
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014625
ARTICLE
Comparative Metabolomics Analysis between Red- and White-Flowered Common Buckwheat Cultivars
1Research Center of Buckwheat Industry Technology, School of Life Sciences, Guizhou Normal University, Guiyang, 550001, China
2State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, 430026, China
3Affiliated Hospital of Jianghan University, Wuhan, 430015, China
*Corresponding Authors: Qingfu Chen. Email: cqf1966@163.com; Pingfang Yang. Email: yangpf@hubu.edu.cn
Received: 13 October 2020; Accepted: 05 December 2020
#These authors contributed equally to this study
Abstract: Common buckwheat (Fagopyrum esculentum), a specialty crop in southwest China, is not only used as a supplement to primary grain crops but also to produce beverages, such as tea and wine. To fully exploit the products made from common buckwheat flower, ultra-performance liquid chromatography–electrospray ionization–tandem mass spectrometry (UPLC–ESI–MS/MS) was conducted to analyze the metabolites in red- (‘Guihong 2’) and white-flowered (‘Fengtian 1’) buckwheat cultivars. A total of 784 metabolites were identified of which flavonoids were the largest group with 191 components, followed by organic acids and derivatives (126), and amino acids and derivatives (95). Besides, dozens of phenylpropanoids, nucleotides and derivates, lipids, alkaloids as well as several kinds of indole derivatives and sterides were detected. Among these rich varieties of metabolites, 24 metabolites were only detected in the red flower that mainly included 8 anthocyanins and 6 flavones, while 22 metabolites were only detected in the white flower, which mainly contained 5 lipids, 5 flavonoids, and 5 organic acids and derivatives. Our results enrich the metabolites’ information of buckwheat and may be helpful for the exploitation of products from common buckwheat flowers.
Keywords: Common buckwheat; flowers; metabolites; flavonoid metabolites; anthocyanins
The flower is the plants’ most important organ, which indicates the start of the reproductive phase and determines the next generation. The colorful flowers not only attract insects that facilitate pollination but are also used for aesthetic value worldwide. Besides their ornamental value, many flowers can be used as herbal medicines due to their high content of bioactive components, such as on Saussurea involucrate [1], lonicera japonica [2], Carthamus tinctorius L. [3], Chrysanthemum [4], among others.
Common buckwheat (Fagopyrum esculentum) and tartary buckwheat (Fagopyrum tataricum) are the most extensively cultivated species of buckwheat, both belonging to the family Polygonaceae and originated from southwest China [5]. The former is mainly distributed around the world while the latter is widely cultivated in the highlands of the Himalayas and China. As one kind of important minor crops, buckwheat is usually utilized for several food types, including noodles, bread, cookies, pasta, tea, as well as liquor [6–8]. Buckwheat has been considered as the most nutritious among the major cereals, due to the seeds’ high quantity proteins, as well as being rich in minerals, vitamins, and especially the abundance of bioactive flavonoids like rutin, quercetin, orientin, homoorientin, vitexin, isovitexin and tannin [6,9]. These flavonoids have been documented to have beneficial functions, such as being anti-inflammatory, antioxidant, antidiabetic, and antihypertension [10,11]. Therefore, buckwheat is considered to have nutritional, prophylactic as well as faddish green functional food values [7,8].
Unlike the flowers of tartary buckwheat which only have a white or light green color with little nectar, common buckwheat flowers are multicolored (e.g., white, pink and red), and produce more nectar that is also beneficial for human health [12,13]. Therefore, common buckwheat is usually planted not only for food but also for supporting the beekeeping industry. Besides, due to its flourished and colorful flowers, common buckwheat can be utilized to create rural tourism, and this has become a buckwheat cultural festival that is held every year in some parts of China and Korea.
Buckwheat metabolites from seeds, sprouts as well as flowers have been studied up-to-date [14–18]. These studies focused mainly on phenolic metabolites, particularly flavonoids. However, as the source of buckwheat honey, flower tea, and other flower products, it’s quite essential to comprehensively assess the metabolites of common buckwheat flowers. In the present study, nine major substrates were identified from white and red common buckwheat flowers by ultra-performance liquid chromatography coupled with electrospray ionization-triple quadrupole-linear ion trap mass spectrometer (UPLC-ESI-Q TRAP-MS/MS). These metabolites mainly included polyphenols, amino acids and derivatives, alcohols, nucleotides and derivates, alkaloids, carbohydrates, terpenes, lipids and vitamins and derivatives. Meanwhile, a comparative analysis of these metabolites between these two cultivars with different flower colors was also carried out. This study will display the fully detected metabolites of common buckwheat flowers, and aims to completely reveal its nutritional composition and provide vital foundation for further product development from these flowers.
Two common buckwheat cultivars ‘Fengtian 1’ and ‘Guihong 2’, with white and red colored flowers, respectively (shown in Fig. 1), were planted in the experimental field of the Research Center of Buckwheat Industry Technology, Guizhou Normal University (1060 58’ E, 260 48’ N, Alt. 1245 m.a.s.l.). In September of 2018, fresh flowers without sepal were collected in 10-ml tubes on the onset of blooming and immediately frozen in liquid N2 and stored at –80°C for further use. Three biological replicates were collected for each sample.

Figure 1: Flowers of two common buckwheat cultivars, ‘Guihong 2’ (left) and ‘Fengtian 1’ (right)
2.2 Extraction of Metabolites from Common Buckwheat Flowers
The metabolites extraction was carried out according to previous reports [19]. Briefly, the freeze-dried flowers were crushed into a fine powder by a mixed mill (MM 400, Retsch) with a zirconia bead for 1.5 min at 30 Hz. About 100 mg of the frozen flower powder was extracted with 1.0 mL 70% aqueous methanol at 4°C overnight. This was followed by centrifugation at 10,000 g for 10 min. Then the extracts were absorbed with CNWBOND Carbon-GCB SPE Cartridge (250 mg, 3 ml; ANPEL, Shanghai, China) and filtrated with 0.22 μm pore size filters (SCAA-104, ANPEL, Shanghai, China) before LC-MS analysis.
A 2 μL aliquot of each extracted sample above was analyzed using a UPLC system (Shim-pack UFLC SHIMADZU CBM30A) by waters ACQUITY UPLC HSS T3 C18 column (1.8 μm, 2.1 mm × 100 mm) according to the method described by Wang et al. [20]. The solvent system contained two solutions, A and B. Solution A was Milli-Q water containing 0.04% acetic acid and solution B was acetonitrile (HPLC grade) containing 0.04% acetic acid. The gradient program was performed as follows: 0 min, 5% B; 0–11 min 95% B; 11–12 min, 95% B, and 12–15 min, 5% B, at a flow rate of 0.40 mL/min; the column temperature was kept at 30°C.
Identification of metabolites was carried on the API 6500 Q TRAP LC/MS/MS System, providing linear ion trap (LIN) and triple quadrupole (QQQ), and equipped with an ESI Turbo Ion-Spray interface. The ESI was performed in a positive ion mode and controlled by Analyst 1.6.3 software (AB Sciex). The parameters were set as follows: Turbo spray ion source; source temperature was 500°C; ion spray voltage was set at 5500 V; ion source gas I, gas II and curtain gas were set at 55, 60, and 25.0 psi, respectively; the collision gas was high. Instrument tuning and mass calibration were 10 and 100 μmol/L polypropylene glycol solutions in QQQ and LIT modes, respectively. QQQ scans were obtained by multiple reaction monitoring (MRM) experiments with collision gas (nitrogen) set to 5 psi. Decluttering potential (DP) and collision energy (CE) for individual MRM transitions were done with further DP and CE optimization. A specific set of MRM transitions were monitored for each period according to the metabolites eluted within this period [18,20].
2.5 Qualitative and Quantitative Analysis of Metabolites of Common Buckwheat Flowers
Qualitative analysis of metabolites was carried out using a widely targeted metabolome method by Wuhan Metware Biotechnology Co., Ltd. (Wuhan, China) (http://www.metware.cn/) based on the public databases and the company’s self-built database MWDB. Quantification analysis of metabolites was carried out using a multiple reaction monitoring (MRM) method.
Three biological replicates were performed in each experiment. Cluster analysis, PCA, and OPLS-DA were carried out by using R software. The differentially expressed metabolites were decided based on the VIP value (variable importance in project) ≥1.
3.1 Metabolites Profile of Flowers of Two Common Buckwheat Cultivars
Compared with the previous study, where only 144 metabolites were identified [15], here, a total of 784 metabolites were identified in the flowers of two common buckwheat cultivars. These metabolites were grouped into 16 groups (Fig. 2). Among them, the number of flavonoids was the most abundant (191, 24.4%), followed by organic acids and derivatives (126), and amino acids and derivatives (95). Meanwhile, common buckwheat flowers contained many types of phenylpropanoids, nucleotides and derivates, lipids, as well as alkaloids. Indole derivatives and sterides were the least (≤10). 24 metabolites only detected in the red flowers that mainly included anthocyanins, flavones, and a small amount of syringic acid and alkaloids. Meanwhile, 22 metabolites were only found in the white flowers with lipids, flavonoids, organic acids and derivatives being the most abundant with 5 components in each type.

Figure 2: Classification of the identified metabolites in flowers of two common buckwheat cultivars
3.2 Differential Metabolites Analysis between Two Common Buckwheat Cultivars
Two principal components (PC1, 66.52%, PC2, 9.32%, respectively) were chosen to display a clear separation and repeatability between these two common buckwheat cultivars (Fig. 3A). Meanwhile, orthogonal signal correction and partial least squares-discriminant analysis (OPLS-DA) were performed to distinguish maximumly between groups and facilitate the search of the differential metabolites. Parameters for evaluation in this model were R2X, R2Y and Q2; the closer their values to 1, they were more stable and reliable. Among them, Q2 represents the predictive ability of this model with a value > 0.9 being regarded as an excellent model. The OPLS-DA was used to compare the differences between these two cultivars (R2X = 0.671, R2Y = 0,997, Q2 = 0.984, Fig. 3B). Since Q2 were > 0.9, it demonstrated that this model was stable and reliable, and could be used for further screening of different metabolites.

Figure 3: Differential metabolites analysis is on the basis of principal component (PCA), and orthogonal signal correction and partial least squares-discriminant analyses (OPLS-DA). (A) PCA score plot, (B) OPLS-DA score plot. FT1, ‘Fengtian 1’; GH2, ‘Guihong 2’
The fold change and variable importance in the project (VIP) value of the OPLS-DA model were combined to screen differential metabolites between these two common buckwheat cultivars. A fold change value of ≥2 or ≤0.5 with a VIP ≥1 were used as the criteria for screening. Totally, 127 significantly different metabolites were screened with 78 up-regulated and 49 down-regulated (Fig. 4A), and the detailed information of these differential metabolites are shown in Appendix A. The content of metabolites showed quite differences between these two common buckwheat varieties based on the heatmap; most of the components were richer in red ‘Guihong 2’ than in ‘Fengtian 1’ (Fig. 4B).

Figure 4: (A) Volcanic plots of differential metabolites. The green dots represent down-regulated, differentially-expressed metabolites; the red dots the mean up-regulated, differentially-expressed metabolites, and the gray dots represent metabolites detected but showed no significant difference. (B) Clustering heat map of all differential metabolites with three biological repeats of each sample. Red and green colors indicate higher or lower contents of metabolites, respectively
Among these 127 differential metabolites, flavonoids were the most abundant (55, 43%), followed by organic acids and derivatives (14, 11%), phenylpropanoids (12, 10%) and amino acids and derivatives (11, 9%) (Fig. 5A). Other metabolites including lipids, indole derivatives, vitamins and derivatives, terpenes, carbohydrates, nucleotides and derivates, amines, alcohols as well as polyphenols were all less than 10 (among 2%~4%, respectively).
Except for five kinds of metabolites that included indole derivatives, vitamins and derivatives, amines, and amino acids and derivatives, which were richer on ‘Fengtian 1’ than on ‘Guihong 2’, the numbers of up-/down-regulated metabolites belonging to lipids and terpenes were equal in the two cultivars. Other metabolites, especially, flavonoids were more abundant in ‘Guihong 2’ (Fig. 5B).

Figure 5: (A) Categorization of the differential metabolites and percentage of each group. (B) Comparison of the numbers of differential metabolites belonging to each category between ‘Guihong 2’ and ‘Fengtian 1’ cultivars. Red and green boxes represent up-/down-regulated expressed metabolites in ‘Guihong 2’, respectively
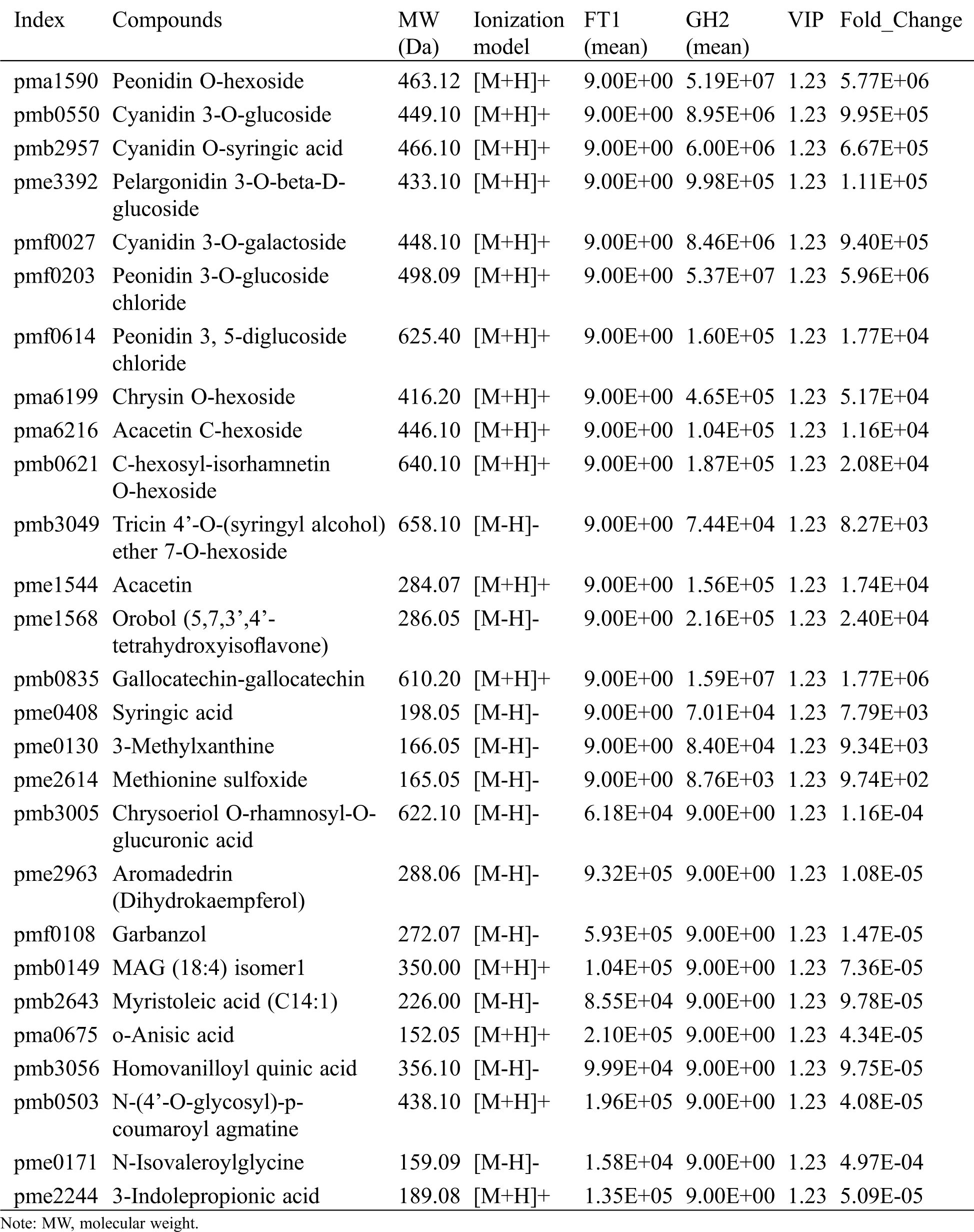
Seventeen metabolites could only be detected in the red flowers of ‘Guihong 2’, including 7 anthocyanins, 5 flavones, 1 isoflavone, 1 polyphenol, 1 phenylpropanoid, 1 nucleotide and derivates and 1 amino acid and derivatives (Tab. 1). ‘Fengtian 2’ contained only 10 specific metabolites that consisted of 3 flavonoids, 2 lipids, 2 organic acids and derivatives, 1 phenolamide, 1 amino acids and derivatives and 1 indole derivatives.
Table 1: Differential metabolites that were only identified in GH2 or FT1
3.3 Functional Annotation and Enrichment Analysis of Differential Metabolites
The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used to annotate the differential metabolites. The results of the KEGG classification indicated that most of the differential metabolites were involved in many metabolism biosynthesis, especially in biosynthesis of secondary metabolites (33) and metabolic pathways (27) (Fig. 6A). The minority were related to organismal system, human diseases, environmental information, processing, drug development as well as genetic information processing. Meanwhile, KEGG enrichment analysis (number > 5) showed that the flavonoid biosynthesis pathway had the largest number of enriched metabolites which was followed by flavone and flavonol biosynthesis pathways, anthocyanin biosynthesis as well as isoflavonoid biosynthesis (Fig. 6B), all being components of flavonoid compounds. Moreover, some metabolites enriched in aminobenzoate degradation, ubiquinone and other terpenoid-quinone biosynthesis, folate biosynthesis and carbohydrate digestion and absorption. Few metabolites belonged to the monoterpenoid biosynthesis, clavulanic acid biosynthesis, benzoic acid family, biotin metabolism, cutin, suberine and wax biosynthesis, to name a few.

Figure 6: (A) KEGG pathway assignment of the differential metabolites (top 50 pathways according to enrichment factor). (B) The significantly enriched KEGG pathways (P < 0.05) from the differential metabolites with the number >5
Common buckwheat usually is consumed as grains and despite having a lower yield than that of tartary buckwheat, it possesses abundant flower resources [21], which provide a high quality nectar source. In this study, we found that flowers of common buckwheat contained numerous types of metabolites, with most of them being beneficial secondary metabolites for humans, such as flavonoids, alkaloids, polyphenols, organic acids and derivatives, vitamins and derivatives, etc. Among them, like on seeds and leaves of buckwheat, flavonoids were rich bioactive compounds in flowers [17,22]. A total of 191 flavonoids were detected in two common buckwheat flowers, including 101 flavones, 38 flavanols, 21 anthocyanins, 18 flavanones, 10 isoflavone, and 3 proanthocyanins. It was similar with flavonoids in tartary buckwheat seed which had flavones and flavanols as the major flavonoid compounds with most of them belonging to glycosides [17]. Flavonoids are the main medicinal composition in many Chinese medicinal herbs, such as Scorzonera austriaca [23], lotus [24], and Epimedium brevicornu [25].
Sixteen anthocyanins showed differential accumulation between the white and red flowers which included 7 types of cyanidins, 3 peonidins, 1 pelargonidin, 1 petunidin, 1 delphinidin, 1 malvidin, 1 rosinidin and 1 apigeninidin chloride. Except for delphinidin which was not modified by glycosyl, all the other anthocyanins were modified (glycosyls, organic acid or chlorine). Previous studies reported that cyanidin 3-O-rutinoside was the main anthocyanin in tartary buckwheat sprouts [26], and only two (cyanidin 3-O-rutinoside and cyanidin 3-O-glucoside) were found in red-flower common buckwheat. However, no anthocyanin was detected in the white-flower cultivar by Kim et al. [15]. Our study, nevertheless, showed that both white and red flowers contained anthocyanins, and cyanidin 3-O-rutinoside was the highest, which was up-regulated in the red flower. This result differs with a previous study, which reported that cyanidin 3-O-rutinoside showed no differences among white, pink and red flowers of common buckwheat [17].
Despite the white and colorless color of flowers and seeds of white-colored common buckwheat, respectively, it was remarkable to detect certain kinds of anthocyanins [17,18]. In the present study, anthocyanins were also detected in white-flower buckwheat. We speculated that those anthocyanins that were only detected in the red flowers play the main role in flower pigmentation, including 3 cyanidins (cyanidin 3-O-glucoside, cyanidin O-syringic acid, cyanidin 3-O-galactoside), 3 peonidins (peonidin O-hexoside, peonidin 3, 5-diglucoside chloride, peonidin 3-O-glucoside chloride) and 1 pelargonidin (pelargonidin 3-O-beta-D-glucoside).
Besides, other bioactive and nutritious compounds in common buckwheat flowers were flavonoids. As the second abundant metabolites, organic acids and derivatives from plant have been reported to be useful for the treatment of diseases and infections [27,28], especially chlorogenic acid that can protect against liver fibrosis through high antioxidant activity [29,30]. These compounds had a high content in both red and white flowers. Plant lipids are essential nutrients for humans. Phenylpropanoids also have an antitumor function [31]. Alkaloids are also an important composition of many Chinese herbs, which have anti-inflammatory and antitumor, anticancer and anticholinergic activities [32,33].
Additionally, both common buckwheat flowers had unique metabolites. For example, 10 metabolites were only detected in the white flowers, including 3 flavonoids, 2 lipids, 2 organic acids and derivatives, 1 amine, 1 amino acid and derivative and 1 indole derivatives. While the red flowers of ‘Guihong 2’ had 17 unique metabolites with most of them being flavonoids, including 7 anthocyanins, 5 flavones and 1 isoflavone.
Therefore, from the above obtained metabolite data, common buckwheat flowers have the potential of being utilized in healthcare and food industries. Additionally, they can be used to make flower tea and buckwheat nectar, which has gained popularity in the recent past.
In addition to the nutritious seeds, common buckwheat flowers contain abundant metabolites, particularly flavonoids. A total of 784 metabolites were identified by UPLC–ESI–MS/MS in the flowers of two common buckwheat cultivars, of which, flavonoids were the most abundant (191). Besides, there were numerous organic acids and derivatives, amino acids and derivatives, phenylpropanoids, nucleotides and derivates, lipids, alkaloids, among others. One hundred and twenty-seven metabolites showed significant differences between these two common buckwheat cultivars. Meanwhile, flowers from two cultivars contained specific biologically active metabolites: the red flower contained more kinds of anthocyanins, while the white flower had several specific lipids, organic acids and derivatives. The present study suggested that common buckwheat flowers have abundant metabolites, especially flavonoids. Therefore, they could be utilized as potential sources in the pharmaceutical and food industries. For example, buckwheat flower tea or filled in some cakes to make flower cakes, or spread on the cake, which is similar to matcha. In addition, these results lay the foundation for further studies on the nutrition of common buckwheat and their potential functions.
Acknowledgement: We thank Dr. Rebecca Njeri Damaris for her help in English proof-reading.
Funding Statement: This work was funded by the National Natural Science Foundation of China-Project of Karst Science Research Center of Guizhou Provincial People’s Government (U1812401), the Science and Technology Foundation of Guizhou Province (QianKeHeJiChu [2020]1Y095), Guizhou provincial department of education youth science and technology talent growth project (Qianjiaohe KY Zi [2018]128), the National Natural Science Foundation of China (31701494, 31760419), the Initial Fund for Doctor Research in Guizhou Normal University (11904/0516026) and the Earmarked Fund for construction of the Key Laboratory for Conservation and Innovation of Buckwheat Germplasm in Guizhou (QianJiaoHe KY Zi [2017]002).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report the present study.
1. Han, X., Su, D., Xian, X., Zhou, M., Li, X. et al. (2016). Inhibitory effects of Saussurea involucrata (Kar. et Kir.) Sch. -Bip. on adjuvant arthritis in rats. Journal of Ethnopharmacology, 194, 228–235. [Google Scholar]
2. Miao, H., Zhang, Y., Huang, Z., Lu, B., Ji, L. (2019). Lonicera japonica attenuates carbon tetrachloride-induced liver fibrosis in mice: Molecular mechanisms of action. American Journal of Chinese Medicine, 47(02), 351–367. DOI 10.1142/S0192415X19500174. [Google Scholar] [CrossRef]
3. Cui, D., Zhao, D., Wang, B., Liu, B., Yang, L. et al. (2018). Safflower (Carthamus tinctorius L.) polysaccharide attenuates cellular apoptosis in steroid-induced avascular necrosis of femoral head by targeting caspase-3-dependent signaling pathway. International Journal of Biological Macromolecules, 116, 106–112. DOI 10.1016/j.ijbiomac.2018.04.181. [Google Scholar] [CrossRef]
4. Jeong, S. C., Kim, S. M., Jeong, Y. T., Song, C. H. (2013). Hepatoprotective effect of water extract from Chrysanthemum indicum L. flower. Chinese Medicine, 8(1), 7. DOI 10.1186/1749-8546-8-7. [Google Scholar] [CrossRef]
5. Kreft, I., Germ, M. (2008). Organically grown buckwheat as a healthy food and a source of natural antioxidants. Agronomski glasnik: Glasilo Hrvatskog agronomskog društva, 70(4), 397–406. [Google Scholar]
6. Joshi, D. C., Zhang, K., Wang, C., Chandora, R., Khurshid, M. et al. (2020). Strategic enhancement of genetic gain for nutraceutical development in buckwheat: A genomics-driven perspective. Biotechnology Advances, 39, 107479. DOI 10.1016/j.biotechadv.2019.107479. [Google Scholar] [CrossRef]
7. Christa, K., Soral-Śmietana, M. (2008). Buckwheat grains and buckwheat products-nutritional and prophylactic value of their components–A review. Czech Journal of Food Sciences, 26(3), 153–162. DOI 10.17221/1602-CJFS. [Google Scholar] [CrossRef]
8. Zhang, Z. L., Zhou, M. L., Tang, Y., Li, F., Tang, Y. et al. (2012). Bioactive compounds in functional buckwheat food. Food Research International, 49(1), 389–395. DOI 10.1016/j.foodres.2012.07.035. [Google Scholar] [CrossRef]
9. Jiang, P., Burczynski, F., Campbell, C., Pierce, G., Austria, J. et al. (2007). Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum, and F. homotropicum and their protective effects against lipid peroxidation. Food Research International, 40(3), 356–364. [Google Scholar]
10. Kreft, S. (2002). Rutin in buckwheat herbs grown at different UV-B radiation levels: Comparison of two UV spectrophotometric and an HPLC method. Journal of Experimental Botany, 53(375), 1801–1804. DOI 10.1093/jxb/erf032. [Google Scholar] [CrossRef]
11. Li, P. C., Piao, C. H., Zhang, L., Zhang, Y., Li, X. et al. (2017). Antidiabetic effect and mechanism of flavonoids extracted from bunckwheat hulls in type 2 diabetic rats. Food Science, 12(5), 335–344. [Google Scholar]
12. Zhou, J., Li, P., Cheng, N., Gao, H., Wang, B. et al. (2012). Protective effects of buckwheat honey on DNA damage induced by hydroxyl radicals. Food and Chemical Toxicology, 50(8), 2766–2773. DOI 10.1016/j.fct.2012.05.046. [Google Scholar] [CrossRef]
13. Hammond, N. A., Duster, M., Musuuza, J. S., Safdar, N. (2016). Effect of united states buckwheat honey on antibiotic-resistant hospital acquired pathogens. Pan African Medical Journal, 25, 212. DOI 10.11604/pamj.2016.25.212.10414. [Google Scholar] [CrossRef]
14. Kim, H. J., Park, K. J., Lim, J. H. (2011). Metabolomic analysis of phenolic compounds in buckwheat (Fagopyrum esculentum M.) sprouts treated with methyl jasmonate. Journal of Agricultural and Food Chemistry, 59(10), 5707–5713. DOI 10.1021/jf200396k. [Google Scholar] [CrossRef]
15. Kim, Y. B., Park, S. Y., Thwe, A. A., Min, J., Tastsuro, S. et al. (2013). Metabolomic analysis and differential expression of anthocyanin biosynthetic genes in white- and red-flowered buckwheat cultivars (Fagopyrum esculentum). Journal of Agricultural and Food Chemistry, 61(44), 10525–10533. DOI 10.1021/jf402258f. [Google Scholar] [CrossRef]
16. Kim, D. S., Kim, M. B., Lim, S. B. (2017). Enhancement of phenolic production and antioxidant activity from buckwheat leaves by subcritical water extraction. Preventive Nutrition and Food Science, 22(4), 345–352. DOI 10.3746/pnf.2017.22.4.345. [Google Scholar] [CrossRef]
17. Li, H., Lv, Q., Ma, C., Qu, J., Cai, F. et al. (2019). Metabolite profiling and transcriptome analyses provide insights into the flavonoid biosynthesis in the developing seed of tartary buckwheat (Fagopyrum tataricum). Journal of Agricultural and Food Chemistry, 67(40), 11262–11276. DOI 10.1021/acs.jafc.9b03135. [Google Scholar] [CrossRef]
18. Li, J., Hossain, M. S., Ma, H., Yang, Q., Gong, X. et al. (2020). Comparative metabolomics reveals differences in flavonoid metabolites among different coloured buckwheat flowers. Journal of Food Composition and Analysis, 85, 103335. DOI 10.1016/j.jfca.2019.103335. [Google Scholar] [CrossRef]
19. Dong, X., Chen, W., Wang, W., Zhang, H., Liu, X. et al. (2014). Comprehensive profiling and natural variation of flavonoids in rice. Journal of Integrative Plant Biology, 56(9), 876–886. DOI 10.1111/jipb.12204. [Google Scholar] [CrossRef]
20. Wang, A., Li, R., Ren, L., Gao, X., Zhang, Y. et al. (2018). A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas, (L.) Lam). Food Chemistry, 260, 124–134. DOI 10.1016/j.foodchem.2018.03.125. [Google Scholar] [CrossRef]
21. Amjad, K. W., Xilin, H., Ke, H., Nadeem, K., Dong, H. et al. (2018). Lipidomic study reveals the effect of morphological variation and other metabolite interactions on the lipid composition in various cultivars of Bok choy. Biochemical and Biophysical Research Communications, 56(3), 755–764. [Google Scholar]
22. Halbrecq, B., Romedenne, P., Ledent, J. F. (2005). Evolution of flowering, ripening and seed set in buckwheat (Fagopyrum esculentum MoenchQuantitative analysis. European Journal of Agronomy, 23(3), 209–224. DOI 10.1016/j.eja.2004.11.006. [Google Scholar] [CrossRef]
23. Zhu, F. (2016). Chemical composition and health effects of Tartary buckwheat. Food Chemistry, 203, 231–245. DOI 10.1016/j.foodchem.2016.02.050. [Google Scholar] [CrossRef]
24. Zhang, S., Xie, Y., Wang, J., Geng, Y., Zhou, Y. et al. (2018). Simultaneous determination of six bioactive components of total flavonoids of Scorzonera austriaca in rat tissues by LC-MS/MS: Application to a tissue distribution study. Revista Brasileira de Farma-cognosia, 28(2), 156–164. DOI 10.1016/j.bjp.2018.01.004. [Google Scholar] [CrossRef]
25. Zhu, M. Z., Wu, W., Jiao, L., Yang, P. F., Guo, M. Q. (2015). Analysis of flavonoids in lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules, 20(6), 10553–10565. DOI 10.3390/molecules200610553. [Google Scholar] [CrossRef]
26. Watanabe, M. (2014). An anthocyanin compound in buckwheat sprouts and its contribution to antioxidant capacity. Bioscience Biotechnology and Biochemistry, 71(2), 579–582. DOI 10.1271/bbb.60471. [Google Scholar] [CrossRef]
27. Pang, X., Yin, S. S., Yu, H. Y., Zhang, Y., Wang, T. et al. (2018). Prenylated flavonoids and dihydrophenanthrenes from the leaves of Epimedium brevicornu and their cytotoxicity against HepG2 cells. Natural Product Research, 32(19), 2253–2259. DOI 10.1080/14786419.2017.1405410. [Google Scholar] [CrossRef]
28. Shen, Q. P., Xu, X. M., Li, L., Zhao, W., Xiang, N. et al. (2016). ChemInform abstract: Sesquiterpenes from the leaves of Nicotiana tabacum and their anti-tobacco mosaic virus activity. Cheminform, 30(22), 2545–2550. [Google Scholar]
29. Igwe, O. (2014). Characterization of bioactive sesquiterpenes, organic acids and their derivatives from the leaves of Psidium guajava Linn. International Research Journal of Pure and Applied Chemistry, 4(4), 456–467. DOI 10.9734/IRJPAC/2014/8592. [Google Scholar] [CrossRef]
30. Shi, H. T., Shi, A., Dong, L., Lu, X., Wang, Y. et al. (2016). Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clinical Nutrition, 35(6), 1366–1373. DOI 10.1016/j.clnu.2016.03.002. [Google Scholar] [CrossRef]
31. Carvalho, A. A., Andrade, L. N., de Sousa, É. B. V., de Sousa, D. P. (2015). Antitumor phenylpropanoids found in essential oils. Biomed Research International, 2015, 1–21. [Google Scholar]
32. Lai, Y. W., Wang, S. W., Hu, Y. Y., Hwang, T., Cheng, M. et al. (2020). Anti-inflammatory alkaloids from the root bark of Hernandia nymphaeifolia. Phytochemistry, 173, 112326. DOI 10.1016/j.phytochem.2020.112326. [Google Scholar] [CrossRef]
33. Rajbir, K., Saroj, A. (2015). Alkaloids-important therapeutic secondary metabolites of plant origin. Journal of Critical Reviews, 2(3), 1–8. [Google Scholar]
Appendix A: The detail informations of differential metabolites between flowers of ‘Guihong 2’ and ‘Fengtian 1’.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |