
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.012726
ARTICLE
Genome-wide Analysis of a Plant AT-rich Sequence and Zinc-binding Protein (PLATZ) in Triticum Aestivum
1Engineering Research Center of Ecology and Agricultural Use of Wetland, Ministry of Education, Hubei Collaborative Innovation Center for Grain Industry, College of Agriculture, Yangtze University, Jingzhou, 434025, China
2Shanxi Key Laboratory of Integrated Pest Management in Agriculture, Institute of Plant Protection, Taiyuan, 030031, China
3State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, 100193, China
*Corresponding Authors: Dongfang Ma. Email: madf@yangtzeu.edu.cn; Yilin Zhou. Email: ylzhou@ippcaas.cn
Received: 10 July 2020; Accepted: 29 December 2020
Abstract: Plant AT-rich sequence and zinc-binding protein (PLATZ) is a plant transcription factor that has been studied in corn. PLATZ can non-specifically bind to sequences rich in A/T bases to induce transcriptional repression. It is involved in the regulation of dehydration tolerance in seeds. In this study, we performed bioinformatics analysis to identify and characterize wheat PLATZ(TaPLATZ)genes. We identified 49 wheat PLATZ genes by searching the wheat genome by using known PLATZ gene sequences from rice, Arabidopsis, and maize. Phylogenetic analysis on PLATZ gene sequences from different species was performed. We found that PLATZs could be divided into three groups. The chromosome (chr) distribution analysis revealed that the 49 identified wheat PLATZ genes are distributed in 15 chrs. Gene structure and motif analyses indicated that most PLATZ genes possess conserved exon/intron arrangements and motif compositions. Our analysis of transcriptional data indicated that several wheat PLATZ genes may play an important role in abiotic stress resistance given that they are expressed under salt stress. The results of qRT-PCR further confirmed that TaPLATZ is involved in plant abiotic stress and is also related to the cell differentiation of plant tissues. Our results lay the foundation for further studies on the function of the wheat PLATZ gene family.
Keywords: Transcription factor; PLATZ; abiotic stress; gene expression
Several families of transcription factors (TFs) exist. TFs specifically bind to cis-acting elements in the promoter region to regulate the expression of multiple downstream genes and act as key regulators of transcriptional expression in biological processes [1,2]. A total of 84 putative TF families and other transcriptional regulators (TRs) have been identified from 19 plant species with genomes that have been completely sequenced and annotated [3]. TFs are proteins that bind to cis-elements in their target promoters in a sequence-specific manner, whereas TRs exert their regulatory function through protein–protein interactions or chromatin remodeling. TFs play an important role in plant-specific processes, including secondary metabolism and response to phytohormones, as well as in the development of the specific characteristics of different cell types. In Arabidopsis thaliana, TF gene families are interspersed throughout the genome, and genes related to TFs account for approximately 50% of the total number of major TF families [4]. Additionally, 45% of TFs in corn are plant specific [5]. The MYB, bHLH, and bZIP gene families are large TF families present in different plant species [6–8]. TF-IIIA was first discovered by Miller et al. in Xenopus laevis. It has since been identified in plants, such as Arabidopsis, petunia, soybean, rice, cotton, and wheat [9,10]. Zinc finger proteins are well-researched nucleic acid-binding proteins that are widely distributed in diverse eukaryote species where they play important regulatory roles [11]. They can be classified as C2H2, C4, C6, C4HC3, C3HC4, C2HC, C3H, and combination types in accordance with the numbers and positions of their cysteine and histidine residues [12]. Zinc finger proteins are involved in cell differentiation, proliferation, apoptosis, and other important life processes. Moreover, some zinc finger proteins can regulate plant-stress tolerance [13]. Zinc finger proteins can play numerous roles in various plant tissues at different developmental stages under diverse abiotic stresses [10].
The zinc finger protein TaZNF can drastically improve the ability of transgenic plants to excrete Na+ [13]. In Arabidopsis, the stress-associated zinc finger protein gene AtSAP5 is mainly expressed in the roots and is induced by high salt, drought, and low temperatures. The overexpression of AtSAP5 in Arabidopsis promotes the expression of other drought stress-related genes and enhances the drought tolerance of transgenic plants under normal growth conditions or drought stress [14]. The rice zinc finger protein genes OsZFP and OsZF19 may participate in drought stress given that their expression is strongly induced under drought [15,16]. Jain et al. found that Arabidopsis transfected with the rice zinc finger protein gene OsTOP6A1 exhibited increased salt tolerance and enhanced expression levels of numerous stress response genes after exposure to stress [17]. The wheat zinc finger protein gene TaCHP is mainly expressed in the roots of seedlings in the three-leaf stage. The overexpression of TaCHP can enhance the salt tolerance of wheat and Arabidopsis and increase the expression of various stress genes, such as AtCBF3, AtDREB2 A, AtABI2, and AtABI1, under salt stress [18]. TaCHP overexpression increases NaCl tolerance in the presence of exogenous 16-carbon DAG and 16-carbon PA. The K ion content and K+/Na+ ratio of TaDSU-overexpressing Arabidopsis considerably increased under stress conditions. The absolute salt ion content of plants affects salt tolerance. In contrast to salt-sensitive plants, salt-tolerant plants can reduce the toxic effects of ions by limiting the variation in ionic content under salt stress [19].
PLATZs represent a new class of plant-specific, zinc-dependent DNA-binding TFs that contain a conserved PLATZ domain [20]. They are novel zinc finger proteins that individually possesses a finger-like domain with two zinc finger domains. PLATZ1 is the first reported PLATZ and was isolated from pea. PLATZ binds nonspecifically to A/T-rich sequences and affects transcription inhibition [9]. Arabidopsis has 12 PLATZ members. In Arabidopsis, PLATZ5 is expressed in various tissues under salt stress and localizes in the nucleus and cytoplasm. The transcriptional activity of PLATZ5 in Arabidopsis may be similar to that of PLATZ1 in peas [21]. The maize PLATZ protein is involved in the regulation of the RNA PIII-mediated transcription of small noncoding RNAs in different tissues, including endosperm in maize [20,22]. The expression of Chinese narcissus NtPLATZ1 gene could be induced by the treatment of paclobutrazol. The results of semi-quantitative interpretation RT-PCR showed that the expression of NtPLATZ1 gene could be detected in leaves of Chinese narcissus from long leaf stage to blooming [23]. Transgenic tobacco growth is inhibited by NtPLATZ1 overexpression and promoted by the inhibition of NtPLATZ1expression [24].
Wheat is an important food crop in the world, accounting for more than half of the total human consumption [25,26]. It has wide adaptability and highly resistant to storage degradation and damage. It is one of the most important food crops in China. Thus, the development of the wheat industry in China is directly related to national food security and social stability [27]. Wheat is affected by various organisms (such as wheat stripe rust) and abiotic stresses such as drought, salinity and high temperature during its growth and development [28–30]. Therefore, improving the stress resistance of wheat is crucial for ensuring high and stable wheat yields. Zinc finger TFs play an important role in plant stress signal transduction and abiotic stress responses. Although the function of several TFs has been understood, that of PLATZ remains unclear. The completion of the genome-wide sequencing of wheat has enabled the size identification and functional analysis of the wheat PLATZ gene. In the present study, we systematically identified and structurally characterized the PLATZ gene from the wheat reference genome. We also described the expression pattern exhibited by the PLATZ gene under salt stress.
2.1 Identification of PLATZ in the Wheat Genome
A computer-based method was used to identify members of the PLATZ family from the wheat (IWGSC v1.0, https://wheat-urgi.versailles.inra.fr/Seq-Repository/Annotations). We first collected information on previously reported PLATZ family members from model plants, including Arabidopsis (TAIR database1, http://www.arabidopsis.org/index.jsp), rice (http://rice.plantbiology.msu.edu/index.shtml) [31], and maize (https://www.maizegdb.org/) [1,32]. We queried the wheat genome [33] by using BLASTp (E < 10−5) and known PLATZ sequences from model plants as seed sequences. We used a profiled Hidden Markov Model (HMM) implemented with default parameters in HMMER 3.0 to search for TaPLATZ proteins with the PLATZ domain in the Pfam (v32.0, http://pfam.xfam.org/) database. Finally, we determined by Pfam whether an obtained sequence contained a PLATZ-specific structural con-served domain and finally determined the number of PLATZ gene family members.
2.2 Phylogenetic Analysis of Wheat TaPLATZs
Phylogenetic relationships were inferred through the Neighbor-Joining (NJ) method with 1000 replicated-bootstraps in MEGA7.0 software [34]. A midpoint rooted base tree was produced using the Interactive Tree of Life (iTOL, version 3.2.317, http://itol.embl.de). The scale bar used corresponded to 0.1 amino acid substitutions.
2.3 Feature Analysis of TaPLATZ Proteins
PLATZ protein sequences were characterized by using the protein identification and analysis tools available on the ExPASy Server10 (https://prosite.expasy.org/). The characteristics of protein length, molecular weight (MW), isoelectric point (pI), stability, and grand average of hydropathicity (GRAVY) were predicted [35].
2.4 Protein Motif and Gene Structure Analysis of TaPLATZs
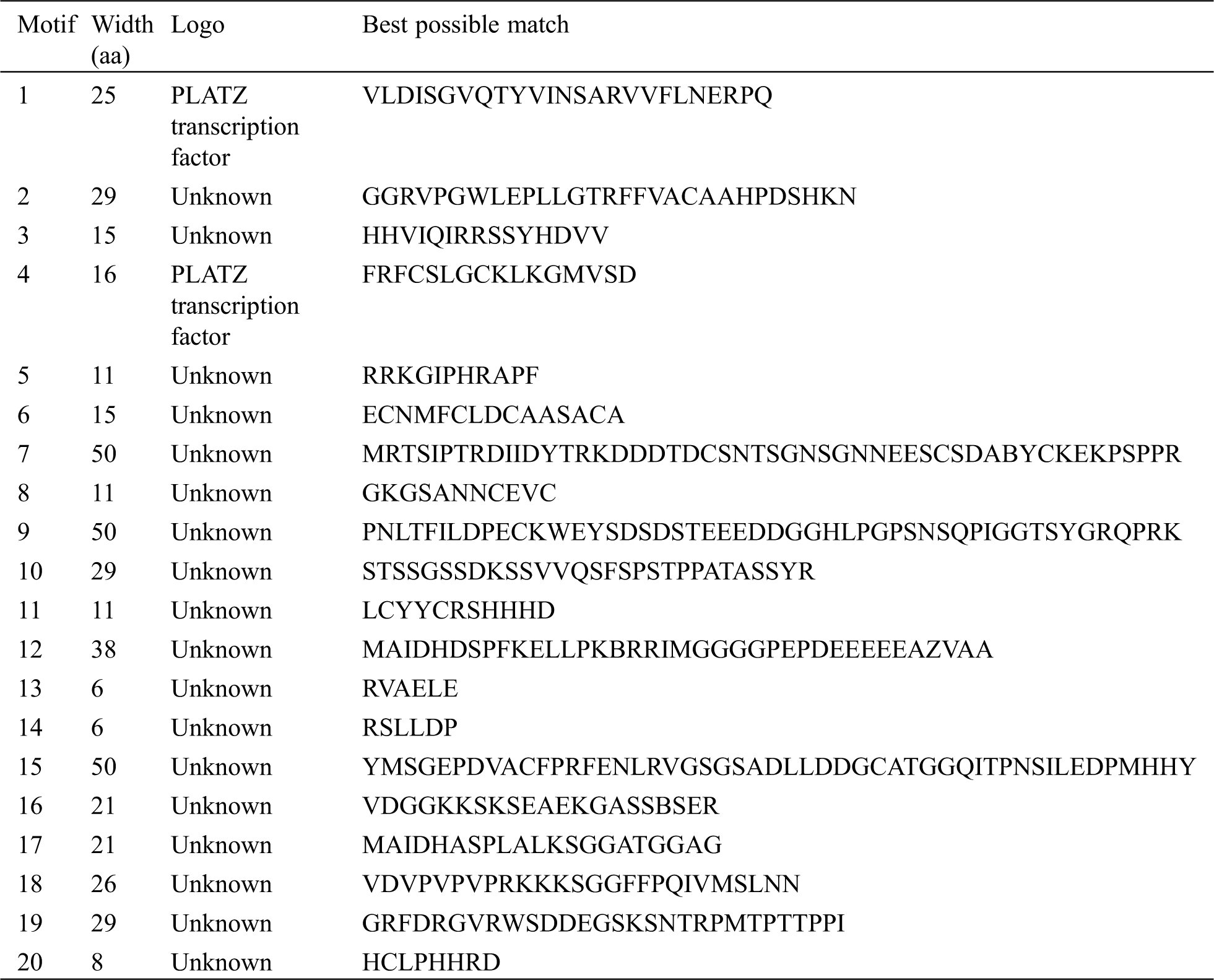
The protein sequences of 49 PLATZs were scanned for conserved motifs by using the MEME suite analysis tool version 4.9.1 (http://meme-suite.org/index.html) [36] and SMART motif search tool. The known PLATZs protein sequence was used as control sequences. Then the control sequences were applied to identify the conserved TaPLATZ motifs by the following criteria: each sequence may contain any number of non-overlapping occurrences of each motif, the number of different motifs as 20, and a range of motif widths from 6 to 50 aa. These motif patterns were drawn by TBtools software. According to GFF3 of TaPLATZs, using GSDS 2.0 (http://gsds.cbi.pku.edu.cn/index.php) to draw the genetic structures [37].
2.5 Chromosomal Localization of Gene Sequences
The wheat genome GFF3 gene annotation file was obtained from the wheat database IWGSC v1.0 and gene annotation of wheat TaPLATZs were extracted from the GFF3 file. MapInspect software was used to draw a physical map on the basis of the start and end location information of the PLATZs in corresponding chromosomes (chrs) [37].
2.6 Multiconditional Transcriptome Analysis of TaPLATZs
Original multiple RNA-seq data original from multiple conditional transcriptome analyses were download from National Center for Biotechnology Information (NCBI) Short Read Archive (SRA) database and mapped to wheat reference genome by hisat2. Then genes were assembled by Cufflinks to inspect the expression levels of TaPLATZs (normalized by TPM, transcripts per kilobase of exon model per million mapped reads). The R package “pheatmap” was used to generate the heatmap of TaPLATZs.
2.7 Growth and Stress Treatment of Wheat Seedlings
This study used hexaploid common wheat “Yangmai 20” as the experimental material. The selected standard seeds were surface sterilized with 1% hydrogen peroxide, rinsed thoroughly with distilled water, and germinate with water saturation at 25°C for 2 days in Petri dishes on three-layer of filter paper. The seedlings were then transferred and cultured in a quarter-strength Hoagland nutrient solution with continuous ventilation and increased to half-strength after 3 days [38]. When the wheat grows to one leaf and one heart, the plants are subsequently treated with 150 mM sodium chloride (NaCl) and 20% polyethylene glycol (PEG). PEG is used to simulate drought treatment. Use 1 M KOH or 0.2 M H2SO4 to adjust the pH of the nutrient solution to 6.0 every 2 days. Conditions during treatment are 25/20°C temperature and 16 h/8 h (day/night) photoperiod. Three biological replicates were set for each treatment. Collect the leaves and roots of wheat seedlings at 2, 6, 12, 24, 48 and 72 h after treatment. For IAA (indole-3-acetic acid) treatment, wheat seedlings were cultured in a 1/2 concentration Hoaglan nutrient solution containing 100 μM/L IAA. Collect the leaves and roots of wheat seedlings at 2, 6, 12 and 24 h after treatment. For 6-BA (6-benzylaminopurine) treatment, wheat seedlings were cultured in a 1/2 concentration Hoaglan nutrient solution containing 5 mg/L 6-BA. Collect the leaves and roots of wheat seedlings at 2, 6, 12, 24, 48 and 72 h after treatment. Each treatment included three biological replications. After sampling, the samples were quickly frozen in liquid nitrogen and stored at –80°C [39].
2.8 Quantitative Real-Time PCR and Data Analysis
In order to further clarify the functional specificity of PLATZ, quantitative real-time polymerase chain reaction (qRT-PCR) was performed to detect the expression level of TaPLATZ genes. TRizol reagent (Invi-trogen, USA) is used for the extraction of total RNA from the sample, and then the 5X all-in-one reverse transcription master mix kit (perfect real-time) is used to reverse transcribe the total RNA into cDNAs for quantitative polymerase chain reaction analysis. Primer 5.0 software was used to design gene-specific primers. The primers were used for PCR, the amplified products were detected by gel electrophoresis, and the desired bands were selected. For qRT-PCR experiments, the reaction system contains 10 μL of 2 × SYBR Green Mix, 0.4 μL of each primer (10 μM), 2 μL of template (about 400 ng/μL) and 7.2 μL of ddH2O to make the total volume reach 20 μL. The protocol was carried out as follows: Pre-denaturation at 95°C for 3 min (Step 1), denaturation at 95°C for 5 s (Step 2), primer annealing/extension and collection of fluorescence signal at 58°C for 30 s (Step 3). The next 40 cycles started at Step 2. Three technical replicates were used for each sample. ADP-ribosylation factor Ta2291 (forward: GCTCTCCAACAACATTGCCAAC, reverse: GCTTCTGCCTGTCACATACGC) was stably expressed under various conditions and was used as an internal reference gene for qRT-PCR analysis. Relative expression level was calculated by 2-ΔΔCt method [39,40].
3.1 Identification of PLATZ in the Wheat Genome
We acquired 48 PLATZ gene sequences from model plants to identify members of the PLATZ gene family in wheat. Specifically, we collected 12, 21, and 15 PLATZ sequences from Arabidopsis, maize, and rice, respectively [24]. We used these sequences and BLASTp (standard E < 10−5) to search the wheat genome database. We screened 51 candidate wheat amino acid sequences of TaPLATZs to ensure the reliability of the protein sequences. The obtained sequences were further screened by using Pfam. Finally, we obtained 49 wheat PLATZ (TaPLATZ) amino acid sequences.
3.2 Phylogenetic Analysis of PLATZ
To better understand the evolutionary history and evolutionary relationships of the PLATZ gene family in wheat, a phylogenetic tree was generated using the Neighbor-Joining (NJ) method with the full-length amino acid sequences (Fig. 1). The 97 identified PLATZ genes were divided into Subfamilies I, II and III. Subfamilies I, II and III contained 15, 13 and 21TaPLATZs, respectively. Moreover, we also found that the dicot PLATZs (Arabidopsis) have more closely phylogenetic relationship related to monocot PLATZs (wheat, rice and maize) in each clade with all plants. Members of the same family often have similar functions, so grouping in phylogenetic trees may reflect differences in gene function during evolution [41].
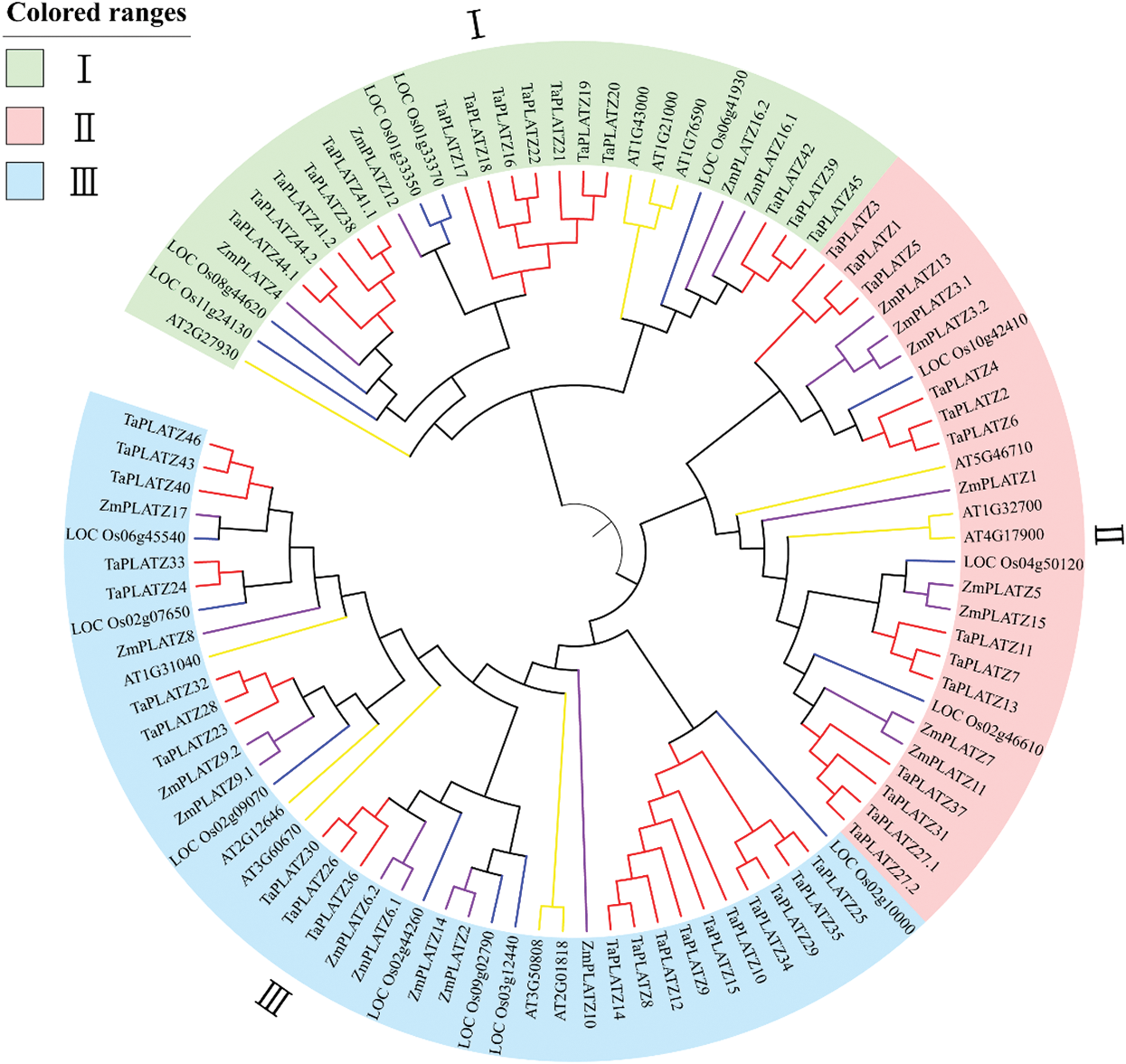
Figure 1: Phylogenetic analysis of the predicted amino acid sequences of TaPLATZs and PLATZ genes of other plant species. Predicted amino acid sequences were aligned by using the ClustalW2 sequence alignment program, and the phylogenetic tree was constructed with MEGA7.0 software through the bootstrap NJ tree method (1,000 replicates). Different colors indicate different groups, and PLATZs from wheat, rice, maize, and Arabidopsis are distinguished by lines with different colors: red-wheat, purple-corn, yellow-Arabidopsis and blue-rice
3.3 Protein Features and Domain Analysis of TaPLATZs
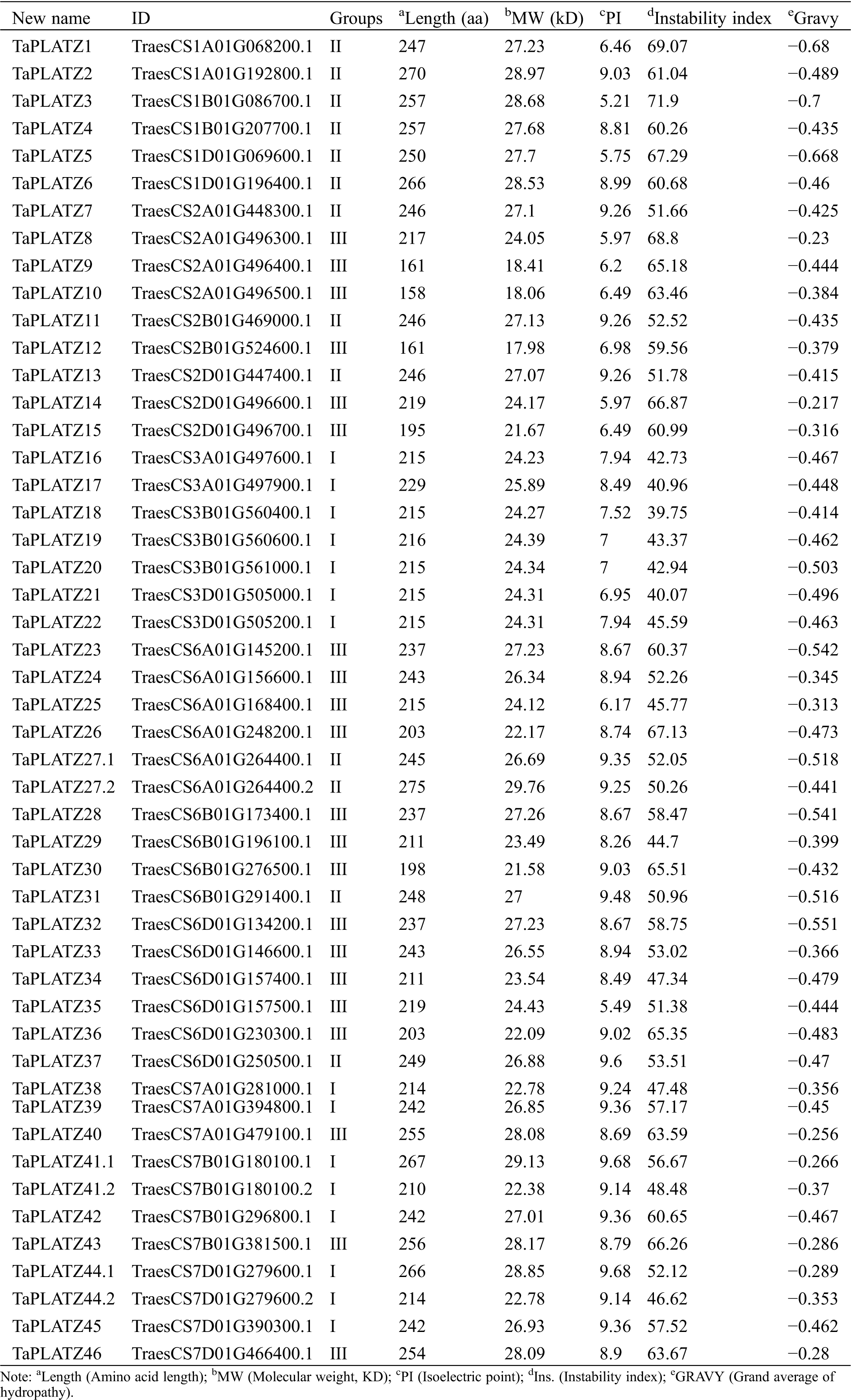
We used Pfam to confirm the existence of conserved sequence domains in PLATZ sequences. Analysis revealed that PLATZ proteins have an average isoelectric point of 8.19 (5.21–9.68). Protein feature analysis showed that the TaPLATZs have an average length of 229.63 aa (158–275 aa), an average molecular weight of 25.38 kD (17.98–29.76 kD) (Tab. 1), and average instability index of 55.58. Only TaPLATZ18 was identified as a stable protein.
Table 1: Predicted sequence features of PLATZ proteins
3.4 Analyses of TaPLATZ Motifs and Gene Structures
We analyzed the structure of wheat PLATZ genes by using the annotated gff3 file. We found that wheat PLATZ genes contain 1–4 introns (Fig. 2). These introns all contain exons. Moreover, the genes have different structures. Seven gene contains 1 introns, 20 genes contain 2 introns, 20 genes contain 3 introns, and 2 genes contain 4 introns. These data indicate that there are intron acquisition and deletion in PLATZ gene. TaPLATZ1, TaPLATZ2, TaPLATZ3, TaPLATZ5, TaPLATZ6, TaPLATZ41.1, and TaPLATZ44.1 contain only one intron. These genes may have diverged early in evolution and lost introns in the process. We applied MEME software InterPro Scan 5 to detect and annotate the structural diversity and predicted function of TaPLATZs. We detected 20 conserved motifs in TaPLATZs. Among them, motif 1 and motif 4 were identified as PLATZ transcription factors, and the others showed unknown. The sequences, positions and markers of the conserved motifs of each sub-family of TaPLATZ are shown in Supplementary Tab. S1. Proteins with relatively close evolutionary relationships are similar in motif composition mode, which indicates that the functions of PLATZ proteins in the same branch are similar [38,40]. The results show that all of the identified wheat PLATZ proteins contain a motif 1and motif 5 domain. While motif 19 only exist in Group I, motif 12 and motif 17 only exist in Group II, motif 9, motif 15 and motif 18 only exist in Group III, the existence of these special motifs may cause differences in PLATZ gene function to some extent. The phylogenetic analysis results and conserved motifs in wheat PLATZ proteins are depicted in Fig. 2.
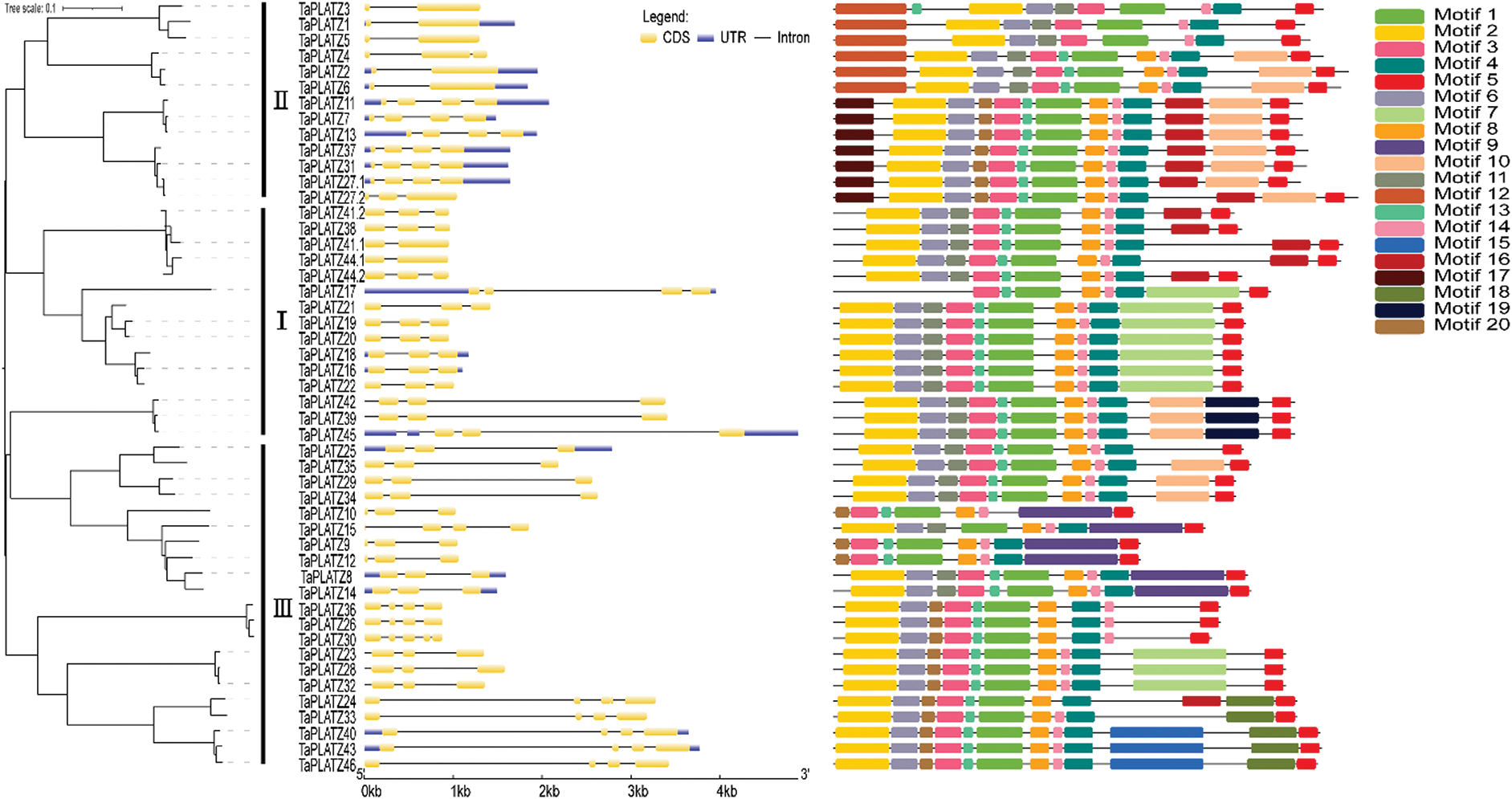
Figure 2: The tree was created with 1,000 bootstraps through the NJ method in MEGA7.0. The composition of motifs in TaPLATZ amino acid sequences was modeled and illustrated by using MAST. Exon-intron structure analyses were conducted using the GSDS. Lengths of exons and introns were displayed proportionally. The untranslated regions (UTRs) were indicated by blue boxes, the exons were indicated by yellow boxes, and the introns were indicated by black lines
3.5 Chromosomal Location and Gene Duplication Events Analysis of TaPLATZ
We applied Mapinspect software to construct chromosomal maps. We found that 49 TaPLATZ genes are unevenly distributed on 15 chromosomes, and chromosomes 6A and 6D contain the largest number of TaPLATZ genes. Chromosomes containing four TaPLATZ genes are 2A, 6B, 7B and 7D; and chromosomes containing two TaPLATZ genes are 1A, 2B, 1D, 2B, 3A and 3D. However, there is no gene distribution on chromosomes 4A, 5A, 4B, 5B, 4D and 5D (Fig. 3). We found gene clusters on 9 chromosomes (2A, 3A, 6A, 3B, 7B, 2D, 3D, 6D and 7D). There are 20 tandem repeat genes: TaPLATZ8/TaPLATZ9/TaPLATZ, TaPLATZ16/TaPLATZ17, TaPLATZ27.1/TaPLATZ27.2, TaPLATZ18/TaPLATZ19/TaPLATZ20, TaPLATZ41.1/TaPLATZ41.2, TaPLATZ14/TaPLATZ15, TaPLATZ21/TaPLATZ22, TaPLATZ34/TaPLATZ35, TaPLATZ44.1/TaPLATZ44.2. In addition, we also discovered the polyploidization of chromosomes. Therefore, we speculate that tandem duplication and chromosome polyploidization may be important ways of TaPLATZ genes amplification [42].
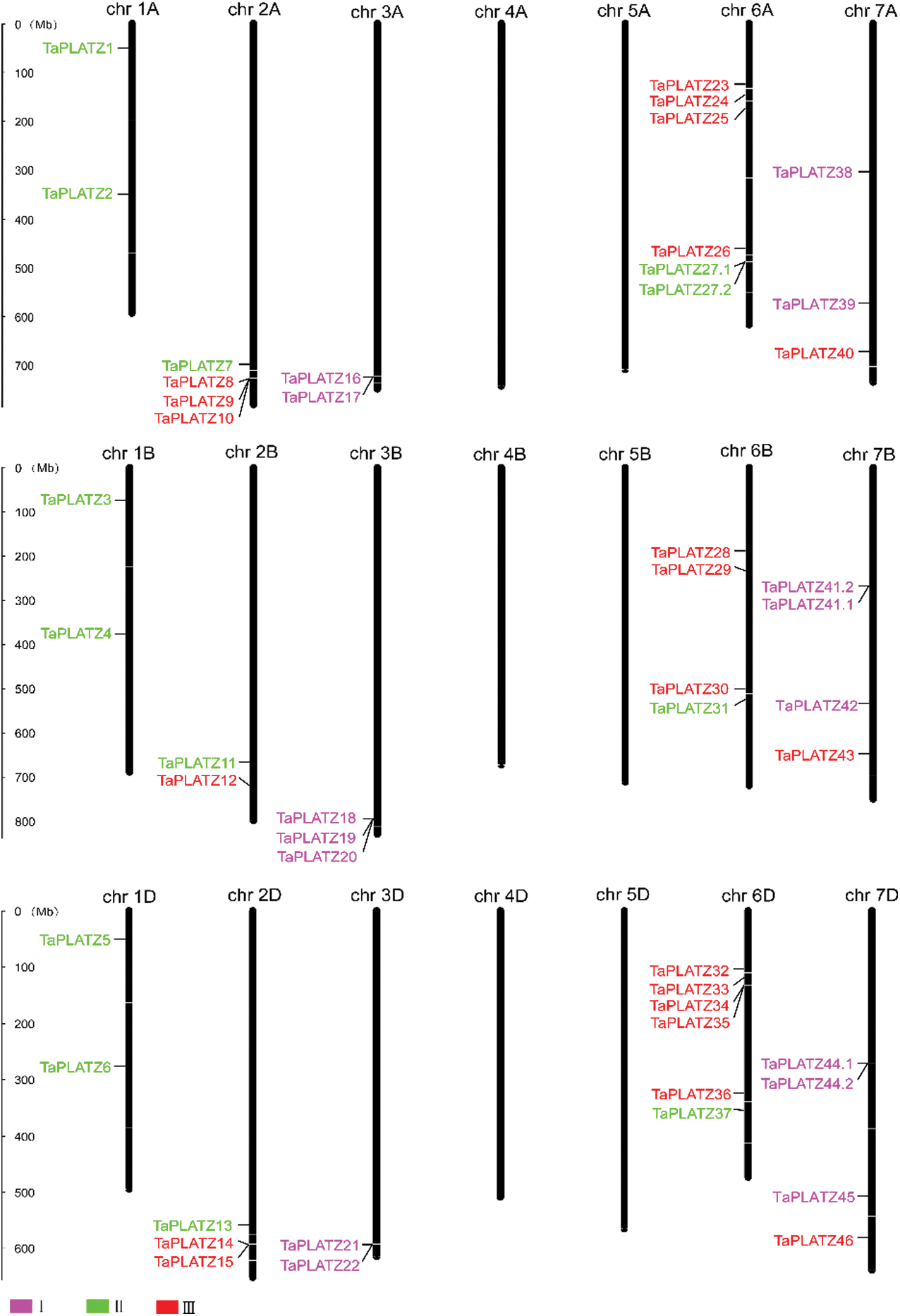
Figure 3: TaPLATZ distributions on wheat chromosomes. The chromosome name is indicated at the top of each bar. The rules on the left indicates the physical map distance among genes (Mbp). Rose, green, red colors represent for Groups I, II and III, respectively
3.6 Multiple Conditional Transcriptome Analysis of TaPLATZs
We downloaded original RNA sequence data from multiple conditional transcriptome analyses from NCBI. We then mapped these sequences to the wheat reference genome by applying hisat2. Then, we assembled these data by using cufflinks to inspect the expression levels of TaPLATZs. Expression levels were normalized on the basis of the fragments per kilobase of exon model per million mapped reads. We used the R package “pheatmap” to draw the Heat map of wheat TaPLATZ genes (Fig. 4). Different genes showed various responses under diverse conditions. For example, wheat TaPLATZ genes, TaPLATZ2, TaPLATZ6, TaPLATZ27.1, TaPLATZ31 and TaPLATZ37 were significantly up-regulated under Na treatment. In addition, TaPLATZ7, TaPLATZ11, TaPLATZ13, TaPLATZ39, TaPLATZ42 and TaPLATZ45 were up-regulated under salt treatment. However, more than half of the genes in this study are not expressed under abiotic stress, and these genes may play a role in biotic stress or other growth and development processes [43].
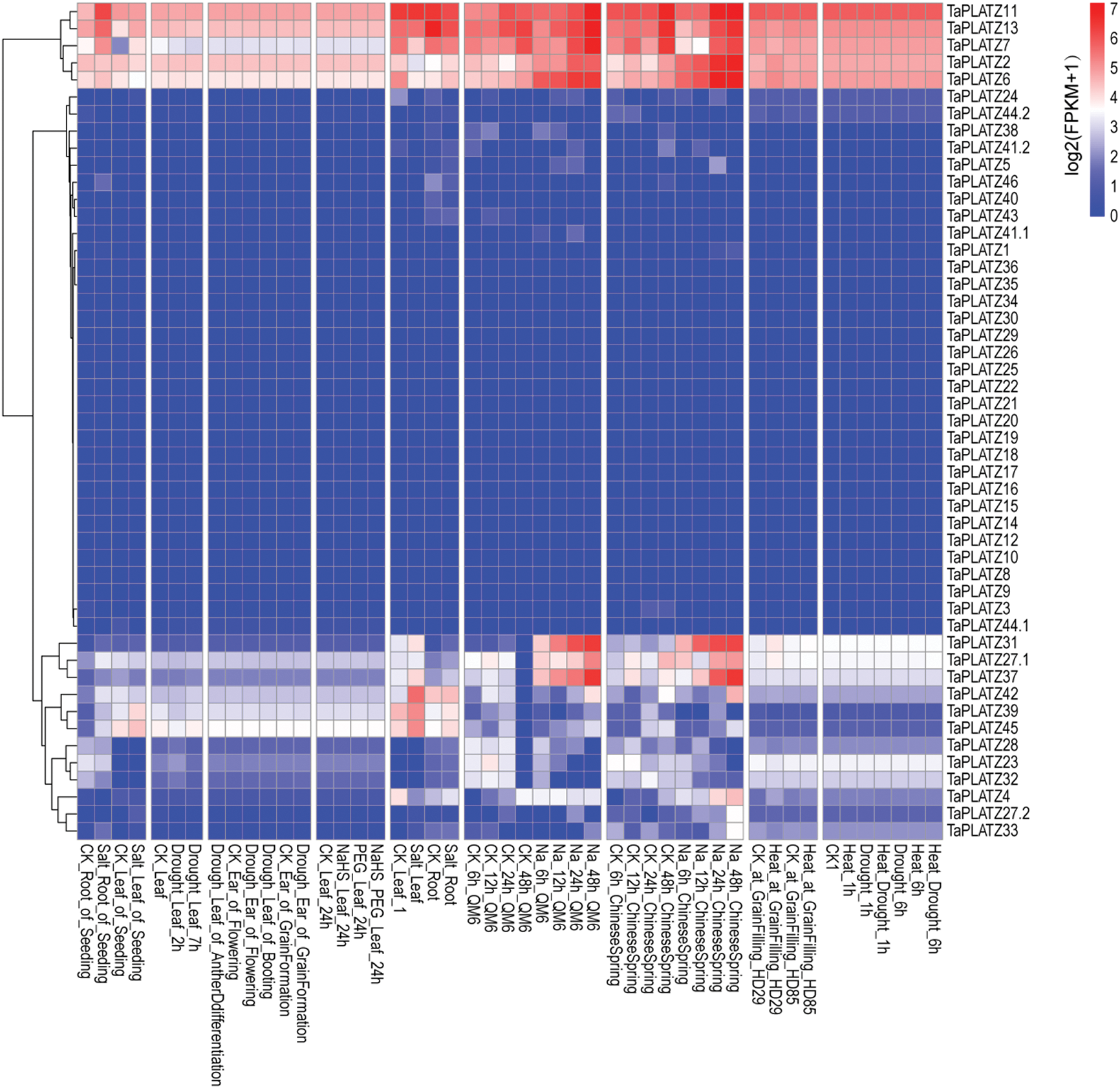
Figure 4: Transcriptome analyses of 49 TaPLATZs under different abiotic stress, including salt stress, Na stress, PEG stress, heat stress, drought stress, heat and drought stress (SRA numbers: PRJNA422010, PRJNA293629, PRJNA378325, PRJNA257938, PRJNA391522 and PRJNA257938). Red color indicates increased expression levels; blue color indicates decreased expression levels
3.7 Quantitative-Real Time PCR Analysis (qRT-PCR)
To further reveal the potential functions of TaPLATZ under abiotic stress (NaCl, PEG, IAA and 6-BA), we used qRT-PCR to explore their expression patterns in different tissues of wheat. According to transcriptome analysis, 6 TaPLATZs with high expression levels (TaPLATZ6, TaPLATZ7, TaPLATZ11, TaPLATZ13, TaPLATZ27.1, TaPLATZ31) were selected. The results showed that the expression levels of the six genes had no significant difference after IAA application in roots and leaves at different stages (Fig. 5). Under NaCl treatment, compared with the control, 5 TaPLATZs (TaPLATZ6, TaPLATZ7, TaPLATZ13, TaPLATZ27.1, TaPLATZ31) were highly expressed at 48 h. In the root, TaPLATZ6 at 48 h, TaPLATZ7 at 12 h, and TaPLATZ31 at 2 h and 72 h showed up-regulation. However, TaPLATZ6, TaPLATZ7, TaPLATZ11, TaPLATZ13, and TaPLATZ27.1 were down-regulated at 72 h. Under PEG treatment, in leaves, only TaPLATZ7 was highly expressed at 6 h and TaPLATZ27.1 at 2 h; in roots, all 6 TaPLATZs were up-regulated at 12 h. Under the 6-BA treatment, in the leaves, the expression levels of TaPLATZ6 at 2 h, TaPLATZ7 at 12 h and TaPLATZ13 at 48h increased. In addition, the expression levels of TaPLATZ27.1 and TaPLATZ31 were low before 72 h. However, in the roots, the expression levels of the 6 TaPLATZs were up-regulated at 12 h and 48 h.
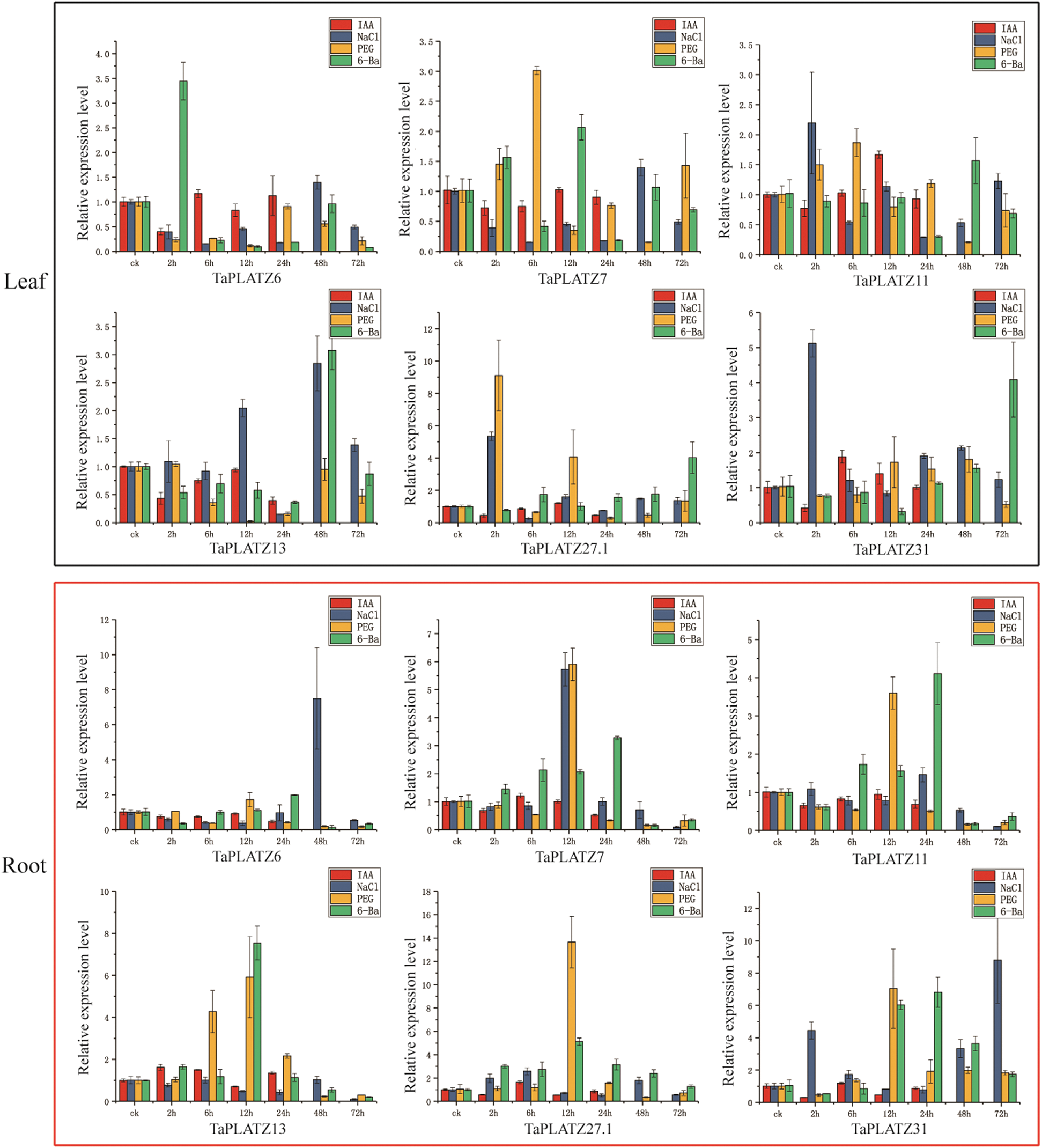
Figure 5: The qPCR analyses of 6 TaPLATZ genes under NaCl, PEG, IAA and 6-BA treatments. The qRT-PCR analysis of the TaPLATZ genes in the leaves and roots of plants treated with NaCl, PEG, IAA and 6-BA during the specified time period shown on the x-axis, the expression level is on the y-axis. The black square represents the leaf tissue, and the red square represents the root tissue. The standard deviation is shown with error bars. The expression levels of TaPLATZ genes were plotted using Origin software
PLATZ protein is a novel class of plant specific zinc dependent DNA binding protein, a transcription factor (TFs), which plays an important role in gene expression and regulation [10,22]. We comprehensively analyzed the PLATZ gene family in wheat by identifying the complex functions and characteristics of PLATZ genes from model plants. Phylogenetic tree shows that all groups have formed clade. This clustering pattern indicates that these genes are evolutionarily conserved. The phylogenetic tree also shows coevolutionary relationships between species, and the relationships among wheat, rice, and maize are closer than those among wheat, rice, maize, and Arabidopsis. Therefore, we speculated that the common ancestors of PLATZ may have evolved independently during the evolution of plants from monocots, such as wheat, rice, and maize, to dicots (Arabidopsis) [40].
Our research found that all TaPLATZs contain a PLATZ domain. Through analysis of gene structure and protein motifs, we found that TaPLATZ gene has intron deletion and acquisition. Studies have shown that the evolution of introns is affected by genetic mutation and selection, and the loss of introns determines evolution rather than gain of them. Genes contain more introns in the early stages of amplification, and will lose introns after differentiation [44]. Generally speaking, the number of introns in the same subfamily is specific, and the difference in the number of introns between different genes may be caused by insertion and deletion events, and this difference may have a driving effect on evolution. At the same time, this may also be an important reason for the diversity of gene structure and functional complexity. In addition, introns have positive or negative regulatory functions on gene expression. Therefore, the conservative and variant gene structure may affect the function of TaPLATZs and its functional diversity [45,46]. The existence of special motifs (For example: motif 9, motif 12, motif 15, motif 17, motif 18, motif 19) may also cause differences in the function of TaPLATZ genes.
Gene amplification is one of the most important driving forces in the evolution of the genome. Genes can be amplified in many ways, including genome-wide duplication, tandem duplication, fragment duplication, and so on. Among them, genome-wide duplication and tandem duplication have an important influence on the evolution of the genome and even the evolution of biological species [47]. Gene duplication events are considered to be frequent events in the evolution of organisms, and are also a manifestation of biological evolution. Homologous genes are more adaptable to environmental changes than single-copy genes [48,49]. Through chromosome mapping, we found some tandem duplications and chromosome polyploidization, which may be an important way for TaPLATZ genes family amplification. Studies have shown that tandem duplication tends to amplify genes related to biotic and abiotic stress. For example, Thellungiella parvula can withstand harsh environments, such as cold, drought, and salinity. This is because there are genes in the T. parvula genome that can resist extreme environments, and the cause of this gene is the existence of tandem duplication [50]. Therefore, we speculate that PLATZ gene is related to abiotic stress related genes.
Transcriptome analysis showed that TaPLATZ gene is related to abiotic stress. The results of qRT-PCR showed that 6 TaPLATZs (TaPLATZ6, TaPLATZ7, TaPLATZ11, TaPLATZ13, TaPLATZ27.1, TaPLATZ31) were up-regulated at different times under NaCl and PEG stress. Especially after PEG treatment of roots for 12 h, these 6 TaPLATZs showed high expression. We speculate that TaPLATZ genes participate in the abiotic stress response of plants and plays an important role in coping with environmental stress. Previous studies reported that PLATZ1 is related to the cell differentiation of plant tissues [9]. It is worth noting that under the 6-BA stress treatment, the 6 TaPLATZs in the leaves were up-regulated after 48 h of the stress treatment. In the roots, the stress treatment increased for 12 h and 24 h respectively, and then showed a downward trend. Therefore, TaPLATZ genes may also be involved in the cell differentiation of plant tissues.
PLATZ gene has been reported in Arabidopsis thaliana and maize [20,21]. Previously identified PLATZ sequences are important for identifying the PLATZ sequences of a particular species. Our systematic analysis identified 49 TaPLATZ genes in the wheat genome. Our initial result was further supported by phylogenetic, gene structural, and conserved protein motif analyses. By comparing the expression profiles of TaPLATZ genes in wheat, we found that some TaPLATZ genes may promote plant resistance to salt stress. At the same time, the qRT-PCR verification of some TaPLATZ genes showed that these genes respond significantly to abiotic stress and hormones. This also shows that the TaPLATZ genes plays an important role in the growth and development of plants. Our data provide new insights into the control of TaPLATZ genes expression on the transcriptional level and new clues for the further functional identification of PLATZ genes and the genetic improvement of wheat.
Acknowledgement: We are very grateful to Dr. Luo Yong for the language polishing of this manuscript. (Luo Yong, Department of Plant Pathology, China Agricultural University, Beijing, 100193, China).
Author Contributions: Junliang Yin and Dongfang Ma guided the design of the experiment. Junliang Yin and Zhengwu Fang directed the data analysis. He Xiaohang conducted experiments, data analysis, and manuscript writing. Minjie Liu and Yilin Zhou participated in the experiment and contributed to the manuscript writing. Dongfang Ma supervised the experiment and confirmed the manuscript. Xiaohang He is the guarantor of this work, so she can have full access to all the data in the research and is responsible for the integrity of the data and the accuracy of the data analysis. Thank all the above staff for the help of this study. The authors thank the reviewers for their valuable suggestions during the revision of the early manuscripts.
Funding Statement: This work was supported by the “National Key R&D Program of China (2018YFD0200500)”, “Open Project Program of Engineering Research Center of Ecology and Agricultural Use of Wetland, Ministry of Education (KF201802)” and “Open Project Program of Shanxi Key Laboratory of Integrated Pest Management in Agriculture, Institute of Plant Protection (YHSW2018002)”.
Conflicts of Interest: The authors declare no conflict of interest.
1. Yamasaki, K., Kigawa, T., Seki, M., Shinozaki, K., Yokoyama, S. (2013). DNA-binding domains of plant-specific transcription factors: Structure, function, and evolution. Trends in Plant Science, 18(5), 267–276. DOI 10.1016/j.tplants.2012.09.001. [Google Scholar] [CrossRef]
2. Fan, Z. Q., Yan, J. F., Lu, W. J., Chen, J. Y. (2015). Progress in the regulation of fruit ripening and senescence by transcription factors. Journal of Horticulture, 42, 1649–1663, DOI 10.16420/j.issn.0513-353x.2015-0356. [Google Scholar]
3. Pérez-Rodríguez, P., Riaño-Pachón, D. M., Corrêa, L. G., Rensing, S. A., Kersten, B. et al. (2010). PlnTFDB: Updated content and new features of the plant transcription factor database. Nucleic Acids Research, 38(suppl_1), D822–D827. DOI 10.1093/nar/gkp805. [Google Scholar] [CrossRef]
4. Riechmann, J. L., Heard, J., Martin, G., Reuber, L., Jiang, C. et al. (2000). Arabidopsis transcription factors: Genome-Wide comparative analysis among eukaryotes. Science, 290(5499), 2105–2110. DOI 10.1126/science.290.5499.2105. [Google Scholar] [CrossRef]
5. Jiang, Y., Zeng, B., Zhao, H. N., Zhang, M., Xie, S. J. et al. (2012). Genome-wide transcription factor gene prediction and their expressional tissue-specificities in maize. Journal of Integrative Plant Biology, 54(09), 616–630. DOI 10.1111/j.1744-7909.2012.01149.x. [Google Scholar] [CrossRef]
6. Nijhawan, A., Jain, M., Tyagi, A. K., Khurana, J. P. (2008). Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiology, 146(2), 333–350. DOI 10.1104/pp.107.112821. [Google Scholar] [CrossRef]
7. Carretero-Paulet, L., Galstyan, A., Roig-Villanova, I., Martínez-García, J. F., Bilbao-Castro, J. R. et al. (2010). Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, Poplar, Rice, Moss, and Algae. Plant Physiology, 153(3), 1398–1412. DOI 10.1104/pp.110.153593. [Google Scholar] [CrossRef]
8. Katiyar, A., Smita, S., Lenka, S., Rajwanshi, R., Chinnusamy, V. et al. (2012). Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genomics, 13(1), 544. DOI 10.1186/1471-2164-13-544. [Google Scholar] [CrossRef]
9. Yukio, N., Hirofumi, F., Takehito, I., Yukiko, S. (2001). A novel class of plant-specific zinc-dependent DNA-binding protein that binds to A/T-rich DNA sequences. Nucleic Acids Research, 29(20), 4097–4105. DOI 10.1093/nar/29.20.4097. [Google Scholar] [CrossRef]
10. Wang, W. Y., Li, H. M., Dai, Y. M., Lin, J. B. (2016). Progress in the function of plant zinc finger proteins. Chinese Horticultural Digest, 32(7), 3–5. [Google Scholar]
11. Song, B., Hong, Y., Wang, P. W., Wang, H., Fu, Y. P. et al. (2010). Research progress of plant C2H2 zinc finger protein. Genomics and Applied Biology, 29, 1133–1141. DOI 10.3969/gab.029.001133. [Google Scholar] [CrossRef]
12. Zhang, J., Liu, J. F., Zhao, T. T., Ren, J., Xu, X. Y. (2018). Research progress of plant C2H2 zinc finger protein. Molecular Plant Breeding, 29(6), 1133–1141. DOI 10.3969/gab.029.001133. [Google Scholar] [CrossRef]
13. Ma, X., Liang, W., Gu, P., Huang, Z. (2016). Salt tolerance function of the novel C2H2-type zinc finger protein TaZNF in wheat. Plant Physiology and Biochemistry, 106, 129–140. DOI 10.1016/j.plaphy.2016.04.033. [Google Scholar] [CrossRef]
14. Kang, M., Fokar, M., Abdelmageed, H., Allen, R. D. (2011). Arabidopsis SAP5 functions as a positive regulator of stress responses and exhibits E3 ubiquitin ligase activity. Plant Molecular Biology, 75(4–5), 451–466. DOI 10.1007/s11103-011-9748-2. [Google Scholar] [CrossRef]
15. Huang, Y. M., Hu, H. H., Wu, C. Q. (2006). Isolation and identification of a stress inducible zinc finger gene in rice. Journal of Huazhong Agricultural University, 25, 581–585. DOI 10.3321/j.issn:1000-2421.2006.06.001. [Google Scholar] [CrossRef]
16. Islam, M. S., Hur, J. H., Wang, M. H. (2009). The influence of abiotic stresses on expression of zinc finger protein gene in rice. Russian Journal of Plant Physiology, 56(5), 695–701. DOI 10.1134/S1021443709050161. [Google Scholar] [CrossRef]
17. Jain, M., Tyagi, A. K., Khurana, J. P. (2008). Constitutive expression of a meiotic recombination protein gene homolog, OsTOP6A1, from rice confers abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Reports, 27(4), 767–778. DOI 10.1007/s00299-007-0491-8. [Google Scholar] [CrossRef]
18. Tian, L. M., Huang, C. L., Zhang, X. H., Zhang, L. S., Wu, Z. Y. (2005). Advances in research on zinc finger proteins in stress-related plants. Biotechnology Bulletin, 6, 12–16. [Google Scholar]
19. Zhang, H. J. (2014). Functional study of wheat stress response genes TaCHP and TaDSU. Shandong University, China. DOI 10.7666/d.Y2594373. [Google Scholar] [CrossRef]
20. Wang, J. C., Ji, C., Li, Q., Zhou, Y., Wu, Y. R. (2018). Genome-wide analysis of the plant-specific PLATZ proteins in maize and identification of their general role in interaction with RNA polymerase III complex. BMC Plant Biology, 18(1), D822. DOI 10.1186/s12870-018-1443-x. [Google Scholar] [CrossRef]
21. Yang, R. (2017). Functional study of Arabidopsis thaliana zinc finger transcription factor PLATZ5 in salt stress response. Shandong Agricultural University, China. [Google Scholar]
22. Ye, J., Zheng, X., Xiang, X., Li, C., Fu, M. et al. (2017). The maize imprinted gene Floury3 encodes a PLATZ protein required for tRNAs and 5S rRNA transcription through interaction with RNA polymerase III. Plant Cell, 29(10), 2661–2675. DOI 10.1105/tpc.17.00576. [Google Scholar] [CrossRef]
23. Lin, J. B., Wang, W. Y., Li, H. M., Wu, J. S., Zou, H. et al. (2016). Cloning and expression analysis of zinc finger protein NtPLATZ1 from Chinese narcissus. Journal of Northwest A&F University (Natural Science Edition), 44(10), 165–170. DOI 10.13207/j.cnki.jnwafu.2016.10.023. [Google Scholar] [CrossRef]
24. Wang, W. Y., Li, H. M., Dai, Y. M., Lin, J. B. (2017). Construction of expression vectors of NtPLATZ1 positive and antisense plants in Chinese narcissus and transformation of tobacco. Journal of Horticulture, 44(12), 2399–2407. [Google Scholar]
25. Yin, J. L., Fang, Z. W., Sun, C., Zhang, P., Zhang, X. et al. (2018). Rapid identification of a stripe rust resistant gene in a space-induced wheat mutant using specific locus amplified fragment (SLAF) sequencing. Scientific Reports, 8(1), 157. DOI 10.1038/s41598-018-21489-5. [Google Scholar] [CrossRef]
26. Sun, C., Zhang, P., Fang, Z., Zhang, X., Yin, J. et al. (2018). Genetic analysis and molecular mapping of stripe rust resistance in an excellent wheat line sanshumai1. Journal of Plant Pathology, 101, 235–241. DOI 10.1007/s42161-018-0166-z. [Google Scholar] [CrossRef]
27. Wang, Y. Q., Cheng, L. F. (2011). Restricting factors and countermeasures of wheat industry development. Barley and Cereal Science, (4), 80–81. DOI 10.3969/j.issn.1673-6486.2011.04.028. [Google Scholar] [CrossRef]
28. Sun, C., Liu, Y. K., Chao, K. X., Fang, Z. W., Wang, S. P. et al. (2019). Characterization and molecular mapping of stripe rust resistance in wheat-psathyrostachys huashanica introgression line h9015-17-1-9-6. Canadian Journal of Plant Pathology, 41(1), 65–75. DOI 10.1080/07060661.2018.1523230. [Google Scholar] [CrossRef]
29. Ma, D. F., Fang, Z. W., Yin, J. L., Chao, K. X., Jing, J. X. et al. (2016). Molecular mapping of stripe rust resistance gene YrHu derived from Psathyrostachys huashanica. Molecular Breeding, 36(6), 80. DOI 10.1007/s11032-016-0487-6. [Google Scholar] [CrossRef]
30. Ma, D. M., Li, Q., Tang, M. S., Chao, K. X., Li, J. C. et al. (2015). Mapping of gene conferring adult-plant resistance to stripe rust in Chinese wheat landrace Baidatou. Molecular Breeding, 35(8), 385. DOI 10.1007/s11032-015-0244-2. [Google Scholar] [CrossRef]
31. Maser, P., Thomine, S., Schroeder, J. I., Ward, J. M., Hirschi, K. et al. (2001). Phylogenetic relationships within cation transporter families of arabidopsis. Plant Physiology, 126(4), 1646–1667. DOI 10.1104/pp.126.4.1646. [Google Scholar] [CrossRef]
32. Andorf, C. M., Cannon, E. K., Portwood, J. L., Gardiner, J. M., Harper, L. C. et al. (2016). MaizeGDB update: New tools, data and interface for the maize model organism database. Nucleic Acids Research, 44(D1), D1195–D1201. DOI 10.1093/nar/gkv1007. [Google Scholar] [CrossRef]
33. Clavijo, B. J., Venturini, L., Schudoma, C., Accinelli, G. G., Kaithakottil, G. et al. (2017). An improved assembly and annotation of the allohexaploid wheat genome identifies complete families of agronomic genes and provides genomic evidence for chromosomal translocations. Genome Research, 27(5), 885–896. DOI 10.1101/gr.217117.116. [Google Scholar] [CrossRef]
34. Kumar, S., Stecher, G., Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. DOI 10.1093/molbev/msw054. [Google Scholar] [CrossRef]
35. Li, R., An, J. P., You, C. X., Shu, J., Wang, X. F. et al. (2018). Identification and expression of the CEP gene family in apple (Malus × domestica). Journal of Integrative Agriculture, 17(2), 348–358. DOI 10.1016/S2095-3119(17)61653-8. [Google Scholar] [CrossRef]
36. Zhang, X. W., Yi, D. X., Shao, L. H., Li, C. (2017). In silico genome-wide identification, phylogeny and expression analysis of the R2R3-MYB gene family in Medicago truncatula. Journal of Integrative Agriculture, 16(7), 1576–1591. DOI 10.1016/S2095-3119(16)61521-6. [Google Scholar] [CrossRef]
37. Jiang, W. Q., Yang, L., He, Y. Q., Zhang, H. T., Li, W. et al. (2019). Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). PeerJ, 7(2), e8062. DOI 10.7717/peerj.8062. [Google Scholar] [CrossRef]
38. Jiang, W. Q., Geng, Y. P., Liu, Y. K., Chen, S. H., Cao, S. L. et al. (2020). Genome-wide identification and characterization of SRO gene family in wheat: Molecular evolution and expression profiles during different stresses. Plant Physiology and Biochemistry, 154(7), 590–611. DOI 10.1016/j.plaphy.2020.07.006. [Google Scholar] [CrossRef]
39. He, Y. Q., Huang, W. D., Yang, L., Li, Y. T., Lu, C. et al. (2020). Genome-wide analysis of ethylene-insensitive3 (EIN3/EIL) in Triticum aestivum. Crop Science, 60(4), 2019–2037. DOI 10.1002/csc2.20115. [Google Scholar] [CrossRef]
40. Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25(4), 402–408. DOI 10.1006/meth.2001.1262. [Google Scholar] [CrossRef]
41. Jiang, W. Q., Yin, J. L., Zhang, H. T., He, Y. Q., Shuai, S. M. et al. (2020). Genome-wide identification, characterization analysis and expression profiling of auxin-responsive GH3 family genes in wheat (triticum aestivum l.). Molecular Biology Reports, 47(1), 3885–3907, DOI 10.1007/s11033-020-05477-5. [Google Scholar]
42. Zhou, X. Y., Zhu, X. G., Shao, W. N., Song, J. H., Jiang, W. Q. et al. (2020). Genome-wide mining of wheat DUF966 gene family provides new insights into salt stress responses. Frontiers in Plant Science, 11, 569838. DOI 10.3389/fpls.2020.569838. [Google Scholar] [CrossRef]
43. Fang, Z. W., He, Y. Q., Liu, Y. Y., Jiang, W. Q., Song, J. H. et al. (2020). Bioinformatic identification and analyses of the non-specific lipid transfer proteins in wheat. Journal of Integrative Agriculture, 19(5), 1170–1185. DOI 10.1016/S2095-3119(19)62776-0. [Google Scholar] [CrossRef]
44. Knowles, D. G., Aoife, M. L. (2006). High rate of recent intron gain and loss in simultaneously duplicated Arabidopsis genes. Molecular Biology and Evolution, 23(8), 1548–1557. DOI 10.1093/molbev/msl017. [Google Scholar] [CrossRef]
45. Zheng, B., Zhan, X. M. (2005). Group III intron and its effect on gene expression. Biotechnology, 15(4), 77–79. DOI 10.3969/j.issn.1004-311X.2005.04.035. [Google Scholar] [CrossRef]
46. Cao, J., Shi, F. (2012). Dynamics of arginase gene evolution in metazoans. Journal of Biomolecular Structure and Dynamics, 30(4), 407–418. DOI 10.1080/07391102.2012.682207. [Google Scholar] [CrossRef]
47. Fang, L., Cheng, F., Wu, J., Wang, X. W. (2012). Gene preservation of cabbage after whole genome and tandem duplication. Biotechnology Bulletin, 11, 9–14. [Google Scholar]
48. Mehan, M. R., Freimer, N. B., Ophoff, R. A. (2004). A genome-wide survey of segmental duplications that mediate common human genetic variation of chromosomal architecture. Human Genomics, 1(5), 335–344. DOI 10.1186/1479-7364-1-5-335. [Google Scholar] [CrossRef]
49. Lockton, S., Gaut, B. S. (2005). Plant conserved non-coding sequences and paralogue evolution. Trends in Genetics, 21(1), 60–65. DOI 10.1016/j.tig.2004.11.013. [Google Scholar] [CrossRef]
50. Dassanayake, M., Oh, D. H., Haas, J. S., Hernandez, A., Hong, H. et al. (2011). The genome of the extremophile crucifer Thellungiella parvula. Nature Genetics, 43(9), 913–918. DOI 10.1038/ng.889. [Google Scholar] [CrossRef]
Table S1: Identification of conserved motifs in these TaGH3 proteins
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |