
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013305
ARTICLE
Non-Polar Chemical Constituents of Atemoya and Evaluation of the Cytotoxic and Antimicrobial Activity
1Post-Graduate Program in Biotechnology (RENORBIO), Federal Rural University of Pernambuco, Recife, Pernambuco, 52171-900, Brazil
2Post-Graduate Program in Biotechnology, State University of Feira de Santana, Feira de Santana, Bahia, 44036-900, Brazil
3Center for Studies and Research of Medicinal Plants (NEPLAME), Federal University of San Francisco Valley, Petrolina, Pernambuco, 56304-205, Brazil
4Multidisciplinary Center of Chemical, Biological and Agricultural Research (CPQBA), Campinas State University, Campinas, São Paulo, 13083-970, Brazil
5National Laboratory of Experimental Oncology (LabNOE), Federal University of Ceará, Fortaleza, Ceará, 60020-181, Brazil
6Departament of Chemistry, Federal University of Amazonas, Manaus, Amazonas, 69067-005, Brazil
*Corresponding Author: Jackson Roberto Guedes da Silva Almeida. Email: jackson.guedes@univasf.edu.br
Received: 01 August 2020; Accepted: 01 December 2020
Abstract: This study aimed to identify the chemical composition of essential oil from fruits (EOAF) and the hexanic crude extract from aerial parts (At-Hex) of atemoya (Annona cherimola x Annona squamosa), a hybrid belonging to the Annonaceae family. Cytotoxic and antimicrobial activity was also evaluated. OEAF was obtained by hydrodistillation using a Clevenger apparatus, and their composition was analyzed by gas chromatography-mass spectrometry (GC-MS) analyses. Cytotoxicity was tested against human tumor cell lines HCT-116 (colon carcinoma), SF-295 (glioblastoma), OVCAR-8 (ovarian carcinoma) and HL60 (leukemia) using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, while antimicrobial activity was conducted by bioauthography method against eleven microorganisms strains. Twenty-four compounds were identified in the EOAF and twenty-nine in At-Hex. The monoterpenes linalool (25.70%), α-pinene (10.38%), β-pinene (9.12%), trans-ocimene (7.43%), and the sesquiterpene bicyclogermacrene (12.58%) were the major constituents of EOAF, whereas the sesquiterpene spathulenol (13.91%) was the main compound of At-Hex. At-Hex showed a high cytotoxicity against SF-295 (glioblastoma). These findings show an important chemotaxonomic contribution for the Annonaceae family, mainly for the Annona genus. Atemoya proved to be a promising source of substances with potential cytotoxic activity.
Keywords: Annona; GC-MS; volatile compounds; terpenoids; biologic activities
Atemoya is a hybrid resulting from the crossing between two species of the Annona genus, belonging to the Annonaceae family [1]. This family comprises about 2500 species in about 135 genera [2], in which the Annona genus stands to produce very tasty and nutritious fruit, which add great commercial value [3].
From the phytochemical point of view, both the family and genus, are considered good sources of bioactive substances steaming from their secondary metabolism. These components mainly include alkaloids, diterpenes, acetogenins, and essential oils that exhibit significant activities, such as cytotoxic, antimicrobial, antioxidant, and anti-parasitic [4].
Cancer is characterized by abnormal cell proliferation and differentiation [5], especially malignant tumors, which is responsible for a significant and increasing number of patients around the world and represents the second leading cause of death in the world population [6]. The latest worldwide estimate, year 2018, points out that 18 million new cases of cancer and 9.6 million deaths occurred worldwide [7]. That increasing incidence and the several limitations in conventional therapy, including high cost and toxicity of current anticancer drugs, have faced a serious challenge for all researchers to design and develop a strategic, economical, and effective alternative in the identification and procurement process of new drugs. In this sense, gas chromatography coupled to mass spectrometry (GC-MS) represents a faster and useful tool to identify substances biologically active plant oil and extracts faster and, under this scenario, phytomolecules are expected to revolutionize cancer treatment in the next decade [8].
Natural products currently play a fundamental role as a source of new drugs or leads for drug development. These focus not only on the treatment of cancer but also as an alternative to the fight against pathogenic microorganisms and multiple drugs due to the indiscriminate use of antimicrobials [9]. In this context, Brazil holds great potential for biodiversity exploitation and, among its regions, the Northeast has areas characterized by high solar radiation values, elevated temperatures, and the irregularity in rainfall regime. These characteristics provide the existing vegetation, abundant periods of water stress affecting the synthesis of secondary metabolites [10].
Thus, this work presents for the first time the chemical composition of the essential oil of fruits (EOAF) and of the crude hexanic extract from aerial parts (At-Hex) of atemoya grown in the Northeast Brazil, and the results of their cytotoxic activities. The antimicrobial activity of the essential oil also was tested.
For essential oil extraction, atemoya immature fruits were collected in Petrolina, Pernambuco State, Brazil (Coordinates: 9°23’19” S, 40°29’3” W), in May 2012, while the aerial parts were collected in the same locality, in September 2014. The botanical material (fruits) was identified by Prof. José Alves de Siqueira Filho and the voucher specimen (#16310) was deposited in the Herbarium of Vale do São Francisco (HVASF) at Federal University of Vale do São Francisco (UNIVASF). On its turn, the plant (aerial parts) was identified by Professor José Alves de Siqueira Filho by comparison with the voucher specimen #16310. All procedures for access to genetic patrimony and associated traditional knowledge were carried out and the project was registered in SisGen (Register #ABD9AA7).
2.2 Preparation and Characterization of Essential Oil
The essential oil from atemoya fruits was obtained by hydrodistillation using a Clevenger-type apparatus for 2 h, from 1503.5 g of fruits. The initial time was recorded from the steam condensing in the Clevenger apparatus. Residual water of the extraction process was removed by decreasing the temperature to the freezing point of water so that, once solidified, the residue was separated from this the essential oil that remains in the liquid phase.
The characterization was carried out by gas chromatography coupled to mass spectrometry (GC-MS). The analysis was conducted on a Hewlett-Packard chromatograph 5890 series II, equipped with a Hewlett-Packard 5971 mass selective detector, split/splitless injector, using a capillary column HP-5 (25 m × 0.20 mm × 0.33 diameter). Temperatures: injector = 220°C, detector = 280°C, column = 60°C, 3 °C/min, 240°C (7 min). Carrier gas flow rate (super dry He) = 1.0 mL/min. The GC-MS electron ionization system was 70 eV. The retention indices were determined by co-injection of hydrocarbons standards. The oil components were identified by comparison with published data from the profiles 138 and Wiley Nist 98 libraries and by co-injection of a series of n-alkanes [11]. The sample was diluted with ethyl acetate (10 mg/mL).
2.3 Preparation and Characterization of Hexanic Crude Extract of Atemoya Aerial Parts
The aerial parts (2.200 g) were dried at 45°C by seven days in stove with air circulation, and then powdered in a mechanical mill obtain 707 g of powder. The powder was extracted with hexane (3 L, three times) yielding 84.1 g of hexane extract after solvent removal under reduced pressure in rotavapor. The hexane extract was transferred to a glass flask, which was kept open for evaporation of traces of the solvent.
The compounds in the hexanic crude extract were investigated on a Shimadzu QP-2010 (Shimadzu Corporation, Kyoto, Japan) gas chromatograph coupled to a mass spectrometer (GC-MS). The following conditions were used: DB-5MS column Agilent Technologies (30 m × 0.25 mm × 0.25 μm); helium (99.999%) carrier gas at a constant flow of 1.1 mL/min; injection volume of 1.0 μL; injector split ratio of 1:10; injector temperature of 250°C; electron impact mode at 70 eV; ion-source temperature of 280°C and transfer line temperature of 260°C. The oven temperature was programmed from 60°C, with an increase of 3 °C/min to 240°C. A mixture of linear hydrocarbons (C8H18–C20H42) was injected under the same experimental conditions. The identification of the constituents in the At-Hex was performed by comparing the spectra obtained with those of the equipment database (Wiley 7 lib and Nist 08 lib) and by using the Retention Index (RI), calculated for each constituent as previously described [11]. The data were acquired and processed on a PC with Shimadzu GC-MS Solution software (Shimadzu Corporation, Kyoto, Japan).
2.4 Evaluation of Cytotoxic Activity of Essential Oil and At-Hex
The cytotoxic study was conducted by the MTT method [12], which has the capacity to analyze the viability and the metabolic state of the cell. The oil was diluted in sterile DMSO and the substances were tested in the concentration of 5 µg/mL. The tumor cell lines used, HCT-116 (colon carcinoma), OVCAR-8 (ovarian carcinoma) and SF-295 (glioblastoma) were provided by the National Cancer Institute (USA), and were maintained in RPMI 1640, supplemented with 10% fetal bovine serum and 1% antibiotics, which were incubated at 37°C under 5% CO2. Cell lines were seeded at concentrations of 0.7 × 106 cels/mL (HCT-116), 0.1 × 106 cels/mL (OVCAR-8) e 0.1 × 105 cels/mL (SF-295).
The plates were incubated for 72 hours in an oven at 5% CO2 at 37°C. Then, they were centrifuged and the supernatant was removed. Then, 150 µL of the solution of MTT (tetrazolium salt) were added, and the plates were incubated for 3 h. The absorbance was read after dissolution of the precipitate with 150 µL of DMSO pure in a plate spectrophotometer at 595 nm. The experiments were analyzed according to the mean of the percentage of inhibition of cell growth using GraphPad Prism. The sample was tested in triplicate in two independent experiments. An intensity scale was used to assess the cytotoxic potential of the tested sample: No activity sample (NA), with low activity (LA, inhibiting cell growth varying from 1 to 50%), with moderate activity (MO, inhibition of cell growth ranging from 50 to 75%) and much activity (MA inhibition growth ranging 75 to 100%). At-Hex was tested against the HCT-116, SF-295 e HL60 cells in the same conditions describe previously. HL60 cells were prepared at 0.3 × 106 cells/mL concentration.
2.5 Evaluation of Antimicrobial Activity of Essential Oil
Antimicrobial activity tests were conducted against 11 strains of bacteria and fungi: Escherichia coli, Pseudomonas aeruginosa, Salmonella choleraesuis, Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus hirae, Candida albicans, Candida dubliniensis, Candida krusei, Candida glabrata e Candida tropicalis. The bacteria were preserved on nutrient agar and C. albicans yeast on sabouraud dextrose agar.
Preliminary tests of antimicrobial activity were conducted by bioauthography method [13]. A suspension of microorganisms was inoculated by pour plate technique on culture media, using 1:1000 (v/v) rate. A 0.5 mL aliquot of 1 mg/mL of triphenyltetrazolium chloride (TTC) was added to the inoculated media and then the media was poured into Petri plates, on thin layer chromatography (TLC) plates where oils were applied. After homogenization, the material was incubated at 37°C for 24 h for the bacterial strains. For C. albicans yeast was directly determined the minimum inhibitory concentration (MIC) of oil.
The MIC was determined in a solid medium in a Petri plate through the addition of the revelator 2,3,5-triphenyltetrazolium chloride. The concentration was determined in a solid medium in a Petri dish through the addition of the developer 2,3,5-triphenyltetrazolium chloride and different oil concentrations up to 2.0 mg/mL, to the media. The oil dilution was made in sterile water containing Tween 80. As positive standards Chloramphenicol was used for bacteria, and Nystatin, for yeast. The MIC was defined as the lowest concentration of oil able to prevent the appearance of red color, conferred on the cells by TTC when they have respiratory activity.
The analysis of EOAF by GC-MS identified a total of 24 compounds, representing 96.23% of the constituents of the sample. The oil was characterized by the presence of monoterpenes (60.84%), and sesquiterpenes (34.54%). Only one diterpene was identified in the sample, corresponding to 0.85% of the chemical composition. More than 60% of the oil consists of only five components: the monoterpenes linalool (25.70%), α-pinene (10.38%), β-pinene (9.12%), trans-ocimene (7.43%), and the sesquiterpene bicyclogermacrene (12.58%) (Tab. 1).
Table 1: Chemical composition of the essential oil extracted from atemoya fruits (EOAF) and of crude hexanic extract from aerial parts (At-Hex)
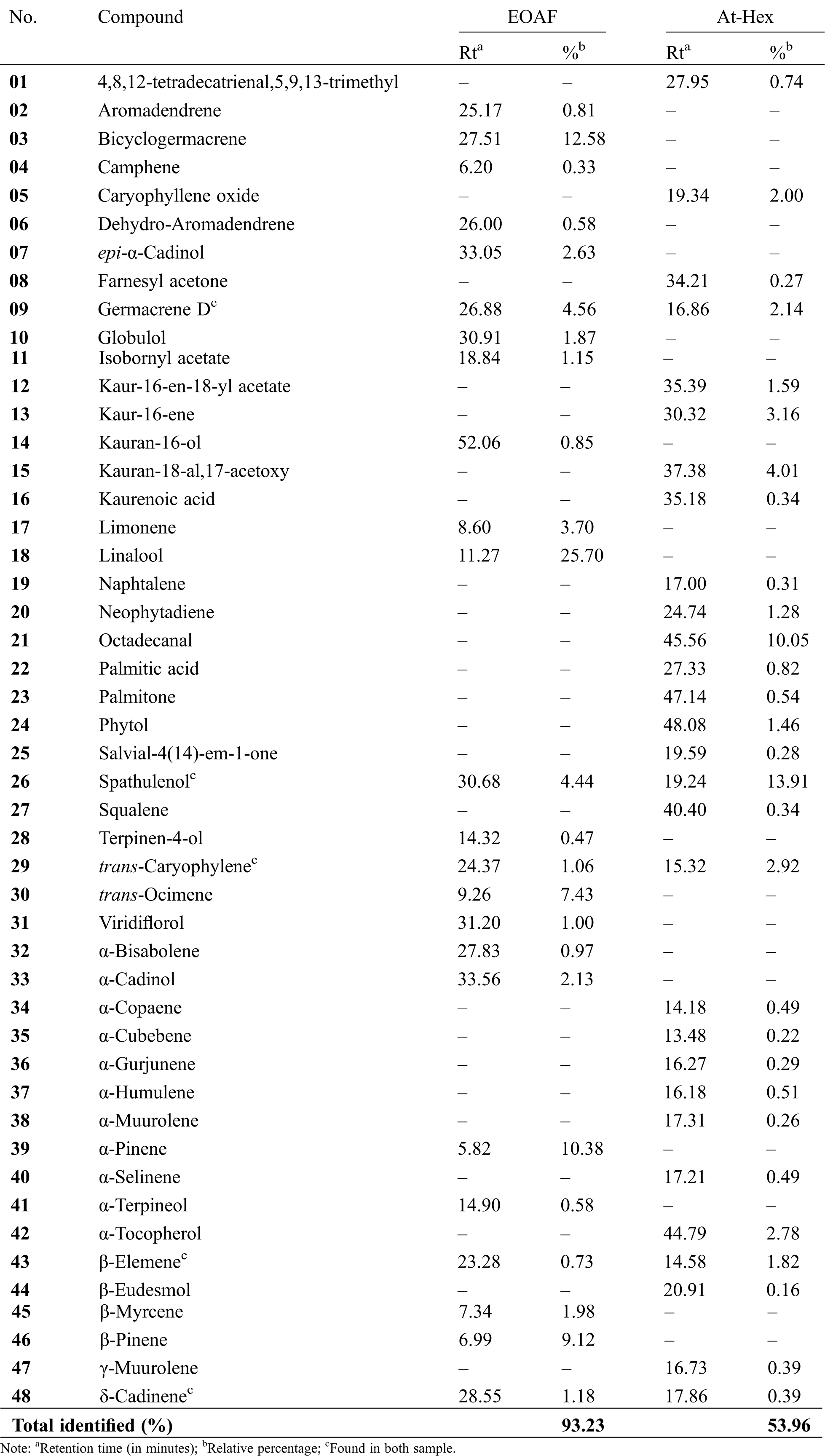
These same components were also identified in essential oils of leaves, flowers, and fruits of Annona cherimola [14] and oils from atemoya fruits from Australia and Havana [15,16]. The larger proportion of α-pinene and bicyclogermacrene has been observed in many essential oils of Annona species, indicating that they may be considered as chemotaxonomic markers of the genus [17].
Linalool, the major constituent of EOAF, is an open-chain tertiary monoterpene alcohol widely used in the perfumery and cosmetics industry [18]. It presents depressant effect on the central nervous system, resulting in hypnotic properties, hypothermic, and anticonvulsants [19,20], and also has antihelmintic efficacy [21]. The enantiomers of this substance when inhaled separately have opposite effects on the human cardiovascular system. While the (+)-linalool stimulates the cardiovascular system, (-)-linalool has a depressive effect [22]. This oil is common in essential oils extracted from the Annona genus, it has also been identified in A. muricata [16] and A. senegalensis [23].
Likewise, the presence of monoterpenes such as α-pinene, β-pinene, (E)-caryophyllene or sesquiterpenes as germacrene D and bicyclogermacrene are quite common in the oils from species of the genus Annona, with the occurrence of at least two of these constituents in A. muricata, A. senegalensis, A. ermaginata, A. squamosa, A. pickeli and A. salzmanii [24–26].
In addition, the chemical composition of At-Hex was also done analyzed employed hyphenated technique of GC-MS. This is often used in the identification of compounds that are suitably volatile, small and stable at high temperatures, by interpreting the fragmentations and comparing with data from a library [27]. Thus, this analysis made it possible to identify 29 compounds representing 54% of the volatile compounds of At-Hex. Among them, the majority spathulenol (13.91%), octadecanal (10.05%) and kauran-18-al-17-acetoxy (4.01%). All compounds identified are shown in Tab. 1 and are frequently found in species of the Annona genus as can be observed in species A. vepretorum [28], A. reticulata [4], A. squamosa [29] and A. leptopetala [30].
Spathulenol, present in most quantity in At-Hex, is a sesquiterpene alcohol which was considered to be the main responsible for antioxidant, anti-inflammatory, and antimycobacterial activities, as well as antiproliferative potential against ovarian (OVCAR-3) cell line shown by essential oil from Psidium guineense Sw., Myrtaceae [31]. This compound was also identified in EOAF, but in different quantity, just 4.44%. Moreover, spathulenol was the major constituent of essential oil extracted from immature atemoya fruits collected in Taiwan so as the α-cubebene, α-copaene, germacrene D, δ-cadinene, and caryophyllene oxide [32]. In this research, the results showed β-elemene, germacrene D, and δ-cadinene were identified in both samples analyzed here, EOAF and At-Hex.
In EOAF, cytotoxicity tests showed that cell growth inhibition percentage of the tumor cells varied from 2.47% to OVCAR-8 to 49.67% for HCT-116, and for SF-295, the inhibition percentage was 10.39% (Tab. 2).
Table 2: Cytotoxic activity of the essential oil of atemoya fruit (EOAF) and hexanic crude extract from aerial parts (At-Hex)a
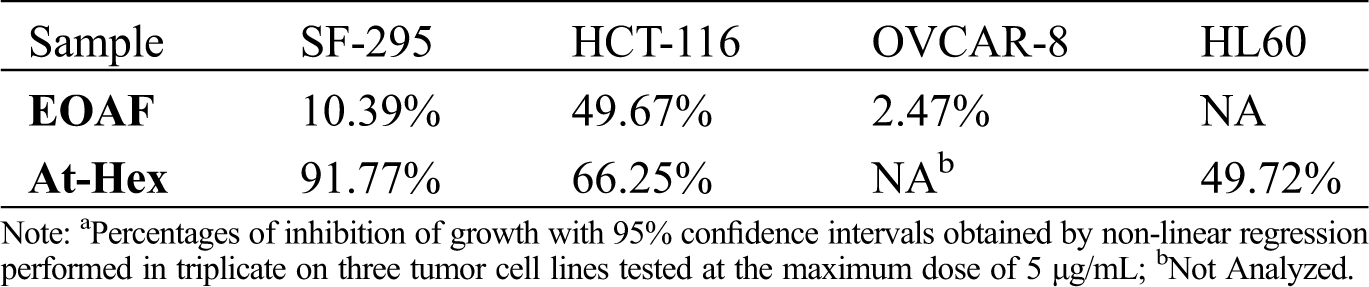
Studies with other Annonaceae species showed higher cytotoxic potential than the obtained in this study, such as fruit essential oil of Annona vepretorum, that demonstrate moderate activity against the HCT-116 cells, with inhibition percentage of 65.57%. On the other hand, cell growth inhibition was 13.15% and 30.32% against OVCAR-8 cells and NS-295, respectively [33].
Another work on cytotoxicity of essential oil and extracts from the leaves of Annona senegalensis found that the moderate cytotoxicity results would be related to the presence of caryophyllene oxide, the main constituent identified in fraction considered moderately cytotoxic (64.5% of the composition) [34]. The essential oil extracted from leaves of Annona muricata showed noticeable cytotoxic activity in vitro on MCF-7 cells (breast) with 99.2% cell death (100 µg/mL). Cytotoxicity data were assigned to the major constituents of essential oil: (E)-caryophyllene (38.9%), and eugenol (30.2%) [35].
Taking into consideration that the percentage of (E)-caryophyllene in the essential oil of fruits atemoya was 1.06%, and eugenol and caryophyllene oxide were not identified. The low cytotoxic activity observed may be related in part to the absence of a greater amount of these constituents, and the way of interaction of the chemical constituents present.
At-Hex was tested against HCT-116 (colon carcinoma), SF-295 (glioblastoma) and HL60 (leukemia) cells. The results, presented in Tab. 2, showed the antiproliferative effect of the extract against the lineage of SF-295, with a mean of 91.77% inhibition (Tab. 2). The At-Hex was not considered cytotoxic against the other tumor lines tested since the percentages of growth inhibition were below 75%.
The SF-295 cells correlate with human glioblastoma cell lines, which is the most common, aggressive and lethal type of brain tumor [36] with increasing estimates of mortality and new cases each year [8]. Recent studies have shown that less than 1% of patients with this type of cancer can survive 10 years or more [37] and that, despite advances in surgery and chemotherapy, patients have an average survival rate of only year [38]. Therefore, the search for less aggressive and more effective drugs against this disease is an important scientific contribution.
The high cytotoxic potential presented by At-Hex against SF-295 demonstrates that atemoya hybrid is a promising source of active molecules important in the treatment of cancer diseases. Among the constituents identified spathulenol (the major in At-Hex) is frequently associated with cytotoxic activity and is common in species of Annona [15–17]. Kaurane diterpenes (kaur-16-en-18-yl acetate, kaur-16-ene, kauran-18-al,17-acetoxy and kaurenoic acid) were found only in the hexanic extract, which showed better cytotoxic activity than the essential oil against SF-295 and HCT-116 cells. These diterpenes are found in some species of the genus Annona and show potent cytotoxic activity [39,40]. As some of these constituents here showed have not yet been tested, it is important that these molecules be isolated for further evaluation of the cytotoxic potential.
Antimicrobial activity analysis (Tab. 3), the oil showed minimum inhibitory concentration values (MIC) greater than 2 mg/mL against all the tested strains, indicating low activity.
Table 3: Antimicrobial activity of the essential oil of atemoya fruit (Annona cherimola Mill x Annona squamosa L.)
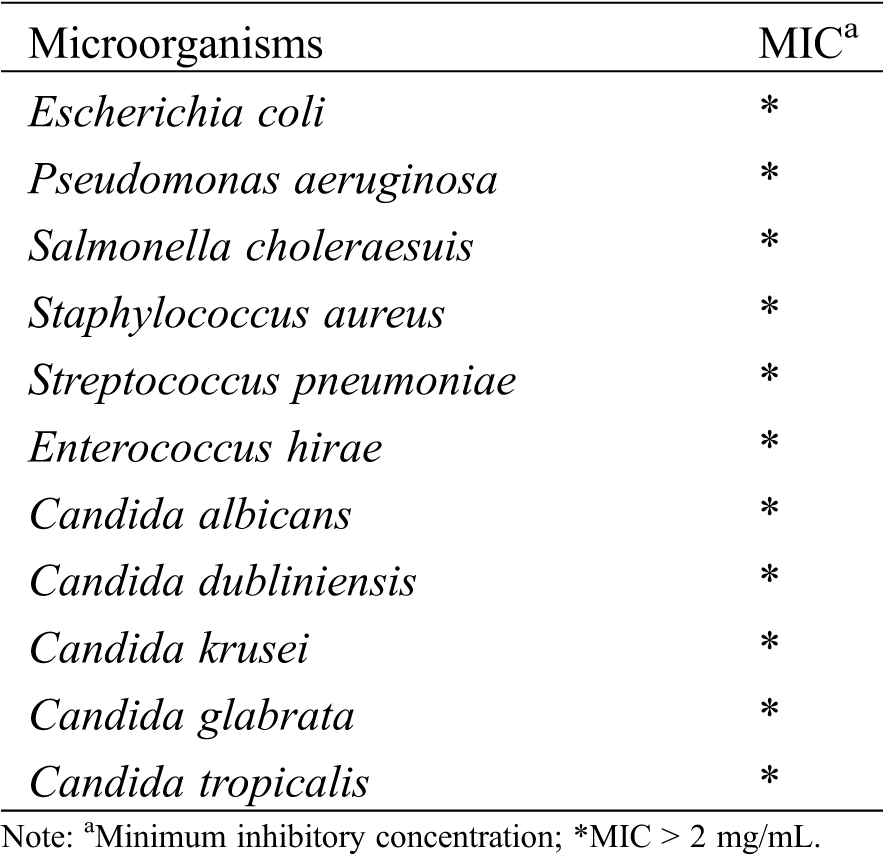
The antimicrobial activity of essential oils of other species and its individual constituents has been widely studied. There are studies indicating that oxygenated compounds, phenolic or alcoholic or ester groups tend to be more potentially active [41]. A study with the species Annona cherimola indicates that different parts exhibit significant and moderate antimicrobial activity. These activities are justified by the presence of linalool and trans-caryophyllene [19].
The EOAF presented 36.06% of oxygenated compounds of which 25.70% just of linalool. By that, it was expected a good antimicrobial activity, but the low observed activity from EOAF must be related to the way of interaction of its various constituents.
In summary, this work shown an interesting analysis of the chemical composition of essential oil from fruits (EOAF) and hexanic extract of aerial parts (At-Hex) of atemoya. Twenty-four compounds were identified in atemoya fruits, and defined the main components as linalool, α-pinene, β-pinene, trans-ocimene, and bicyclogermacrene. On the other hand, twenty-nine compounds were observed in At-Hex, to be spathulenol was major compound. Compounds as β-elemene, germacrene D, and δ-cadinene were recorded on both samples (EOAF and At-Hex). Notwithstanding essential oil revealed low cytotoxic activity against tumor lines tested, hexanic extract of aerial parts showed high cytotoxicity for gliobastoma cells. This work displayed that atemoya hybrid is a promising source of active compounds, that may act as anti-tumor agents.
Acknowledgement: The authors wish to express their thanks to Centro de Referência para Recuperação de Áreas Degradadas (CRAD) for botanical identification of the plant material.
Funding Statement: This work was supported by grants from Brazilian agency CNPq (Process 470594/2013-6).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Oliveira, M. C., Ferreira, G., Guimarães, V. F., Dias, G. B. (2010). Germinação de sementes de atemoia (Annona cherimola Mill. x A. squamosa L.) cv ‘Gefner’ submetidas a tratamentos com ácido giberélico (GA3) e ethephon. Revista Brasileira de Fruticultura, 32(2), 544–554. DOI 10.1590/S0100-29452010005000062. [Google Scholar] [CrossRef]
2. Chatrou, L. W., Rainer, H., Maas, P. J. M. (2004). Annonaceae (Soursop, Family). In: Smith, N., Mori, S. A., Henderson, A., Stevenson, D. W., Heald, S. V. (eds.Flowering plants of neotropics. pp. 18–20. New Jersey: Princeton University Press. [Google Scholar]
3. Lemos, E. E. P. (2014). The production of Annona fruits in Brazil. Revista Brasileira de Fruticultura, 36(spe1), 77–85. DOI 10.1590/S0100-29452014000500009. [Google Scholar] [CrossRef]
4. Rabêlo, S. V., Quintans, J. S. S., Costa, E. V., Almeida, J. R. G. S., Quintans-Júnior, L. J. (2016). Annona species (Annonaceae) oils. In: Preedy, V. R. (eds.Essential oils in food preservation, flavor and safety, pp. 221–228. London: Academic Press. [Google Scholar]
5. Doll, R., Peto, R. (1981). The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. Journal of the National Cancer Institute, 66(6), 1191–1308. DOI 10.1093/jnci/66.6.1192. [Google Scholar] [CrossRef]
6. Costa-Lotufo, L. V., Montenegro, R. C., Alves, A. P. N. N., Madeira, S. V. F., Pessoa, C. et al. (2010). A contribuição dos produtos naturais como fonte de novos fármacos anticâncer: Estudos no Laboratório Nacional de Oncologia Experimental da Universidade Federal do Ceará. Revista Virtual de Química, 2, 47–58. [Google Scholar]
7. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal of Clinicians, 68(6), 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
8. Iqbal, J., Abbasi, B. A., Mahmood, T., Kanwal, S., Ali, B. et al. (2017). Plant-derived anticancer agents: A green anticancer approach. Asian Pacific Journal of Tropical Biomedicine, 7(12), 1129–1150. DOI 10.1016/j.apjtb.2017.10.016. [Google Scholar] [CrossRef]
9. Guimarães, D. O., Momesso, L. S., Pupo, M. T. (2010). Antibiotics: Therapeutic importance and perspectives for the discovery and development of new agents. Química Nova, 33, 667–679. [Google Scholar]
10. Gobbo-Neto, L., Lopes, N. P. (2007). Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Química Nova, 30, 374–381. [Google Scholar]
11. Adams, R. P. (2001). Identification of essential oils components by gas chromatography/quadrupole mass spectroscopy. Illinois: Allured Publishing Corporation. [Google Scholar]
12. Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65(1–2), 55–63. DOI 10.1016/0022-1759(83)90303-4. [Google Scholar] [CrossRef]
13. Slusarenko, A. J., Longland, A. C., Whitehead, I. M. (1989). A convenient, sensitive and rapid assay for antibacterial activity of phytoalexins. Botanica Helvetica, 99, 203–207. [Google Scholar]
14. Ríos, M. Y., Castrejón, F., Robledo, N., León, I., Rojas, G. et al. (2003). Chemical composition and antimicrobial activity of the essential oils from Annona cherimola (Annonaceae). Journal of the Mexican Chemical Society, 47, 139–142. [Google Scholar]
15. Wyllie, S. G., Cook, D., Brophy, J., Richter, K. M. (1987). Volatile flavor components of Annona atemoya (custard apple). Journal of Agricultural and Food Chemistry, 35(5), 768–770. DOI 10.1021/jf00077a029. [Google Scholar] [CrossRef]
16. Pino, J. A., Rosado, A. (1999). Volatile constituents of custard apple (Annona atemoya). Journal of Essential Oil Research, 11(3), 303–305. DOI 10.1080/10412905.1999.9701139. [Google Scholar] [CrossRef]
17. Costa, E. V., Dutra, L. M., Salvador, M. J., Ribeiro, L. H., Gadelha, F. R. et al. (2013). Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceaeand their antitumour and trypanocidal activities. Natural Product Research, 27(11), 997–1001. DOI 10.1080/14786419.2012.686913. [Google Scholar] [CrossRef]
18. Lapczynski, A., Letizia, C. S., Api, A. M. (2008). Fragrance material review on d-linalool. Food and Chemical Toxicology, 46(11), S193–S194. DOI 10.1016/j.fct.2008.06.044. [Google Scholar] [CrossRef]
19. Elisabetsky, E., Brum, L. F. S., Souza, D. O. (1999). Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine, 6(2), 107–113. DOI 10.1016/S0944-7113(99)80044-0. [Google Scholar] [CrossRef]
20. Re, L., Barocci, S., Sonnino, S., Mencarelli, A., Vivani, C. et al. (2000). Linalool modifies the nicotinic receptor–ion channel kinetics at the mouse neuromuscular junction. Pharmacological Research, 42(2), 77–82. DOI 10.1006/phrs.2000.0671. [Google Scholar] [CrossRef]
21. Katiki, L., Evangelista, A., Canova, E., Piza, A., Fornazari, B. et al. (2014). Anthelmintic activity of anethole, carvone, carvacrol, thymol, linalool, limonene, eucalyptol, vanillin, cinnamaldehyde and eugenol in in vitro tests. Planta Medica, 80, 1415. [Google Scholar]
22. Santos, M. R. V., Moreira, F. V., Fraga, B. P., Souza, D. P., Bonjardim, L. R. et al. (2011). Cardiovascular effects of monoterpenes: A review. Revista Brasileira de Farmacognosia, 21(4), 764–771. DOI 10.1590/S0102-695X2011005000119. [Google Scholar] [CrossRef]
23. Jirovetz, L., Buchbauer, G., Ngassoum, M. B. (1998). Essential oil compounds of the Annona muricata fresh fruit pulp from Cameroon. Journal of Agricultural and Food Chemistry, 46(9), 3719–3720. DOI 10.1021/jf980204n. [Google Scholar] [CrossRef]
24. Ameen, O. M., Usman, L. A., Oganija, F. S., Hamid, A. A., Muhammed, N. O. et al. (2011). Chemical composition of leaf essential oil of Annona senegalensis Pers. (Annonaceae) growing in North Central Nigeria. International Journal of Biological and Chemical Sciences, 5(1), 375–379. DOI 10.4314/ijbcs.v5i1.68117. [Google Scholar] [CrossRef]
25. Campos, F. G., Baron, D., Marques, M. O., Ferreira, G., Boaro, C. S. F. (2014). Characterization of the chemical composition of the essential oils from Annona emarginata (Schltdl.) H. Rainer ‘terra-fria’ and Annona squamosa L. Revista Brasileira de Fruticultura, 36, 202–208. DOI 10.1590/S0100-29452014000500024. [Google Scholar] [CrossRef]
26. Costa, E. V., Dutra, L. M., Nogueira, P. C., Moraes, V. R., Salvador, M. J. et al. (2012). Essential oil from the leaves of Annona vepretorum: Chemical composition and bioactivity. Natural Product Communications, 7, 265–266. [Google Scholar]
27. Patel, K. N., Patel, J. K., Patel, M. P., Rajput, G. C., Patel, H. A. (2010). Review: Introduction to hyphenated techniques and their applications in pharmacy. Pharmaceutical Methods, 2(1), 2–13. DOI 10.4103/2229-4708.72222. [Google Scholar] [CrossRef]
28. Bomfim, L. M., Menezes, L. R. A., Rodrigues, A. C. B. C., Dias, R. B., Gurgel-Rocha, C. A. et al. (2015). Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic & Clinical Pharmacology & Toxicology, 118(3), 208–213. DOI 10.1111/bcpt.12488. [Google Scholar] [CrossRef]
29. Quílez, A. M., Fernández-Arche, M. A., García-Giménez, M. D., De la Puerta, R. (2018). Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. Journal of Ethnopharmacology, 225(2), 244–270. DOI 10.1016/j.jep.2018.06.014. [Google Scholar] [CrossRef]
30. Brito, M. T., Ferreira, R. C., Beltrão, D. M., Moura, A. P. G., Xavier, A. L. et al. (2018). Antitumor activity and toxicity of volatile oil from the leaves of Annona leptopetala. Brazilian Journal of Pharmacognosy, 28(5), 602–609. DOI 10.1016/j.bjp.2018.06.009. [Google Scholar] [CrossRef]
31. Do Nascimento, K. F., Moreira, F. M. F., Santos, J. A., Kassuya, C. A. L., Croda, J. H. R. et al. (2018). Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. Journal of Ethnopharmacology, 210(2), 351–358. DOI 10.1016/j.jep.2017.08.030. [Google Scholar] [CrossRef]
32. Liu, T. T., Chao, L. K., Peng, C. W., Yang, T. S. (2016). Effects of processing methods on composition and functionality of volatile components isolated from immature fruits of atemoya. Food Chemistry, 202(2), 176–183. DOI 10.1016/j.foodchem.2016.01.111. [Google Scholar] [CrossRef]
33. Araújo, C. S. (2013). Estudo fitoquímico e atividade biológica in vitro de Annona vepretorum Mart. (Annonaceae) (Dissertation). Federal University of San Franciso Valey, Brazil. [Google Scholar]
34. Ahmed, A. L., Bassem, S. E. M., Mohamed, Y. H., Gamila, M. W. (2010). Cytotoxic essential oil from Annona sengalensis Pers. leaves. Pharmacognosy Research, 2(4), 211–214. DOI 10.4103/0974-8490.69105. [Google Scholar] [CrossRef]
35. Owolabi, M. S., Ogundajo, A. L., Dosoky, N. S., Setzer, W. N. (2013). The cytotoxic activity of Annona muricata leaf oil from Badagary. Nigeria American Journal of Essential Oils and Natural Products, 1, 1–3. [Google Scholar]
36. Basso, J., Miranda, A., Sousa, J., Pais, A., Vitorino, C. (2018). Repurposing drugs for glioblastoma: From bench to bedside. Cancer Letters, 428(4), 173–183. DOI 10.1016/j.canlet.2018.04.039. [Google Scholar] [CrossRef]
37. Tykocki, T., Eltayeb, M. (2018). Ten-year survival in glioblastoma. A systematic review. Journal of Clinical Neuroscience, 54(Suppl 5), 7–13. DOI 10.1016/j.jocn.2018.05.002. [Google Scholar] [CrossRef]
38. Nunes, F., Silva, L. B., Winter, E., Silva, A. H., Melo, L. J. et al. (2018). Tacrine derivatives stimulate glioma SF-295 cell death and alter important proteins related to disease development: An old drug for new targets. Biochimica et Biophysica Acta (BBA)-General Subjects, 1862(7), 1527–1536. DOI 10.1016/j.bbagen.2018.04.019. [Google Scholar] [CrossRef]
39. Dutra, L. M., Bomfim, L. M., Rocha, S. L. A., Nepel, A., Soares, M. B. P. et al. (2014). ent-Kaurane diterpenes from the stem bark of Annona vepretorum (Annonaceae) and cytotoxic evaluation. Bioorganic & Medicinal Chemistry Letters, 24(15), 3315–3320. DOI 10.1016/j.bmcl.2014.06.005. [Google Scholar] [CrossRef]
40. Joy, B., Remani, P. (2008). Antitumor constituents from Annona squamosa fruit pericarp. Medicinal Chemistry Research, 17(2–7), 345–355. DOI 10.1007/s00044-007-9070-3. [Google Scholar] [CrossRef]
41. Henriques, A. T., Simões-Pires, C. A., Apel, M. A. (2009). Óleos essenciais: Importância e perspectivas terapêuticas. In: Yunes, R. A., Cechinel Filho, V. (eds.Química de Produtos Naturais, novos fármacos e a moderna farmacognosia, pp. 216–256. Itajaí: Universidade do Vale do Itajaí. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |