
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.016015
ARTICLE
Phenotypic and Molecular Assessment of Wheat Genotypes Tolerant to Leaf Blight, Rust and Blast Diseases
1Bangladesh Wheat and Maize Research Institute (BWMRI), Dinajpur, 5200, Bangladesh
2Department of Botany and Plant Physiology, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences Prague, Kamycka 129, Prague, 16500, Czechia
3Regional Agricultural Research Station, BWMRI, Jashore, 7400, Bangladesh
4Department of Biology, College of Science, Taif University, Taif, 21944, Saudi Arabia
5Department of Agronomy, Faculty of Agriculture, University of Poonch Rawalakot, Hajira Road, Shamsabad, 12350, Pakistan
6Department of Plant Physiology, Slovak University of Agriculture, Nitra, Tr. A. Hlinku 2, Nitra, 94901, Slovak Republic
7Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
8Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
9Department of Agronomy, Faculty of Agriculture, Kafrelsheikh University, Kafrelsheikh, 33516, Egypt
*Corresponding Authors: Akbar Hossain. Email: akbarhossainwrc@gmail.com; Ayman EL Sabagh. Email: ayman.elsabagh@agr.kfs.edu.eg
Received: 31 January 2021; Accepted: 06 April 2021
Abstract: Globally among biotic stresses, diseases like blight, rust and blast constitute prime constraints for reducing wheat productivity especially in Bangladesh. For sustainable productivity, the development of disease-resistant lines and high yielding varieties is vital and necessary. This study was conducted using 122 advanced breeding lines of wheat including 21 varieties developed by Bangladesh Wheat and Maize Research Institute with aims to identify genotypes having high yield potential and resistant to leaf blight, leaf rust and blast diseases. These genotypes were evaluated for resistance against leaf blight and leaf rust at Dinajpur and wheat blast at Jashore under field condition. Out of 122 genotypes tested, 20 lines were selected as resistant to leaf blight based on the area under the diseases progress curve under both irrigated timely sown and irrigated late sown conditions. Forty-two genotypes were found completely free from leaf rust infection, 59 genotypes were identified as resistant, and 13 genotypes were identified as moderately resistant to leaf rust. Eighteen genotypes were immune against wheat blast, 42 genotypes were categorized as resistant, and 26 genotypes were identified as moderately resistant to wheat blast. Molecular data revealed that the 16 genotypes showed a positive 2NS segment among the 18 immune genotypes selected against wheat blast under field conditions. The genotypes BAW 1322, BAW 1295, and BAW 1203 can be used as earlier maturing genotypes and the genotypes BAW 1372, BAW 1373, BAW 1297 and BAW 1364 can be used for lodging tolerant due to short plant height. The genotypes WMRI Gom 1, BAW 1349 and BAW 1350 can be selected for bold grain and the genotypes WMRI Gom 1, BAW 1297, BAW 1377 can be used as high yielder for optimum seeding condition but genotypes BAW 1377 and BAW 1366 can be used for late sown condition. The selected resistant genotypes against specific diseases can be used in the further breeding program to develop wheat varieties having higher disease resistance and yield potential.
Keywords: Wheat blast; leaf blight; leaf rust; 2NS marker; grain yield
Wheat (Triticum aestivum L.) is the staple food of over half of the human population and ranks second only after rice in terms of production and acreage [1,2]. In South Asia, its consumption is multiplying due to the rapidly increasing population [3–5]. Wheat has attained the status of a profitable and secure crop for farmers in Bangladesh during the last four decades [6,7]. A variety of factors including high yielding varieties which are resistant to biotic and abiotic stresses, modernized production technology packages and effectively disseminated research outputs to the farming community are the attributes that have assisted in boosting wheat productivity (3.49 t ha−1) [8,9]. However, a sharp decline in wheat area and production has been recorded in the country recently due to its competition with other cash crops as well as the incidence of different diseases [7].
Diseases play an important role in wheat production all over the world including Bangladesh. There are over 200 diseases of the wheat crop but in Bangladesh, among them, three diseases including leaf rust (Puccinia triticina), leaf blight and seedling blight (Bipolaris sorokiniana) are considered economically drastic through reducing wheat growth and yield [10,11]. Moreover, rust caused by Puccinia triticina Eriks is also posing a major threat to the sustainable production of wheat worldwide [12]. Leaf rust incidence usually occurs in mid-February, while its severity attains peak at the end of March under Bangladesh’s agro-climatic conditions. These diseases get optimal environment (hot and humid) and ultimately the severity of this disease has reached an alarming level having the potential to inflict yield losses by 40%–100% [13–15]. The existing wheat varieties in Bangladesh have no absolute resistant gene against these diseases; while other abiotic stresses (i.e., heat, drought, waterlogging, weed infestation and poor plant nutrition) tend to increase the severity of these diseases [16,17]. Generally, yield losses of wheat depend on the susceptibility level of genotypes, agro-environmental conditions and crop growth stage [18]. These diseases have the potential to impart drastic effects including complete crop failure especially in case of susceptible varieties under hot climate. There exist a considerable research gap and limited information regarding susceptibility and resistance/tolerance reaction of the cultivated wheat varieties and germplasm against different diseases under agro-ecological conditions of Bangladesh.
In February 2016, the wheat blast (WB) caused by Magnaporthe oryzae Triticum [19] has emerged a great threat for the sustainability of wheat production in Bangladesh [20]. The initial year, the WB was spotted in eight districts and affected 15,000 hectares (about 16%) wheat area in Bangladesh, with upto 100% yield losses in some [21]. The disease was first reported in 1985 in Paraná, Brazil and has since spread throughout many of the important wheat-producing areas of Brazil and to the neighboring countries of Bolivia and Paraguay. Blast is now considered a major threat to wheat production in South America.
Scientists observed that CIMMYT line Milan appeared to contain high levels of resistance against WB under field condition [22]. Although, other cultivars with this resistance source are now being widely deployed, but the genetic basis of the resistance in Milan has not yet been established [22]. Therefore, there is an urgent need for the documentation of new sources of resistance to WB. It is well-documented that WB resistance genes have come from Aegilops ventricosa (Zhuk.) Chennav on wheat. This translocation carries a 25 to 38 cM distal segment of chromosome arm 2NS from Aegilops ventricosa to the distal region of chromosome arm 2AS in wheat. The A. ventricosa 2NS/2AS translocation carries resistance genes Rkn3 against root-knot nematodes (Meloidogyne spp.), Cre5 against the French pathotype Ha12 of the cereal cyst nematode (Heterodera avenae Wollenweber), and Lr37, Sr38, and Yr17 against some races of wheat leaf, stem and stripe rust [23,24].
To meet the threat of WB, Bangladesh Wheat and Maize Research Institute (BWMRI), with the technical support of the International Maize and Wheat Improvement Center (CIMMYT), Mexico, has developed and released a new wheat ‘BARI Gom 33’ [25]. Before releasing the variety, several multilocation trials, and laboratory screening were done both in 2016 and 2017 at Jashore (natural condition)-Bangladesh, Bolivia (natural condition), and in the Maryland USA in Laboratory condition and found WB resistant. The wheat variety also provides 5%–8% more yield and 40–45 ppm Zn-enriched than exiting varieties than exiting wheat varieties Bangladesh [7,25]. Besides these, the variety is moderately resistant against leaf blight and leaf rust diseases [7,25].
The researchers of BWMRI-Bangladesh are trying to find out the resistance sources for the development of wheat varieties for the sustainability of wheat production in the modern era to find out the resistant wheat genotypes against blight, rust and WB diseases through phenotyping and molecular assessment, and to identify high yielding promising genotypes based on yield and yield attributing traits.
2.1 Plant Materials and Locations of Evaluation
One hundred twenty-two high yielding advanced lines of wheat including 21 varieties developed from 1968 to 2018 by Bangladesh Wheat and Maize Research Institute BWMRI were evaluated against Bipolaris leaf blight (BpLB) and leaf rust under field condition at Dinajpur, Bangladesh (Tab. 1). The same genotypes were evaluated against wheat blast at Regional Agricultural Research Station (RARS), Jashore, one of the wheat blast hotspots area of Bangladesh.
Table 1: List of the tested BWMRI wheat varieties/genotypes and their pedigrees
Weather information such as daily maximum (Max.), minimum (Min.) and mean temperatures, as well as rainfall, were recorded in each location (Fig. 1) from November 2018 to March 2019. For recording the weather data during crop stages, a HOBO U12 Family of Data Loggers was set in both locations.
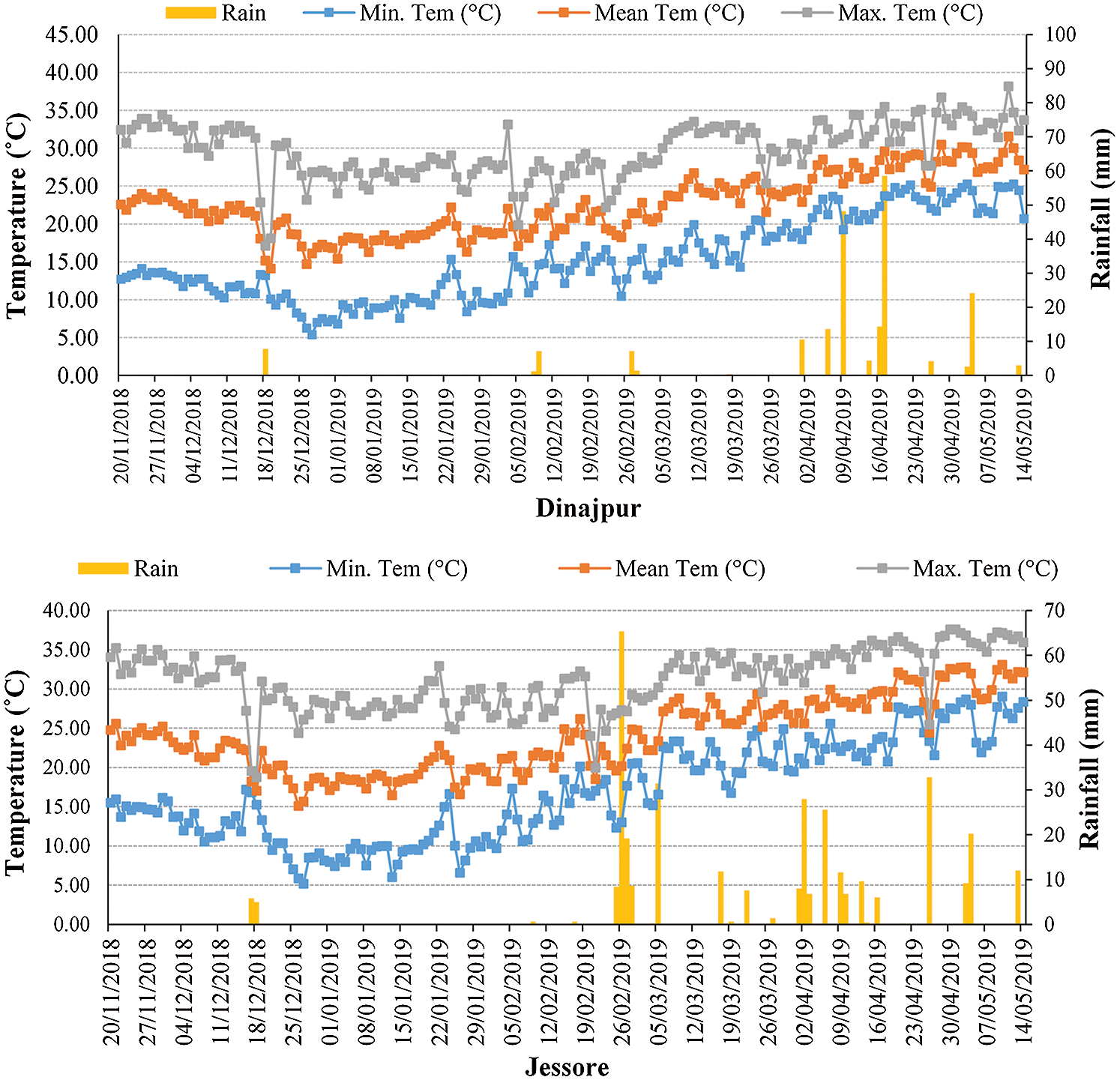
Figure 1: Average daily maximum (Max.), minimum (Min.) and mean temperatures as well as rainfall data recorded in both locations during the wheat season
2.3 Seed Sowing and Agronomic Management
The genotypes were planted in 5 m long 8 row-plots with 20 cm spacing between rows and 60 cm between entries. The materials were planted following an alpha-lattice design with two replications. The trial was set under irrigated timely sown (ITS) on 21 November 2018 and irrigated late sown (ILS) conditions on 25 December 2018 at Dinajpur. In the case of Jashore, the genotypes were sown on 23 November 2018 for ITS and 26 December 2018 for ILS condition. Seed rate for all genotypes was120 kg ha−1. For minimizing seedling infection and also ensuring better germination, seeds of all wheat genotypes were treated with a fungicide namely ‘Provax-200 WP’ (containing carboxin and thiram). For controlling soil-borne insects, Furadan 5G at 10 kg ha−1 (Carbofuran) was incorporated with soil at final land preparation. For proper growth and development, BWMRI recommended fertilizers, namely N, P, K, S and B, respectively were applied at the rate of 100, 27, 40, 20, and 1 kg ha−1 during final land preparation. In the case of nitrogen fertilizer, 2/3 was applied as basal dose during final land preparation with other fertilizers. The remaining 1/3 N fertilizer was applied as top dress immediately after the first irrigation (20 days after sowing (DAS)). Three irrigations were applied during the whole cropping season. The first irrigation was applied at 20 DAS, while the second and third irrigation were applied at 55 and 75 DAS, respectively. Weeds were controlled by a post-emergence herbicide namely ‘Affinity 50.75 WP’ (Carfentrazone + Isoproturon (ready-mix formulation) at 1.25 kg ha−1, which was applied at 10 days after first irrigation.
A mixture of susceptible varieties was planted surrounding the experimental plots spreader rows at Dinajpur. The susceptible mixture serves as a substrate for multiplication as well as the distribution of BpLB and rust inoculum. The inoculation of spreader rows was done by spraying the aqueous suspension of uredospores of Puccinina triticina for disease development of leaf rust at the booting stage of the crop at Dinajpur. The highly blast susceptible variety ‘BARI Gom 26’ was sown surrounded by the experiment at Jashore for wheat blast spreader. At Jashore, the spreader rows were inoculated with Magnaporthe oryzae pathotype Triticum (MoT) spores (20000 spores per mL) for blast symptom development starting from three weeks after sowing and continued until the primary infection was observed. The inoculum of MoT was multiplied at plant pathology laboratory of Regional Agricultural Research Station, Jashore.
2.5 Assessment Procedures of Diseases
The severity of leaf blight was estimated thrice on the double-digit scale (00–99) [26], which started from water ripe to dough stages of Zadoks growth scale [27,28].
2.5.1 Assessment of Leaf Blight
The data about leaf blight were converted to diseased leaf area (DLA) and then the area under the disease progress curve (AUDPC) was calculated as suggested by [17].
The assessment of leaf rust was executed at the dough stage with the help of a modified Cobb scale [29].
2.5.3 Assessment of Wheat Blast
Wheat blast severity was recorded as per the following equation:
Disease severity or index (%) = (spike incidence (%)/100) × (diseased area on spike (%)/100) × 100
2.5.4 Molecular Screening of Wheat Blast
To amplify 2NS translocation region, genomic DNA was extracted from 10 days old seedlings using the modified CTAB method [15]. Briefly, about 200 mg fresh wheat leaves were cut into small pieces (∼2 mm) and collected in 2 mL centrifuge tube which contained two sterile steel balls, frozen in liquid nitrogen, crushed the leaves to powder by the high-speed vortex. The cap of the centrifuge tube was carefully opened, added 1 ml (0.8%) of warmed (65°C) CTAB buffer, added 0.4 ml of chloroform and mixed thoroughly, removed the steel balls from the tube by magnet suction; heated in a water bath at 60°C for 15 min. and then incubated at −20°C for 15 min. The sample was centrifuged at 12000 rpm for 10 min at 4°C temperature; the clear supernatant was transferred into a new 1.5 mL centrifuge tube. An equal volume of isopropyl alcohol (isopropanol) was added and mixed gently by inverting (2–3 times). The sample was incubated on ice for 10 min for precipitating the DNA and centrifuged at 12000 rpm for 15 min at 4°C. A very small gel-like pellet was visible on the side and bottom of the tube. The pellet was washed twice with 0.4 ml of 75% chilled ethanol. The final pellet was air-dried and then dissolved in 100 to 500 μl of 1×TE (100 mM Tris-HCl, 10 mM EDTA, pH = 8.0).
PCR Amplification and gel Electrophoresis
Two PCR primers (VENTRUIP 5’-AGGGGCTACTGACCA AGGCT-3’), LN2 (5’-TGCAGCTACAGCAGTATGTACACAAAA-3’) and Yr17F (5’-TTATTACCTTGATGAGAAATACAGF-3’) Yr17-R (5’-CTGAAATTGGGACTAGCGAAATTA-3’) were used for screening wheat blast resistance genes in 2NS segment of wheat germplasms. PCR was performed in a volume of 10 μL using a Verity Thermal Cycler (Applied Biosystems, USA). The reaction mixture contained 40 to 100 ng of genomic DNA, 2× PCR master mix, 10 μM each Primer and ddH2O up to 10 μL. The amplification program of VENTRUIP-F/LN2-R was as follows: 94°C for 3 min (enzyme activation); 30 cycles of 94°C for 45 sec (melting), 65°C (depending on the specific primers) for 30 sec (annealing) and 72°C for 60 sec (extension); and a final extension at 72°C for 7 min. The amplification program of Yr17-F/Yr17-R was as follows: 94°C for 3 min (enzyme activation); 26 cycles of 94°C for 45 sec (melting), 57°C (depending on the specific primers) for 45 sec (annealing) and 72°C for 45 sec (extension); and a final extension at 72°C for 8 min. PCR products (10 μl each) were run on 1.5% agarose gel and stained with ethidium bromide.
Data on days to heading, days to maturity, plant height, spike m−2, spikelet spike−1, grain spike−1, 1000-grain weight and grain yield were recorded in the location of Dinajpur. At maturity, the middle five-meter long six rows were harvested to estimate yield.
Data collected during this study were statistically analyzed using R software [30]. Duncan’s new multiple range test (DMRT) at a 5% probability level was used to test differences among mean values [31].
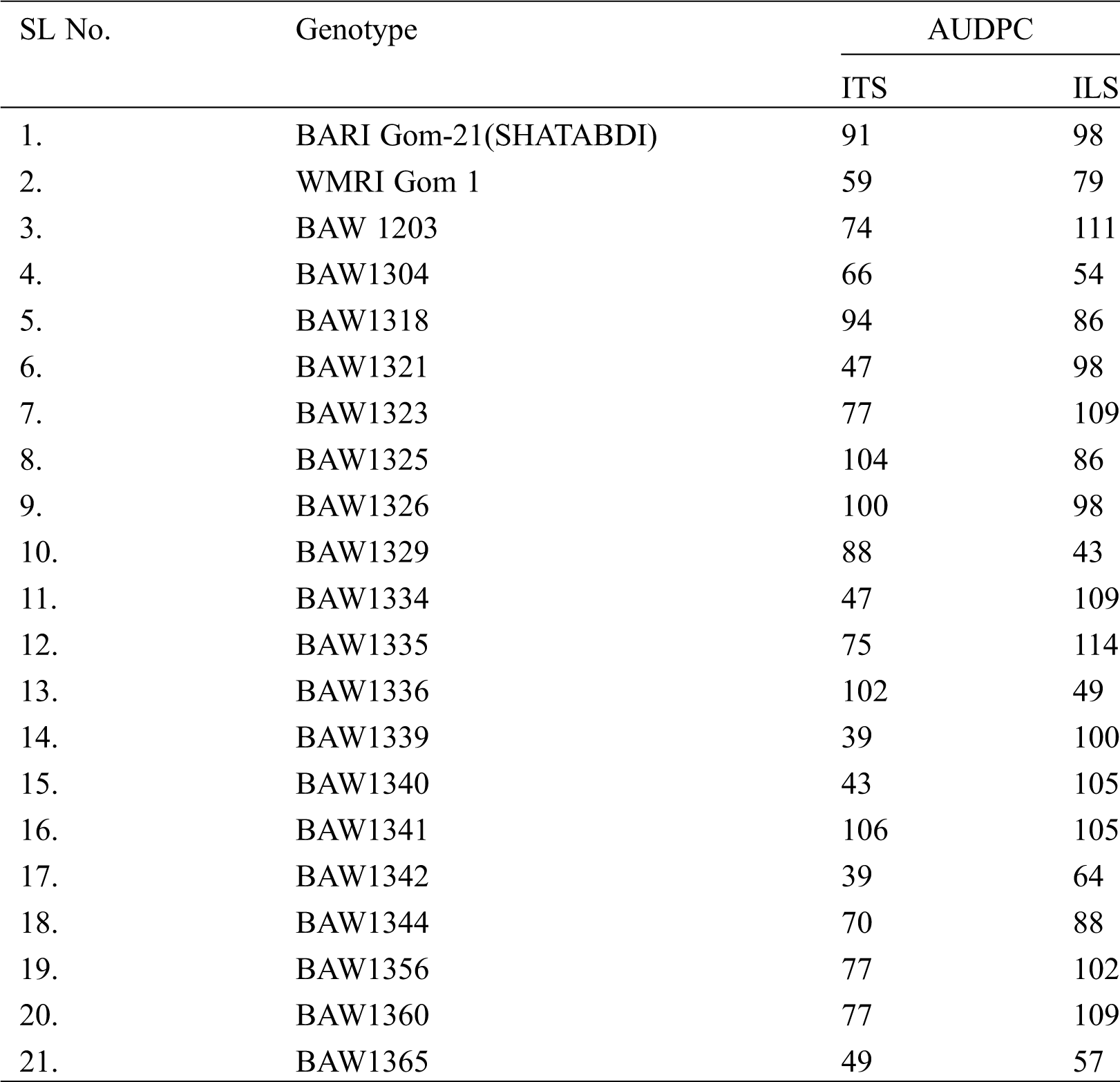
Out of 122 genotypes tested, 20 lines were selected as resistant based on AUDPC under both in ITS and ILS conditions. The AUDPC of the selected lines ranged from 39 to 106 in ITS and 43 to 114 in ILS condition (Tab. 2). The lowest AUDPC was found from the genotype of BAW 1339 and BAW 1342 int ITS and BAW 1329 in ILS condition.
Table 2: Performance of resistant wheat lines selected against Bipolaris leaf blight
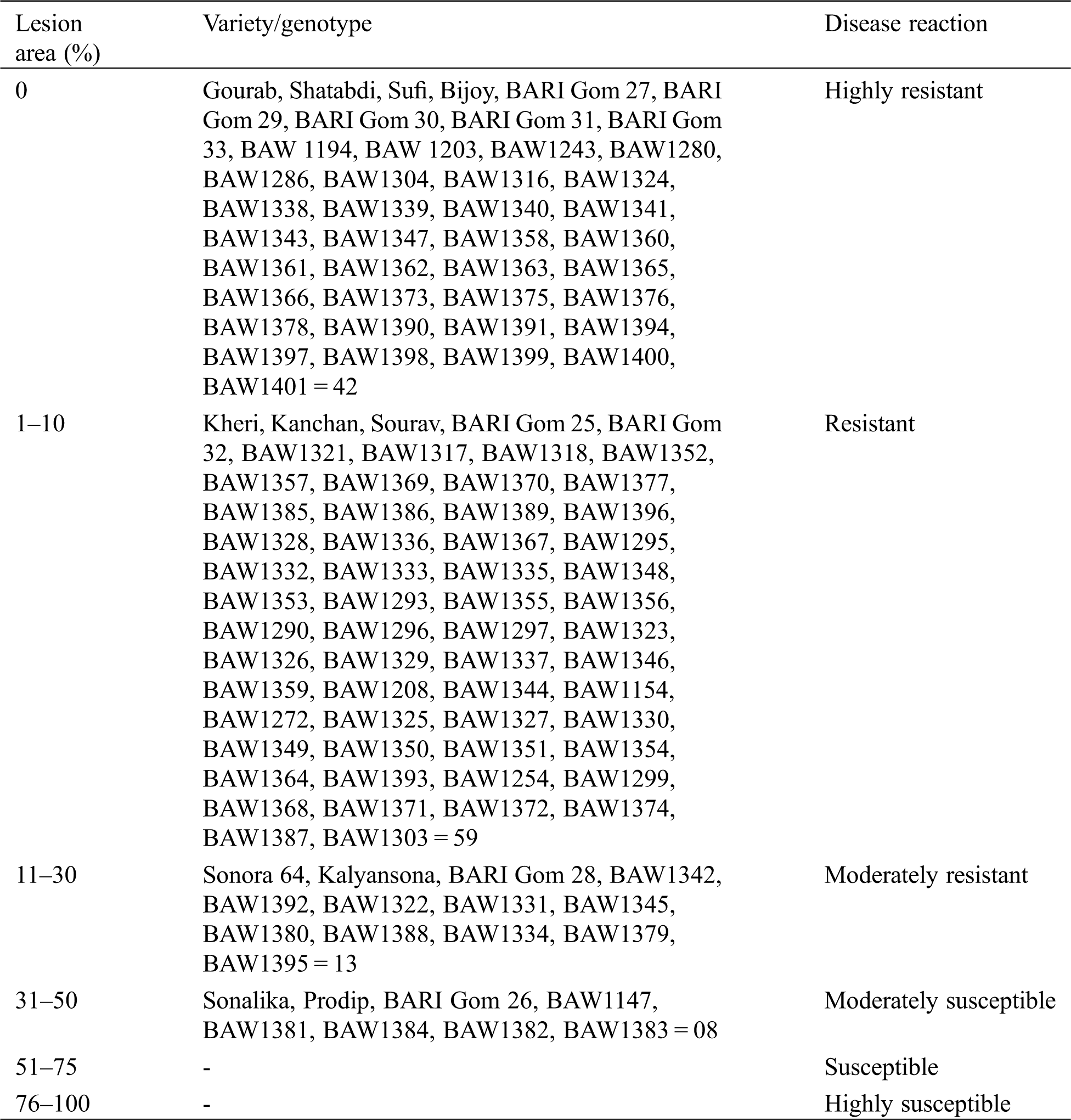
The severity of leaf rust varied among different advanced genotypes and varieties tested under field condition (Tab. 3). The varieties/advanced lines showed 0% to 40% severity with different types of disease reaction, while 80% severity with susceptible reaction was displayed in spreader rows. Based on rust severity, genotypes were categorized in highly resistant (0% severity), resistant (1%–10% severity), moderately resistant (11–30% severity), moderately susceptible (31%–50% severity), susceptible (51%–75% severity) and highly susceptible (76%–100% severity) groups. Out of 122 genotypes, 42 genotypes including 9 varieties were completely free from leaf rust infection, 59 genotypes showed resistance, 13 genotypes showed moderate resistance and 8 genotypes showed moderately susceptible reactions against leaf rust diseases.
Table 3: The response of wheat genotypes to leaf rust under field condition and their classification based on disease severity
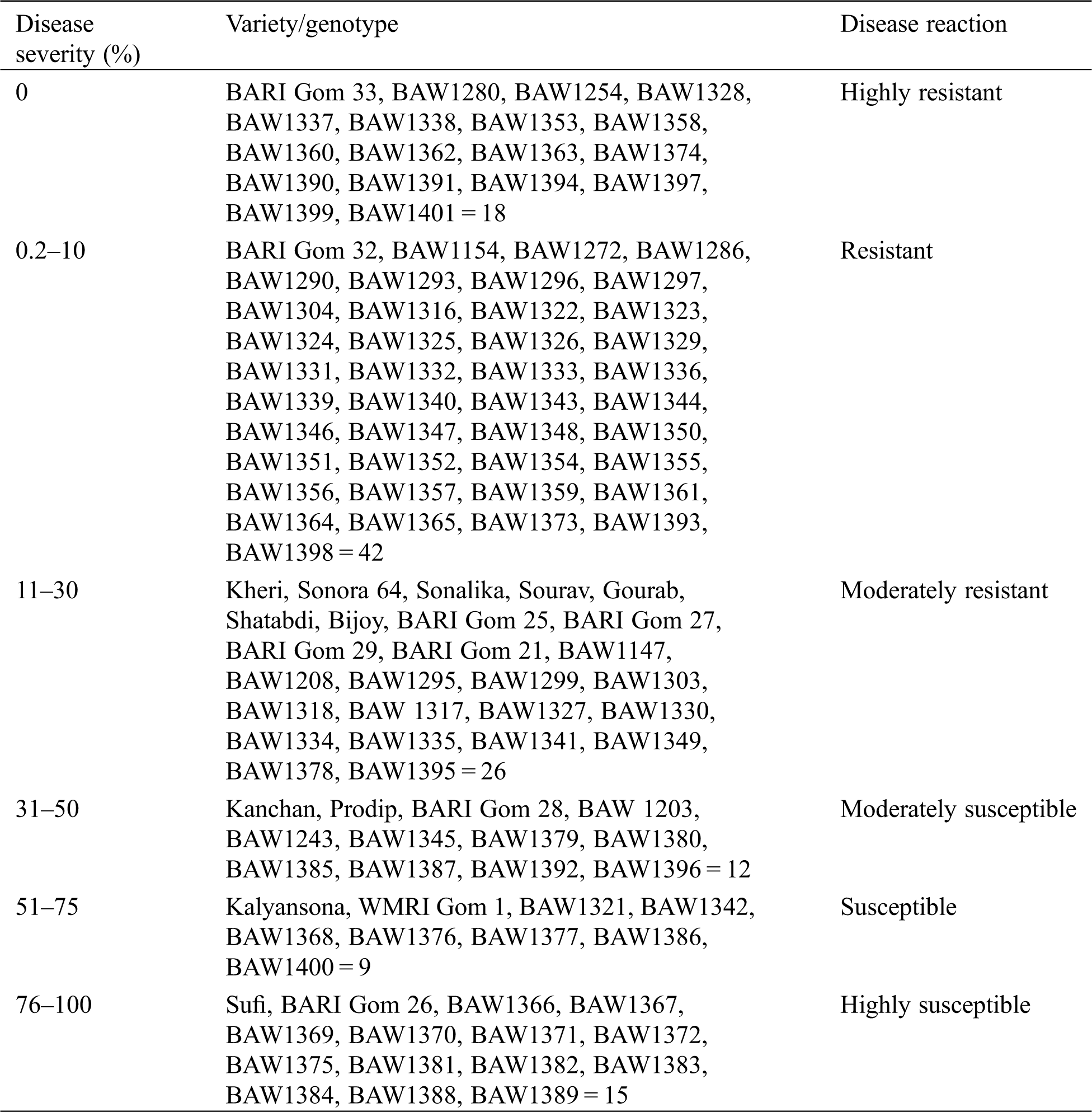
Out of 122 genotypes, 18 genotypes including BARI Gom 33 were immune against wheat blast under field condition, 42 genotypes including BARI Gom 30 and BARI Gom 32 were categorized as resistant (0.2%–10% disease index), 26 genotypes were categorized as moderately resistant (11%–30% disease index), 12 genotypes were identified as moderately susceptible (31%–50% disease index), 9 genotypes were classified as susceptible (51%–75% disease index), 15 genotypes were classified as highly susceptible (76%–100% disease index) to blast disease (Tab. 4).
Table 4: The response of wheat genotypes to blast under field condition and their classification based on disease severity
3.3.1 Molecular Screening of Wheat Genotypes for Blast Resistance Using 2NS Markers
Development and dissemination of resistant/tolerant wheat varieties would be the most effective way to control the fearsome wheat blast disease. It has been proved that 2NS translocation from Aegilops ventricosa expresses resistance to wheat blast in the most background. Therefore all of the selected genotypes were screened to identity wheat blast-resistant genotypes using the 2NS marker. Out of 122 genotypes, 16 genotypes including one variety (BARI Gom 33, BAW 1254, BAW 1280, BAW 1328, BAW 1338, BAW 1358, BAW 1360, BAW 1362, BAW 1363, BAW 1374, BAW 1390, BAW 1391, BAW 1394, BAW 1397, BAW 1399 and BAW 1401) showed positive 2NS segment (Figs. 2, 3). Importantly, genotypes with 2NS translocation showed immune disease reaction under field condition.
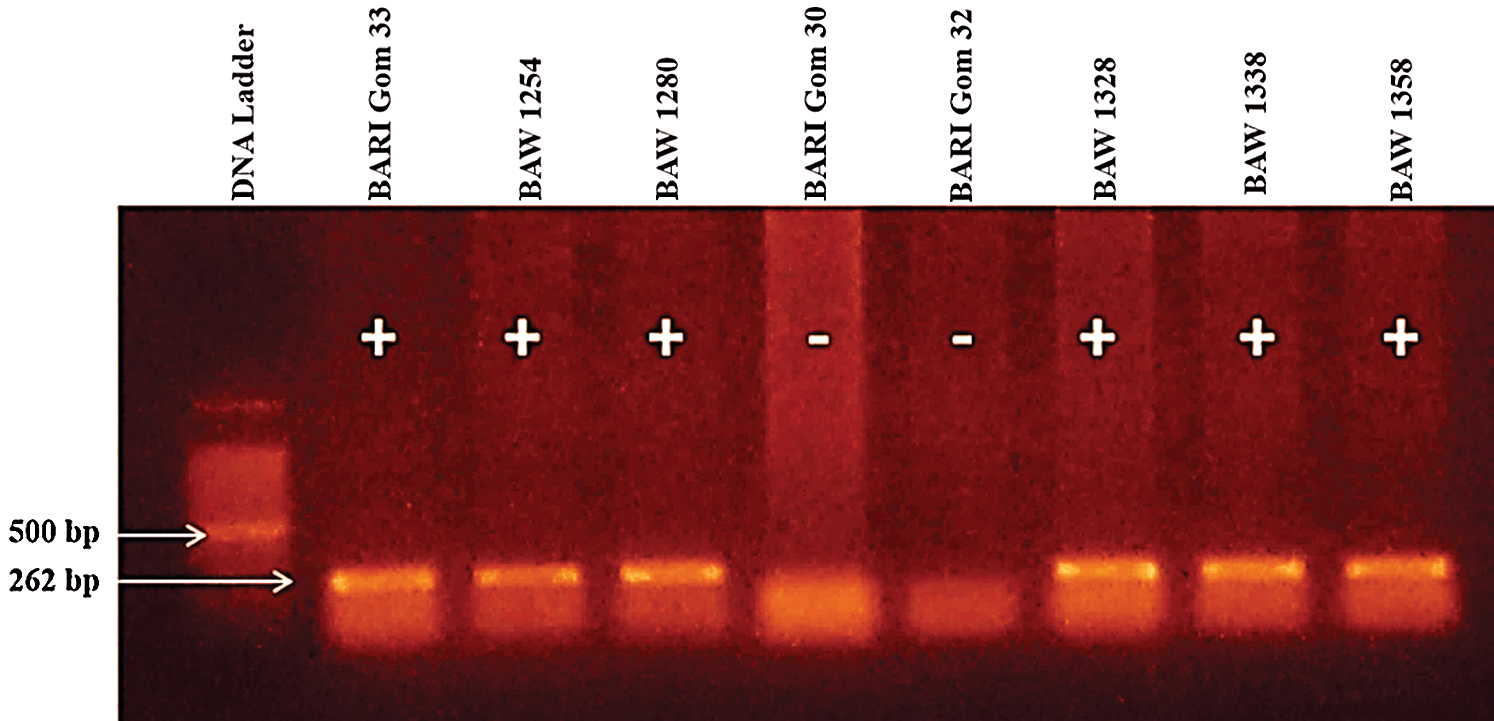
Figure 2: PCR amplification with 2NS specific primers VENTRUIP-F/LN2-R
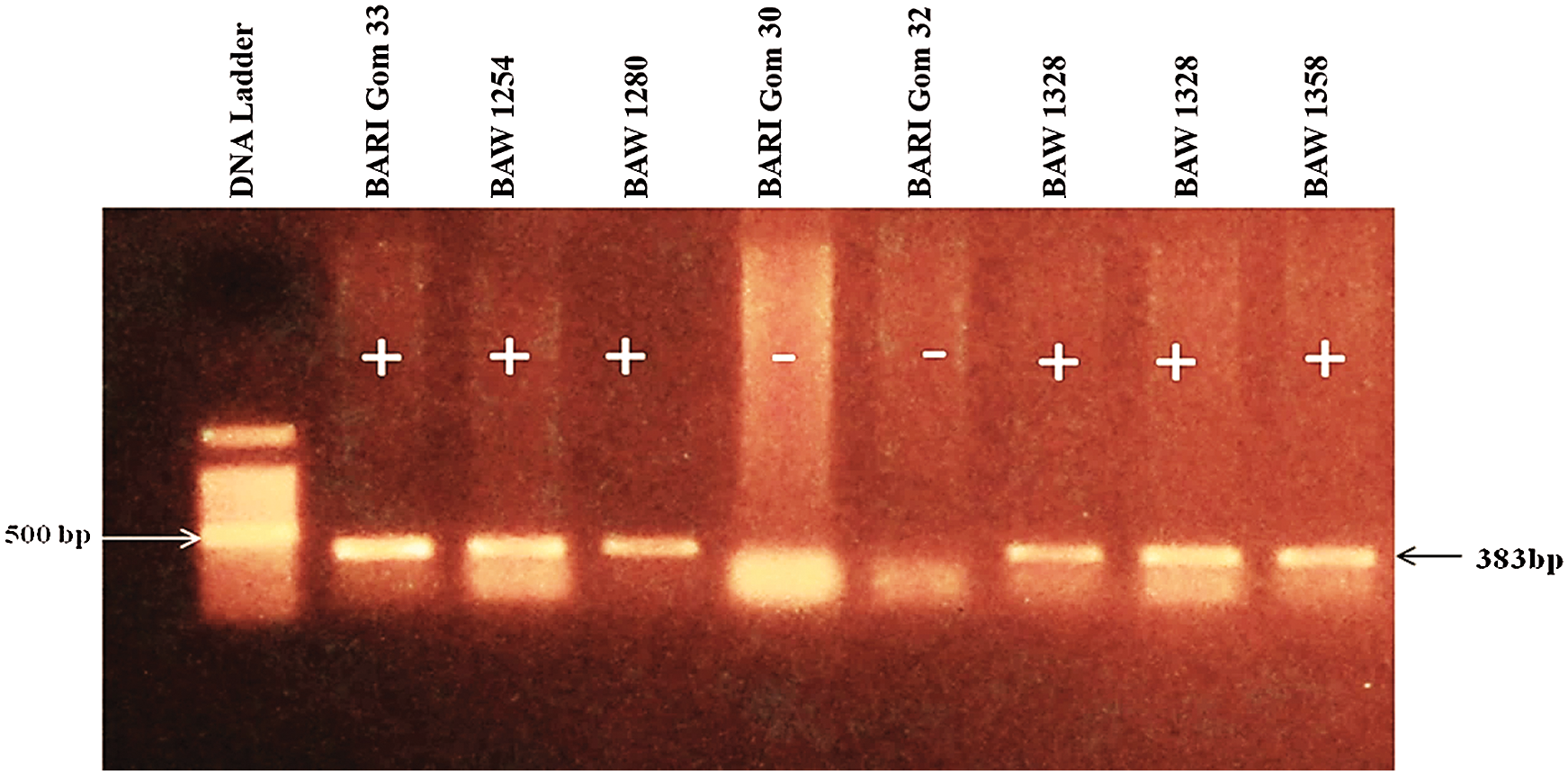
Figure 3: PCR amplification with 2NS specific primers Yr17-F/Yr17-R
The results revealed that significant variation was present among wheat varieties for grain yield-related parameters (Tab. 5).
Table 5: Mean square from the analysis of variance of 122 wheat genotypes evaluated for eight yield and yield attributing traits at BWMRI, Dinajpur, Bangladesh in 2018–2019
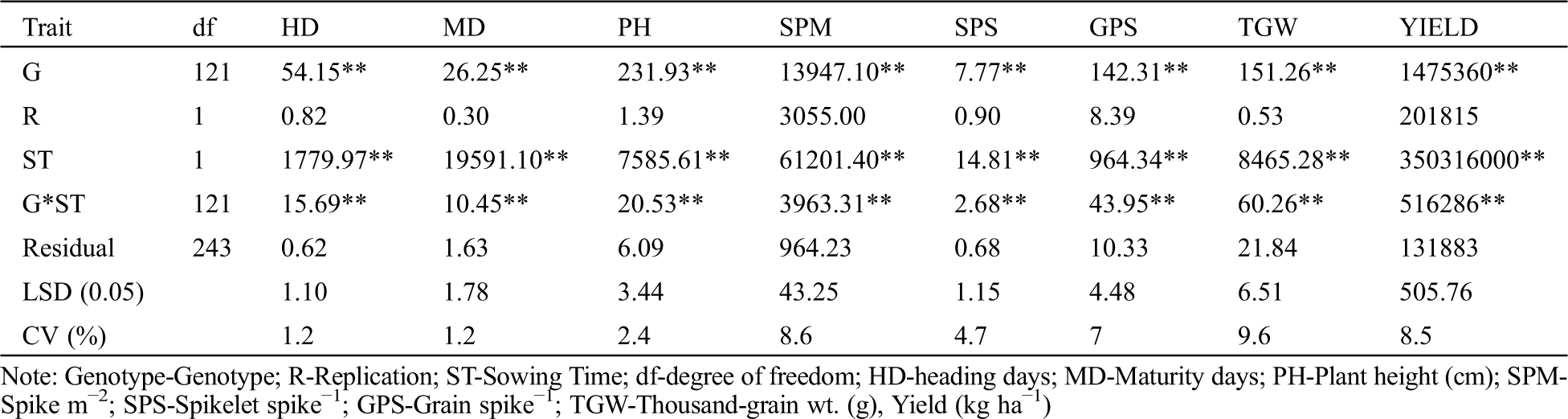
The results revealed significant differences among the wheat genotypes regarding plant height, heading days, maturity days and other yield attributes (Tab. 5). Ample variability among wheat genotypes for yield attributes offers an excellent opportunity to exploit their genetic potential to breed new cultivars having a higher potential for grain yield. The coefficient of variation was low for days to heading and days to maturity (1.2%), plant height (2.4%), and Spikelet spike-1 (4.7%). Thirteen genotypes had higher thousand-grain weight than the involved released 3 check varieties Fentalle, Amibara and Werer-2. Genotypes able to maintain high 1000-grain weight under high-temperature stress may possess a high level of heat tolerance.
The effect of seeding times revealed that all traits were significantly influenced by seeding time (Supplementary Tab. 2). Days to heading and days to maturity were earlier at late sowing condition. Plant height, number of spikes m−2, grain spike−1 was significantly higher at ITS condition. The TGW was higher at ITS condition compared to ILS conditions. The higher yield was achieved under ITS (5102 kg/ha) than ILS (3414 kg/ha) condition.
The minimum time to heading and maturity was found in WMRI Gom 1, BAW 1203 and BAW 1295. On the other hand, the highest days to heading and maturity were found in BAW 1386 and most of the genotypes headed and matured earlier than the long duration variety Shatabdi (Supplementary Table 3). The lowest plan height was found in BAW 1372 (72 cm) and BAW 1373 (72 cm). There are fifty genotypes sowed lower plant height (<100 cm). The cultivars with short stature are generally tolerant to lodging and result in a significant increase in crop yield. The highest spike m−2 was recorded in Khery (623) but the yield was low. The highest spikelet spike−1 was found in BAW 1358 and grain spike−1 in BAW 1396. Out of 122 genotypes, the highest 1000-grain weight was recorded in BAW 1293 followed by BAW 1380. The lowest 1000-grain weight was showed in Kalyansona. The highest yield was achieved in BAW 1377 (5668 kg/ha), followed by BAW 1397 (5412 kg/ha) and WMRI Gom 1 (5276 kg/ha).
A significant effect was found for seeding time, genotypes and their interactions on yield and yield contributing characters (Supplementary Tab. 4). The lowest heading days found in BAW 1322 (56 days), BARI Gom 32 (56 days) and BAW 1295 (56 days) both under ITS and ILS condition. The lowest maturity days was found in WMRI Gom 1 (96 days) and BAW 1203 (96 days) in ITS condition and BAW 1332 (93 days), BAW 1280 (93 days) in ILS condition. The short stature plant was observed in BAW 1372 (74 cm), BAW 1373 (77 cm), BAW 1297 (88 cm) and BAW 1364 (88 cm) both in ITS and ILS condition. The highest 1000-grain weight was found in BAW 1357 (66.1 g) followed by BAW 1293 (65 g) and BAW 1380 (65 g) in ITS condition but variety WMRI Gom 1 showed the highest 1000-grain weight (62.6 g) followed by BAW 1349 (56.8 g) and BAW 1350 (56.4 g) in ILS condition. The highest yield was achieved in WMRI Gom 1 (6261 kg ha−1) followed by BAW 1297 (6260 kg ha−1), BAW 1377 (6234 kg ha−1) under ITS condition. In ILS condition, the highest yield was found in BAW 1377 (5102 kg ha−1) followed by BAW 1366 (4948 kg ha−1). The genotype BAW 1322, BAW 1295, BAW 1203 can be used as earlier maturing genotypes for the further breeding program. The genotype BAW 1372, BAW 1373, BAW 1297 and BAW 1364 can be used for tolerance to lodging due to short plant height. The genotype BAW WMRI Gom 1, BAW 1349 and BAW 1350 can be used for bold grain. The genotype WMRI Gom 1, BAW 1297, BAW 1377 can be used as a high yielder for optimum seeding condition but genotype BAW 1377 and BAW 1366 for the late sown condition. The genotype BAW 1385, BAW 1366, BAW 1359 and BAW 1394 can be used as terminal heat tolerant.
Reduction of wheat yield due to different diseases including spot blotch, rust and blast pose a serious threat to sustainable wheat production worldwide. A couple of research experiments were conducted both under field and laboratory conditions following standard procedures to identify the best genotypes resistant to the above-mentioned diseases including high yield potential by assessing a large number of promising genotypes including varieties. Calculation of the area under the disease-progress curve (AUDPC) as a measure of quantitative disease resistance entails repeated disease assessments [32]. Trails related to diseases assessment require work and time to a large extent. There are some limitations (weather, space) on how frequently assessments can be made. The use of the calculated AUDPC has increased in recent years and can certainly be recommended when, because of either host phenology or growth, monotonically increasing disease progress is unlikely [32]. The field and molecular study tested 122 wheat genotypes for their tolerance against B. sorokiniana at the Bangladesh Wheat and Maize Research Institute (BWMRI), Dinajpur location on the field showed different reactions and 20 lines were selected based on AUDPC under both in ITS and ILS condition, indicating that the genetic variability/variations for the response to leaf blight among the entries. Earlier, it was concluded that wheat cultivars especially cv. Kanchan having higher potential against fungal diseases have been developed through the targeted breeding program and new commercial cultivars have performed better in terms of grain yield [33,34]. On similar lines, a study was executed to identify and screen out spot blotch resistant wheat genotypes from the pool of 1387 genotypes and subsequent performance evaluation was conformed through the field and molecular analyses [35]. Similar to our results, wheat genotypes were sorted out into low and moderate resistance potential against spot blotch disease [36]. It was also reported a set of recombinant inbred lines were screened for spot blotch disease under the natural condition at three hot spot locations in India and few resistant lines were isolated [37]. Recently, it was concluded that only 20 genotypes out of 100 were found to be highly resistant to the disease, whereas others were resistant (28 genotypes), moderately resistant (22 genotypes), moderately susceptible (15 genotypes) and susceptible (15 genotypes) [38].
In Bangladesh, leaf rust which is caused by Puccinia triticina has become one of the most pertinent diseases of wheat [39]. The disease becomes very serious if susceptible varieties are sown later than the optimum time and wheat crop experiences terminal heat stress which is congenial for rust development under Bangladesh condition. Genetic resistance is the most economic and effective means of reducing yield losses caused by leaf rust disease [40]. Breeding for disease resistance genotypes is a continuous process and plant breeders need to add new effective genes to their breeding materials. The present investigation deals with new sources of resistance that can be incorporated into wheat to escape yield losses wreaked by the leaf rust disease. In this study, out of 122 genotypes, 42 genotypes including 9 varieties showed completely free from leaf rust infection, 59 genotypes showed resistance, 13 genotypes showed moderate resistance and 8 genotypes showed moderately susceptible reactions. It was concluded that resistant wheat lines might contain any resistant set of Lr genes. These new sources of leaf rust resistance can be incorporated into wheat to avoid yield losses and crop failure [41–44].
Previously, wheat blast remained restricted to South America’s tropical regions [45], however recently it has spread to South Asian countries especially Bangladesh [46]. The current scenario is that over 15% of wheat area in Bangladesh is under serious threat of this disease. Recently, it is reported that disease-resistant genotypes at both stages (vegetative and reproductive) have been found promising to be used in wheat breeding programs [47,48].
The blast resistance genes come from Aegilops ventricosa (Zhuk.) Chennav on wheat [22,49]. In this experiment, conidia of M. oryzae pathotype triticum inoculated at the reproductive stage, 18 genotypes including BARI Gom 33 were immune against wheat blast under field condition, 42 genotypes including BARI Gom 30 and BARI Gom 32 were categorized as resistant, 26 genotypes were categorized as moderately resistant under field conditions. Among the identified 18 immune genotypes against wheat blast under field test, 16 genotypes including one variety (BARI Gom 33, BAW 1254, BAW 1280, BAW 1328, BAW 1338, BAW 1358, BAW 1360, BAW 1362, BAW 1363, BAW 1374, BAW 1390, BAW 1391, BAW 1394, BAW 1397, BAW 1399 and BAW 1401) showed positive 2NS segment of Aegilops ventricosa. This finding indicates a good correlation between the phenotypic and genotypic assessment of the genotypes against blast diseases. Similar research was executed and results revealed that few wheat varieties (BR 18-Terena) showed better performance as compared to others in terms of grain yield and disease tolerance owing to better genetic makeup [50]. Other studies have elaborated considerable differences among wheat genotypes regarding resistance to wheat blast [51–55]. Our findings are in agreement with previously reported results where wheat varieties having lower resistance to the wheat blast were identified and reported in Brazil to develop a genetic pool for developing more resistant varieties [56].
Out of 122 genotypes tested, 20 genotypes were found resistant to leaf blight, 42 were found completely free from leaf rust infection, while 59 were identified as resistant, and 13 were identified as moderately resistant to leaf rust both in irrigated timely and late sown conditions. Eighteen genotypes were immune against wheat blast (WB), 42 were characterized as resistant, and 26 were recognized as moderately resistant to WB. Molecular data revealed that the 16 genotypes showed a positive 2NS segment among the 18 immune genotypes selected against wheat blast under field conditions. Besides these, genotypes BAW 1322, BAW 1295, and BAW 1203 were found as earlier maturing genotypes and genotypes BAW 1372, BAW 1373, BAW 1297 and BAW 1364 were recorded as lodging tolerant due to short stature. Three genotypes namely WMRI Gom 1, BAW 1349 and BAW 1350 were categorized as bold grain. The genotypes WMRI Gom 1, BAW 1297, BAW 1377 were found suitable for optimum seeding condition and genotypes BAW 1377 and BAW 1366 were selected for late sown heat stress condition. The selected resistant genotypes against specific diseases and the other selected genotypes for yield and yield attributing traits can be used in a further breeding program to develop disease-resistant high yielding wheat varieties.
Acknowledgement: The authors sincerely acknowledge to Bangladesh Wheat and Maize Research Institute (BWMRI), Dinajpur 5200, Bangladesh, for providing the necessary laboratory facility during the investigation. The authors are also highly grateful to the Taif University Researchers Supporting Project (TURSP–2020/143), Taif, Saudi Arabia.
Funding Statement: The current reserach was funded by Bangladesh Wheat and Maize Research Institute (BWMRI), Dinajpur 5200, Bangladesh, and the Taif University Researchers Supporting Project (TURSP-2020/143), Taif, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ahmad, Z., Waraich, E. A., Barutçular, C., Hossain, A., Erman, M. (2020). Enhancing drought tolerance in wheat through improving morpho-physiological and antioxidants activities of plants by the supplementation of foliar silicon. Phyton-International Journal of Experimental Botany, 89, 529–539. DOI 10.32604/phyton.2020.09143. [Google Scholar] [CrossRef]
2. El Sabagh, A., Hossain, A., Barutcular, C., Islam, M. S., Awan, S. I. (2019). Wheat (Triticum aestivum L.) production under drought and heat stress-adverse effects, mechanisms and mitigation: A review. Applied Ecology and Environmental Research, 17, 8307–8332. DOI 10.15666/aeer. [Google Scholar] [CrossRef]
3. Siddiqui, M. H., Iqbal, M. A., Wajid, N., Imtiaz, H., Abdul, K. (2019). Bio-economic viability of rainfed wheat (Triticum aestivum L.) cultivars under integrated fertilization regimes in Pakistan. Custos e Agronegocio, 15, 81–96. http://www.custoseagronegocioonline.com.br/numero3v15/OK%205%20bioeconomic.pdf. [Google Scholar]
4. Iqbal, M. A., Imtiaz H., Muzammil H. S., Essa, A., Zahoor, A. (2018). Probing profitability of irrigated and rainfed bread wheat (Triticum aestivum L.) crops under foliage applied sorghum and moringa extracts in Pakistan. Custos e Agronegocio, 14, 2–16. http://www.custoseagronegocioonline.com.br/numero2v14/OK%201%20profitability.pdf. [Google Scholar]
5. Afzal, M. I., Iqbal, M. A., Cheema, Z. A. (2016) Triggering growth and boosting economic yield of late-sown wheat (Triticum aestivum L.) with foliar application of allelopathic water extracts. World Journal of Agricultural Science, 11, 94–100. DOI 10.5829/idosi.wjas.2015.11.2.12650. [Google Scholar] [CrossRef]
6. Hussain, M., Iqbal, M. A., Till, B. J., Rahman, M. U. (2018). Identification of induced mutations in hexaploid wheat genome using exome capture assay. PLoS One, 13(8), e0201918. DOI 10.1371/journal.pone.0201918. [Google Scholar] [CrossRef]
7. Barma, N. C. D., Hossain, A., Hakim, M. A., Mottaleb, K. A., Alam, M. A., et al. (2019). Progress and challenges of wheat production in the era of climate change: A Bangladesh perspective. Springer Nature Singapore, pp. 616–671. DOI 10.1007/978-981-13-6883-7. [Google Scholar] [CrossRef]
8. Ahmed, S. M., Meisner, C. (1996). Wheat research and development in Bangladesh. Bangladesh Australia wheat improvement project and CIMMYT-Bangladesh; pp. 74–79. Bangladesh Australia Wheat Improvement Project and CIMMYT-Bangladesh, https://libcatalog.cimmyt.org/Download/cim/66681.pdf. [Google Scholar]
9. Hossain, A., Teixeira da Silva, J. A. (2013). Wheat production in Bangladesh: its future in the light of global warming. AoB Plants, 5, pls042. DOI 10.1093/aobpla/pls042. [Google Scholar] [CrossRef]
10. Talukdar, M. J. (1974). Plant diseases in Bangladesh. Bangladesh Journal of Agricultural Research, 1, 61–86. [Google Scholar]
11. Ahmed, S. M. (1986). An overview of wheat research and development in Bangladesh. 3rd National Wheat Training Workshop. pp. 29–33. Wheat Research Center Bangladesh Agricultural Research Institute Dinajpur. [Google Scholar]
12. Saari, E. E., Prescott, J. M. (1985). World distribution in relation to economic losses. In: Roelfs, A. P., Bush-nell, W. R. (Eds.The cereal rusts. Diseases, distribution, epidemiology and control. pp. 259–298. Orlando: Academic Press. [Google Scholar]
13. Hossain, I., Azad, A. K. (1992). Reaction of wheat to Helminthosporium sativum in Bangladesh. Hereditas, 116, 203–205. DOI 10.1111/j.1601-5223.1992.tb00824.x. [Google Scholar] [CrossRef]
14. Hossain, I., Azad, A. K. (1994). Bipolaris sorokiniana: Its reaction and effect on grain yield of wheat. Progressive Agriculture, 5, 63–65. [Google Scholar]
15. Alam, K. B., Shaheed, M. A., Ahmed, A. U., Malaker, P. K. (1994). Bipolaris leaf blight (spot blotch) of wheat in Bangladesh. In: Saunders D. A., Hettel, G. P. (Eds.Wheat in Heat-Stressed Environments: Irrigated, Dry Areas and Rice-Wheat Farming Systems. pp. 23–29. Mexico. [Google Scholar]
16. Singh, V., Singh, R. N. (2006). Effect of mineral nutrition and environmental variables on the intensity of wheat spot blotch under rice-wheat system. Indian Phytopathology, 59(4), 417–426. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.888.7058&rep=rep1&type=pdf. [Google Scholar]
17. Sharma, R. C., Duveiller, E. (2003). Effect of stress on Helminthosporium leaf blight in wheat. In: Rasmussen, J. B., Friesen, T. L., Ali, S. (Eds.Proceedings of 4th International Wheat Tan Spot and Spot Blotch Workshop. pp. 140–144. North Dakota State University, Fargo, USA, [Google Scholar]
18. Singh, G., Sheoran, S., Chowdhury, A. K., Tyagi, B. S., Bhattacharya, P. M. et al. (2014). Phenotypic and marker aided identification of donors for spot blotch resistance in wheat. Journal of Wheat Reserach, 6, 98–100. https://sawbar.in/wp-content/uploads/2018/07/41956-97942-1-SM.pdf. [Google Scholar]
19. Duveiller, E., He, X., Singh, P. K. (2016). Wheat Blast: An Emerging Disease in South America Potentially Threatening Wheat Production. In: Bonjean, A., van Ginkel, M. (Eds.World wheat book, a history of wheat. pp. 1107–1122. France: Lavoisier, Paris. [Google Scholar]
20. Malaker, P. K., Barma, N. C. D., Tiwari, T. P., Collis, W. J., Duveiller, E. et al. (2016). First report of wheat blast caused by Magnaporthe oryzae pathotype triticum in Bangladesh. Plant Disease, 100, 2330. DOI 10.1094/PDIS-05-16-0666-PDN. [Google Scholar] [CrossRef]
21. Islam, M. T., Gupta, D. R., Hossain, A., Roy, K. K., He, X. et al. (2020). Wheat blast: A new threat to food security. Phytopathology Research, 2(1), 1–13. DOI 10.1186/s42483-020-00067-6. [Google Scholar] [CrossRef]
22. Kohli, M. M., Mehta, Y. R., Guzman, E., Viedma, L., Cubilla, L. E. (2011). Pyricularia blast-a threat to wheat cultivation. Czech Journal of Genetic and Plant Breeding, 47, 130–134. DOI 10.17221/CJGPB. [Google Scholar] [CrossRef]
23. Williamson, V. M., Thomas, V., Ferris, H., Dubcovsky, J. (2013). An Aegilops ventricosa translocation confers resistance against root-knot nematodes to common wheat. Crop Science, 53(4), 1412–1418. DOI 10.2135/cropsci2012.12.0681. [Google Scholar] [CrossRef]
24. Cruz, C. D., Peterson, G. L., Bockus, W. W., Kankanala, P., Dubcovsky, J. et al. (2016). The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae. Crop Science, 56(3), 990–1000. DOI 10.2135/cropsci2015.07.0410. [Google Scholar] [CrossRef]
25. Hossain, A., Mottaleb, K. A., Farhad, M., Barma, N. C. D. (2019). Mitigating the twin problems of malnutrition and wheat blast by one wheat variety, ‘BARI Gom 33’, in Bangladesh. Acta Agrobotanica, 72(2), 1775. DOI 10.5586/aa.1775. [Google Scholar] [CrossRef]
26. Sousa, P. G. (2002). BR 18-Terena: Cultivar de trigo para o Brasil. Pesquisa Agropecuária Brasileira, 37, 1039–1043. DOI 10.1590/S0100-204X2002000700019. [Google Scholar] [CrossRef]
27. Saari, E. E., Prescott, J. M. (1975). A scale for appraising the foliar intensity of wheat diseases. Plant Disease Reporter, 59, 377–380. https://eurekamag.com/research/000/008/000008657.php. Accessed 15 September 2020. [Google Scholar]
28. Zadoks, J. C., Chang, T. T., Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Research, 14, 415–421. DOI 10.1111/j.1365-3180.1974.tb01084.x. [Google Scholar] [CrossRef]
29. Stubbs, R. W., Prescott, J. M., Saari, E. E., Dubin, H. J. (1986). Cereal disease methodology manual. pp. 123–129. CIMMYT, Mexico. [Google Scholar]
30. R-Core Team (2013). R-language and environment for statistical computing. R Foundation for Statistical Computing, pp. 83–99. Vienna, Austria. [Google Scholar]
31. Steel, R. G. D., Torrie, J. H. (1984). Principles and procedures of statistics, pp. 172–177. Singapore: Mc Graw Hill Book C., Inc. [Google Scholar]
32. Jeger, M. J., Viljanen-Rollinson, S. L. H. (2001). The use of the area under the disease-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theoretical and Applied Genetic, 102, 32–40. DOI 10.1007/s001220051615. [Google Scholar] [CrossRef]
33. Shefazadeh, M. K., Mohammadi, M., Karimizadeh, R., Mohammadinia, G. (2012) Tolerance study on bread wheat genotypes under heat stress. Annals of Biological Research, 3, 4786–4789. https://www.researchgate.net/publication/259391765_Tolerance_study_on_bread_wheat_genotypes_under_heat_stress. [Google Scholar]
34. Siddique, A. B., Hossain, M. H., Duveiller, E., Sharma, R. C. (2006). Progress in wheat resistance to spot blotch in Bangladesh. Journal of Phytopathology, 154, 16–22. DOI 10.1111/j.1439-0434.2005.01049.x. [Google Scholar] [CrossRef]
35. Chaurasia, S., Joshi, A. K., Dhari, R., Chand, R. (1999). Resistance to foliar blight of wheat: A search. Genetic Resources and Crop Evolution, 46(5), 469–475. DOI 10.1023/A:1008797232108. [Google Scholar] [CrossRef]
36. Dubin, H. J. (1998). Results of the south Asia regional Helminthosporium leaf blight and yield experiment, 1993–94. In: Duveillar, E., Dubin, H. J., Reeves, J., McNab, A. (Eds.Helminthosporium blights of wheat: Spot blotch and tan spot, pp. 182–187. CIMMYTMexico. [Google Scholar]
37. Singh, R. P., Huerta-Espino, J., Roelfs, A. P. (2002). The wheat rusts. In: Curtis, B. C., Rajaram, S., MacPher-son, H. G. (Eds.Bread wheat: Improvement and pro-duction, plant production and protection. Series No. 30, pp. 227–249. Rome: FAO. [Google Scholar]
38. Ojha, A., Singh, G., Tyagi, B. S., Rajita, V. S., Kumar, P. (2016) Screening of resistance source against spot blotch disease caused by Bipolaris sorokiniana in Triticum aestivum L. International Journal of Advanced Research, 5, 23–28. DOI 10.21474/IJAR01. [Google Scholar] [CrossRef]
39. Malaker, P. K., Reza, M. M. A. (2011). Resistance to rusts in Bangladeshi wheat (Triticum aestivum L.). Czech Journal of Genetic and Plant Breeding, 47, 155–159. DOI 10.17221/CJGPB. [Google Scholar] [CrossRef]
40. Liu, J. Q., Kolmer, J. A. (1997). Genetics of leaf rust resistance in Canadian spring wheat AC Domain and AC Taber. Plant Disease, 81, 757–760. DOI 10.1094/PDIS.1997.81.7.757. [Google Scholar] [CrossRef]
41. Stepień, L., Chen, Y., Chełkowski, J., Kowalczyk, K. (2001). Powdery mildew resistance genes in wheat: verification of STS markers. Journal of Applied Genetics, 42(4), 413–423. https://pubmed.ncbi.nlm.nih.gov/14564018/. [Google Scholar]
42. Jerzy Chelkowski, J., Stepien, L. (2001). Molecular markers for leaf rust resistance genes in wheat. Journal of Applied Genetic, 42(2), 117–126. https://pubmed.ncbi.nlm.nih.gov/14564046/. [Google Scholar]
43. Helguera, M., Khan, I. A., Dubcovsky, J. (2000). Development of PCR markers for the wheat leaf rust resistance gene Lr47. Theoretical and Applied Genetics, 100(7), 1137–43. DOI 10.1007/s001220051397. [Google Scholar] [CrossRef]
44. Vanzetti, L. S., Campos, P., Demichelis, M., Lombardo, L. A., Aurelia, P. R. et al. (2011). Identification of leaf rust resistance genes in selected Argentinean bread wheat cultivars by gene postulation and molecular markers. Electronic Journal of Biotechnology, 14(3), 9. DOI 10.2225/vol14-issue3-fulltext-14. [Google Scholar] [CrossRef]
45. Hussain, W., Inamullah Ahmad, H., Iqbal, M. S., Abbassi, F. M. et al. (2011). Identification of leaf rust resistant gene Lr10 in Pakistani wheat germplasm. African Journal of Biotechnology, 10, 8578–8584. https://www.ajol.info/index.php/ajb/article/view/95451. [Google Scholar]
46. Kolmer, J. A., Long, D. L., Hughes, M. E. (2007). Physiological specialization of Puccinia triticina on wheat in the United States in 2005. Plant Disease, 91, 979–984. DOI 10.1094/PDIS-91-8-0979. [Google Scholar] [CrossRef]
47. Stepien, L., Golka, L., Chelkowski, J. (2003) Leaf rust resistance genes of wheat: identification in cultivars and resistance sources. Journal of Applied Genetic, 44, 139–149. https://pubmed.ncbi.nlm.nih.gov/12773791/. [Google Scholar]
48. Muhammad, S., Khan, A. I., Aziz, R., Awan, F. S., Rehman, A. (2015). Screening for leaf rust resistance and association of leaf rust with epediomological factors in wheat (Triticum aestivum L.). Pakistan Journal Agricultural Science, 52, 691–700. [Google Scholar]
49. Islam, M. T., Croll, D., Gladieux, P. (2016). Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biology, 14, 84–91. DOI 10.1186/s12915-016-0309-7. [Google Scholar] [CrossRef]
50. de Paula, I. G., Gloria, H. B., Pimentel, A. J. B., Ribeiro, G. and de Souza, M. A. (2019). Screening wheat genotypes for resistance to wheat blast disease in the vegetative and reproductive stages. Euphytica, 215(3), 59–69. DOI 10.1007/s10681-019-2382-9. [Google Scholar] [CrossRef]
51. Urashima, A. S., Lavorent, N. A., Goulart, A. C. P., Mehta, Y. R. (2004). Resistance spectra of wheat cultivars and virulence diversity of Magnaporthe grisea isolates in Brazil. Fitopatol Brasileira, 29, 511–518. DOI 10.1590/S0100-41582004000500007. [Google Scholar] [CrossRef]
52. Pagani, A. P. S., Dianese, A. C., Café-Filho, A. C. (2014). Management of wheat blast with synthetic fungicides, partial resistance and silicate and phosphite minerals. Phytoparasitica, 42, 609–617. DOI 10.1007/s12600-014-0401-x. [Google Scholar] [CrossRef]
53. Goulart, A. C. P., Sousa, P. G., Urashima, A. S. (2007). Danos em trigo causados pela infecção de Pyricularia grisea (In Spanish). Summa Phytopathol, 33, 358–363. DOI 10.1590/S0100-54052007000400007. [Google Scholar] [CrossRef]
54. Alam, M. A., Xue, F., Ali, M., Wang, C., Ji, W. (2013). Identification and molecular mapping of powdery mildew resistance gene PmG25 in common wheat originated from wild emmer (Triticum turgidum var. dicoccoides). Pakistan Journal of Botany, 45, 203–208. https://www.pakbs.org/pjbot/PDFs/45(1)/28.pdf. [Google Scholar]
55. Cruz, C. D., Peterson, G. L., Bockus, W. W., Kankanala, P., Dubcovsky, J. et al. (2016). The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae. Crop Science, 56, 990–1000. DOI 10.2135/cropsci2015.07.0410. [Google Scholar] [CrossRef]
56. Maciel, J. L. N., Danelli, A. L. D., Boaretto, C., Forcelini, C. A. (2013). Diagrammatic scale for the assessment of blast on wheat spikes. Summa Phytopathol, 39, 162–166. DOI 10.1590/S0100-54052013000300003. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |