
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015476
ARTICLE
Improved Tolerance of Three Saudi Pearl Millet Cultivars (Pennisetum spicatum) to Salt Stress by Mycorrhiza
Biology Department, Faculty of Science, Taibah University, Almadinah Almunawwarah, 41311, Saudi Arabia
*Corresponding Author: Abdulkhaliq Alshoaibi. Email: Ashoaibi@taibahu.edu.sa
Received: 21 December 2020; Accepted: 09 February 2021
Abstract: Seeds of three Saudi pearl millet cultivars (Pennisetum spicatum) from three regions (Madinah, Khulais and Jaizan) were inoculated with arbuscular mycorrhizal fungus Glomus mosseae obtained from the Agriculture Research Center of Giza, Egypt to enhance their salt tolerance. Five different NaCl concentrations (0, 30, 60, 90, and 120 mM) were used for treating cultivars with and without mycorrhiza. Growth rates, chlorophyll content, chlorophyll fluorescence (Fv/Fm), proline content and gas exchange were measured to determine the effect of salinity on these cultivars. The results indicated that compared to cultivars without mycorrhiza, all cultivars with mycorrhiza had enhanced growth and physiological parameters including shoot and root length, area and number of leaves, fresh and dry weights of shoots and roots, chlorophyll contents and gas exchanges at 0 and 30 mM of salinity. In addition, the measurements of the different growth rates showed higher growth performance of the cultivars from Madinah and Khulais than the cultivar from Jaizan. However, all cultivars with and without mycorrhiza showed significant reductions in growth rates, chlorophyll contents and gas exchanges at a salinity of 60 mM than those grown at 0 and 30 mM. Moreover, the values of Fv/Fm were significantly reduced in all cultivars with and without mycorrhiza grown at 60 mM than in those grown at 0 mM and 30 mM. Proline contents indicated a progressive increase with the elevation of NaCl concentration stress. The proline contents in the mycorrhiza-inoculated cultivars were significantly higher than those in the non-inoculated cultivars. On the other hand, all cultivars with and without mycorrhiza underwent senescence within four weeks of growth at salinity concentrations of 90 mM and 120 mM. Therefore, relatively low salinity must be maintained to achieve high growth rates and gas exchanges of these inoculated cultivars.
Keywords: NaCl salinity; pearl millet; mycorrhiza; CO2 uptake; photochemistry; chlorophyll content
One of the most common agricultural problems in arid and semi-arid regions is soil salinity, which reduces the productivity of crops in these regions [1]. The main factor responsible for increasing soil salinity is the use of saline water for irrigation in agricultural lands [2]. Therefore, improving plant tolerance to salinity is very important for increasing plant growth in agricultural areas [3]. Salinity has double effects on the physiological processes of plants. It lowers the water available to the roots and accumulates ions in specific tissues to toxic levels [4]. As a result, salinity limits most commercial crop growth and productivity worldwide [5,6].
Salinity is known for its adverse effects on plant growth and development [7–9]. It reduces leaf development and extension, the number of leaves, leaf area, plant height and the dry weights of roots and shoots [10]. Ashoaibi et al. [11] found that salinity of 400 mM NaCl significantly reduced leaf extension, leaf area, number of leaves, plant height, fresh and dry weights of shoots and roots, and the chlorophyll of Pennisetum purpureum compared to the control plants. The average reductions of all growth parameters ranged between 45% to 72% when NaCl was increased from 0 mM to 400 mM. Furthermore, photosynthesis is another considerable factor affecting the productivity of crops. Saline stress can limit photosynthesis by lowering the availability of CO2 by restricting diffusion through stomata and the mesophyll, consequently breaking down the reaction centre of Photosystems II and disrupting electron transfer of electrons from Photosystem II to Photosystem I [12,13]. These adverse results reduce the capacity of photosynthesis and inhibit the growth of crops. Furthermore, saline stress inhibits the synthesis of photosynthetic pigment and, as a result, chlorophyll content decreases for many crops in different regions when they grow under saline conditions [14–16].
Incorporating factors that enable plants to tolerate salt stress could improve plant growth and production under saline conditions. One of the most common form of symbiotic relationship between a fungus and the roots of a vascular plant is arbuscular mycorrhiza (AMF). It can create symbiotic relationship with 90% of plant species [17,18]. The fungal hyphae infiltrate plant cells and build within the cells branching structures, enabling both organisms to share a vast exchange surface. As a result, plant root access to a wide area of the soil, thus enhancing plant growth and productivity [19,20]. Many studies have demonstrated that AMF improves plant growth by promoting the uptake of water and nutrients under salt stress [21–23]. Gao et al. [24] found that the symbiosis between cotton and AMF has enhanced photosynthesis, number of bolls per plant, the maturity of fibre and growth of the plant. They also found that statistical analysis showed a highly significant yield increase for inoculated plots relative to non-inoculated controls, with a percentage increase of 28.54% [24]. Similarly, Ebrahim et al. [25] found that vesicular arbuscular mycorrhizal fungus enhanced the photosynthesis and productivity of tomato cultivar (Sultana-7) grown under NaCl stress in comparison to non-inoculated controls. Additionally, numerous reports have suggested that AMF can increase plant growth under saline conditions by increasing the accumulation of osmotic regulators, such as proline, polyamines, betaine, and soluble sugar [21,22,26]. Proline is one of the most osmotic regulators accumulated in the plant under salt stresses and is often considered to be involved in stress resistance mechanisms [27,28]. The proline concentration is more remarkable in plants when inoculated with AMF [29]. AMF can also improve leaf tolerance to salinity by decreasing non-photochemical quenching under salinity conditions [30]. Furthermore, the capacity of photosynthesis and stomatal conductance could be improved by AMF for different plants under salt stress [6,22,30].
Pearl millet (Pennisetum spicatum) belongs to the Poaceae family and is grown as a cereal crop for feeding humans in many parts of Asia and Africa [31]. In Saudi Arabia, pearl millet is cultivated in many regions, such as Madinah, Khulais, and Jaizan. Salinity resulting from irrigation presents a large problem for the growth and productive establishment of pearl millet in these regions [32]. The successful development of crops in saline areas is one of the most cost-effective soil salinity management strategies and is an important factor affecting crop production and sustainable agriculture in dry regions [17,33]. Currently, several studies have presented that inoculation with AMF improves the tolerance of plants to salinity, but there are few studies on pearl millet growth under salt stress, particularly photosynthesis function. Therefore, the purpose of this research is to investigate the effect of AMF inoculation on the growth, Fv/Fm, chlorophyll content, proline content and gas exchange of three Saudi pearl millet cultivars under different NaCl concentrations to determine the potential of mycorrhiza to improve their tolerance to salt stress. This information will be useful in determining the cultivar that achieves high growth and gas exchanges rates under different salinity conditions.
2.1 Plant Material and Mycorrhizal Inoculation
Seeds of pearl millet (Pennisetum spicatum L. cv. Khulais), (Pennisetum spicatum L. cv. Madinah) and (Pennisetum spicatum L. cv. Jaizan) were obtained commercially from Khulais, Madinah and Jaizan (Saudi Arabia). The seeds of the three cultivars were sterilized on the surface by soaking in 0.1% mercury chloride for ten minutes. After five rinses in sterile distilled water, the seeds were washed and transferred to pot experiments. On the other hand, mycorrhizal spores of Glomus mosseae (Nicol. & Gerd.) Gerdemann and Trappe were kindly provided by professor El-Nagar from the Agriculture Research Center, Giza, Egypt. The original inoculum was propagated several times on maize plants grown in a sandy soil for ten weeks according to Ortas [34]. The inoculum consisted of spores, chlamydospores, hyphae and heavily colonized corn roots cut into small pieces, blend with sterile distilled water in order to form a dense liquid paste (25 g corn roots + 100 ml water/approximately 80 fungal spores/g).
Seeds of the three pearl millet cultivars were sown in 2-liter plastic pots (12 cm × 20 cm) filled with 2 kg washed and sterilized sandy soil. Eight seeds were sown in each pot, and after emergence, the seedlings were thinned to two per pot. The pots for each cultivar were grouped into two groups (I and II). Each pot in Group I was inoculated with approximately 30 g of sterilized inoculum of G. mosseae (non-mycorrhizal, NM), while those in Group II were inoculated with 30 g of G. mosseae spores (mycorrhizal, M). The inoculums were located adjacent to each seedling root, and the plants were irrigated with equal amounts of water. For two months, the experimental pots were placed in growth chambers (Sanyo Gallenkamp PLC, Fitotron SGC066, Leicester, UK) at a temperature of 25°C/20°C (day/night). The density of the photon flux at leaf height was 600 µmol m-2s-1 (14 h photoperiod), and the relative humidity was 60%. The experiments included ten treatments crossing two mycorrhizal inoculation levels (NM and M) with five salt levels (0, 30, 60, 90, and 120 mM NaCl) in soil. Four replicates for each cultivar of each treatment were applied, leading to a total of 240 pots. The seedlings were harvested after 60 days for further analyses.
Roots from each cultivar were collected, cleaned, stained as previously described by Phillips et al. [35], and microscopically tested for colonization of the vesicular arbuscular mycorrhizal fungus. The grid-line-intersect method previously defined by Giovannetti et al. [36] was used to calculate colonization.
Growth parameters, including shoot length, number of leaves, and fresh weights of shoots and roots, were calculated 60 days after planting. Leaf extension was measured every other day by a ruler, while the leaf area was measured by a Licor Area Meter (LICOR Inc., Lincoln, Nebraska, USA). To evaluate dry weight, the shoot and root samples were oven-dried at 80°C for 48 h.
2.5 Determination of Photosynthesis
The gas exchange rates were measured on the current completely expanded leaf of pearl millet cultivars using an infrared gas analyser LICOR 6400XT (LI-6400, LICOR Inc., Lincoln, US). The 4th youngest fully extended leaves were used for measurements of photosynthesis and dark respiration. Light response curves were determined at a leaf temperature of 25°C and an atmospheric CO2 concentration of 360 μmol mol-1. Gradually, the leaves were illuminated with densities of photon flux between 0 and 1500 μmol m-2s-1. The Von Caemmerer and Farquar equations were used to determine net photosynthesis per unit leaf area and intercellular CO2 concentration ci [37]. Asat was estimated at saturating PPFD (1500 μmol m-2s-1) and at an ambient CO2 concentration of 360 µmol mol-1. Curves of the carbon dioxide response (A/Ci) were measured inside the range of 50-550 µmol mol-1 using 1500 μmol m-2s-1 PPFD at 25°C leaf temperature.
2.6 Determination of Chlorophyll Fluorescence
The chlorophyll fluorescence of the fourth youngest leaves of pearl millet cultivars was measured using a portable fluorimeter (PEA, Hansatech, Norfolk, Kings Lynn). The leaves were dark adapted for 20 min before measuring the fluorescence. The ratio of variable to maximum fluorescence (Fv/Fm) was measured four times for each cultivar as defined by Al-shoaibi [38].
2.7 Determination of Chlorophyll Content
A handheld chlorophyll content meter was used to measure the content of leaf chlorophyll (CCM-200, USA Opti-Sciences). The chlorophyll content was measured four times for each leaf, and the average was used for analysis.
2.8 Determination of Proline Content
To evaluate the proline level content, 0.5 g of leaf samples in 3% (w/v) sulfosalicylic acid was homogenized and then filtered homogeneously through filter paper, as described by Bates et al. [39]. Glacial acetic acid and ninhydrin were applied to the mixture and then heated in a water bath at 100°C for one hour. Four millilitres of toluene was added to the mixture, and the reaction was terminated by placing the mixture in an ice bath. The mixture with toluene was read at 520 nm, and a calibration curve was used to assess the proline concentration, which was expressed as μmol/g fresh weight.
Statistical analyses of the data collected from the several analyses and measurements were conducted using Two-way and Three-way Analysis of Variance (ANOVA), with a general linear model to test main effects and interactions of the factors examined in this study (Salinity; Cultivar; Mycorrhiza). Multiple comparisons using Tukey’s test were also conducted to assess significance between different levels of the factors. All the analyses were carried out using version 15 of Minitab (Brandon Court, Unit E1-E2, Progress Path, Coventry CV3 2TE, UK). Four replicates were used for each treatment, and the standard deviations and standard errors were calculated using Microsoft Excel 2016 from Microsoft.
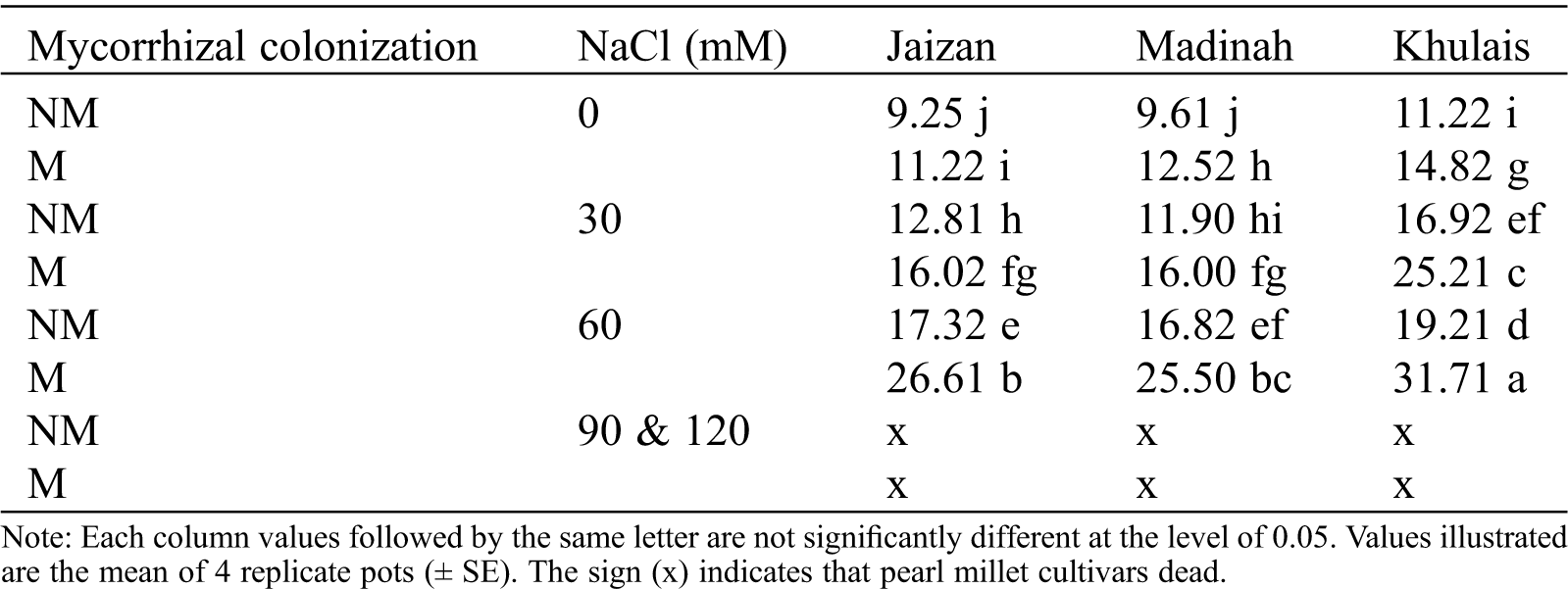
None of the pearl millet cultivars from the non-inoculated treatments were colonized by G. mosseae. On the other hand, the infection in the different cultivars inoculated with G. mosseae was found to decline significantly (p < 0.001, Tab. 1) with increased salinity, although it remained fairly high even when salinity was moderate (22%–24% at 60 mM of salinity).
Table 1: The effect of NaCl concentrations (0, 30, 60, 90 and 120 mM NaCl) on root colonization of Jaizan, Madinah and Khulais pearl millet cultivars inoculated with AMF
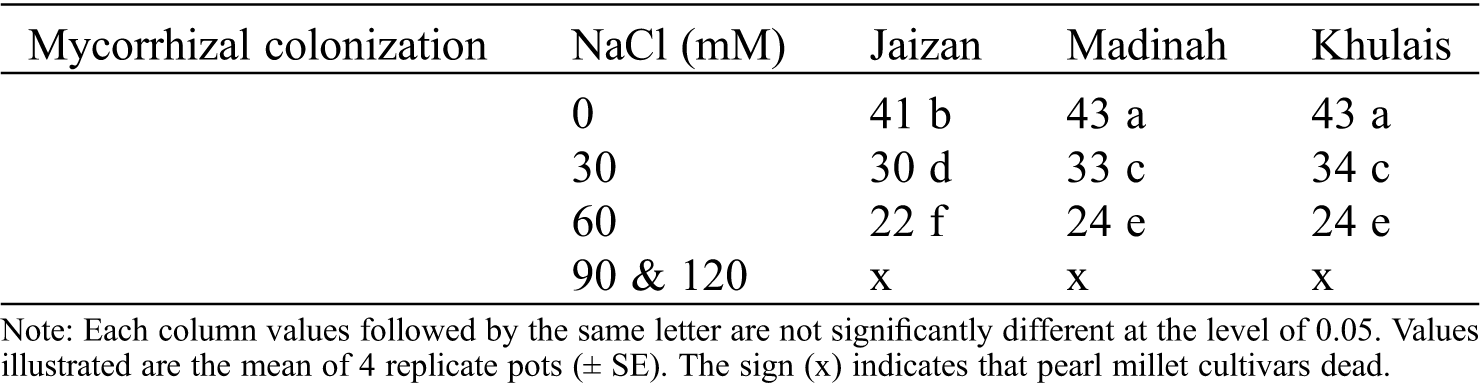
The effect of salinity treatments on the leaf extension of inoculated or non-inoculated pearl millet cultivars with mycorrhiza is illustrated in Fig. 1A. In general, the leaf extension rates significantly reduced in the non-mycorrhizal cultivars at 30 mM and 60 mM of salinity than in the control (p < 0.05). In addition, the leaf extension of mycorrhizal cultivars showed higher extension rates at 0 mM and 30 mM of salinity than in non-mycorrhizal cultivars grown under the same salinity levels. However, the 60 mM of salinity reduced the leaf extension of all mycorrhizal cultivars compared to non-mycorrhizal cultivars grown at the same level of salinity. Additionally, leaf areas and plant heights followed nearly the same pattern of leaf extension (Fig. 1B, 1C, p < 0.05). On the other hand, all cultivars with and without mycorrhiza showed senescence within four weeks of growth at salinity concentrations of 90 and 120 mM. The length of the roots for all cultivars grown with mycorrhiza at 0 mM and 30 mM of salinity were higher than those grown without mycorrhiza at the same levels of salinity (Fig. 1D, p < 0.05). At 60 mM, most mycorrhizal cultivars showed significantly lower root lengths than non-mycorrhizal cultivars (Fig. 1D, p < 0.05). In addition, fresh and dry weights of shoots and roots for most mycorrhizal cultivars grown at 0 mM and 30 mM were significantly increased compared to non- mycorrhizal cultivars (Fig. 2A–2D, p < 0.05). However, most mycorrhizal cultivars showed slightly lower fresh and dry weights of shoots and roots at 60 mM than non-mycorrhizal cultivars.
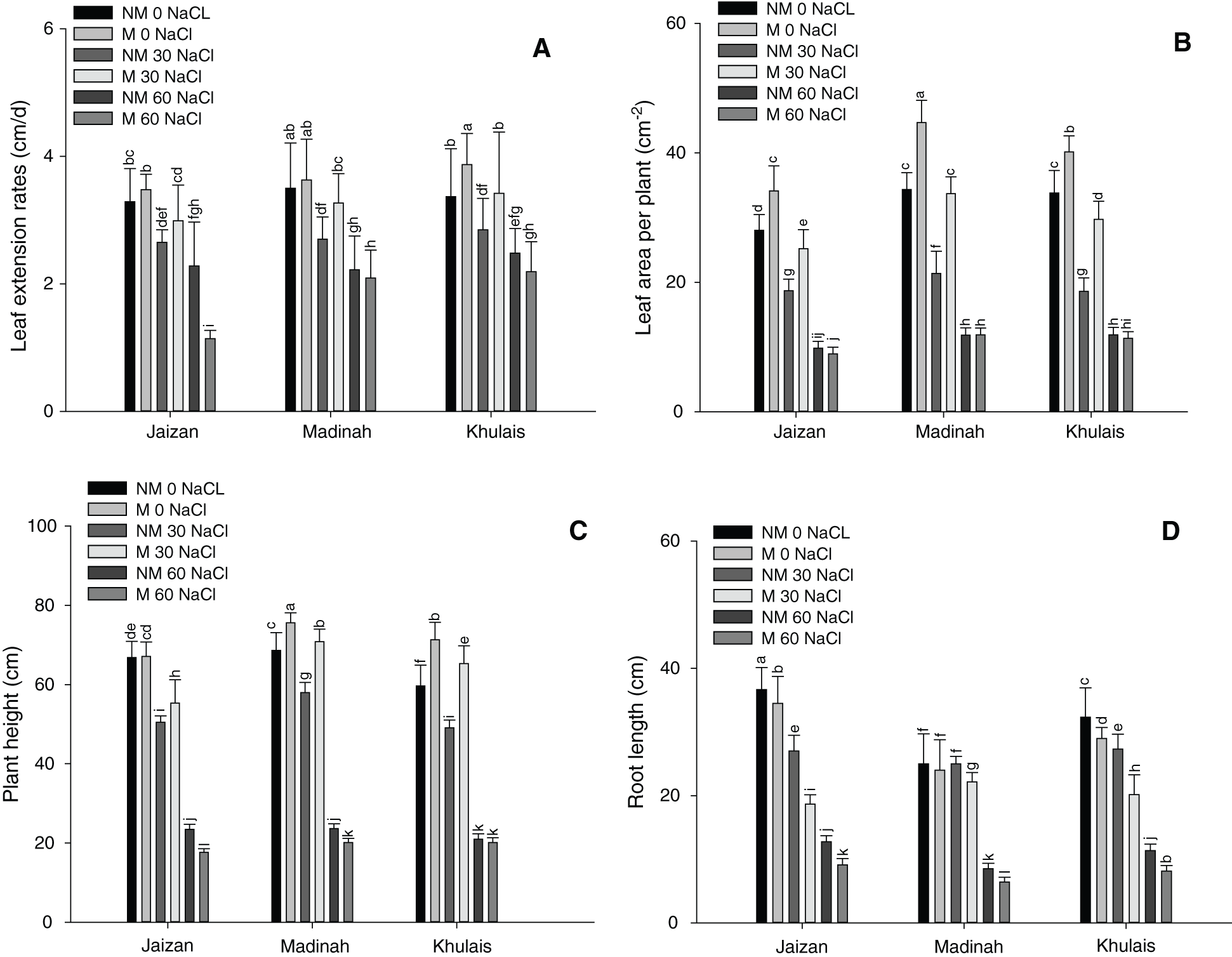
Figure 1: Effect of different NaCl levels on plant growth parameters of Jaizan, Madinah and Khulais pearl millet cultivars inoculated and non-inoculated with AMF, (A) Leaf extension rates, (B) Leaf area, (C) Plant height and (D) Root length. The data is the mean of leaves n = 4 (± SE). Different letters indicate significance of two-way interactions between salinity and mycorrhiza. Means that do not share a letter are significantly different
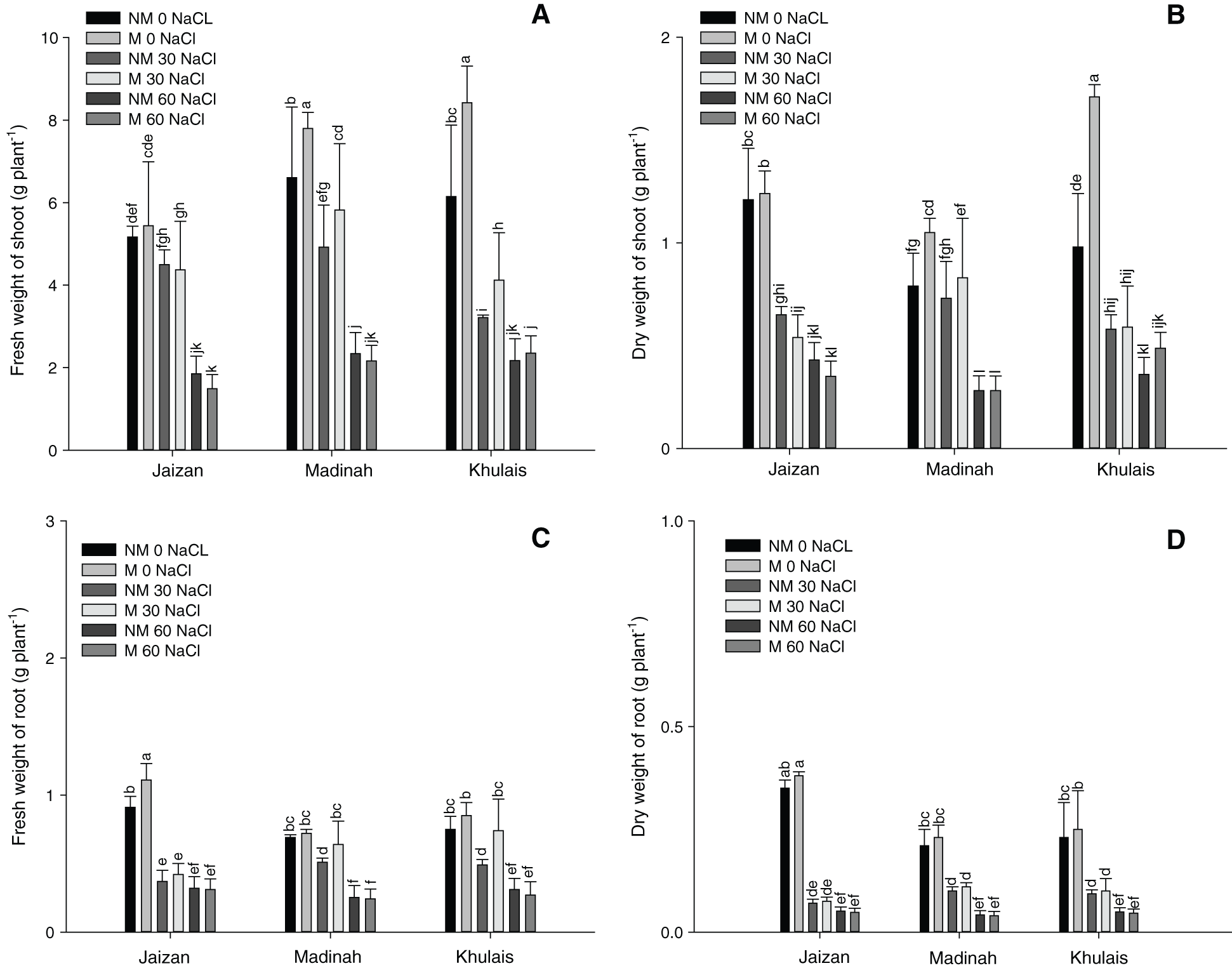
Figure 2: Effect of different NaCl levels on plant growth parameters of Jaizan, Madinah and Khulais pearl millet cultivars inoculated and non-inoculated with AMF, (A) Fresh weight of shoot, (B) Dry weight of shoot, (C) Fresh weight of root and (D) Dry weight of root. The data is the mean of leaves n = 4 (± SE). Different letters indicate significance of two-way interactions between salinity and mycorrhiza. Means that do not share a letter are significantly different
3.3 Chlorophyll Content and Chlorophyll Fluorescence
Fig. 3A illustrates the effect of the salinity treatments on the chlorophyll content of pearl millet cultivars inoculated or non-inoculated with mycorrhiza. Compared to the control, the chlorophyll contents were significantly reduced by increasing the salinity level to 30 mM and 60 mM for all non-mycorrhizal cultivars (p < 0.01). In addition, the chlorophyll contents of mycorrhizal cultivars were higher at 0 mM and 30 mM of salinity compared to non-mycorrhizal cultivars grown at the same levels of salinity (p < 0.01). However, at 60 mM, mycorrhizal cultivars did not induce a significant change in the chlorophyll content compared to the corresponding non-mycorrhizal cultivars (Fig. 3A). On the other hand, the maximum quantum efficiency of the PSII photochemistry (Fv/Fm) of the leaves of the three pearl millet cultivars inoculated or non-inoculated with mycorrhiza is shown in Fig. 3B. The Fv/Fm values of most inoculated cultivars grown at 0 and 30 mM of salinity were almost similar to those of non-inoculated cultivars grown at the same salinity levels. However, the parameters of chlorophyll fluorescence were significantly decreased at 60 mM of salinity in all pearl millet cultivars in the presence or absence of mycorrhiza compared to control plants (p < 0.01).
Figure 3: Effect of different NaCl levels on (A) chlorophyll index and (B) chlorophyll fluorescence (Fv/Fm) of Jaizan, Madinah and Khulais pearl millet cultivars inoculated and non-inoculated with AMF. The data is the mean of leaves n = 4 (± SE). Different letters indicate significance of two-way interactions between salinity and mycorrhiza. Means that do not share a letter are significantly different
The results shown in Fig. 4 reveal the response of photosynthetic CO2 uptake (A) to photon flux (Q) for the three-mycorrhiza inoculated or non-inoculated pearl millet cultivars at different levels of salinity. Compared to the control, there was a sharp decrease in CO2 uptake in 60 mM of salinity application under different flux values in both inoculated and non-inoculated cultivars (Fig. 5A, p < 0.001). However, an increase in CO2 uptake was noticed in most cultivars inoculated with mycorrhiza at 0 and 30 mM of salinity compared to non-inoculated cultivars grown at the same salinity stress levels (p < 0.01). In addition, the quantum yields of most inoculated cultivars were not significantly affected by 0 mM and 30 mM of salinity compared to non-inoculated cultivars grown at the same salinity levels (Fig. 5B). However, the 60 mM of salinity concentration significantly reduced the light-limited photosynthetic capacity of both inoculated or non-inoculated cultivars (p < 0.01).
The responses of CO2 uptake A, per unit leaf area, to changes in intercellular CO2 concentration ci for leaves of pearl millet cultivars grown at different salinity treatments with and without mycorrhiza are illustrated in Fig. 5C. There was a significant increase in the CO2 assimilation rates for all inoculated cultivars grown at 0 and 30 mM compared to the same non-inoculated cultivars grown at the same salinity levels (p < 0.01). However, the maximum photosynthetic capacity levels were significantly reduced (p < 0.01) at 60 mM of salinity compared to other salinity treatments for both inoculated and non-inoculated cultivars (Fig. 5C). In addition, compared to growth under the other salinity treatments, growth at the 60 mM of salinity had a significant effect (p < 0.01) on the carboxylation efficiency of all cultivars (Fig. 5D).
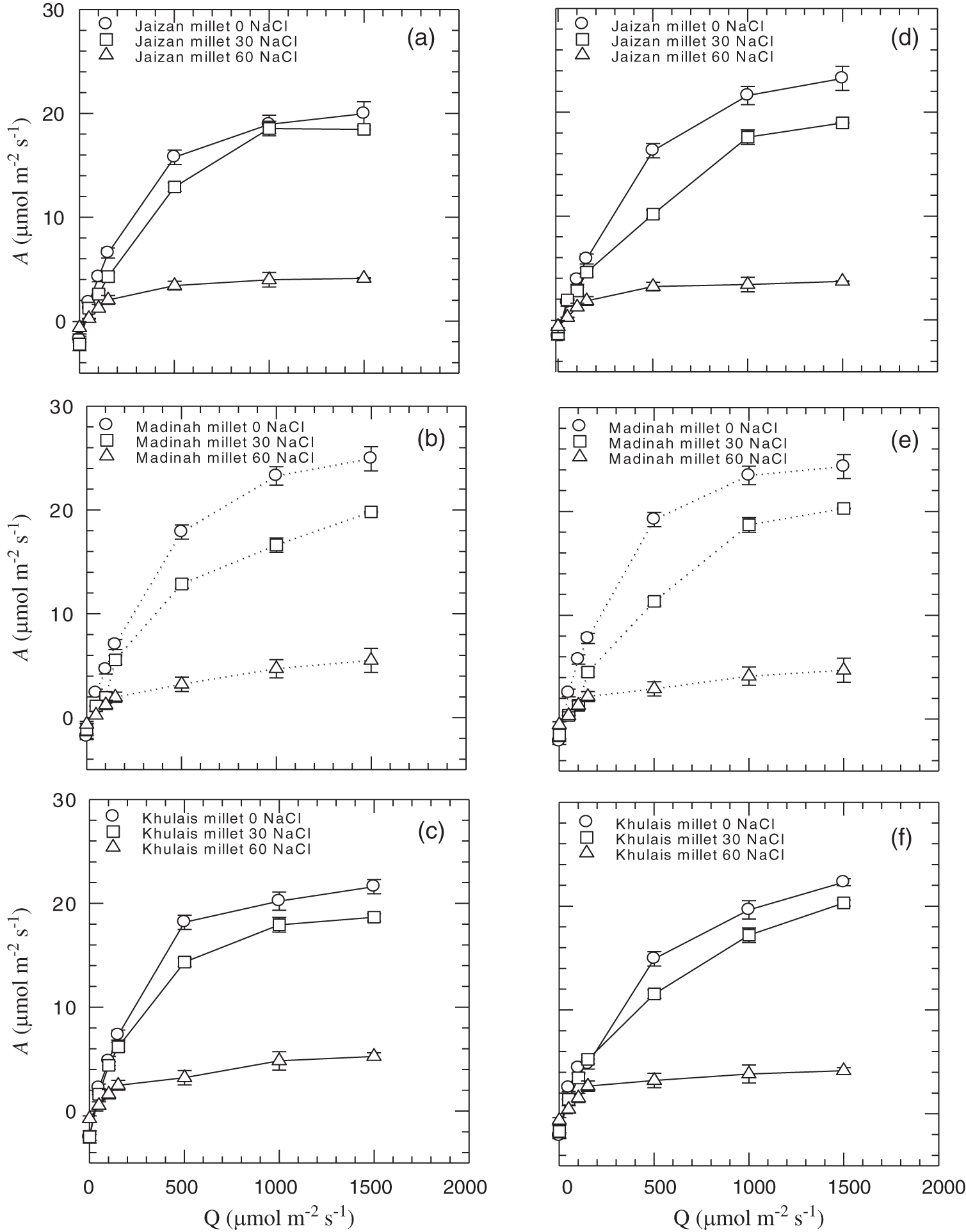
Figure 4: The response of photosynthetic CO2 uptake, per unit leaf area (A), to photon flux (Q) for leaves of Jaizan, Madinah and Khulais pearl millet cultivars non-inoculated (a, b, c) and inoculated (d, e, f) with AMF. The data is the mean of leaves n = 4 (± SE)
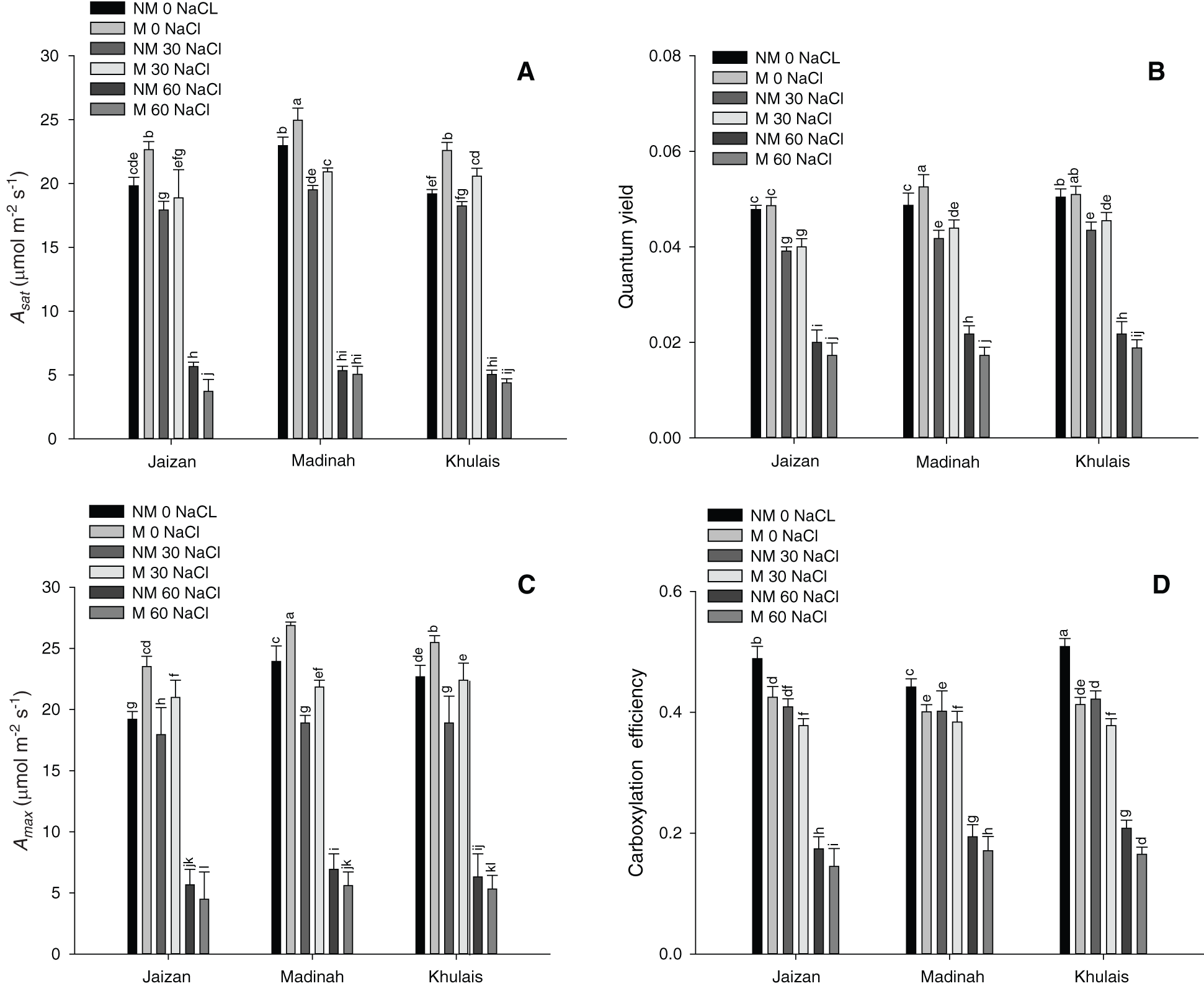
Figure 5: Effect of different NaCl levels on (A) The light-saturated photosynthetic rate (Asat), (B) The quantum yield (φ), (C) The plateau (Amax) and (D) The carboxylation efficiency of Jaizan, Madinah and Khulais pearl millet cultivars inoculated and non-inoculated with AMF. The data is the mean of leaves n = 4 (± SE). Different letters indicate significance of two-way interactions between salinity and mycorrhiza. Means that do not share a letter are significantly different
The free proline content in shoots indicated a progressive increase with the elevation of NaCl concentration, reaching 26.61, 25.5 and 31.71 µmol/g fresh weight at 60 mM in the inoculated cultivars from Jaizan, Madinah and Khulais, respectively (Tab. 2). Under all salinity concentrations, the proline contents of the inoculated cultivars were significantly higher than those of non-inoculated cultivars (p < 0.01).
Table 2: The effect of NaCl concentrations (0, 30, 60, 90 and 120 mM NaCl) on free proline content (µmol/g fresh weight) in shoots of non-mycorrhizal (NM) and mycorrhizal (M) Jaizan, Madinah and Khulais pearl millet cultivars
Salinity has traditionally been perceived as a severe problem facing agricultural areas. Therefore, improving plant tolerance to salinity is very important for increasing plant growth in these areas [3,23]. The present research examined the effect of AMF inoculation on plant growth, Fv/Fm, chlorophyll content, proline content and gas exchange of three Saudi pearl millet cultivars under five different salinity concentrations (0, 30, 60, 90 and 120 mM NaCl). Inoculation of mycorrhizal fungus, Glomus mosseae, to pearl millet cultivars markedly improved the growth of the different cultivars at low concentrations of salinity. This stimulation was pronounced with Khulais and Madinah cultivars (Figs. 1 and 2). It is observed that the pearl millet cultivars developed in soil inoculated with mycorrhiza were taller with more leaf area and root length than the control. These growth criteria were better in plants grown in soil treated with salinity and mycorrhiza than those grown in saline soil free of mycorrhiza. This finding indicates that the mycorrhiza is effective in protecting the test cultivars when grown under lower salinity stress. Mycorrhizal application was more beneficial with the Khulais cultivar, with regard to leaf extension, and the Madinah cultivar, with respect to leaf area and plant height. The variable responses of the two cultivars to salinity could be due to genetic diversity and variations in heredity between cultivars [40]. Many studies have demonstrated that inoculation with AMF improves the growth of plants under salt stress [17,25,33]. The improved growth of AMF plants has been attributed to the enhanced acquisition of mineral nutrients, such as P, Zn, Cu, and Fe [25,33]. Moreover, all cultivars inoculated with mycorrhiza showed higher root length at 0 mM and 30 mM than non-inoculated cultivars. However, all cultivars showed a significant reduction in all growth parameters at 60 mM in inoculated or non-inoculated soil. The reduction in all growth parameters could be due to toxicities of a specific-ion or imbalances in nutrition or a combination of these factors [41,42]. Furthermore, the reduction in growth under unfavourable conditions allows the conservation of energy, thereby launching the appropriate defence response and reducing the risk of heritable damage, as reported in Calendula officinalis plants under salinity [43]. On the other hand, all cultivars with and without mycorrhiza showed senescence within four weeks of growth at salinity concentrations of 90 mM and 120 mM. This finding indicated that relatively low salinity must be maintained to achieve high growth rates of these cultivars when inoculating with mycorrhiza. Moreover, the results of this study found that the shoots of most cultivars are more sensitive to low and moderate salinity than the roots. Munns and Sharp found that shoots are more sensitive to salinity than roots [44].
The present study showed that the chlorophyll contents of all pearl millet cultivars inoculated with mycorrhiza were higher than those grown at 0 and 30 mM of salinity (Fig. 3A). Similar findings have been observed in many plants at various salinity levels [13,17]. The reason for this rise could be an increase in the number of chloroplasts in the leaves of inoculated pearl millet cultivars or could be a result of the reduction in the area of leaves [11,45]. On the other hand, large and significant declines in chlorophyll content have been observed in all cultivars inoculated or non-inoculated with mycorrhiza grown at 60 mM of salinity compared to those grown in the control. This decrease in the content of chlorophyll might be a consequence of losing grana stacking or could be due to changes in the thylakoid structure [46,47].
Measurement of chlorophyll fluorescence has been used as a means to evaluate the integrity of Photosystem II upon exposure to stress [48]. In this research, the efficiency of light harvesting of PSII, measured by Fv/Fm, was generally unaffected by low salinity. All tested cultivars maintained the same Fv/Fm levels in inoculated or non-inoculated cultivars, indicating strong performance in 30 mM when compared to the control (Fig. 3B). These high Fv/Fm values provided strong evidence that all pearl millet cultivars were resistant to photoinhibition when the cultivars were grown at low salinity levels. Similar findings were previously reported for two different wheat cultivars that differed in their salinity tolerance [49]. Therefore, the current study indicates that chlorophyll fluorescence parameters cannot be considered one of the factors for controlling the net assimilation rate of CO2 in pearl millet cultivars when grown under low salinity treatments. On the other hand, compared to the control, plants grown under 60 mM of salinity had significantly lower Fv/Fm values across all cultivars. This drop in Fv/Fm might result from oxidative damage to the photosynthetic organs of cultivars, especially under salinity stress [50].
Salinity can negatively affect photosynthesis [13]. It can restrict the electron transport ability of the thylakoid membrane and decrease the amount of ribulose-1,5-bisphosphate carboxylase/oxygenase [51–54]. In many plants under salt stress, stomatal limitations and degradation in carotenoid and chlorophyll contents are associated with a decrease in the photosynthesis phase [51–54]. The results of this study showed that all inoculated cultivars that were grown under the control and 30 mM of salinity had higher light-saturated photosynthetic rates (Asat) and similar quantum yields (φ) than non-inoculated cultivars (Figs. 4 and 5). Asat and φ of all pearl millet cultivars were close to those previously recorded for healthy leaves in various C4 grasses belonging to the NADP-malic enzyme [55]. This result indicates that all pearl millet cultivars inoculated or non-inoculated with mycorrhiza grown under the previous salinity conditions were unstressed and did not suffer any photoinhibition. Furthermore, these results show that the photosynthetic apparatus of the Madinah and Khulais cultivars is more tolerant to salinity than that of the Jaizan cultivar. Amax and carboxylation efficiency of all pearl millet cultivars inoculated or non-inoculated with mycorrhiza grown under 0 mM and 30 mM of salinity followed nearly the same performance of Asat and φ. On the other hand, the rates of Asat, φ, Amax and carboxylation efficiency of all pearl millet cultivars inoculated or non-inoculated with mycorrhiza grown under 60 mM of salinity were lower than those for healthy leaves previously noted in C4 grasses belonging to the NADP-malic enzyme, suggesting that all cultivars of pearl millet were stressed and suffered photoinhibition. This decrease in photosynthetic output among pearl millet cultivars may have been caused by stomatal behaviour, ion toxicity, or both [17,56,57]. Furthermore, the reduction in the activity of enzymes such as Rubisco in bundle sheath cells may be a reason for this decrease in the photosynthetic efficiency of all pearl millet cultivars [52,54]. Another reason for this reduction in the photosynthetic capacity of all pearl millet cultivars grown at 60 mM of salinity may also result from a reduction in the content of chlorophyll or inhibition of leaf growth [52,53].
The results of this research indicated a considerable rise in the accumulation of the amino acid proline in the shoots of pearl millet under different concentrations of salinity. The highest accumulation of proline was recorded at 60 mM in the different cultivars. The proline contents in the shoots of all pearl millet cultivars inoculated with mycorrhiza under different salinity levels were significantly higher than those of non-inoculated cultivars (Tab. 2). This result indicated that mycorrhiza amendment plays a substantial role in lowering salt stress in the plant. Amino acid proline accumulation is one of the most frequently reported modifications induced by water and salt stresses in plants and is often considered to be involved in stress resistance mechanisms. A previous study documented a significant proline accumulation in sorghum grown under salt stress [57]. The accumulation of proline in plant leaves will decrease the water potential and help to maintain the water content in the leaves [42,58].
This study indicated that all three Saudi pearl millet cultivars inoculated with mycorrhiza had higher growth rates, chlorophyll contents and gas exchanges at 0 and 30 mM of salinity compared to cultivars without mycorrhiza. In addition, the measurements of the different growth rates showed higher growth performance of the cultivars from Madinah and Khulais than the cultivar from Jaizan. However, all cultivars with and without mycorrhiza underwent senescence within four weeks of growth at salinity concentrations of 90 and 120 mM. Therefore, relatively low salinity must be maintained to achieve high growth rates and gas exchanges of these inoculated cultivars.
Funding Statement: The author received no specific funding for this study.
Conflicts of Interest: The author declare that he has no conflicts of interest to report regarding the present study.
1. Mahajan, S., Tuteja, N. (2005). Cold, salinity and drought stresses: An overview. Archives of Biochemistry and Biophysics, 444(2), 139–158. DOI 10.1016/j.abb.2005.10.018. [Google Scholar] [CrossRef]
2. Gibberd, M. R., Turner, N. C., Storey, R. (2002). Influence of saline irrigation on growth, ion accumulation and partitioning, and leaf gas exchange of carrot (Daucus carota L.). Annals of Botany, 90(6), 715–724. DOI 10.1093/aob/mcf253. [Google Scholar] [CrossRef]
3. Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I. et al. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Frontiers in Plant Science, 10, 495. DOI 10.3389/fpls.2019.01068. [Google Scholar] [CrossRef]
4. Munns, R., James, R. A., Läuchli, A. (2006). Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany, 57(5), 1025–1043. DOI 10.1093/jxb/erj100. [Google Scholar] [CrossRef]
5. Ashraf, M., Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, 59(2), 206–216. DOI 10.1016/j.envexpbot.2005.12.006. [Google Scholar] [CrossRef]
6. Chandrasekaran, M., Chanratana, M., Kim, K., Seshadri, S., Sa, T. (2019). Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress-A meta-analysis. Frontiers in Plant Science, 10, 228. DOI 10.3389/fpls.2019.00457. [Google Scholar] [CrossRef]
7. Egamberdieva, D., Wirth, S., Bellingrath-Kimura, S. D., Mishra, J., Arora, N. K. (2019). Salt-tolerant plant growth promoting Rhizobacteria for enhancing crop productivity of saline soils. Frontiers in Microbiology, 10, 1303. DOI 10.3389/fmicb.2019.02791. [Google Scholar] [CrossRef]
8. Tian, F., Hou, M., Qiu, Y., Zhang, T., Yuan, Y. (2020). Salinity stress effects on transpiration and plant growth under different salinity soil levels based on thermal infrared remote (TIR) technique. Geoderma, 357(1–2), 113961. DOI 10.1016/j.geoderma.2019.113961. [Google Scholar] [CrossRef]
9. Dehnavi, A., Zahedi, M., Ludwiczak, A., Perez, S., Piernil, A. (2020). Effect of salinity on seed germination and seedling development of Sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy, 10(6), 859. DOI 10.3390/agronomy10060859. [Google Scholar] [CrossRef]
10. Saleh, B. (2012). Salt stress alters physiological indicators in cotton (Gossypium hirsutum L.). Soil Environment, 31, 113–118. [Google Scholar]
11. AL-Shoaibi, A. A., AL-Sobhi, O. A. (2004). The effect of salinity on growth of elephant grass (Pennisetum purpureum). Proceedings of 2nd Saudi Science Conference, pp. 141–147. Jeddah, Saudi Arabia. [Google Scholar]
12. Talaat, N. B., Shawky, B. T. (2014). Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environmental and Experimental Botany, 98, 20–31. DOI 10.1016/j.envexpbot.2013.10.005. [Google Scholar] [CrossRef]
13. Xu, H., Lu, Y., Tong, S. (2018). Effects of arbuscular mycorrhizal fungi on photosynthesis and chlorophyll fluorescence of maize seedlings under salt stress. Emirates Journal of Food and Agriculture, 30, 199–204. DOI 10.9755/ejfa.2018.v30.i3.1642. [Google Scholar] [CrossRef]
14. Turan, M. A., Türkmen, N., Taban, N. (2007). Effect of NaCl on stomatal resistance and proline, chlorophyll, Na, Cl and K concentrations of lentil plants. Journal of Agronomy, 6(2), 378–381. DOI 10.3923/ja.2007.378.381. [Google Scholar] [CrossRef]
15. Taffouo, V. D., Nouck, A. H., Amougou, A., Dibong, S. D. (2010). Effects of salinity stress on seedlings growth, mineral nutrients and total chlorophyll of some tomato (Lycopersicum esculentum L.) cultivars. African Journal of Biotechnology, 9, 5366–5372. [Google Scholar]
16. Taïbi, K., Taïbi, F., Abderrahima, L. A., Ennajah, A., Belkhodja, M. et al. (2016). Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South African Journal of Botany, 105, 306–312. DOI 10.1016/j.sajb.2016.03.011. [Google Scholar] [CrossRef]
17. Wang, J., Fu, Z., Ren, Q., Zhu, L., Lin, J. et al. (2019). Effects of Arbuscular Mycorrhizal Fungi on growth, photosynthesis, and nutrient uptake of Zelkova serrata (Thunb.) makino seedlings under salt stress. Forests, 10(2), 186. DOI 10.3390/f10020186. [Google Scholar] [CrossRef]
18. Birhane, E., Sterck, F. J., Fetene, M., Bongers, F., Kuyper, T. W. (2012). Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia, 169(4), 895–904. DOI 10.1007/s00442-012-2258-3. [Google Scholar] [CrossRef]
19. Bowles, T. M., Jackson, L. E., Loeher, M., Cavagnaro, T. R. (2017). Ecological intensification and arbuscular mycorrhizas: A meta-analysis of tillage and cover crop effects. Journal of Applied Ecology, 54(6), 1785–1793. DOI 10.1111/1365-2664.12815. [Google Scholar] [CrossRef]
20. Ye, L., Zhao, X., Bao, E., Cao, K., Zou, Z. (2019). Effects of Arbuscular Mycorrhizal Fungi on watermelon growth, elemental uptake, antioxidant, and Photosystem II activities and stress-response gene expressions under salinity-alkalinity stresses. Frontiers in Plant Science, 10, 644. DOI 10.3389/fpls.2019.00863. [Google Scholar] [CrossRef]
21. Zhang, T., Hub, Y., Zhang, K., Tian, C., Gu, J. (2018). Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Industrial Crops and Products, 117(11), 13–19. DOI 10.1016/j.indcrop.2018.02.087. [Google Scholar] [CrossRef]
22. Evelin, H., Devi, T. S., Gupta, S., Kapoor, R. (2019). Mitigation of salinity stress in plants by Arbuscular Mycorrhizal Symbiosis: Current understanding and new challenges. Frontiers in Plant Science, 10, 274. DOI 10.3389/fpls.2019.00470. [Google Scholar] [CrossRef]
23. Romero-Munar, A., Baraza, E., Gulías, J., Cabot, C. (2019). Arbuscular mycorrhizal fungi confer salt tolerance in giant reed (Arundo donax L.) plants grown under low phosphorus by reducing leaf Na+ concentration and improving phosphorus use efficiency. Frontiers in Plant Science, 10, 225. DOI 10.3389/fpls.2019.00843. [Google Scholar] [CrossRef]
24. Gao, X., Guo, H., Zhang, Q., Guo, H., Zhang, L. et al. (2020). Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Scientific Reports, 10(1), 199. DOI 10.1038/s41598-020-59180-3. [Google Scholar] [CrossRef]
25. Ebrahim, M. K. H., Saleem, A. R. (2018). Alleviating salt stress in tomato inoculated with mycorrhizae: Photosynthetic performance and enzymatic antioxidants. Journal of Taibah University for Science, 11(6), 850–860. DOI 10.1016/j.jtusci.2017.02.002. [Google Scholar] [CrossRef]
26. Liang, Q., Li, B., Wang, J., Ren, P., Yao, L. et al. (2019). PGPBS, a mitogen-activated protein kinase kinase, is required for vegetative differentiation, cell wall integrity, and pathogenicity of the barley leaf stripe fungus Pyrenophora graminea. Gene, 696(4), 95–104. DOI 10.1016/j.gene.2019.02.032. [Google Scholar] [CrossRef]
27. Medina, A., Roldán, A., Azcón, R. (2010). The effectiveness of arbuscularmycorrhizal fungi and Aspergillus niger or Phanerochaete chrysosporium treated organic amendments from olive residues upon plant growth in a semi-arid degraded soil. Journal of Environmental Management, 91(12), 2547–2553. DOI 10.1016/j.jenvman.2010.07.008. [Google Scholar] [CrossRef]
28. Miransari, M. (2010). Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biology, 10, 51. DOI 10.1111/j.1438-8677.2009.00308.x. [Google Scholar] [CrossRef]
29. Porcel, R., Redondo-Gómez, S., Mateos-Naranjo, E., Aroca, R., Garcia, R. et al. (2015). Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. Journal of Plant Physiology, 185, 75–83. DOI 10.1016/j.jplph.2015.07.006. [Google Scholar] [CrossRef]
30. Lin, J., Wang, Y., Sun, S., Mu, C., Yan, X. (2017). Effects of arbuscular mycorrhizal Fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Science of the Total Environment, 576(3), 234–241. DOI 10.1016/j.scitotenv.2016.10.091. [Google Scholar] [CrossRef]
31. Fabbrin, E. G., Gogorcena, Y., Mogor, A. F., Garmendia, I., Goicoechea, N. (2015). Pearl millet growth and biochemical alterations determined by mycorrhizal inoculation, water availability and atmospheric CO2 concentration. Crop and Pasture Science, 66(8), 831. DOI 10.1071/CP14089. [Google Scholar] [CrossRef]
32. Migahid, A. M. (1990). Flora of Saudi Arabia, 4th Ed., Riyadh, Saudi Arabia. [Google Scholar]
33. Liang, S. C., Jiang, Y., Li, M. B., Zhu, W. X., Xu, N. et al. (2019). Improvin plant growth and alleviating photosynthetic inhibition from salt stress using AMF in alfalfa seedlings. Journal of Plant Interactions, 14(1), 482–491. DOI 10.1080/17429145.2019.1662101. [Google Scholar] [CrossRef]
34. Ortas, I. (2008). The influence of use of different rates of mycorrhizal inoculums on root infection plant growth and phosphorus uptake. Communications in Soil Science and Plant Analysis, 27(18–20), 2935–2946. DOI 10.1080/00103629609369753. [Google Scholar] [CrossRef]
35. Phillips, J. M., Hayman, D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizalfungi for rapid assessment of infection. Transactions of the British Mycological Society, 55(1), 158–161. DOI 10.1016/S0007-1536(70)80110-3. [Google Scholar] [CrossRef]
36. Giovannetti, M., Mosse, B. (1980). An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist, 84(3), 489–500. DOI 10.1111/j.1469-8137.1980.tb04556.x. [Google Scholar] [CrossRef]
37. Von Caemmerer, S., Farquhar, G. D. (1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta, 153(4), 376–387. DOI 10.1007/BF00384257. [Google Scholar] [CrossRef]
38. AL-Shoaibi, A. A. (2008). Photosynthetic response of Elephant grass (Pennisetum purpureum) to NaCl salinity. Journal of Biological Sciences, 8(3), 610–615. DOI 10.3923/jbs.2008.610.615. [Google Scholar] [CrossRef]
39. Bates, L. S., Waldren, R. P., Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
40. Almodares, A., Hadi, M. R., Dosti, B. (2007). Effects of salt stress on germination percentage and seedling growth in sweet sorghum cultivars. Journal of Biological Sciences, 7(8), 1492–1495. DOI 10.3923/jbs.2007.1492.1495. [Google Scholar] [CrossRef]
41. Läuchli, A., Epstein, E. (1990). Plant responses to saline and sodic conditions. Agricultural salinity assessment and management, vol. 71, pp. 113–137. Tanji, K. K., Reston, VA, USA: American Society of Civil Engineers. DOI 10.1061/9780784411698.ch06. [Google Scholar] [CrossRef]
42. Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59(1), 651–681. DOI 10.1146/annurev.arplant.59.032607.092911. [Google Scholar] [CrossRef]
43. Chaparzadeh, N., D’Amico, M. L., Khavari-Nejad, R. A., Izzo, R., Navari-Izzo, F. (2004). Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiology and Biochemistry, 42(9), 695–701. DOI 10.1016/j.plaphy.2004.07.001. [Google Scholar] [CrossRef]
44. Munns, R., Sharp, R. E. (1993). Involvement of abscise acid in controlling plant growth in soils of low water potential. Functional Plant Biology, 20(5), 425–437. DOI 10.1071/PP9930425. [Google Scholar] [CrossRef]
45. Misra, A. N., Sahu, S. M., Misra, M., Singh, P., Meera, I. et al. (1997). Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biologia Plantarum, 39(2), 257–262. DOI 10.1023/A:1000357323205. [Google Scholar] [CrossRef]
46. Boutraa, T., Akhkha, A., Al-Shoaibi, A. A. (2015). Evaluation of growth and gas exchange rates of two local Saudi wheat cultivars grown under heat stress conditions. Pakistan Journal of Botany, 47, 27–34. [Google Scholar]
47. Purnama, P. R., Purnama, E. R., Manuhara, Y. S. W., Hariyanto, S., Purnobasuki, H. (2018). Effect of high temperature stress on changes in morphology, anatomy and chlorophyll content in tropical seagrass Thalassia hemprichii. AACL Bioflux, 11, 6. https://www.researchgate.net/publication/. [Google Scholar]
48. Shabala, S., Shabala, L. (2002). Kinetics of net H+, Ca2+, K+, Na+, NH4+, and Cl– fluxes associated with post-chilling recovery of plasma membrane transporters in Zea mays leaf and root tissues. Physiologia Plantarum, 114(1), 47–56. DOI 10.1046/j.0031-9317.2001.1140108.x. [Google Scholar] [CrossRef]
49. Arfan, M., Athar, H. R., Ashraf, M. (2007). Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? Journal of Plant Physiology, 164(6), 685–694. DOI 10.1016/j.jplph.2006.05.010. [Google Scholar] [CrossRef]
50. Aro, E. M., Virgin, I., Andersson, B. (1993). Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochimica et Biophysica Acta (BBA)–Bioenergetics, 1143(2), 113–134. DOI 10.1016/0005-2728(93)90134-2. [Google Scholar] [CrossRef]
51. Qu, C., Liu, C., Gong, X., Li, C., Hong, M. et al. (2012). Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environmental and Experimental Botany, 75(2), 134–141. DOI 10.1016/j.envexpbot.2011.08.019. [Google Scholar] [CrossRef]
52. Sharma, A., Kumar, V., Shahzad, B., Ramakrishnan, M., Sidhu, G. P. S. et al. (2020). Photosynthetic response of plants under different abiotic stresses: A Review. Journal of Plant Growth Regulation, 39(2), 509–531. DOI 10.1007/s00344-019-10018-x. [Google Scholar] [CrossRef]
53. Morales, F., Ancín, M., Fakhet, D., González-Torralba, J., Gámez, A. L. et al. (2020). Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement: A review. Plants, 9(1), 88. DOI 10.3390/plants9010088. [Google Scholar] [CrossRef]
54. Yang, Z., Li, J. L., Liu, L. N., Xie, Q., Sui, N. (2020). Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Frontiers in Plant Science, 10, 772. DOI 10.3389/fpls.2019.01722. [Google Scholar] [CrossRef]
55. Ehleringer, J., Pearcy, R. W. (1983). Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiology, 73(3), 555–559. DOI 10.1104/pp.73.3.555. [Google Scholar] [CrossRef]
56. Sudhir, P., Murthy, S. D. S. (2004). Effects of salt stress on basic processes of photosynthesis. Photosynthetica, 42(4), 481–486. DOI 10.1007/S11099-005-0001-6. [Google Scholar] [CrossRef]
57. Yan, K., Chen, P., Shao, H., Zhao, S., Zhang, L. et al. (2012). Responses of photosynthesis and Photosystem II to higher temperature and salt stress in Sorghum. Journal of Agronomy and Crop Science, 198(3), 218–225. DOI 10.1111/j.1439-037X.2011.00498.x. [Google Scholar] [CrossRef]
58. Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J. et al. (2014). Role of proline under changing environments. Plant Signaling & Behavior, 7(11), 1456–1466. DOI 10.4161/psb.21949. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |