
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014951
ARTICLE
Integrated Fertilization Regimes Boost Heavy Metals Accumulation and Biomass of Sedum alfredii Hance
1State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Lin’an, 311300, China
2Key Laboratory of Soil Contamination Bioremediation of Zhejiang Province, School of Environmental Sciences and Resources, Zhejiang A&F University, Lin’an, 311300, China
3Plantation Bureau of Ningguo County, Ningguo, 242300, China
*Corresponding Authors: Zhengqian Ye. Email: yezhq@zafu.edu.cn; Weijun Fu. Email: fuweijun@zafu.edu.cn
Received: 10 November 2020; Accepted: 22 January 2021
Abstract: The hyperaccumulator Sedum alfredii Hance (S. alfredii) may be employed for zinc (Zn) and cadmium (Cd)-polluted soil remediation. However, the low phytoremediation efficiency, related to the low biomass production, limits its use with that purpose. In this experiment, nitrogen (N), phosphorus (P), and potassium (K) fertilizers, and organic manure were applied to investigate the phytoremediation ability of S. alfredii. Hydroponic and pot experiments were conducted using Zn-Cd polluted soil. The hydroponic experiment indicated that appropriate fertilizer application could increase (p < 0.05) the amount of accumulated Zn and Cd in S. alfredii. When N supply ranged from 0.5 to 2.5 mmol L−1, it could improve growth and accumulation of Zn and Cd in whole plants of S. alfredii. The 1 mmol L-1 N was an optimal N dosage for shoot biomass production and Cd accumulation in shoots, while the 2.5 mmol L-1 was an optimal N dosage for Zn accumulation in shoots. Both low (<0.05 mmol L-1) and high (>0.8 mmol L-1) P supply decreased growth, and Zn/Cd accumulation in whole plants of the studied species. The 0.1 mmol L-1 P was an optimal dosage for S. alfredii biomass production and Zn/Cd accumulation in shoots. The supply levels within the range from 0.3 to 1 mmol L-1 K could significantly improve the biomass production of S. alfredii and its capability to accumulate Zn and Cd in the biomass. The 0.5 mmol L-1 K was an optimal dosage for the whole biomass production and Zn accumulation in shoots, while the 1 mmol L-1 was an optimal K dosage for Zn accumulation in shoots, which was 17.2% higher than the control. Moreover, the soil pot experiment showed that the combination of organic (fermented manure) and inorganic fertilizers made significant effects on the Zn and Cd-polluted soil remediation by S. alfredii. These effects varied, however, with the application of different proportions of N, P, K and organic matter. The Zn accumulation by S. alfredii reached the highest efficiency ability under the highest fertilizer mixing rate (N: 50 mg kg-1, P: 40 mg kg-1, K: 100 mg kg-1, organic matter: 1%). Even more, S. alfredii showed the strongest ability to accumulate Cd with a lower fertilizer mixing rate (N: 25mg kg-1, P: 20mg kg-1, K: 50 mg kg-1, organic matter: 0.5%).
Keywords: Sedum alfredii Hance; zinc; cadmium; phytoremediation; fertilizer application
Potentially hazardous metals (PHMs) contamination in soils continue to attract researchers concern, as they impose detrimental effects on environment, and further threaten sustainable use of agro-environmental resources, which affects human being’s health [1]. The PHMs in the soils are hard to remove because of its non-biodegradation [2]. Traditional physico-chemical methods for PHMs-contaminated soils’ remediation include the replacement of the polluted soils, combination with clean soils and washing with soil chelating agents [3]. However, such measures could destroy soil structure, reduce soil stability and lead to new PHMs into the soils. Hyperaccumulators can effectively absorb soil heavy metals and accumulate them in roots and shoots. Therefore, phytoremediation regarded as an economical and environment friendly option is being constantly applied to reduce PHMs in agricultural soils [4–7].
Sedum alfredii Hance characterizing its high Zn/Cd uptake ability, is a perennial hyperaccumulator [8]. This species can use secreted rhizosphere protons and special organic matter to acidify rhizosphere environments, chelate and dissolve heavy metals in soil, and increase accumulation [9]. Previous research has proved that S. alfredii possesses many advantages such as perennial growth and a strong enrichment ability for soil PHMs remediation [10]. However, the low biomass production and low phytoremediation efficiency limit use of most hyperaccumulators [11,12]. Up to date, a study reported that the application of 1 mmol L−1 N in the form of Ca(NO3)2 significantly increased the shoot of dry weight yield of Sedum plumbizincicola seedlings grown in Cd contaminated soils [13]. Similarly, Zn accumulation significantly raised under the 2.5 mmol L−1 N concentration in Pentas lanceolata [14].
As one of the most traditional agronomic management measures, fertilization is considered vital to improve soil nutrient status and promote crops growth, development and yield [15]. Soil nutrients can affect their bioavailability by influencing the translocation, adsorption and the forms of PHMs in soils, which will further affect the absorption and accumulation of PHMs by plants [16]. Organic fertilizers (e.g., composted pig manure) can increase the content of soil nutrients, promote plant growth and also play a vital role in the PHMs bioavailability in soils [17]. However, previous researches related to nutrient regulation by hyperaccumulators mainly focus on the selection of conventional fertilizers and individual element application rates [18,19]. Liu et al. showed that the application of chicken manure significantly increased Cd uptake in the shoots of Carpobrotus rossii compared to the control [20]. A similar study showed that the application of pig manure compost (15 g kg-1) significantly increased shoot Cd accumulation in Sedum alfredii [21]. Few studies were carried out to study the PHMs uptake efficiency ability under the combination of different levels of organic manure and chemical fertilizers.
In this study, hydroponic and pot experiments were carried out containing different application rates of N, P and K fertilizers with organic manure. The aims of this research were (1) to reveal the biomass production of S. alfredii, and its absorption and accumulation of Zn and Cd, (2) to explore an optimum fertilization practice for improving the absorption efficiency of Zn and Cd, and (3) to improve the effective use of S. alfredii on heavy metal-contaminated soils. Specifically, the following hypotheses were tested: (1) Increasing the supply of N, P and K improve the biomass production of S. alfredii and its capability to accumulate Zn and Cd in its tissues; (2) The combination of organic (fermented manure) and inorganic fertilizers make significant effect on Zn and Cd-polluted soil remediation by S. alfredii.
The seedlings of S. alfredii were collected from the trial field of the Institute of Soil Science, Chinese Academy of Sciences in Fuyang (30°05′N, 119°95′E), Zhejiang Province. They were kept under greenhouse conditions before the experiment. After pre-culture, homogeneous S. alfredii individuals with a length of ~5 cm, and uniform size, leaves, and buds were chosen as testing materials for this study.
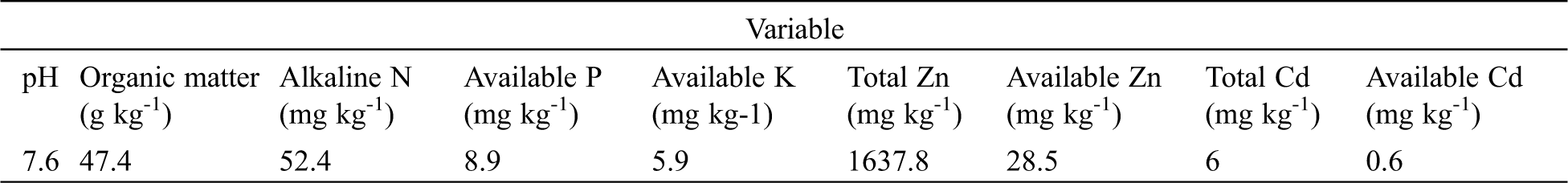
In this study, contaminated soil was collected from polluted agriculture lands close to the mining factory in Fuyang County, Zhejiang Province, Southeastern China. Soil samples were collected from the topsoil (0–20 cm) within an area was 20 m × 20 m. The double diagonal method was used for soil sampling at 5 points. Then soil samples were stored in sealed plastic bags [22]. The farmland was infertile due to the contamination from the nearby smelting plant. All soil samples were ground and sieved through a 5-mm mesh. Physico-chemical characteristics of the tested soils are shown in Tab. 1.
Table 1: Available nutrients, and total Zn and Cd values on the collected soils. Each value is the mean of n = 5
The components of the complete nitrogenous nutrient solution used in this study were the same as those described in Deng et al. study [23]. The treatments consisted of five concentrations of N: 0, 0.5, 1, 2.5, 5, 10 mol L-1. The nitrogenous element was added in the form of NH4NO3. Each treatment had 4 replicates.
The components of the complete phosphorus nutrient solution followed those as described by Chen and Lin’s work [24]. The treatments included eight concentrations of P: 0, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, and 3.2 mol L-1. P was added in the form of NaH2PO4. Each treatment had 4 replicates.
The components of the complete potassic nutrient solution were as in the Kashem and Kawai’s study [25]. The treatments consisted of five concentrations of K: 0, 0.3, 0.5, 1, and 2 mol L-1. Potassium was added in the form of KCl. Each treatment had 4 replicates.
The hydroponic experiment was performed in a greenhouse. After 7 days of pre-culture in a nutrient solution, plants were treated with different N, P and K application levels. Part of the nutrient solution was collected daily to measure the pH values, which were adjusted to pH 5.8 with 0.1 mol L-1 NaOH/0.1 mol L-1 HCl. It was continuously aireated during any 24 h period. Each solution was refreshed every 5 days. The plant growth conditions and disease symptoms were recorded over the whole growing period. The study lasted 40 days. Both before and after the experiments, whole plant samples were obtained. They were repeatedly washed using deionized water. Thereafter, samples were divided into aboveground parts and roots, and rinsed using tap water repeatedly. Roots were treatedwith Na-EDTA (20 mmol L-1) for 15 min to remove Zn2+ and Cd2+ from the root surface. The dry weight of plant parts was measured. They were ground to pass through a 0.25 mm sieve.
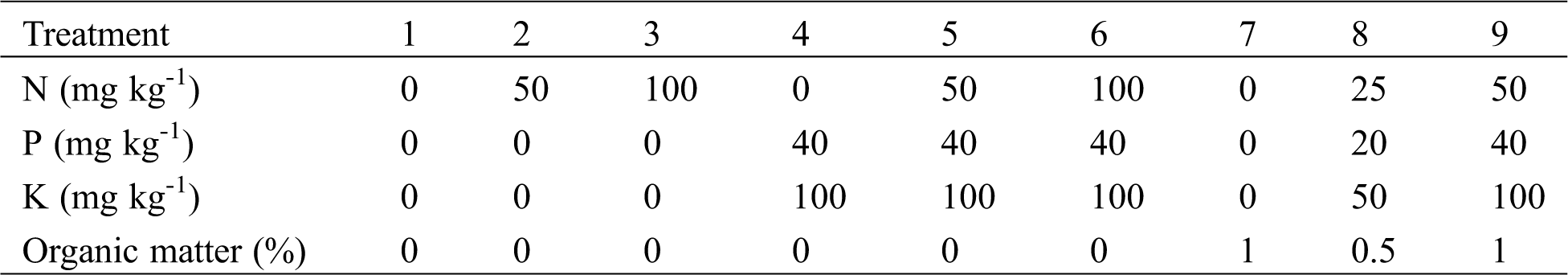
Different treatments regarding N, P, K and organic matter levels are described in Tab. 2. Each treatment had 4 replicates. Nitrogen was added as NH4NO3, while P and K were applied in the form of K2HPO4. The nutrient concentration of the organic fertilizer (i.e., fermented pig manure) was 1.36, 2.31 and 0.84 g kg-1 for N, P, and K, respectively. The concentrations of total Zn and Cd in soils were 1500 mg kg-1 and 19 mg kg-1, respectively. The base fertilizer was applied mixed in plastic pots. Each pot had 3 uniform-size plants. After 5 months, the shoot and root plant parts were collected. The weight of plant samples was measured, and then they were treated as mentioned above.
Table 2: Treatments of the soil pot experiment
2.4 Sample Determination Methods
Plant samples were treated with a mixture of HNO3 and HClO4. The total Zn and Cd concentrations of plants were determined by an ICP-OES.
One-way ANOVAs were applied to determine the effects of various nutrient concentrations on dry weight yield, and Zn and Cd concentrations and contents of S. alfredii. The Duncan’s multiple comparison test was used when F tests were significant at p < 0.05 [26]. Data were analysed using SPSS 20.0 software.
3.1 Biomass Production, and Zinc and Cadmium Absorption by S. alfredii at Different N Doses
The results showed that the dry weights of the aboveground parts (shoots) varied from 0.020 g to 0.301 g, and the dry weights of the roots were in the range of 0.005 g to 0.0301 g (Fig. 1). The highest shoot dry weight was observed under 1 mmol L-1 N, while treatment of 2.5 mmol L-1 N showed the highest root biomass. In addition, total biomass (shoot+root) of S. alfredii plants was significantly reduced by N application of 10 mmol L-1.
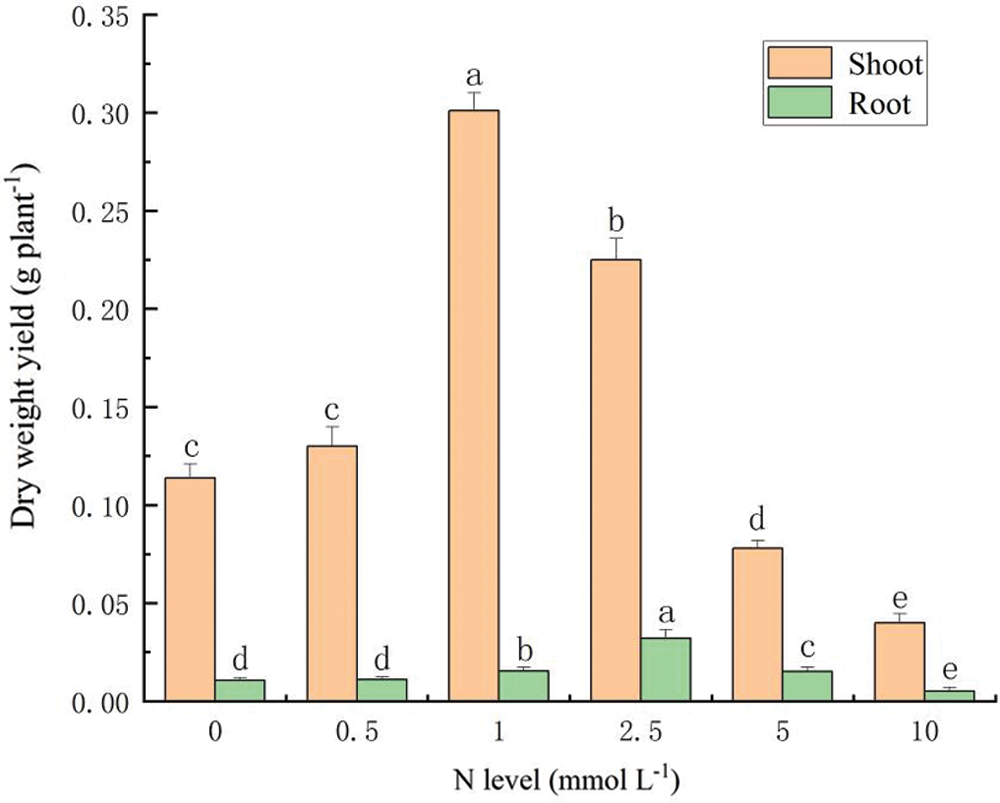
Figure 1: Biomass production of S. alfredii at different N application rates. Data are average + standard deviation of n = 4. Different letters in the same color-histogram indicate significant differences at ɑ = 0.05
At 2.5 and 5 mmol L-1 N, Cd concentrations in shoots of S. alfredii reached the highest value of 2680 mg kg-1, while Zn concentrations in the root had the highest value of 14876 mg kg-1 at 10 mmol L-1 N (Figs. 2A and 2B). With increasing N supply, Zn accumulation in the different plant parts of S. alfredii and Cd accumulation in the shoot increased first, and then started to decrease (Figs. 2C and 2D). The highest accumulation of Zn (2.1735 mg per plant) was in the shoot under 2.5 mmol L-1 N. Meanwhile, the accumulation of Cd in the shoot of S. alfredii reached the peak of 0.5513 mg per plant under the N level of 1 mmol L-1.
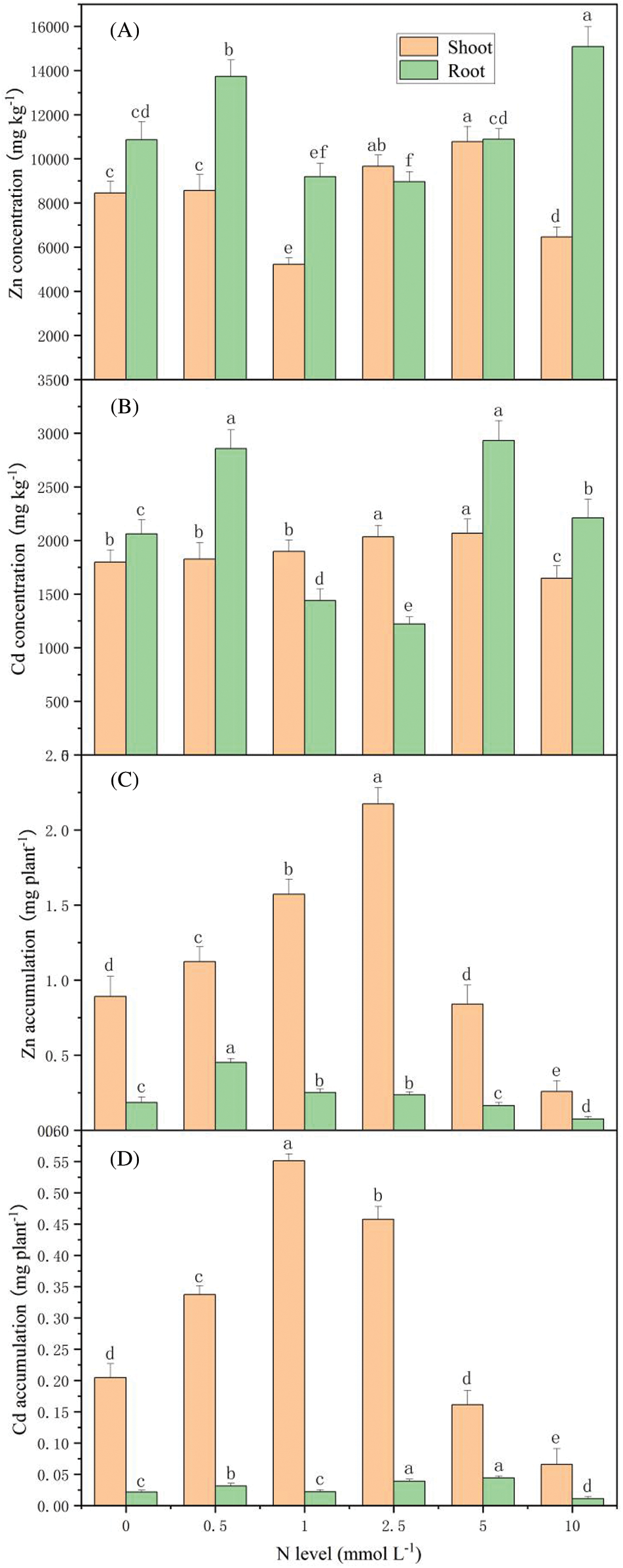
Figure 2: Zn and Cd concentration and accumulation by S. alfredii at different N applications. Data are average + standard deviation of n = 4. Different letters in the same color-histogram indicate significant differences at ɑ = 0.05
3.2 Biomass Production, and Zinc and Cadmium Absorption by S. alfredii at Different P Doses
With the increasing P concentration in solution, the dry weight of S. alfredii increased first then decreased (Fig. 3). The biomass production of both the shoot and root reached a peak at 0.1 mmol L-1 P, which were 0.559 g per plant and 0.129 g per plant, respectively. Compared with the control (shoot biomass was 0.4366 g plant-1, root biomass was 0.0856 g plant-1), the P concentration from 0.05 mmol L-1 to 1.6 mmol L-1, kept S. alfredii growing normally without toxic effects.
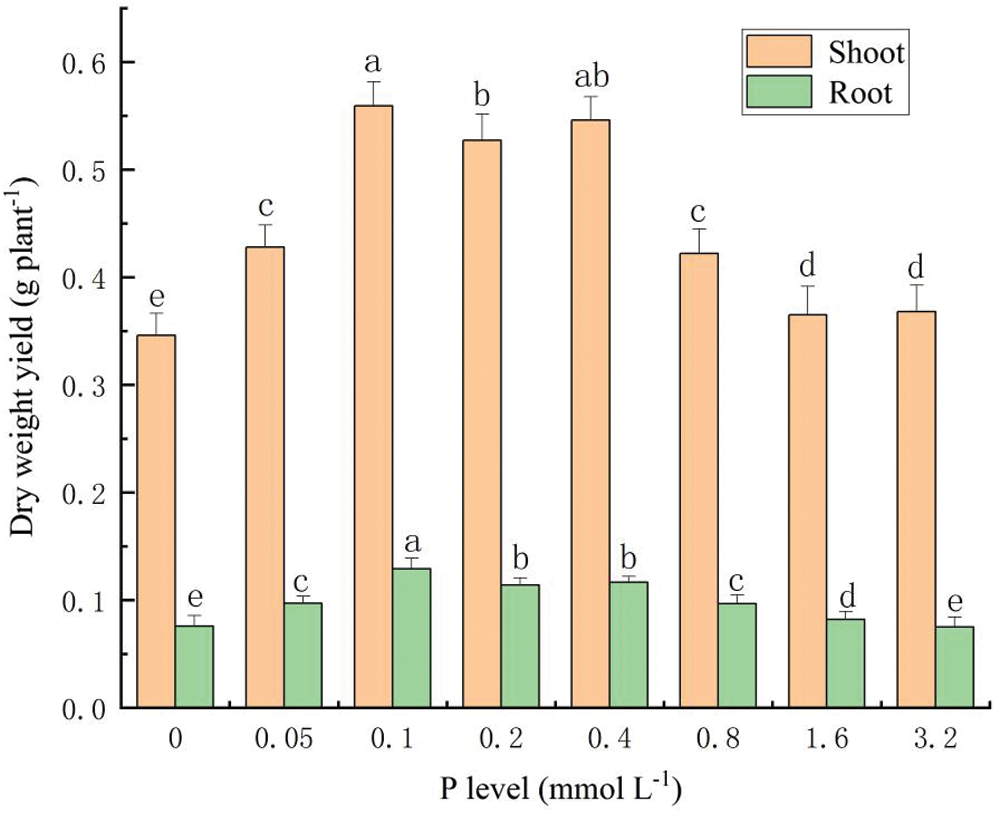
Figure 3: Biomass production of S. alfredii at different P application rates. Data are average + standard deviation of n = 4. Different letters in the same color-histogram indicate significant differences at ɑ = 0.05
The increasing amount of P application improved the studied heavy metal contents in the plant’s roots (Fig. 4). Furthermore, when the P supply level was 0.4 mmol L-1, the concentrations of Zn and Cd in the roots peaked at 15035 and 5334 mg kg-1, respectively. Moreover, with the increasing concentrations of P, the uptake of heavy metals in the shoots and roots of S. alfredii presented a trend of increasing firstly and then decreasing. The accumulation of Zn and Cd on the plant’s shoots reached the maximum values of 9.746 and 4.192 mg per plant, respectively, when the P concentration was 0.1 mmol L-1. The highest amounts of Zn and Cd in the roots were 1.579 and 0.513 mg per plant, respectively, when the P concentration was 0.4 mmol L-1.
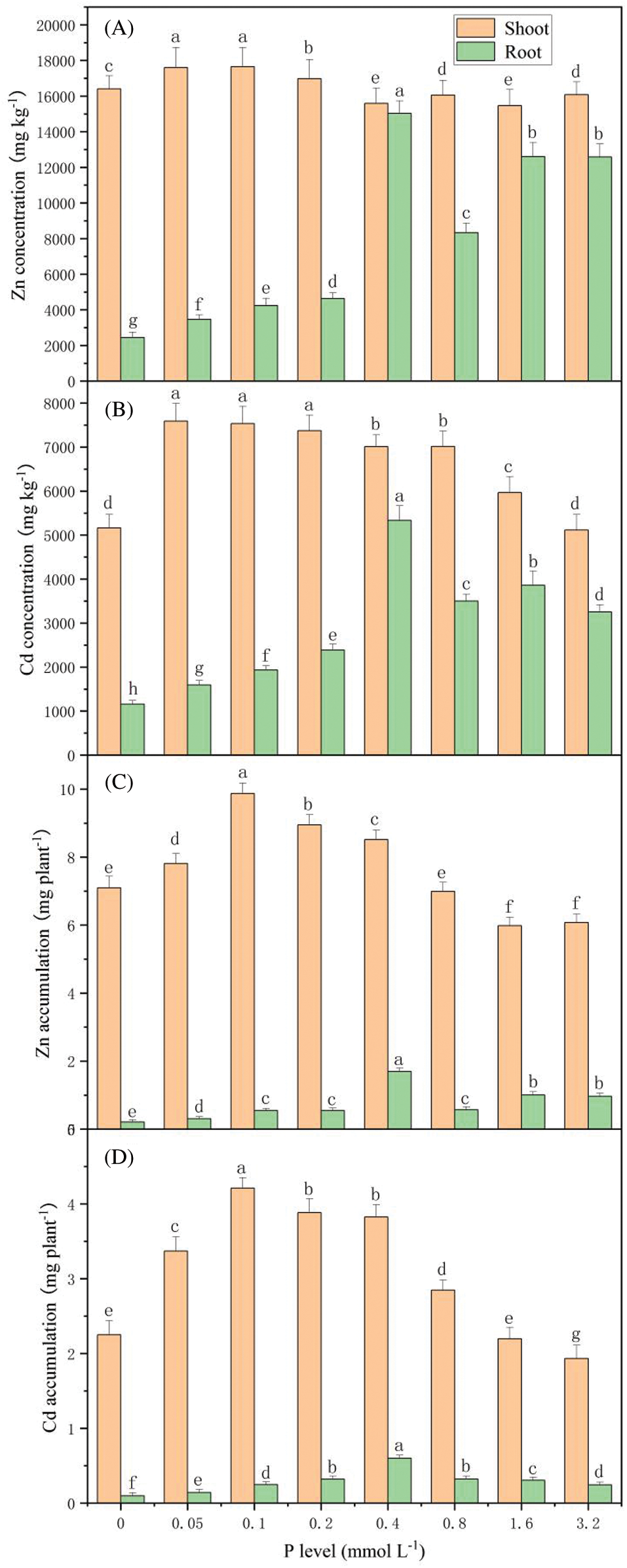
Figure 4: Concentration and accumulation of Zn and Cd on S. alfredii with different P applications. Data are average + standard deviation of n = 4. Different letters in the same color-histogram indicate significant differences at ɑ = 0.05
3.3 Biomass Production, and Zinc and Cadmium Absorption by S. alfredii at Different K Doses
The different K supplies significantly influenced biomass production of the different parts of S. alfredii (Fig. 5). When the K concentration was 0.5 mmol L-1, shoot and root biomasses reached a peak of 0.576 g per plant and 0.0918 g per plant, respectively. However, under the K treatment of 2 mmol L-1, the shoot biomass was reduced to 0.405 g per plant.
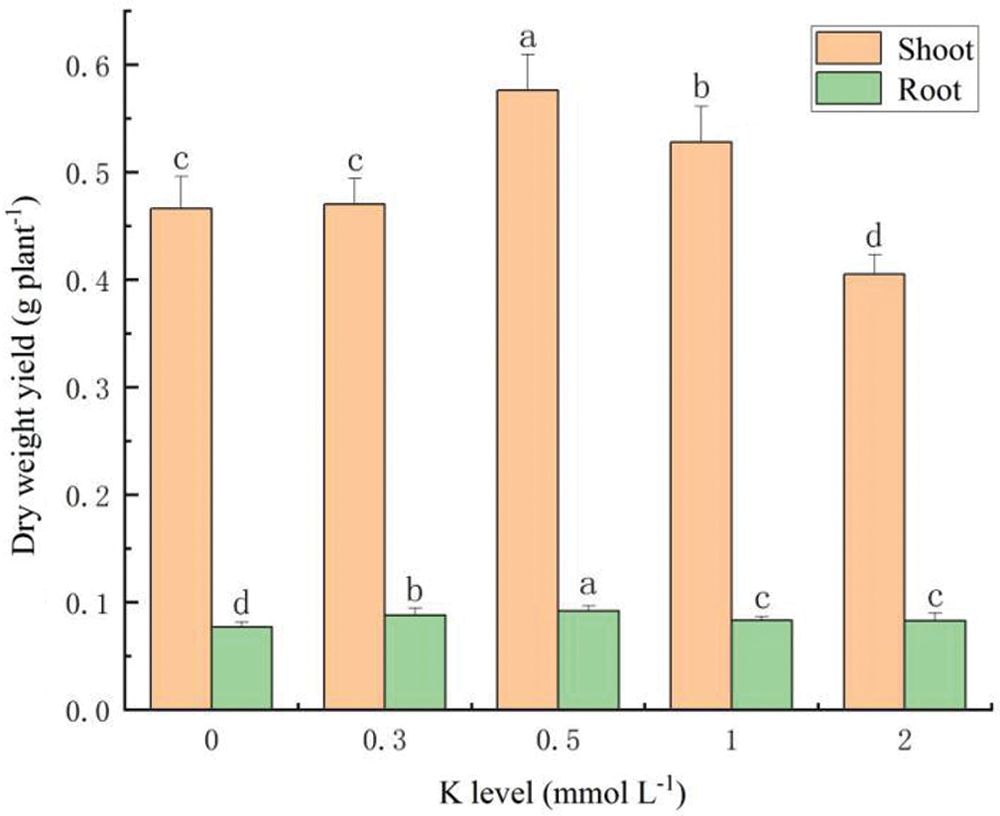
Figure 5: Biomass production of S. alfredii at different K application rates. Data are average + standard deviation of n = 4. Different letters in the same color-histogram indicate significant differences at ɑ = 0.05
In the K treatment of 1 mmol L-1, Zn and Cd shoot concentrations reached the highest values of 17015 mg kg-1 and 7710 mg kg-1, respectively. With the K application of 0.3 mmol L-1, the highest Zn and Cd root concentrations were 7725 mg kg-1 and 2658 mg kg-1, respectively (Figs. 6A and 6B). However, root Cd concentrations were similar at 0.3 and 0.5 mmol L-1. With the increasing K supply, Zn and Cd accumulations on shoots and roots showed the same trend, which initially increased but then decreased (Figs. 6C, 6D). The Zn accumulation of the shoot reached a peak value of 9.239 mg per plant, at a K concentration of 0.5 mmol L-1. However, the shoot Cd accumulation reached the maximum of 4.071 mg per plant under the treatment of 1 mmol L-1 K. Zn and Cd accumulations on roots reached their greatest values at 0.5 mmol L-1 K.
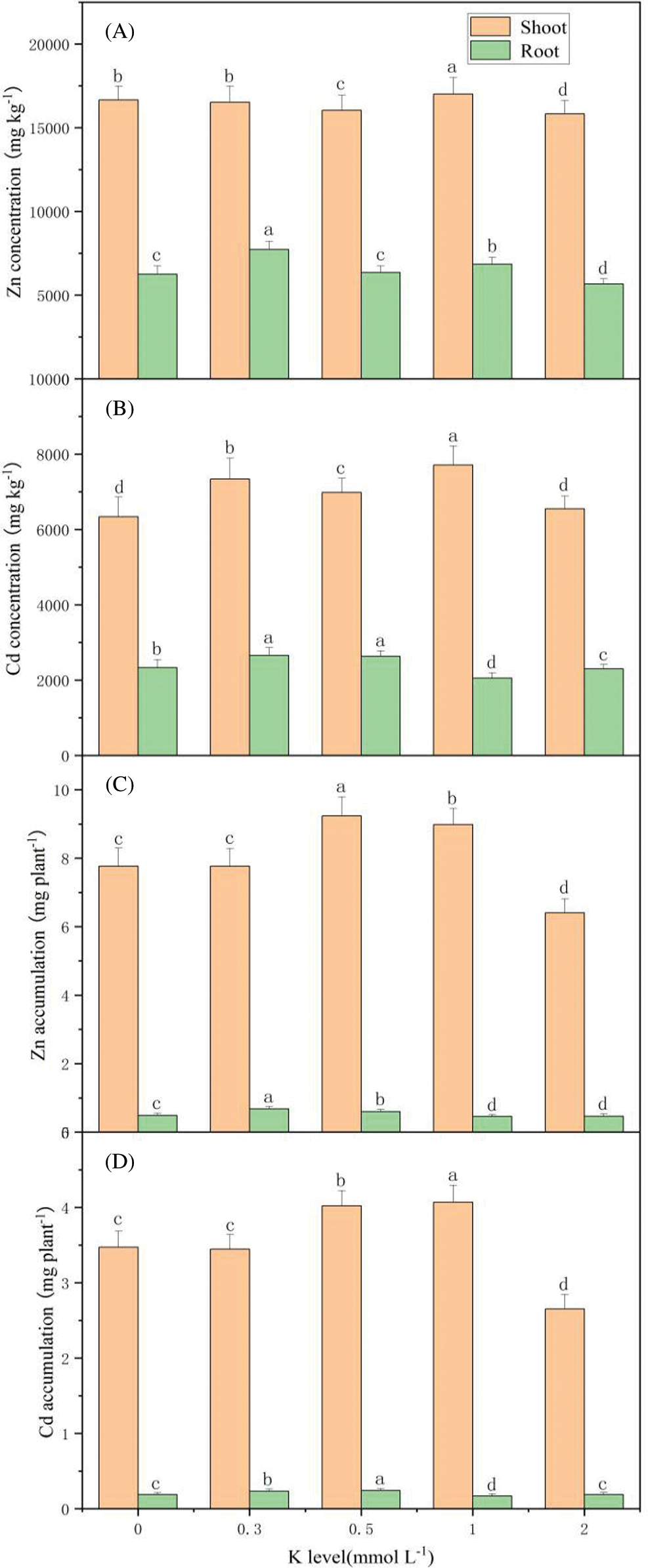
Figure 6: Zn and Cd concentration and accumulation on S. alfredii at different K applications. Means are average + standard deviation of n = 4. Different letters in the same color-histogram indicate significant differences at ɑ = 0.05
3.4 Effects of Different Fertilization Treatments on Growth of and Zn and Cd Removal by S. alfredii Grown on Soil Pots
When only N was applied as a fertilizer, the dry matter yield of S. alfredii varied significantly and gradually decreased with increasing levels of that fertilizer (Fig. 7, see Tab. 2). Compared with the control treatment (2.44 g pot-1), growth of S. alfredii was significantly reduced under the high N level (100 mg kg-1) to 1.23 g pot-1. In the treatments where phosphate and potassium fertilizers were combined with different concentrations of nitrogen fertilizers (i.e., treatments 4–6: Tab. 2), the additional application of P and K significantly improved the biomass yield of S. alfredii Dry matter yield of S. alfredii in treatment 5 (50 mg kg-1 N combined with P and K fertilizers) reached a value of 2.86 g pot-1, which did not differ with that in treatment 4. At the same levels of P and K concentrations, however, biomass yield of S. alfredii was once again reduced at the highest N concentration (i.e., 100 mg kg-1) in treatment 6.
The organic fertilizer was applied as composted pig manure with different concentrations of the inorganic fertilizers (treatments 7–9: Tab. 2). The dry matter of S. alfredii reached a maximum of 4.36 g pot-1 in treatment 9 [mixed application of fertilizers at the highest concentrations (i.e., N: 50 mg kg-1, P: 40 mg kg-1, K: 100 mg kg-1, organic matter: 10 g kg-1)], indicating that the organic fertilizer application significantly promoted growth of S. alfredii.
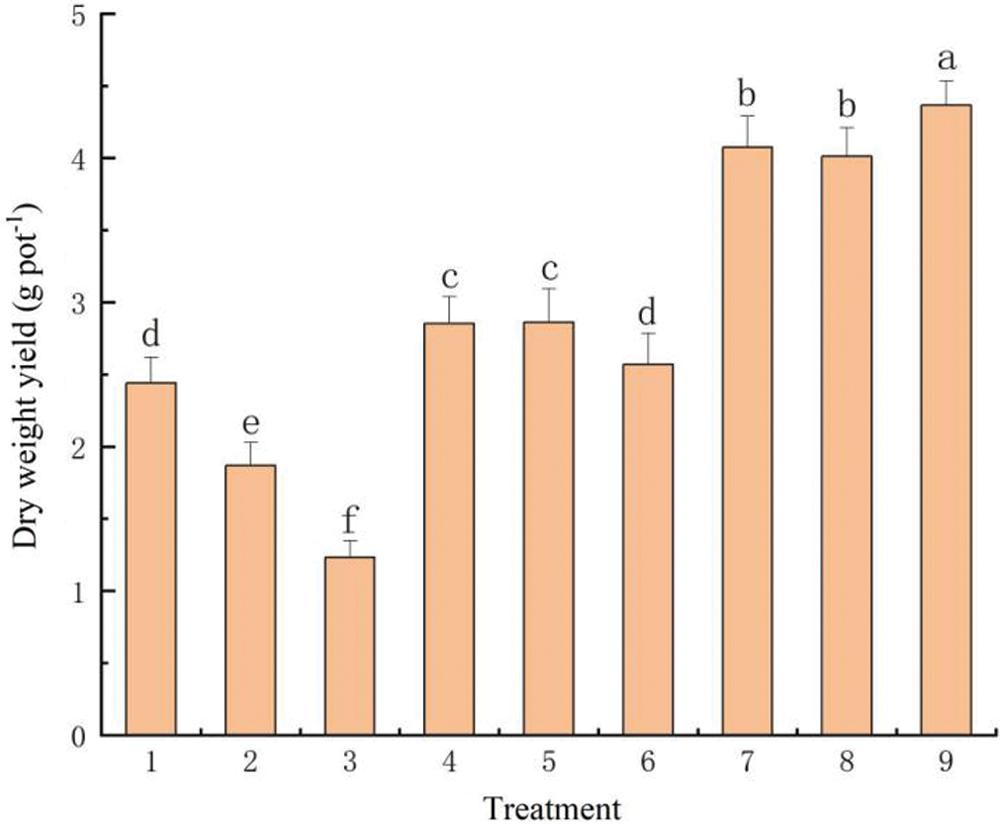
Figure 7: Effect of different fertilizer treatments on biomass of S. alfredii. Data are average + standard deviation of n = 4. Different letters indicate significant differences at ɑ = 0.05
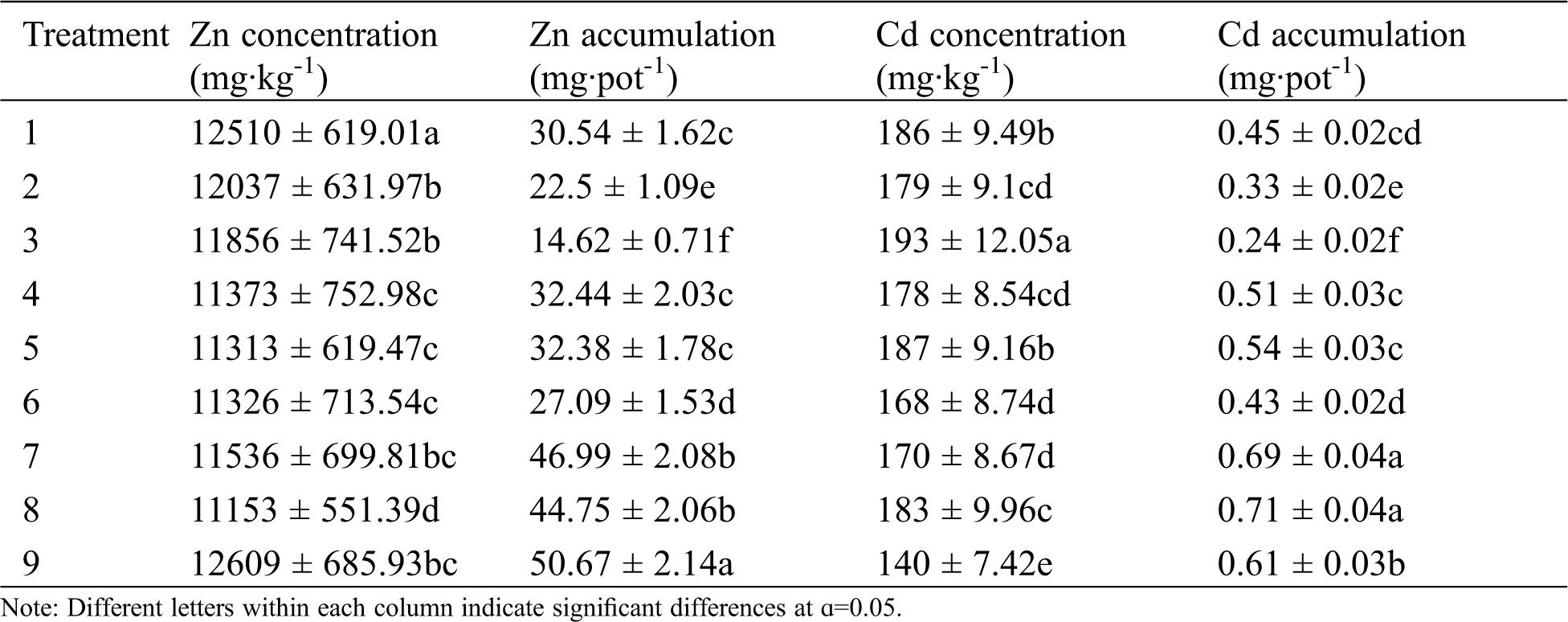
Compared with the control treatment (treatment 1), the Zn concentration of different fertilization (treatments 2–9) decreased in S. alfredii (Tab. 3). With a high amount of N (treatment 3) application, the Cd concentration of S. alfredii reached a maximum of 193 mg kg-1; in mixed applications of fertilizers at the highest concentrations (treatment 9), the Cd concentration was only of 140 mg kg-1. When only N fertilizer was applied (treatments 1–3), increasing N concentrations reduced the Zn concentration and accumulation of Zn and Cd in S. alfredii. In the treatments of mixed application of inorganic fertilizers (treatments 4–6), K and P fertilizer application (under the same N application) increased significantly the accumulation of heavy metals in plants. Organic fertilizer application (treatments 7–9) increased the accumulation of Zn and Cd in plants. In treatment 9 [mixed application of fertilizers at the highest concentrations (N: 50 mg kg-1, P: 40 mg kg-1, K: 100 mg kg-1, organic matter: 10 g kg-1)], Zn accumulation reached the highest value of 50.67 mg pot-1. Meanwhile, Cd accumulation reached a maximum of 0.71 mg pot-1 in treatment 8 [mixed application of fertilizers (N: 25 mg kg-1, P: 20 mg kg-1, K: 50 mg kg-1, organic matter: 5 g kg-1)]. However, this value did not differ from that when only the organic fertilizer was applied (i.e., treatment 7).
Table 3: Effect of different fertilizer treatments on Zn and Cd concentrations and accumulations by S. alfredii
Optimal applications of N improve the chlorophyll level in plant leaves, strengthen N assimilation and consequently enhance photosynthetic rates. However, excessive amounts of N application can decrease the assimilation rate and further reduce photosynthetic rates [27,28]. In our experiment, N was added in the form of NH4NO3, which included nitrate-N and ammonium-N. Our results agree with previous findings that the demand of N for growth of S. alfredii was low [29–31]. The biomass production and Zn and Cd accumulations were not in line with the increasing N doses. We found that the biomass of shoots and roots of S. alfredii was obviously enhanced within the suitable N concentration range (0.5 to 2.5 mmol L-1), while the biomass production of whole plants and their plant parts decreased at higher levels of N concentration (5 to 10 mmol L-1). Our finding was in line with that of Liu et al. [20], who reported that the translocation ability of Cd by Carpobrotus rossii significantly increased under an optimal concentration of nitrogenous fertilizer. Similarly, Cd accumulation significantly increased under the 32 mM nitrogen treatment, obtaining the maximum concentration of Cd (622.9 mg kg-1) in the roots of the Pentas lanceolata with a total Cd amount of 27.9 μg [32]. Similarly, this may also be related to the pH value caused by suitable N dosages in the nutrient solution, which further influenced the root function for nutrient uptake [33]. In this study, both low and high N concentrations adversely affected Zn and Cd uptake in S. alfredii, which was in agreement with the results of Hao et al. [34]. These authors also showed that both low and high N concentrations could severely restrict Mn uptake in rice (IR68144). Additionally, both low and high N concentrations altered Fe uptake in brown rice (IR64). These results were mainly attributed to the fact that high N concentrations decreased plant growth, and the low N concentrations did not meet the N requirements for plant growth [35].
The level of phosphorus in plants affects the metabolism of organic molecules such as nucleic acids, proteins, sugars and organic acids [36]. Clarkson et al. [37] proposed that the optimal P demand of general plants was at the range of 3–5 g kg-1, which could promote photosynthesis, respiration and energy storage in plants as to help healthy plant growth. At the same time, Singh et al. [38] reported that when the P concentrations of leaves and shoots are 12-45 g kg-1 and 9-30 g kg-1, respectively, plants may be poisoned for the application of excessive phosphorus fertilizer. This is because of it can consume a lot of sugar and energy due to the strong respiration of plants, and further lead to the imbalance between the aboveground part and the root growth ratio [39]. When the root quantity is very large, but the root length is short, itwill also have adverse effects on crop growth, yield and quality [39]. However, our experimental results reported that the growth of S. alfredii was not highly impacted at high P concentrations. This is mainly due to the fact that there is a regulating mechanism of P concentration in S. alfredii, which helps healthy growth of this species under high P concentrations [40]. Millikan et al. [41] reported that Zn demand increased with rising P doses. Similarly, we found that the addition of P (0–0.8 mmol L-1) significantly improved the absorption of Zn and Cd, and their translocation and accumulation from the root to shoot of S. alfredii. This further suggested that the increase of P dosage could satisfy the high demands of Zn in the shoots of the studied plant species as it was found in other studies [42]. However, our experiment indicated that the ability to accumulate Zn and Cd was weakened in the shoot under a relatively high P concentration (≥1.6 mmol L-1), which differed with the tendency of the plant biomass production of S. alfredii at these P concentrations. The availability of Zn and Cd in the plant might have been limited by insoluble phosphate existing in the shoot and leaves [42].
Potassium is a necessary component for plant growth. Plants selectively absorb water-soluble potassium ions from the soil through roots. Potassium is concentrated in the most active organs and tissues, such as buds and young leaves [43]. Potassium absorption from soil is mainly used to promote enzyme activation and protein synthesis, thus promoting plant growth and development [44]. In our results, K fertilization (from 0 to 0.5 mmol L-1) enhanced total biomass production of S. alfredii. However, S. alfredii’s growth was reduced at higher K concentrations (>1 mmol L-1). Excessive application of potassium fertilizer leads to a decrease in the absorption of magnesium and calcium by plants, causing plant pathological symptoms and affecting plant development [45]. However, at 1 mmol L-1 K in our study, Zn and Cd contents of the shoots reached the maximum values. This is in agreement with the results of Wang et al. [46], who found that the shoot growth of Solanum nigrum L. was restrained under high K concentrations, while the content of Cd increased significantly. However, what was different with Wang’s findings is that in the high K treatment (2 mmol L-1), our results showed that the content of dry matter declined, and Zn and Cd accumulations of the plant were reduced. This was probably because different kinds of hyperaccumulators have different demands for K amounts. A previous study reported that K fertilization induced the increase of Cd content in the soil and therefore enhanced the ability of the plant in absorbing Cd [47]. In addition, some studies reported that the addition of K could increase the content of Cd at the exchangeable state, by competing adsorption sites with Cd in soil particles [48]. Our results showed that the optimal concentration of K was 0.3-1 mmol L-1 for S. alfredii. Proper K application can significantly promote the absorption of Zn and Cd and their transport from the root to the shoot. This might be due because of the K fertilizer could induce Cl- and SO42- combination with heavy metal ions in the soil [49].
Previous studies indicated that fertilization could greatly improve soil fertility, thereby increasing crop yields [50,51]. Fertilization was also a key method to improve the accumulation of heavy metals in plants. Similarly, plants respond differently to different doses of Nie et al. [52] reported that appropriate amounts of N and K fertilizers can increase the absorption of the heavy metal Pb in the soil by increasing plant chlorophyll content, strengthening plant photosynthesis, and then increasing plant biomass yield. Sun et al. [53] found that appropriate addition of P can increase the biomass of our studied plant species and the uptake of heavy metals. However, in the present study, with sufficient nutrients in the potting soil, the application of inorganic N,and P and K fertilizers did not significantly promote the growth of S. alfredii and its capability of accumulating Zn and Cd. Under high N concentrations, the plant's biomass and heavy metal accumulation decreased significantly. Therefore, in the remediation of soil heavy metal pollution, it is necessary to keep an appropriate level of N in the soil [53]. Zhang et al. [54] identified that the application of organic fertilizer could increase soil nutrients and decrease the content of exchangeable Cd, and then weakened the Cd activity. The treatment of organic fertilizer in this experiment promoted the growth of S. alfredii, thus increasing Zn and Cd accumulation. Meanwhile, the heavy metal concentrations in the plant all declined to some extent. However, the variation in the concentrations of Zn and Cd were unnoticeable with the application of inorganic and organic fertilizers. This differs from the results of Guo [55], who found that the organic fertilizer is more capable of strengthening the mobility of heavy metals, thus enhancing their accumulation compared with the inorganic fertilizers in soils. It is likely that decomposition and activation of various organic fertilizers lead to different degrees of transformation of heavy metals to an exchangeable form in soils, thus affecting the availability of soil Zn and Cd [56,57]. Therefore, selecting the proper type of organic fertilizer that can promote the enrichment of heavy metals in S. alfredii is particularly critical for remediation of heavy metal contaminated soils.
In the current study, hydroponics and pot experiments were used to reveal the effects of different nutrient supplies on growth of S. alfredii and its uptake of Zn and Cd. In the hydroponics experiment, the increasing N, P and K supplies (within a suitable range of concentrations) could accelerate the growth of S. alfredii, thus enhancing the absorption of Zn and Cd. However, the result of the pot experiment showed that P and K fertilizers did not significantly promote growth and accumulation of the target heavy metals by S. alfredii, while the N fertilizer significantly reduced plant growth and the removal of Zn and Cd. The results of different experiments are diverse, owing to the difference in the available nutrient amounts and Zn/Cd in soil and hydroponics experiments, or the nutrient interactions in soils [58]. Therefore, more research needs to be conducted to remediate Zn/Cd-polluted soils by hyperaccumulators.
The hydroponic experiment showed that suitable amounts of N fertilizer (0.5–2.5 mmol L-1) improved Zn and Cd uptake by S. alfredii. However, an excessive amount of N application resulted in a decrease in growth of S. alfsredii. Similarly, appropriate P addition within a range of 0.05-0.8 mmol L-1 increased plant growth and the studied heavy metals uptake of S. alfredii. The optimum K supply level was between 0.3 and 1 mmol L-1, which significantly improved the biomass and the ability to enrich heavy metals in the plant.
The soil experiment revealed that the single inorganic nitrogen fertilizer application did not improve the biomass and the ability to enrich Zn and Cd in the studied plant species. The application of inorganic phosphate and potassium fertilizers or the organic fertilizer could promote plant growth and the ability to absorb Zn and Cd. The combination of the organic and inorganic fertilizers made the most significant effect on Zn and Cd phytoremediation. For Zn, the highest accumulation value was found at a high fertilizer application dose (N: 50 mg kg-1, P: 40 mg kg-1, K: 100 mg kg-1, organic matter: 1%), while the strongest ability to accumulate Cd was found at a relatively low fertilizer application rate (N: 25 mg kg-1, P: 20 mg kg-1, K: 50 mg kg-1, organic matter: 0.5%).
Acknowledgement: We thank Wei Fang for his help in the laboratory analysis.
Funding Statement: This work was financially supported by the National Key Research and Development Project (2017YFD0801302)
Conflicts of Interest: There are no conflict of interest regarding this research work.
1. Zhao, K. L., Fu, W. J., Qiu, Q. Z., Ye, Z. Q., Li, Y. F. et al. (2019). Spatial patterns of potentially hazardous metals in paddy soils in a typical electrical waste dismantling area and their pollution characteristics. Geoderma, 337(2–3), 453–462. DOI 10.1016/j.geoderma.2018.10.004. [Google Scholar] [CrossRef]
2. Zhao, K. L., Fu, W. J., Liu, X. M., Zhang, C. S., Ye, Z. Q. et al. (2014). Spatial variations of concentrations of copper and its speciation in the soil-rice system in Wenling of southeastern China. Environmental Science and Pollution Research, 21(11), 7165–7176. DOI 10.1007/s11356-014-2638-9. [Google Scholar] [CrossRef]
3. Wang, F. (2014). Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth, 1(5), 2155–2179. [Google Scholar]
4. Liu, X. B., Xing, B. S., Zhou, K. Q., Wang, G. H., Liu, J. D. (2005). Phytoremediation and its mechanisms for contaminated soils. Chinese Journal of Eco-Agriculture, 13(1), 134–138. [Google Scholar]
5. Nouri, J., Khorasani, N., Lorestani, B., Karami, M., Hassani, A. H. et al. (2009). Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environmental Earth Sciences, 59(2), 315–323. DOI 10.1007/s12665-009-0028-2. [Google Scholar] [CrossRef]
6. Luo, Y. M. (1999). Phytoremediation of metal contaminated soil. Soil, 31(5), 261–265. [Google Scholar]
7. Ye, H. B., Yang, X. E., He, B., Long, X. X., Shi, W. Y. (2003). Growth response and metal accumulation of Sedum alfredii to Cd/Zn complex-polluted ion levels. Acta Botanica Sinica, 45(9), 1030–1036. [Google Scholar]
8. Yang, X. E., Long, X. X., Ye, H. B., He, Z. L., Calvert, D. V. et al. (2004). Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant and Soil, 259(1/2), 181–189. DOI 10.1023/B:PLSO.0000020956.24027.f2. [Google Scholar] [CrossRef]
9. Chen, Y. P. (2008). Research progress of heavy metal superaccumulating plants. Environmental Science and Management, 33(3), 20–24. [Google Scholar]
10. Liu, L., Wu, L. H., Li, N., Cui, L. Q., Li, Z. et al. (2009). Effect of planting densities on yields and zinc and cadmium uptake by Sedum plumbizincicola. Enviromental Science, (11), 304–308. [Google Scholar]
11. Agnello, A. C., Bagard, M., van Hullebusch, E. D., Esposito, G., Huguenot, D. (2016). Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Science of The Total Environment, 563–564(2), 693–703. DOI 10.1016/j.scitotenv.2015.10.061. [Google Scholar] [CrossRef]
12. Yavari, S., Malakahmad, A., Sapari, N., Yavari, S. (2018). Nutrients balance for improvement of phytoremediation ability of teak seedlings (Tectona grandis). Taylor & Francis. Journal of Plant Nutrition, 41(5), 1–7. [Google Scholar]
13. Hu, P., Yin, Y. G., Ishikawa, S., Suzui, N., Kawachi, N. et al. (2013). Nitrate facilitates cadmium uptake, transport and accumulation in the hyperaccumulator Sedum plumbizincicola. Environmental Science and Pollution Research, 20(9), 6306–6316. DOI 10.1007/s11356-013-1680-3. [Google Scholar] [CrossRef]
14. Lin, Z. W., Dou, C. Y., Li, Y. F., Wang, H. L., Niazi, N. K. et al. (2020). Nitrogen fertilizer enhances zinc and cadmium uptake by hyperaccumulator Sedum alfredii Hance. Journal of Soils and Sediments, 20(1), 320–329. DOI 10.1007/s11368-019-02405-4. [Google Scholar] [CrossRef]
15. Gupta, D. K., Chatterjee, S., Datta, S., Veer, V., Walther, C. (2014). Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere, 108(2), 134–144. DOI 10.1016/j.chemosphere.2014.01.030. [Google Scholar] [CrossRef]
16. Zhao, B. Z., Morihiro, M., Zhang, J. B., Zhu, A. N., Yasuo, O. (2006). Accumulation and chemical fractionation of heavy metals in andisols after a different, 6-year fertilization management (8pp). Environmental Science and Pollution Research-International, 13(2), 90–97. DOI 10.1065/espr2005.06.268. [Google Scholar] [CrossRef]
17. Zhao, Q. G., Huang, G. Q., Qian, H. Y. (2007). Ecological agriculture and food safety. Acta Pedologica Sinica, 44(6), 1127–1134. [Google Scholar]
18. Yu, S. Y., Chen, Z. L., Zhao, K. L., Ye, Z. Q., Zhang, L. Y. et al. (2019). Spatial patterns of potentially hazardous metals in soils of Lin’an City, Southeastern China. International Journal of Environmental Research and Public Health, 16(2), 246. DOI 10.3390/ijerph16020246. [Google Scholar] [CrossRef]
19. Sun, Q., Mi, W. Z., Yang, X. E., Ding, S. M. (2003). Effects of phosphorus on the growth, zinc absorption and accumulation in hyperaccumulator-Sedum alfredii Hance. Acta scientiae Circumstantiae, 23(6), 818–824. [Google Scholar]
20. Liu, W. X., Zhang, C. J., Hu, P. J., Luo, Y. M., Wu, L. H. et al. (2016). Influence of nitrogen form on the phytoextraction of cadmium by a newly discovered hyperaccumulator Carpobrotus rossii. Environmental Science and Pollution Research, 23(2), 1246–1253. DOI 10.1007/s11356-015-5231-y. [Google Scholar] [CrossRef]
21. Xiao, W., Li, D., Ye, X. Z., Xu, H. Z., Yao, G. H. et al. (2017). Enhancement of Cd phytoextraction by hyperaccumulator Sedum alfredii using electrical field and organic amendments. Environmental Science & Pollution Research, 24(5), 5060–5067. DOI 10.1007/s11356-016-8277-6. [Google Scholar] [CrossRef]
22. Wang, H. B., Jin, J., Fu, W. J., Morrison, L., Lin, H. P. et al. (2020). Converting evergreen broad-leaved forests into tea and Moso bamboo plantations affects labile carbon pools and the chemical composition of soil organic carbon. Science of the Total Environment, 711(3), 135225. DOI 10.1016/j.scitotenv.2019.135225. [Google Scholar] [CrossRef]
23. Deng, D., Shu, W., Zhang, J., Zou, H. L., Lin, Z. et al. (2007). Zinc and cadmium accumulation and tolerance in populations of Sedum alfredii. Environmental Pollution, 147(2), 381–386. DOI 10.1016/j.envpol.2006.05.024. [Google Scholar] [CrossRef]
24. Chen, J., Lin, Y. (2010). Effect of aluminum on variations in the proteins in pineapple roots. Soil Science and Plant Nutrition, 56(3), 438–444. DOI 10.1111/j.1747-0765.2010.00479.x. [Google Scholar] [CrossRef]
25. Kashem, M. A., Kawai, S. (2007). Alleviation of cadmium phytotoxicity by magnesium in Japanese mustard spinach. Soil Science and Plant Nutrition, 53(3), 246–251. DOI 10.1111/j.1747-0765.2007.00129.x. [Google Scholar] [CrossRef]
26. Zhao, K. L., Zhang, L. Y., Dong, J. Q., Wu, J. S., Ye, Z. Q. et al. (2020). Risk assessment, spatial patterns and source apportionment of soil heavy metals in a typical Chinese hickory plantation region of southeastern China. Geoderma, 360(1–4), 114011. DOI 10.1016/j.geoderma.2019.114011. [Google Scholar] [CrossRef]
27. Ding, L., Wang, K. J., Jiang, G. M., Biswas, D. K., Xu, H. et al. (2005). Effects of nitrogen deficiency on photosynthetic traits of maize hybrids released in different years. Annals of Botany, 96(5), 925–930. DOI 10.1093/aob/mci244. [Google Scholar] [CrossRef]
28. Cao, C. L., Li, S. X., Fang, M. (1998). The research situation about effects of nitrogenous certain physiological and biochemical process in plants. Journal of Northwest A&F University (Natural Science Edition), 27(4), 96–101. [Google Scholar]
29. Xue, Q. W., Chen, P. Y. (1997). Effects of Nitrogen Nutrition on Water Status and Photosynthesis in Wheat Under Soil Drought. Plant Physiology Journal, 151(1), 79–82. DOI 10.1016/S0176-1617(97)80040-5. [Google Scholar] [CrossRef]
30. Hu, P., Yin, Y., Ishikawa, S., Suzui, N., Kawachi, N. et al. (2013). Nitrate facilitates cadmium uptake, transport and accumulation in the hyperaccumulator Sedum plumbizincicola. Environmental Science and Pollution Research, 20(9), 6306–6316. DOI 10.1007/s11356-013-1680-3. [Google Scholar] [CrossRef]
31. Liu, W., Wang, Q., Wang, B., Hou, J., Luo, Y. et al. (2015). Plant growth-promoting rhizobacteria enhance the growth and Cd uptake of Sedum plumbizincicola in a Cd-contaminated soil. Journal of Soils and Sediments, 15(5), 1191–1199. DOI 10.1007/s11368-015-1067-9. [Google Scholar] [CrossRef]
32. Chang, Y. S., Chang, Y. J., Lin, C. T., Lee, M. C., Wu, C. W. (2013). Nitrogen fertilization promotes the phytoremediation of cadmium in Pentas lanceolata. International Biodeterioration & Biodegradation, 85, 709–714. DOI 10.1016/j.ibiod.2013.05.021. [Google Scholar] [CrossRef]
33. Gazzarrini, S., Lejay, L., Gojon, A., Ninnemann, O., Frommer, W. B. et al. (1999). Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into arabidopsis roots. Plant Cell, 11(5), 937–947. DOI 10.1105/tpc.11.5.937. [Google Scholar] [CrossRef]
34. Hao, H. L., Wei, Y. Z., Yang, X. E., Feng, Y., Wu, C. Y. (2007). Effects of different nitrogen fertilizer levels on Fe, Mn, Cu and Zn concentrations in shoot and grain quality in rice (Oryza sativa). Chinese Journal of Rice Science, 14(4), 289–294. DOI 10.1016/S1672-6308(08)60007-4. [Google Scholar] [CrossRef]
35. Wiszniewska, A., Hanus-Fajerska, E., Muszyska, E., Ciarkowska, K. (2016). Natural organic amendments for improved phytoremediation of polluted soils: a review of recent progress. Pedosphere, 26(1), 1–12. DOI 10.1016/S1002-0160(15)60017-0. [Google Scholar] [CrossRef]
36. Ramos-Artuso, F., Galatro, A., Lima, A. (2019). Early events following phosphorus restriction involve changes in proteome and affects nitric oxide metabolism in soybean leaves. Environmental and Experimental Botany, 161(417), 203–217. DOI 10.1016/j.envexpbot.2019.01.002. [Google Scholar] [CrossRef]
37. Clarkson, D. T., Hanson, J. B. (1980). The mineral nutrition of higher plants. Annual Review of Plant Physiology, 31(1), 239–298. DOI 10.1146/annurev.pp.31.060180.001323. [Google Scholar] [CrossRef]
38. Singh, J. P., Karamanos, R. E., Stewart, J. W. B. (1986). Phosphorus-induced Zinc deficiency in wheat on residual phosphorus plots. Agronomy Journal, 78(4), 668–675. DOI 10.2134/agronj1986.00021962007800040023x. [Google Scholar] [CrossRef]
39. Modupeola, T. O., Olaniyi, J. O., Abdul-Rafi, A. M., Taylor, O. O., Fariyike, T. A. et al. (2013). Effect of organic phosphorus fertilizer and plant density on the growth, yield and nutritional value of ginger (Zingiber officinale). International Journal of Agricultural Research, 8(2), 94–100. DOI 10.3923/ijar.2013.94.100. [Google Scholar] [CrossRef]
40. Yan, N., Zhang, Y., Xue, H. M., Zhang, X. H., Wang, Z. D. et al. (2015). Changes in plant growth and photosynthetic performance of Zizania latifolia exposed to different phosphorus concentrations under hydroponic condition. Photosynthetica, 53(4), 630–635. DOI 10.1007/s11099-015-0149-7. [Google Scholar] [CrossRef]
41. Millikan, C. R., Hanger, B. C., BJarnason, E. N. (1968). Effect of phosphorus and zinc levels in the substrate on 65Zn distribution in subterranean clove and flax. Australian Journal of Biological Sciences, 21(4), 619–640. DOI 10.1071/BI9680619. [Google Scholar] [CrossRef]
42. Cakmark, I., Marschner, H. (1987). Mechanism of phosphorus-induced zinc deficiency in cotton. III. Changes in physiological availability of zinc in plants is mail. Physiologia Plantarum, 70(1), 13–20. DOI 10.1111/j.1399-3054.1987.tb08690.x. [Google Scholar] [CrossRef]
43. Sawana, Z. M., Hafez, S. A., Alkassas, A. R. (2007). Nitrogen, potassium and plant growth retardant effects on oil content and quality of cotton seed. Grasas y Aceites, 58(3), 243–251. DOI 10.3989/gya.2007.v58.i3.179. [Google Scholar] [CrossRef]
44. Shabala, S., Cuin, T. A. (2008). Potassium transport and plant salt tolerance. Physiologia Plantarum, 133(4), 651–669. DOI 10.1111/j.1399-3054.2007.01008.x. [Google Scholar] [CrossRef]
45. Naby, H. M. A., El-Gamily, E. L., Allah, A. G. (2018). Response of potato plants to sources and rates of potassium fertilizer. Journal of Plant Production, 9(1), 67–71. DOI 10.21608/jpp.2018.35254. [Google Scholar] [CrossRef]
46. Wang, L., Zhou, Q. X., Sun, Y. B. (2008). Intensification of Solanum nigrum L. remedying cadmium contaminated soils by nitrogen and potassium fertilizers. China Environmental Science, 28(10), 915–920. [Google Scholar]
47. Smolders, E., Lambregts, R. M., Mclaughlin, M. J., Tiller, K. G. (1998). Effect of soil solution chloride on cadmium availability to Swiss Chard. Journal of Environmental Quality, 27(2), 426–431. DOI 10.2134/jeq1998.00472425002700020025x. [Google Scholar] [CrossRef]
48. Tu, C., Zheng, C. R., Chen, H. M. (2000). Effect of applying chemical fertilizers on forms of lead and cadmium in red soil. Chemosphere, 41(1–2), 133–138. DOI 10.1016/S0045-6535(99)00400-2. [Google Scholar] [CrossRef]
49. Zhao, Z. Q., Zhu, Y. G., Li, H. Y., Smith, S. E., Smith, F. A. (2004). Effects of forms and rates of potassium fertilizers on cadmium uptake by two cultivars of spring wheat (Triticum aestivum, L.). Environment International, 29(7), 973–978. DOI 10.1016/S0160-4120(03)00081-3. [Google Scholar] [CrossRef]
50. Li, X. G., Liu, X. P., Liu, X. J. (2020). Long-term fertilization effects on crop yield and desalinized soil properties. Agronomy Journal, 112(5), 4321–4331. DOI 10.1002/agj2.20338. [Google Scholar] [CrossRef]
51. Fayiga, A. O., Ma, L. Q., Rathinasabapathi, B. (2008). Effects of nutrients on arsenic accumulation by arsenic hyperaccumulator Pteris vittata L. Environmental and Experimental Botany, 62(3), 231–237. DOI 10.1016/j.envexpbot.2007.09.001. [Google Scholar] [CrossRef]
52. Nie, J. H., Liu, X. M., Wang, Q. R. (2004). Effect of nutrient elements on the lead uptake by hyperaccumulators. Ecology and Environment, 20(5), 262–265. [Google Scholar]
53. Jacobs, A., de, B., Léna, D., Sterckeman, T., Noret, T. et al. (2018). Phytoextraction of Cd and Zn with Noccaea caerulescens for urban soil remediation: influence of nitrogen fertilization and planting density. Ecological Engineering, 116, 178–187. DOI 10.1016/j.ecoleng.2018.03.007. [Google Scholar] [CrossRef]
54. Zhang, Y. L., Shen, Q. R., Jiang, Y. (2001). Effects of organic manure on the amelioration of Cd-polluted soil. Acta Pedologica Sinica, 38(2), 212–218. [Google Scholar]
55. Guo, J. M., Lei, M., Yang, J. X., Yang, J., Wan, X. M. et al. (2017). Effect of fertilizers on the Cd uptake of two sedum species (Sedum spectabile Boreau and Sedum aizoon L.) as potential Cd accumulators. Ecological Engineering, 106(2), 409–414. DOI 10.1016/j.ecoleng.2017.04.069. [Google Scholar] [CrossRef]
56. Yang, J. Y., Yang, X. E., He, Z. L. (2005). Resource and bio-availability of lead in soil. Chinese Journal of Soil Science, 36(5), 127–134. [Google Scholar]
57. Chen, H., Chen, Y. C., Yang, X. H. (2004). Regulation of phyto-availability of soil lead by chemical additives. Ecology and Environment, 13(1), 9–10. [Google Scholar]
58. Wang, X., Chen, C., Wang, J. (2017). Cs phytoremediation by Sorghum bicolor cultivated in soil and in hydroponic system. International Journal of Phytoremediation, 19(4), 402–412. DOI 10.1080/15226514.2016.1244158. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |