
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015109
ARTICLE
Suppression Effects on Pineapple Soil-Borne Pathogens by Crotalaria juncea, Dolomitic Lime and Plastic Mulch Cover on MD-2 Hybrid Cultivar
1Fitopatología, Colegio de Postgraduados, Montecillo-Texcoco, 56230, México
2CONACyT-Universidad Autónoma de San Luis Potosí, CIACYT, San Luis Potosí, 78000, México
3Edafología, Colegio de Postgraduados, Montecillo-Texcoco, 56230, México
4Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Veracruz, 94270, México
5Rebouças São José, A. UESB/DFZ, Vitória da Conquista, 95, Brasil
*Corresponding Authors: Luis Alfonso Aguilar Pérez. Email: aguilar.luis@colpos.mx; Daniel Nieto Ángel. Email: dnieto@colpos.mx
Received: 22 November 2020; Accepted: 26 January 2021
Abstract: The development and implementation of sustainable and environmentally friendly agricultural practices are indispensable as alternatives to pesticide use and to keep populations of soil-borne plant pathogens at levels that do not affect crop productivity. The present research evaluates the incidence of soil-borne phytopathogens on the pineapple variety MD-2, which was subjected to different treatments: Incorporation of Crotalaria juncea into the soil (organic amendment), application of dolomitic lime to soil (inorganic amendment), and the use of plastic mulch covering the soil. During the crop cycle (15 months), the following variables were evaluated: plant height (cm), fruit weight (kg·plant−1), crop yield (ton·ha−1), the bud root disease incidence caused by Phytophthora nicotianae, number of soil phytoparasitic nematodes and colony-forming-units (CFUs) of soil fungi and oomycetes. The results indicate that Crotalaria juncea treatment reduced the pathogen population (nematode and oomycetes) at levels that did not affect crop development, so that yield increased (18–20%). The incorporation of C. juncea into the soil as an organic amendment favors the populations of fungi disease suppressors (Trichoderma-Aspergillus). The phytoparasitic nematodes (Meloidogyne sp., Pratylenchus sp., and Mesocriconema sp.) and oomycetes (Phytophthora spp., and Pythium spp.) showed a reduction of their population levels by effects of organic amendment (C. juncea). The plastic mulch was also effective, probably due to the maintenance of optimal condition to crop growth and weed control. However, the dolomitic lime application had the poorest effect under the conditions of the study area on the variables analyzed. The described observations are characteristics of a system-based approach for the potential management of soil-borne pathogens of pineapple MD-2 in Veracruz, México.
Keywords: Suppressive soil; integrated pest management; Phytophthora nicotiannae; Phytoparasitic nematodes
Pineapple [Ananas comosus (L.) Merr.] is an important crop in México, and a significant increase in the production area has occurred, with 945,210 tons harvested from 42,679 ha in 2017 [1]. The most important cultivars are ‘Smooth Cayenne’, ‘Champaka’ and the MD-2 hybrid cultivar [2], but consumers prefer MD-2 because it shows superior organoleptic, color, firmness and appearance properties. However, MD-2 is highly susceptible to bud rot disease (BRD) caused by the oomycete Phytophthora nicotianae [3]. Other pathogenic microorganism of pineapple is the bacterium Erwinia chrysanthemi and the phytoparasitic nematodes belonging to the genera Helicotylenchus, Pratylenchus, Rotylenchus, Hoplolaimus, Criconemoides, Tylenchus, Ditylenchus and Meloidogyne [4,5].
The management of soil-borne pathogens is difficult and requires the use of synthetic chemical products for direct soil application or as a fumigant [6]. Nevertheless, the environmental degradation and human health risks associated with pesticides, the escalating crop production costs and pest resistance to pesticides, among others, are factors that have increased the interest in examining alternative approaches for management of soil-borne pathogens [7]. Integrated pest management (IPM) considers the incorporation of agricultural practices into the design and operation of cropping systems by integrating the use of biological, cultural and other control techniques in complementary ways, and IPM operates with pesticide application (action thresholds) only on an as-needed basis [8]. The management of soil-borne pathogens in pineapple crops, by means of the incorporation of organic and inorganic amendments combined with the use of plastic mulch, has high potential due to their properties, but the control efficiency is highly influenced by the soil features. Understanding the mechanisms involved in soil pathogen suppression in amended soils is essential to improve this method to obtain maximum control efficacy [9].
Therefore, the aim of the present study was to evaluate the suppression capacity of organic and inorganic amendments and the use of plastic mulch cover on the populations of soil-borne pathogens of pineapple MD-2 under the edaphoclimatic conditions of a producing area from Veracruz, Mexico.
The research was conducted in the locality of Juan Rodríguez Clara in Veracruz State; this community is located at coordinates 18° 1’ 11.62” N and 95° 24’ 4.31” W and an elevation of 124 meters above sea level. A split-plot experimental design was used, the replications consisted of four beds (experimental unit) per block and the treatments were the following: (1) Dolomitic lime (DL) incorporated into the soil as inorganic amendment, which was identified as the main plot; (2) The incorporation to soil of Crotalaria juncea L. (CJ) as organic amendment and (3) The use of black plastic mulch (PM) covering the soil, these were identified has the subplot factors. Control treatments were included in the experiment (Non-DL, Non-CJ and Non-PM). Each experimental unit (crop bed) consisted of two double-rows of 20 m long. The pineapple plantation was conformed to ‘suckers’ (vegetative material) of the pineapple variety MD-2, and the suckers (820 g on average) were planted in double-rows with a spacing of 0.45 m between rows and 1.25 m between beds. Dolomitic lime (DL) [CaMg (CO3)2] was applied at a dose of 2 ton·ha−1. C. juncea (CJ) was previously planted in rows spaced 0.80 m, with 20 kg·ha−1 of seed, and after 138 days (flowering stage), was incorporated into the soil by two harrow passes. The mulch consisted of black plastic 0.4 mm thick.
The fertilization program was applied according to the recommended doses in the region, and considering a density of 45,000 plants·ha−1, the formula used was 14-8-14-4 g per plant for the elements nitrogen (N), phosphorus (P), potassium (K) and magnesium (Mg). The fertilizers mixture was divided into four applications according to the phenological growth stage of the plant: one application at the time of transplanting, two applications during the vegetative phase and the last during fruit development. Micronutrients were also applied at doses of 46, 35, 20, 40 and 8 g per plant of Zinc (Zn), Boron (B), Manganese (Mn), Copper (Cu) and Sulphur (S), respectively. Calcium carbide (CaC2) was applied for floral induction, with three applications at three-day intervals at a dose of 2 kg dissolved in 180 L·ha−1 of water, and 60 mL of the solution was applied to the bud per plant. Pest and weed control were performed according to the technical recommendations for the region [10].
2.3 Plant Height and Crop Yield
The plant height was measured quarterly from transplant (6 measurements) to harvest, and each evaluation included ten plants per experimental unit (40 plants per treatment). During the harvest, the fruit weight average (FWA) was determined per experimental unit, and crop yield (CY) expressed as ton·ha−1 was also calculated by the following formula:
2.4 Disease Incidence and Pathogen Identification
The Bud Rot Disease (BRD) incidence (Inc) was determined by visual detection of symptomatic plants (leaf chlorosis, apical necrosis and the plant bud becoming water-soaked and rotten with a foul smell), and 30 plants were examined per experimental unit. The observations were carried out monthly and it during a period of time of 15 months. The incidence was calculated according to the formula:
The identification of the causal agent of BRD was carried out by analyzing 30 pineapple plants randomly selected with typical symptoms. Then, 30 fragments (one per plant) of partially rotten tissue from the plant bud were disinfected in 30 mL of 3% sodium hypochlorite solution for three minutes, they were rinsed three times with sterile distilled water and dried with absorbent sterilized paper. Subsequently, the tissue was seeded in a selective medium (PARPH) for oomycetes [3]. The Petri dishes were kept at room temperature (22° to 24°C), and monohyphal cultures were obtained as described by Espinosa et al. [3]. Identification was performed using the [11] keys, with 100 measurements of the reproductive structures (sporangiophores, sporangia and chlamydospores). Additionally, pure colonies were molecularly analyzed by extraction of genomic DNA using the CTAB method [12]. PCR amplification was performed by analyzing the internal transcriber spacer (ITS) regions of fungal ribosomal DNA (rDNA) with the oligonucleotides ITS6 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) according to Bowman et al. [13]. PCR products of expected sizes (900 bp) were purified and sequenced. The BLASTn algorithm was used to search the NCBI GenBank database [14] to confirm the taxonomical assignment of sequence.
2.5 Quantification of Colony-Forming-Units (CFUs) of Fungi and Oomycetes
The counting of colony-forming-units (CFUs) of soil fungi was carried out for each treatment in 10 g of composite sample of soil mixed in 90 mL of sterile distilled water in a flask. Subsequently, serial dilutions up to 10−3 were obtained from the soil suspension, and 0.1 mL of the supernatant suspension was seeded in PDA-PS medium [potato dextrose agar (DB-Bioxon®, USA), with 0.5 mL of Penetrex-F® (FAGRO, Mexico), 0.01 g of streptomycin and 0.0075 g of tetracycline hydrochloride]. Petri dishes were incubated for seven days at room temperature (22° to 24oC). The CFUs of the isolated fungi were counted, based on 10 replicates (Petri dishes) per treatment, and the identification of the isolated fungi was carried out at the genus level using the taxonomic keys of Barnett et al. [15] and Gallegly et al. [11].
2.6 Quantification of Phytoparasitic Nematodes
Soil samples around pineapple roots were taken from each block every three months at a depth of 20 cm in a systematic zig-zag pattern, and 10 subsamples of 100 gr were collected per experimental unit (40 per block); subsamples were placed inside a strong polyethylene bag and homogenized to obtain a composite sample (CS). The nematodes were extracted in 300 g CS by the sieving-centrifugation method [16]. The nematodes were identified to the genus level according to their morphological characteristics [17] and they were quantified.
The results were subjected to analysis of variance and Tukey’s range test (p ≤ 0.05) using the statistical analysis software SAS® [18].
3.1 Plant Height, Fruit Weight and Crop Yield
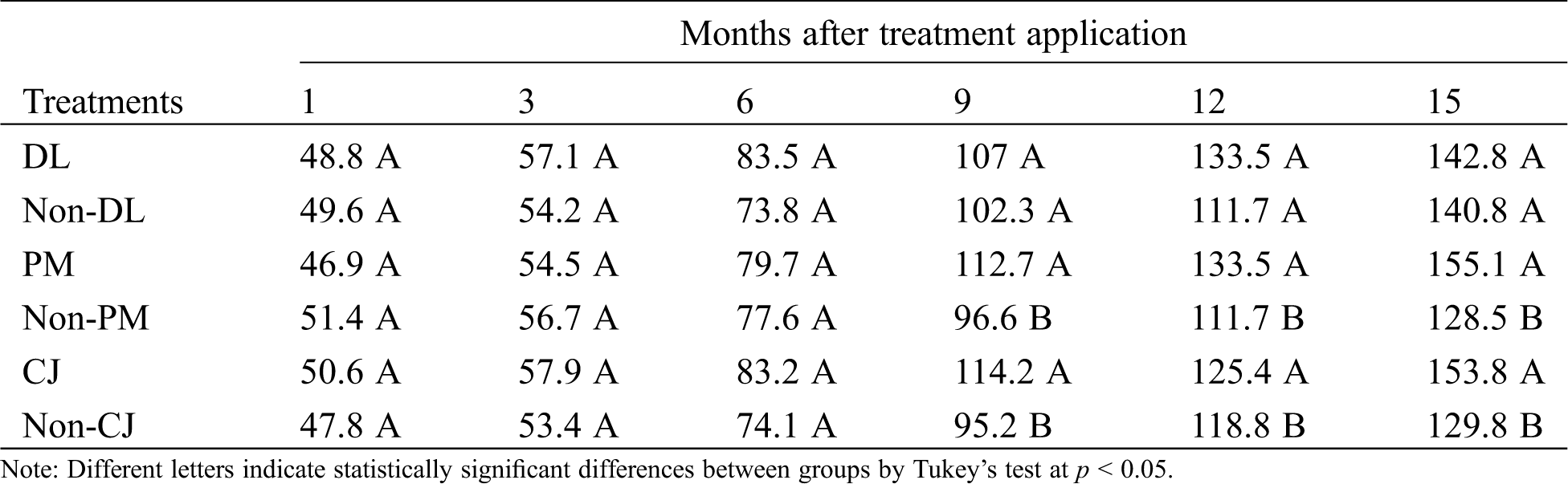
The pineapple plant height did not show statistically significant differences during the evaluation at one (p = 0.275), three (p = 0.292) and six months (p = 0.332) after treatment application. However, during the subsequent evaluations at 9, 12 and 15 months, the taller plants were those from blocks where plastic mulch (PM) was used, which showed height increases of 16, 19 and 20% (for each evaluation) (p < 0.0001), contrary to the observed results in blocks without plastic mulch cover (Non-PM). The same behavior was observed when C. juncea was incorporated into the soil (CJ) because it showed height increases at 9 (20%), 12 (5.5%) and 15 (18%) months, respectively (p < 0.0001). No statistically significant differences were observed in plant height by incorporating dolomitic lime into the soil (DL) (Tab. 1).
Table 1: Effects in plant height (cm) of pineapple MD-2 during the crop growth. Dolomitic lime incorporated as inorganic amendment into the soil (DL), C. juncea incorporated as organic amendment into the soil (CJ) and plastic mulch used as row cover (PM). Control treatments: Non-DL, Non-PM and Non-CJ
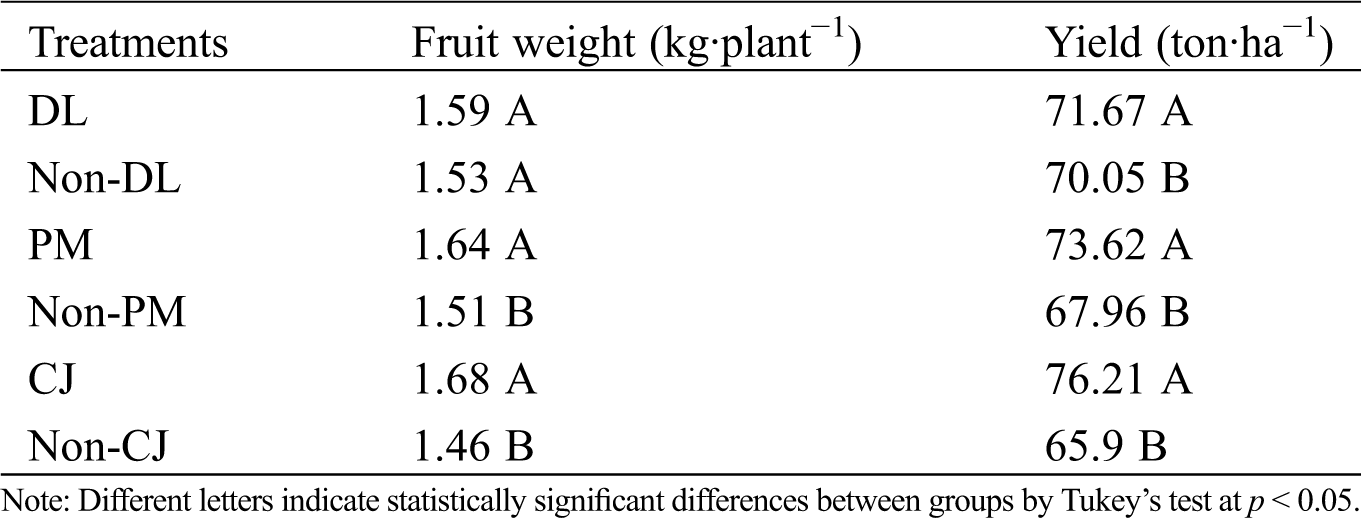
The fruit weight showed statistically significant differences (p < 0.0001). The plastic mulch (PM) when used as a cover produced fruits 8.6 % heavier (1.64 kg·plant−1) compared with the treatment without plastic cover (1.51 kg·plant−1). The incorporation of C. juncea into the soil (CJ) produced fruits of 1.68 kg·plant−1, but the absence of this organic amendment (Non-CJ) produced fruits of 1.46 kg·plant−1 (15% less than CJ treatment) (p < 0.0001). In addition, the incorporation of dolomitic lime did not have a significant effect on fruit weight (Tab. 2). The yield increased simultaneously for the same treatments, which showed yield values of 76.21 ton·ha−1 for CJ and 73.62 ton·ha−1 for PM, but Non-CJ and Non-PM produced 65.9 and 67.96 ton·ha−1 (15.6% and 8% lower), respectively. Finally, the incorporation of dolomitic lime to the soil (DL) showed a marginal yield increase of 2.3 % (Tab. 2).
Table 2: Fruit weight (kg·plant−1) and crop yield (ton·ha−1) obtained from pineapple MD-2. Dolomitic lime incorporated as inorganic amendment into the soil (DL), C. juncea incorporated as organic amendment into the soil (CJ) and plastic mulch used as row cover (PM). Control treatments: Non-DL, Non-PM and Non-CJ
The main oomycete isolated from the symptoms of bud rot disease (BRD) corresponded to Phytophthora nicotianae Breda de Haan (1896) (=P. parasitica), according to their morphological characteristics [11]. The oomycete in V8-medium developed colonies with rosette-shaped cottony growth, coenocytic mycelium with arachnoid growth, predominantly oval-shaped sporangia with pronounced papilla of 44 μm in length, 35 μm in width and the presence of intercalary and terminal chlamydospores of 28 μm in diameter, which is in accordance with the results reported by Espinosa et al. [3].
The PCR amplicons obtained from 15 isolates were of the expected size (~900 pb). The isolates were identified by sequencing the ITS region surrounding the 5.8 rRNA gene [13] and through a search for homology at DNA sequencing level with BLASTn in NCBI [14]. The isolates analyzed were classified to Phytophthora nicotianae (NCBI Accession Number: KJ562359), and the percentage of homology was superior of 99.6% when comparing with the reference sequences (NCBI Accession Number: KR827692, GU111682 and JF792541). The results obtained were in accordance with previous studies [3,19].
3.3 Bud Rot Disease (BRD) Incidence
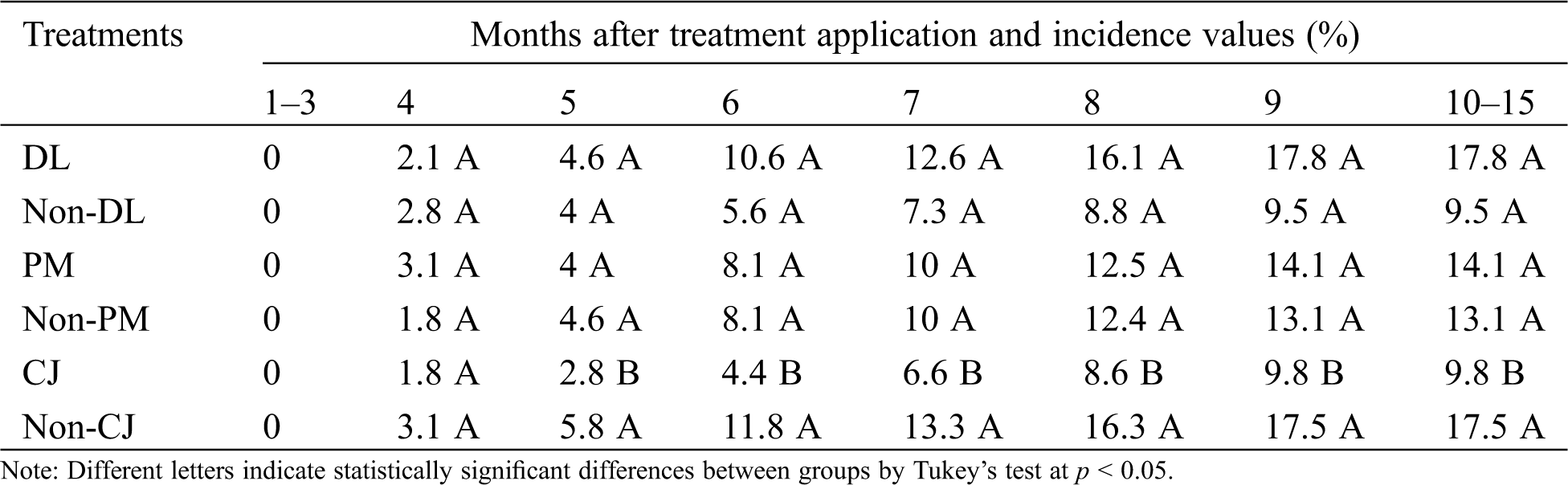
The disease symptoms of BRD were observed from the fourth month. The treatment where C. juncea (CJ) was incorporated into the soil showed statistical significant differences (p = 0.04) when the crop reached the fifth month of growth. The same trend was maintained in subsequent evaluations (6 to 15 months), and the CJ treatment showed the lowest final incidence (9.8%) (p < 0.0001); nevertheless, the higher incidence values were observed in DL (17.8%) and Non-CJ (17.5%) treatments (Tab. 3). The plastic mulch did not affected the incidence of BRD, but the DL treatment showed an increase of the disease incidence.
Table 3: Bud rot disease (BRD) incidence (%) caused by P. nicotianae during the crop cycle of pineapple var. MD-2. Dolomitic lime incorporated as inorganic amendment into the soil (DL), C. juncea incorporated as organic amendment into the soil (CJ) and plastic mulch used as row cover (PM). Control treatments: Non-DL, Non-PM and Non-CJ
3.4 Colony-Forming-Units (CFUs) from Fungi and Oomycetes
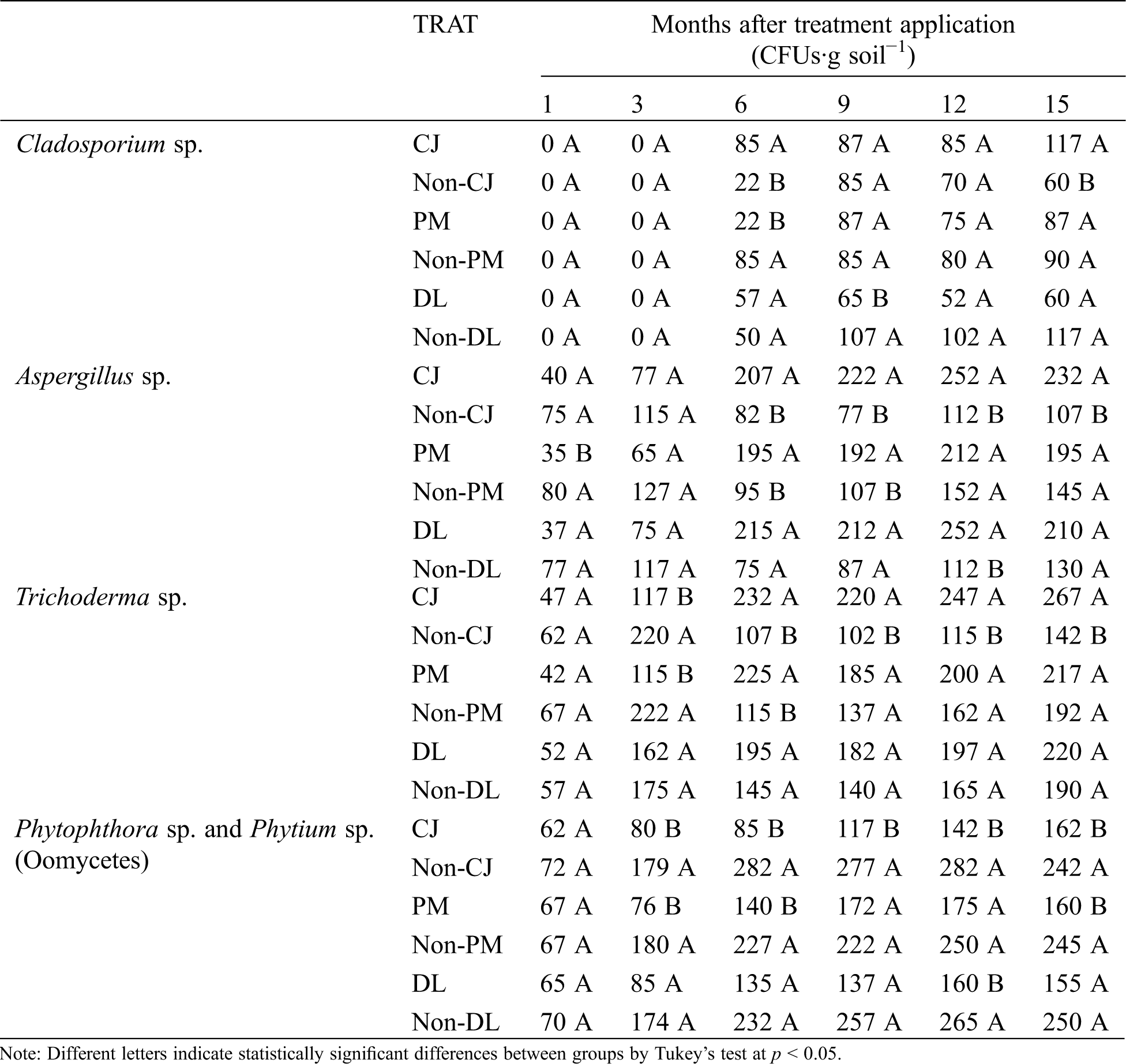
The fungi isolated from the soil samples were identified as Cladosporium spp., Aspergillus spp. and Trichoderma spp., and the oomycetes Phytophthora spp. and Pythium spp., these results were according to their morphological characteristics [11,15]. The treatments evaluated affected the CFUs counts, the fungal genera Cladosporium spp., Aspergillus spp. and Trichoderma spp. changed their population levels by treatment, because Trichoderma sp. increased the CFUs at the end of the experiment by 11–13% as a result of the effect of DL and PM treatments, but the organic amendment (C. juncea) resulted in an 47% increase in CFUs of Trichoderma spp. CFUs showed a similar behavior to Aspergillus sp. counts: the CJ treatment induced an increase of 54%, but DL and PM treatments showed an increases of 38 and 25%, respectively when compared with controls. Cladosporium sp., did not show significant changes in the CFUs count by effect of PM and DL treatments, but the CJ treatment presented 54% higher CFUs counts of this fungus. We noted a statistically significant reduction of CFUs counts of the oomycetes Phytophthora spp. and Pythium spp. with the incorporation of C. juncea (CJ) by about 33%, this from the third month until the end of the crop cycle. The use of plastic cover reduced the CFUs counts about 35%, but the inorganic amendment (dolomitic lime) don’t showed significant statistical differences on the CFUs counts of these oomycetes (Tab. 4).
Table 4: Colony-forming-units (CFUs) of fungi and oomycetes obtained from soil after treatment applications: Dolomitic lime incorporated as inorganic amendment into the soil (DL), plastic mulch used as row cover (PM) and Crotalaria juncea incorporated as organic amendment into the soil (CJ). Control treatments: Non-DL, Non-PM and Non-CJ
3.5 Quantification of Phytoparasitic Nematodes in Soil
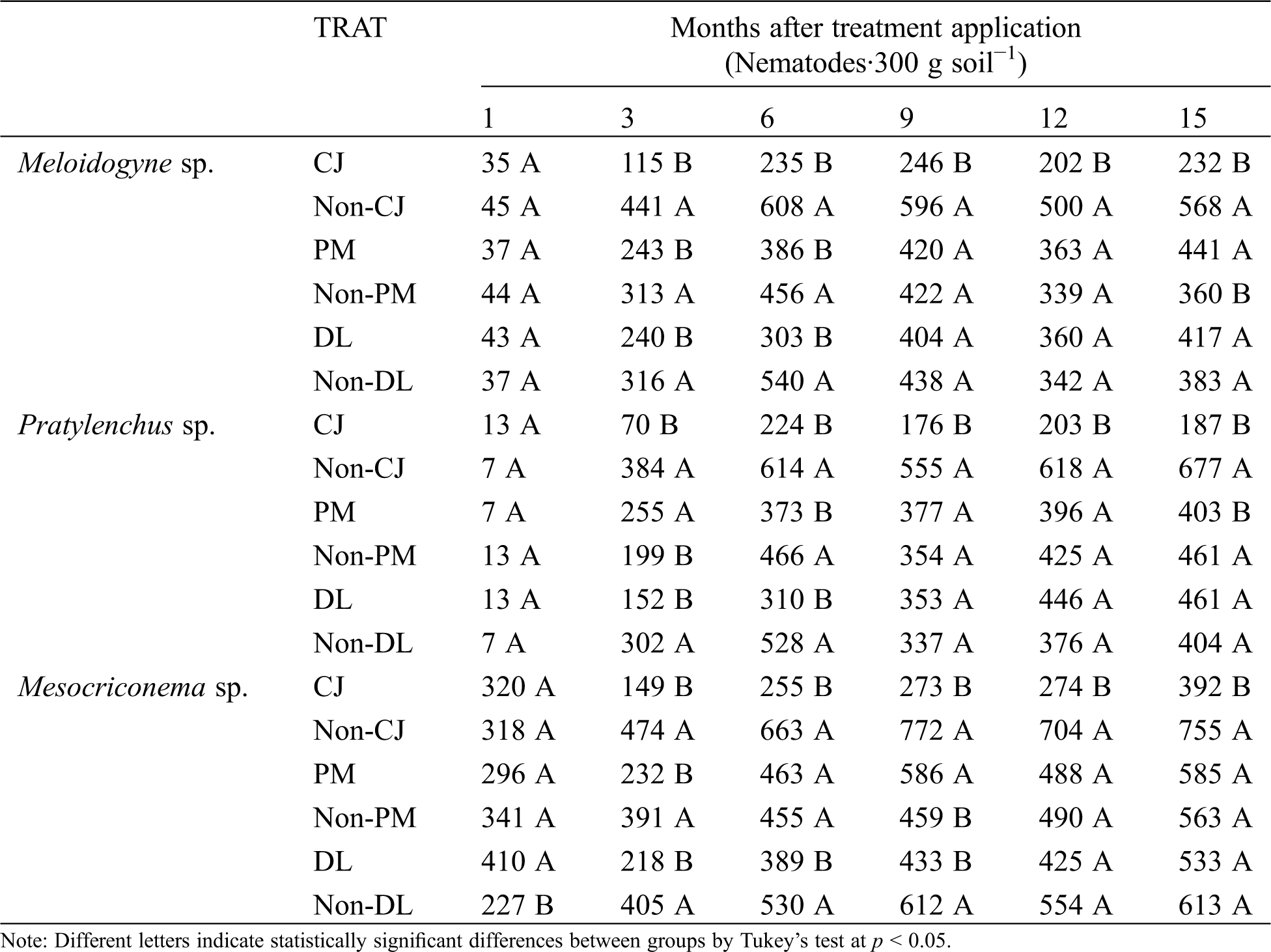
Three genera of phytoparasitic nematodes were identified as Meloidogyne spp., Pratylenchus spp., and Mesocriconema spp., according to previous reports [20]. Population dynamics presented particularities according to the genera of nematodes. Populations of the genus Meloidogyne spp. at the beginning of the experiment ranged between 37–45 individuals per analyzed soil sample (IAS). With respect to Pratylenchus spp., the initial populations were 7–13 IAS, whereas Mesocriconema spp. had a population density of 227–410 IAS.
Meloidogyne spp. showed a reduction of IAS during the second and third evaluations (3, 6 months) due to the effect of the treatments PM (22% and 15% reduction) and DL (24% and 43% reduction) when compared with controls (Non-PM and Non-DL) (p < 0.05), but the most effective treatment was Crotalaria juncea used as organic amendment (CJ), which reduced 60% of Meloidogyne spp. IAS during the 15 months of evaluation. Pratylenchus spp. was not affected by PM and DL from the ninth month to the end of the experiment, but the CJ treatment reduced 68% of the IAS of Pratylenchus spp. during the crop season. Mesocriconema showed variable behavior; the DL treatment resulted in reductions of 46%, 26% and 29% of IAS during the second, third and fourth evaluations, respectively, but no statistically significant differences were observed during the last two evaluations. The effect of PM on Mesocriconema was variable, but the effect of CJ was similar to the observation for Meloidogyne and Pratylenchus because the nematode (Mesocriconema sp.) showed an average reduction of 60% of IAS during the experiment.
Finally, Crotalaria juncea amendment (CJ) maintained lower populations of Meloidogyne spp. (232 IAS), Pratylenchus spp. (187 IAS) and Mesocriconema spp. (392 IAS) at the end of the experiment, in contrast to non-incorporation of C. juncea (Non-CJ) for Meloidogyne spp. (568 IAS), Pratylenchus spp. (677 IAS) and Mesocriconema spp. (755 IAS). The DL treatment showed similar number of IAS at the end of the experiment, so non effects were observed (Tab. 5).
Table 5: Nematode counts of Meloidogyne spp., Pratylenchus spp., Mesocriconema spp., after soil treatments: dolomitic lime incorporated as inorganic amendment into the soil (DL), plastic mulch used as row cover (PM), Crotalaria juncea incorporated as organic amendment into the soil (CJ). Control treatments: Non-DL, Non-PM and Non-CJ
The diseases management of soil-borne pathogens must considers different control strategies, and the present research describes the potential utility of organic and inorganic amendments and the use of plastic mulch. The treatments showed different effects on the variables evaluated; the incorporation of C. juncea into the soil and the use of plastic mulch favored an increase in the fruit size and yield of the MD-2 pineapple variety. Rebolledo et al. [2,21] reported that plastic mulch generates higher relative growth rate, leaf area and dry matter accumulation in pineapple plants. In addition, Perez et al. [22] showed that fruits of pineapple cultivar Smooth Cayenne increased weight when plastic mulch was used, because soil moisture increases, temperature is more stable, the evaporation is lower and weed control, among others [23]. The incorporation of dolomitic lime did not show important effects, although [24] mention that liming increases nutrient uptake by adjusting soil pH, which changes soil structure and infiltration rates; however, these effects are variable according to the agroclimatic zones.
Phytophthora nicotianae was identified as the causal agent of bud rot disease (BRD), the typical morphological features and the molecular analysis (homology at DNA sequencing) confirmed the etiology of the disease (NCBI Accession Number: KJ562359), similar results reported [3,19]. The incorporation of C. juncea reduced the incidence of BRD by up to 44% in comparison with the control treatment (Non-CJ); moreover, this condition was observed from the fifth month of crop development. Different authors mention that the incorporation of organic amendments into the soil can stimulate the development of beneficial microorganisms, because they modify different soil characteristics, including the structure, particle aggregation, pH, salinity, carbon dioxide, oxygen and other chemical compound levels [25], thus affecting the entire structure of the microbial community in the soil ecosystem [26].
The incorporation of C. juncea increased soil population levels of fungi disease suppressors such as Aspergillus spp., and Trichoderma spp., but the phytopathogens Phytophthora spp., and Pythium spp. populations decreased. Khan et al. [27] mentioned that Aspergillus spp., Penicillium spp. and Trichoderma spp., are a complex of fungi that can control other soil pathogenic fungi such as Fusarium oxysporum, Macrophomina phaseolina, Pythium aphanidermatum, Rhizoctonia solani, Sclerotinia sclerotiorum and Phytophthora spp. In addition, Trichoderma spp. is frequently used as an antagonistic controller of phytopathogenic microorganisms [28]. Harman et al. [29] mentioned that Trichoderma spp. efficiently controlled the pathogens Rhizoctonia solani, Pythium ultimum and Sclerotium rolfsii. Native Trichoderma spp. isolates are more effective in inhibiting the development of Phytophthora nicotianae in pineapple [30]. The cover crops when are incorporated into soil, increases the microbial populations, but the use of Crotalaria has an additional suppressive effect by reducing the damage caused by oomycetes and nematodes [31].
Plastic mulch (PM) showed a lower effect in increasing the populations of suppressor fungi, such as Aspergillus spp., and Trichoderma spp., probably due to plastic mulch favors moisture retention, prevents soil compaction and maintains temperature conditions for root growth, which favor crop yield [32]. It is important to mention that the plastic mulch (PM) did not alter the incidence percentage of BRD caused by P. nicotianae. The studied area presented a soil pH of 4.92, and the incorporation of dolomitic lime apparently increased the incidence of the BRD. Soil chemistry greatly affects disease development, and soils at pH values of 5.5 showed a higher incidence of BRD [10].
Pratylenchus spp., Meloidogyne spp., and Criconemoides spp., are considered the main phytopathogenic nematodes affecting pineapple cultivation in México [20] and these nematodes were also identified in the present study. Crotalaria juncea affects the life cycle of these phytoparasitic nematodes [33,34]. Wang et al. [35] indicated that the incorporation of C. juncea into the soil significantly increased populations of competing bacterivorous and fungivorous nematodes and predatory nematophagous fungi. Additionally, the root and exudates from Crotalaria leaves contain allelopathic alkaloids such as monocrotaline and pyrrolizidine, substances that are toxic to various nematodes [36]. During the present study, the incorporation of Crotalaria juncea reduced the populations of Pratylenchus spp., Meloidogyne spp., and Criconemoides spp. in accordance with Wang et al. [37]. Additionally, the suppressive effect was maintained throughout the crop cycle (15 months). C. juncea had a greater effect on the populations of Pratylenchus spp., by reducing the population levels six-fold; but, Meloidogyne spp. and Mesocriconema spp. populations were only halved. The role of nematodes in the development of soil-borne diseases is well documented, because as a result of the nematode feeding process, the plant’s root system is damaged, thereby facilitating the introduction of other soil-borne pathogens, including Phytophthora spp. [9]. The organic amendment consisting of C. juncea incorporated into soil may reduce costs in the use of nematicides and the application of nitrogen fertilizers by increasing the nitrogen content [34,35].
The incorporation of Crotalaria juncea into the soil as an organic amendment showed good effects by reducing the incidence of bud rot disease caused by Phytophthora nicotianae and for maintaining low population levels of the phytoparasitic nematodes Meloidogyne spp., Pratylenchus spp. and Mesocriconema spp. The incorporation of C. juncea increased soil population levels of fungi disease suppressors such as Aspergillus spp., and Trichoderma spp. The reduction of population levels of soil phytopathogens and nematodes led to yield increases of 18–20%. The plastic mulch was also effective, probably due to the maintenance of optimal condition to crop growth and weed control. However, the dolomitic lime application had the poorest effects. The use of C. juncea and plastic mulch have potential utility for the management of soil-borne pathogens of pineapple MD-2 under the local conditions of the studied area. These are preliminary results, future investigations are needed.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. SIAP (2018). Sistema de Información Agrícola y Pecuaria. Cierre de la producción agrícola por cultivo. http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-cultivo/. [Google Scholar]
2. Rebolledo, M. A., Del Ángel, P. A. L., Rebolledo, M. L., Becerril, R. A. E., Uriza, A. D. (2006). Rendimiento y calidad de fruto de cultivares de piña en densidades de plantación. Revista Fitotecnia Mexicana, 29, 55–62. [Google Scholar]
3. Espinosa, R. C. J., Nieto, D., De León, G. C., Villegas, M. A., Aguilar, P. L. A. et al. (2015). Etiología de la pudrición del cogollo de la piña (Ananas comosus. L. Merril) MD2 en Isla, Veracruz, México. Revista Mexicana de Fitopatología, 33, 104–115. [Google Scholar]
4. García, C. R., Palma, L. D., García, E. R., Rodríguez, M., González, H. H. (2005). Effect of legumes rotation on pineapple root diseases in Huimanguillo, Tabasco, Mexico. Acta Horticulturae, 666, 247–256. [Google Scholar]
5. Chan, Y. K., d’Eeckenbrugge, G. C., Sanewski, G. M. (2003). Breeding and variety improvement. In: Bartholomew, D. P., Paull, R. E., Rohrbach, K. G. (eds.The pineapple, botany, production and uses, pp. 33–55. New York: CABI Publishing. [Google Scholar]
6. Anderson, J. M., Pegg, K. G., Scott, C., Drenth, A. (2011). Phosphonate applied as a pre-plant dip controls Phytophthora cinnamomi root and heart rot in susceptible pineapple hybrids. Australasian Plant Pathology, 41(1), 59–68. DOI 10.1007/s13313-011-0090-6. [Google Scholar] [CrossRef]
7. Chellemi, D. O., Wu, T., Graham, J. H., Church, G. (2012). Biological impact of divergent land management practices on tomato crop health. Phytopathology, 102(6), 597–608. DOI 10.1094/PHYTO-08-11-0219. [Google Scholar] [CrossRef]
8. Hawes, C., Begg, G. S., Iannetta, P. P. M., Karley, A. J., Squire, G. R. (2016). A whole-systems approach for assessing measures to improve arable ecosystems sustainability. Ecosystem Health and Sustainability, 12(12), e01252. DOI 10.1002/ehs2.1252. [Google Scholar] [CrossRef]
9. Back, M. A., Haydock, P. P. J., Jenkinson, P. (2002). Disease complexes involving plant parasitic nematodes and soilborne pathogens. Plant Pathology, 51(6), 683–697. DOI 10.1046/j.1365-3059.2002.00785.x. [Google Scholar] [CrossRef]
10. Uriza-Ávila, D. E., Torres-Ávila, A., Aguilar-Ávila, J., Santoyo-Cortés, V. H. Zetina-Lezama, R. et al. (2018). La piña mexicana frente al reto de la innovación. Avances y retos en la gestión de la innovación. Chapingo, México: Colección Trópico Húmedo. [Google Scholar]
11. Gallegly, M. E., Hong, C. X. (2008). Phytophthora: Identifying Species with Morphology and DNA Fingerprints. ST. Paul, MN, USA: APS Press. [Google Scholar]
12. Trzewik, A., Nowak, K. J., Orlikowska, T. (2016). A simple method for extracting DNA from rhododendron plants infected with Phytophthora spp., for use in PCR. Journal of Plant Protection Research, 56(1), 104–109. DOI 10.1515/jppr-2016-0014. [Google Scholar] [CrossRef]
13. Bowman, K. D., Albrecht, U., Graham, J. H., Bright, D. B. (2007). Detection of Phytophthora nicotianae and P. palmivora in citrus roots using PCR-RFLP in comparison with other methods. European Journal of Plant Pathology, 119(2), 143–158. DOI 10.1007/s10658-007-9135-7. [Google Scholar] [CrossRef]
14. Benson, D. A., Karsch-Mizrachi, I., Lipman, D. J., Ostell, J., Sayers, E. W. (2010). GenBank. Nucleic Acids Research, 38, D46–D51. [Google Scholar]
15. Barnett, H. L., Hunter, B. B. (2010). Illustrated genera of imperfect fungi. Fourth edition, American Phytopathological Society Press [Google Scholar]
16. Coyne, D. L., Nicol, J. M., Claudius-Cole, B. (2007). Practical plant nematology: A field and laboratory guide. Cotonou, Benin: SP-IPM Secretariat, International Institute of Tropical Agriculture (IITA). [Google Scholar]
17. Manzanilla, L. R. H., Marbán, M. N. (2012). Practical Plant Nematology. México: Edit. Mundi-Prensa, Colegio de Postgraduados. [Google Scholar]
18. SAS Institute (2002). The SAS system for Windows. Release 6.10. SAS Inst., Cary, NC. [Google Scholar]
19. Hernández, M. A. A., Lina, M. B., Rosón, Á.C., Cazola, G. C. (2011). Hongos y oomycetes fitopatógenos en viveros de piña Ananas comosus (L.) Merril en Ciego de Ávila, Cuba. Fitosanidad, 15, 137–142. [Google Scholar]
20. Uriza, A. D. E. (2011). Paquete Tecnológico Piña MD2 (Ananas comosus var. comosus). Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. México. [Google Scholar]
21. Rebolledo, M. A., Del Ángel, A. L., Becerril, R. A. E., Rebolledo, M. L. (2005). Growth analysis for three pineapple cultivars grown on plastic mulch and bare soil. Interciencia, 30, 758–763. [Google Scholar]
22. Pérez, G. P., García, P. G. M., Rebolledo, M. L., Uriza, A. D., Tinoco, A. C. A. et al. (2005). Planting densities and plastic mulching for smooth cayenne pineapple grown in an AW2 climate and fluvisol soil in Veracruz, Mexico. Acta Horticulturae, 666, 271–275. [Google Scholar]
23. Dusek, J., Ray, C., Alavi, G., Vogel, T., Sanda, M. (2010). Effect of plastic mulch on water flow and herbicide transport in soil cultivated with pineapple crop: A modeling study. Agricultural Water Management, 97(10), 1637–1645. DOI 10.1016/j.agwat.2010.05.019. [Google Scholar] [CrossRef]
24. Mite, F., Espinosa, J., Medina, L. (2010). Liming effect on pineapple yield and soil properties in volcanic soils. Better Crops, 94, 7–9. [Google Scholar]
25. Oka, Y. (2010). Mechanisms of nematode suppression by organic soil amendments—A review. Applied Soil Ecology, 44(2), 101–115. DOI 10.1016/j.apsoil.2009.11.003. [Google Scholar] [CrossRef]
26. Summers, F. C., Park, S., Dunn, A. R., Rong, X., Everts, K. L. et al. (2014). Fungal and oomycete pathogen detection in the rhizosphere of organic tomatoes grown in cover crop-treated soils. Applied Soil Ecology, 80(8), 4–50. DOI 10.1016/j.apsoil.2014.03.012. [Google Scholar] [CrossRef]
27. Khan, M. R., Altaf, F. S., Mohidin, A., Khan, U., Anwer, A. (2009). Biological control of plant nematodes with phosphate solubilizing microorganisms. In: Khan, M. S., Zaidi, A. (eds.pp. 395–426, Phosphate solubilizing microbes for crop improvement. New York, USA: Nova Science Publisher Inc. [Google Scholar]
28. Borrero, C., Trillas, M. I., Delgado, A., Avilés, M. (2012). Effect of ammonium/nitrate ratio in nutrient solution on control of Fusarium wilt of tomato by Trichoderma asperellum T34. Plant Pathology, 61(1), 132–139. DOI 10.1111/j.1365-3059.2011.02490.x. [Google Scholar] [CrossRef]
29. Harman, G. E., Howell, C. R., Viterbo, A., Chet, I., Lorito, M. (2004). Trichoderma species—opportunistic, avirulent plant symbionts. Nature Reviews Microbiology, 2(1), 43–56. DOI 10.1038/nrmicro797. [Google Scholar] [CrossRef]
30. Hernández, M. A. A., Sierra, P. A., Carr, P. A. (2006). Evaluación in vitro del antagonismo de especies de trichoderma sobre hongos fitopatógenos que afectan las vitroplantas de piña (Ananas comosus (L.) Merr.). Fitosanidad, 10, 105–108. [Google Scholar]
31. Schutter, M. E., Dick, R. P. (2002). Microbial community profiles and activities among aggregates of winter fallow and cover-cropped soil. Soil Science Society of America Journal, 66(1), 142–153. DOI 10.2136/sssaj2002.1420. [Google Scholar] [CrossRef]
32. Chen, Y., Liu, T., Tian, X., Wang, X., Li, M. et al. (2015). Effects of plastic film combined with straw mulch on grain yield and water use efficiency of winter wheat in Loess Plateau. Field Crops Research, 172, 53–58. DOI 10.1016/j.fcr.2014.11.016. [Google Scholar] [CrossRef]
33. Wang, K. H., McSorley, R., Gallaher, R. N. (2003). Effects of Crotalaria juncea amendment on nematode communities in soil with different agricultural histories. Journal of Nematology, 35, 294–301. [Google Scholar]
34. Germani, G., Plenchette, C. (2005). Potential of Crotalaria species as green manure crops for the management of pathogenic nematodes and beneficial mycorrhizal fungi. Plant and Soil, 266(1–2), 333–342. DOI 10.1007/s11104-005-2281-9. [Google Scholar] [CrossRef]
35. Wang, K. H., Sipes, B. S., Schmitt, D. P. (2002). Crotalaria as a cover crop for nematode management: A review. Nematropica, 32, 35–57. [Google Scholar]
36. Wang, K. H., Sipes, B. S., Schmitt, D. P. (2002). Management of Rotylenchulus reniformis in Pineapple, Ananas comosus, by intercycle cover crops. Journal of Nematology, 34, 106–114. [Google Scholar]
37. Wang, K. H., McSorley, R., Marshall, A. J., Gallaher, R. N. (2004). Nematode community changes associated with decomposition of Crotalaria juncea amendment in litterbags. Applied Soil Ecology, 27(1), 31–45. DOI 10.1016/j.apsoil.2004.03.006. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |