
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014858
ARTICLE
Mycorrhizal Fungal Effects on Growth, Antioxidant Capacity, and Medicine Quality of Paris polyphylla var. yunnanensis
1Chongqing Engineering Laboratory of Green Planting and Deep Processing of Famous-region Drug in the Three Gorges Reservoir Region, College of Biology and Food Engineering, Chongqing Three Gorges University, Chongqing, 404120, China
2College of Chemistry and Pharmaceutical Sciences, Dali University, Dali, 671000, China
*Correspondence Author: Jingjing Zhao. Email: nl140828@163.com
Received: 03 November 2020; Accepted: 07 December 2020
Abstract: A field experiment was conducted to determine the effects of two commercial strains composed of mulple arbuscular mycorrhizal fungi (AMF) species on plant growth, antioxidant capacity, and medicine quality of Paris polyphylla var. yunnanensis in three subtropical soils from Wanzhou, Anshun and Baoshan in fields. The results showed that AMF inoculation enhanced the fungal colonization rate and activities of both succinate dehydrogenase and alkaline phosphatase, thereby, enhancing the mycorrhizal viability of P. polyphylla var. yunnanensis. The concentrations of photosynthetic pigments (chlorophyll a, b, a + b and carotenoids), soluble sugar, soluble protein and photosynthetic capacity were higher in AMF-inoculated plants than in non-AMF-treated plants in field. AMF-treated plants recorded higher activities of catalase, peroxidase and superoxide dismutase, and caused the reduction in malondialdehyde content, indicating lower oxidative damage, compared with non-AMF plants. Polyphyllin I, Polyphyllin II, Polyphyllin III, Polyphyllin IV and total polyphyllin contents were increased by AMF treatment. In conclusion, AMF improved the plant growth, antioxidant capacity and medicinal quality of P. polyphylla var. yunnanensis seedlings. Hereinto, AMF effects on the soil from Wanzhou was relatively greater than on other soils.
Keywords: Paris polyphylla var. yunnanensis; arbuscular mycorrhizal fungi; growth and development; medicine quality
Paris polyphylla var. yunnanensis is one of the most valuable medicinal herbs mostly distributing in Provinces of Sichuan, Guizhou and Yunnan, the southwest of China, and has been used as a traditional Chinese medicinal plant for its rhizomes containing Polyphyllin I, Polyphyllin II, Polyphyllin VI, Polyphyllin VII [1], pennogenin and diosgenin as the aglycones [2,3]. P. polyphylla var. yunnanensis is used for pain relief, detoxification, analgesic and anti-inflammatory properties [3,4]. More than 80% of traditional Chinese medicines comes from wild resources [5]. Wild individuals have been overexploited for the last few decades because of increasing demand for such medicines. And there is no effective supply available from cultivation. Many medicinal species have been listed as endangered species including P. polyphylla var. yunnanensis [4,6].
Arbuscular mycorrhizal fungi (AMF) are the most common symbiotic association between some soil fungi and plant roots [7]. AMF play a significantly stimulating role in uptake of certain nutrients (especially phosphorous and nitrogen) and water to their host plants, while the fungus obtains photosynthetically derived carbon compounds from their host plants [7]. An increasing number of experiments showed that AMF provided several benefits to their host plants, including promoted plant transplant survival rate [8], increased abiotic stress tolerance [9,10], improving soil structure and fertility [11], and disease resistance, adjusted plant population and community structure, and maintained ecosystem stability [12]. AMF inoculation is also known to have tremendous effects on plant growth by enhancing macronutrient content (P, K, and Ca) and micronutrient content (Cu, Fe, and Zn) [13], increasing biomass of medicinal plants [14], improving photosynthesis [9,15], and inducing change of alkaloids and terpenoids [16].
However, there is little information about successful inoculation of P. polyphylla var. yunnanensis with AMF or about the application of AMF inoculation to commercial P. polyphylla var. yunnanensis production. The aim of this experiment was to investigate the effect of AMF on photosynthetic pigments, membrane lipid peroxidation, antioxidant enzyme activity, and medicine quality of P. polyphylla var. yunnanensis plants, in order to further understand the application of AMF in the production of medicinal plants.
2.1 Mycorrhizal Fungal Inoculums
We selected two mixed AMF inoculums: 1) The AMF biofertilizer was rich in endomycorrhizal fungi Scutellospora calospora, Cetraspora pellucida, Racocetra coralloidea and Racocetra fulgida (S1); 2) The other AMF inoculum was rich in endomycorrhizal fungi Scutellospora calospora, Cetraspora pellucida, Gigaspora margarita, G. gigantea, Septoglomus deserticola and Claroideoglomus claroideum (S2). The mixed AMF treatment exhibited a superior effect on nutrient acquisition and fruit quality of plant in field than single AM fungal inoculation [17]. The two mixed mycorrhizal inoculums were obtained from the International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (INVAM, http://invam.wvu.edu). The inoculum consisted of extraradical hyphae, spores, and infected roots.
2.2 Plant Culture and Experimental Design
One-year-old Paris polyphylla var. yunnanensis seedlings were used, and soils were fertilized with organic sources of nutrients before transplanting seedlings. The study was located in Sichuan, Guizhou and Yunnan provinces, southwest China. The field experiment was carried out according to a completely randomized block design with three treatments (two AMF groups (S1 and S2) and a control group [CK group]) having four replicates per treatment. To examine the effect of AMF on growth of P. polyphylla var. yunnanensis seedlings, the 20 g of each inoculum containing approximately 120 spores was supplied to each plot and then mixed with the soil–sand mixture. Transplanted P. polyphylla var. yunnanensis seedlings were watered once three days to reach around 60% water contents in soils. From July 12 to 20, 2016, the P. polyphylla var. yunnanensis seedlings were inoculated with AMF at the time of transplantation. Growth characteristics, photosynthetic pigments contents and photosynthetic parameters were determined for 10 individual plants in different fields. At the same time, the leaves were harvested directly into liquid nitrogen and stored at −40°C. From November 21 to 30, 2016, the rhizomes and roots of P. polyphylla var. yunnanensis were selected for quality analysis. Meanwhile, the root was cut into 1.0–1.5 cm long root segments in an ice water bath. One part was fixed in FAA fixative and used for the determination of mycorrhizal infection rate; the other part was stored in liquid nitrogen and used for the determination of phosphatase and succinate dehydrogenase activities in mycorrhizal hyphae.
2.3.1 Mycorrhizal Colonization Rate
Assessment of roots for AMF colonization was made on those plants sampled for soil surface (0–200 mm depth). A fraction of the roots (<1 cm long) were carefully washed, and were fixed in formalin acetic acid solution before estimation of mycorrhizal colonization [18]. The AMF colonization was estimated using a modified method of Brundrett et al. [19]. These observations were stained with 0.05% trypan blue, washed in 50% glycerol, and measured by using Olympus BX50 (Olympus, Japan) transmitted-light bright field microscope for mycorrhizal colonization. The rate of AMF colonization was determined according to the method of Trouvelot et al. [20].
2.3.2 Phosphatase and Succinate Dehydrogenase Activity
Sites of alkaline phosphatase (ALP) activity was assayed by the method of Van Aarle et al. [21]. Roots were stained for 30 min at room temperature in the dark after which they were thoroughly washed with a Tris buffer (pH 8.0). Clearing was done at room temperature for 2 h. The clearing solution contains 15 units ml1 cellulase, 0.05% sorbitol, 15 units ml1 pectinase and 0.05 M Tris/citric acid (pH 9.2). Sites of phosphatase activity were revealed by a dark purple precipitate.
Succinate dehydrogenase (SDH) activity was measured according to the method of MacDonald and Lewis [22]. 0.2 g 1 cm viable mycorrhizal fresh roots was incubated at room temperature overnight in an NBT-succinate solution. The NBT solution consist of Tris–HCl buffer (0.05 M; pH 9.2), pectinase (15 U·mL–1), cellulase (15 U·mL–1) and sorbitol (50 g·L–1). Roots were rinsed three times with distilled water, and cleared exclusively with KOH turn dark.
2.3.3 Chlorophyll (Chl) Content
Chlorophyll content was assayed based on the method of Arnon [23]. 100 mg fresh leaf tissue from the third fully expanded leaf with a mixture containing absolute ethanol until the pellets became colorless. The concentration was calculated from the value of A470, A645 and A663, and expressed as mg·g–1 FW.
2.3.4 Photosynthetic Parameters
Net photosynthetic rate (PN), intercellular CO2 concentration (Ci), stomatal conductance (gs) and transpiration rate (E) were measured using a portable photosynthesis system (Li-Cor 6400, Li-Cor Inc., Nebraska, USA). Data were recorded between 9:30 and 11:30 am during the treatment period. Plants were measured under PPFD of 1,000 µmol·m–2·s–1, 25 ± 3°C, 80% humidity and CO2 concentration of 500 μmol·s–1. Five representative plants were randomly selected from each treatment.
The content of malondialdehyde (MDA) was determined by the thiobarbituric acid (TBA) test according to the method of Liu et al. [24] and Dhindsa et al. [25]. Fresh leaf tissues (500 mg) were homogenized in 5 mL of phosphate buffer (0.05 M, pH 7.8) using mortar and pestle, and then centrifuged at 12,000 × g for 20 min. 1 mL of supernatant, 2 mL of 0.5% TBA and 1 mL of PBS buffer (pH 7.8) were incubated in boiling water for 15 min. The concentration was measured using a spectrophotometer at 532 nm and 600 nm.
For determination of soluble sugar content, leaf samples (0.5 g) were ground in liquid nitrogen and homogenized in 10 mL of 80 % (v/v) ethanol [26]. The mixture was extracted in a water bath at 80°C for 15 min, and the supernatant was centrifuged three times (10 000 g, 20 min). Absorbance was recorded at 630 nm to measure soluble sugar content by the colorimetry of sulfuric acid-anthrone method.
The content of protein was determined at 595 nm as described by Bradford [27] using bovine serum albumin as a protein standard. Fresh leaves (0.5 g) were homogenized in distilled water. 1 mL of supernatant and 5 mL of Coomassie’s Brilliant Blue solution were placed in tubes.
2.3.8 Antioxidant Enzymes Activity
A 1.0 g leaves was homogenized in precooled PBS buffer (pH 7.0), and centrifuged at 11 500 g and 4°C for 15 min. The supernatant was used for enzyme activity assay.
The catalase (CAT) activity was measured using the method of Fu et al. [28] by monitoring a change in absorbance at 240 nm for 1 min. The reaction mixture contained 25 mM sodium phosphate buffer (pH 7.0) and 0.1 mL enzyme fraction. The reaction was initiated by adding 10 mM H2O2.
Peroxidase activity was assayed by the method of Polle et al. [29]. The reaction mixture was composed of 50 mM potassium phosphate buffer (pH 6.5), 5 mM hydrogen peroxide (H2O2), 30 mL diluted enzymatic extract and 20 mM pyrogallol (benzene-1,2,3-triol), totaling 1.0 mL. The reaction was initiated by adding 0.2 mL crude enzyme preparations. The activity of POD was expressed as µmol·min–1·g–1 (FM). A change in absorbance was read at 470 nm at every 1 min for 5 min.
The method of El-Shabrawi et al. [30] was followed to measure superoxide dismutase (SOD) activity. The reaction mixture contained 0.2 mL of 13 mM methionine, 0.2 mL of 25 mM nitroblue tetrazolium (NBT), 2.4 mL of 50 mM PBS (pH 7.8), 0.1 mL of EDTA, and 50 mL of the enzyme extract. A change in absorbance was read at 560 nm.
Fresh weight (FW) and dry weight (DW) of P. polyphylla var. yunnanensis were harvested in November 2016, and dried at 35°C for 48 h to obtain dry weight. Drying rate (DR) was defined as the ratio of fresh weight to dry weight.
The healthy rhizomes of P. polyphylla var. yunnanensis were collected in different sites and stored in sealed plastic bags at 4°C. Polyphyllin I (batch No. 111590-201103), Polyphyllin II (batch No. 111591-201103), Polyphyllin VI (batch No. 111592-201103) and Polyphyllin VII (batch No. 111593-200402) were purchased from the National Institute for Food and Drug Control (Beijing, China). Acetonitrile (HPLC-grade) was purchased from Fisher (USA). All regents were all of analytical grade and were passed through membrane filter (0.22 mm) before use to purify. Polyphyllin I, Polyphyllin II, Polyphyllin VI and Polyphyllin VII content were determined using the method previously described by Yuangui et al. [1]. Polyphyllin content was measured under column oven temperature of 40°C, flow rate of 0.25 mL·min–1, and injection volume of 5 mL. The methanol was linearly 50%, and held for 10 min before the next injection. Acetonitrile and water were used as mobile phases. The mass spectrometer (Agilent, Agilent Quick Probe, USA) was set at 350°C of gas temperature and 12 L·min–1 of gas flow.
Statistical analysis was conducted using the SPSS (21.0, International Business Machines Corporation, USA) software. The experimental data of treatment and control were analyzed by using analysis of variance (ANOVA) with Duncan’s multiple range test at 0.05 level. The figure was drawn by the OriginPro 9.1 software (OriginLab, Northampton, MA, USA).
3.1 Mycorrhizal Colonization Rate in Paris polyphylla var. yunnanensis
Under natural environment, the AMF colonization rate of non-inoculated P. polyphylla var. yunnanensis plants was 51.06% (Wanzhou), 25.37% (Anshun) and 44.72% (Baoshan), respectively (Fig. 1). The AMF colonization rate was higher in AMF-inoculated than non-AMF-inoculated plants (Fig. 1). The maximum AMF colonization rate was observed inoculated plants by S1 treatment (149.09%) in Wanzhou, followed by S2 treatment inoculated plants in Wanzhou (148.18%). Exogenous AMF had a promoting effect on the mycorrhizal infection rate, indicating that it is feasible to improve the quality of P. polyphylla var. yunnanensis.
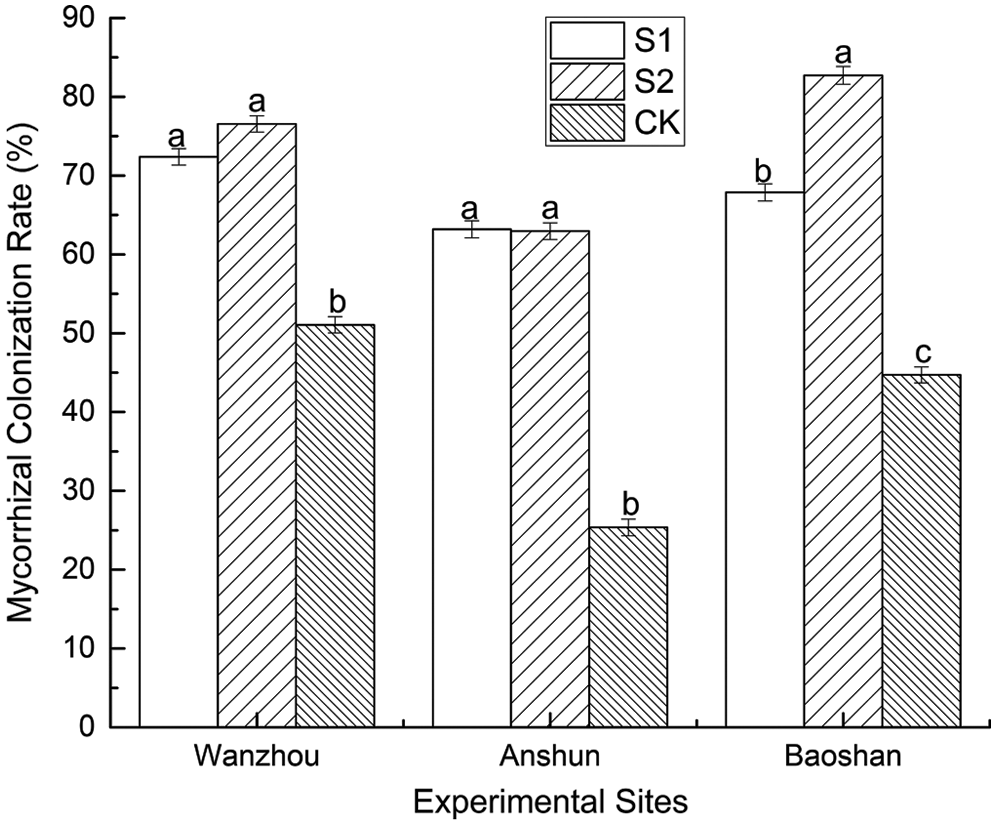
Figure 1: Effects of AMF on mycorrhizal colonization rate of seedlings in Paris polyphylla var. yunnanensis
Data (means ± SE, n = 4) are the difference between treatments. Different letters above horizontal lines indicate significant differences between treatments. The same as below.
3.2 Effects of AMF on the SDH and ALP Activities in Paris polyphylla var. yunnanensis
Succinic dehydrogenase (SDH) and alkaline phosphatase (ALP) activities were significant increased by AMF inoculations (Fig. 2). In Wanzhou, exogenous inoculation of AMF treatment caused the significant increase in the SDH and ALP by 45.52% and 60.06% under S1 conditions and by 36.39% and 60.06% under S2 conditions, respectively. In Anshun, S1 treatment caused 44.50% and 130.13% remarkable increase in the above indicators, while S2 treatment, they were 43.72% and 130.13% compared to non-AM seedlings, respectively. In Baoshan, S1 treatment caused 78.38% and 159.39% prominent (p < 0.05) increase in the above indicators, while S2 treatment, they were 106.90% and 159.39% compared to non-AMF seedlings, respectively.
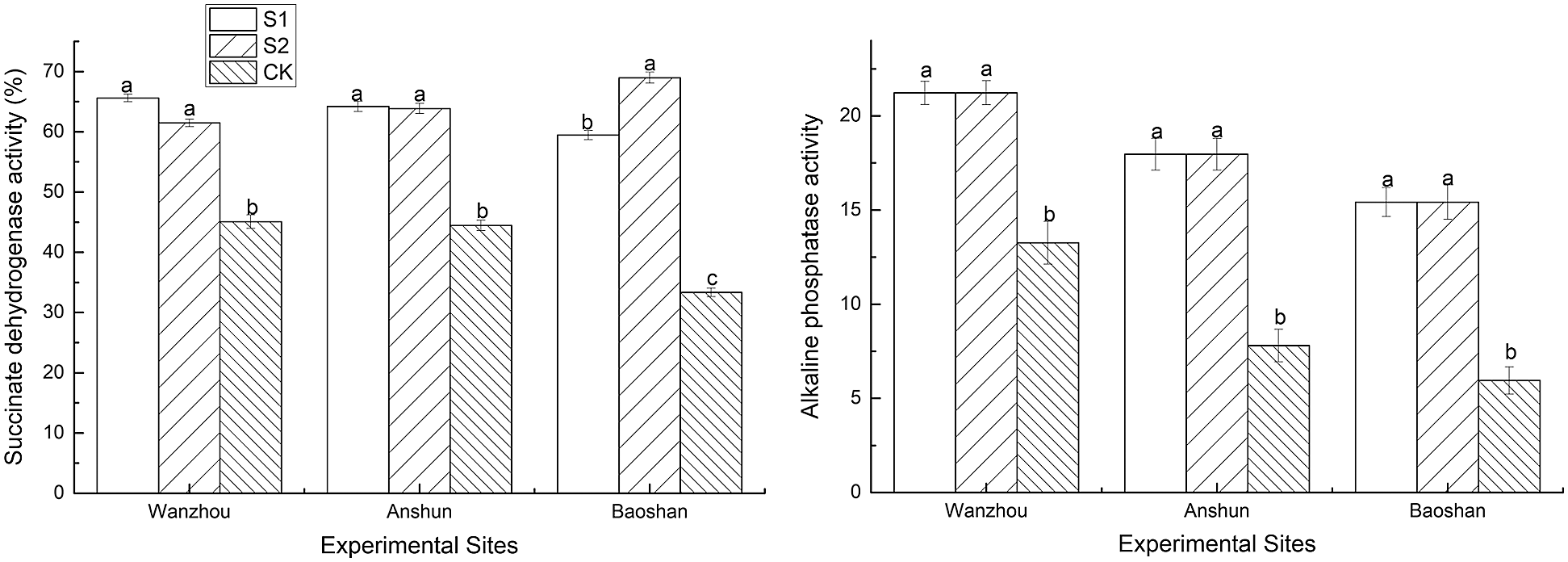
Figure 2: Effects of AMF on SDH and ALP activities of seedlings in Paris polyphylla var. yunnanensis
3.3 Effects of AMF on the Photosynthetic Pigments Content in Paris polyphylla var. yunnanensis
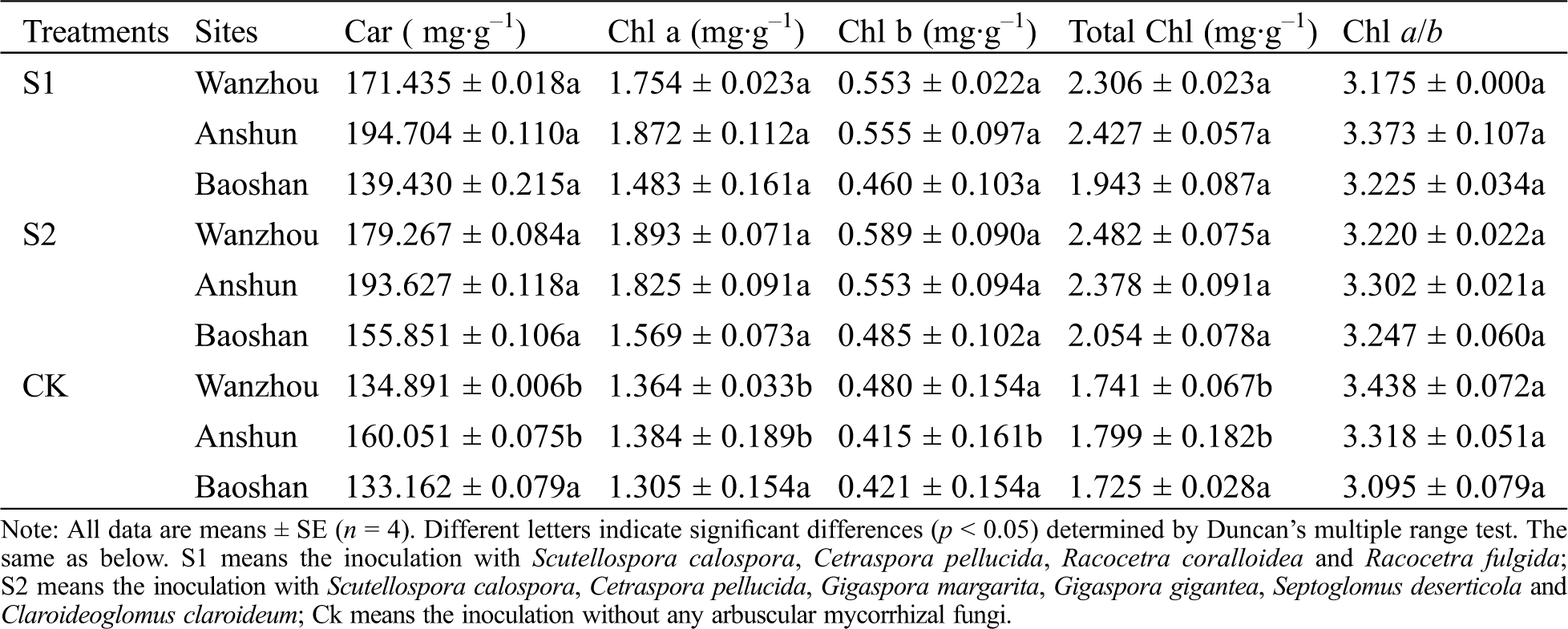
AMF-inoculated seedlings had higher carotenoid (Car), chlorophyll (Chl) a, Chl b and total Chl content than corresponding non-AMF-treated seedlings (Tab. 1). In Wanzhou, AMF treatments caused 27.09%, 28.59%, 15.21% and 32.45% significant (p < 0.05) increase in the Car, Chl a, Chl b and total Chl content under S1 conditions and 32.90%, 38.78%, 22.71% and 42.56% under S2 conditions, compared to non-AM seedlings, respectively. In Anshun, compared with the controls, mycorrhiza-inoculated plants showed 21.65%, 35.26%, 33.73% and 34.91% significantly higher Car, Chl a, Chl b and total Chl content under S1 conditions and 20.98%, 31.86%, 33.25% and 32.18% under S2 conditions, respectively. In Baoshan, compared with the control plants, S1 treatment caused 4.71%, 13.64%, 9.26% and 12.64% increase in the Car, Chl a, Chl b and total Chl content, while S2 caused 17.04%, 20.23%, 15.20% and 19.07% increase in the Car, Chl a, Chl b and total Chl content. However, the difference of Chl a/b was not significant between AM seedlings and non-AM seedlings. Inoculation with AMF can help increase the content of photosynthetic pigments, and then promote the growth and development of P. polyphylla var. yunnanensis.
Table 1: Effects of AMF on photosynthetic pigment content of seedlings in Paris polyphylla var. yunnanensis
3.4 Effects of AMF on the Photosynthetic Parameters in Paris polyphylla var. yunnanensis
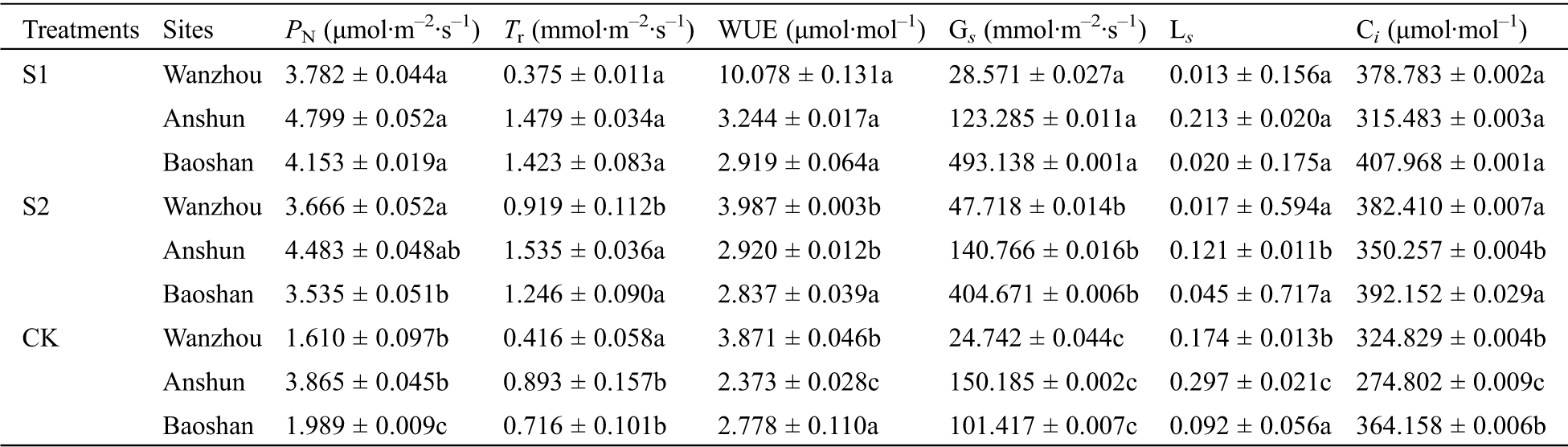
Mycorrhizal fungal treatments significantly improved PN in comparison to the non-mycorrhizal fungal treated plants (Tab. 2). S1 treatment dramatically increased PN by 134.91% in Wanzhou, 24.17% in Anshun and 108.80% Baoshan compared with control, while S2 treatment, they were by 127.70%, 15.99% and 77.73%, respectively. In addition, application of S1 and S2 fungus combination improved other photosynthetic parameters in P. polyphylla var. yunnanensis leaves compared with the plants under natural environment, such as E, Ci, Gs and water use efficiency (WUE).
Table 2: Effects of AMF on photosynthetic parameters of seedlings in Paris polyphylla var. yunnanensis
3.5 Effects of AMF on the Protective Enzymes Activities in Paris polyphylla var. yunnanensis
CAT, POD and SOD activities were increased in inoculated P. polyphylla var. yunnanensis plants by 8.96–14.88%, 37.79–215.32% and 52.03–98.34% in S1 treatment, and by 4.68–13.66%, 84.66–165.51%, and 18.89–33.10% in S2 treatment, respectively, compared to the non-inoculated plants (Fig. 3).Hence, the inoculation of AMF was beneficial to increase the protective enzyme activity of P. polyphylla var. yunnanensis leaves.
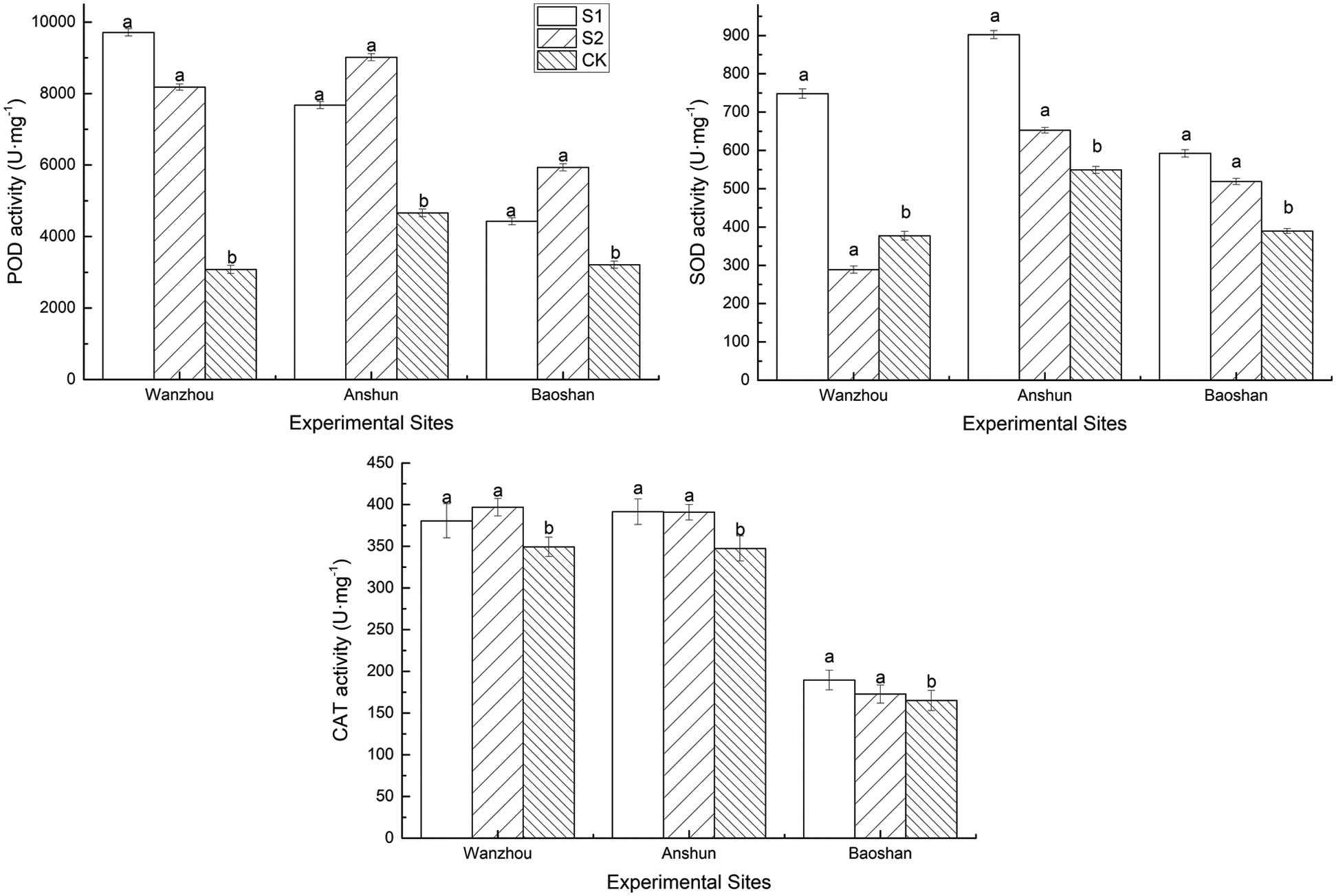
Figure 3: Effects of AMF on CAT, POD and SOD activities of seedlings in Paris polyphylla var. yunnanensis
3.6 Effects of AMF on the MDA, Soluble Sugar and Soluble Protein Content in Paris polyphylla var. yunnanensis
AMF inoculation induced dramatic reduction of MDA (an indicator of lipid peroxidation) (Fig. 4). Exogenous inoculation of AMF significantly reduced the MDA content, thereby reducing the degree of membrane lipid peroxidation.
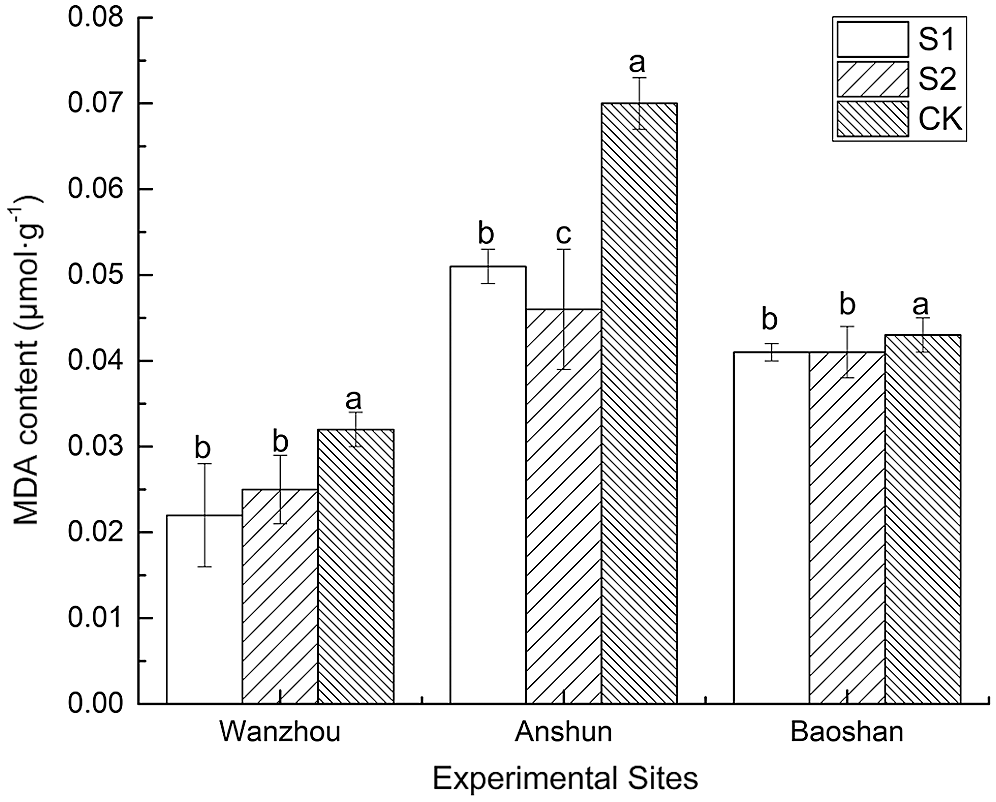
Figure 4: Effects of AMF on MAD content of seedlings in Paris polyphylla var. yunnanensis
The soluble sugar and soluble protein contents in P. polyphylla var. yunnanensis were significantly increased under inoculation AMF environment (Figs. 5a, 5b). Compared to the CK group, the soluble sugar content in Wanzhou, Anshun and Baoshan was increased by 25.91%, 85.24% and 123.93% in the S1 group, and by 9.91%, 60.22% and 95.26% in the S2 group, respectively. The soluble protein content in Wanzhou, Anshun and Baoshan was increased by 22.70%, 15.36% and 4.26% in the S1 group, and by 22.70%, 15.36% and 4.26% in the S2 group, respectively, compared with the CK group plants.
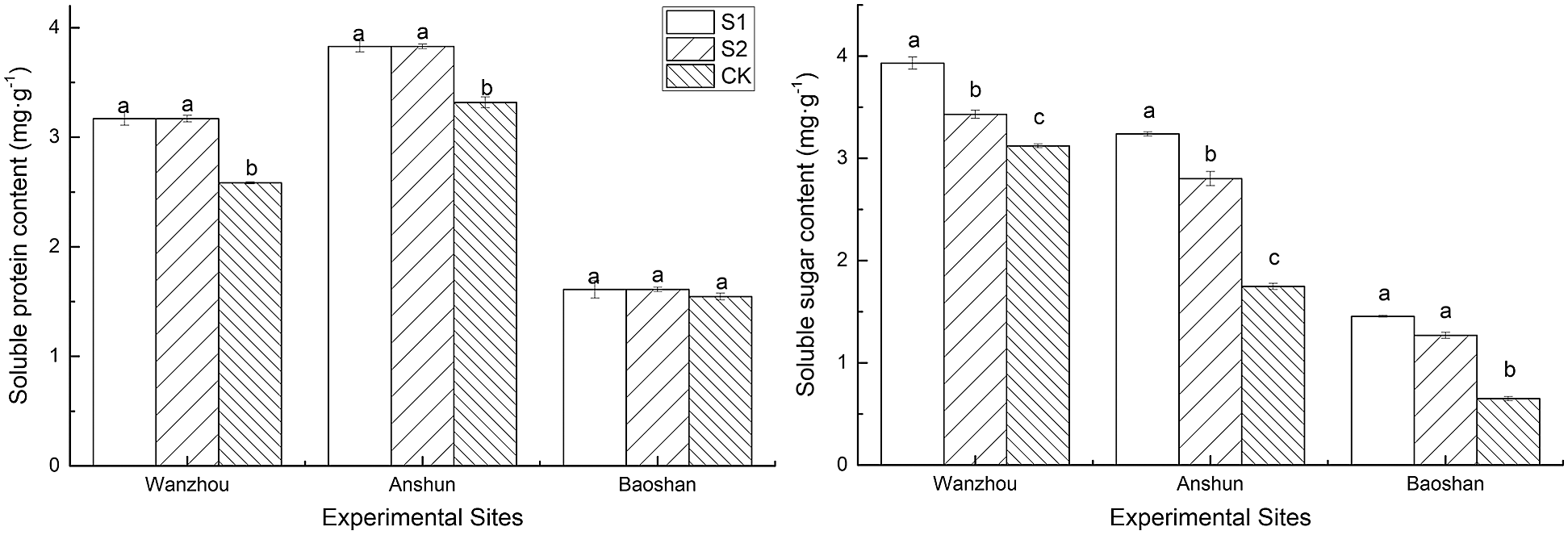
Figure 5: Effects of AMF on soluble sugar and soluble protein content of seedlings in Paris polyphylla var. yunnanensis
3.7 Effects of AMF on the Biomass in Paris polyphylla var. yunnanensis
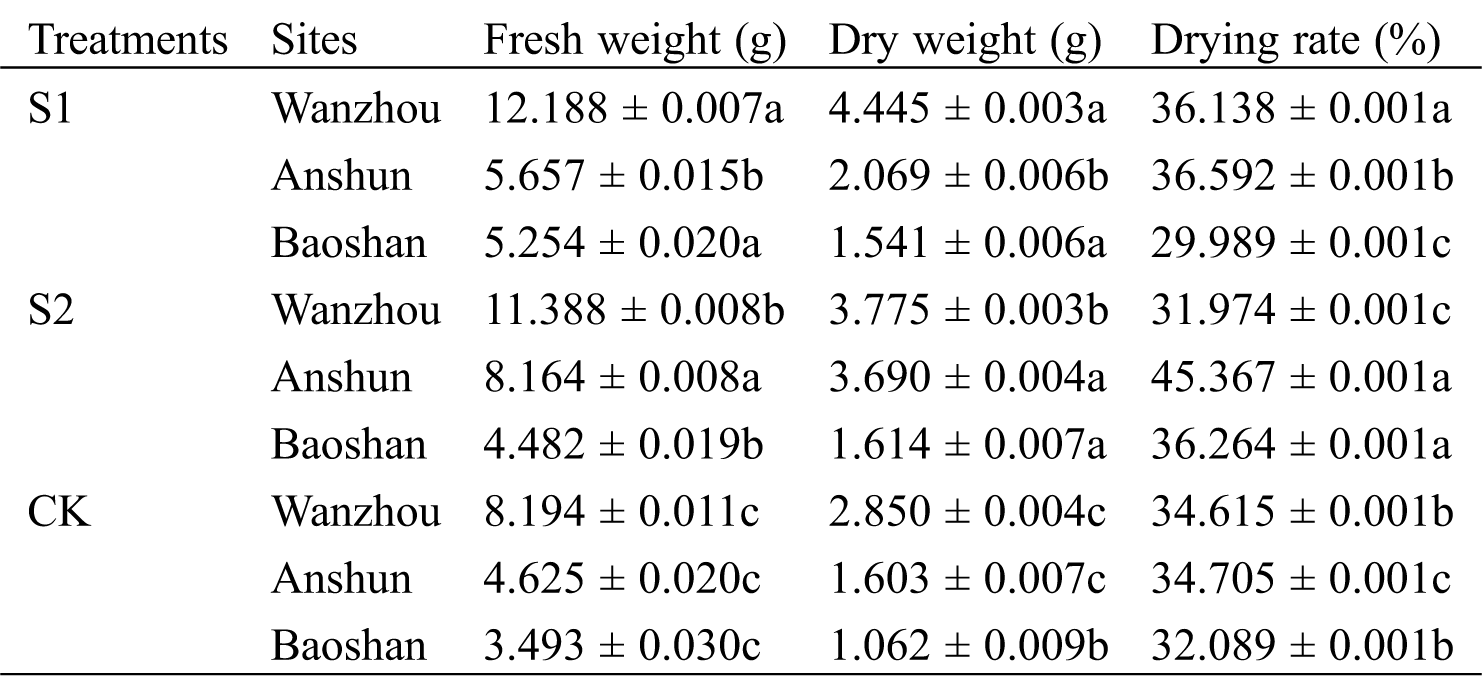
Fresh weight and dry weight were significantly higher in mycorrhizal than non-mycorrhizal plants (Tab. 3). In Wanzhou, S1 treatment caused 48.74% and 55.96% significant (p < 0.05) increase in the above indicators, while S2 treatment, they were 38.98% and 32.46% compared to non-AM seedlings, respectively. In Anshun, compared with the controls, S1 treatment caused 22.31% and 29.07% obvious (p < 0.05) enhancement in the above indicators, while S2 treatment, they were 76.52% and 130.19%, respectively. In Baoshan, compared with the control plants, S1 treatment caused 50.42% and 45.10% increase in the above indicators, while S2 pretreatment they were enhanced by 28.31% and 51.98%, respectively.
Table 3: Effects of AMF on rhizomes biomass and rhizome drying rate of seedlings in Paris polyphylla var. yunnanensis
3.8 Effects of AMF on the Polyphyllin Yield and Content in Paris polyphylla var. yunnanensis
Compared with the non-AMF-inoculated plants under natural environment alone, S1 and S2 treatments effectively improved the yield of polyphyllin by 29.89–135.39% (Fig. 6). The yield of polyphyllin reached maximum values when plants were inoculated by S2 treatment in Anshun.
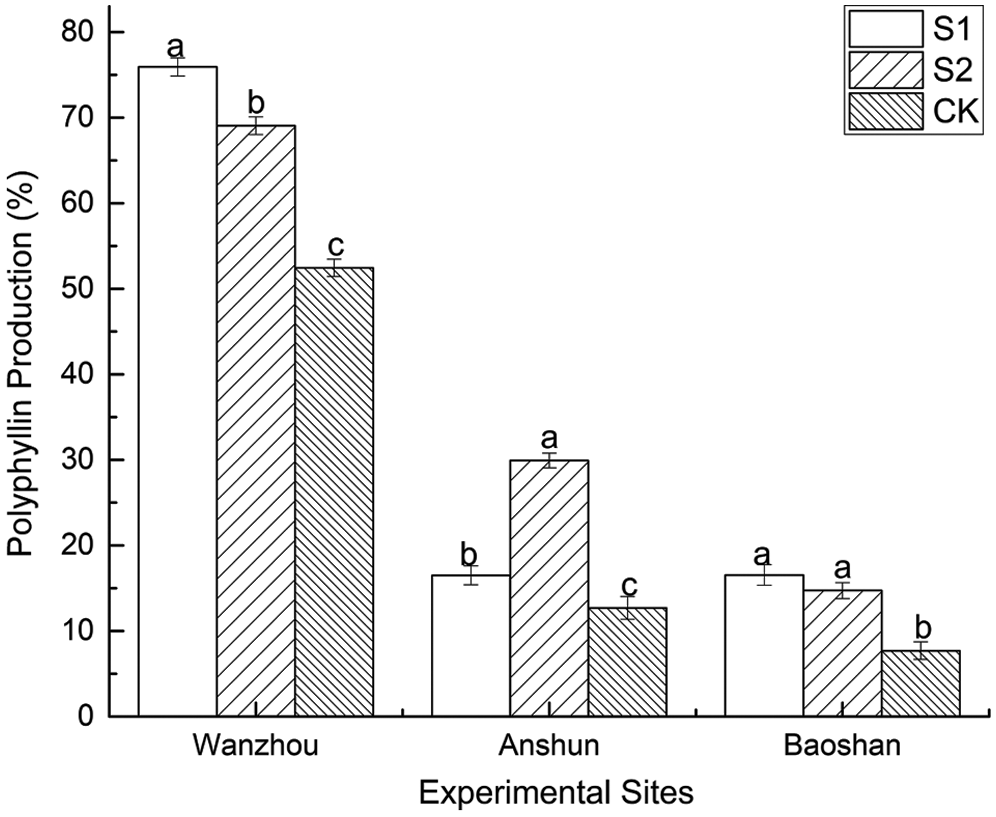
Figure 6: Effects of AMF on polyphyllin production of seedlings in Paris polyphylla var. yunnanensis
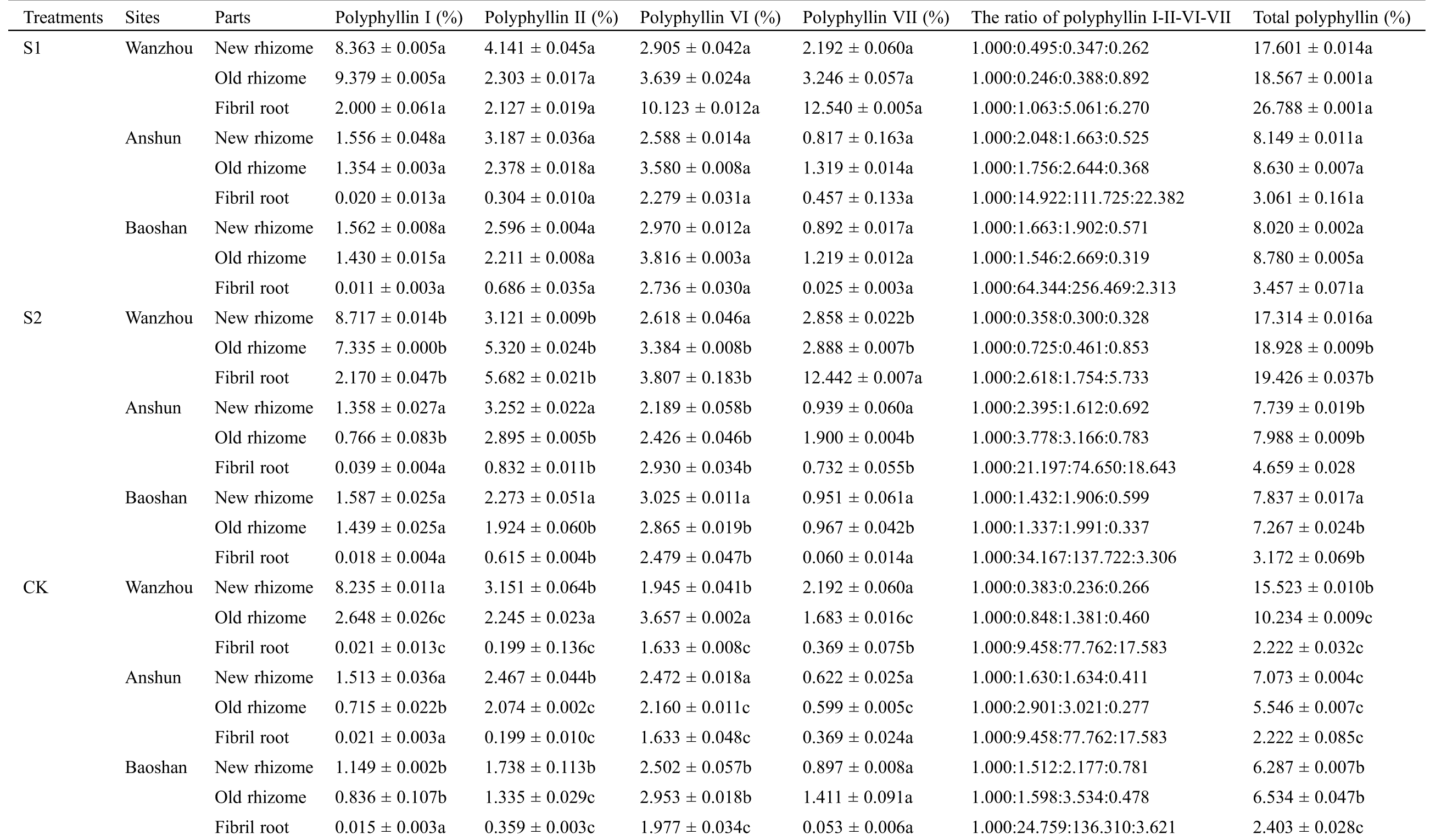
The types of P. polyphylla var. yunnanensis polyphyllin vary with the organs of P. polyphylla var. yunnanensis. A marked increase in Polyphyllin I, Polyphyllin II, Polyphyllin VI, Polyphyllin VII and total polyphyllin content was observed in AMF-treated seedlings of different parts (Tab. 4). In Wanzhou, total polyphyllin content was enhanced by 13.39%~1105.58% in S1 treatment and by 11.54%~774.26% in S2 treatment. In Anshun, total polyphyllin content was enhanced by 15.21%~55.61% in S1 treatment and by 9.42%~109.68% in S2 treatment. In Baoshun, total polyphyllin content was enhanced by 27.56%~43.86% in S1 treatment and by 11.22%~32.00% in S2 treatment. As a result, the plants supplemented with AMF could improve medicinal quality, especially four kinds of polyphyllin and total polyphyllin, in P. polyphylla var. yunnanensis.
Table 4: Effects of AMF on the content of polyphyllin from new rhizome, old rhizome and fibrous roots of seedlings in Paris polyphylla var. yunnanensis
AMF are extremely sensitive to the environment, and its infection status is affected by factors such as crop type, soil fertility, climatic conditions and agricultural measures [7]. In this experiment, the infection rate of P. polyphylla var. yunnanensis seedlings inoculated with AMF was 62.96%~82.72%, indicating that the seedling stage of P. polyphylla var. yunnanensis was the best period for inoculation of exogenous AMF. Compared with the CK group, SDH and ALP activities were significant enhanced by AMF inoculation, indicating that three experimental sites (Wanzhou, Anshun, and Baoshan) could form good mycorrhizas in roots of P. polyphylla var. yunnanensis.
Recent studies indicated that AMF increased chlorophyll content, PN and Gs, improved photosynthetic performance of plants, and enhanced growth and development [9,15]. CAT, POD and SOD as the key enzymes for scavenging free radicals in plants, can play an important role in maintaining the balance of oxygen metabolism. Existing studies have shown that the increase of protective enzyme activity, soluble sugar and soluble protein content is conducive to enhancing plant stress resistance, including salinity [10], drought [11,28], and temperature stress [15,24]. Compared with the non-AMF plants, the Chl a, Chl b, and total Chl contents in the leaves of P. polyphylla var. yunnanensis were increased under mycorrhization. The photosynthesis of P. polyphylla var. yunnanensis leaves inoculated with different AMF mixed treatments in the field was different. Compared with the CK group, the photosynthesis of treatment groups S1 and S2 were enhanced. Among the three field planting sites, P. polyphylla var. yunnanensis in Anshun and Wanzhou had better photosynthetic capacity. In addition, AMF inoculation dramatically enhanced SOD, POD, and CAT activities of P. polyphylla var. yunnanensis, almostly dependent on experimental sites, indicating that field application of AMF could improve antioxidant capacity of host plants, as seen by a low MDA content in AMF-inoculated plants. Similar results were reported by Zhang et al. [9] in trifoliate orange colonizaed by Funneliformis mosseae. Hence, the field test of P. polyphylla var. yunnanensis rhizome inoculated with AMF was relatively successful.
The formation of mycorrhiza not only stimulated the growth of plants, but also increased the accumulation of related active ingredients. Previous studies have showed that AMF greatly improved the medicinal quality in traditional Chinese medicine including Atractylodes lancea [31], Medicago truncatula [32], M. sativa [33] and so on. Polyphyllin is considered to be one of the main active ingredients in Rhizoma Paridis (‘‘Chong-lou’’ in Chinses), the dried rhizomes of P. polyphylla var. yunnanensis, which has various activities, such as detoxification and pain relief (Chinese Pharmacopoeia Committee, 2015). The two mixed microbial agents used in this experiment increased the content of polyphyllin. I guess that AMF inoculation potentially stimulated activity of plant second metabolism, thus, promoting the polyphyllin accumulation in P. polyphylla var. yunnanensis, which still needs to be further studied. The increase of polyphyllin indicates that establishing dominant mycorrhizal fungus populations in the field and accelerating mycorrhizal infection is effective ways to improve its production. According to the records in the latest edition of the Chinese Pharmacopoeia, the amount of 4 kinds of polyphyllin content not be less than 0.60%. In this experiment, the content of polyphyllin in the old and new rhizomes of P. polyphylla var. yunnanensis in all treatment groups reached the standard of medicinal materials.
In summary, different experimental treatments had different influences on the growth and development and medicine quality of P. polyphylla var. yunnanensis. Among the three field test sites, Wanzhou had the better field cultivation effect. Therefore, it is possible to improve the medicinal quality of P. polyphylla var. yunnanensis from small-scale laboratory experiments to inoculation of exogenous AMF in the field environment, which may bring great economic benefits.
Funding Statement: This work was supported by the National Natural Science Foundation of China (81260622), Applied Basic Research Program of Yunnan Province (2011FB081) and Scientific Research Fund Key Project of Yunnan Province (2012Z119).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Yang, Y., Zhang, J., Jin, H., Zhang, J., Wang, Y. (2016). Quantitative analysis in combination with fingerprint technology and chemometric analysis applied for evaluating six species of wild paris using UHPLC-UV-MS. Journal of Analytical Methods in Chemistry, 2016, 1–9. DOI 10.1155/2016/3182796. [Google Scholar] [CrossRef]
2. Li, J., Zhao, J., Xu, L., Zhou, L., Li, X. et al. (2008). Endophytic fungi from rhizomes of Paris polyphylla var. yunnanensis. World Journal of Microbiology and Biotechnology, 24(5), 733–737. DOI 10.1007/s11274-007-9531-3. [Google Scholar] [CrossRef]
3. Qin, X., Sun, D., Ni, W., Chen, C., Hua, Y. et al. (2012). Steroidal saponins with antimicrobial activity from stems and leaves of Paris polyphylla var. yunnanensis. Steroids, 77(12), 1242–1248. DOI 10.1016/j.steroids.2012.07.007. [Google Scholar] [CrossRef]
4. Song, Y., Li, M., Xu, J., Zin, Z., Chen, N. (2015). Polymorphic microsatellite markers in the traditional Chinese medicinal plant Paris polyphylla var. yunnanensis. Genetics and Molecular Research, 14(3), 9939–9942. DOI 10.4238/2015.August.19.29. [Google Scholar] [CrossRef]
5. Vines, G. (2004). Herbal harvests with a future: Towards sustainable sources for medicinal plants. Report for plantlife international salisbury. Wiltshire, UK: The Wild Plant Conservation Charity. [Google Scholar]
6. Huang, Y., Zhou, N., Yang, M., Shen, Y., Zhang, D. (2019). A comparative study of the population genetics of wild and cultivated populations of Paris polyphylla var. yunnanensis based on amplified fragment length polymorphism markers. Ecology and Evolution, 9(18), 10707–10722. DOI 10.1002/ece3.5589. [Google Scholar] [CrossRef]
7. Youpensuk, S., Lordkaewb, S., Rerkasemc, B. (2008). Arbuscular mycorrhizal fungi associated with tangerine (Citrus reticulata) in Chiang Mai province, northern Thailand, and their effects on the host plant. Science Asia, 34, 259–264. [Google Scholar]
8. Subhan, S., Sharmila, P., Pardha, S. (1998). Glomus fasciculatum alleviates transplantation shock of micropropagated Sesbania sesban. Plant Cell Reports, 17(4), 268–272. DOI 10.1007/s002990050390. [Google Scholar] [CrossRef]
9. Zhang, F., Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environmental and Experimental Botany, 171, 103962. DOI 10.1016/j.envexpbot.2019.103962. [Google Scholar] [CrossRef]
10. Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Unraveling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biology, 8, 1–8. DOI 10.1111/plb.13161. [Google Scholar] [CrossRef]
11. He, J. D., Chi, G. G., Zou, Y. N., Shu, B., Wu, Q. S. et al. (2020). Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Applied Soil Ecology, 154, 103592. DOI 10.1016/j.apsoil.2020.103592. [Google Scholar] [CrossRef]
12. Brundrett, C. (2009). Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil, 320(1–2), 37–77. DOI 10.1007/s11104-008-9877-9. [Google Scholar] [CrossRef]
13. Eunju, C., Dojin, L., Chido, W., Honglim, K., Yonghwa, C. et al. (2009). Effects of AMF inoculation on growth of Panax ginseng C.A. Meyer seedlings and on soil structures in mycorrhizosphere. Scientia Horticulturae, 122, 633–637. [Google Scholar]
14. Zubek, S., Mielcarek, S., Turnau, K. (2012). Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza, 22(2), 149–156. DOI 10.1007/s00572-011-0391-1. [Google Scholar] [CrossRef]
15. Heikham, E., Rupam, K., Bhoopander, G. (2018). Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. Journal of Photochemistry and Photobiology B: Biology, 180, 149–154. DOI 10.1016/j.jphotobiol.2018.02.002. [Google Scholar] [CrossRef]
16. Maier, W., Hammer, K., Dammann, U., Schulz, B., Strack, D. (1997). Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae. Planta, 202(1), 36–42. DOI 10.1007/s004250050100. [Google Scholar] [CrossRef]
17. Wu, Q. S., Gao, W. Q., Srivastava, A. K., Zhang, F., Zou, Y. N. (2020). Nutrient acquisition and fruit quality of Ponkan mandarin in response to AMF inoculation. Indian Journal of Agricultural Sciences, 90, 1563–1567. [Google Scholar]
18. Phillips J. M., Hayman D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society, 55(1), 158–IN18. DOI 10.1016/S0007-1536(70)80110-3. [Google Scholar] [CrossRef]
19. Brundrett, M., Piche, Y., Peterson, R. (1984). A new method for observing the morphology of vesicular-arbuscular mycorrhizae. Canadian Journal of Botany, 62(10), 2128–2134. DOI 10.1139/b84-290. [Google Scholar] [CrossRef]
20. Trouvelot, A., Kough, J., Gianinazzi, P. (1986). Mesure du taux de mycorhization VA d'un système radiculaire. Recherche de méthodes d'estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson, V., Gianinazzi, S. (eds.Physiological and genetical aspects of mycorrhizae, pp. 217. Paris: INRA Publications. [Google Scholar]
21. VanAarle, I., Olsson, P., Soderstrom, B. (2001). Microscopic detection of phosphatase activity of saprophytic and arbuscular mycorrhizal fungi using a fluorogenic substrate. Mycologia, 93(1), 17–24. DOI 10.2307/3761601. [Google Scholar] [CrossRef]
22. MacDonald, R., Lewis, M. (1978). The occurrence of some acid phosphatases and dehydrogenases in the vesicular-arbuscular mycorrhizal fungus glomus mosseae. New Phytologist, 80(1), 135–141. DOI 10.1111/j.1469-8137.1978.tb02273.x. [Google Scholar] [CrossRef]
23. Arnon, D. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), 1–15. DOI 10.1104/pp.24.1.1. [Google Scholar] [CrossRef]
24. Liu, Y., Zhang, G., Qi, M., Li, T. (2015). Effects of calcium on photosynthesis, antioxidant system, and chloroplast ultrastructure in tomato leaves under low night temperature stress. Journal of Plant Growth Regulation, 34(2), 263–273. DOI 10.1007/s00344-014-9462-9. [Google Scholar] [CrossRef]
25. Dhindsa, R., Plumb-Dhindsa, P., Thorpe, T. (1981). Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32(1), 93–101. DOI 10.1093/jxb/32.1.93. [Google Scholar] [CrossRef]
26. Buysse, J., Merckx, R. (1993). An improved colorimetric method to quantify sugar content of plant tissue. Journal of Experimental Botany, 44(10), 1627–1629. DOI 10.1093/jxb/44.10.1627. [Google Scholar] [CrossRef]
27. Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
28. Fu, J., Huang, B. (2001). Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environmental and Experimental Botany, 45(2), 105–114. DOI 10.1016/S0098-8472(00)00084-8. [Google Scholar] [CrossRef]
29. Polle, A., Otter, T., Seifert, F. (1994). Apoplastic peroxidases and lignification in needles of Norway (Picea abies L.). Plant Physiology, 106(1), 53–60. DOI 10.1104/pp.106.1.53. [Google Scholar] [CrossRef]
30. El-Shabrawi, H., Kumar, B., Kaul, T., Reddy, M., Singla-Pareek, S. et al. (2010). Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma, 245(1–4), 85–96. DOI 10.1007/s00709-010-0144-6. [Google Scholar] [CrossRef]
31. Yang, T., Dai, C. (2013). Interactions of two endophytic fungi colonizing Atractylodes lancea and effects on the host's essential oils. Acta Ecologica Sinica, 33(2), 87–93. DOI 10.1016/j.chnaes.2013.01.004. [Google Scholar] [CrossRef]
32. Aloui, A., Dumas-Gaudot, E., Daher, Z., Tuinen, D., Aschi-Smit, S. et al. (2012). Influence of arbuscular mycorrhizal colonisation on cadmium induced Medicago truncatula root isoflavonoid accumulation. Plant Physiology and Biochemistry, 60, 233–239. DOI 10.1016/j.plaphy.2012.08.014. [Google Scholar] [CrossRef]
33. Larose, G., Chênevert, R., Moutoglis, P., Gagné, S., Piché, Y. et al. (2002). Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. Journal of Plant Physiology, 159(12), 1329–1339. DOI 10.1078/0176-1617-00896. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |