further development of millet resilience. This will provide them with an extra characteristic plant resistance to withstand environmental pressures, besides their excellent nutritional value over the conventional staple crops like rice, wheat and maize.
Keywords: Millets; adaptation; abiotic stress; osmoprotectants; antioxidants; transcriptomics
Millets are small seeded, annual C4 grasses grown for both food and fodder [1]. They belong to the family Poaceae, comprising Pearl millet (Pennisetum glaucum (L.) R.Br.), Foxtail millet (Setaria italica (L.) P. Beauvois), Common millet (Panicum miliaceum L.), Finger millet (Eleusine coracana Gaertn.), Barnyard Millet (Echinochloa utilis Ohwi & Yabuno; Echinochloa frumentacea Link), Little Millet (Panicum sumatrense Roth. ex Roem. & Schultz), Kodo millet (Paspalum scrobiculatum L.) and Teff millet (Eragrostis tef (Zucc.) Trotter). Millets are adapted to marginal and dry lands of arid and semi-arid regions, and show exceptional tolerance to various abiotic stresses [2]. The total world production of millets in 2018 was estimated to be 31,019,370 tonnes (FAO 2020) [3]. Millets account for only 2% of the world cereal production, and 95% of the world millet production comes from Asia and Africa (Fig. 1). Millets are a good source of energy and essential nutrients, and thus serve as the food source of for millions of people across the globe. They are superior to cereals in various beneficial components such as dietary fibre, micro and macro nutrients and bioactive components. Millets are the chief food of small farmer communities of India, Africa, China, and some parts of Central America, and ensure food security to low income generating countries of Asia and Africa [4]. To bring millets back to the mainstream and gain benefits from their nutritious properties, 2018 was declared as the “National Year of Millets” by the Indian Government. Also, FAO declared that the year 2023 willbe the “International Year of Millets” to increase the production and productivity of millets throughout the globe [5]. Major cereals such as rice, wheat, and maize have a significant global warming potential due to their high carbon emission rates. Millets, on the other hand, have comparatively lower carbon footprints [6]. They are considered as models for studying C4 photosynthesis, stress biology, and biofuel traits; this has led to studies on structural and functional genomics of foxtail millet [7]. Currently, this is the age of an agrarian crisis which has called for crop improvement under the detrimental effects of climate change. Intensive agriculture of a few crops for food requirements has led to inadequate nutrition, and genetic erosion, and has forced to neglect local nutritionally-rich crops. “Millets” are neglected and underutilized crops which are nutritionally-rich and gluten free. Being the 6th most important crop in the world, Millets are used for the purpose of food, feed and fodder. These are known as poor man’s crops and sustain about one-third of the world’s population [8]. Among the various elite traits of foxtail millet are its tolerance to various abiotic stresses (e.g., drought and salinity), less fertilizer requirement, and higher photosynthetic efficiency than C3 plants, and its ability to grow on less fertile lands [9]. Finger millet can resist storage pests for as long as 10 years and hence it has earned thepopular name of ‘’Famine Crop’’ [10]. Millets provide the poor people with nutritional security in these regions, but lacks adequate scientific attention which restricts it mainly to regions of major cereal crops. Millets, popularly known as minor cereals, have been given very little attention for their improvement, however, this could be easily done by the development of genetic resources [11]. Drought, temperature extremes (e.g., heat, cold), nutrient deficiency, salinityand heavy metals are categorized as abiotic stresses. These factors threaten the food security and plant production [12]. Abiotic stress conditions lead to the accumulation of reactive oxygen species (ROS), causing extreme cell damage and inhibition of photosynthesis [13]. With the increasing population, agriculture is currently facing a tough time due to the unavailability of land and water, and climatechange. The problem can be solved to a large extent by the use of naturally stress resistant plants (NSRP’s), which ensures yield stability, global food security and health. These NSRP’s (minor crops) should be genetically improved for increasing their productivity [14]. Millets are agronomically beneficial because ofthey are tolerant to drought, heat, salt and biotic stresses, and survive in marginal lands under rainfed conditions [15]. These plants are classified as glycophytes and have an average salt tolerance threshold of 6 (ECe) (dS/m) [16].
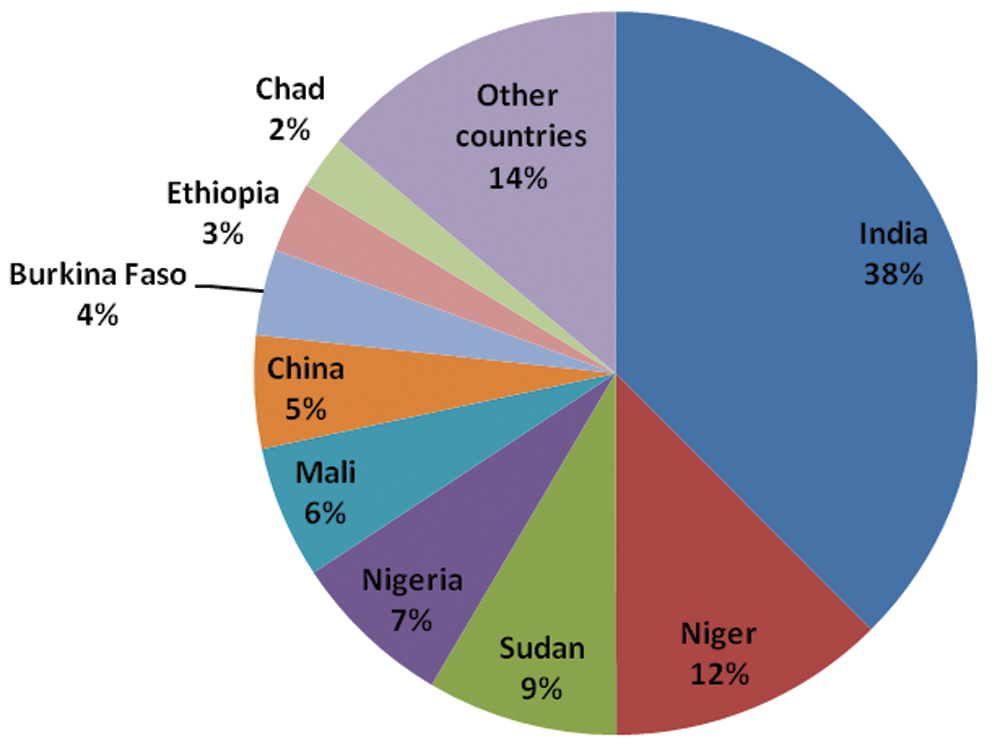
Figure 1: Millet production (%) in different countries of the world (FAO 2018)
Water requirements for producing 1 gm of dry biomass of maize and wheat are 470 g and 510 g, respectively, while those of some drought tolerant varieties of Seteria italica are of only 257 g [17]. Millets are adapted to low rainfall and poor soils. Pearl millet is probably the most drought and heat resistant among the millets, and is preferably grown in well drained sands or sandy loams. Lighter soils are best suited, and sometimes a mixture of black, red and light coloured gravelly soils of Deccan are well suited for their growth. Finger millet is adapted to various temperature and moisture ranges, and is mostly grown on reddish brown lateritic soils having good drainage and adequate water holding capacity [18]. Foxtail millet is grown on water deficient black cotton soils, and also on loamy or alluvial or clayey soils. Kodo millet is extremely drought resistant, and it is grown on hard-gravelly soils where other crops cannot grow. Fonio is mainly grown on plateau savanna lands with slightly heavier soils and moist conditions [19]. Among the millets, finger millet is the most stress resilient crop in terms of stress conditions such as high temperature, low moisture, and poor soils. As a result, itcan be used in the improvement of other economically important crops. So, millets are a treasure of important genes and regulatory proteins which are responsible for their adaptive traits, and can be used in the development of stress resistant crops. Transgenic crops with desired traits can be developed by inactivation or over-expression of transcription factor genes with desirable stress tolerance traits. These can be identified by genome wide expression profiling [20]. The pearl millet varieties have been reported to have a better drought tolerance capacity than maize showing a better relative water content (RWC); photosynthetic rate; upregulated expression of CBF, PIP2;3 transcripts, and a repressed expression of RubSc on leaves which provide drought resistance [21]. Among the minor millets, the drought tolerance was found to be highest in barnyard millet followed by finger millet and little millet when they are in the reproductive developmental morphology stage. Barnyard millet performed better in terms of number of reproductive tillers, ear heads, grain weight, ear head weight, grain yield and straw yield [22]. Furthermore, pearl millet ishighly nutritious and it has been called a perfect solution under water stress conditionsbecause of its drought tolerance [23].
2 Impact of Abiotic Stress in Millets
Salinization of arable lands due to improper water drainage systems, underlying high salt content rocks, irrigation of crops in arid and semi-arid regions with saline water, and lack of good quality water due to shortage of rainfall affect soil characteristics. Soil salinity has rendered in valueless agricultural lands, and has hadhazardous impact on growth of many plants. Na+ and Cl− ions present in poor quality water are at excess levels and cause osmotic damage, ion-specific toxicity and nutritional disorders in plants leading to salinity stress [24,25]. Thirty three percent of the global irrigated agricultural lands and 20% of the cultivable land areimpacted by salinity, and this can increase up to 50% by the year 2050 [26]. Salinity stress causes a reduction in RWC which might be due to the osmotic stress in roots caused by high salt content. This restricts water absorption and leads to dehydration [27]. Finger millet which is moderately tolerant to salt stress, has displayed a decrease in (1) shoot dry weight, leaf number, leaf surface area, and leaf chlorophyll content, and an increase in (2) leaf succulence, destruction of chloroplast, leaf chlorosis, severe damage oftissues, lignification of xylem vessels, electrolyte leakage, hydrogen peroxide and proline content under increased salinity [28]. Also, salinity stress in finger millet resulted in reduced germination rates, root and shoot growth, chlorophyll content, leaf relative water content, and K+ concentration of leaves, and chlorosis, and increased salt and malonaldehyde contents [29]. Salinization and alkalization resulted in reduction of plant dry weight, relative growth rate (RGR), net assimilation rate (NAR), leaf area ratio (LAR), RWC and nitrogen in foxtail millet and proso millet. However, the detrimental effects were greateron foxtail than proso millet which indicates that proso millet is more tolerant to both stresses [30]. It was reported that the tolerant accessions of proso millet were high in chlorophyll a under saline conditions. Chlorophyll a content can be related to salt tolerance in proso millet (Panicum miliaceum). The salt tolerance of a plant species determines the degree of reduction in total chlorophyll [31].
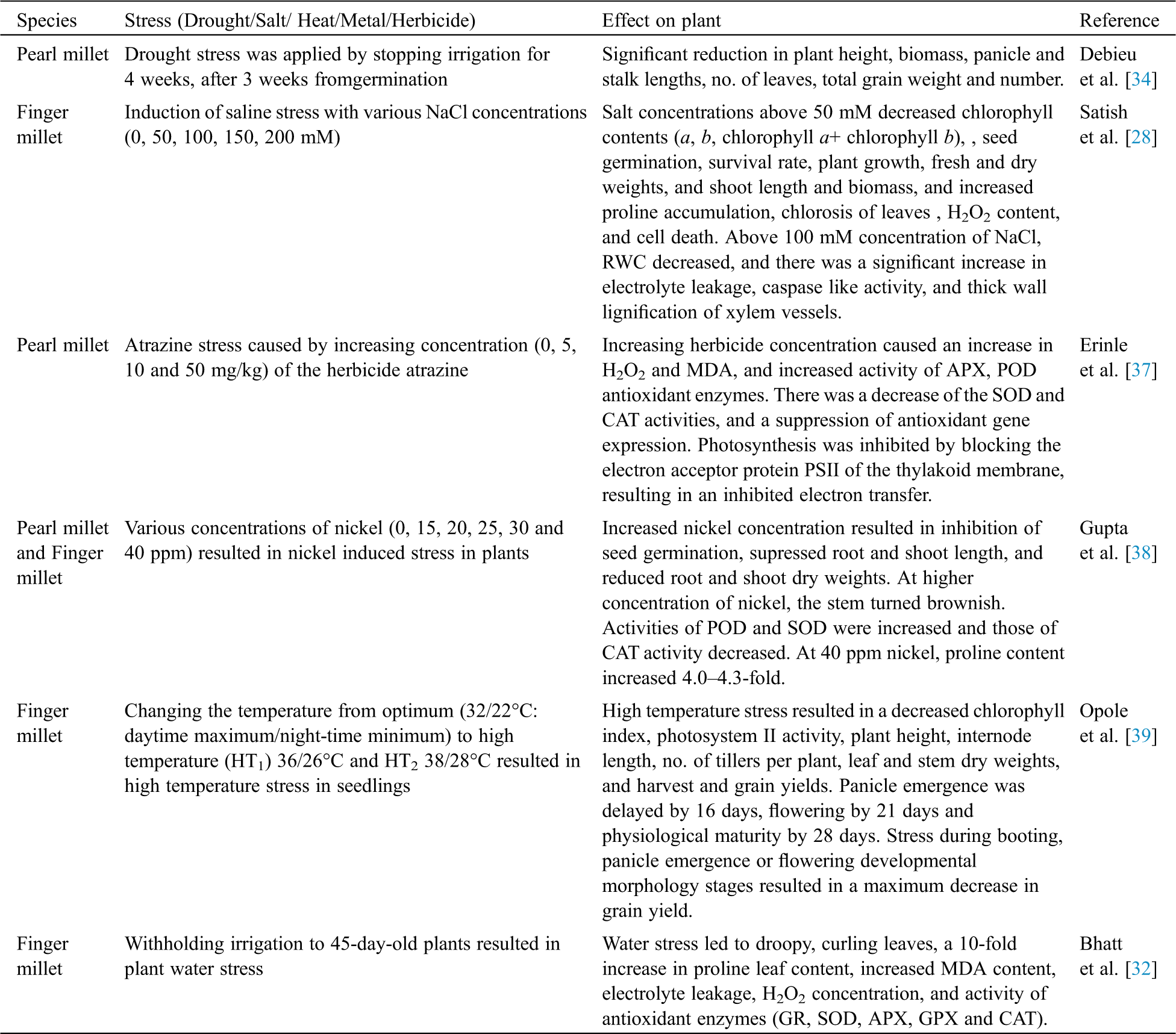
Drought, which leads to a severe water deficiency, has devastating effects on crop productivity. The stomatal closure due to drought leads to an excessive accumulation of ROS leading to oxidative stress. This stress results in lipid peroxidation and damage to other bio-molecules [32]. Abscisic Acid (ABA) and Ethylene (ET) are among the phytohormones which are often involved in drought stress signalling and tolerance. Salicylic acid (SA) and jasmonic acid (JA) enhance plant tolerance against drought, salinity and heat stresses [33]. Drought stress in pearl millet has led to a significant reduction in plant height, plant biomass, plant weight and grain number [34]. Water stress on black and brown finger millet resulted in a decreased chlorophyll, photosynthesis, and RWC, and an increased proline content; brown finger millet showed higher levels of tolerance than black finger millet [35]. Drought-induced oxidative stress in finger millet led to droopy shoots, curling of leaves, increased proline and malondialdehyde (MDA) contents, electrolyte leakage, damaged membrane integrity, and a significant increase in H2O2. Drought resulted in increased activities of antioxidant enzymes such as glutathione reductase (GR), superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione peroxidase (GPX) and catalase (CAT) [32]. At the sight of drought induction, this stress promoted an increase of the endophyte Actinobacteria in roots, therhizosphere, and the bulk soil communities of millet species, which might benefit the host [36]. Increasing atrazine herbicide concentration caused a physiological distress in pearl millet seedlings by inhibiting photosynthesis. Furthermore, it blocked the electron acceptor in photosynthesis and led to an excessive production of ROS causing an oxidative damage to proteins, lipids and pigments [37]. Nickel toxicity in pearl millet and finger millet resulted in inhibition of seed germination with supressed root and shoot length, and reduced root and shoot dry weights, and a 4-5-fold increase in proline content, with roots and stem turning brownish [38]. Heat stress resulted in reduced chlorophyll index, grain yield, harvest index and photosystem II activity in finger millet. Furthermore, the maximum impact of heat stress occurredduring the booting, panicle emergence and flowering developmental morphology stages, where genetic variability played a significant role in plant stress tolerance [39]. Linoleate 9S-lipoxygenase (LOX) which encodes an enzyme involved in lipid peroxidation, was induced by the water and heat stresses in foxtail millet [40]. The impact of various abiotic stresses on different millet varieties is given in Tab. 1. The overview of the biological plant responses tovarious abiotic stresses is depicted in Fig. 2.
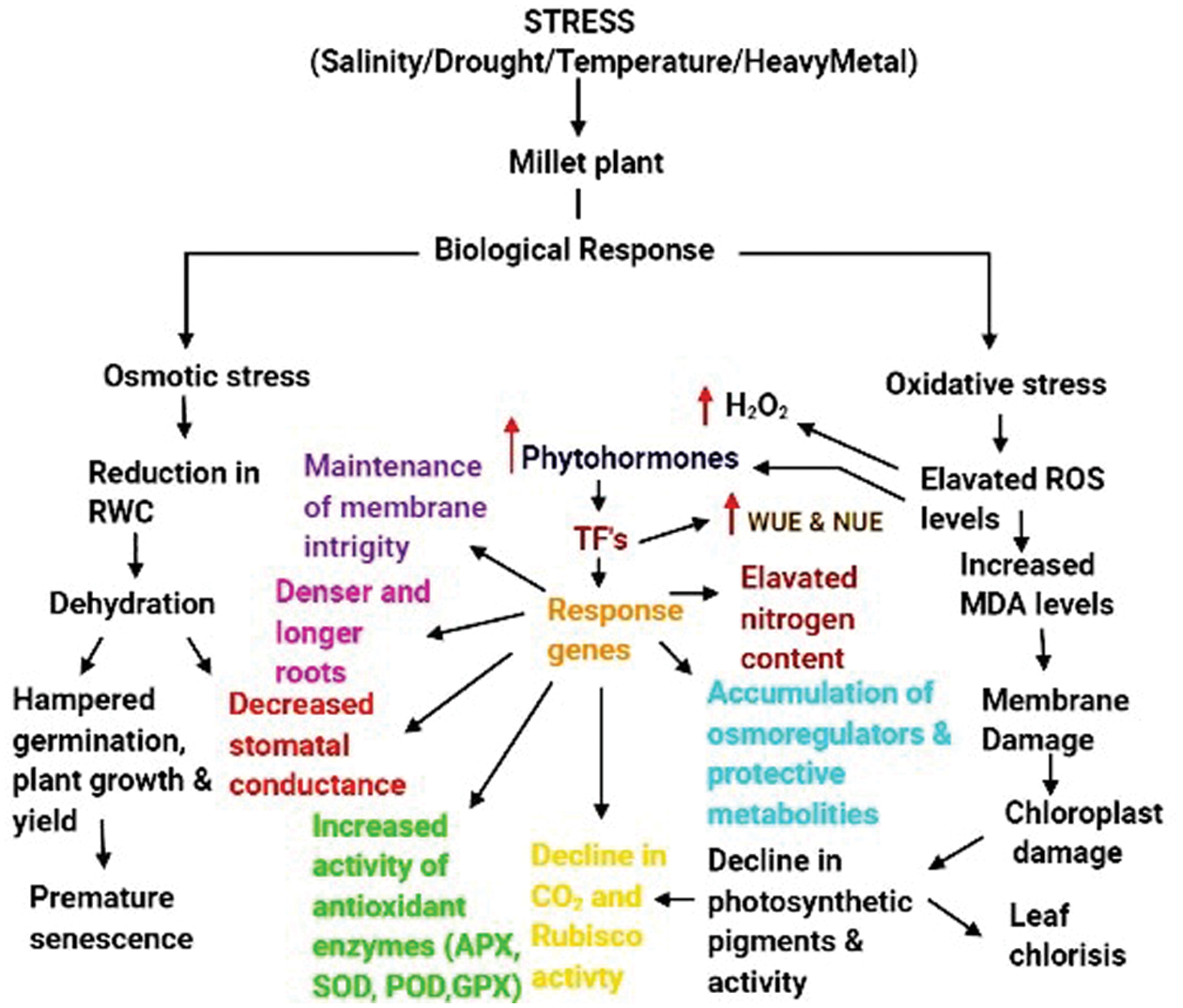
Figure 2: Plant biological responses to various abiotic stresses. (Created with BioRender.com)
Table 1: Impact of abiotic stresses on millets
Plants have evolved various morphological, biochemical, physiological and molecular mechanisms to withstand various environmental stresses.Stress stimuli are perceived by plant cells via various sensors which then activate various signalling pathways. These involve various plant hormones, secondary messengers, transcription regulators and signal transducers [41]. These adaptations affect the plant’s vegetative growth, reproductive development, yield and quality [42].
3 Morphological Adaptations to Stress
Short life cycle, and plant heights, small leaf areas, thickened cell walls, and dense root systems are various traits that help millets in resistingstress. Being C4 plants is highly advantageous as it increases the water use efficiency (WUE) and nitrogen use efficiency (NUE) [43]. Flowering in pearl millet mightchange with the rainfall pattern [44]. An increased root length and a decreased shoot length were seen in Panicum sumatrense undergoing drought treatments [45]. It was further reported that pearl millet is composed of a fast-growing primary root system and a rapid colonization of deeper soil horizons [46]. Pearl millet yields are reduced because of water stress after flowering that effects both grain filling and seed setting. High tillering varieties having small-size grains and small panicles minimize drought-related grain filling impairments [47]. Foxtail millet plants produce longer and denser root hairs forming a large rhizosheath that produces more root biomass which mighthelp in penetrating deep into dry soils [48]. It is reported that the farmers in dry areas preferred short duration, high tillering varieties of pearl millet which ensured better yield and fodder value. The drought escape mechanism of pearl millet is the short “flowering time” which is completed with little available water [49]. Pearl millet has a varying root system depending on the water limitation, with a root depth ranging from 140 cm to 3 m with lateral root spreading. The transpiration rate is kept high by adjusting the stomatal movements with a maximisation of carbon fixation while water is available [50]. The adaptive responses of pearl millet to drought stress include an increase in its root length to increase water uptake [51]. Stay green is a drought tolerance trait which is a characteristic of some genotypes where active photosynthesis is extended by delaying leaf senescence via a complex signalling network. The pearl millet semi-dwarf, inbred lines developed in USA have this “stay green” characteristics. This allowsplants to continue with photosynthesis regardless of the soil water content, and maintain a good grain yield under drought stress conditions [52]. Drought tolerant pearl millet accessions showed various morphological and physiological responses to stress such as upright folding of leaves that reduces surface area of evaporation, greater osmotic adjustment capacity of young leaves and stems, and higher accumulation of NO3–, K+, amino acids, proline, sucrose, glucose and ammonium compounds [53]. It was reported that an increased leaf tensile strength leads to an increased drought tolerance among three species of Eragrotis [54].
Metal tolerance in finger millet was reported to be higher than that in pearl millet and oats. Finger millet had the maximum build-up of nickel (Ni) in roots which indicates that Ni accumulation in the roots helps the plant to mitigate the effects of metal toxicity [38]. Phosphorus (P) limitation in plants lead to a phenotypic adaption of large root systems. In foxtail millet, phosphorus adaptation leads to lateral root proliferation by increasing rootnumber, density and length, and thus enlarging the root absorptive surface area. Auxin and gibberellin stimulate root development understress conditions [55].
4 Biochemical and Physiological Adaptations to Stresses in Millets
Osmoprotectants play a vital role in improving hyperosmolarity which is caused by salinity stress and establishes cellular ionic homeostatic conditions. The biochemical adaptive response to salt stress in finger millet included anelevated proline content, increased reduced sugar concentration and total leaf proteins [29]. Proline which is an important amino acid, plays a vital role as a compatible osmotic molecule and in osmotic potential adjustment; it thus helps in improving drought tolerance. It also acts in antioxidative defense, metal chelation and stress signalling [56]. Antioxidant enzymes represent the adaptive mechanism of plants exposed to oxidative damage caused by stress. This consists of SOD, CAT, peroxidase (POD) and APX. Metal stress in millets resulted in elevated activities of POD and SOD with a reduction in CAT activity [38]. A pearl millet variety well-adapted to saline environments showed goodphysiological and biochemical responses to increased salinity such as increased proline, total soluble proteins, and epicuticular wax content [27]. It was seen that the salt tolerant varieties of finger millet and rice had lower shoot Na+/K+ ratios and much higher leaf carbohydrate contents; it was concluded that ion regulation along with carbohydrate metabolism led to salt tolerance in rice and finger millet [57]. Ascorbate is a water-soluble antioxidant in plants that is necessary for the efficient activity of APX, which plays an important role in the scavenging process ofconverting H2O2 into H2O. A 200% ascorbate increase was reported in finger millet drought tolerant varieties which implies that ascorbate increases tolerance against drought stress [58]. A higher expression of secondary metabolite genes associated with alkaloid, terpenoid, flavanols, lignin, wax, mevalonic acid (MVA) and Shikimic acid (SA) metabolic pathways, was seen in drought stressed pearl millet at the flowering than at the vegetative stage; this helped in maintaining osmotic potential and membrane integrity. A higher accumulation of secondary metabolites was found in drought tolerant pearl millet genotypes [59]. Phytohormones such as auxin, cytokinin, ABA, gibberellin and ethylene play a vital role in stress adaptive responses.
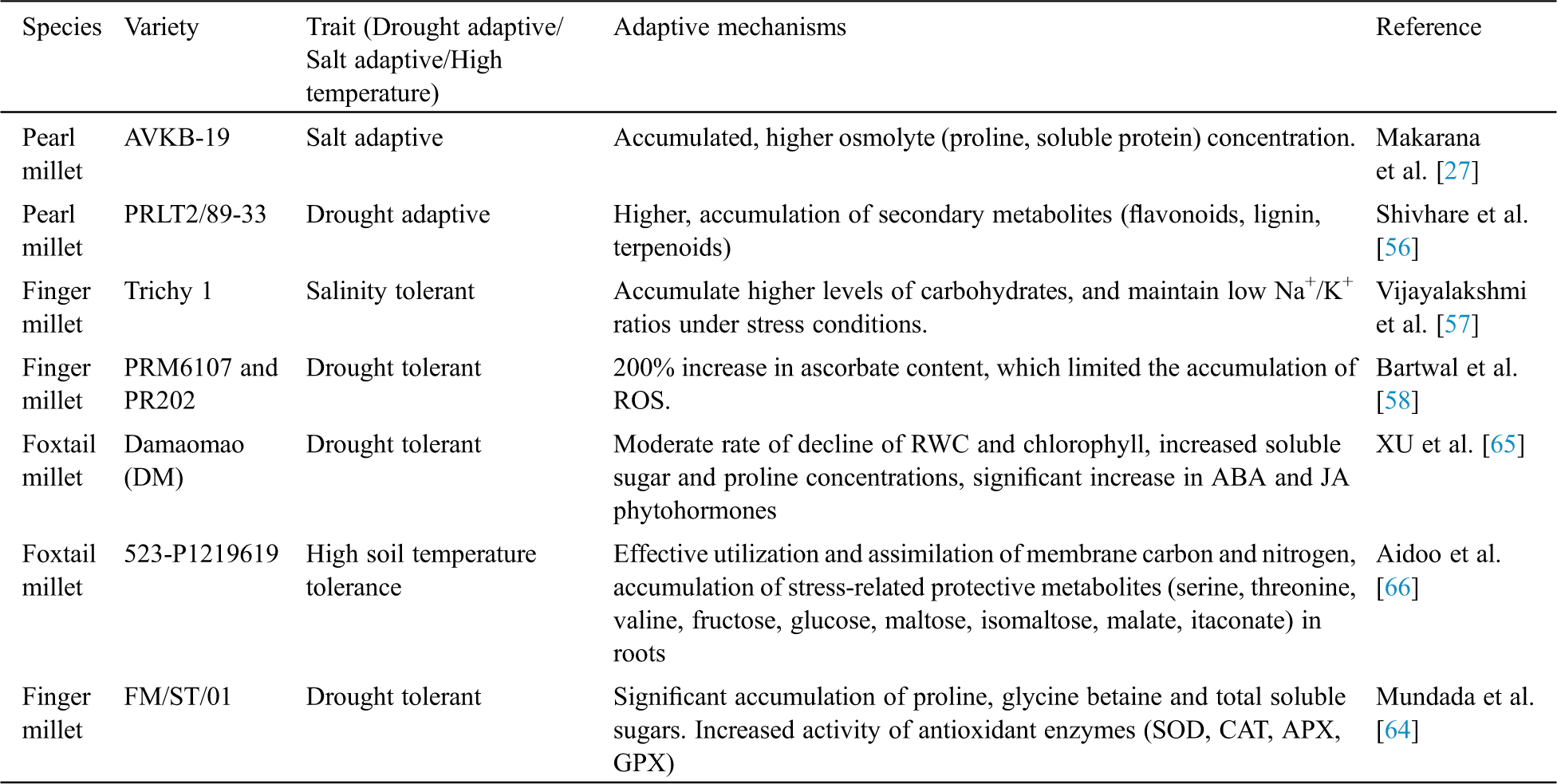
An increased lipoxygenase enzyme activity during water stress in millets indicate that it might provide a better drought tolerance to plants [60]. The foremost physiological adaptations of pearl millet to drought stress were stomatal closure to prevent transpiration water loss, reduced stomatal conductance, reduced photosynthetic rate and ultimately decreased CO2 and rubisco activities [61]. A total of 2474 differentially expressed proteins, identified by proteomic analysis, were found to be involved in various plant processes (photosynthesis; stress and defense responses; ATP synthesis; carbon metabolism; protein biosynthesis, folding and degradation; cellular organization) and had up to a 4-fold increased expression underdrought stress, which indicates their possible role in the response and adaption of foxtail millet todrought stress [62]. Pearl millet has expanded the gene families forwax, suberin, and cutin biosynthesis, and transporters for secondary metabolites as compared to other cereal crops. It has been proposed that these deposits might provide the plants with drought and heat tolerances [34]. High levels of osmotic adjustment and transpiration were found in resistant races of millet to drought stress. Osmotic adjustment is greater in millet races with smaller plants having small organs and cells; hence, having smaller plants in these races is a drought adaptive trait [63]. It was concluded that higher excised leaf water retention capacity (ELWRC), plant water relations, proline accumulation, leaf area index (LAI), total biomass (TB) and an efficient antioxidant (AOX) system contribute to the dehydration stress tolerance in pearl millet [61]. It was reported that the drought tolerance capacity of a finger millet genotype included alower MDA content, higher osmolyte accumulation (proline, glycine betaine and total soluble sugars) and an increased activity of antioxidant enzymes (SOD, CAT, APX and GPX) [64]. A drought tolerant foxtail millet variety had a moderate rate of decline RCW and chlorophyll, increased soluble sugar and proline concentrations, and a significant increase of the stress hormones ABA and JA. These phytohormones are involved in drought adaptive responses such as the regulation of gene expression whichhelp plants in the adaption to stress [65]. A high temperature tolerant variety of foxtail millet showed low photosynthetic and stomatal conductance rates, reduced root respiration, accumulation of protective metabolites (serine, threonine, valine, fructose, glucose, maltose, isomaltose, malate, itaconate) in roots with a better utilization of carbon and nitrogen [66]. The effects of water stress and heat stress are reported to be key regulators of abscisic acid (ABA) biosynthesis, and led to a 7–8 fold increase of ABA in foxtail millet [40]. The various biochemical and physiological adaptations of millets to various abiotic stresses are summarizedin Tab. 2.
Table 2: Biochemical and physiological adaptations to stress in millets
5 Molecular Adaptations of Millets to Stresses
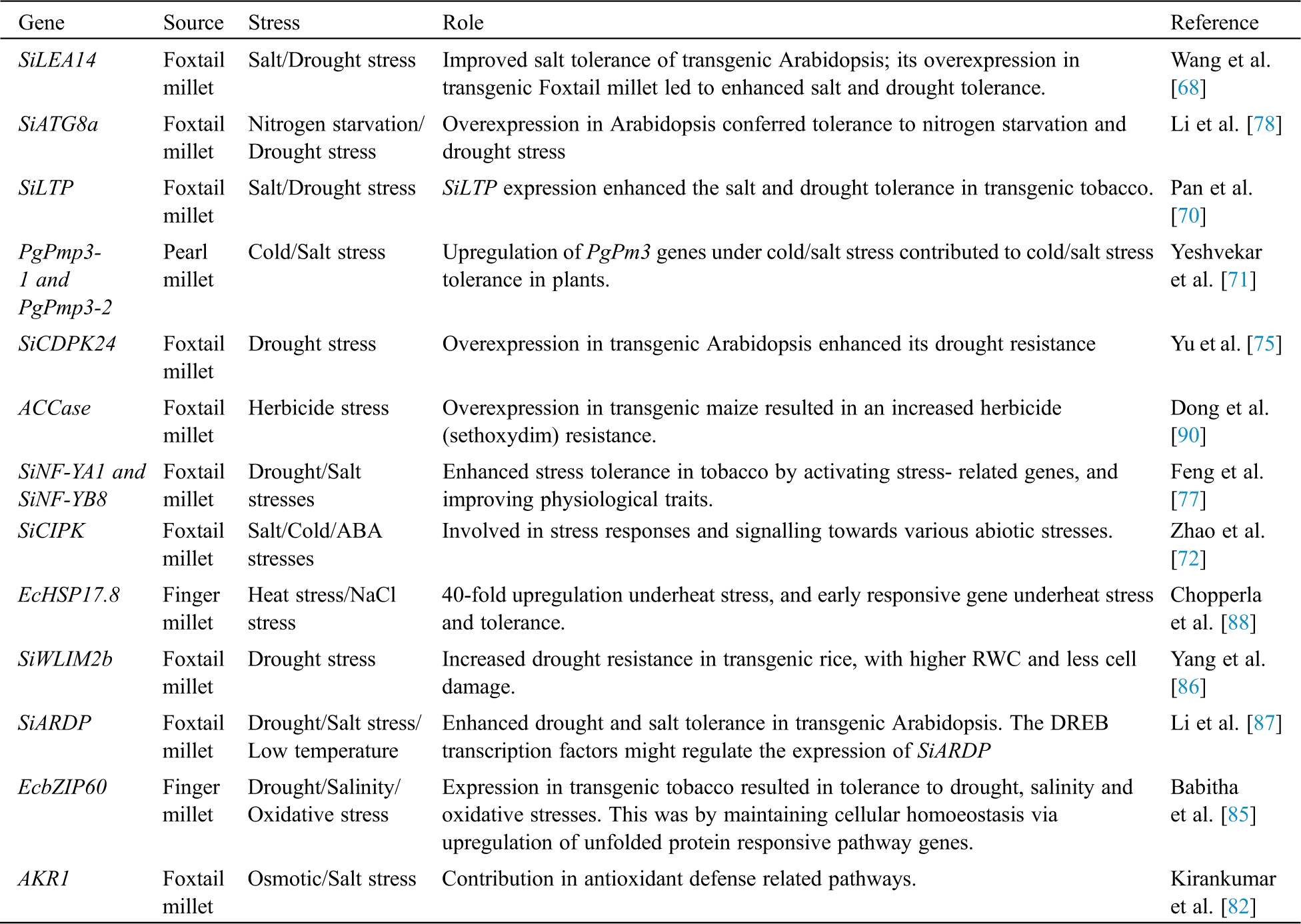
The plant response to various environmental factors is differentially perceived and expressed at the molecular level. In a study on phosphorus limitation in foxtail millet the molecular adaptions include the upregulated expression of SiPHT1;1, SiPHT1;4 in roots and that of SiPHT1;2 in roots and shoots for anenhanced uptake and translocation of phosphorus under stress conditions [51]. Drought tolerance QTL of pearl millet helped in a reduced salt uptake and enhanced growth undersalt stress [67]. Late embryogenesis abundant (LEA) gene, namely SiLEA14, from foxtail millet was induced by osmotic, NaCl stress and ABA. It increased salt tolerance in transgenic Arabidopsis and when overexpressed in transgenic foxtail millet, it enhanced tolerance tosalt and drought stresses [68]. Stress induced EcNAC1 (NAM, ATAF1/2, and CUC2) transcription factor from finger millet, which is induced by salinity and drought, was characterised and expressed in transgenic tobacco plants. It resulted in an increased tolerance to various abiotic stresses such as osmotic stress and salinity stress [69]. Lipid transfer gene (SiLTP) expressed in all foxtail millet tissues improved the drought and salt tolerance in this speciesby increasing the proline and total soluble sugar contents. This gene can be used for anenhanced drought and salt stress tolerance in crop plants [70]. Plasma membrane proteolipid genes in pearl millet (PgPmp3-1 and PgPmp3-2), in association with other proteins showed enhanced expression during cold, drought and salt stresses and provided abiotic stress tolerance to plants by encoding hydrophobic proteins and maintaining cellular ion homeostasis [71]. 35 CBL-interacting protein kinase (CIPK) genes reported in foxtail millet are involved in stress signalling pathways and play an important role in stress responses and plant development. Most SiCIPK genes are strongly induced by salt and cold stresses, and others by ABA and PEG treatments [72]. There are three abiotic stress-inducible promoters in pearl millet which are induced under high temperature, drought and salt stresses that confer high abiotic stress tolerance and can be used in developing stress tolerant crops [73]. These promoters include (1) Cytoplasmic Apx1 (Ascorbate peroxidase)- potential candidate in the elimination of H2O2; (2) Dhn (Dehydrin)-stabilization and protection of cellular membrane and enzymes from low temperature and ROS; and (3) Hsc70 (Heat shock cognate)-play chaperone function by proper folding and translocation of newly synthesised proteins. The SiMYB42 (Myeloblastosis) transcription factor in foxtail millet was upregulated under low nitrogen, salt, and drought stresses; it regulated the expression of nitrate transporter genes which enhanced the plant tolerance to low nitrogen conditions [74]. Calcium-dependent protein kinases (CDPK) genes of foxtail millet play a vital role in signalling pathways, and enhance drought resistance in the plant and transgenic Arabidopsis. Foxtail millet has 29 CDPK genes, and SiCDPK24 had the highest transcript levels underdrought conditions; it was concluded that CDPK’s play an important role in drought stress resistance [75]. NAC (NAM, ATAF, and CUC) like transcription factor SiNAC110 in foxtail millet, localized in the nucleus, is induced by drought, salt and other abiotic stresses. Its over expression led to an increased drought and salt tolerance in Arabidopsis by enhancing the gene expression for proline biosynthesis, Na+/K+ transport, and aqueous transport proteins [76]. Under salt and drought stresses in foxtail millet, NF-Y (Nuclear Factor Y) genes, SiNF-YA1 and SiNF-YB8 were highly induced by ABA and H2O2. These led to stress tolerance by activating stress related genes, RWC, chlorophyll contents, and SOD, POD, and CAT, thus enhancing the antioxidant system [77]. In foxtail millet, the autophagy-related gene SiATG8a, which is localized in the membrane and cytoplasm, is involved in plant responses to nitrogen starvation and drought stress. Overexpression of SiATG8a in transgenic Arabidopsis, resulted in plant tolerance to nitrogen starvation and drought, as the plants having higher nitrogen content showed higher drought tolerance [78]. The three antioxidant genes, i.e., APX, glutathione reductase (GluR) and SOD had a higher expression level in pearl millet genotypes during polyethylene glycol (PEG)-induced drought stress conditions, resulting in a higher osmotic stress tolerance in the seedlings [56]. Sevendrought-responsive genes may be involved in the drought tolerance of minor millets as their expression was up regulated by water stress treatment, and can be used for the development of transgenic drought tolerant crops [79]. These genes included (1) NAC2; (2) CDPK; (3) U2-snRNP (small nuclear RiboNucleoProtein particles)-regulates gene expression; (4) plant synaptotagmin- maintains plasma membrane intrigrity; (5) Aquaporin- membrane channel; (6) MPK17-1 (Mitogen activated Protein Kinase)-signalling and (7) Scythe protein-regulates apoptosis Transcriptome analysis of finger millet showed upregulation of various drought stress signalling cascade genes such as Protein Phosphatase 2A (PP2A)-2 fold increase in drought stressed finger millet; Calcineurin B-Like protein (CBL) Interacting Protein Kinase 31 (CIPK31)- highly stress responsive; Farnesyl Pyrophosphate Synthase (FPS)- which facilitates farnesylation of proteins which are involved in ABA signalling; Signal Recognition Particle Receptor (SRPR α); and basal regulatory gene TBP (Tata Binding Protein) Associated Factor6 (TAF6). It was concluded that drought activates the genes associated with housekeeping or basal regulatory processes in finger millet [80].
Terpene synthase (TPS) genes in foxtail millet especially SiTPS19 showed a significantly higher expression under both biotic and abiotic stresses, indicating that it can improve crop resilience by having a possible function in defense and environmental adaptation [81]. There was an abundant upregulation of the AKR1 gene (Aldo Keto Reductases) in roots and leaves of foxtail millet with increasing drought and salt stress; it was concluded that the AKR1 gene is associated with aphysiological defense against oxidative stress [82]. The PgPAP18 gene onpearl millet, belonging to the purple acid phosphatase (PAP) family, showed a 2-3-fold upregulation underheat, drought, salt and metal stresses. In addition to having a role in the phosphatase activity, genes of this family may play a vital role in tolerance against various abiotic stresses by scavenging ROS and crosstalk between stress signalling pathways [83]. Ninety seven pgWRKY genes were identified in pearl millet with the presence of 127 cis regulatory elements, specific to various biotic and abiotic stresses. This indicated the likelyinvolvement of pearl millet WRKY transcription factors in providing resistance against plant biotic and abiotic stresses [84]. The transcription factor EcbZIP60 belonging to the family of basic leucine Zippers (bZIPs) from finger millet was highly upregulated underdrought, osmotic and salinity stresses. EcbZIP60 plays an important role in adaptation to various stresses by improving growth and upregulation of unfolded protein-protein responsive pathway genes [85]. Ten LIM genes were reported in foxtail millet with cis acting elements related to abiotic stresses. SiWLIM2b was highly upregulated in foxtail millet under abiotic stress; when it was overexpressed in transgenic rice under drought conditions led to a higher survival rate with higher relative water content and less cell damage in the plant. Therefore, it was concluded that SiWLIM2b is involved in the phenylpropane pathway, gene regulation and enhances drought stress tolerance [86]. Increased transcription levels of the stress-induced SiARDP (ABA- responsive DREB-binding protein gene) gene after drought, salinity, and low temperature stresses, and ABA treatment, was seen in foxtail millet seedlings. SiARDP gene expression might be regulated by SiAREB1 and SiAREB2 (ABA responsive element binding) transcription factors; SiARDP is involved in signalling pathways and plays an important role in stress response and increased stress tolerance in plants [87]. The heat-shock protein gene EcHSP17.8 in finger millet was induced by heat, NaCl, and oxidative stresses, and mannitol, and the maximum expression was found in root tissues. An upregulation of up to 40-folds was found underheat stress, and hence this gene is characterised for heat stress tolerance in plants [88]. Cold stress resulted in upregulation of the SiSET14 gene in foxtail millet. SET [(Su(var)3–9, E(Z) and Trithorax)] domain proteins are putative candidates for histone lysine methyltransferases. When expressed in a yeast system, it conferred abiotic stress tolerance to transgenic yeast cells. This suggests a possible role of SiSET genes in conferring abiotic stress tolerance in foxtail millet [89]. The Acetyl-Coenzyme A Carboxylase (ACCase) gene in foxtail millet provides herbicide (sethoxydim) resistance, and can be used in the development of transgenic maize with herbicide resistance and higher oil content [90].The various genes involved in stress adaption of millets are summarized in Tab. 3. An account of the millet adaptation mechanisms is depicted in Fig. 3.
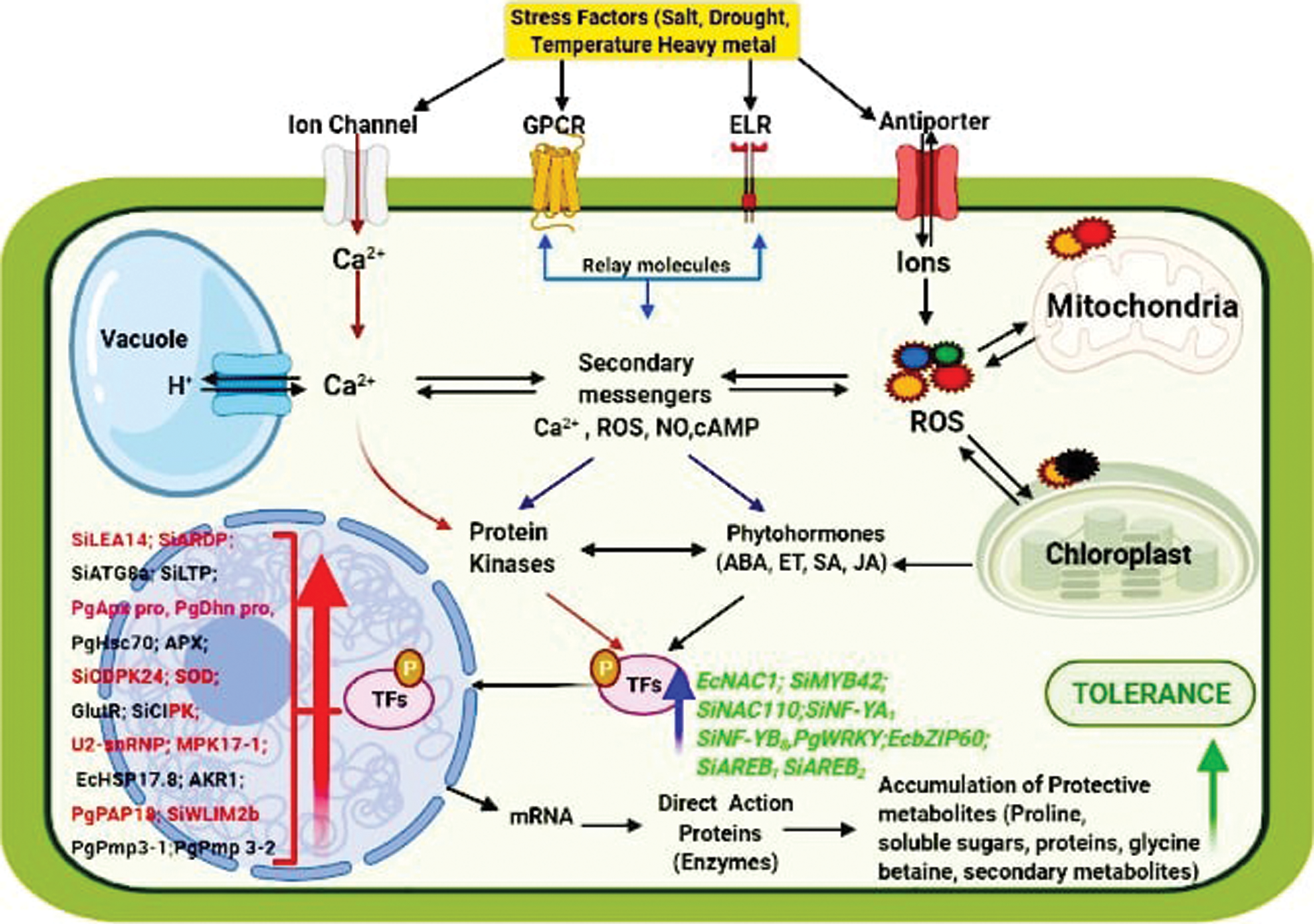
Figure 3: Molecular mechanism of adaptation in millets at the functional gene level (Created with BioRender.com)
Table 3: Genes involved in the stress adaptation of millets
The abiotic stress signalling cascade is activated with the recognition of stress signal by the various cell membrane receptors and transporters including GPCR (G-Protein Coupled Receptor), Enzyme Linked Receptor (ELR), Calcium channels and Ion transporters. The cytosolic Ca2+ increases in response to hyperosmotic stress, and oxidative stress causes an elevation in the ROS levels. The signal is then transmitted downstream through the relay molecules and is converted into intracellular signal by the secondary messengers [i.e., Ca2+, ROS, cAMP, cGMP, Nitric oxide (NO)]. These secondary messengers further activate the kinase cascade (protein kinases; i.e., CDPK; MAPK) and elevate phytohormone signalling (Abscisic acid-ABA; Ethylene-ET; Salicylic acid-SA; Jasmonic acid-JA). These kinases are responsible for the sequential phosphorylation/dephosphorylation of proteins, and activation of cascade components. The phosphorylation/dephosphorylation of transcription factors (TF’s) results in their upregulation/downregulation. Various upregulated TF’s from various millets include [Eleusine coracana (EcNAC1,EcbZIP60); Setaria italica (SiMYB42, SiNAC110, SiNF-YA1, SiNF-YB8, SiAREB1, SiAREB2); Pennisetum glaucum (pgWRKY)] which are involved inregulating the expression of stress responsive/defensive genes in various millets [Setaria italica (SiLEA14; SiARDP; SiCDPK24, SiCIPK, SiATG8a, SiLTP, SiWLIM2b); Pennisetum glaucum (PgApx pro, PgDhn pro, PgHsc70, PgPAP18, PgPmp3-1, PgPmp3-2); Eleusine coracana (EcHSP17.8); APX; SOD; GlutR; U2-snRNP; MPK17-1;AKR1]. These later genes are involved in the various abiotic stress responses and tolerance such as the accumulation of protective metabolites, osmoregulators, decreased transpiration, reduced stomatal conductance, reduced photosynthetic rate, increased root length and denser roots, enhanced activity of antioxidant enzymes and phytohormones, elevated nitrogen content, maintenance of membrane integrity, increased epicuticular wax content, increased nitrogen use efficiency (NUE) and water use efficiency (WUE).
6 Transcriptomic Analysis of Millets
Transcriptomic analysis of a salt tolerant and a susceptible foxtail millet cultivars, revealed that 159 differentially expressed transcripts produced >2-fold change in response to salinity stress. Among these, 115 were upregulated and 44 were down regulated. It was concluded that the expression of transcription factors and signalling genes was greater in the tolerant than in the susceptible variety which contribute to their signal perception mechanisms under saline conditions [91]. Eighty one conserved and 14 novel differentially-regulated miRNAs were identified during a small RNA sequencing on the salinity tolerant pearl millet genotypes. A total of 448 genes were identified as target genes, and 122 among these encoded transcription factors. These miRNA’s and their target genes can regulate the auxin response pathway, and hence have a role in salinity stress tolerance in pearl millet [92]. Twenty-nine upregulated and downregulated differentially expressed proteins (involved in various energy, lipid, nitrogen, carbohydrate, nucleotide, stress related metabolism, signal transduction, and photosynthesis) were identified in foxtail millet seedlings, and they seemed to be involved in Providing tolerance against salt stress [93]. The transcriptome changes in a drought tolerant foxtail millet were analysed after a PEG-induced drought stress. Among the identified 327 differentially expressed transcripts, the reverse northern technique identified 86 upregulated transcripts, which suggested their possible function in dehydration adaption. Most of the upregulated transcripts were involved in metabolism, transcription regulation, signalling, protein degradation and stress. A 5-11-fold induction of the DREB2 (Dehydration Responsive Element Binding type) protein was seen by qRT-PCR analysis [94]. Comparative transcriptome analysis of pearl millet salinity tolerant and susceptible cultivars identified 11,627 DEG’s, 1,287 upregulated unigenes and 1,451 downregulated unigenes that were common in both cultivars. Among the differentially-expressed genes, there were the genes encoding for transcription factors, ion transporters, and metabolic pathways involved in stress responses. The tolerant line had an upregulation of the ubiquitin-mediated proteolysis and phenylpropanoid biosynthesis pathway genes. On the other hand, glycolysis/gluconeogenesis unigenes and ribosome genes were downregulated in the susceptible variety [95]. Three thousand and sixty six differentially-expressed genes (DEGs) were identified (1404 upregulated and 1,662 downregulated) in a drought tolerant variety of foxtail millet, which lead to the formation of regulatory networks involving photosynthesis, signal transduction, osmotic regulation, redox regulation, hormonal signalling, cuticle and wax biosynthesis and enhanced drought tolerance [96]. Leaf transcriptome of two contrasting pearl millet varieties differing in terminal drought tolerance was examined. A total of 40,880 genes were differentially-expressed in both varieties; 13,260 and 8,799 DEGs were significantly-expressed in the sensitive and tolerant varieties, respectively. The tolerant variety had a higher expression than the sensitive one in receptor kinases, genes involved in regulation of detoxification enzymes, phytohormone biosynthesis, secondary metabolites, and stress-related transcription factors [59]. Root transcriptome of the drought tolerant and sensitive pearl millet lines led to the identification of 6,799 and 1,253 DEGs, respectively, under both control and drought conditions. The tolerant variety had an upregulation of 2,846 genes, and 3,169 genes were downregulated, while the sensitive line had an upregulation of 371 genes, and 96 genes were downregulated. The upregulated DEGs were involved in photosynthesis, plant hormone signal transduction, and mitogen-activated protein kinase signalling [97]. Integrated transcriptomic and metabolomics of two foxtail millet cultivars implied that salinity tolerance is attributed to higher ion channel efficiencies and the antioxidant system. A total of 8,887 and 12,249 DEGs were identified in the salt tolerant and salt sensitive varieties, respectively. A total of 4,830 and 4,057 genes were upregulated and downregulated, respectively, on the tolerant cultivar. The sensitive cultivar had an upregulation of 6,339 genes, and 5,910 genes were downregulated [98]. The transcriptome analysis of various millets is listed in Tab. 4.
Table 4: Transcriptomic analysis of millets
7 Alleviation of Stress in Millets
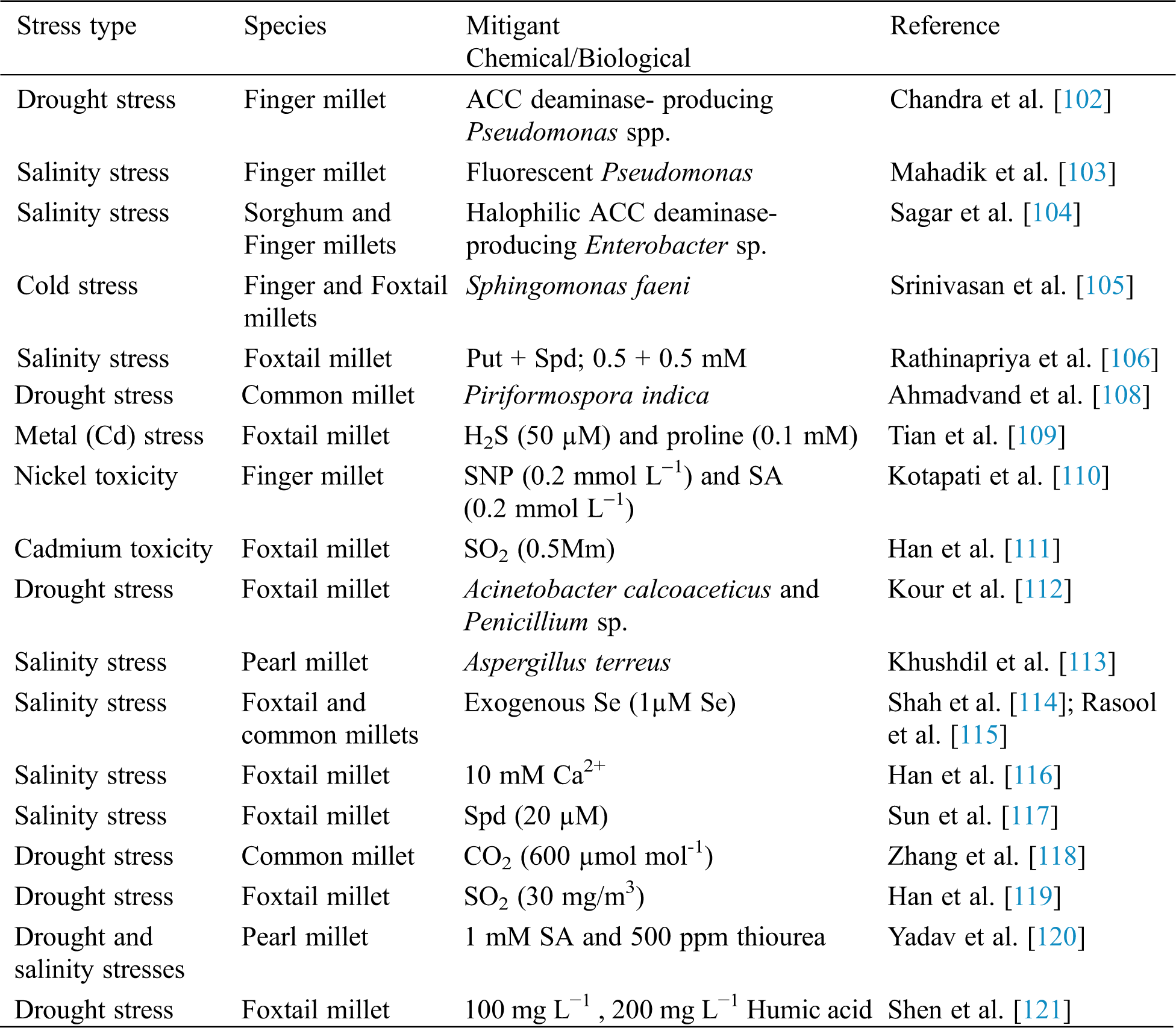
One economically feasible option to tackle the effects of stress on plants is the application of plant growth promoting bacteria (PGPB). Finger millet inoculated with 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase-producing drought tolerant Pseudomonas spp. resulted in improved growth, and enhanced antioxidant activity in both well-watered and drought stressed plants. The ACC deaminase converts the immediate precursor of ethylene (ACC) into α-ketobutyrate (α-KB) and ammonia, thereby reducing the ethylene level of plants and promoting growth [102]. Florescent Pseudomonads (SPF-33, SPF-37, SPF-5), which are plant growth promoting rhizobacteria (PGPR) have been reported to alleviate salt stress in salinity-sensitive finger millet. This was done by increasing its plant height and spikelet number, germination, total chlorophyll, phenolics, flavonoids, proteins, activity of enzymatic antioxidants, andproline content, and decreasing its lipid peroxidation and H2O2 [103]. Millets inoculated with halophilic rhizobacteria Enterobacter sp. PR14 showed growth promoting traits such as indole acetic acid (IAA), aminocyclopropane-1-carboxylate deaminase (ACCD), phosphate solubilization and antioxidant enzymes. This led to an increased seed germination, root and shoot length, and dry weight, hence ameliorating salinity stress in millets [104]. Inoculated finger and foxtail millets with Sphingomonas faeni bacterial mutants carrying the ACC deaminase gene, which is known to regulate ethylene, evolved during cold stress which in turn hampered plant growth. Blocking of ethylene resulted in improved root and shoot length, biomass content, and increased antioxidant activity, thus alleviating cold stress in finger and foxtail millets [105]. NaCl-stressed foxtail millet had an enhanced antioxidant enzyme system when treated with biogenic amines putrescine (Put) and spermidine (Spd). Plants of foxtail millet showed a reduced hydrogen peroxide level and electrolyte leakage, allowing an increased biomass content, relative water content, total chlorophyll, carotenoid levels, and a greater activity of in SOD, CAT, APX and GPX [106]. It was reported that the endophytic, salt tolerant, plant growth promoting Bacillus amyloliquefaciens EPP90 from pearl millet is a potential multi stress reliever and growth promoter. These halophilic bacteria were obtained from the roots, leaves and stem of the host pearl millet [107]. Inoculation of Panicum miliaceum with the root colonising endophytic fungi Piriformospora indica, resulted in an increased number of grains, plant height, and pinnacle length, and a greater grain nitrogen, protein, phosphorus, and chlorophyll contents under both well-watered and drought conditions [108]. Hydrogen sulphide (H2S) in combination with proline alleviate cadmium (Cd) damage in foxtail millet [109]. Nickel overloaded finger millet seedlings reduced the toxic effect of Ni when treated with sodium nitroprusside (SNP) and Salicylic Acid (SA) by improving root and shoot length, chlorophyll content, mineral concentration and dry mass [110]. Cadmium (Cd2+)-induced oxidative damage in foxtail millet was alleviated by sulphur dioxide (SO2) by enhancing the activities of antioxidant enzymes, increasing the contents of glutathione and phytochelatins, and reducing the uptake and translocation of Cd2+ [111]. Drought tolerant, phosphorus solubilizing microbes Acinetobacter calcoaceticus and Penicillium sp. mitigated the adverse effects of drought stress in foxtail millet by enhancing the accumulation of glycine betaine, sugars, and proline [112]. Pearl millet seedlings improved their tolerance to salt stress when they were inoculated with the endophyte Aspergillus terreus; this was because of increased chlorophyll content, RWC, soluble sugar, phenol and flavonoids [113]. Fig. 4 depicts the abiotic stress amelioration in millets by plant growth promoting bacteria (PGPB). Furthermore, the role of exogenously applied selenium was elucidated in Seteria italica and Panicum miliaceum after exposure to salt stress. It was concluded that Se amplified the antioxidant enzyme activities and the osmolyte concentrations, and lowered the H2O2 production. Hence, Se alleviated salt stress in millets [114,115]. Various stress mitigants in millets are mentioned in Tab. 5.
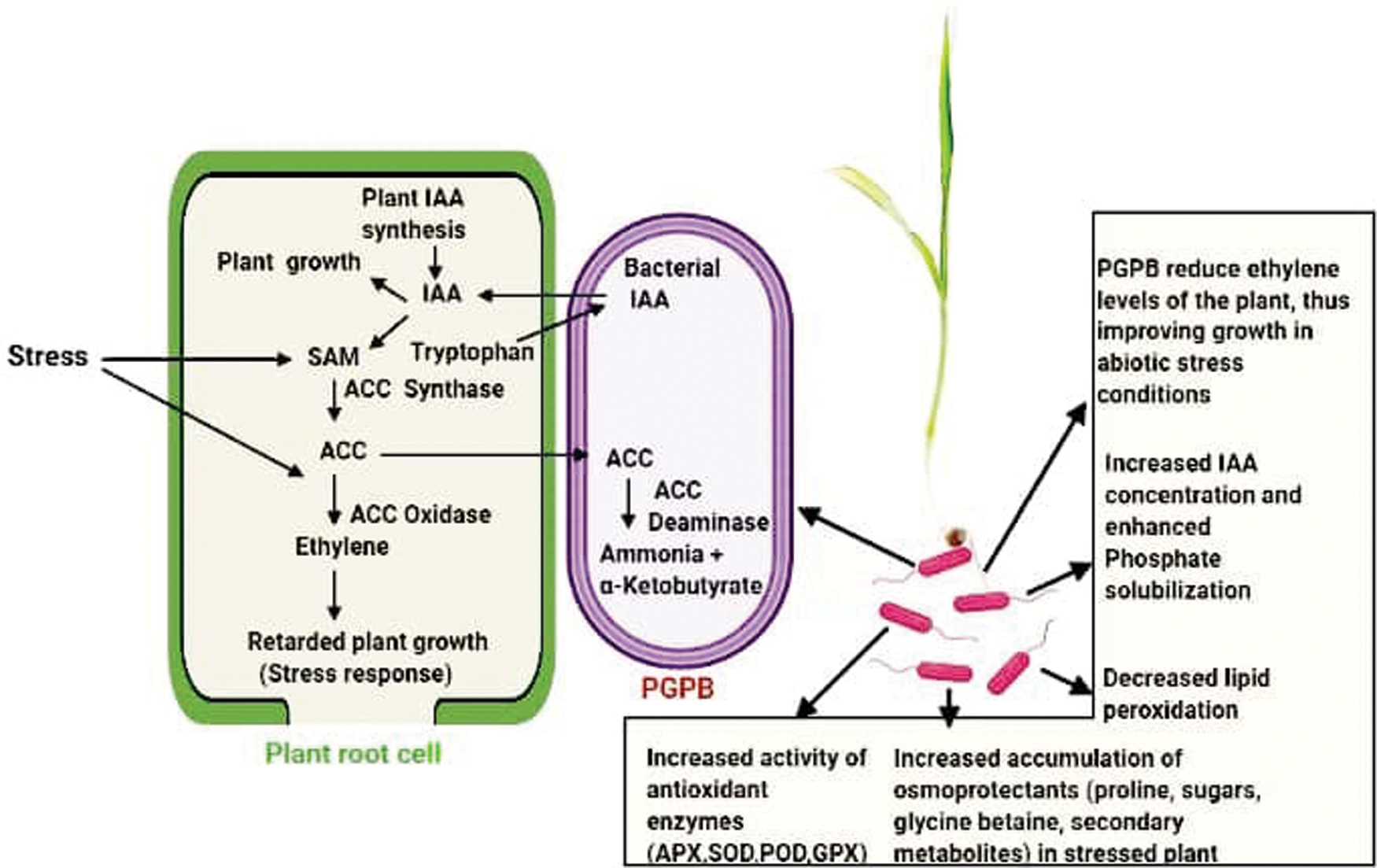
Figure 4: Abiotic stress amelioration in millets by plant growth promoting bacteria (PGPB) (Created with BioRender.com)
PGPBs help plants in surviving various stress conditions because of their growth promoting traits. Rhizobacteria associated with plants take up tryptophan and other exudates from the plant. They utilize tryptophan (trp) to synthesise the phytohormone indole-3-acetic acid (IAA) which is utilized by plants (along with its own synthesized IAA) to regulate plant development via cell proliferation and elongation, and development of lateral and adventitious roots. IAA activates the transcription of the plant enzyme 1-aminocyclopropane-1-carboxylic acid (ACC) synthase which catalyses the production of ACC from S-Adenosyl Methionine (SAM). ACC is further converted to ethylene with the aid of the enzyme ACC oxidase (ACCO). After perceiving a stress signal, the ethylene level inside the plant is increased as a stress response, and growth is retarded. PGPBs take up a large portion of the ACC synthesised by root cells and limit the ethylene production by the root cells. This is accomplished by the enzyme 1-aminocyclopropane-1-carboxylic acid deaminase (ACCD) present in the bacteria that hydrolyses ACC into ammonia and α- Ketobutyrate. Thus, PGPB promote growth by IAA production and ACC deamination. The various PGPB studies in millets include Pseudomonas sp., Florescent pseudomonads, Enterobacter sp. PR14, Sphingomonas faeni mutants, Acinetobacter calcoaceticus, and Bacillus amyloliquefaciens EPP90. These bacteria help in mitigating the effects of various abiotic stresses by an increased phosphate solubilization, and antioxidant activity of enzymes, and accumulation of osmoprotectants, and a decreased lipid peroxidation.)
Abiotic restrains like salinity and drought are the foremost preventive factors for the development and productivity of plants. However, millets have a broad range of adaptive measures to deal with those stresses. Millets are well adapted to marginal regions, and thus they can be suitable crops for food security as demanded by the year 2050. So far, we have various studies on millets like stress tolerance mechanisms, adaptations, genetic manipulation, targeted expression of enzymes and transporters, contribution of proline etc. However, the proteomic and metabolic investigations on millets in response to various abiotic stresses are still limited. Additional molecular studiesand gene transfer methods are required to develop new and proficient cultivars with boosted natural osmolytes and raised tolerance for crop production. This will aid in attaining sustainable development efforts. The application of ‘omics’ approaches can be useful in enhancing tolerance ofabiotic stress in millets. There is a need to focus on the crosstalk between various stress responses and signalling pathways to understand the precise mechanisms used by plants to adjust in the fluctuating environments. This will help to obtaincrop varieties that are more resistant to stress conditions, thus producing a better yield of increasedquality.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report the present study.
1. Singh, R. K., Muthamilarasan, M., Prasad, M. (2021). Biotechnological approaches to dissect climate-resilient traits in millets and their application in crop improvement. Journal of Biotechnology, 327, 64–73. DOI 10.1016/j.jbiotec.2021.01.002. [Google Scholar] [CrossRef]
2. Taylor, J. R. (2019). Sorghum and millets: taxonomy, history, distribution and production. In: Taylor, J. R. N., Duodu, K. G. (eds.Sorghum and millets, pp. 1–21. Washington DC, USA: AACC International Press. [Google Scholar]
3. FAO (2018). FAOSTAT database Rome: Food and Agriculture Organization. http://www.fao.org/faostat/en/#data/QC Accessed 04-July- 2020. [Google Scholar]
4. Taylor, J. R. N., Taylor, J. (2017). Proteins from sorghum and millets. In: Nadathur, S., Wanasundara, J. P. D., Scanlin, L. (eds.Sustainable protein sources, pp. 79–104. Academic Press. [Google Scholar]
5. Ashwini, I. B., Aparna, B., Vani, N., Naidu, G. M. (2019). Growth and instability of major millets in andhra pradesh India. International Journal of Current Microbiology and Applied Sciences, 8(7), 985–993. DOI 10.20546/ijcmas.2019.807.118. [Google Scholar] [CrossRef]
6. Saxena, R., Vanga, S. K., Wang, J., Orsat, V., Raghavan, V. (2018). Millets for food security in the context of climate change: A review. Sustainability, 10(7), 2228. DOI 10.3390/su10072228. [Google Scholar] [CrossRef]
7. Muthamilarasan, M., Prasad, M. (2015). Advances in Setaria genomics for genetic improvement of cereals and bioenergy grasses. Theoretical and Applied Genetics, 128(1), 1–14. DOI 10.1007/s00122-014-2399-3. [Google Scholar] [CrossRef]
8. Patil, D. A. (2020). Agrobiodiversity and advances in the development of millets in changing environment. In: Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S. (eds.Sustainable agriculture in the era of climate change, pp. 643–673. Switzerland: Springer. [Google Scholar]
9. Peng, R., Zhang, B. (2020). Foxtail millet: A new model for C4 plants. Trends in Plant Science. https://doi.org/10.1016/j.tplants.2020.12.003 [Google Scholar]
10. Mgonja, M. A., Lenne, J. M., Manyasa, E., Sreenivasaprasad, S. (2007). Finger millet blast management in East Africa creating opportunities for improving production and utilization of finger millet. Proceedings of the First International Finger Millet Stakeholder Workshop, Projects R8030 & R8445 UK Department for International Development-Crop Protection Programme. International Crops Research Institute for the Semi-Arid Tropics. [Google Scholar]
11. Lata, C. (2015). Advances in omics for enhancing abiotic stress tolerance in millets. Proceedings of the Indian National Science Academy, 81, 397–417. [Google Scholar]
12. Zhu, J. K. (2016). Abiotic stress signaling and responses in plants. Cell, 167(2), 313–324. DOI 10.1016/j.cell.2016.08.029. [Google Scholar] [CrossRef]
13. Baxter, A., Mittler, R., Suzuki, N. (2014). ROS as key players in plant stress signalling. Journal of Experimental Botany, 65(5), 1229–1240. DOI 10.1093/jxb/ert375. [Google Scholar] [CrossRef]
14. Zhang, H., Li, Y., Zhu, J. K. (2018). Developing naturally stress-resistant crops for a sustainable agriculture. Nature Plants, 4(12), 989–996. DOI 10.1038/s41477-018-0309-4. [Google Scholar] [CrossRef]
15. Tadele, Z. (2016). Drought adaptation in millets. In: Shanker, A., Shanker, C. (eds.Abiotic and biotic stress in plants: recent advances and future perspectives, pp. 639–662. Rijeka: InTech. [Google Scholar]
16. Mushtaq, N. U., Saleem, S., Rasool, A., Shah, W. H., Hakeem, K. R. et al. (2021). Salt stress threshold in millets: perspective on cultivation on marginal lands for biomass. Phyton-International Journal of Experimental Botany, 90(1), 51–61. DOI 10.32604/phyton.2020.012163. [Google Scholar] [CrossRef]
17. Li, P., Brutnell, T. P. (2011). Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. Journal of Experimental Botany, 62(9), 3031–3037. DOI 10.1093/jxb/err096. [Google Scholar] [CrossRef]
18. Prasad, P. V., Staggenborg, S. A. (2009). Growth and production of sorghum and millets. In: Verheye, Willy H. (eds.Soils, plant growth and crop production, pp. 1–27. Oxford, UK: Eolss Publishers. [Google Scholar]
19. Rachie, K. O. (1975). The millets: importance, utilization and outlook. International Crops Research Institution for the Semi-Arid Tropics, pp. 1–62. Hyderabad. [Google Scholar]
20. Gupta, S. M., Arora, S., Mirza, N., Pande, A., Lata, C. et al. (2017). Finger millet: A certain crop for an uncertain future and a solution to food insecurity and hidden hunger under stressful environments. Frontiers in Plant Science, 8, 643. DOI 10.3389/fpls.2017.00643. [Google Scholar] [CrossRef]
21. Iwuala, E., Odjegba, V., Umebese, C., Sharma, V., Alam, A. (2019). Physiological and gene expression studies of selected Zea mays L. and Pennisetum glaucum (L.) R. Br. Genotypes to simulated drought stress condition. Vegetos, 32(3), 397–406. DOI 10.1007/s42535-019-00030-7. [Google Scholar] [CrossRef]
22. Ashok, S., Senthil, A., Sritharan, N., Punitha, S., Divya, K. et al. (2018). Yield potential of small millets under drought condition. Madras Agricultural Journal, 105(7–9), 370–372. DOI 10.29321/MAJ.2018.000163. [Google Scholar] [CrossRef]
23. Ullah, A., Ahmad, A., Khaliq, T., Akhtar, J. (2017). Recognizing production options for pearl millet in Pakistan under changing climate scenarios. Journal of Integrative Agriculture, 16(4), 762–773. DOI 10.1016/S2095-3119(16)61450-8. [Google Scholar] [CrossRef]
24. Neocleous, D., Ntatsi, G., Savvas, D. (2017). Physiological, nutritional and growth responses of melon (Cucumis melo L.) to a gradual salinity built-up in recirculating nutrient solution. Journal of Plant Nutrition, 40(15), 2168–2180. DOI 10.1080/01904167.2017.1346673. [Google Scholar] [CrossRef]
25. Flowers, T. S., Yeo, A. R. (1989). Effects of salinity on plant growth and crop yields. In: Cherry, J. H. (ed.Environmental stress in plants, pp. 101–119. Berlin, Heidelberg: Springer. [Google Scholar]
26. Mustafa, G., Akhtar, M. S., Abdullah, R. (2019). Global concern for salinity on various agro-ecosystems. In: Akhtar, M. (ed.Salt stress, microbes, and plant interactions: Causes and solution, pp. 1–19. Singapore: Springer. [Google Scholar]
27. Makarana, G., Kumar, A., Yadav, R. K., Kumar, R., Soni, P. G. et al. (2019). Effect of saline water irrigations on physiological, biochemical and yield attributes of dual purpose pearl millet (Pennisetum glaucum) varieties. Indian Journal of Agricultural Sciences, 89(4), 624–633. [Google Scholar]
28. Satish, L., Rathinapriya, P., Rency, A. S., Ceasar, S. A., Prathibha, M. et al. (2016). Effect of salinity stress on finger millet (Eleusine coracana (L.) Gaertnhistochemical and morphological analysis of coleoptile and coleorhizae. Flora-Morphology, Distribution, Functional Ecology of Plants, 222, 111–120. DOI 10.1016/j.flora.2016.04.006. [Google Scholar] [CrossRef]
29. Mukami, A., Ng’etich, A., Syombua, E., Oduor, R., Mbinda, W. (2020). Varietal differences in physiological and biochemical responses to salinity stress in six finger millet plants. Physiology and Molecular Biology of Plants, 26(8), 1569–1582. DOI 10.1007/s12298-020-00853-8. [Google Scholar] [CrossRef]
30. Islam, M. S., Akhter, M. M., El Sabagh, A., Liu, L. Y., Nguyen, N. T. et al. (2011). Comparative studies on growth and physiological responses to saline and alkaline stresses of Foxtail millet (‘Setaria italica’ L.) and Proso millet (‘Panicum miliaceum’ L.). Australian Journal of Crop Science, 5(10), 1269. [Google Scholar]
31. Sabir, P., Ashraf, M., Hussain, M., Jamil, A. (2009). Relationship of photosynthetic pigments and water relations with salt tolerance of proso millet (Panicum miliaceum L.) accessions. Pakistan Journal of Botany, 41(6), 2957–2964. [Google Scholar]
32. Bhatt, D., Negi, M., Sharma, P., Saxena, S. C., Dobriyal, A. K. et al. (2011). Responses to drought induced oxidative stress in five finger millet varieties differing in their geographical distribution. Physiology and Molecular Biology of Plants, 17(4), 347. DOI 10.1007/s12298-011-0084-4. [Google Scholar] [CrossRef]
33. Tiwari, S., Lata, C., Singh Chauhan, P., Prasad, V., Prasad, M. (2017). A functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Current Genomics, 18(6), 469–482. DOI 10.2174/1389202918666170605083319. [Google Scholar] [CrossRef]
34. Debieu, M., Sine, B., Passot, S., Grondin, A., Akata, E. et al. (2018). Response to early drought stress and identification of QTLs controlling biomass production under drought in pearl millet. PLoS One, 13(10), e0201635. DOI 10.1371/journal.pone.0201635. [Google Scholar] [CrossRef]
35. Khatoon, H., Singh, V. (2016). Impact of water stress on physiological and biochemical parameters of finger millet (Eleusine coracana L.). Research in Environment and Life Science, 9(12), 1474–1477. [Google Scholar]
36. Simmons, T., Styer, A. B., Pierroz, G., Gonçalves, A. P., Pasricha, R. et al. (2020). Drought drives spatial variation in the millet root microbiome. Frontiers in Plant Science, 11, 599. DOI 10.3389/fpls.2020.00599. [Google Scholar] [CrossRef]
37. Erinle, K. O., Jiang, Z., Ma, B., Ur-Rehman, K., Shahla, A. et al. (2018). Physiological and molecular responses of pearl millet seedling to atrazine stress. International Journal of Phytoremediation, 20(4), 343–351. DOI 10.1080/15226514.2017.1393385. [Google Scholar] [CrossRef]
38. Gupta, V., Jatav, P. K., Verma, R., Kothari, S. L., Kachhwaha, S. (2017). Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environmental Science and Pollution Research, 24(30), 23915–23925. DOI 10.1007/s11356-017-0057-4. [Google Scholar] [CrossRef]
39. Opole, R. A., Prasad, P. V. V., Djanaguiraman, M., Vimala, K., Kirkham, M. B. et al. (2018). Thresholds, sensitive stages and genetic variability of finger millet to high temperature stress. Journal of Agronomy and Crop Science, 204(5), 477–492. DOI 10.1111/jac.12279. [Google Scholar] [CrossRef]
40. Saha, P., Sade, N., Arzani, A., Wilhelmi, M., Coe, K. M. et al. (2016). Effects of abiotic stress on physiological plasticity and water use of Setaria viridis (L.). Plant Science, 251, 128–138. DOI 10.1016/j.plantsci.2016.06.011. [Google Scholar] [CrossRef]
41. Zandalinas, S. I., Mittler, R., Balfagón, D., Arbona, V., Gómez-Cadenas, A. (2018). Plant adaptations to the combination of drought and high temperatures. Physiologia Plantarum, 162(1), 2–12. DOI 10.1111/ppl.12540. [Google Scholar] [CrossRef]
42. Trivedi, A. K. (2015). Adaptations and mechanisms of heat stress tolerance of plants. Academic Research Journal of Agriculture Science and Research, 3(7), 151–160. [Google Scholar]
43. Bandyopadhyay, T., Muthamilarasan, M., Prasad, M. (2017). Millets for next generation climate-smart agriculture. Frontiers in Plant Science, 8, 1266. DOI 10.3389/fpls.2017.01266. [Google Scholar] [CrossRef]
44. Bidinger, F. R., Nepolean, T., Hash, C. T., Yadav, R. S., Howarth, C. J. (2007). Quantitative trait loci for grain yield in pearl millet under variable postflowering moisture conditions. Crop Science, 47(3), 969–980. DOI 10.2135/cropsci2006.07.0465. [Google Scholar] [CrossRef]
45. Ajithkumar, I. P., Panneerselvam, R. (2014). ROS scavenging system, osmotic maintenance, pigment and growth status of Panicum sumatrense roth. under drought stress. Cell Biochemistry and Biophysics, 68(3), 587–595. DOI 10.1007/s12013-013-9746-x. [Google Scholar] [CrossRef]
46. Passot, S., Gnacko, F., Moukouanga, D., Lucas, M., Guyomarc’h, S. et al. (2016). Characterization of pearl millet root architecture and anatomy reveals three types of lateral roots. Frontiers in Plant Science, 7, 829. DOI 10.3389/fpls.2016.00829. [Google Scholar] [CrossRef]
47. Aparna, K., Hash, C. T., Yadav, R. S., Vadez, V. (2014). Seed number and 100-seed weight of pearl millet (Pennisetum glaucum L.) respond differently to low soil moisture in genotypes contrasting for drought tolerance. Journal of Agronomy and Crop Science, 200(2), 119–131. DOI 10.1111/jac.12052. [Google Scholar] [CrossRef]
48. Liu, T. Y., Ye, N., Song, T., Cao, Y., Gao, B. et al. (2019). Rhizosheath formation and involvement in foxtail millet (Setaria italica) root growth under drought stress. Journal of Integrative Plant Biology, 61(4), 449–462. DOI 10.1111/jipb.12716. [Google Scholar] [CrossRef]
49. van Oosterom, E. J., Whitaker, M. L., Weltzien, E. (1996). Integrating genotype by environment interaction analysis, characterization of drought patterns, and farmer preferences to identify adaptive plant traits for pearl millet. In: Cooper, M., Hammer, G. L. (eds.Plant adaptation and crop improvement, pp. 282–402. Wallingford: CAB International. [Google Scholar]
50. Vadez, V., Hash, T., Kholova, J. (2012). Phenotyping pearl millet for adaptation to drought. Frontiers in Physiology, 3, 386. DOI 10.3389/fphys.2012.00386. [Google Scholar] [CrossRef]
51. Faye, A., Sine, B., Chopart, J. L., Grondin, A., Lucas, M. et al. (2019). Development of a model estimating root length density from root impacts on a soil profile in pearl millet (Pennisetum glaucum (L.) R. Br). Application to measure root system response to water stress in field conditions. PLoS One, 14(7), e0214182. DOI 10.1371/journal.pone.0214182. [Google Scholar] [CrossRef]
52. Serba, D. D., Yadav, R. S. (2016). Genomic tools in pearl millet breeding for drought tolerance: Status and prospects. Frontiers in Plant Science, 7, 1724. DOI 10.3389/fpls.2016.01724. [Google Scholar] [CrossRef]
53. Kusaka, M., Ohta, M., Fujimura, T. (2005). Contribution of inorganic components to osmotic adjustment and leaf folding for drought tolerance in pearl millet. Physiologia Plantarum, 125(4), 474–489. DOI 10.1111/j.1399-3054.2005.00578.x. [Google Scholar] [CrossRef]
54. Balsamo, R. A., Willigen, C. V., Bauer, A. M., Farrant, J. (2006). Drought tolerance of selected Eragrostis species correlates with leaf tensile properties. Annals of Botany, 97(6), 985–991. DOI 10.1093/aob/mcl068. [Google Scholar] [CrossRef]
55. Nadeem, F., Ahmad, Z., Ul Hassan, M., Ruifeng, W., Diao, X. et al. (2020). Adaptation of foxtail millet (Setaria italica L.) to abiotic stresses: A special perspective of responses to nitrogen and phosphate limitations. Frontiers in Plant Science, 11, 187. DOI 10.3389/fpls.2020.00187. [Google Scholar] [CrossRef]
56. Shivhare, R., Lata, C. (2019). Assessment of pearl millet genotypes for drought stress tolerance at early and late seedling stages. Acta Physiologiae Plantarum, 41(3), 39. DOI 10.1007/s11738-019-2831-z. [Google Scholar] [CrossRef]
57. Vijayalakshmi, D., Ashok, S. K., Raveendran, M. (2014). Screening for salinity stress tolerance in rice and finger millet genotypes using shoot Na+/K+ ratio and leaf carbohydrate contents as key physiological traits. Indian Journal of Plant Physiology, 19(2), 156–160. DOI 10.1007/s40502-014-0090-y. [Google Scholar] [CrossRef]
58. Bartwal, A., Pande, A., Sharma, P., Arora, S. (2016). Intervarietal variations in various oxidative stress markers and antioxidant potential of finger millet (Eleusine coracana L.) subjected to drought stress. Journal of Environmental Biology, 37(4), 517. [Google Scholar]
59. Shivhare, R., Asif, M. H., Lata, C. (2020). Comparative transcriptome analysis reveals the genes and pathways involved in terminal drought tolerance in pearl millet. Plant Molecular Biology, 103(6), 639–652. DOI 10.1007/s11103-020-01015-w. [Google Scholar] [CrossRef]
60. Kotapati, K. V., Palaka, B. K., Anithamma, K., Pamuru, R. R., Ampasala, D. R. (2014). Response of antioxidative enzymes and lipoxygenase to drought stress in finger millet leaves (Eleusine coracana (L.) Gaertn). International Journal of Plant, Animal and Environmental Sciences, 4(3), 644–653. [Google Scholar]
61. Vijayalakshmi, T., Varalaxmi, Y., Jainender, S., Yadav, S. K., Vanaja, M. et al. (2012). Physiological and biochemical basis of water-deficit stress tolerance in pearl millet hybrid and parents. American Journal of Plant Science, 3, 1730–1740. DOI 10.4236/ajps.2012.312211. [Google Scholar] [CrossRef]
62. Pan, J., Li, Z., Wang, Q., Garrell, A. K., Liu, M. et al. (2018). Comparative proteomic investigation of drought responses in foxtail millet. BMC Plant Biology, 18(1), 315. DOI 10.1186/s12870-018-1533-9. [Google Scholar] [CrossRef]
63. Blum, A., Sullivan, C. Y. (1986). The comparative drought resistance of landraces of sorghum and millet from dry and humid regions. Annals of Botany, 57(6), 835–846. DOI 10.1093/oxfordjournals.aob.a087168. [Google Scholar] [CrossRef]
64. Mundada, P. S., Nikam, T. D., Kumar, S. A., Umdale, S. D., Ahire, M. L. (2020). Morpho-physiological and biochemical responses of finger millet (Eleusine coracana (L.) Gaertn.) genotypes to PEG-induced osmotic stress. Biocatalysis and Agricultural Biotechnology, 23, 101488. DOI 10.1016/j.bcab.2019.101488. [Google Scholar] [CrossRef]
65. Xu, B. Q., Gao, X. L., Gao, J. F., Jing, L. I., Pu, Y. A. et al. (2019). Transcriptome profiling using RNA-seq to provide insights into foxtail millet seedling tolerance to short-term water deficit stress induced by PEG-6000. Journal of Integrative Agriculture, 18(11), 2457–2471. DOI 10.1016/S2095-3119(19)62576-1. [Google Scholar] [CrossRef]
66. Aidoo, M. K., Bdolach, E., Fait, A., Lazarovitch, N., Rachmilevitch, S. (2016). Tolerance to high soil temperature in foxtail millet (Setaria italica L.) is related to shoot and root growth and metabolism. Plant Physiology and Biochemistry, 106, 73–81. DOI 10.1016/j.plaphy.2016.04.038. [Google Scholar] [CrossRef]
67. Sharma, P. C., Singh, D., Sehgal, D., Singh, G., Hash, C. T. et al. (2014). Further evidence that a terminal drought tolerance QTL of pearl millet is associated with reduced salt uptake. Environmental and Experimental Botany, 102, 48–57. DOI 10.1016/j.envexpbot.2014.01.013. [Google Scholar] [CrossRef]
68. Wang, M., Li, P., Li, C., Pan, Y., Jiang, X. et al. (2014). SiLEA14, a novel atypical LEA protein, confers abiotic stress resistance in foxtail millet. BMC Plant Biology, 14(1), 290. DOI 10.1186/s12870-014-0290-7. [Google Scholar] [CrossRef]
69. Ramegowda, V., Senthil-Kumar, M., Nataraja, K. N., Reddy, M. K., Mysore, K. S. et al. (2012). Expression of a finger millet transcription factor, EcNAC1, in tobacco confers abiotic stress-tolerance. PLoS One, 7(7), e40397. DOI 10.1371/journal.pone.0040397. [Google Scholar] [CrossRef]
70. Pan, Y., Li, J., Jiao, L., Li, C., Zhu, D. et al. (2016). A non-specific Setaria italica L. lipid transfer protein gene plays a critical role under abiotic stress. Frontiers in Plant Science, 7, 1752. DOI 10.3389/fpls.2016.01752. [Google Scholar] [CrossRef]
71. Yeshvekar, R. K., Nitnavare, R. B., Chakradhar, T., Bhatnagar-Mathur, P., Reddy, M. K. et al. (2017). Molecular characterization and expression analysis of pearl millet plasma membrane proteolipid 3 (Pmp3) genes in response to abiotic stress conditions. Plant Gene, 10, 37–44. DOI 10.1016/j.plgene.2017.05.002. [Google Scholar] [CrossRef]
72. Zhao, J., Yu, A., Du, Y., Wang, G., Li, Y. et al. (2019). Foxtail millet (Setaria italica (L.) P. Beauv) CIPKs are responsive to ABA and abiotic stresses. PLoS One, 14(11), e0225091. DOI 10.1371/journal.pone.0225091. [Google Scholar] [CrossRef]
73. Divya, K., Kishor, P. K., Bhatnagar-Mathur, P., Singam, P., Sharma, K. K. et al. (2019). Isolation and functional characterization of three abiotic stress-inducible (Apx, Dhn and Hsc70) promoters from pearl millet (Pennisetum glaucum L.). Molecular Biology Reports, 46(6), 6039–6052. DOI 10.1007/s11033-019-05039-4. [Google Scholar] [CrossRef]
74. Ding, Q. Q., Wang, X. T., Hu, L. Q., Qi, X., Ge, L. H. et al. (2018). MYB-like transcription factor SiMYB42 from foxtail millet (Setaria italica L.) enhances Arabidopsis tolerance to low-nitrogen stress. Hereditas, 40(4), 327–338. [Google Scholar]
75. Yu, T. F., Zhao, W. Y., Fu, J. D., Liu, Y. W., Chen, M. et al. (2018). Genome-wide analysis of CDPK family in foxtail millet and determination of SiCDPK24 functions in drought stress. Frontiers in Plant Science, 9, 541. DOI 10.3389/fpls.2018.00651. [Google Scholar] [CrossRef]
76. Xie, L. N., Ming, C., Min, D. H., Lu, F. E., Xu, Z. S. et al. (2017). The NAC-like transcription factor SiNAC110 in foxtail millet (Setaria italica L.) confers tolerance to drought and high salt stress through an ABA independent signaling pathway. Journal of Integrative Agriculture, 16(3), 559–571. DOI 10.1016/S2095-3119(16)61429-6. [Google Scholar] [CrossRef]
77. Feng, Z. J., He, G. H., Zheng, W. J., Lu, P. P., Chen, M. et al. (2015). Foxtail millet NF-Y families: genome-wide survey and evolution analyses identified two functional genes important in abiotic stresses. Frontiers in Plant Science, 6, 1142. DOI 10.3389/fpls.2015.01142. [Google Scholar] [CrossRef]
78. Li, W. W., Chen, M., Zhong, L., Liu, J. M., Xu, Z. S. et al. (2015). Overexpression of the autophagy-related gene SiATG8a from foxtail millet (Setaria italica L.) confers tolerance to both nitrogen starvation and drought stress in Arabidopsis. Biochemical and Biophysical Research Communications, 468(4), 800–806. DOI 10.1016/j.bbrc.2015.11.035. [Google Scholar] [CrossRef]
79. Patil Arun, H., Dubey, M., Chandel, G. (2017). Transcript analysis of differentially expressed genes in minor millets under water stress. Internation Journal of Chemical Sciences, 5(6), 1564–1568. [Google Scholar]
80. Parvathi, M. S., Nataraja, K. N., Reddy, Y. N., Naika, M. B., Gowda, M. C. (2019). Transcriptome analysis of finger millet (Eleusine coracana (L.) Gaertn.) reveals unique drought responsive genes. Journal of Genetics, 98(2), 46. DOI 10.1007/s12041-019-1087-0. [Google Scholar] [CrossRef]
81. Karunanithi, P. S., Berrios, D. I., Wang, S., Davis, J., Shen, T. et al. (2020). The foxtail millet (Setaria italica L.) terpene synthase gene family. Plant Journal, 103(2), 781–800. DOI 10.1111/tpj.14771. [Google Scholar] [CrossRef]
82. Kirankumar, T. V., Madhusudhan, K. V., Nareshkumar, A., Kiranmai, K., Lokesh, U. et al. (2016). Expression analysis of Aldo-Keto Reductase 1 (AKR1) in foxtail millet (Setaria italica L.) subjected to abiotic stresses. American Journal of Plant Sciences, 7(3), 500–509. DOI 10.4236/ajps.2016.73044. [Google Scholar] [CrossRef]
83. Reddy, C. S., Kim, K. M., James, D., Varakumar, P., Reddy, M. K. (2017). PgPAP18, a heat-inducible novel purple acid phosphatase 18-like gene (PgPAP18-like) from Pennisetum glaucum, may play a crucial role in environmental stress adaptation. Acta Physiologiae Plantarum, 39(2), 54. DOI 10.1007/s11738-017-2348-2. [Google Scholar] [CrossRef]
84. Chanwala, J., Satpati, S., Dixit, A., Parida, A., Giri, M. K. et al. (2020). Genome-wide identification and expression analysis of WRKY transcription factors in pearl millet (Pennisetum glaucum) under dehydration and salinity stress. BMC Genomics, 21(1), 1–16. DOI 10.1186/s12864-020-6622-0. [Google Scholar] [CrossRef]
85. Babitha, K. C., Ramu, S. V., Nataraja, K. N., Sheshshayee, M. S., Udayakumar, M. (2015). EcbZIP60, a basic leucine zipper transcription factor from Eleusine coracana L. improves abiotic stress tolerance in tobacco by activating unfolded protein response pathway. Molecular Breeding, 35(9), 181. DOI 10.1007/s11032-015-0374-6. [Google Scholar] [CrossRef]
86. Yang, R., Chen, M., Sun, J. C., Yu, Y., Min, D. H. et al. (2019). Genome-wide analysis of LIM family genes in Foxtail Millet (Setaria italica L.) and characterization of the role of SiWLIM2b in drought tolerance. International Journal of Molecular Sciences, 20(6), 1303. DOI 10.3390/ijms20061303. [Google Scholar] [CrossRef]
87. Li, C., Yue, J., Wu, X., Xu, C., Yu, J. (2014). An ABA-responsive DRE-binding protein gene from Setaria italica, SiARDP, the target gene of SiAREB, plays a critical role under drought stress. Journal of Experimental Botany, 65(18), 5415–5427. DOI 10.1093/jxb/eru302. [Google Scholar] [CrossRef]
88. Chopperla, R., Singh, S., Tomar, R. S., Mohanty, S., Khan, S. et al. (2018). Isolation and allelic characterization of finger millet (Eleusine coracana L.) small heat shock protein EcHSP17. 8 for stress tolerance. Indian Journal of Genetics and Plant Breeding, 78(1), 95–103. DOI 10.5958/0975-6906.2018.00011.1. [Google Scholar] [CrossRef]
89. Yadav, C. B., Muthamilarasan, M., Dangi, A., Shweta, S., Prasad, M. (2016). Comprehensive analysis of SET domain gene family in foxtail millet identifies the putative role of SiSET14 in abiotic stress tolerance. Scientific Reports, 6(1), 1–13. DOI 10.1038/srep32621. [Google Scholar] [CrossRef]
90. Dong, Z., Zhao, H., He, J., Huai, J., Lin, H. et al. (2011). Overexpression of a foxtail millet Acetyl-CoA carboxylase gene in maize increases sethoxydim resistance and oil content. African Journal of Biotechnology, 10(20), 3986–3995. [Google Scholar]
91. Puranik, S., Jha, S., Srivastava, P. S., Sreenivasulu, N., Prasad, M. (2011). Comparative transcriptome analysis of contrasting foxtail millet cultivars in response to short-term salinity stress. Journal of Plant Physiology, 168(3), 280–287. DOI 10.1016/j.jplph.2010.07.005. [Google Scholar] [CrossRef]
92. Shinde, H., Dudhate, A., Anand, L., Tsugama, D., Gupta, S. K. et al. (2020). Small RNA sequencing reveals the role of pearl millet miRNAs and their targets in salinity stress responses. South African Journal of Botany, 132, 395–402. DOI 10.1016/j.sajb.2020.06.011. [Google Scholar] [CrossRef]
93. Veeranagamallaiah, G., Jyothsnakumari, G., Thippeswamy, M., Reddy, P. C. O., Surabhi, G. K. et al. (2008). Proteomic analysis of salt stress responses in foxtail millet (Setaria italica L. cv. Prasad) seedlings. Plant Science, 175(5), 631–641. DOI 10.1016/j.plantsci.2008.06.017. [Google Scholar] [CrossRef]
94. Lata, C., Sahu, P. P., Prasad, M. (2010). Comparative transcriptome analysis of differentially expressed genes in foxtail millet (Setaria italica L.) during dehydration stress. Biochemical and Biophysical Research Communications, 393(4), 720–727. DOI 10.1016/j.bbrc.2010.02.068. [Google Scholar] [CrossRef]
95. Shinde, H., Tanaka, K., Dudhate, A., Tsugama, D., Mine, Y. et al. (2018). Comparative de novo transcriptomic profiling of the salinity stress responsiveness in contrasting pearl millet lines. Environmental and Experimental Botany, 155, 619–627. DOI 10.1016/j.envexpbot.2018.07.008. [Google Scholar] [CrossRef]
96. Shi, W. P., Cheng, J. Y., Wen, J. X., Wang, J. X., Shi, G. Y. et al. (2018). Transcriptomic studies reveal a key metabolic pathway contributing to a well-maintained photosynthetic system under drought stress in foxtail millet (Setaria italica L.). PeerJ, 6, e4752. DOI 10.7717/peerj.4752. [Google Scholar] [CrossRef]
97. Dudhate, A., Shinde, H., Tsugama, D., Liu, S., Takano, T. (2018). Transcriptomic analysis reveals the differentially expressed genes and pathways involved in drought tolerance in pearl millet [Pennisetum glaucum (L.) R. Br]. PLoS One, 13(4), e0195908. DOI 10.1371/journal.pone.0195908. [Google Scholar] [CrossRef]
98. Pan, J., Li, Z., Dai, S., Ding, H., Wang, Q. et al. (2020). Integrative analyses of transcriptomics and metabolomics upon seed germination of foxtail millet in response to salinity. Scientific Reports, 10(1), 1–16. DOI 10.1038/s41598-020-70520-1. [Google Scholar] [CrossRef]
99. Hou, S., Sun, Z., Li, Y., Wang, Y., Ling, H. et al. (2017). Transcriptomic analysis, genic SSR development, and genetic diversity of proso millet (Panicum miliaceum; Poaceae). Applications in Plant Sciences, 5(7), 1600137. DOI 10.3732/apps.1600137. [Google Scholar] [CrossRef]
100. Tang, S., Li, L., Wang, Y., Chen, Q., Zhang, W. et al. (2017). Genotype-specific physiological and transcriptomic responses to drought stress in Setaria italica L. (an emerging model for Panicoideae grasses). Scientific Reports, 7(1), 1–15. DOI 10.1038/s41598-017-08854-6. [Google Scholar] [CrossRef]
101. Shan, Z., Jiang, Y., Li, H., Guo, J., Dong, M. et al. (2020). Genome-wide analysis of the NAC transcription factor family in broomcorn millet (Panicum miliaceum L.) and expression analysis under drought stress. BMC Genomics, 21(1), 1–13. DOI 10.1186/s12864-020-6479-2. [Google Scholar] [CrossRef]
102. Chandra, D., Srivastava, R., Glick, B. R., Sharma, A. K. (2018). Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere, 28(2), 227–240. DOI 10.1016/S1002-0160(18)60013-X. [Google Scholar] [CrossRef]
103. Mahadik, S., Kumudini, B. S. (2020). Enhancement of salinity stress tolerance and plant growth in finger millet using fluorescent pseudomonads. Rhizosphere, 15, 100226. DOI 10.1016/j.rhisph.2020.100226. [Google Scholar] [CrossRef]
104. Sagar, A., Sayyed, R. Z., Ramteke, P. W., Sharma, S., Marraiki, N. et al. (2020). ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiology and Molecular Biology of Plants, 26(9), 1847–1854. DOI 10.1007/s12298-020-00852-9. [Google Scholar] [CrossRef]
105. Srinivasan, R., Mageswari, A., Subramanian, P., Maurya, V. K., Sugnathi, C. et al. (2017). Exogenous expression of ACC deaminase gene in psychrotolerant bacteria alleviates chilling stress and promotes plant growth in millets under chilling conditions. Indian Journal of Experimental Botany, 55, 463–468. [Google Scholar]
106. Rathinapriya, P., Pandian, S., Rakkammal, K., Balasangeetha, M., Alexpandi, R. et al. (2020). The protective effects of polyamines on salinity stress tolerance in foxtail millet (Setaria italica L.an important C4 model crop. Physiology and Molecular Biology of Plants, 26(9), 1815–1829. DOI 10.1007/s12298-020-00869-0. [Google Scholar] [CrossRef]
107. Kushwaha, P., Kashyap, P. L., Kuppusamy, P., Srivastava, A. K., Tiwari, R. K. (2020). Functional characterization of endophytic bacilli from pearl millet (Pennisetum glaucum) and their possible role in multiple stress tolerance. Plant Biosystems–An International Journal Dealing with all Aspects of Plant Biology, 154(4), 503–514. DOI 10.1080/11263504.2019.1651773. [Google Scholar] [CrossRef]
108. Ahmadvand, G., Hajinia, S. (2018). Effect of endophytic fungus Piriformospora indica on yield and some physiological traits of millet (Panicum miliaceum) under water stress. Crop and Pasture Science, 69(6), 594–560. DOI 10.1071/CP17364. [Google Scholar] [CrossRef]
109. Tian, B., Qiao, Z., Zhang, L., Li, H., Pei, Y. (2016). Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiology and Biochemistry, 109, 293–299. DOI 10.1016/j.plaphy.2016.10.006. [Google Scholar] [CrossRef]
110. Kotapati, K. V., Palaka, B. K., Ampasala, D. R. (2017). Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop Journal, 5(3), 240–250. DOI 10.1016/j.cj.2016.09.002. [Google Scholar] [CrossRef]
111. Han, Y., Wu, M., Hao, L., Yi, H. (2018). Sulfur dioxide derivatives alleviate cadmium toxicity by enhancing antioxidant defence and reducing Cd2+ uptake and translocation in foxtail millet seedlings. Ecotoxicology and Environmental Safety, 157, 207–215. DOI 10.1016/j.ecoenv.2018.03.084. [Google Scholar] [CrossRef]
112. Kour, D., Rana, K. L., Yadav, A. N., Sheikh, I., Kumar, V. et al. (2020). Amelioration of drought stress in Foxtail millet (Setaria italica L.) by P-solubilizing drought-tolerant microbes with multifarious plant growth promoting attributes. Environmental Sustainability, 3, 23–34. DOI 10.1007/s42398-020-00094-1. [Google Scholar] [CrossRef]
113. Khushdil, F., Jan, F. G., Jan, G., Hamayun, M., Iqbal, A. et al. (2019). Salt stress alleviation in Pennisetum glaucum through secondary metabolites modulation by Aspergillus terreus. Plant Physiology and Biochemistry, 144, 127–134. DOI 10.1016/j.plaphy.2019.09.038. [Google Scholar] [CrossRef]
114. Shah, W. H., Rasool, A., Tahir, I., Rehman, R. U. (2020). Exogenously applied selenium (Se) mitigates the impact of salt stress in Setaria italica L. and Panicum miliaceum L. Nucleus, 63, 327–339. DOI 10.1007/s13237-020-00326-z. [Google Scholar] [CrossRef]
115. Rasool, A., Shah, W. H., Tahir, I., Alharby, H. F., Hakeem, K. R. et al. (2020). Exogenous application of selenium (Se) mitigates NaCl stress in proso and foxtail millets by improving their growth, physiology and biochemical parameters. Acta Physiologiae Plantarum, 42(7), 1–13. DOI 10.1007/s11738-020-03109-w. [Google Scholar] [CrossRef]
116. Han, F., Sun, M., He, W., Cui, X., Pan, H. et al. (2019). Ameliorating effects of exogenous Ca2+ on foxtail millet seedlings under salt stress. Functional Plant Biology, 46(5), 407–416. DOI 10.1071/FP18314. [Google Scholar] [CrossRef]
117. Sun, M., Wang, T., Fan, L., Wang, H., Pan, H. et al. (2020). Foliar applications of spermidine improve foxtail millet seedling characteristics under salt stress. Biologia Plantarum, 64, 353–362. DOI 10.32615/bp.2019.158. [Google Scholar] [CrossRef]
118. Zhang, D., Li, A., Lam, S. K., Li, P., Zong, Y. et al. (2021). Increased carbon uptake under elevated CO2 concentration enhances water-use efficiency of C4 broomcorn millet under drought. Agricultural Water Management, 245, 106631. DOI 10.1016/j.agwat.2020.106631. [Google Scholar] [CrossRef]
119. Han, Y., Yang, H., Wu, M., Yi, H. (2019). Enhanced drought tolerance of foxtail millet seedlings by sulfur dioxide fumigation. Ecotoxicology and Environmental Safety, 178, 9–16. DOI 10.1016/j.ecoenv.2019.04.006. [Google Scholar] [CrossRef]
120. Yadav, T., Kumar, A., Yadav, R. K., Yadav, G., Kumar, R. et al. (2020). Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi Journal of Biological Sciences, 27(8), 2010–2017. DOI 10.1016/j.sjbs.2020.06.030. [Google Scholar] [CrossRef]
121. Shen, J., Guo, M. J., Wang, Y. G., Yuan, X. Y., Wen, Y. Y. et al. (2020). Humic acid improves the physiological and photosynthetic characteristics of millet seedlings under drought stress. Plant Signaling and Behavior, 15(8), 1774212. DOI 10.1080/15592324.2020.1774212. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |