
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014740
ARTICLE
Mycorrhizal Networks Interacting with Litter Improves Nutrients and Growth for One Plant through the Vary of N/P Ratio under Karst Soil
1Forestry College, Forest Ecology Research Center, Guizhou University, Guiyang, 550025, China
2College of Landscape Architecture and Life Science, Chongqing University of Arts and Sciences, Chongqing, 402160, China
3School of Pharmacy, Guizhou University of Traditional Chinese Medicine, Guiyang, 550025, China
*Corresponding Author: Yuejun He. Email: hyj1358@163.com
Received: 26 October 2020; Accepted: 24 November 2020
Abstract: Arbuscular mycorrhizae (AM) fungi affect nutrient uptake for host plants, while it is unclear how AM fungi interacting with soil litter affect plant growth and nutrient utilization through mycorrhizal networks in karst soil of deficient nutrients beyond the rhizosphere. An experiment was conducted in a microcosm composed of a planting compartment for Cinnamomum camphora seedlings with or without Glomus mosseae fungus (M+ vs. M−) and an adjacent litter compartment containing or not containing additional litter material of Arthraxon hispidus (L+ vs. L−), where the compartments are connected either by nylon mesh of 20 μm or 0.45 μm which either allow available mycorrhizal networks within the litter compartment or prevent mycelium entering into the litter compartment (N+ vs. N−). Plant biomass and nutrients were measured. The results showed that the addition of litter changed the symbiotic process in mycorrhizal colonization, spore, and hyphal density, which when in association with the host plant then affected the biomass, and accumulations of N (nitrogen) and P (phosphorus) in the individual plant as well as root, stem, and leaf respectively. AM fungi increased N and P accumulations and N/P ratio in individual plants and plant tissues. A decrease of the N/P ratio of the individual plant was observed when AM fungus interacted significantly with litter through mycorrhizal networks in the litter compartment. The results indicate that the C. camphora seedlings benefited from litter in nutrient utilization of N and P through the vary of N/P ratio when accessing mycorrhizal networks. These findings suggest that mycorrhizal networks interacting with litter improve growth and nutrients of N and P for plants through the vary of N/P ratio in order to alleviate nutrient limitation under karst soil.
Keywords: Arbuscular mycorrhizae; Cinnamomum camphora; litter; mycelium; nutrient utilization
Arbuscular mycorrhizal (AM) fungi as functional microbes form a symbiosis with the roots of 80% of terrestrial plant species [1–3] and play essential roles in exchanging carbohydrates and soil nutrients via root hyphae for host plant growth [4–6]. AM fungi are widely distributed in the natural ecosystem, including unique karst environments [7]. Generally, Glomus is the dominant genus that influences plant growth and community succession [8]. However, whether AM fungi are or are not able to supply their host with mineral nutrients through saprotrophic nutrition, that is directly from organic residues such as from releases from decomposing litter, remains uncertain [9,10]. For instance, AM fungi may find organically bound nutrients in litter even more accessible than inorganic forms in the underlying soil [11,12]. AM fungi have been found to promote decomposition of litter [13–15] and further Leigh et al. [16] discovered that AM networks could transport up to 1/3 of N from litter nutrients to host plant; He et al. [10] also confirmed that mycorrhizal networks could transfer more N from labeled leaf litter to the host. However, AM networks scavenging for mineral nutrients and the deposition of fungal material can increase soil aggregation and then may prevent organic matter decomposition [17,18]. Additionally, AM mycelium by networks may reduce the decomposition of litter due to competition with other litter decomposers [19,20], increasing negative impacts on plant nutrient uptakes. Therefore, the degree to which AM fungi by mycorrhizal networks increases plant growth by facilitating litter nutrient absorption remains unclear at present.
Litter is an essential component in soil nutritional transformation supporting plant growth and is also the critical component of nutrient cycles in returning nutrients to the soil in the forest ecosystem [21–23], especially in nutrient-deficient habitat. For instance, it is speculated that plant growth is still maintained within some karst ecosystems even under conditions of severe soil erosion because litter releases compensating nutrients through decomposition that plants can utilize via their AM fungi symbionts instead of nutrients plants would otherwise obtain from soil [24]. Litter decomposition is a fundamental process that links plant and microbial communities [25,26]. Under nutrient limitation, the microorganism can enhance soil enzyme activities to accelerate nutrient release from organic matter [27]. For example, Atul-Nayyar et al. [13] found that AM fungi could promote organic residues decomposition by regulating soil microbial biomass and enhancing soil enzyme activities.
Furthermore, Kong et al. [15] found that under low nutrient conditions, mycorrhizal fungi can regulate the rate of N mineralization to accelerate the decomposition of litter. These processes changed soil microbial compositions [28], which are essential for plant growth. AM fungi have been demonstrated to absorb and transport N and P from soil to plants [29]. Additionally, Thirkell et al. [30] discovered that mycorrhizal mycelium as a network could enter into organic matter patches and thereby increase plant N and P and increase plant biomass. However, Loydi et al. [31] argued that litter primarily affected seedling emergence as well as reduced seedling biomass, and Li et al. [32] found litter had a neutral effect on early growth of native plants. Thus, AM fungi via mycorrhizal networks interacting with litter may affect soil properties, nutrient conversion, and plant growth within nutrient-deficient soil and thus may be relevant within typical karst habitats, which are often nutrient deficient with lower P and N due to severe soil erosion.
Karst soil is developed from carbonatite and contains relatively low quantities of N and P nutrients [33–35], and is found in a range of microhabitats such as stone facings, stone trenches, swallets and abundant rocky outcrops [36] distributed widely in southwest China [37]. Karst ecosystems provide many niches for microbes, such as mycorrhizal fungi [38]. Restoring vegetation in the karst areas of China is a high priority for ecological reconstruction. Therefore, the potential application of AM fungi for the enhancement of plant growth and restoration of plants and soil within the karst ecosystem is worth exploring in more detail. Litter decomposition can maintain soil fertility [39], further suggesting the possibility of plant growth through nutrient uptake via AM fungi symbionts through the above-mentioned pathways. However, how AM fungi interacting with soil litter through mycorrhizal networks affects plants in terms of their growth and nutrient utilization in nutrient-deficient karst soil remains unclear. According to previous research works, we hypothesized that: (H1) litter could increase plant growth and nutrient absorption via AM fungi in karst soil; (H2) AM fungi colonization and litter addition would increase the N/P ratio of the plant through mycorrhizal networks.
A microcosm experiment was conducted using a black perspex container (Fig. 1) divided into a planting compartment and litter compartment (each 95 mm × 100 mm × 115 mm; length × width × height) in the west campus of Guizhou University, Guiyang city, China (106°22′E, 29°49′N, 1,120 m above the sea level). The Cinnamomum camphora seedlings were planted into the planting compartment, and litter was added into the litter compartment. Four circular holes of 20 mm diameter were drilled in the center of the baffle between the planting compartments and the litter compartment, and each interval was 1 cm. A hole of 20 mm diameter was opened at the bottom of each compartment to permeate water.
The experimental treatments included AM fungus treatments (M) within the planting compartment, the litter addition treatments (L) within the litter compartment, and the nylon mesh treatments (N) attached on both sides of baffle separating the planting and litter compartments for either forming mycorrhizal networks or no available mycelium. The AM fungus treatments included the inoculation with 50 g Glomus mosseae inoculum (M+) and the control without AM fungal inoculum (M−). AM inoculum was purchased from the Institute of Nutritional Resources, Beijing Academy of Agricultural and Forestry Sciences (BGGAM0046). The plant species supplying the litter for the experiment is Arthraxon hispidus, which is a widespread Gramineae plant co-existing with tree species generally in the same habitats of southwest China. Field surveys preceding this experiment revealed that there is less litter remaining in natural habitat in C. camphora stand companying with A. hispidus plant. Therefore, we speculated that A. hispidus litter might have a substantial effect on the growth and nutrition of C. camphora as a critical nutrient carrier on the soil. Thus, the A. hispidus litter was used in this experiment, which had contents of nitrogen (N) 4.38 mg.g−1, phosphorus (P) 1.17 mg.g−1, potassium (K) 9.91 mg.g−1 and carbon (C) 396 mg.g−1 (collected from natural karst habitat near to Guiyang). A. hispidus litter was immersed into 3% H2O2 for 10 minutes and washed three times with sterile water. Approximately 2 g of the litter of 0.5 cm−1 cm fragments were wrapped within a 20 μm nylon bag (5 cm × 2.5 cm) and was then added into the litter compartment as the L+ treatment; the L− treatment did not receive the bag of litter. The mycorrhizal networks treatments by as nylon mesh included the N+ treatment, which had a double 20 μm nylon mesh attached to the baffle for forming mycorrhizal networks in the planting compartment, the N− treatment, which had a double 0.45 μm mesh separating the two compartments to prevent mycelium and roots as the control. The 20 μm nylon mesh of the N+ treatment allows AM mycelium to pass through the baffle plate separating the planting compartment and litter compartment while plant roots cannot pass. However, the 0.45 μm nylon mesh of the N− treatment prevents both mycelium and roots from accessing the litter compartment from the planting compartment, which was shown by Hodge et al. [9]. Each treatment contained four replicates.
The initial substrate of 2.65 kg was created by mixing karst soil and sand (1:1 by volume) and filled into each compartment, and was sterilized at 0.14 Mpa at 126°C for one hour, and had pH 6.90, total N of 0.403 g.kg−1, available N of 148.75 mg.kg−1, total P of 0.699 g.kg−1 and available P of 18.73 mg.kg−1, using the measurement of methods from Zhang [40]. Five seeds of C. camphora were planted into the substrate of the planting compartment, and one plant was kept after germination. All treatment materials were cultivated in a greenhouse for 18 weeks. The mycorrhizal colonization rate was determined after the roots were cleaned with distilled water; then the C. camphora seedlings were divided into roots, stems and leaves, and put into the oven to be sterilized at 105°C for 30 min, dried at 65°C to constant weight for determining biomass, N and P accumulations. The soil was packed with bags and taken back to the laboratory, removing the impurities in the soil and then determining hyphal density and spore density.
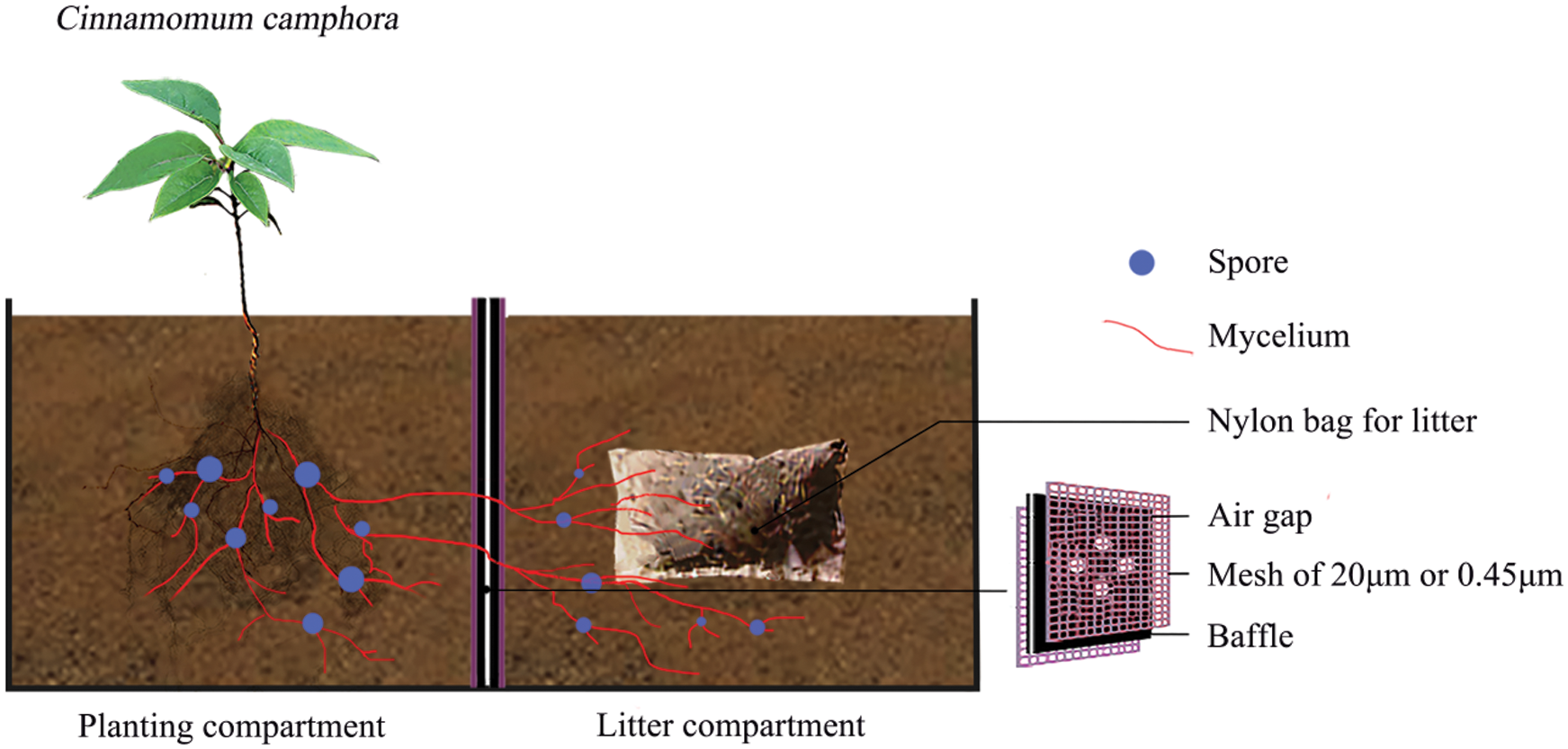
Figure 1: A diagram of the plant growth experimental microcosm. The setup was composed of a black perspex container which consisted of two adjacent compartments (95 mm × 100 mm × 115 mm; length × width × height). Within the planting compartment C. camphora seedlings were planted and were either inoculated with Glomus mosseae (M+ treatment) or not (M− treatment), and litter was added to the adjacent litter compartment (L+ treatment) or not (L− treatment). Four circular holes of 20 mm diameter in the center of the baffle were drilled between the planting compartments and litter compartment, and each interval was 1 cm. A 20 mm diameter hole was opened at the bottom of each compartment to permeate water. A double nylon mesh of 20 μm or 0.45 μm was attached on both sides of the compartmental baffle separating the planting and litter compartments; the mycorrhizal networks treatment using 20 μm nylon mesh (N+ treatment) allows mycelium to pass through from one compartment to the adjacent compartment but does not allow roots to pass, while the 0.45 μm nylon mesh (N− treatment) prevents both mycelium and roots from passing between the planting and litter compartments according to a comparable experimental setup from Hodge et al. [9]
2.2 Determinations of Root Mycorrhizal Colonization Rate, Spore Density, Hyphal Density, Plant Biomass, and Concentrations of Nitrogen and Phosphorus
Root mycorrhizal colonization rate was determined by the methods of Brundrett et al. [41]. Spore density was determined by the methods of Khakpour et al. [42], and hyphal density was measured according to Abbott et al. [43]. Plant biomass was obtained by weighing roots, stems and leaves respectively dried at 105°C for 24 h. N concentration was determined by the Kjeldahl method, and P concentration was determined by anti-colorimetric methods [40]. Accumulations of N and P were calculated through the concentration multiplied by biomass.
Statistical analysis was performed with SPSS 22.0 software (Company: International Business Machines Corp; Origin: www.ibm.com/legal/copytrade.shtml). All of the data were tested for normality and homogeneity of variance before analysis. Three-way ANOVAs were applied for the effects of AM fungus (M+ vs. M−) and litter addition (L+ vs. L−) and nylon mesh treatment (N+ vs. N−) and their interactions on plant biomass production and the accumulation of N and P and N/P ratio. The t-test was applied to compare differences between M+ and M−, N+ and N−, L+ and L− treatments on plant biomass, accumulations of N and P and the N/P ratio at p ≤ 0.05 levels. All statistical photographs were drawn by Origin 2018 software.
3.1 Root Mycorrhizal Colonization, Spore Density and Hyphal Density of C. camphora Seedlings
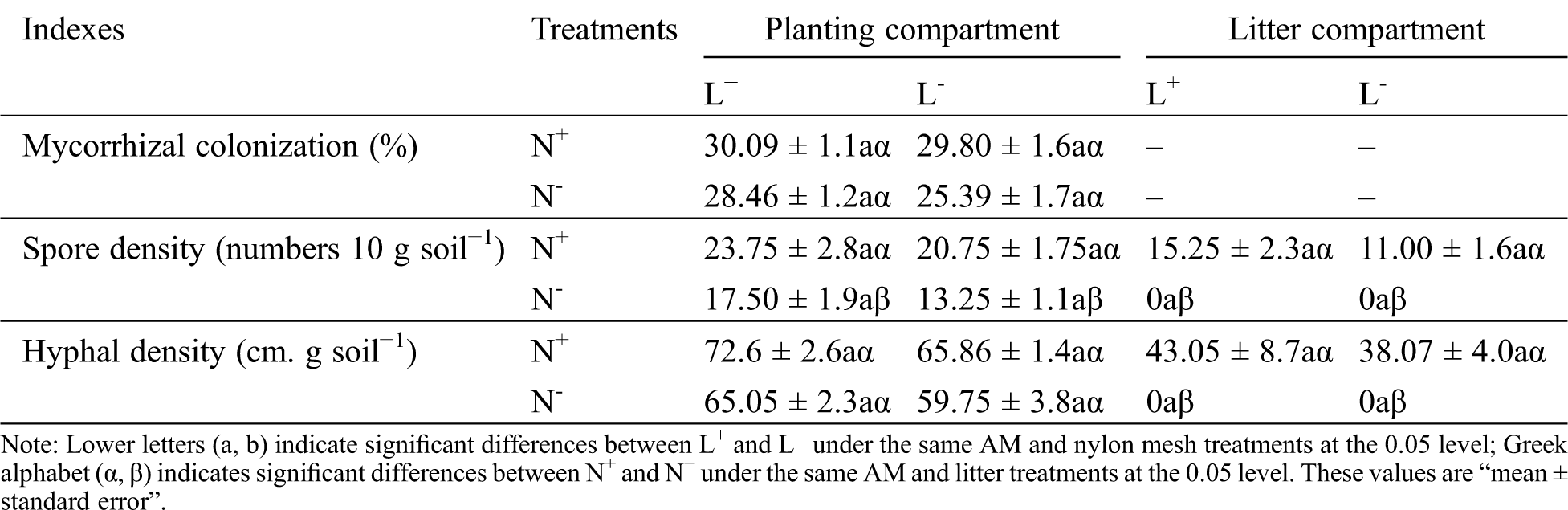
When considering the planting and litter compartments together, differences in root mycorrhizal colonization, spore density, and hyphal density between L+ and L− as well as N+ and N−, were not significant, with the exception of spore density which was significantly greater under N+ than under N− (Tab. 1). In particular, spore and hyphal density were found to be lower in the litter compartment soil than in the planting compartment soil under N+, as no spores and hyphae were detected in litter compartment soil under N−. This result showed that AM mycelium passed through 20 μm mesh from the planting compartment to the litter compartment forming mycorrhizal networks for further propagation, indicating that the addition of litter can change the symbiotic process in the soil far away from the rhizosphere by external root mycelia of the host plant; meanwhile, the 0.45 μm mesh effectively separated mycelium growth from the litter compartment.
Table 1: Root mycorrhizal colonization of C. camphora seedlings, spore density and hyphal density of soil in the planting compartment and litter compartment under the M+ treatment
3.2 Plant Biomass of C. camphora Seedlings and Allocation to Root, Stem and Leaf
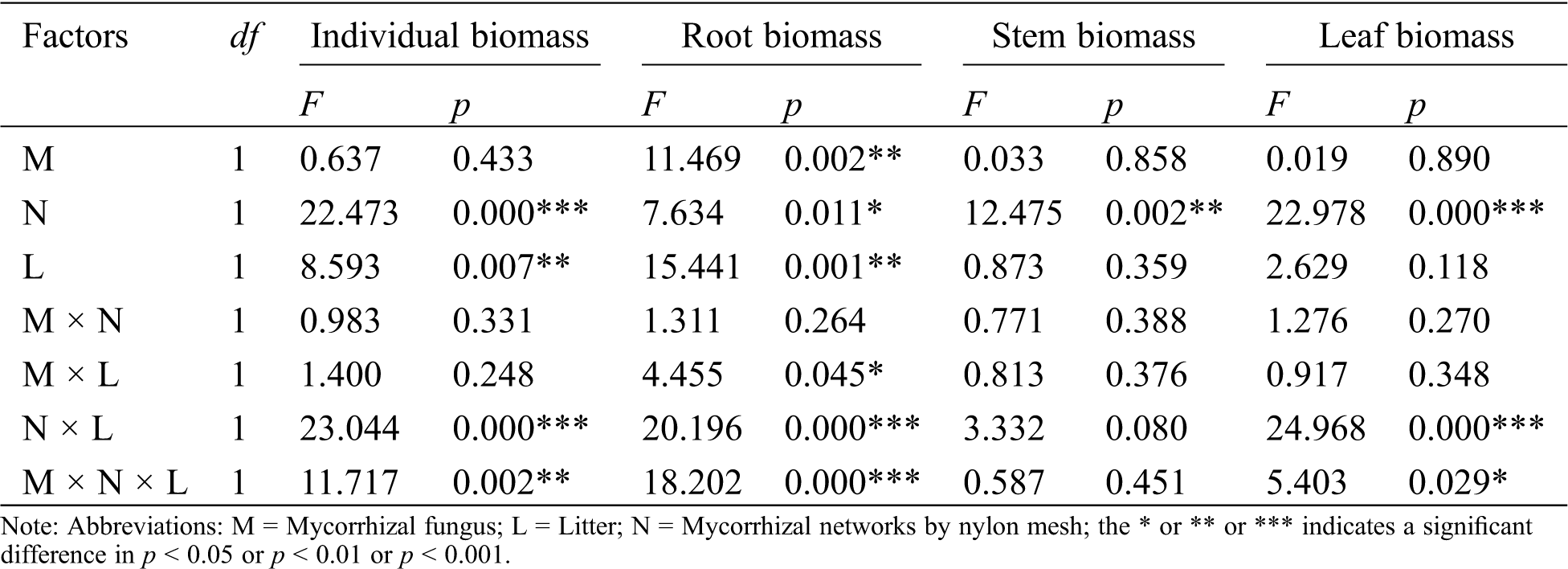
The AM fungus treatment (M) significantly affected root biomass of C. camphora seedlings (Tab. 2), but not stem or leaf or individual plant; for individual biomass, the M+ treatment was significantly greater than the M− treatment under L+ of the N+ treatment in comparison to L− of the N+ treatment (Fig. 2a); a significant M+ > M− difference was found under L+ in root biomass and whereas a significant M+ < M− difference was found under L− in root and leaf biomass (Figs. 2b and 2d), however, others treatments were not significantly different in the same conditions in stem biomass (Fig. 2c). Moreover, the nylon mesh treatment (N), namely the mycelium litter access condition, affected the biomass of C. camphora seedlings significantly including its individual plant biomass and allocations to root, stem and leaf (Tab. 2); for the individual plant, the N+ biomass was significantly greater than N− under L+ under both the M+ and M− treatments and under L− of the M− treatment (Fig. 2a); for root, the biomass of N+ was significantly higher than N− under L+ of N+ while the N+ was significantly lower than N− under L− of M+ (Fig. 2b); the stem and leaf biomass of N+ was significantly greater than N− under L+ under both the M+ and M− treatments (Figs. 2c and 2d). Furthermore, the litter addition treatment (L) significantly affected the biomass of C. camphora seedlings including individual plant and root (Tab. 2); L+ was significantly greater than L− under M+ of N+ in individual, root and stem biomass (Figs. 2a–2c); for leaf, the biomass of L+ was significantly greater than L− under M+ of N+, but L+ was significantly lower than L− under M+ of N− (Fig. 2d). In addition, the interactions of N × L and M × N × L significantly affected the biomass of individual, root and leaf but not the stem; however, M × L only significantly affected the root biomass while M × N did not significantly affect the biomass of C. camphora seedlings (Tab. 2). These results indicate that the biomass accumulation of C. camphora seedlings was affected by mycorrhizal networks interacting with soil litter in soil far away from the adjacent rhizosphere compartment.
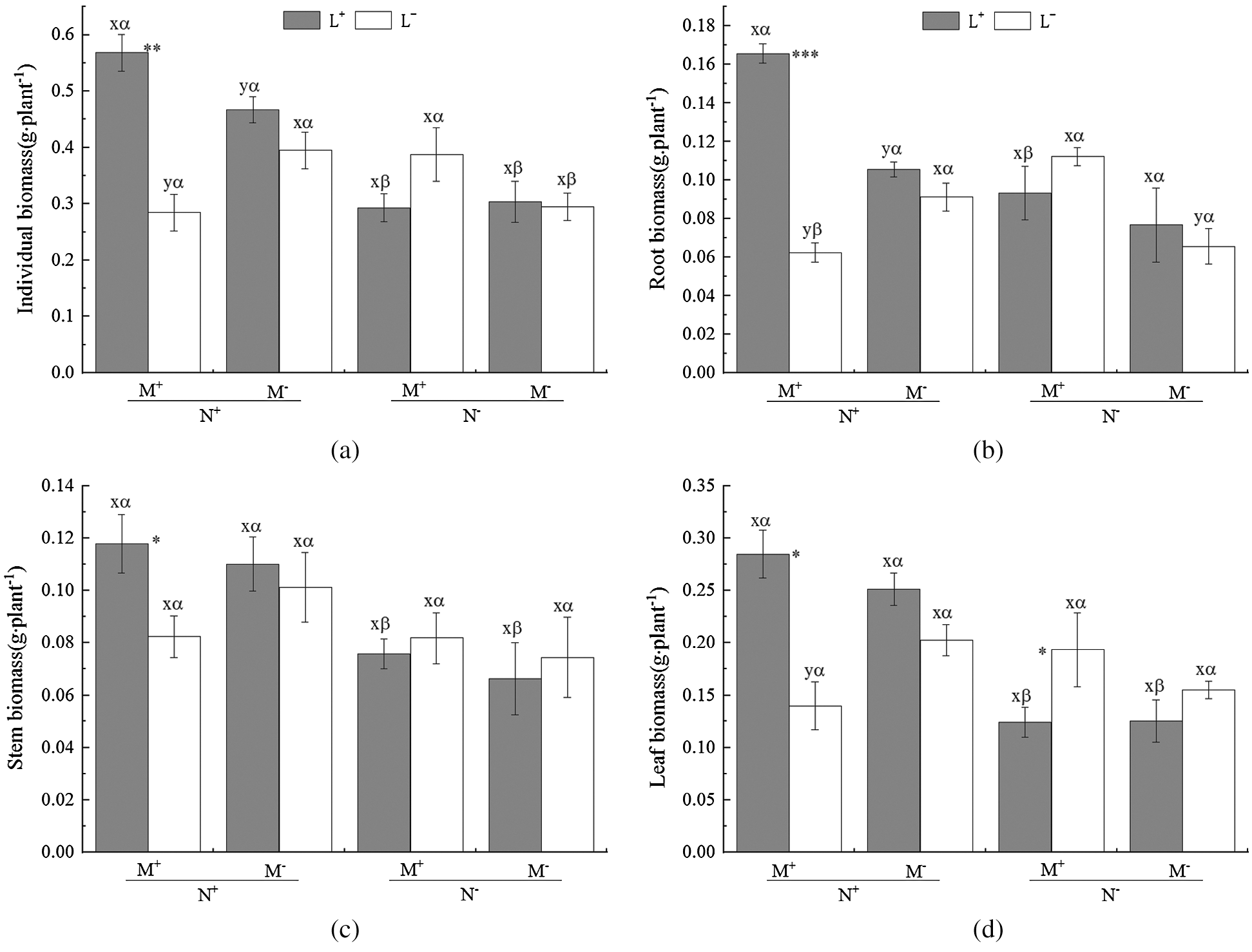
Figure 2: The biomass of C. camphora seedling and its allocation to root, stem and leaf. Abbreviation: M+ = Mycorrhizal fungus was used to inoculate C. camphora seedlings; M− = Mycorrhizal fungus was not used to inoculate C. camphora seedlings; L+ = Litter was added in the litter compartment; L− = Litter was not added in the litter compartment; N+ = mycorrhzial networks forming which AM mycelium passing through the compartment baffle attached by 20 μm nylon mesh; N− = none mycorrhzial networks which AM mycelium could not pass through the compartment baffle attached by 0.45 μm nylon mesh. The means ± standard error values are shown in the figure. Lower letters (x, y) indicate significant differences between M+ and M− under the same litter and nylon mesh treatments at the 0.05 level; Greek alphabet (α, β) indicates significant differences between N+ and N− under the same AM and litter treatments at the 0.05 level; *, **, and *** indicate significant differences in p ≤ 0.05, ≤ 0.01, ≤ 0.001 and no asterisk indicates p > 0.05 between L+ and L− under the same AM and nylon mesh treatments
Table 2: ANOVAS for effects of AM fungus (M+ vs. M−), mycorrhizal networks by nylon mesh (N+ vs. N−) and litter (L+ vs. L−) on the biomass of C. camphora seedlings
3.3 N Accumulation of C. camphora Seedlings and Allocation to Root, Stem and Leaf
The AM fungus treatment (M) significantly affected the N accumulation of C. camphora seedlings including individual, root, stem and leaf (Tab. 3); in individual plant, a significant M+ > M− difference was found, ranked leaf > root > stem under L+ of N+, and also under L+ and L− of N− in root and leaf (Figs. 3a–3d).
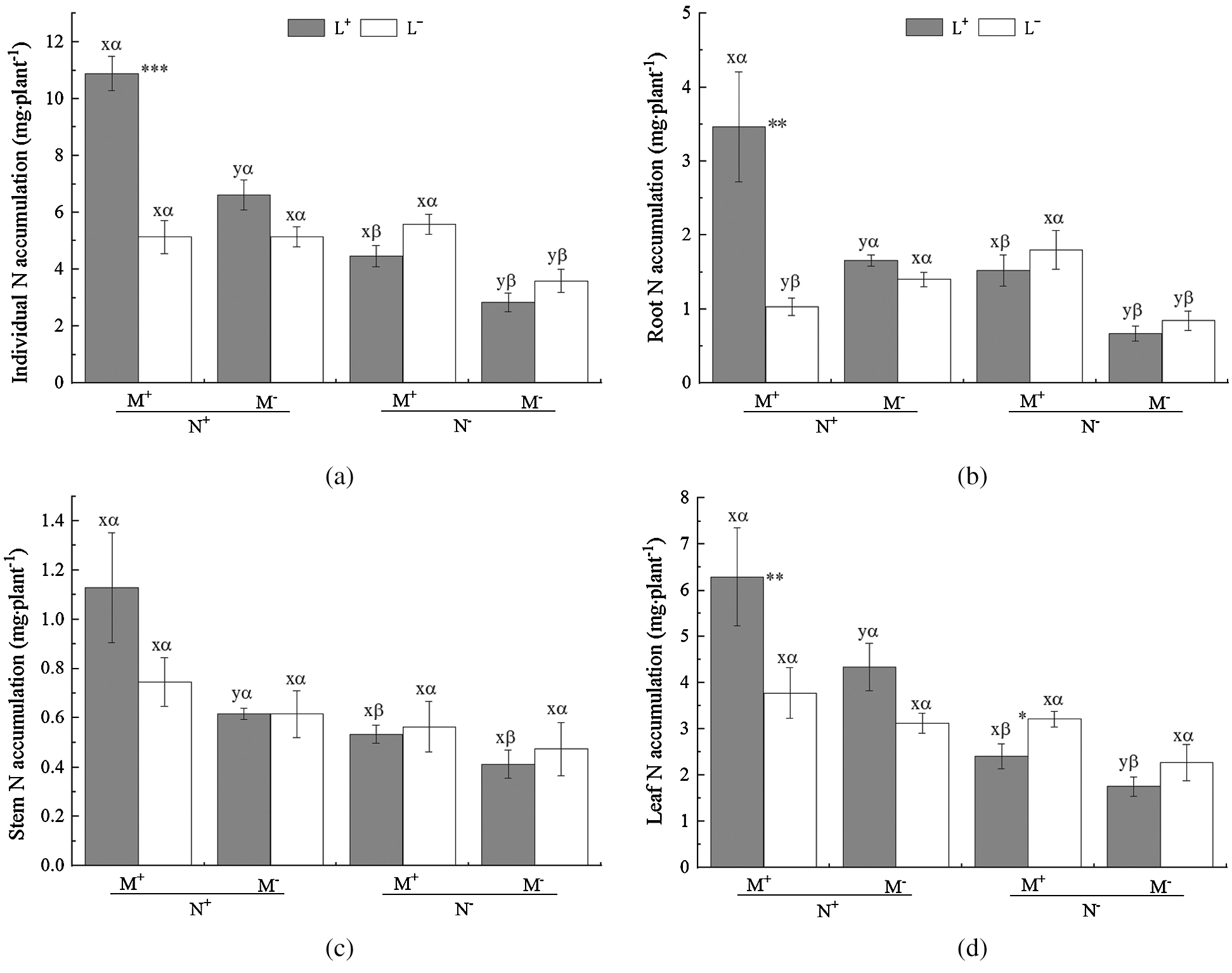
Figure 3: N accumulation of C. camphora seedling and allocation to root, stem and leaf. See Fig. 2 for an explanation of M+, M−, L+, L−, N+ and N−, lowercase letters, *, **, and ***
Under L− of N+ the opposite difference (M+ < M−) in root plant biomass was observed (Fig. 3b). In addition, the nylon mesh treatment (N) significantly affected N accumulation of C. camphora seedlings including its individual, root, stem and leaf (Tab. 3); the N+ treatment significantly increased N accumulation of individual plant and all plant parts for both L+ and L− except for a significant decrease under M+ of L− in the root and a non-significant difference in individual, stem, and leaf (Figs. 3a–3d). Moreover, the litter addition treatment (L) significantly affected N accumulation of C. camphora seedlings in individual, root and leaf but not for stem (Tab. 3), where L+ significantly increased N accumulation compared to L− under M+ of N+ (Figs. 3a, 3b and 3d) while the L+ < L− was observed in leaf N accumulation (Fig. 3d); however, there were no significant differences between L+ and L− under other treatments (Figs. 3a–3d). Furthermore, the interactions of M × L and N × L and M × N × L significantly affected N accumulations for individual and root but not for stem. Additionally, the interactions of N × L and M × N × L also significantly affected leaf N accumulations (Tab. 3). Overall, these results indicate that AM fungi significantly promoted N accumulation of C. camphora seedlings through mycorrhizal networks interacting litter in the soil far away from the adjacent rhizosphere compartment.
Table 3: ANOVAS for effects of AM fungus (M+ vs. M−), mycorrohizal networks by nylon mesh (N+ vs. N−) and litter (L+ vs. L−) on the N accumulation of C. camphora seedlings
3.4 P Accumulation of C. camphora Seedlings and Allocation to Root, Stem and Leaf
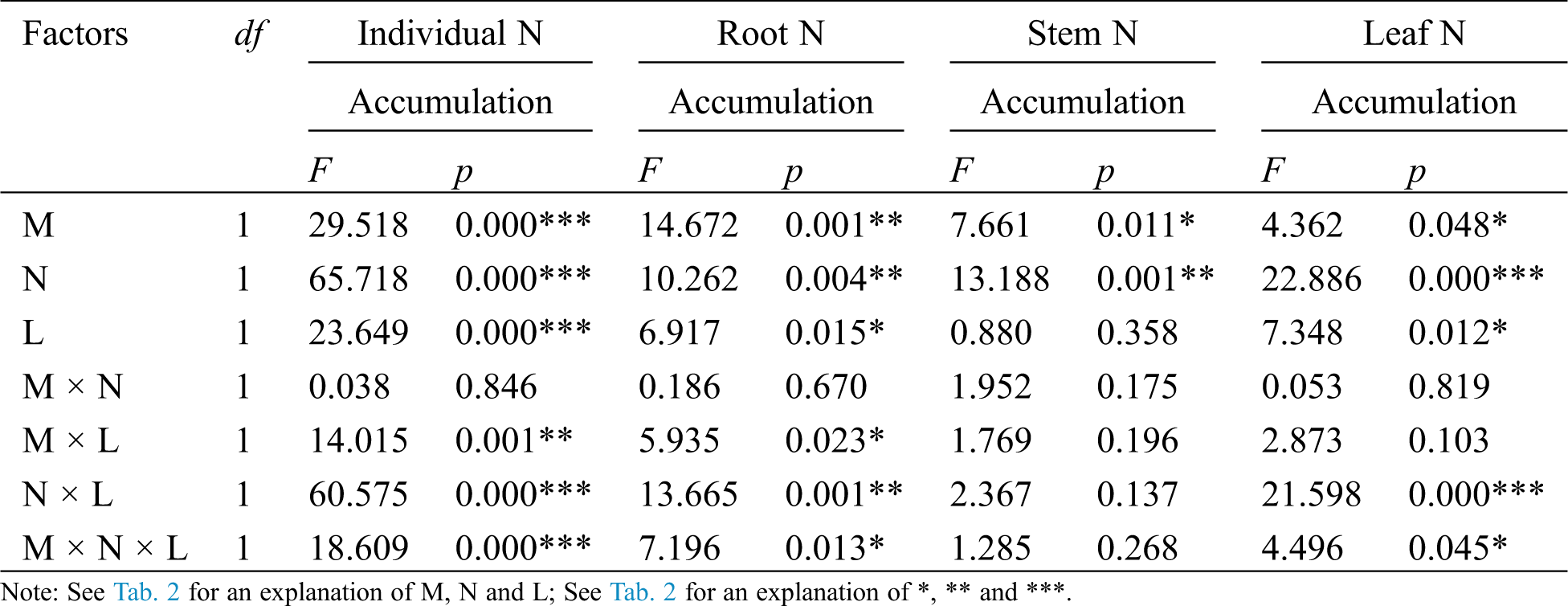
On the whole the AM fungus treatment (M) had a non-significant effect on the P accumulation of C. camphora seedlings including root and stem and leaf (Tab. 4); however, the M+ treatment significantly increased P accumulation compared to the M− treatment under L+ of the N+ treatment (Figs. 4a–4d), but significantly decreased P accumulation in root and stem (Figs. 4b and 4c). In addition, the nylon mesh treatment (N) significantly affected P accumulation in the individual plant, stem and leaf but not root (Tab. 4) where N+ > N− under L+ of M+ and L− of M− (Figs. 4a and 4c); a significant N+ < N− difference in P accumulation was observed in root under L− of M+ (Fig. 4b). Moreover, the litter addition treatment (L) significantly affected P accumulation of C. camphora seedlings in individual plant, root, stem and leaf (Tab. 4), where L+ > L− under all M+ of N+ (Figs. 4a–4d) and M− of N+ in leaf (Fig. 4d) and N− in the root (Fig. 4b). However, L+ was significantly lower than L− under M+ of N− (Fig. 4d). Furthermore, the significant interaction of M × N affected leaf P accumulation, and M × L significantly affected individual P accumulation; meanwhile N × L significantly affected individual and leaf P accumulations when M × N × L significantly affected individual, root, stem and leaf (Tab. 4). Overall, these results indicate AM fungi interacting with litter in the soil far away from the adjacent rhizosphere compartment significantly promoted P accumulation of C. camphora seedlings via mycorrhizal networks.
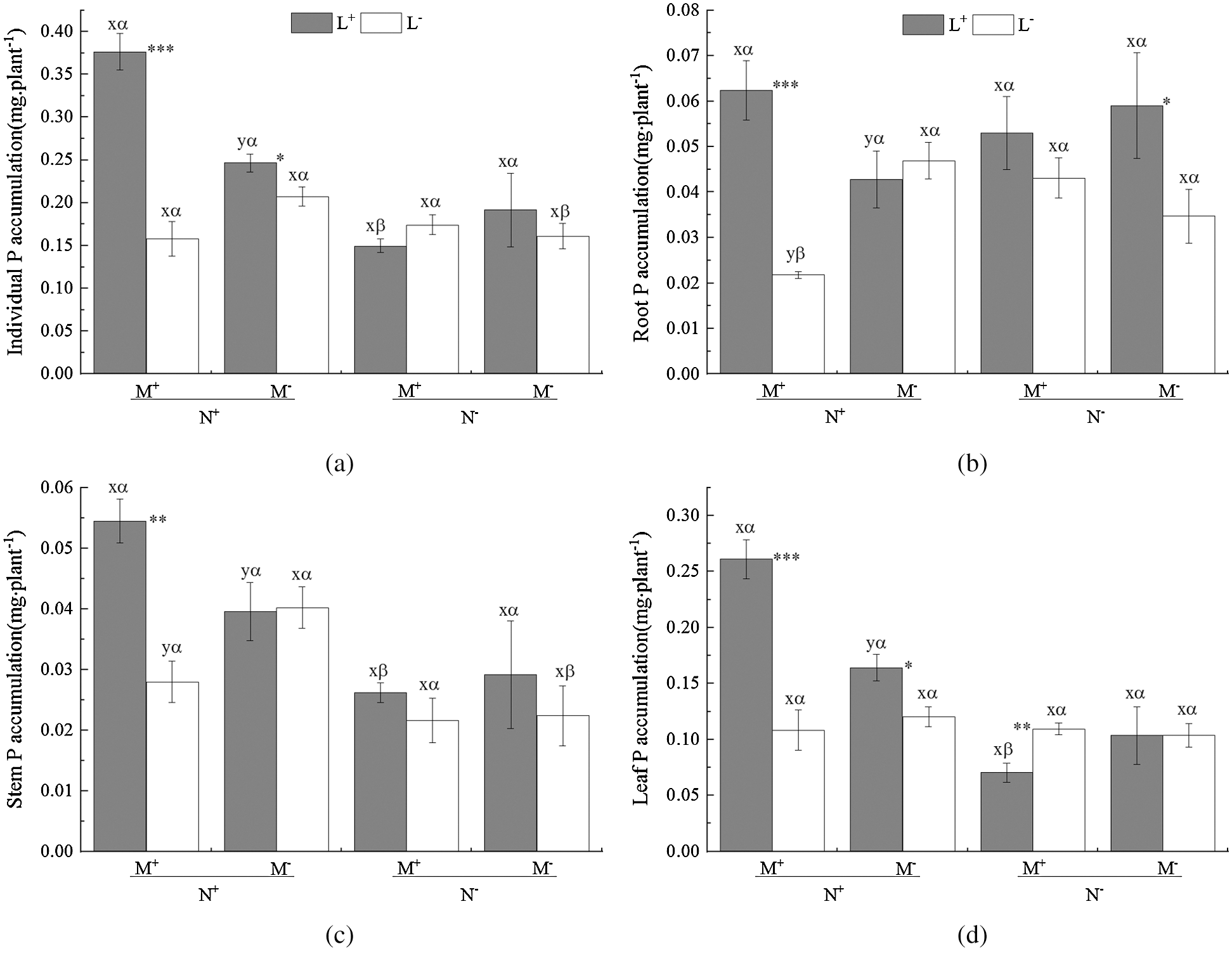
Figure 4: P accumulation of C. camphora seedling and allocation to root, stem and leaf. See Fig. 2 for an explanation of M+, M−, L+, L−, N+ and N−, lowercase letters, *, **, and ***
Table 4: ANOVAS for effects of AM fungus (M+ vs. M−), mycorrohizal networks by nylon mesh (N+ vs. N−) and litter (L+ vs. L−) on the P accumulation of C. camphora seedlings
3.5 N/P Ratios of C. camphora Seedlings, Root, Stem and Leaf
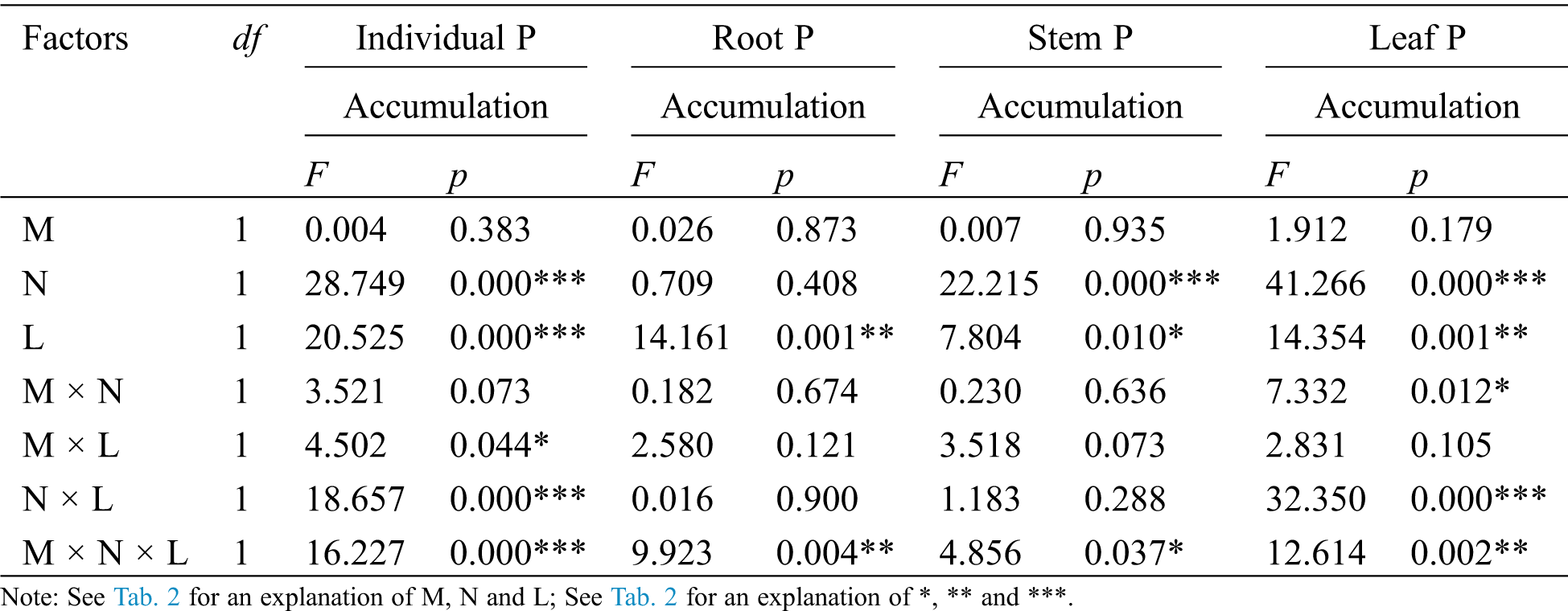
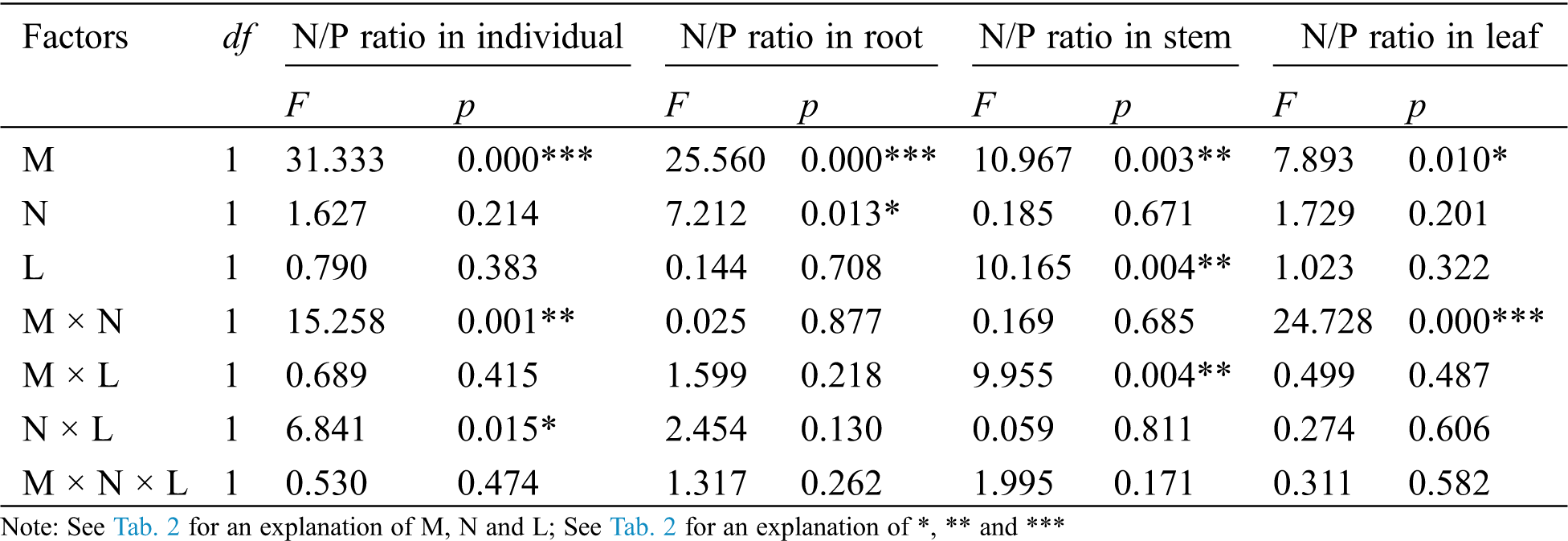
The AM fungus treatment (M) significantly affected the individual plant, root, stem and leaf N/P ratio (Tab. 5), where significant increases were observed in the individual under N− under both L+ and L− (Fig. 5a), and in root under N+ and N− of L− (Fig. 5b), and in stem under L− (Fig. 5c) as well as in leaf under N− (Fig. 5d) when comparing M+ with M−. The nylon mesh treatment (N) did not significantly affect N/P ratio of C. camphora seedlings except for root (Tab. 5); for the individual plant, the N/P ratio of M+ was significantly lower under L− and were significantly higher under M− of L+ although L+ > L− (Fig. 5a); the N/P ratio in root of N+ was significantly greater than N− under L+ of both M+ and M− treatments (Fig. 5b), leaf N/P ratio was significantly lower under N+ than N− under both the L+ and L− treatments of M+ (Fig. 5d). Moreover, the litter addition treatment (L) significantly affected the N/P ratio of the stem (Tab. 5), where L+ < L− under M+ but not under M− (Fig. 5c). Furthermore, significant interactions were observed in the individual plant for M × N and N × L, in the stem for M × L and in leaf for M × N (Tab. 5). Overall, these results indicate that AM fungi increased the N/P ratio of C. camphora seedlings, but decreased plant N/P ratio when it interacted with litter via mycelium in the soil far away from the adjacent rhizosphere compartment of this experiment.
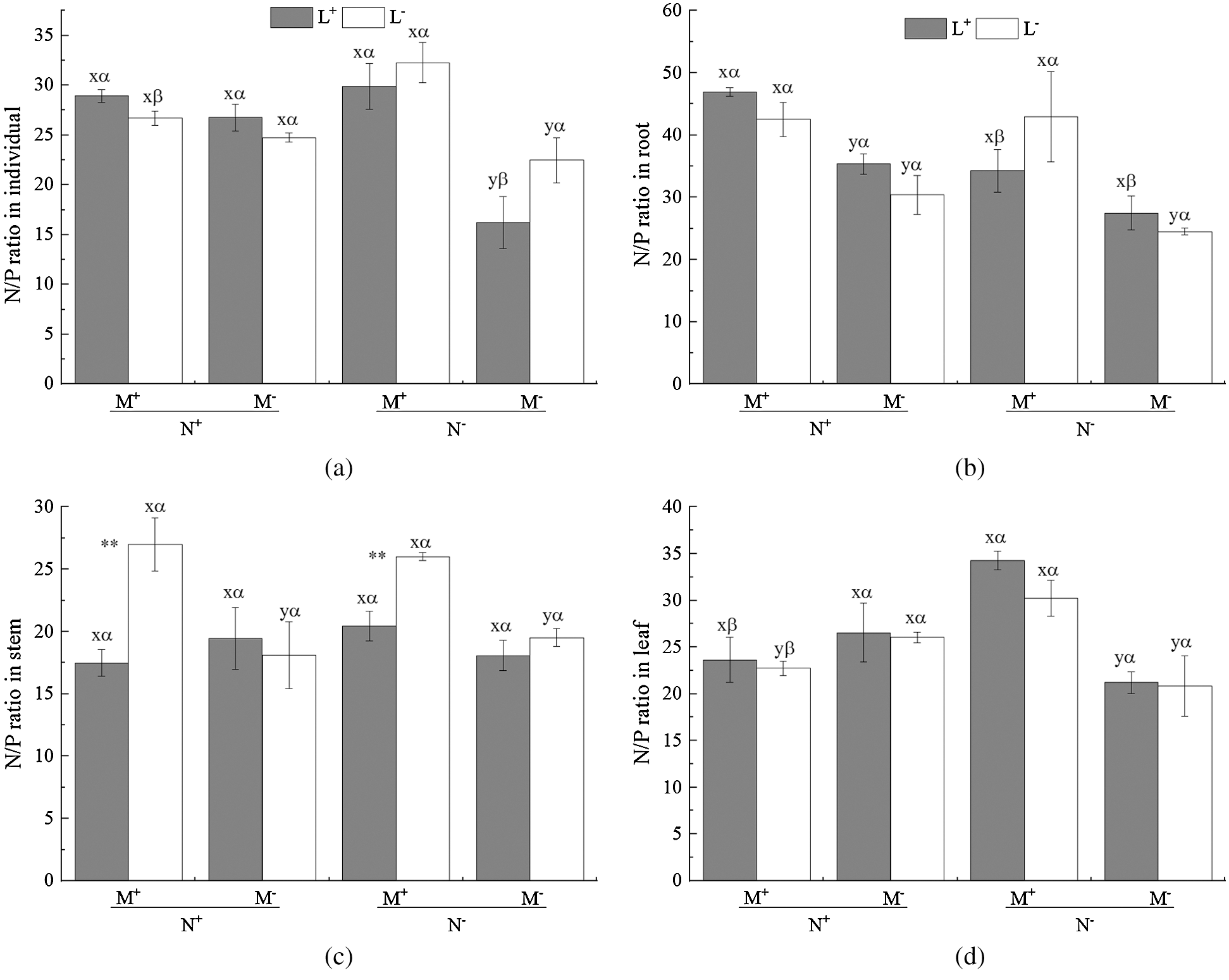
Figure 5: N/P ratio of C. camphora seedlings, root, stem and leaf. See Fig. 2 for an explanation of M+, M−, L+, L−, N+ and N−, lowercase letters, *, **, and ***
Table 5: ANOVAS for effects of AM fungus (M+ vs. M−), mycorrohizal networks by nylon mesh (N+ vs. N−) and litter (L+ vs. L−) on the N/P ratio of C. camphora seedlings
In our study, AM fungus differentially increased N accumulation of C. camphora seedlings with the following rank order of leaf > root > stem (Figs. 3b–3d). These results are consistent with Zhang et al. [44] regarding how AM fungi promoted differential N allocation in parts of plant tissues. Sun et al. [45] reviewed research showing that increased leaf N can improve the photosynthetic capacity for biomass production. Consistent with those findings, we found that the M+ biomass was significantly greater than M− biomass under L+ of N+ (Fig. 2a), indicating that the biomass enhancement was caused by the increased N accumulation obtained by AM fungus interacting with the added litter through associating mycelium. AM fungi can enlarge the absorption area for nutrient utilization via root external mycelium [46], and can transfer nutrients for C. camphora seedlings [47,48], and AM fungi can provide additional nutrients for roots [5], which is mainly as a result of their hyphae facilitating the accumulation and rapid delivery of ammonium N to the plants [49]. However, AM fungus did not have significant effects on the P accumulation of C. camphora seedlings tissue as a whole (Tab. 4), which differs from previous studies which showed that AM symbiosis significantly increased the P accumulation [50,51]. The present study did obtain higher P accumulation under the M+ treatment compared to the M− under L+ of N+ (Fig. 3). Simultaneously, the interactions of M × L and N × L and M × N × L significantly affected plant individual P accumulation (Tab. 4). These results show that interaction is essential to plant P utilization. Plants exude a wide range of organic compounds and inorganic ions into the rhizosphere, activating mineral nutrients [52] in P accumulations. In this study, substrate soil had a relatively low concentration with a total P of 0.699 g.kg−1 and available P of 18.73 mg.kg−1, which is lower than the average level of soil N and P in China [53], as litter is a potential carrier of nutrients, litter may increase available P nutrients in the soil by release through decomposition [54]. However, the release of P from litter requires a process that alone probably leads to largely non-significant effects, but may increase decomposition when AM mycelium is present and interacting with litter in the early stages of plant development. This experiment demonstrated that AM plays an important role in N and P nutrient acquisition for the host plant C. camphora seedlings through mutualist mycorrhizal networks extending from the planting compartment to the litter in the adjacent compartment.
Wang et al. [55] showed that leaf N/P ratio could be used as a predictor for determining nutrient limits on plant growth. Low N/P ratio (<14) reflects an N limitation, whereas a high N/P ratio (>16) likely reflects P limitation, and an N/P ratio between 14 and 16 reflects co-limitation by both N and P [56]. In this study, the N/P ratio of C. camphora seedlings tissue was greater than 16 in the individual plants (22.47 ± 2.3) and root (24.53 ± 0.5) and leaf (20.83 ± 3.3) and stem (19.52 ± 0.7) under the condition without litter and fungi and mycorrhizal networks (Fig. 5), indicating plant seedlings were extremely restricted by P in the experiment substrate. However, the N/P ratio on average was increased when plants were inoculated with AM fungi and received added litter, meaning that AM fungi and litter increased the ability of the plants to accumulate N and P nutrients, even when the N/P value was greater than 16 which was suggested to reflect P limitation. This result can be explained by the fact that AM fungi significantly affected N accumulation but not P accumulation. Nevertheless, it is interesting that the N/P ratio of N+ was lower than N− under M+ and the N/P ratio of L+ was greater than L− under N+ for individual plants (Fig. 5a), indicating that mycorrhizal networks alleviated P limitation according to Wang et al. [55] and that litter compensated for N and P for seedlings. Zhang et al. [57] found that plants are P limited in karst habitat. C. camphora is a common plant species in karst regions, generally in P limited soil. Given that karst soil is often low in soil nutrients such as N and P, AM fungi may play an essential role by increasing access to N and P by accessing surrounding litter through their mycorrhizal networks. P deficiency may also cause compensating promotion of utilization of other nutrients such as N to maintain growth [58], a scenario which is consistent with our results where mycelia are associated with and accessing litter. The litter addition also significantly reduced the stem N/P ratio from (27.00 ± 2.1) to (17.48 ± 1.0) (Fig. 5c) under M+ treatments indicating that litter relieved in tissues or compensated the P nutrient in individual level, because AM fungi can likely promote P mineralization in soil or litter [59]. The results also verified our hypotheses H1 and H2. Overall, AM fungi combined with litter have a positive effect on plant growth in nutrient deficient karst soil.
Previous studies has shown that leaf litter continuously releases C, N and P nutrients into the surrounding soil solution during the decomposition process; AM mycelium can transform these nutrients and provide them to host plants [60]. Additionally, Kong et al. [15] found that when soil nutrient levels decreased, the promotion effect of AM fungi on litter decomposition began to appear, especially for N mobilization, and then AM mycelium transfers these newly mobilized nutrients from litter decomposition to supply their host plants when grown under low nutrient conditions. Barrett et al. [61] found that AM mycelium networks enhanced plant (including both root and shoot) biomass and plant P content at a suitable temperature and also transferred 15N to host plant tissues. This is consistent with our results. AM fungi can absorb mineral nutrients in the soil [62], or can transfer nutrients from organic residues (e.g., litter) via mycelium, thereby promoting nutrient accumulation via AM mycelium networks and increasing plant biomass [10]. Additionally, Shu et al. [63] found that AM fungi release organic acids, soil enzymes, and other substances by themselves or by inducing symbiotic plants, which activate the fixed mineral nutrients in the soil and improve the effective concentration of mineral nutrients, which is beneficial for plant nutrient absorption. Furthermore, the mineral nutrient transporters on AM mycelium ensure the efficiency of nutrient transport from soil to mycorrhizal mycelium [64] making nutrient transfer to plants faster [65]. The promoting effect of AM fungi on litter decomposition is related to the nutrient content of the soil in low nutrient conditions, e.g., in karst soil, AM fungi can regulate the rate of N mineralization and accelerate the decomposition of litter [15]. Additionally, the AM mycelium exudates organic acids and enzymes into soil, a process that will enhance soil organic matter decomposition and nutrient release [66] then the AM mycelium transfers nutrients from litter to their host affecting the host plant’s growth. For example, Xu et al. [67] found that the host plant can acquire more nutrients (P and N) through the AM mycelium from organic matter when soil P availability was low. This result was also confirmed by He et al. [10] who found that AM mycelium networks can promote litter decomposition and transfer N from litter to host plants in karst habitat. In this experiment, the AM mycelium contacted litter patches and then promoted the absorption of nutrients of C. camphora seedlings. This pattern probably means that AM networks transferred nutrients from litter patches to the host plant. In this study, C. camphora seedlings were likely P limited as the N/P ratio was greater than 16 in all N− treatments of plant tissues, with the rank order being root > leaf > stem (Fig. 5). When AM mycelium forming networks had access to the litter compartment (N+) differentially reduced the N/P ratio in individual and leaf (Figs. 5a and 5d); the possible explanation may be that the microbial community was altered under litter addition [68] forming a microbial flora distinct from the rhizosphere [69], and causing the contribution of mycorrhizal networks to P uptake to differ from N uptake [70], by reducing the N/P ratio, thus alleviating the P limitation of karst soil. In summary, one of the functions of AM networks in karst habitats may include the promotion of nutrients mobilization and acquisition from leaf litter. However, further studies are required to determine the mechanism underlying AM fungi’s role in nutrient regulation released into soil from litter. Specifically, isotope trace studies are needed to determine whether AM mycelium networks directly decompose litter or if AM fungi indirectly regulates microbes in the decomposition of litter.
In this experiment, available litter in adjacent soils can change the symbiotic process by the extend mycelium networks from host plant roots for spores and hyphal proliferation. Litter interacting through AM affected C. camphora plants by significantly increasing accumulations of biomass and N and P in root, stem and leaf, when in association with mycorrhizal networks of G. mosseae fungus. AM fungi also increased N accumulations and N/P ratio on the whole. P accumulations were increased in the individual and plant tissues of root, stem, and leaf; the individual N/P ratio was decreased when the available litter through interacting mycorrhizal networks. In conclusion, we suggest that plants in the growth and nutrition utilization benefit from mycorrhizal networks interacting with litter in order to alleviate nutrient limitation via the vary of N/P ratio under deficient nutrient karst soil.
Acknowledgement: We thank Yuejun He for designing this experiment, Jianpeng Si and Ying Yang for conducting the experiment. We also thank Kaiping Shen, Tingting Xia, Qiyu Tan, Yun Guo, Bangli Wu for their assistance during the data analysis and thankLipeng Zang, Wei Wang, Qin Liang for the revision.
Funding Statement:This study was supported by the National Natural Science Foundation of China (NSFC: 31660200; 31660156; 31360106; 31700539), the First-class Disciplines Program on Ecology of Guizhou Province (GNYL[2017]007), the Guizhou High level (Hundred-level) Innovative Talents Project (Qian-ke-he platform talents [2020] 6004), the Provincial Key Technologies R&D Program of Guizhou Province of China (NY[2014]3029; [2016] Zhi-cheng 2805), the Talent-platform Program of Guizhou Province ([2017]5788; [2018]5781), and the Doctor starts Fund Project of Guizhou University of Traditional Chinese Medicine ([2020]15).
Conflicts of Interest:The authors declare that they have no conflicts of interest to report regarding the present study.
1. Augé, R. M. (2001). Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza, 11(1), 3–42. DOI 10.1007/s005720100097. [Google Scholar] [CrossRef]
2. Brundrett, M. C. (2009). Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil, 320(1–2), 37–77. DOI 10.1007/s11104-008-9877-9. [Google Scholar] [CrossRef]
3. Strack, D., Fester, T., Hause, B., Schliemann, W., Walter, M. H. (2003). Arbuscular mycorrhiza: Biological, chemical, and molecular aspects. Journal of Chemical Ecology, 29(9), 1955–1979. DOI 10.1023/A:1025695032113. [Google Scholar] [CrossRef]
4. Chen, K., Shi, S., Yang, X., Huang, X. (2014). Contribution of arbuscular mycorrhizal inoculation to the growth and photosynthesis of mulberry in Karst Rocky Desertification Area. Applied Mechanics and Materials, 488–489, 769–773. DOI 10.4028/www.scientific.net/AMM.488-489.769. [Google Scholar] [CrossRef]
5. Van Der Heijden, M. G. A., Martin, F. M., Selosse, M. A., Sanders, I. R. (2015). Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytologist, 205(4), 1406–1423. DOI 10.1111/nph.13288. [Google Scholar] [CrossRef]
6. Wilkinson, T. D. J., Ferrari, J., Hartley, S. E., Hodge, A. (2019). Aphids can acquire the nitrogen delivered to plants by arbuscular mycorrhizal fungi. Functional Ecology, 33(4), 576–586. DOI 10.1111/1365-2435.13283. [Google Scholar] [CrossRef]
7. Wei, Y., Wang, S. J., Liu, X. M., Huang, T. Z. (2012). Molecular diversity and distribution of arbuscular mycorrhizal fungi in Karst ecosystem, Southwest China. African Journal of Biotechnology, 11(80), 14561–14568. DOI 10.5897/AJB12.587. [Google Scholar] [CrossRef]
8. Bavaresco, L., Cantu, E., Trevisan, M. (2000). Chlorosis occurrence, natural arbuscular-mycorrhizal infection and stilbene root concentration of ungrafted grapevine rootstocks growing on calcareous soil. Journal of Plant Nutrition, 23(11–12), 1685–1697. DOI 10.1080/01904160009382133. [Google Scholar] [CrossRef]
9. Hodge, A., Campbell, C. D., Fitter, A. H. (2001). An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature, 413(6853), 297–299. DOI 10.1038/35095041. [Google Scholar] [CrossRef]
10. He, Y., Cornelissen, J. H. C., Zhong, Z., Dong, M., Jiang, C. (2017). How interacting fungal species and mineral nitrogen inputs affect transfer of nitrogen from litter via arbuscular mycorrhizal mycelium. Environmental Science and Pollution Research, 24(10), 9791–9801. DOI 10.1007/s11356-017-8649-6. [Google Scholar] [CrossRef]
11. Bunn, R. A., Simpson, D. T., Bullington, L. S., Lekberg, Y., Janos, D. P. (2019). Revisiting the ‘direct mineral cycling’ hypothesis: Arbuscular mycorrhizal fungi colonize leaf litter, but why? ISME Journal, 13(8), 1891–1898. DOI 10.1038/s41396-019-0403-2. [Google Scholar] [CrossRef]
12. Sheldrake, M., Rosenstock, N. P., Revillini, D., Olsson, P. A., Mangan, S. et al. (2017). Arbuscular mycorrhizal fungal community composition is altered by long-term litter removal but not litter addition in a lowland tropical forest. New Phytologist, 214(1), 455–467. DOI 10.1111/nph.14384. [Google Scholar] [CrossRef]
13. Atul-Nayyar, A., Hamel, C., Hanson, K., Germida, J. (2009). The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza, 19(4), 239–246. DOI 10.1007/s00572-008-0215-0. [Google Scholar] [CrossRef]
14. Gui, H., Hyde, K., Xu, J., Mortimer, P. (2017). Arbuscular mycorrhiza enhance the rate of litter decomposition while inhibiting soil microbial community development. Scientific Reports, 7(1), 42184. DOI 10.1038/srep42184. [Google Scholar] [CrossRef]
15. Kong, X., Jia, Y., Song, F., Tian, K., Lin, H. et al. (2018). Insight into litter decomposition driven by nutrient demands of symbiosis system through the hypha bridge of arbuscular mycorrhizal fungi. Environmental Science and Pollution Research, 25(6), 5369–5378. DOI 10.1007/s11356-017-0877-2. [Google Scholar] [CrossRef]
16. Leigh, J., Hodge, A., Fitter, A. H. (2009). Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist, 181(1), 199–207. DOI 10.1111/j.1469-8137.2008.02630.x. [Google Scholar] [CrossRef]
17. Rillig, M. C., Wright, S. F., Eviner, V. T. (2002). The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant and Soil, 238(2), 325–333. DOI 10.1023/A:1014483303813. [Google Scholar] [CrossRef]
18. Wilson, G. W. T., Rice, C. W., Rillig, M. C., Springer, A., Hartnett, D. C. (2009). Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecology Letters, 12(5), 452–461. DOI 10.1111/j.1461-0248.2009.01303.x. [Google Scholar] [CrossRef]
19. Brzostek, E. R., Dragoni, D., Brown, Z. A., Phillips, R. P. (2015). Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytologist, 206(4), 1274–1282. DOI 10.1111/nph.13303. [Google Scholar] [CrossRef]
20. Leifheit, E. F., Verbruggen, E., Rillig, M. C. (2015). Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biology and Biochemistry, 81, 323–328. DOI 10.1016/j.soilbio.2014.12.003. [Google Scholar] [CrossRef]
21. Melillo, J. M., Aber, J. D., Muratore, J. F. (1982). Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology, 63(3), 621–626. DOI 10.2307/1936780. [Google Scholar] [CrossRef]
22. Sayer, E. J., Tanner, E. V. J. (2010). Experimental investigation of the importance of litterfall in lowland semi-evergreen tropical forest nutrient cycling. Journal of Ecology, 98(5), 1052–1062. DOI 10.1111/j.1365-2745.2010.01680.x. [Google Scholar] [CrossRef]
23. Vitousek, P. M. (1984). Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology, 65(1), 285–298. DOI 10.2307/1939481. [Google Scholar] [CrossRef]
24. Pan, F., Zhang, W., Liang, Y., Liu, S., Wang, K. (2018). Increased associated effects of topography and litter and soil nutrients on soil enzyme activities and microbial biomass along vegetation successions in Karst ecosystem, southwestern China. Environmental Science and Pollution Research, 25(17), 16979–16990. DOI 10.1007/s11356-018-1673-3. [Google Scholar] [CrossRef]
25. Idbella, M., Zotti, M., Cesarano, G., Fechtali, T., Mazzoleni, S. et al. (2019). Fungal endophytes affect plant response to leaf litter with contrasting chemical traits. Community Ecology, 20(2), 205–213. DOI 10.1556/168.2019.20.2.10. [Google Scholar] [CrossRef]
26. Li, Y., Bezemer, T. M., Yang, J., Lü, X., Li, X. et al. (2019). Changes in litter quality induced by N deposition alter soil microbial communities. Soil Biology and Biochemistry, 130, 33–42. DOI 10.1016/j.soilbio.2018.11.025. [Google Scholar] [CrossRef]
27. Phillips, R. P., Finzi, A. C., Bernhardt, E. S. (2011). Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecology Letters, 14(2), 187–194. DOI 10.1111/j.1461-0248.2010.01570.x. [Google Scholar] [CrossRef]
28. Nuccio, E. E., Hodge, A., Pett-Ridge, J., Herman, D. J., Weber, P. K. et al. (2013). An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environmental Microbiology, 15(6), 1870–1881. DOI 10.1111/1462-2920.12081. [Google Scholar] [CrossRef]
29. Hodge, A., Fitter, A. H. (2010). Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proceedings of the National Academy of Sciences of the United States of America, 107(31), 13754–13759. DOI 10.1073/pnas.1005874107. [Google Scholar] [CrossRef]
30. Thirkell, T., Cameron, D. D., Hodge, A. (2016). Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant, Cell and Environment, 39(8), 1683–1690. DOI 10.1111/pce.12667. [Google Scholar] [CrossRef]
31. Loydi, A., Donath, T. W., Otte, A., Eckstein, R. L. (2015). Negative and positive interactions among plants: Effects of competitors and litter on seedling emergence and growth of forest and grassland species. Plant Biology, 17(3), 667–675. DOI 10.1111/plb.12287. [Google Scholar] [CrossRef]
32. Li, K., Li, H., Huangfu, C., Yang, D., Liu, H. et al. (2016). Species-specific effects of leaf litter on seedling emergence and growth of the invasive Flaveria bidentis and its co-occurring native species: A common garden test. Plant Ecology, 217(12), 1457–1465. DOI 10.1007/s11258-016-0657-y. [Google Scholar] [CrossRef]
33. Song, X., Gao, Y., Green, S. M., Dungait, J. A. J., Peng, T. et al. (2017). Nitrogen loss from karst area in China in recent 50 years: An in-situ simulated rainfall experiment’s assessment. Ecology and Evolution, 7(23), 10131–10142. DOI 10.1002/ece3.3502. [Google Scholar] [CrossRef]
34. Tian, X., Jian, N. I. (2010). Comprehensive treatment of rocky desertification in Karst mountainous areas, southwestern China: Fundamental, approach and issues. Arid Land Geography, 33, 532–539. [Google Scholar]
35. Wang, S. J., Liu, Q. M., Zhang, D. F. (2004). Karst rocky desertification in southwestern China: Geomorphology, landuse, impact and rehabilitation. Land Degradation & Development, 15(2), 115–121. DOI 10.1002/ldr.592. [Google Scholar] [CrossRef]
36. Zhang, X. Y., Sui, Y. Y., Zhang, X. D., Meng, K., Herbert, S. J. (2007). Spatial variability of nutrient properties in black soil of Northeast China. Pedosphere, 17(1), 19–29. DOI 10.1016/S1002-0160(07)60003-4. [Google Scholar] [CrossRef]
37. Zhang, Z., Hu, B., Hu, G. (2014). Spatial heterogeneity of soil chemical properties in a subtropical Karst Forest, Southwest China. Scientific World Journal, 2014, 1–9. DOI 10.1155/2014/473651. [Google Scholar] [CrossRef]
38. Martinezgarcia, L. B., Pugnaire, F. I. (2011). Arbuscular mycorrhizal fungi host preference and site effects in two plant species in a semiarid environment. Applied Soil Ecology, 48(3), 313–317. DOI 10.1016/j.apsoil.2011.04.003. [Google Scholar] [CrossRef]
39. Tamayo-Vélez, Á., Osorio, N. W. (2018). Soil fertility improvement by litter decomposition and inoculation with the fungus Mortierella sp. in Avocado Plantations of Colombia. Communications in Soil Science and Plant Analysis, 49(2), 139–147. DOI 10.1080/00103624.2017.1417420. [Google Scholar] [CrossRef]
40. Zhang, W. (2011). Course on physical and chemical analysis of soil, water and plants (in Chinese). Beijing: Forestry Press. [Google Scholar]
41. Brundrett, M., Bougher, N., Dell, B., Grove, T., Malajczuk, N. (1996). Working with mycorrhizas in forestry and agriculture. Canberra: Australian Centre for International Agricultural Research. [Google Scholar]
42. Khakpour, O., Khara, J. (2012). Spore density and root colonization by arbuscular mycorrhizal fungi in some species in the northwest of Iran. International Research Journal of Applied and Basic Sciences, 3, 977–982. [Google Scholar]
43. Abbott, L. K., Robson, A. D., De Boer, G. (1984). The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytologist, 97(3), 437–446. DOI 10.1111/j.1469-8137.1984.tb03609.x. [Google Scholar] [CrossRef]
44. Zhang, X., Wang, L., Ma, F., Yang, J., Su, M. (2017). Effects of arbuscular mycorrhizal fungi inoculation on carbon and nitrogen distribution and grain yield and nutritional quality in rice (Oryza sativa L.). Journal of the Science of Food and Agriculture, 97(9), 2919–2925. DOI 10.1002/jsfa.8129. [Google Scholar] [CrossRef]
45. Sun, J., Ye, M., Peng, S., Li, Y. (2016). Nitrogen can improve the rapid response of photosynthesis to changing irradiance in rice (Oryza sativa L.) plants. Scientific Reports, 6(1), 31305. DOI 10.1038/srep31305. [Google Scholar] [CrossRef]
46. Cornejo, P., Seguel, A., Aguilera, P., Meier, S., Larsen, J. et al. (2017). Arbuscular mycorrhizal fungi improve tolerance of agricultural plants to cope abiotic stress conditions. In: Singh, D. P., Singh, H. B. Prabha (eds.Plant-microbe interactions in agro-ecological perspectives: Volume 2: Microbial interactions and agro-ecological impacts, pp. 55–80. Springer Singapore: Singapore. [Google Scholar]
47. Chen, S., Zhao, H., Zou, C., Li, Y., Chen, Y. et al. (2017). Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Frontiers in Microbiology, 8, 2516. DOI 10.3389/fmicb.2017.02516. [Google Scholar] [CrossRef]
48. He, Y., Cornelissen, J. H. C., Wang, P., Dong, M., Ou, J. (2019). Nitrogen transfer from one plant to another depends on plant biomass production between conspecific and heterospecific species via a common arbuscular mycorrhizal network. Environmental Science and Pollution Research, 26(9), 8828–8837. DOI 10.1007/s11356-019-04385-x. [Google Scholar] [CrossRef]
49. Pérez-Tienda, J., Corrêa, A., Azcón-Aguilar, C., Ferrol, N. (2014). Transcriptional regulation of host NH4+ transporters and GS/GOGAT pathway in arbuscular mycorrhizal rice roots. Plant Physiology and Biochemistry, 75, 1–8. DOI 10.1016/j.plaphy.2013.11.029. [Google Scholar] [CrossRef]
50. Zhang, S., Wang, L., Ma, F., Bloomfield, K. J., Yang, J. et al. (2014). Is resource allocation and grain yield of rice altered by inoculation with arbuscular mycorrhizal fungi? Journal of Plant Ecology, 8(4), 436–448. DOI 10.1093/jpe/rtu025. [Google Scholar] [CrossRef]
51. Gao, X., Guo, H., Zhang, Q., Guo, H., Zhang, L. et al. (2020). Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Scientific Reports, 10(1), 2084. DOI 10.1038/s41598-020-59180-3. [Google Scholar] [CrossRef]
52. Marschner, P., Solaiman, Z. M., Rengel, Z. (2006). Rhizosphere properties of poaceae genotypes under P-limiting conditions. Plant and Soil, 283(1), 11–24. DOI 10.1007/s11104-005-8295-5. [Google Scholar] [CrossRef]
53. Tian, H., Chen, G., Zhang, C., Melillo, J. M., Hall, C. A. S. (2010). Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry, 98(1), 139–151. DOI 10.1007/s10533-009-9382-0. [Google Scholar] [CrossRef]
54. Koorem, K., Price, J. N., Moora, M. (2011). Species-specific effects of woody litter on seedling emergence and growth of herbaceous plants. PLoS One, 6(10), 1–7. DOI 10.1371/journal.pone.0026505. [Google Scholar] [CrossRef]
55. Wang, Z., Lu, J., Yang, M., Yang, H., Zhang, Q. (2015). Stoichiometric characteristics of carbon, nitrogen, and phosphorus in leaves of differently aged lucerne (Medicago sativa) stands. Frontiers in Plant Science, 6(e109052), 1062. DOI 10.3389/fpls.2015.01062. [Google Scholar] [CrossRef]
56. Güsewell, S. (2004). N:P ratios in terrestrial plants: Variation and functional significance. New Phytologist, 164(2), 243–266. DOI 10.1111/j.1469-8137.2004.01192.x. [Google Scholar] [CrossRef]
57. Zhang, W., Zhao, J., Pan, F., Li, D., Chen, H. et al. (2015). Changes in nitrogen and phosphorus limitation during secondary succession in a Karst region in southwest China. Plant and Soil, 391(1), 77–91. DOI 10.1007/s11104-015-2406-8. [Google Scholar] [CrossRef]
58. Treseder, K. K., Vitousek, P. M. (2001). Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology, 82(4), 946–954. DOI 10.1890/0012-9658(2001)082[0946:EOSNAO]2.0.CO;2. [Google Scholar] [CrossRef]
59. Zhang, L., Shi, N., Fan, J., Wang, F., George, T. S. et al. (2018). Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environmental Microbiology, 20(7), 2639–2651. DOI 10.1111/1462-2920.14289. [Google Scholar] [CrossRef]
60. Flindt, M. R., Lillebø, A. I. (2005). Determination of total nitrogen and phosphorus in leaf litter. Methods to study litter decomposition, pp. 53–59. Netherlands: Springer. [Google Scholar]
61. Barrett, G., Campbell, C. D., Hodge, A. (2014). The direct response of the external mycelium of arbuscular mycorrhizal fungi to temperature and the implications for nutrient transfer. Soil Biology and Biochemistry, 78, 109–117. DOI 10.1016/j.soilbio.2014.07.025. [Google Scholar] [CrossRef]
62. Smith, S. E., Dickson, S., Smith, F. A. (2001). Nutrient transfer in arbuscular mycorrhizas: How are fungal and plant processes integrated? Functional Plant Biology, 28(7), 685–696. DOI 10.1071/PP01033. [Google Scholar] [CrossRef]
63. Shu, B., Wang, P., Xia, R.X. (2014). Effects of mycorrhizal fungi on phytate-phosphorus utilization in trifoliate orange (Poncirus trifoliata L. Raf) seedlings. Acta Physiologiae Plantarum, 36(4), 1023–1032. DOI 10.1007/s11738-013-1480-x. [Google Scholar] [CrossRef]
64. Benedetto, A., Magurno, F., Bonfante, P., Lanfranco, L. (2005). Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza, 15(8), 620–627. DOI 10.1007/s00572-005-0006-9. [Google Scholar] [CrossRef]
65. Neumann, E., Schmid, B., Römheld, V., George, E. (2009). Extraradical development and contribution to plant performance of an arbuscular mycorrhizal symbiosis exposed to complete or partial rootzone drying. Mycorrhiza, 20(1), 13–23. DOI 10.1007/s00572-009-0259-9. [Google Scholar] [CrossRef]
66. Clarholm, M., Skyllberg, U., Rosling, A. (2015). Organic acid induced release of nutrients from metal-stabilized soil organic matter–The unbutton model. Soil Biology and Biochemistry, 84, 168–176. DOI 10.1016/j.soilbio.2015.02.019. [Google Scholar] [CrossRef]
67. Xu, J., Liu, S., Song, S., Guo, H., Tang, J. et al. (2018). Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biology and Biochemistry, 120, 181–190. DOI 10.1016/j.soilbio.2018.02.010. [Google Scholar] [CrossRef]
68. Jacobs, L. M., Sulman, B. N., Brzostek, E. R., Feighery, J. J., Phillips, R. P. (2018). Interactions among decaying leaf litter, root litter and soil organic matter vary with mycorrhizal type. Journal of Ecology, 106(2), 502–513. DOI 10.1111/1365-2745.12921. [Google Scholar] [CrossRef]
69. Lerat, S., Lapointe, L., Gutjahr, S., Piché, Y., Vierheilig, H. (2003). Carbon partitioning in a split-root system of arbuscular mycorrhizal plants is fungal and plant species dependent. New Phytologist, 157(3), 589–595. DOI 10.1046/j.1469-8137.2003.00691.x. [Google Scholar] [CrossRef]
70. Mei, L., Yang, X., Zhang, S., Zhang, T., Guo, J. (2019). Arbuscular mycorrhizal fungi alleviate phosphorus limitation by reducing plant N:P ratios under warming and nitrogen addition in a temperate meadow ecosystem. Science of the Total Environment, 686, 1129–1139. DOI 10.1016/j.scitotenv.2019.06.035. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |