
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014411
ARTICLE
Laboratory-and Field-Phenotyping for Drought Stress Tolerance and Diversity Study in Lentil (Lens culinaris Medik.)
Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
*Corresponding Authors: Md. Amir Hossain. Email: amirgpb@bau.edu.bd; Mohammad Anwar Hossain. Email: anwargpb@bau.edu.bd
Received: 24 September 2020; Accepted: 01 December 2020
Abstract: Drought susceptibility and low genetic variability are the major constraints of lentil (Lens culinaris Medik.) production worldwide. Development of an efficient pre-field drought phenotyping technique and identification of diversified drought tolerant lentil genotype(s) are therefore vital and necessary. Two separate experiments were conducted using thirty diverse lentil genotypes to isolate drought tolerant genotype(s) as well as to assess their diversity. In both of the experiments, significant (p ≤ 0.01) variation in genotype (G), treatment (T) and G X T was observed for most of the studied traits. In experiment I, genotypes were examined for drought tolerance at the seedlings stage under hydroponic conditions by assessing root and shoot traits. Among the 30 genotypes studied, BM-1247, BM-1227 and BM-502 were selected as highly tolerant to drought stress as they showed maximum seedling survivability and minimum reduction in growth parameters under drought stress. In experiment II, the genotypes were assayed for diversity and drought stress tolerance based on morphological traits grown under field condition. Drought stress caused a substantial reduction in yield attributing traits, however, the genotypes BM-1247, BM-981, BM-1227 and BM-502 were categorized as drought tolerant genotypes with less than 20% yield reduction. The field screening result of drought stress tolerance was coincided well with the results of laboratory screening. Genetic divergence study reflected the presence of considerable diversity among the genotypes. Considering laboratory and field screening results, the genotypes, BM-1247, BM-1227, BM-981 and BM-502 were selected as the best drought tolerant genotypes. This information can be exploited for further breeding in developing drought tolerance in lentil.
Keywords: Lentil; water stress; seedling survival; yield attributing traits; phenotypic diversity
Lentil (Lens culinaris Medik.) is an important legume crop of rain-fed agriculture and significantly contributes to food and nutritional security worldwide. It is considered as an important source of proteins, carbohydrate, minerals and fiber [1,2]. It is also highly valuable as feed and fodder for livestock. Moreover, lentil plays an important role in crop rotation due to its atmospheric nitrogen fixing ability [3] and has appeared as a viable opportunity for expanding of cereal-based cropping systems around the globe. Lentil, popularly known as Masur, is one of the most essential pulse crops in Bangladesh. It is the second most important pulse crop both in acreage and production but stands first in consumer’s preference [4]. It is considered as the poor man’s meat, a substitute of animal protein for the underprivileged people of Bangladesh who cannot afford to buy animal protein [5,6]. Currently, there is a huge gap in between the annual demand and production due to lack of higher yield potential variety, occurrence of terminal drought stress at pod filling stage, poor technological intervention during production and competition with other profitable winter crops in Bangladesh. In the year 2016, we had a demand for 2.5 million MT lentil but we produced only 0.158 million MT with an average yield of 415 kg/acre [7]. Bangladesh imports a significant amount of lentil every year involving a huge amount of foreign currency. Development of high yielding, stable and drought tolerant variety is therefore urgently needed to ensure nutritional food security as well as to save the foreign currency.
Lentil is usually cultivated in moderately dry ecosystem where yield is mostly affected by various abiotic stresses. Water or low moisture stress is one of the major abiotic stresses limiting lentil production all over the world including Bangladesh [1,2,8]. Lentil is cultivated in different parts of Bangladesh during Rabi season from the month of November to February under the rain-fed condition on the soils that conserve moisture from preceding monsoon season. But it is extensively cultivated in mid-western parts of Bangladesh and invariably encounters terminal drought stress that leads to the forced maturity and lower yield. During Rabi season, rainfall in Bangladesh is very low which is frequently insufficient for successful lentil production. Both the seedling and flowering stages are the most sensitive stages to drought in lentil [9,10]. A reduction in economic yield by drought stress in lentil during the reproductive stage is about 24% [11] and at the pod development stage is around 70% [12]. Global climate change model predicts that the frequency of drought stress will be increased in the near future that will negatively impact on crop growth and productivity in various cropping season of Bangladesh. Therefore, it is urgently needed to conduct research to identify drought-tolerant lentil variety to ensure food security as well as to predict yield potential of lentil under drought stress condition for increasing both production and profitability.
Lack of phenotypic and genetic variability and narrow genetic base are the chief restrictive factors in genetic improvement of lentil [13]. Importantly, the success of any crop improvement program depends upon the magnitude and nature of genetic diversity existing in breeding materials [14]. As superior genotypes are used as parent materials in a hybridization program, information on the nature and magnitude of phenotypic and genetic divergence in the population facilitates in choosing the diverse parents for effective hybridization for further improvement of lentil genotypes [15,16]. In contrast, the genetic relation among the accessions can be explained by cluster analysis which facilitates the selection of genetically diverse parents in hybridization program resulting in significant extent of heterosis and wide range of segregation.
To date, several physiological, morphological and biochemical indicators have been identified in lentil conferring drought stress tolerance under field conditions [2,17,18]. Additionally, various screening techniques have been proposed based on morpho-physiological and biochemical traits in various crop species to screen drought tolerant lentil genotypes under soil/field conditions [19–21]. There is no comprehensive report regarding the assessment of drought tolerance at the seedling stage (under hydroponic conditions) and reproductive stage (under field conditions) by using the same genotypes. While field screening is the most appropriate approach to identify the stress tolerant genotypes however it can be affected in open fields due to variable extent of weather condition during the experimental period [21]. Additionally, field screening is very expensive and time-consuming and difficult to investigate a huge number of genotypes. A robust, economic and reliable laboratory-based drought screening technique at the early phases of plant growth is therefore essential to speed-up the selection breeding program for maximizing genetic gain. Considering above facts in mind, the present research studies have been conducted to investigate the suitability of a cost effective and rapid laboratory-based drought screening techniques at the seedling stage in relation to field screening at the reproductive stage using diverse lentil genotypes. The diversity of the genotypes was also studied to identify the best genotypes to be hybridized for higher genetic gain and improvement of lentil for drought stress tolerance.
2.1 Experiment I: Hydroponic Screening of Lentil Germplasm for Drought Tolerance at the Seedling Stage
A total of thirty diverse lentil genotypes were used as plant materials for this study. Eighteen genotypes viz., BM-1247, BM-1227, BM-1222, BM-1220, BM-1181, BM-981, BM-941, BM-908, BM-868, BM-728, BM-680, BM-512, BM-507, BM-502, BM-477, BM-135, BM-120, BM-119 were exotic lines collected from International Center for Agricultural Research in the Dry Areas (ICARDA), India and rest of the twelve locally released varieties viz., Binamasur-1, Binamasur-5, Binamasur-6, Binamasur-8, Binamasur-9, BARI Masur-1, BARI Masur-2, BARI Masur-3, BARI Masur-4, BARI Masur-5, BARI Masur-6, BARI Masur-7 were collected from Bangladesh Institute of Nuclear Agriculture (BINA) and Bangladesh Agricultural Research Institute (BARI), respectively.
2.1.2 Experimental Site and Design
The experiment was carried-out during the period of August-November 2015 in plant growth chamber of the Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh, Bangladesh, by maintaining controlled temperature and humidity (25 ± 2°C and 60% RH). A completely randomized design with three replications was followed to conduct the experiment.
2.1.3 Seedling Establishment and Drought Stress Treatments
The seeds of the selected 30 genotypes were sterilized with NaOCl and placed in moistened filer paper for germination. Three-day-old seedlings were transferred to hydroponic set-up and placed into the holes of the lids carefully in order to avoid root injury (Fig. 1). Each hydroponic tank contained 8L nutrient solution (0.75 g Peter professional per liter and 0.15 g FeSO4 per liter). The pH of the solution was adjusted to the range of 5.3–5.5 by adding HCl and NaOH. The nutrient solution was changed after every 7 days. Drought stress was imposed by taken-out 10-day-old seedlings from the nutrient solution and exposed them to air for 4 h at every alternate day for 3 times following the methods of Singh et al. [21]. The drought-stressed plants were then allowed for grow for 7 days in new nutrient solution under control conditions.
2.1.4 Data on Root-Shoot Traits under Drought Stress
After drought stress and seven days recovery, data on six quantitative traits viz., shoot length (SL), root length (RL), shoot fresh weight (SFW), root fresh weight (RFW), shoot dry weight (SDW) and root dry weight (RDW) were recorded from 21-day-old seedlings. For dry weight, samples were dried at 80° for 72 h and weight was taken with a fine electric balance.
2.1.5 Seedling Survivability (%)
Seedling survivability was calculated in percentage for each replication by dividing the total number of alive seedlings in each replication with the total number of seedlings placed in each replication.
Drought stress injuries were characterized by collecting 21-day-old seedlings and drought scoring was done by rating the plants on a 0–4 scale for each plant separately and then averaged to generate mean values of each genotype for drought tolerance level as described by Singh et al. [21] and Idrissi et al. [22]. Genotypes with the lowest and highest scores were considered to be the most tolerant and sensitive to drought stress, respectively. The criteria for different scale are as ‘0’ for “healthy plants with no visible symptoms of drought stress”, ‘1’ for “green plants with slight wilting”, ‘2’ for leaves turning yellowish green with moderate wilting, “3’ for “leaves yellow-brown with severe wilting”, and “4’ represents “completely dried leaves and/ or stems”.
2.2 Experiment II: Assessment of Drought Stress Tolerance under Field Conditions and Diversity Studies
Same as experiment I.
2.2.2 Experimental Site and Design
The experiment was conducted during rabi season (November 2015 to April 2016) at the experimental farm of the Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh. In conducting the experiment, a Randomized Complete Block Design (RCBD) with three replicates was followed. Unit plot size was 0.6 m2 with row to row distance 30 cm and plant to plant distance 5 cm.
2.2.3 Imposition of Drought Stress
The control plants were grown under rain-fed condition, however, one irrigation was applied at the pre-flowering stage. The drought-stressed plants were grown under rain-fed condition without any irrigation. Other intercultural operations were done whenever necessary in both control and drought-stressed plot following the standard procedures of BARI and BINA.
2.2.4 Data on Yield and Yield Attributing Traits
Data on various yield contributing and yield traits viz., days to 50% flowering (DFF), days to maturity (DM), plant height (cm) (PH), number of primary branches per plant (NPB), number of secondary branches per plant (NSB), number of pods per plant (NPP), number of seeds per plant (NSP), number of seeds per pod (NSPP), 100-seed weight (g) (HSW) and yield per plant (g) (YPP) were recorded from ten randomly selected plants for each replication of each genotype.
2.3 Statistical Analysis of Data
For experiment I, data on six shoot and root traits were analyzed following a CRD design. In case of experiment II, data were analyzed following RCBD design. The separation of means was done following least significant differences (LSD) test at 5% level of probability. Diversity analysis through clustering was performed to find out the desirable drought tolerant lines from various diversified genotypes using the MSTATC and PLABSTAT R and SAS software. The genotypes were arranged in different cluster following the methods suggested by Ward [23] based on Euclidean distance and hierarchical cluster analysis. The D2 values were calculated from transformed uncorrelated means of characters according to Rao [24] and Singh et al. [25]. Average intra- and inter-cluster distances were calculated following the methods suggested by Rao [24].
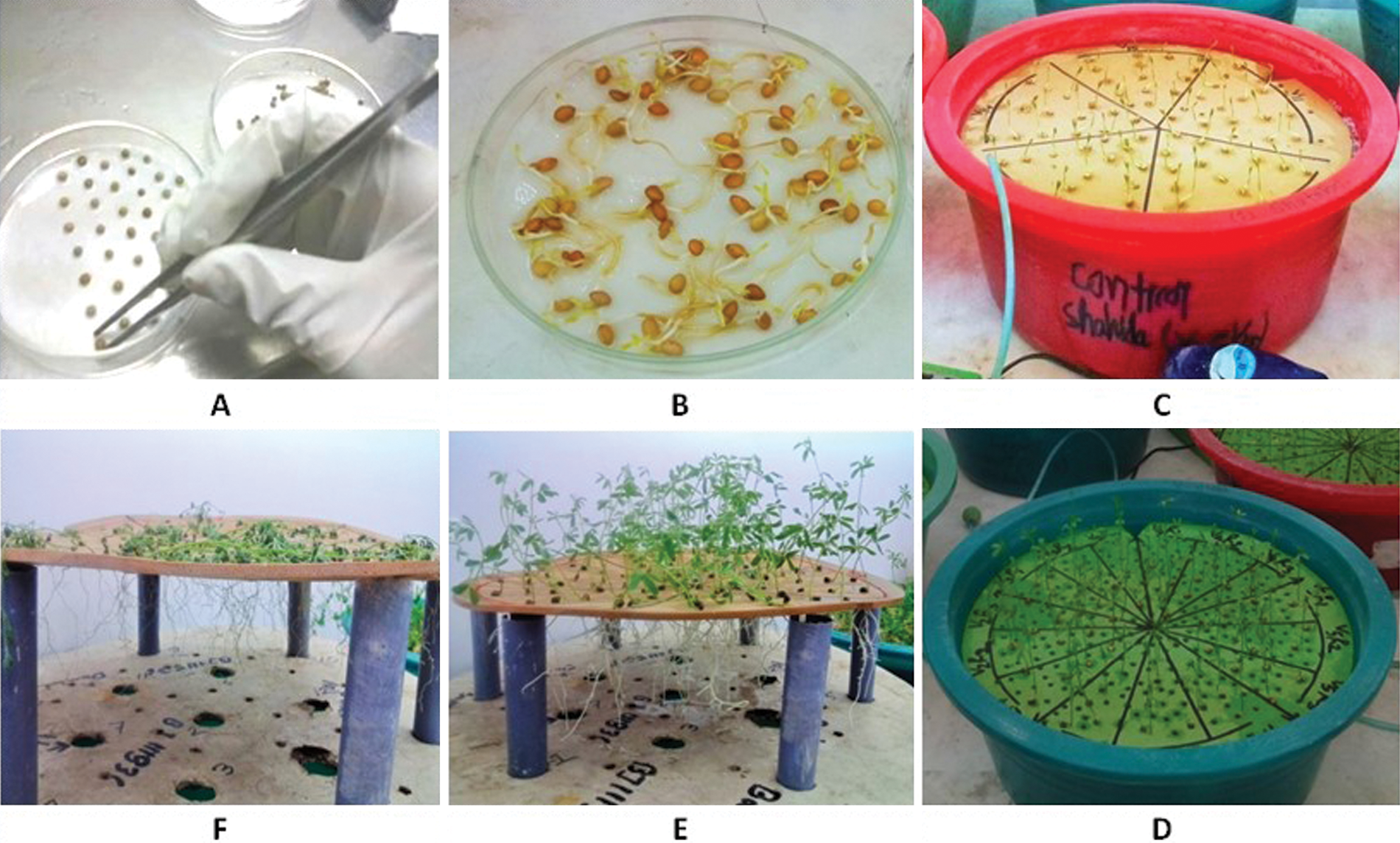
Figure 1: Hydroponic culture of lentil genotypes for drought stress tolerance: (A) seed placement, (B) seed germination, (C) setting of hydroponic culture, (D) seedling phenotype just before the imposition of drought stress, (E) control seedlings at the end of growth period, (F) drought-stressed seedling at the end of stress imposition
The results of combined analyses of variance (ANOVA) of thirty lentil genotypes for six root-shoot traits in hydroponic screening (Appendix A) and ten yield attributing traits in field screening (Appendix B) were showed highly significant (p < 0.01) variation for genotype (G), Treatment (T) and G X T interaction for all of the studied traits except DFF and DM. G X T interaction of DFF and DM was showed significant at 5% level of probability.
3.1 Effect of Drought Stress on Root-Shoot Traits at the Seedling Stage
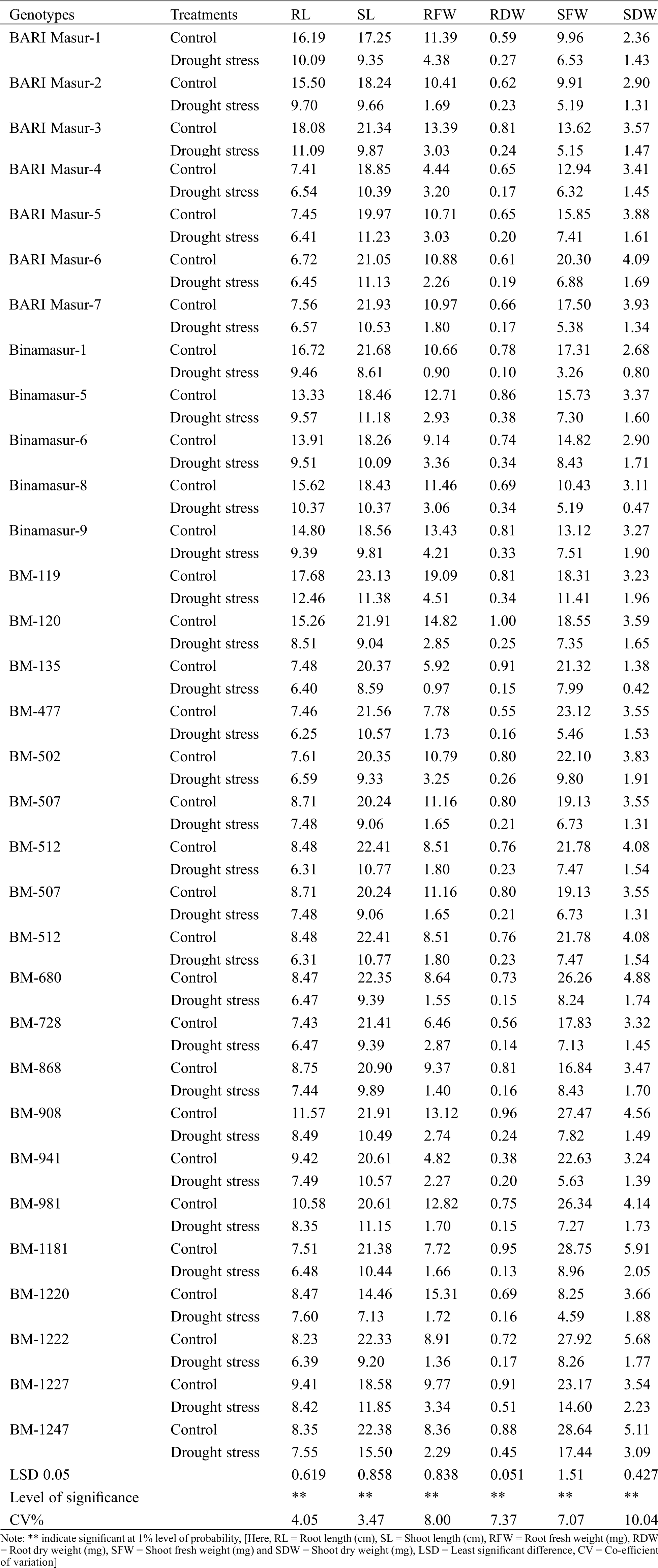
Plenty of variation was observed in root length among the lentil genotypes studied in response to treatments. The highest RL (18.08 cm) under control condition was recorded in BARI Masur-3 whereas the lowest RL (6.72 cm) was recorded in BARI Masur-6 (Tab. 1). Under drought stress, the highest (12.46 cm) RL was recorded in BM-119 whereas the lowest RL (6.25 cm) was recorded in BM-477. Drought stress caused a substantial decrease in RL for most of the studied genotypes; the highest reduction (44.23%) was recorded for BM-120 whereas the lowest reduction (4.02%) was observed in BARI Masur-6 (Tab. 1).
The genotype BM-119 showed the highest SL (23.13 cm) under control condition whereas the lowest SL (14.46 cm) was recorded in BM-1220 (Tab. 1). Under drought stress, the highest (11.85 cm) SL was recorded in BM-1227 and the lowest SL (7.13 cm) in BM-1220. A significant decrease of SL was recorded in most of the studied genotypes; the highest reduction (60.29%) was recorded in Binamasur-1 whereas the lowest reduction (30.74%) was observed in BM-1247(Tab. 1).
The maximum RFW (19.09 mg) under control condition was recorded in BM-119 whereas the minimum RFW (4.44 mg) was recorded in BARI Masur-4 (Tab. 1). Under drought stress, the highest (4.51 mg) RFW was recorded in BM-119 whereas the lowest RFW (0.9 mg) in Binamasur-1. A substantial reduction in RFW was observed under drought stress, the highest reduction (91.56%) was recorded for Binamasur-1 whereas the lowest reduction (27.93%) was observed in BARI Masur-4 (Tab. 1).
The highest RDW (1.0 mg) under control condition was recorded in BM-120 but the lowest RDW (0.38 mg) was recorded in BM-941 (Tab. 1). Under drought stress, the highest (0.51 mg) RDW was recorded in BM-1227 whereas the lowest RDW (0.1 mg) was recorded in Binamasur-1. Drought stress resulted in a significant decrease in RDW, the highest reduction (87.18%) was recorded for Binamasur-1 whereas the lowest reduction (43.95%) was observed in BM-1227 (Tab. 1).
Under control condition, the highest SFW (28.78 mg) was recorded in BM-1181, however, the lowest SFW (8.25 mg) was recorded in BM-1220 (Tab. 1). Under drought stress, the highest (17.44 mg) SFW was recorded in BM-1247 whereas the lowest SFW (3.26 mg) was recorded in Binamaur-1. A significant decrease in SFW was observed upon imposition of drought, the highest reduction (81.17%) was recorded for Binamasur-1 whereas the lowest reduction (34.44%) was observed in BARI Masur-1 (Tab. 1).
The genotype BM-1181 showed the highest SDW (5.91 mg) under control condition, however, the lowest SDW (1.38 mg) was recorded in BM-135 (Tab. 1). Under drought stress, the highest (3.09 mg) SDW was recorded in BM-1247 whereas the lowest SDW (0.42 mg) was recorded in BM-135. A sharp decrease in SDW was found under drought, the highest reduction (84.89%) was recorded for Binamasur-8 whereas the lowest reduction (37.01%) was observed in BM-1227 (Tab. 1).
Table 1: Effect of drought stress on root-shoot traits of lentil at the seedling stage grown under hydroponic conditions. Data represented in the table are the treatment means of three replicates (10 plants per replication)
3.2 Classification of Lentil Genotypes for Drought Tolerance at the Seedling Stage
After drought stress and recovery treatment, several stress symptoms were recorded such as wilting of seedlings, yellowing of leaves, drying leaves, leaf curling, reduced rate of growth and finally the death of the seedling while the seedlings grown under controlled conditions displayed normal growth and development. Among 30 lentil genotypes, three genotypes viz., BM-1247, BM-1227 and BM-502 were found highly-tolerant to drought stress (Tab. 2) showing a drought score of 0.27, 0.33, and 0.33, respectively with 93% seedling survivability. Genotype BM-1222, BM-981 and BM-477 were categorized as tolerant genotypes. In contrast, nine genotypes viz., BARI Masur-3, BARI Masur-6, BARI Masur-7, Binamasur-1, Binamasur-5, Binamasur-6, BM-507, BM-908 and BM-941 were found highly-sensitive to drought stress as they showed less than 30% seedling survivability. Rest of the genotypes showed average to moderate performance under drought stress. Phenological appearance clearly separated the susceptible and tolerant genotype (Fig. 2).
Table 2: Categorization of lentil genotypes based on germination percentage, percent seedling survivability, and drought score at the end of drought stress imposition under hydroponic condition
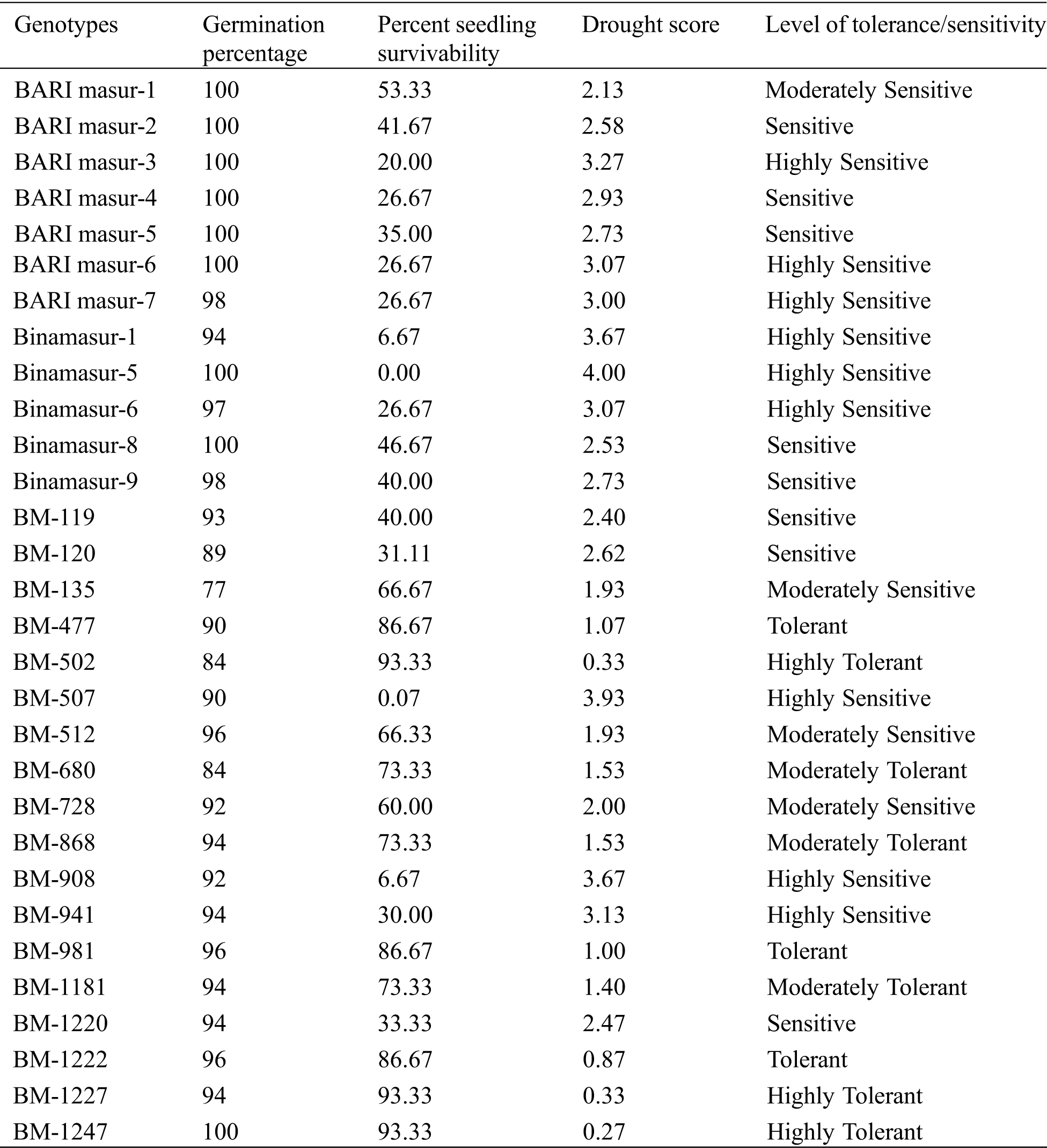

Figure 2: Phenological appearance of highly-tolerant and highly-sensitive genotypes after drought stress and recovery treatment
3.3 Performance of 30 Lentil Genotypes for 10 Yield Contributing Traits
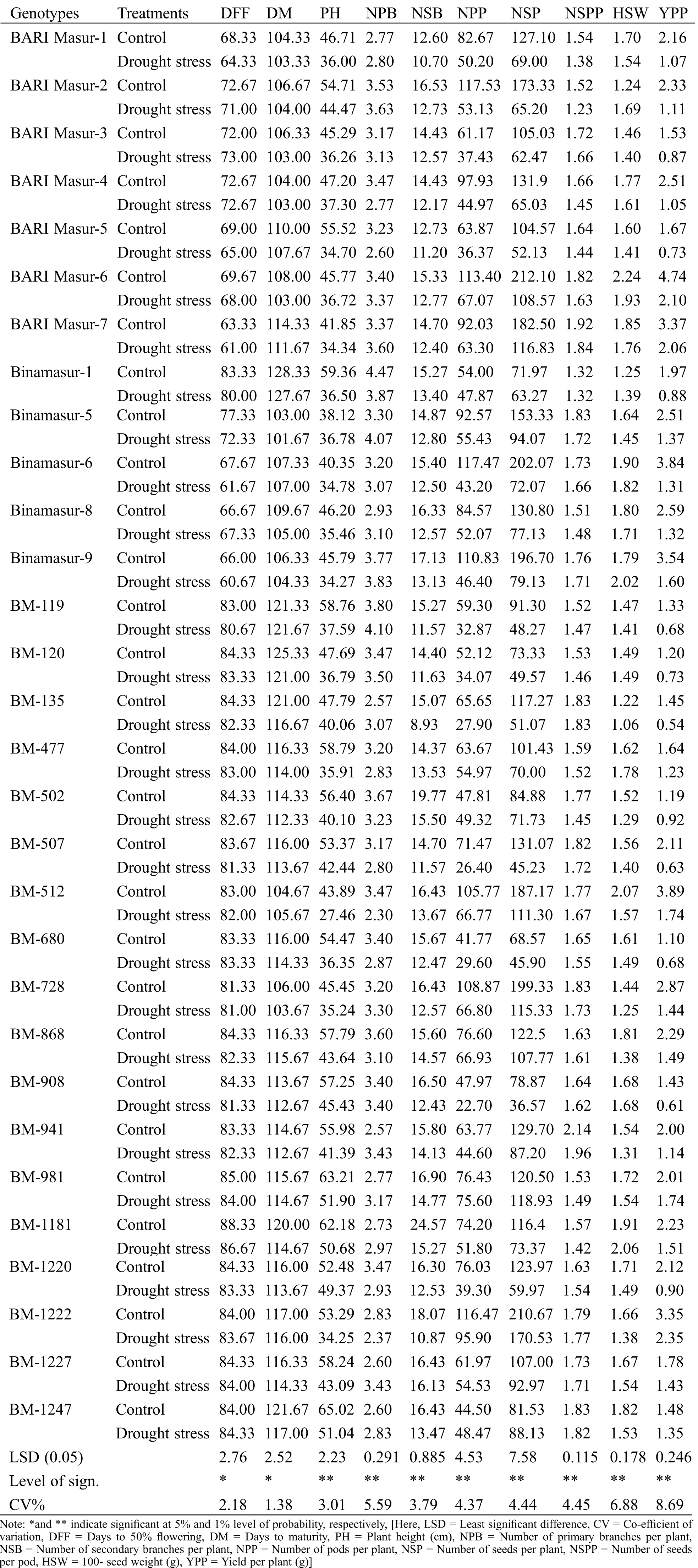
Under control condition, the genotype BM-1811 required maximum days (88.33) to flower whereas the genotype Binamasur-7 required the minimum days (63.33) for flowering (Tab. 3). Under drought stress, the genotype BM-1811 required maximum days (86.67) to flower whereas the genotype Binamasur-9 required minimum days (60.67) to flower. A reduction in days to first flowering was observed under drought stress, the highest decrease (9.73%) was recorded for Binamasur-6 whereas the lowest decrease (0.39%) was observed in BM-1222, BM-1227, BM-1247, respectively (Tab. 3).
The genotype Binamasur-1 required maximum days (128.33) to mature whereas the genotype Binamasur-5 required the minimum days (103.00) to mature (Tab. 3) under control condition. Under drought stress, the genotype Binamasur-1 required maximum days (127.67) to mature whereas the genotype Binamasur-5 required the minimum days (101.67) to mature. A decreasing trend in days to maturity under drought stress was recorded in most of the studied genotypes; the highest reduction (4.63%) was recorded for BARI Masur-6 whereas the lowest reduction (0.28%) was observed in the genotype BM-119 (Tab. 3).
The highest PH (65.02 cm) under control condition was recorded in BM-1247 whereas the lowest PH (38.12 cm) was recorded in BARI Masur-5 (Tab. 3). Under drought stress, the highest PH (51.9 cm) was recorded in BM-981 whereas the lowest PH (27.46 cm) was recorded in BM-512. Imposition of drought stress resulted in a substantial reduction in PH, the highest reduction (38.92%) was recorded for BM-477 whereas the lowest reduction (3.52%) was observed in Binamasur-5 (Tab. 3).
3.3.4 Number of Primary Branches Per Plant
Under control condition, the highest NPB (4.47) under control condition was recorded in Binamasur-1 whereas the lowest NPB (2.57) was recorded in BM-941 (Tab. 3). Under drought stress, the highest (4.1) NPB was recorded in BM-119 whereas the lowest NPB (2.3) was recorded in BM-512. A noteworthy decrease in NPB for most of the studied genotypes was found in response to drought, the highest decrease (33.72%) was recorded for BM-512 whereas the lowest decrease (0.88%) was observed in Binamasur-6. Importantly, the genotype BM-908 showed the same NPB under drought stress and control condition (Tab. 3).
3.3.5 Number of Secondary Branches per Plant
The highest NSB (24.57) under control condition was recorded in BM-1181 whereas the lowest NSB (12.60) was recorded in Binamasur-1 (Tab. 3). Under drought stress, the highest (16.13) NSB was recorded in BM-1227 whereas the lowest NSB (8.93) was recorded in BM-135. Drought stress caused a substantial decrease in NSB; the highest decrease (40.74%) was recorded for BM-135 whereas the lowest decrease (1.83%) was observed in BM-1227 (Tab. 3).
3.3.6 Number of Pods Per Plant
Under control conditions, the highest NPP (117.53) under control condition was recorded in BARI Masur-2 whereas the lowest NPP (68.57) was recorded in BM-680 (Tab. 3). Under drought stress, the highest (95.90) NPP was recorded in BM-1222 whereas the lowest NPP (22.70) was recorded in BM-908. Drought stress resulted in a substantial decrease in NPP in most of the studied genotypes; the highest reduction (63.22%) was recorded in Binamasur-6 whereas the lowest reduction (1.09%) was observed in BM-981(Tab. 3).
3.3.7 Number of Seeds Per Plant
The highest NSP (212.10) under control condition was recorded in BARI Masur-6 whereas the lowest NSP (68.57) was recorded in BM-680 (Tab. 3). Under drought stress, the highest (170.53) NSP was recorded in BM-1222 whereas the lowest NSP (36.57) was recorded in BM-908. In response to drought stress a significant decrease in NSP was observed, the highest reduction (65.49%) was recorded for BM-507 whereas the lowest reduction (1.30%) was observed in BM-981(Tab. 3).
The genotype, BM-941 showed the highest NSPP (2.14) under control condition whereas the lowest NSPP (1.32) was recorded in Binamasur-1 (Tab. 3). Under drought stress, the highest (1.96) NSPP was recorded in BM-941 whereas the lowest NSPP (1.23) was recorded in BARI Masur-2. A significant decrease in NSPP was recorded in response to drought stress; the highest reduction (19.08%) was recorded for BARI Masur-2 whereas the lowest reduction (0.55%) was observed in BM-1247. Importantly the genotype Binamasur-1 and BM-135 showed no difference in 100-seed weight irrespective of treatments (Tab. 3).
The highest HSW (2.24 g) under control condition was recorded in BARI Masur-6 whereas the lowest HSW (1.22 g) was recorded in BM-135 (Tab. 3). Under drought stress, the highest (2.06) HSW was recorded in BM-1181 whereas the lowest HSW (1.06) was recorded in BM-135. HSW showed a substantial decrease under drought, the highest reduction (36.00%) was recorded for BARI Masur-2 whereas the lowest reduction (4.08%) was observed in BM-119. The genotype BM-120 and BM-908 showed no difference in HSW irrespective of control or drought stress treatments (Tab. 3).
The studied genotype showed plenty of variability in YPP under drought and control conditions. The highest YPP (4.74 g) under control condition was recorded in BARI Masur-6 whereas the lowest YPP (1.10 g) was recorded in BM-680 (Tab. 3). Under drought stress, the highest YPP (2.35 g) was recorded in BM-1227 whereas the lowest YPP (0.54 g) was recorded in BM-135. Drought stress resulted in a significant decrease in YPP, the highest reduction (70.01%) was recorded for BM-507 whereas the lowest reduction (9.01%) was observed in BM-1247 followed by BM-981, BM-1227, BM-502, and BM-477 (13.20%, 19.95%, 22.21% and 25.09%, respectively) (Tab. 3).
Table 3: Effect of drought stress on different morphological traits related to yield under field conditions. Data represented in the table are the treatment means of three replicates (10 plants per replication)
3.4 Study of Genetic Divergence among 30 Lentil Genotypes for Yield Related Traits
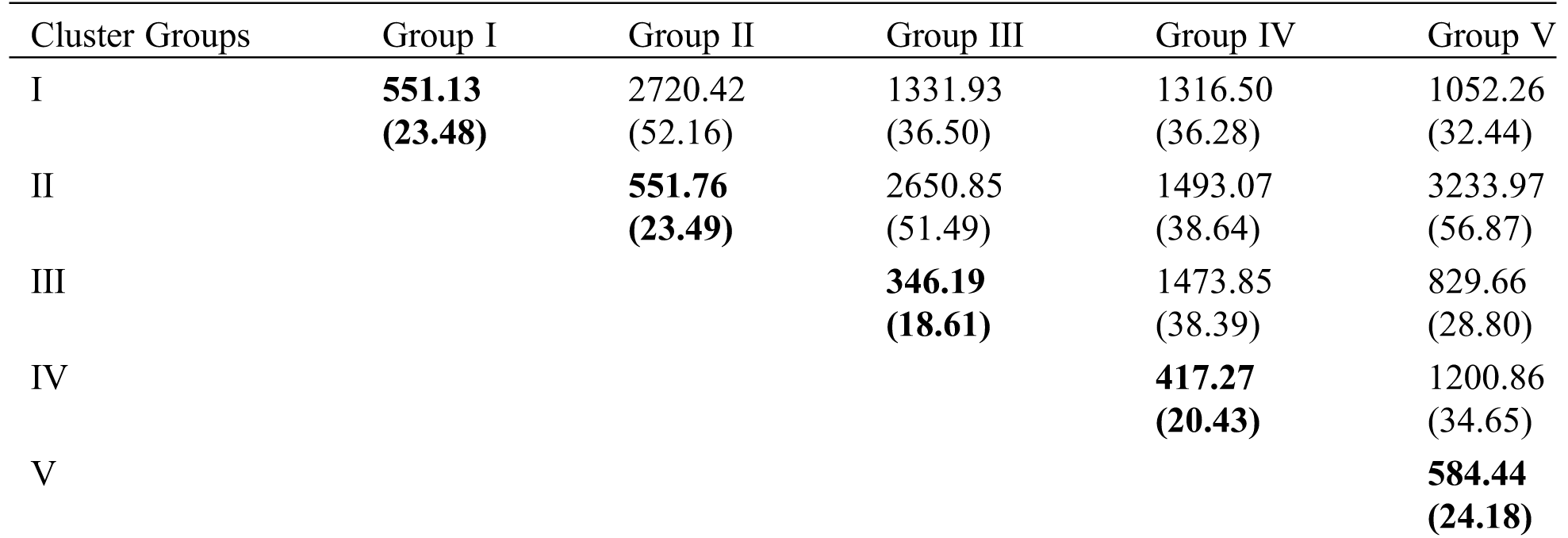
Based on Euclidean distance following Ward’s method and D2-value, 30 genotypes were grouped into 5 distinct clusters for 10 yield attributing traits (Tab. 4 and Fig. 3). Cluster II was the largest group containing ten genotypes (33.3% of total lentil genotypes) whereas the cluster IV was the smallest group containing one genotype. The cluster I, III and V contained five, eight and six genotypes, respectively. The average intra- and inter-cluster distances are presented in Tab. 5. It is observed that the inter-cluster distances were always higher than those of intra-cluster distances (Tab. 5). Maximum intra-cluster distance (24.18) was recorded among the genotypes of cluster V. Cluster II, the largest group containing the highest number of genotypes showed the second highest (23.49) intra-cluster distances. The lowest intra-cluster distance (18.61) was recorded for the genotypes in cluster III. Regarding inter-cluster distance, cluster II showed maximum genetic distance (56.87) from cluster V followed by cluster II (52.16) from cluster I and cluster II (51.49) from cluster III (Tab. 5). Minimum distance was found between the genotypes of the cluster III and V (28.80).
Table 4: Number, percent and name of the genotypes in different cluster for yield attributing traits
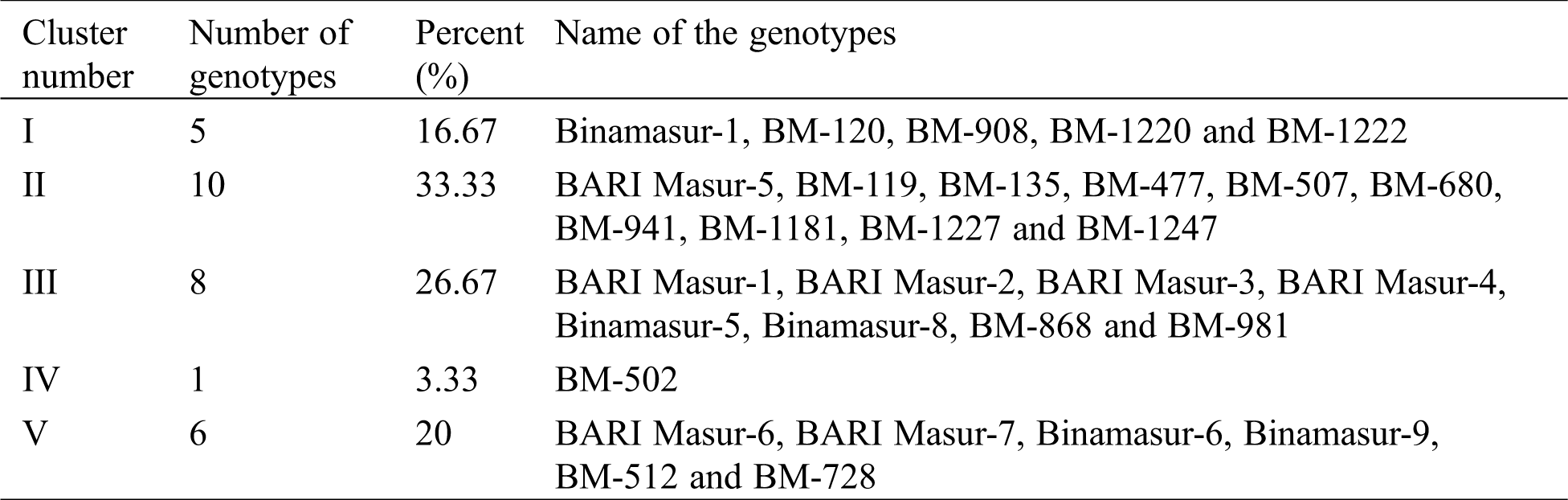
Figure 3: Dendrogram showing differentiation among 30 lentil genotypes for yield components according to Ward's method (red colored rectangle indicates the individual cluster group viz., cluster I, cluster II, cluster III, cluster IV and cluster V from the left to right, respectively)
Table 5: Average intra- and inter-cluster distance among 30 lentil genotypes for yield and yield attributing traits. Values within the bracket ‘( )’ indicates intra-cluster distance
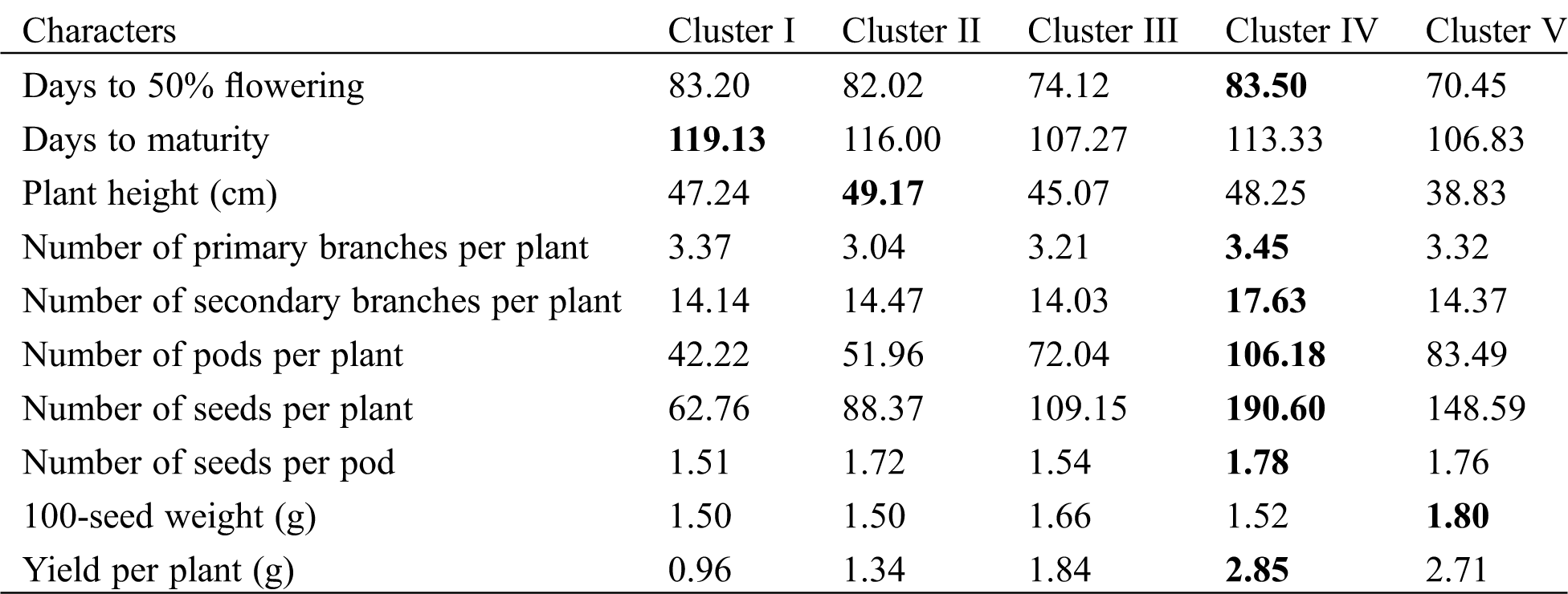
The results of cluster mean (Tab. 6) reflected that the early flowering (70.45 days) genotypes were grouped into cluster V whereas the late flowering (83.50 days) genotypes grouped in cluster IV. Cluster I included long duration (119.13 days) genotype whereas the cluster V included the short duration (106.83 days) genotypes. Short-height genotypes (38.83 cm) were grouped into V cluster whereas the tall-height (38.83 cm) genotypes grouped into cluster II. Cluster IV had the genotypes with high abundance of NPB (3.45), NSB (17.63), NPP (106.18), NSP (190.60), NSPP (1.78), and YPP (2.85) while the genotypes included in cluster I had the lowest value for most of these traits. Additionally, cluster V included the bold-seeded genotype and clusters I and II included the small-seeded genotype.
Table 6: Cluster mean for ten characters related to yield of 30 lentil genotypes
Lentil is a major crop in rained areas and an apposite candidate for drought stress tolerance research [26]. Tolerance to drought is a quantitative trait and largely depends on plant developmental stages. Lack of a simple reliable phenotyping technique severely handicapped the speed of breeding for drought stress tolerance in lentil. An efficient and economic pre-field phenotyping may save time and money and play a crucial role in the selection of candidates for inclusion in field tests to be conducted in a particular environment [27]. As far as our knowledge, this is the first report by using the same genotypes where we showed the suitability of a laboratory based cost effective screening to omit the expensive field screening as we found a good inter-relationship between the drought tolerances in two important plant developmental stages in lentil, i.e., genotype(s) showed drought tolerant reaction at the seedlings stage in pre-field condition were also showed tolerant reaction at the reproductive stage under field screening. It is well well-known that water stress considerably reduced the shoot and root traits. Variations in root and shoot characters have been connected with enhanced drought or water stress tolerance through dehydration avoidance, a mechanism that allows a crop plant to thrive in water-limited environments in pulse crops including lentil [28–30]. In several studies, significant correlations have also been found between a deep root system and shoot growth and seed yield [30–32]. As a common response to drought stress, plants augmented the root growth characteristics to collect more water from the root zone. Well-developed root system and high early biomass conferred water stress tolerance in lentil that could be used in breeding programs targeting root and shoot traits [21,30–32].
Drought stress severely restricted development and growth of plants leading to demising yield and yield attributing traits. The results of present study showed that the genotypes exhibited a substantial variation in germination percentage, percent seedling survivability, shoot and root traits (Tabs. 1 and 2, Appendix A) and drought stress resulted in a noteworthy decreased in root and shoot traits (Tab. 1). Maximum reduction percentages were recorded for shoot length, shoot dry weight, root fresh weight and root dry weight in the drought sensitive genotypes in comparison with the tolerant genotypes. Similar outcomes in root and shoot in response to drought stress was also reported in lentil by several research investigations [21,26,29,30]. The findings of the present study showed that water stress substantially impacted root and shoot biomass development indicating that these traits were negatively affected by drought stress as plant growth and development in general. Reduced shoot and root traits as a response of drought stress has also been demonstrated in rice (Oryza sativa L.) [33,34], wheat (Triticum durum) [35] and barley (Hordeum vulgare) [36]. This type of growth inhibition ensued due to lower level photosynthetic pigments synthesis, cell dehydration, osmotic imbalance, reactive oxygen species production, and improper nutrient uptake [34,37]. However, root characters increased under drought stress in response to less favorable conditions for aboveground biomass development and probably for more belowground (root) biomass development to explore more space for more moisture and nutriment uptake in early stages of drought. In contrast, under favorable conditions (well-watered treatment) aboveground biomass development increased. These results were in agreement with the findings of Aswaf et al. [38]. High values of root and shoot traits under water-limited conditions may be important for selection of drought tolerant genotypes. Based on the results of our hydroponic experiment, three genotypes (BM-1247, BM-1227 and BM-502) categorized as highly tolerant with drought scoring values of 0.27, 0.33, and 0.33 respectively with 93% seedling survivability. Genotypes BM-1222, BM-981 and BM-477 were categorized as drought tolerant genotypes. Nine genotypes (BARI Masur-3, BARI Masur-6, BARI Masur-7, Binamasur-1, Binamasur-5, Binamasur-6, BM-507, BM-908 and BM-941) were categorized as categorized as highly sensitive to drought stress showing seedling survivability less than 30%. The phenological appearance clearly separated the susceptible and tolerant genotypes (Fig. 2) indicating the suitability of the phenotyping techniques to separate the drought tolerant genotypes from a large and diverse germplasm at the early stages of plant growth.
The outcomes of the field experiment reflected that the genotypes displayed significant (p < 0.01) variations in performance for all of the studied traits related to yield (Appendix B) grown under control and drought stress environments. These results were fully in consent with the findings of other research groups [4,39–42]. In this study, genotypes showed maximum variations for the traits number of pods per plant, seeds per plant, 100-seed weight and yield per plant under drought stress condition and those variations might be created due to the result of genotypic or due to environmental factors. Water stress during grain filling significantly reduced yield and yield contributing traits (Tab. 3). Plants encountered drought stress through multiple approaches through cell to ecosystem level. Drought escape is the one of the classical adaptive mechanisms employ by plants to overcome drought stress. In this experiment, drought stress conferred a substantial reduction in days to 50% flowering as well as days to maturity. This is because when a crop plant is exposed to drought stress condition, it requires less time to complete its normal life cycle since stress condition shortens the life cycle compared to control conditions due to many factors competing with the growth of the plants such as insufficient nutrient, unavailability of soil moisture etc. which ceases the normal growth and development of crop leading to the forced earliness in flowering and maturity. Similar results have also been reported in other crops by several research groups [43,44]. Importantly, the genotypes identified as high tolerant and tolerant showed little changes in days to 50% flowering and days to maturity as compared to the drought sensitive genotypes which indicates that the genotypes might have different drought tolerance mechanism except drought escape.
In the present experiment, imposition of drought stress also significantly reduces the yield and yield attributing traits such as number of primary branches per plant, plant height, number of secondary branches per plant, number of seeds per plant, number of pods per plant, 100-seed weight and number of seeds per pod. A similar decrease in yield contributing traits in lentil under the condition of drought stress was also observed by other researchers [45,46]. Significant reduction in plant height was also observed by Bayoumi [47] and Hussain et al. [41]; number of pods per plant [9]; number of seeds per plant [48–50]; number of seeds per pod [48]; 100-seed weight [47–49,51] and yield per plant [9,52] in lentil. The reduction in seed weight due to stress is primarily attributed to a reduction in starch accumulation in leaves and seeds, which was linked to a reduction in the activity of the starch-synthesizing enzyme as well as poor availability of sucrose to seeds that ultimately leads to the yield per plant [45,46]. Importantly, approximately 40% to 70% yield reduction occurred in local cultivars as well as most of the exotic genotypes under drought stress. On the other hand, lower yield reduction might be desirable for drought stress tolerance. Based on this criterion, the genotypes BM-1247, BM-981 and BM-1227 categorized as high drought tolerant genotypes as these three genotypes showed lower yield reduction (9%, 13.20% and 19.95%, respectively) in comparison with other genotypes. Importantly these genotypes also showed a less reduction in root and shoot attributes under hydroponic conditions, indicating the correlation of drought tolerance between lab and field screening in two developmental stages. BM-507 (70%) was the most sensitive to drought stress followed by Binamasur-6 (65.83%), BM-135 (62.58%) and BARI Masur-4 (58.3%). This result showed consistency with Salehi et al. [9], Ali Akbar et al. [53], Babayeva et al. [51] and Hoque [49]. Importantly, these genotypes also showed higher root and shoot reduction traits and lower seedling survival under hydroponic condition. Thus, indicated the relationship of drought sensitivity between lab and field screening at the seedling and maturity stages.
Cluster analysis of ten yield and yield contributing traits and their relative contribution towards the total genetic divergence are presented in Tab. 4 and Fig. 3. Based on D2 value, the genotypes of the present experiments were grouped into five clusters (Tab. 4, Fig. 3). The distribution pattern disclosed that cluster II contained 10 genotypes (one cultivated variety from Bangladesh and 9 genotypes from ICARDA) which was the largest one contained 33.3% of the total genotypes. This cluster also contained the maximum number of drought tolerant genotypes (BM-1227 and BM-1247). Importantly, maximum Bangladeshi cultivars were placed in the cluster III including two genotypes from ICARDA. Cluster IV was the smallest group containing only one genotype (BM-502) of the total genotypes which was tolerant to drought stress at the seedlings stage. These results indicated that there is no relationship between cluster groups based on geographic distribution and genetic diversity of lentil in this study. Surprisingly in the same cluster several genotypes from different geographical origins as well as tolerant and susceptible genotypes were found. Thus indicated that the genetic divergence is an outcome of several factors such as changing of breeding material, parent’s selection, genetic drift, natural variation and artificial selection other than ecological and geographical diversification. It is noted that most of the released varieties of Bangladesh were developed through the collaboration with ICARDA and most of the cases the ancestors are probably the same. However, they might be different for few plant specific root, shoot or yield contributing traits. These findings also suggesting that parental selection should be made on the basis of systematic assessment of genetic distance in a specific population rather than on geographic difference. Similar clustering pattern in lentil was also reported by others [4,47,54].
The results of the present study indicated that the magnitude of inter-cluster distance was higher than intra-cluster distance in most of the cases indicating wider divergence among the studied genotypes of this group (Tab. 5, Fig. 3). The finding was in harmony with the findings of other researchers [55,56]. The highest intra-cluster distance was found in cluster V indicating that the genotypes belonged to the cluster V was more heterogeneous. In cluster III, genotypes were comparatively more closely related as intra-cluster distance among the genotypes was low. Importantly, the genotypes gathered in one cluster are not sharply diversified. In case of inter-cluster distance, cluster V and cluster II showed maximum inter-cluster distance followed by cluster II and cluster I (Tab. 5, Fig. 3) which indicated that efficient breeding program could be formulated to improve yield potential by hybridization and selection of superior genotypes. Hence, genotypes included in these clusters were genetically diverse and might give rise to high heterotic response in segregating generation. The finding was in line with Paliya et al. [57] and Gupta et al. [39]. Minimum inter-cluster distance was found between cluster III and cluster V which suggested that the genotypes of these clusters were genetically least diverse and almost of the same genetic architecture. The crosses between the genotypes of theses cluster are unlikely to generate promising recombinants in segregating generations. However, genotypes with minimum inter-cluster distances might also be used in breeding programs for bi-parental crosses between the most diverse and the closest groups to break the awful linkages between yield and its associated traits.
From cluster mean, maximum good characters were accumulated in cluster IV and importantly the highest seed yield (2.85 g/plant) was obtained in this cluster. But it was interesting that in the entire cases cluster II produced the highest inter cluster-value with all other clusters. Therefore, the genotypes of cluster II and IV can be used in hybridization program to produce higher yielding genotypes. Mean values of cluster groups revealed that secondary branches per plant, pods per plant, seeds per plant, 100-seed weight and yield per plant were the high significant divergent traits contributing to the total divergence suggesting that these traits were the most important traits under drought condition that was positively correlated with yield per plant [58] and the additive gene action was predominant among the genotypes for these traits. Again, days to maturity, days to 50% flowering, number of primary branches and seeds per pod had a moderate contribution towards the total divergence indicating the non-additive gene action controlling these traits. The findings of the present study suggested that the material involved in this study had sufficient amount of diversity for important agronomic traits and may be exploited with great extent by resorting to hybridization which subsequently would result into the development of superior varieties.
Our studies clearly demonstrated that the imposition of drought stress at the seedlings stage and/or reproductive stages significantly impacted plant growth and development, and reduced the yields and yield attributing traits. A close correlation was observed between the laboratory- and field-based screenings for drought stress tolerance. Significant variability and diversity were observed among the studied genotypes. The findings of the present study might be useful for lentil breeding programs to enhance productivity under drought stress.
Acknowledgement: Authors gratefully acknowledge the help of BARI, BINA and ICARDA for providing the seeds.
Funding Statement: This project was funded by the Ministry of Education, Government of the People’s Republic of Bangladesh (Grant No. 2018/518/MOE).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Houasli, O., Udupa, C., De Keyser, S. M., Van Damme, E., De Riek, P. et al. (2015). Genetic variability for root and shoot traits in a lentil (Lens culinaris Medik) recombinant inbred line population and their association with drought tolerance. Euphytica, 204(3), 693–709. DOI 10.1007/s10681-015-1373-8. [Google Scholar] [CrossRef]
2. Sinha, R., Pal, A. K., Singh, A. K. (2018). Physiological, biochemical and molecular responses of lentil (Lens culinaris Medik.) genotypes under drought stress. Indian Journal of Plant Physiology, 23(4), 772–784. DOI 10.1007/s40502-018-0411-7. [Google Scholar] [CrossRef]
3. Lombardi, M., Materne, M., Cogan, N. O., Rodda, M., Daetwyler, H. D. et al. (2014). Assessment of genetic variation within a global collection of lentil (Lens culinaris Medik) cultivars and landraces using SNP markers. BMC Genetics, 15, 150. [Google Scholar]
4. Roy, S., Islam, M. A., Sarker, A., Malek, M. A., Raffi, M. Y. et al. (2013). Determination of genetic diversity in lentil germplasm based on quantitative traits. Australian Journal of Crop Science, 7(1), 14–21. [Google Scholar]
5. Datta, S. K., Sarkar, M. A. R., Uddin, F. M. J. (2013). Effect of variety and level of phosphorus on the yield and yield components of lentil. International Journal of Agricultural Research Innovation and Technology, 3(1), 78–82. DOI 10.3329/ijarit.v3i1.16097. [Google Scholar] [CrossRef]
6. Nath, U. K., Rani, S., Paul, M. R. (2014). Selection of superior lentil genotypes by assessing character association and genetic diversity. Scientific World Journal, 4(7), 1–6. DOI 10.1155/2014/372405. [Google Scholar] [CrossRef]
7. BBS (2017). Bangladesh Bureau of Statistics. Year book of agricultural statistics of bangladesh.Statistics Division, Ministry of Planning, Government of Bangladesh. [Google Scholar]
8. Roy, S., Roy, D. C., Noor, M. M. A., Ghosh, S. R., Ahmed, F. et al. (2019). Binamasur-10, the first drought tolerant lentil variety registered in Bangladesh. Research in Agriculture Livestock and Fisheries, 6(2), 253–262. DOI 10.3329/ralf.v6i2.43048. [Google Scholar] [CrossRef]
9. Salehi, M., Iaghnazari, Z. A., Sherri, Z. I. T., Faramarzi, C. A. (2008). The study of seed yield and seed components of lentil (Lens culinaris Medik.) under drought stress conditions. Pakistan Journal of Ecological Science, 11(5), 758–762. DOI 10.3923/pjbs.2008.758.762. [Google Scholar] [CrossRef]
10. Salehi, M. (2012). The study of drought tolerance of lentil (Lens culinaris Medik) in seedling growth stages. International Journal of Agronomy & Plant Production, 3, 38–41. [Google Scholar]
11. Allahmoradi, P., Mansourifar, C., Saiedi, M., Jalali Honar-mand, S. (2013). Water deficiency and its effects on grain yield and some physiological traits during different growth stages in lentil (Lens culinaris L.) cultivars. Annals of Biological Research, 4, 139–145. [Google Scholar]
12. Turner, R. N. C., Siddique, K. H. M., Turner, D. W., Speijers, J. (2006). A water deficit during pod development in lentils reduces flower and pod numbers but not seed size. Australian Journal of Agricultural Research, 57(2), 427–438. DOI 10.1071/AR05130. [Google Scholar] [CrossRef]
13. Udupa, O. S. M., Houasli, C., De Keyser, E., Van Damme, P. et al. (2015). Genetic diversity analysis of Moroccan lentil (Lens culinaris Medik.) landraces using simple sequence repeat and amplified fragment length polymorphisms reveals functional adaptation towards agro-environmental origins. Plant Breeding, 134(3), 322–332. DOI 10.1111/pbr.12261. [Google Scholar] [CrossRef]
14. Govindaraj, M., Vetriventhan, M., Srinivasan, M. (2015). Importance of genetic diversity assessment in crop plants and its recent advances: An overview of its analytical perspectives. Genetics Research International, 2015(2), 431487–14. DOI 10.1155/2015/431487. [Google Scholar] [CrossRef]
15. Gautam, N. K., Singh, N., Iquebal, M. A., Singh, M., Akhtar, J. et al. (2014). Genetic diversity analysis for quantitative traits in lentil (Lens culinaris Medik.) germplasm. Legume Research, 37(2), 139–144. DOI 10.5958/j.0976-0571.37.2.021. [Google Scholar] [CrossRef]
16. Kumar, J., Solanki, R. K. (2014). Evaluation of lentil germplasm for agro-morphological traits. Journal of Food Legume, 27, 302–306. [Google Scholar]
17. El-Nahas, A., El-Shazly, H., Ahmed, S., Omran, A. (2011). Molecular and biochemical markers in some lentil (Lens culinaris Medik.) genotypes. Annals of Agricultural Sciences, 56(2), 105–112. DOI 10.1016/j.aoas.2011.11.001. [Google Scholar] [CrossRef]
18. Kumar, J., Basu, P. S., Srivastava, E., Chaturvedi, S. K., Nadarnjan, N. et al. (2012). Phenotyping of traits imparting drought tolerance lentil. Crop and Pasture Science, 63(6), 547–554. DOI 10.1071/CP12168. [Google Scholar] [CrossRef]
19. Kumar, A., Singh, D. P. (1998). Use of physiological indices as a screening technique for drought tolerance in oilseed Brassica species. Annals of Botany, 81(3), 413–420. DOI 10.1006/anbo.1997.0573. [Google Scholar] [CrossRef]
20. Hura, T., Hura, K., Grzesiak, S. (2009). Physiological and biochemical parameters for identification of QTLs controlling the winter triticale screening for drought tolerance in lentil drought tolerance at the seedling stage. Plant Physiology and Biochemistry, 47(3), 210–214. DOI 10.1016/j.plaphy.2008.11.004. [Google Scholar] [CrossRef]
21. Singh, D., Dikshit, H. K., Singh, R. (2013). A new phenotyping technique for screening for drought tolerance in lentil (Lens culinaris Medik). Plant Breeding, 132(2), 185–190. DOI 10.1111/pbr.12033. [Google Scholar] [CrossRef]
22. Udupa, O., Houasli, S. M., De Keyser, C., van Damme, E., De Riek, P. et al. (2016). Functional genetic diversity analysis and identification of associated simple sequence repeats and amplified fragment length polymorphism markers to drought tolerance in lentil (Lens culinaris ssp. culinaris Medikus) landraces. Plant Molecular Biology Reports, 34(3), 659–680. DOI 10.1007/s11105-015-0940-4. [Google Scholar] [CrossRef]
23. Ward, J. H., Jr. (1963). Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association, 58(301), 236–244. DOI 10.1080/01621459.1963.10500845. [Google Scholar] [CrossRef]
24. Rao, C. R. (1952). Advanced statistical methods in biometrical research. New York: John Wiley and Sons, 328. [Google Scholar]
25. Singh, R. K., Choudhury, B. D. (1985). Biometrical method in quantitative genetic analysis. Ludhiana, New Delhi: Kalyani Publishers, 54-57. [Google Scholar]
26. Singh, D., Singh, C. K., Taunk, J., Tomar, R. S. S., Chaturvedi, A. K. et al. (2017). Transcriptome analysis of lentil (Lens culinaris Medikus) in response to seedling drought stress. BMC Genomics, 18(1), 206. DOI 10.1186/s12864-017-3596-7. [Google Scholar] [CrossRef]
27. Moshelion, M., Altman, A. (2015). Current challenges and future perspectives of plant and agricultural biotechnology. Trends in Biotechnology, 33(6), 337–342. DOI 10.1016/j.tibtech.2015.03.001. [Google Scholar] [CrossRef]
28. Subbarao, G. V., Johansen, C., Slinkard, A. E., Nageswara Rao, R. C., Saxena, N. P. et al. (1995). Strategies for improving drought resistance in grain legumes. Critical Reviews in Plant Sciences, 14(6), 469–523. DOI 10.1080/07352689509701933. [Google Scholar] [CrossRef]
29. Foti, C., Khah, E., Pavli, O. (2018). Response of lentil genotypes under peg-induced drought stress: Effect on germination and growth. Plant, 6, 75–83. [Google Scholar]
30. Roy, S., Ghanim, A. M. A., Nunekpeku, W., Islam, R., Rasel, M. et al. (2020). Phenotyping of lentil genotypes for drought tolerance using polyethylene glycol. Indian Journal of Natural Sciences, 10, 18145–18159. [Google Scholar]
31. Sponchiado, B. N., White, J. W., Castillo, J. A., Jones, P. G. (1989). Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Experimental Agriculture, 25(2), 249–257. DOI 10.1017/S0014479700016756. [Google Scholar] [CrossRef]
32. Sarker, D. K. R. (2005). Study on the growth, yield and yield attributes of advanced mutants of lentil (M.S. Bangladesh: Thesis). Bangladesh Agricultural University. [Google Scholar]
33. Sohag, A. A., Tahjib-Ul-Arif, M., Brestič, M., Afrin, S., Sakil, M. A. et al. (2020). Exogenous salicylic acid and hydrogen peroxide attenuates drought stress in rice. Plant Soil and Environment, 66(1), 7–13. DOI 10.17221/472/2019-PSE. [Google Scholar] [CrossRef]
34. Sohag, A. A. M., Tahjib-Ul-Arif, M., Polash, M. A. S., Chowdhury, M. B., Afrin, A. et al. (2020). Exogenous glutathione-mediated drought stress tolerance in rice (Oryza sativa L.) is associated with lower oxidative damage and favorable ionic homeostasis. Iranian Journal of Science and Technology, Transactions A: Science, 44(4), 955–971. DOI 10.1007/s40995-020-00917-0. [Google Scholar] [CrossRef]
35. Boutraa, T., Akhkha, A., Al-Shoaibi, A. A., Alhejeli, A. M. (2010). Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. Journal of Taibah University for Science, 3(1), 39–48. DOI 10.1016/S1658-3655(12)60019-3. [Google Scholar] [CrossRef]
36. Hellal, F. A., El-Shabrawi, H. M., Abd El-Hady, M., Khatab, I. A., El-Sayed, S. A. A. et al. (2018). Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. Journal of Genetic Engineering and Biotechnology, 16(1), 203–212. DOI 10.1016/j.jgeb.2017.10.009. [Google Scholar] [CrossRef]
37. Forni, C., Duca, D., Glick, B. R. (2017). Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant and Soil, 410(1–2), 335–356. DOI 10.1007/s11104-016-3007-x. [Google Scholar] [CrossRef]
38. Aswaf, A., Blair, M. (2012). Quantitative trait loci for rooting pattern traits of common beans grown under drought stress versus non-stress conditions. Molecular Breeding, 30(2), 681–695. DOI 10.1007/s11032-011-9654-y. [Google Scholar] [CrossRef]
39. Gupta, R., Begum, S. N., Islam, M. M., Alam, M. S. (2012). Characterization of lentil (Lens culinaris M.) germplasm through phenotypic marker. Journal of Bangladesh Agricultural University, 10(2), 197–204. DOI 10.3329/jbau.v10i2.14682. [Google Scholar] [CrossRef]
40. Cristóbal, M. D., Pando, V., Herrero, B. (2014). Morphological characterization of lentil (Lens culinaris Medik.) landraces from Castilla Y León. Spain Pakistan Journal Botany, 46, 1373–1380. [Google Scholar]
41. Hussain, N., Yaqoob, M., Rashid, A. (2014). Genetic Competition among lentil (Lens culinaris) candidate lines for yield and yield components under rainfed conditions. Pakistan Journal of Agricultural Research, 52, 54. [Google Scholar]
42. Rahman, M. M., Islam, M. M., Hoque, M. E., Ahmed, B., Rahman, M. M. (2015). Performance of advanced lentil genotypes in different pulse growing regions of Bangladesh. Eco-Friendly Agriculture Journal, 8, 116–120. [Google Scholar]
43. Gorim, L. Y., Vandenberg, A. (2017). Evaluation of wild lentil species as genetic resources to improve drought tolerance in cultivated lentil. Frontiers in Plant Science, 8, 1129. DOI 10.3389/fpls.2017.01129. [Google Scholar] [CrossRef]
44. Mishra, B. K., Srivastava, J. P., Lal, J. P. (2018). Drought resistance in lentil (Lens culinaris Medik.) in relation to morphological, physiological parameters and phenological developments. International Journal of Current Microbiology and Applied Sciences, 7(1), 2288–2304. DOI 10.20546/ijcmas.2018.701.277. [Google Scholar] [CrossRef]
45. Awasthi, R., Gaur, P., Turner, N. C., Vadez, V., Siddique, K. H. et al. (2017). Effects of individual and combined heat and drought stress during seed filling on the oxidative metabolism and yield of chickpea (Cicer arietinum) genotypes differing in heat and drought tolerance. Crop and Pasture Science, 68(9), 823–841. DOI 10.1071/CP17028. [Google Scholar] [CrossRef]
46. Sehgal, A., Sita, K., Kumar, J., Kumar, S., Singh, S. et al. (2017). Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Frontiers in Plant Science, 8, 1776. DOI 10.3389/fpls.2017.01776. [Google Scholar] [CrossRef]
47. Bayoumi, T. Y. (2008). Genetic diversity among lentil genotypes for drought tolerance. Journal of Agriculture Investment, 3, 25–35. [Google Scholar]
48. Panahyan-e-Kivi, M., Ebadi, A., Ahmad, T., Sh, Jamaati-e-Somarin (2009). Evaluation of yield and yield components of lentil genotypes under drought stress. Research Journal of Environmental Sciences, 3(4), 456–460. DOI 10.3923/rjes.2009.456.460. [Google Scholar] [CrossRef]
49. Hoque, M. A. E. (2016). Development of an efficient phenotypic technique for screening lentil genotypes for drought tolerance (M.S. Bangladesh:Thesis). Bangladesh Agricultural University. [Google Scholar]
50. Sehgal, A., Sita, K., Bhandari, K., Kumar, S., Kumar, J. et al. (2019). Influence of drought and heat stress, applied independently or in combination during seed development, on qualitative and quantitative aspects of seeds of lentil (Lens culinaris Medikus) genotypes, differing in drought sensitivity. Plant Cell & Environment, 42(1), 198–211. DOI 10.1111/pce.13328. [Google Scholar] [CrossRef]
51. Babayeva, S., Akparov, Z., Damania, A., Izzatullayeva, V., Aslanova, G. et al. (2014). Genetic diversity for drought tolerance in lentils from Central Asia and the Caucasus: CAC Lentil. Albanian Journal of Agricultural Sciences, 13, 1–8. [Google Scholar]
52. Sinha, R. P., Singh, R. N. (2002). Correlation of seed yield with other traits in lentil. Journal of Applied Biology, 12, 1–3. [Google Scholar]
53. Ali Akbar, I., Seyed, S. M., Ali, M. K., Yousef, A. (2014). Evaluation of seed yield, yield components and some main crop traits in 25 lentil genotypes under rain fed and irrigated in various environments. Bulletin of Environmental, Pharmacology and Life Sciences, 3, 9–13. [Google Scholar]
54. Chauhan, M. P., Nath, M., Srivastava, R. K. (2005). Classification of genetic diversity in Indian lentil germplasm. Legume Research, 28, 125–127. [Google Scholar]
55. Pandey, S., Bhatore, A. (2018). Genetic diversity analysis for quantitative traits in indigenous germplasm of lentil in Madhya Pradesh. Journal of Pharmacognosy and Phytochemistry, 7, 279–283. [Google Scholar]
56. Kumar, A. (2019). Genetic diversity of yield attributing components and seed yield in lentil (Lens culinaris Medik.). Current Journal of Applied Science and Technology, 33, 1–6. [Google Scholar]
57. Paliya, S., Saxena, A., Tikle, A. N., Singh, M., Tilwari, A. (2015). Genetic divergence and character association of yield and yield component traits of lentil (Lens culinaris Medik.). Advances in Bioresearch, 6, 53–59. [Google Scholar]
58. Akter, S., Jahan, I., Hossain, M. A., Hossain, M. A. (2020). Variability for agromorphological traits, genetic parameters, correlation and path coefficient analyses in Lentil (Lens culinaris Medik.). Research in Plant Biology, 10, 1–7. DOI 10.25081/ripb.2020.v10.6237. [Google Scholar] [CrossRef]
Appendix A: Analysis of variance (ANOVA) for six morphological traits of 30 lentil genotypes at the seedling stage grown under laboratory conditions
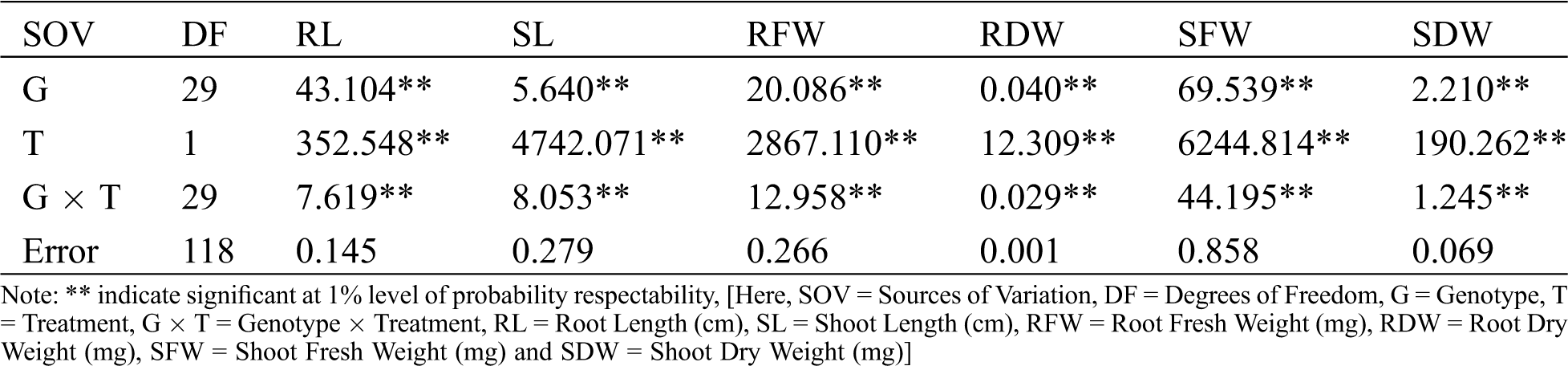
Appendix B: Analysis of variance (ANOVA) for yield and yield contributing characters of 30 lentil genotypes grown under field conditions
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |