
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014505
ARTICLE
Phenotype Analysis and Fine Mapping of the Male Sterile Mutant ms10 in Rice (Oryza sativa L.)
College of Agronomy and Biotechnology, Rice Research Institute, Southwest University, Chongqing, 400715, China
*Corresponding Author: Nan Wang. Email: wangnan_xndx@126.com
Received: 03 October 2020; Accepted: 16 November 2020
#These authors contributed equally to this study
Abstract: There is a positive correlation between fertility and yield, and the decrease of fertility is bound to a greatly reduced crop yield. Male sterile mutants can be used in hybrid rice. Therefore, rice male sterility has an important value in research and application, and the study of related mutants is also very vital. The mutant ms10 (male sterile 10) reported in this study was induced by ethyl methane sulfonate (EMS) in the indica maintainer line Xinong 1B. There was no significant difference between the ms10 and wild type in the vegetative growth stage. However, in the reproductive growth stage, ms10 showed that the plant became shorter, the anther became smaller and the color became lighter, and finally showed the phenotype of male sterility in comparison to the wild type. I2-KI staining showed that the pollen was malformed and only a little was active. Scanning electron microscopy observation showed that the exine waxy layer of the ms10 anther decreased, suggesting that the protective effect on pollen was decreased. This may be one of the reasons leading to the phenotype of male sterility. Finally, the pollen showed shrinkage and collapsed, and the structure of germinating pore cover disappeared. This may be the result of sterility. Genetic analysis showed that the male sterility phenotype of the mutant was controlled by a single recessive nuclear gene. MS10 was mapped between the molecular markers IND37 and IND51 on chromosome 4, with a physical distance of 178.6 kb. These results lay the foundation for further studies on MS10.
Keywords: Gene mapping; male sterility; rice (Oryza sativa L.)
Male sterility is a very common phenomenon in nature. Such sterility is due to the abnormal development or even degeneration of the male reproductive organs. This leads to produce abnormal fertile pollen or even pollen cannot be produced. At the same time, the female reproductive organs are fertile [1]. Rice, as an important food crop, has the advantages of a short growing period and easy genetic transformation compared with other gramineous crops [2]. Studies on the mechanism of male sterility are of great theoretical significance and application value [3]. When making use of heterosis in breeding, people can use the characteristic of male sterility to avoid the process of artificial castration, which can save a lot of manpower and time. Therefore, the study of male sterility is of great significance to agricultural production.
Rice anther development is an extremely complex biological process, and it is also a key process for rice reproduction and yield formation [4]. It involves the sequence and co-expression of a series of genes, as well as the corresponding cellular processes, including meiosis, callose metabolism, tapetum metabolism, anther wall development, mitosis and so on [5]. At present, many genes related to male sterility in rice have been cloned. For example, OsACOS12 is located on chromosome 4; the osacos12 mutant showed the phenotype of male sterility. Even though its vegetative growth and spikelet development were normal, the outer layer of sporopollen was defective, the anther turned white and its length became shorter. Also, the wax content of the anther epidermis decreased and could not produce pollen [6]. EDT1 was located on chromosome 11; the mutant edt1 is characterized by a reduced plant height, thin and whitish anthers, early degradation of tapetum cells and inability to produce active pollen [7]. PTC1 was located on chromosome 9. The mutant ptc1 not only showed a phenotype similar to Arabidopsis ms1, such as delayed tapetum degradation, abnormal pollen wall and pollen abortion, but also a free proliferation of tapetum cells and subsequent necrosis [8]. LEPTO1 was located on chromosome 2; the pollen mother cell chromosome of the mutant lepto1 was stagnated in the pre-leptotene state, and could not be further assembled into the typical leptotene chromosome state. As a result, there was no DNA double strand break (DSB), and no assembly of meiosis specific proteins in pollen mother cells. No accumulation of callose was observed in the mutant, which finally showed the phenotype of being unable to produce pollen [9]. OsDAF1 is located on chromosome 2; the mutant osdaf1 was normal during the vegetative growth period, but showed a thinner anther and a lighter color during the reproductive period. Most mature pollens were aborted due to the defects in the germination pores. In addition, the remaining pollens showed the lack of pore ring structure in the germination pores, resulting in the inability of pollen tubes to germinate and subsequent complete male sterility [10]. OsMSP1 is located on chromosome 1. It not only controls the number of rice pollen mother cells, but also affects the development of the anther wall. Deletion or mutation of OsMSP1 will lead to abnormal development of rice floral organs and lead to complete male sterility [11].
Rice is one of the main staple foods in the world. Maintaining and increasing rice yield has become a major challenge to food security. In highly inbred species such as rice, controlling crop pollen fertility is particularly important for improving grain yield [12]. At present, RAD51C [13], GSL5 [14], DTM1 [15], ABCG15 [16] and other male sterility related genes have been cloned in rice. The functional changes of these genes will lead to abnormal anther development in rice. In the msp1 mutation, the anther wall layer was disordered, which eventually led to complete male sterility, indicating that MSP1 plays an important role in the development of anther wall [11]. The pollen of the mutant osinp1 was completely absent from the germination pore, and the pollen tube could not germinate, which eventually led to male abortion; OsDAF1 encodes a lectin receptor-like kinase, which is essential for the formation of the “ring structure” on the rice pollen germination pores [10].
In this study, the mutant ms10 was induced by EMS from the indica maintainer line Xinong 1B. Compared with the wild type, there was no significant difference in the vegetative growth period of the ms10. In the reproductive growth stage, the ms10 showed that the anther shrank and became smaller, and the pollen was inactive. Finally, it showed the character of male sterility. An interval whose physical distance was 178.6 kb was determined by gene mapping. This mapping interval contained 26 annotated genes, all of which were unreported genes, indicating that the ms10 was a new male sterile mutant. In this paper, the phenotypic analysis, genetic analysis and gene molecular mapping of the mutant were studied, which laid the foundation for the cloning and functional analysis of the gene in the later stage.
The rice male sterile mutant ms10 was mutated by EMS from Xinong 1B, an indica maintainer line cultivated by the Rice Research Institute of Southwest University. After successive generations of stable inheritance of self-crossing characters, F1 seeds were obtained by crossing with the restorer line Jinhui 10 (J10). F1 seeds were harvested in Hainan, and F2 seeds were planted with parents in the experimental base of the Rice Research Institute of Southwest University. F2 plants were used for genetic analysis and gene mapping.
In order to determine the genetic law of mutant traits, we used ms10, a mutant with a background of 1B, as the female parent and J10 as the male parent. Hybrids were prepared to observe the representative type of F1, and the F2 population was obtained by selfing the F1 generation. The segregation ratio of the F2 population was investigated, and the segregation ratio of normal and mutant plants was calculated and tested by chi-square.
2.3 Identification of Pollen Fertility
At the flowering stage of rice, the florets at the mature stage on the main stem of wild type 1B and mutant ms10 were randomly selected. The glumes were peeled off and the anthers were removed. Anthers were crushed on the glass slides dripping with 1% I2-KI staining solution with pointed tweezers to make the pollen flow out. The pollen was observed and photographed using anordinary light microscope, and the anthers of the same period in different panicles were repeated for three times.
2.4 Observation with Asana Mirror
At the flowering stage of rice, the florets of mature stage on the main stem of wild type 1B and mutant ms10 were randomly selected. The intact spikelets and intact anthers were observed and photographed at different rates under the asana microscope after peeling the glumes.
2.5 Observation and Analysis by Scanning Electron Microscopy
During the flowering stage of rice, the florets of mature stage on the main stem of wild type 1B and mutant ms10 were randomly selected. The glumes were carefully peeled off with pointed tweezers to retain the anther, and the anther was cut to expose the pollen by a scalpel. Subsequently, it was placed together with a single complete anther on the base with conductive glue. Thereafter, the glued sample was placed on the scanning electron microscopy loading table, and adjusted to an appropriate height. The anther epidermis, Urinite and pollen grains were observed and photographed under the condition of instantaneous freezing at –20°C.
2.6 Genomic DNA Extraction and PCR Amplification
During the reproductive period, the DNA of parents, gene pool and F2 population were extracted from fresh leaves according to the improved CTAB method [17] for gene mapping.
The total PCR reaction system was 15 μL, including template DNA 1.0 μL, forward and reverse primers 0.5 μL, dNTPs 0.5 μL, 10 × PCR buffer 1.5 μL, ddH2O 10.8 μL and rTaq DNA polymerase 0.2 μL. The PCR program was pre-denatured at 94°C for 5 min; Denatured at 94°C for 30 s, annealing at 55°C for 30 s and extending at 72°C for 30 s, 35 cycles; Final extension was at 72°C for 10 min; Preservation was during 1 min at 25°C.
2.7 Molecular Marker Analysis and Genetic Map Construction
Ten normal and mutant plants were randomly selected from the F2 population of J10/ms10 by the BSA method [18]. The same amount of leaves were cut and mixed to construct a normal gene pool and a mutant gene pool. The genomic DNA was extracted from the gene pool and amplified with 400 pairs of SSR and InDel molecular markers uniformly distributed on rice chromosomes. The PCR products were electrophoretic with 10% non-denaturing polyacrylamide gel, and stained with silver. After rapid silver staining, the results were observed to look for molecular markers that might be linked to the mutant genes. Then, the F2 population was used to verify the molecular markers that might be linked to the mutant genes. Http://www.gramene.org/microsat database and Vector NTI Advance 11.5 software were used to develop new polymorphic SSR and InDel molecular markers between two parents to determine the interval of the target gene. The primers we used were synthesized by the Chengdu Tsingke Biotechnology Co., China.
In the F2 population, the single plant with the J10 banding pattern was marked as A; the single plant with the ms10 banding pattern was marked as B, and the single plant with the J10/ms10 heterozygous banding pattern was marked as H; the population number was expressed by n. The linkage data of the mapping population was analyzed by using the formula
3.1 Phenotypic Characteristic Analysis
At the vegetative growth stage, no significant difference was observed between the mutant ms10 and the wild type. However, at the reproductive growth stage, the spikelet of ms10 was shorter and the spikelet width was not significantly different from that of the wild type (Fig. 1D). At the mature stage, the wild type showed normal seed production, ms10 showed empty glumes and an erect spike, and could hardly produce seeds (Figs. 1A–1C).

Figure 1: Phenotypic observation and analysis of the wild type and ms10. A: WT and ms10 at the mature stage, Bars = 17.5 cm; B: The main panicles of the WT and ms10 plants at maturity, Bars = 4.5 cm; C: Grains of the WT and ms10, Bars = 4.2 cm; D: Spikelets of the WT and ms10, Bars = 0.8 cm
3.2 Observation of Anther Posture under Microscope and Analysis of Pollen Iodine Staining
The mature anthers of the wild type 1B and mutant ms10 were observed under the asana microscope. Results showed that the spikelet of ms10 became shorter (Fig. 2A), and the anthers were obviously shorter and lighter in color than those in the wild type (Figs. 2B and 2E); The anther of ms10 was peeled off to expose the pollen, and it was analyzed by iodine staining. The pollen of ms10 was malformed and its number decreased significantly; it cannot be stained by I2-KI (Figs. 2C and 2D) compared with the wild type. This indicated that the pollen of ms10 was inactive, resulting in the failure to produce seeds normally.
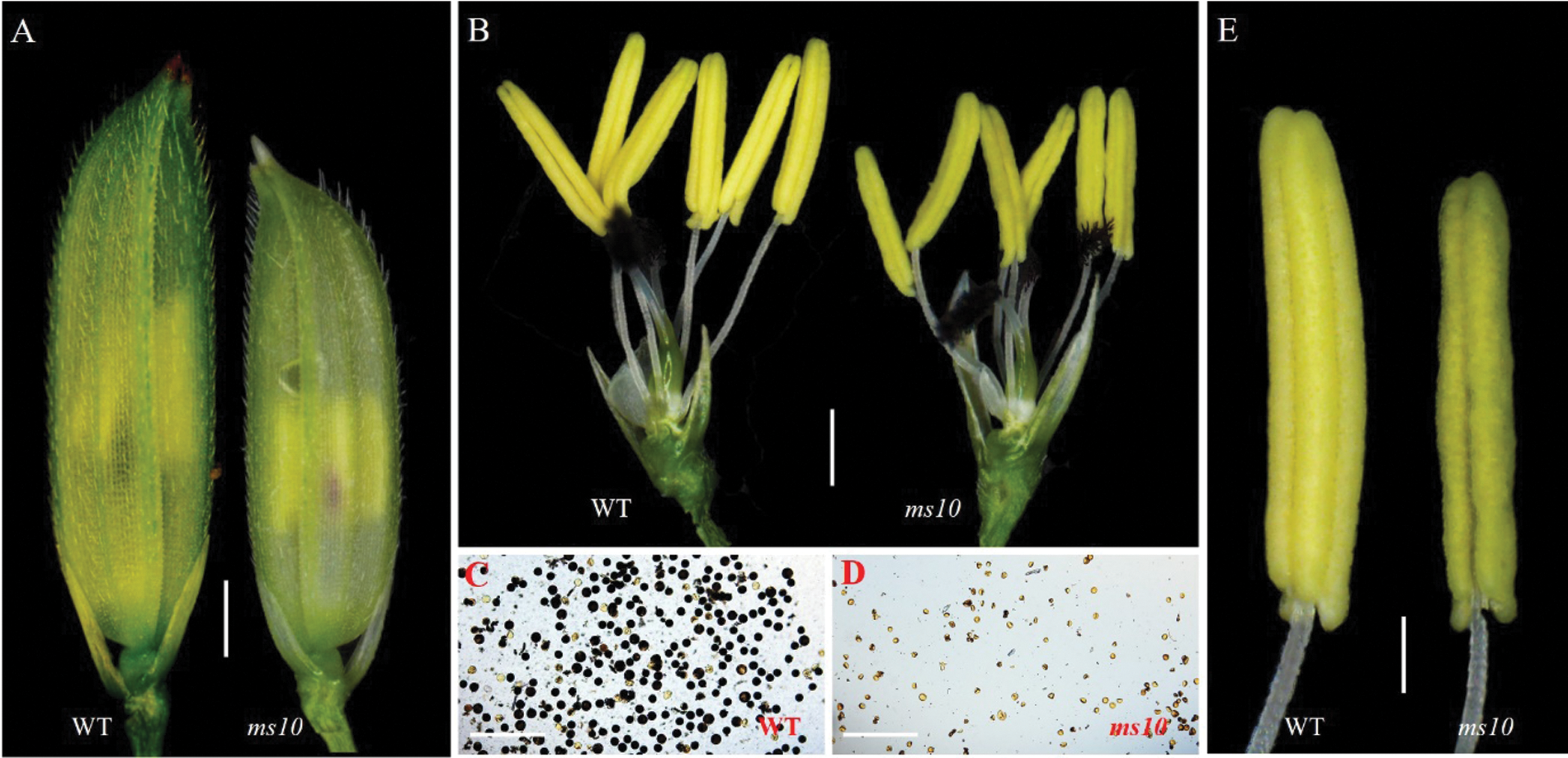
Figure 2: Observation and analysis of the wild type and ms10 by asana mirror. A: Spikelets of WT and ms10, Bars = 1.4 mm; B: Anthers of the WT and ms10, Bars = 1.5 mm; C: Pollen of the WT, Bars = 78 μm; D: Pollen of ms10, Bars = 78 μm; E: Single anther of the WT and ms10, Bars = 0.5 mm
3.3 Statistics and Analysis of Agronomic Characters
Plant height, grain number per panicle, seed setting rate, pollen number per unit area and pollen fertility rate decreased significantly on the ms10 in comparison to the wild type (Figs. 3A, 3E, 3F, 3G and 3H). The decreasing rates were 19.61%, 28.05%, 96.24%, 52.73% and 97.94%, respectively. Panicle and spike lengths decreased significantly by 11.67% and 15.53%, respectively (Figs. 3B and 3C). The WT and ms10 showed a similar (p < 0.05) spike width (Fig. 3D).
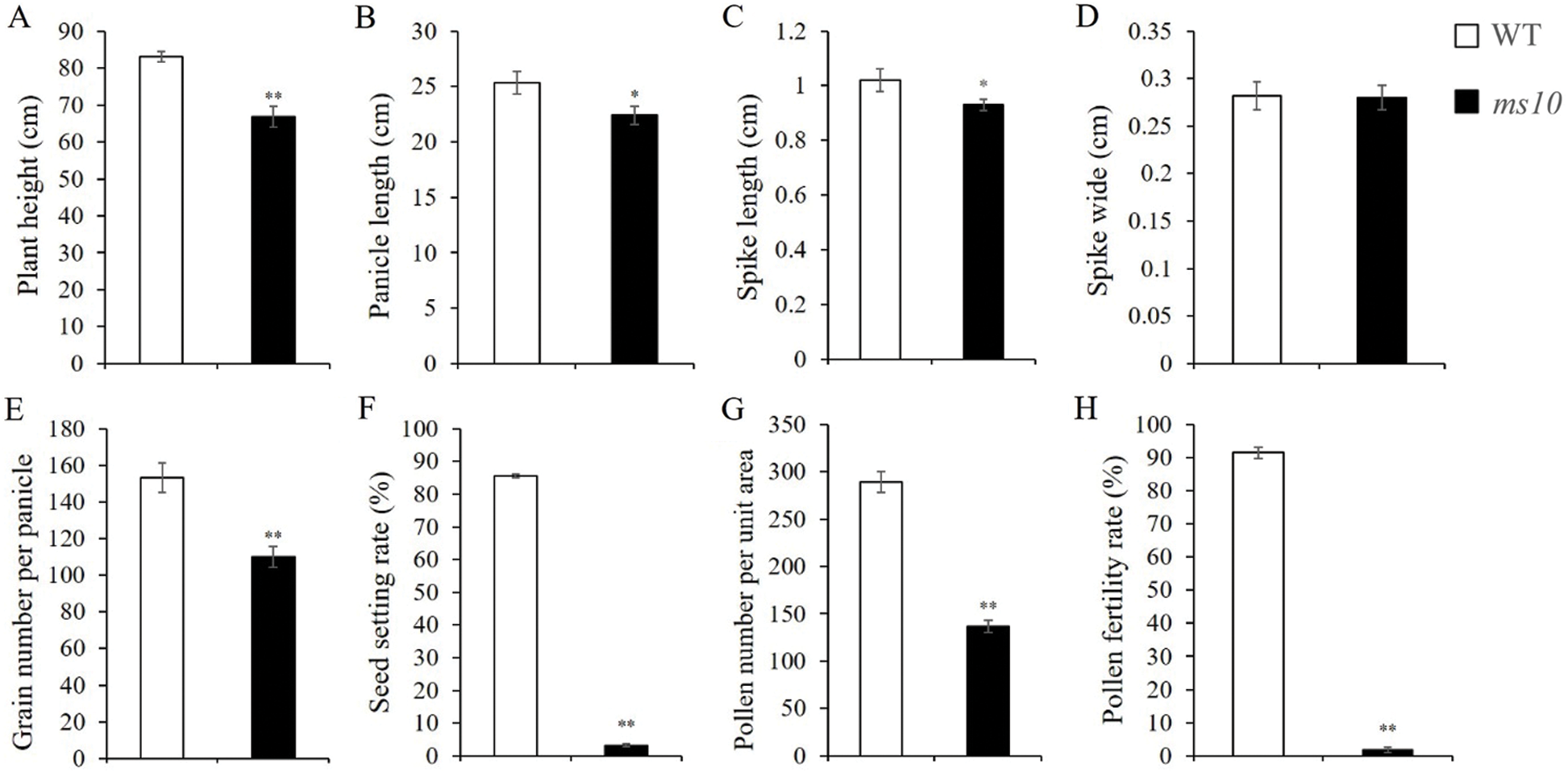
Figure 3: Statistics and analysis of agronomic characters on the wild type and ms10. A: Plant height, B: Panicle length, C: Spike length, D: Spike width, E: Grain number per panicle, F: Seed setting rate, G: Pollen number, H: Pollen fertility rate. In the Y axis of the figure legend replace wide by width
3.4 Observation and Analysis of Anthers by Scanning Electron Microscopy
Scanning electron microscopy analysis showed that the anther of the ms10 was significantly shorter than that of the wild type (Figs. 4A and 4B). Further magnification of the anther surface showed a decrease in the structure of the waxy layer on the anther epidermis of the mutant (Figs. 4C and 4D). This suggests that the protective effect on the pollen was weakened. By peeling off the anther and observing the structure of the inner wall of the anther and pollen, there was no significant difference in the structure of the inner wall of the anther between the ms10 and the wild type (Figs. 4E and 4F). However, it was found that the pollen of the ms10 showed a collapsing shape and shrinkage, and the structure of the germinating hole lid disappeared (Figs. 4G and 4H). According to the results of the scanning electron microscopy, the sterile phenotype of the ms10 may lead to an anther wax layer structure which reduced the pollen germination hole cover structure and it disappeared.
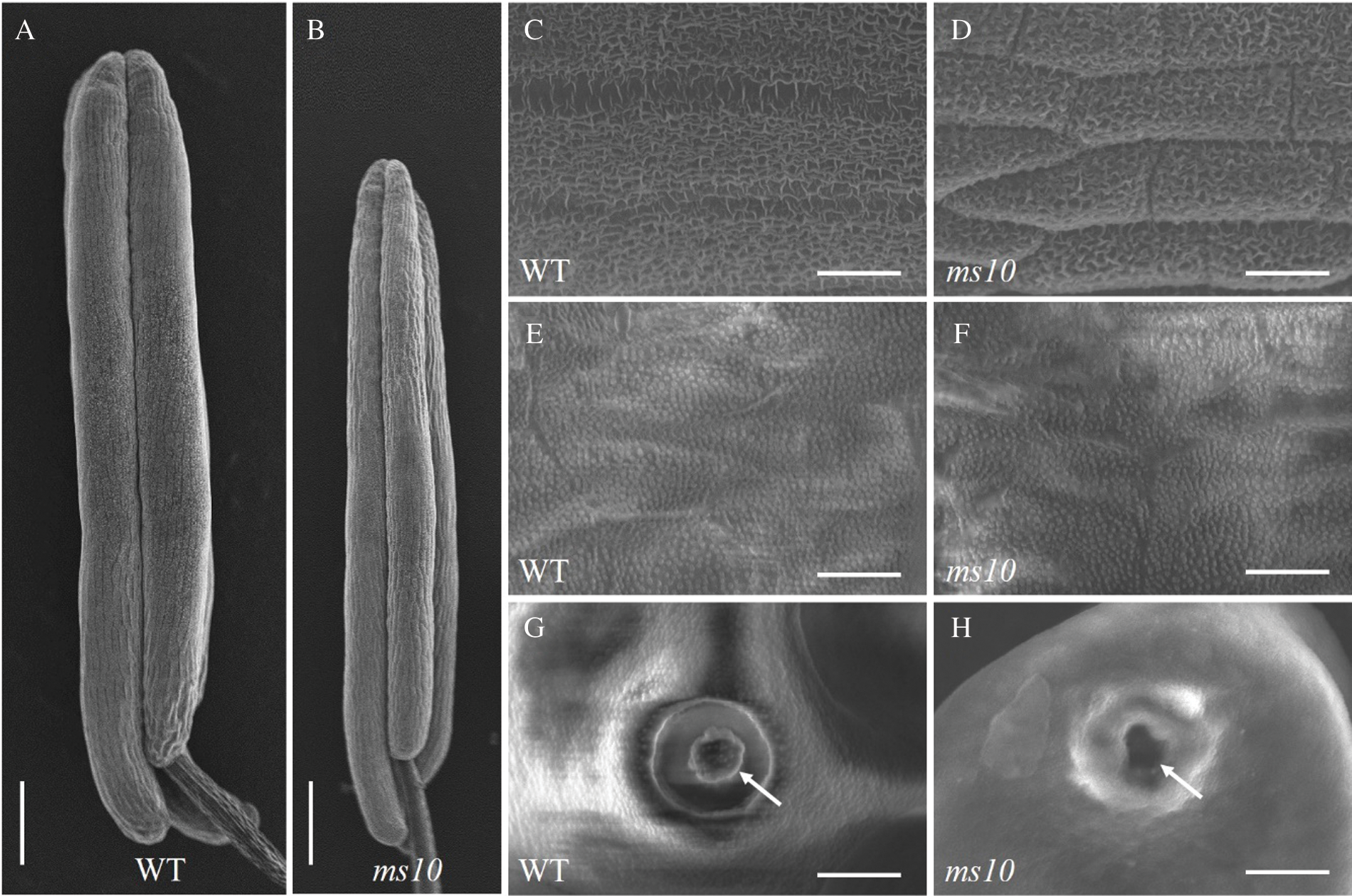
Figure 4: Observation and analysis of the wild type and ms10 by scanning electron microscopy. A: Anthers of WT, Bars = 360 μm; B: Anthers of ms10, Bars = 360 μm; C: The structure of anther epidermis on the WT, Bars = 37 μm; D: The structure of anther epidermis on the ms10, Bars = 37 μm; E: The structure of inner wall on anther of the WT, Bars = 22.2 μm; F: The structure of inner wall on anther of the ms10, Bars = 22.2 μm; G: Pollen germination pores on the WT, Bars = 9.3 μm; H: Pollen germination pores on the ms10, Bars = 9.3 μm
The F1 plants obtained after hybridization between the mutant ms10 and the wild type J10 showed normal fertility, and the F2 population was obtained by self-crossing F1 generation seeds. There were two types of sterility and fertility in the F2 population. Field statistical characters showed that there were 1381 fertile plants and 429 sterile plants in the F2 population. Chi-square test showed that the segregation ratio conformed to the Mendel genetic segregation ratio at 3:1
Table 1: Genetic analysis of MS10

The 429 male sterile plants in the F2 population were used as a mapping population for gene mapping. Three hundred and sixty pairs of primers evenly distributed on the 12 chromosomes of rice were used to analyze the polymorphism of wild type and ms10, and 52 pairs of polymorphic primers were screened. These 52 pairs of polymorphic molecular marker primers were used to analyze the gene pools of J10 and ms10. Finally, it was shown that there was a linkage relationship between the target gene and the molecular marker ZTQ38 on chromosome 4. Further linkage analysis was carried out on the adjacent marker primers at both sides of the marker ZTQ38, and the target gene was initially located between ZTQ38 and LR35. SSR markers and InDel markers were further developed in the initial positioning interval (Tab. 2), and finally MS10 was located between the markers IND37 and IND51, and the physical distance was 178.6 kb (Fig. 5).

Figure 5: Gene mapping of MS10
Table 2: Molecular markers newly designed in this study

3.7 Analysis of Candidate Genes in Interval
Through the analysis of the mapping interval by the National Rice data Center (http://www.ricedata.cn/gene/) and gramene database (http://gramene.org/) and other websites, expressed that the interval contained 26 annotated genes (Tab. 3), including 9 expressed proteins, 2 transposon proteins, 2 AThook family proteins, and 2 proteins containing RNA recognition motifs, 1 encodes acyltransferase, 1 encodes polyphenoloxidase, 1 encodes NBS-LRR resistance protein, 1 contained OsFBL20-F-box domain and LRR, 1 encodes soluble starch synthase 3 and chloroplast precursor protein, 1 encodes OsCHL chlorophyll lipid transport protein, 1 encodes MrBTB2-BTB domain protein, 1 encodes MBTB6-BTB domain protein, 1 protein encodes MBTB7-BTB domain. One protein encodes MBTB8-BTB domain. None of the genes in this interval have been reported, therefore MS10 is likely to be a new male sterile gene.
Table 3: Locate annotated genes in the mapping region

Rice (Oryza sativa L.) is one of the most productive crops in the world and it is also one of the main food supplies for the world’s population [19]. Male sterility is an important character in crop heterosis breeding. In recent years, advances in molecular biology have enabled people to identify genes related to plant reproductive development. There was no significant difference between the mutant ms10 and the wild type during the vegetative growth stage. However, in the reproductive growth stage, the anther of ms10 became smaller and the color became lighter. Finally, the phenotype was male sterility. On the one hand, male sterility in rice can lead to a substantial reduction in yield. On the other hand, it can be applied to cross breeding to avoid the tedious work of artificial castration. Therefore, it is of great significance to study the molecular mechanism of male sterility.
Anther is an important organ affecting pollen development. The study on anther structure and development on male sterile lines is helpful to explore the causes of pollen abortion and to further understand the mechanism of male sterility in plants [20]. Stratum corneum is a layer of fatty substances on the surface of aboveground organs of plants, which is composed of horny and waxy. Stratum corneum has many vital functions in plant physiology and ecology, it can not only prevent the loss of non-stomatal water in plants, but also resist a variety of abiotic stresses and other adverse effects. The stratum corneum of the anther is the protective layer of pollen, and the synthesis of the stratum corneum and exine of the anther is an indispensable condition to ensure the formation, maturation, dispersion, transmission and pollination of pollen [21]. In order to further study the anther development of the mutant ms10, the mature anthers of wild type and ms10 were observed by scanning electron microscopy. The analysis showed that the anther of ms10 became shorter and the wax layer structure of the extine of the anther decreased by magnifying observation. It is suggested that the protective effect on pollen is reduced, which may be caused by infertility. The germination pore is a gap of extine deposition, which is very important for pollen germination. In grain pollen, the germination pore is a single pore located at the far end, surrounded by a ring of germination pores of protuberant and thickened adventitia edge structure, and covered by a cap-like cover of the isolated adventitia region. The morphology of pollen germination pores has been studied for more than a century, but the genes directly involved in the formation of pollen germination pores have not been reported until recently. Therefore, the study of pore size formation is very important to understand the fertility of important crops in agriculture, but the factors that directly form these complex structures are still largely unknown [10]. We further peeled off the anther and observed the pollen by scanning electron microscopy. It was found that the pollen germination pore cap structure of ms10 disappeared, indicating that the pollen germination pore of ms10 developed abnormally. Therefore, the loss of the structure of the germination hole cover may be caused by ms10 male sterility, which may be an important gene for the abnormal germination hole caused by male sterility. The study of ms10 mutants is of great significance to the study of germination pore development.
In this study, we identified a male sterile mutant ms10, whose mutant character is controlled by a single recessive nuclear gene. By gene mapping, MS10 was located between the molecular markers IND37 and IND51 on chromosome 4, and the physical distance was 178.6 kb. This indicates that there were no reported male sterile genes but 26 annotated genes in the interval. Therefore, MS10 may be a new male sterile gene. In mutant ms10, the structure of a wax layer on the anther epidermis decreased, and the development of the pollen germination pore was abnormal; this was characterized by the disappearance of pore cover structure. At present, there are few reports about the related genes of the germination pore involved in fertility. Therefore, the cloning and functional study of MS10 are of great significance for understanding the structure of the wax layer of the anther exine, and the molecular mechanism of male sterility caused by the abnormal pollen germination pore development. In turn, it may be helpful for the genetic improvement of rice.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Mai, J., Luo, Y., Xia, Z., Chen, S. (2008). Molecular mechanism of male sterility in rice. Anhui Agricultural Science, 13, 5338–5341. [Google Scholar]
2. He, S. (2009). Cloning and functional study of Rice DES2 gene (Master’s Thesis). Shanghai Jiao Tong University, China. [Google Scholar]
3. Zhang, P., Zhang, Y., Sun, L., Sinumporn, S., Yang, Z. et al. (2017). The rice AAA-ATPase OsFIGNL1 Is essential for male meiosis. Frontiers in Plant Science, 8, 1639. DOI 10.3389/fpls.2017.01639. [Google Scholar] [CrossRef]
4. Yang, Z., Zhang, Y., Sun, L., Zhang, P., Xuan, D. et al. (2016). Identification and gene mapping of male sterile Mutant gamyb5 in Rice. Rice Science of China, 30, 37–45. [Google Scholar]
5. Li, G. (2011). Cell morphological staging of anther development in rice. Journal of Chongqing Normal University: Natural Science, 28, 56–59. [Google Scholar]
6. Li, Y., Li, D., Guo, Z., Shi, Q., Xiong, S. et al. (2016). OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice. BMC Plant Biology, 16(1), 876. DOI 10.1186/s12870-016-0943-9. [Google Scholar] [CrossRef]
7. Bai, W., Wang, P., Hong, J., Kong, W., Wan, J. (2019). Earlier Degraded Tapetum1 (EDT1) encodes an ATP-citrate lyase required for yapetum programmed cell death. Plant Physiology, 181(3), 1223–1238. DOI 10.1104/pp.19.00202. [Google Scholar] [CrossRef]
8. Li, H., Yuan, Z., Vizcay-Barrena, G., Yang, C., Liang, W. et al. (2011). PERSISTENT TAPETAL CELL1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice. Plant Physiology, 156(2), 615–630. DOI 10.1104/pp.111.175760. [Google Scholar] [CrossRef]
9. Zhao, T., Ren, L., Chen, X., Yu, H., Liu, C. et al. (2019). The OsRR24/LEPTO1 type-B response regulator is essential for the organization of leptotene chromosomes in rice meiosis. Plant Cell, 30(12), 3024–3037. DOI 10.1105/tpc.18.00479. [Google Scholar] [CrossRef]
10. Zhang, X., Zhao, G., Tan, Q., Yuan, H., Betts, N. et al. (2020). Rice pollen aperture formation is regulated by the interplay between OsINP1 and OsDAF1. Nature Plants, 6(4), 394–403. DOI 10.1038/s41477-020-0630-6. [Google Scholar] [CrossRef]
11. Nonomura, K., Miyoshi, K., Eiguchi, M., Suzuki, T., Miyao, A. et al. (2003). The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell, 15(8), 1728–1739. DOI 10.1105/tpc.012401. [Google Scholar] [CrossRef]
12. Tan, H., Liang, W., Hu, J., Zhang, D. (2012). MTR1 encodes a secretory fasciclin glycoprotein required for male reproductive development in rice. Developmental Cell, 22(6), 1127–1137. DOI 10.1016/j.devcel.2012.04.011. [Google Scholar] [CrossRef]
13. Kou, Y., Chang, Y., Li, X., Xiao, J., Wang, S. (2012). The rice RAD51C gene is required for the meiosis of both female and male gametocytes and the DNA repair of somatic cells. Journal of Experimental Botany, 63(14), 5323–5335. DOI 10.1093/jxb/ers190. [Google Scholar] [CrossRef]
14. Shi, X., Sun, X., Zhang, Z., Feng, D., Zhang, Q. et al. (2015). GLUCAN SYNTHASE-LIKE5 (GSL5) plays an essential role in male fertility by regulating callose metabolism during microsporogenesis in rice. Plant and Cell Physiology, 56(3), 497–509. DOI 10.1093/pcp/pcu193. [Google Scholar] [CrossRef]
15. Yi, J., Kim, S. R., Lee, D. Y., Moon, S., Lee, Y. S. et al. (2012). The rice gene DEFECTIVE TAPETUM AND MEIOCYTES1 (DTM1) is required for early tapetum development and meiosis. Plant Journal, 70(2), 256–270. DOI 10.1111/j.1365-313X.2011.04864.x. [Google Scholar] [CrossRef]
16. Qin, P., Tu, B., Wang, Y., Deng, L., Quilichini, T. D. et al. (2013). ABCG15 encodes an ABC transporter protein, and is essential for post-meiotic anther and pollen exine development in rice. Plant & Cell Physiology, 54(1), 138–154. DOI 10.1093/pcp/pcs162. [Google Scholar] [CrossRef]
17. Rogers, S. O., Bendich, A. J. (1985). Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Molecular Biology, 5(2), 69–76. DOI 10.1007/BF00020088. [Google Scholar] [CrossRef]
18. Michelmore, R. W., Paran, I., Kesseli, R. V. (1991). Identification of markers linked to disease-resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions by using segregating populations. Proceedings of the National Academy of Sciences of the United States of America, 88(21), 9828–9832. DOI 10.1073/pnas.88.21.9828. [Google Scholar] [CrossRef]
19. Wang, K., Peng, X., Ji, Y., Yang, P., Zhu, Y. et al. (2013). Gene, protein, and network of male sterility in rice. Frontiers in Plant Science, 4, 92. [Google Scholar]
20. Feng, M. (2016). Mapping cloning and functional research of rice male sterility gene MIL3 (Master’s Thesis). Yangzhou University, China. [Google Scholar]
21. Liu, J. (2017). Effects of endogenous and exogenous ABA on anther development and horny wax in rice (Master’s Thesis). Shanxi Normal University, China. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |