
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015427
REVIEW
Interaction between Earthworms and Arbuscular Mycorrhizal Fungi in Plants: A Review
1College of Horticulture and Gardening, Yangtze University, Jingzhou, 434025, China
2ICAR-Central Citrus Research Institute, Nagpur, Maharashtra, 440033, India
3Department of Chemistry, Faculty of Science, University of Hradec Kralove, Hradec Kralove, 50003, Czech Republic
4Department of Botany, Swami Shraddhanand College, University of Delhi, Delhi, 110007, India
5Natural Science Unit, Tokyo Gakugei University, Koganei, Tokyo, 184-8501, Japan
*Corresponding Author: Qiangsheng Wu. Email: wuqiangsh@163.com
Received: 20 December 2020; Accepted: 23 February 2021
Abstract: Different kinds of soil animals and microorganisms inhabit the plant rhizosphere, which function closely to plant roots. Of them, arbuscular mycorrhizal fungi (AMF) and earthworms play a critical role in sustaining the soil-plant health. Earthworms and AMF belong to the soil community and are soil beneficial organisms at different trophic levels. Both of them improve soil fertility and structural development, collectively promoting plant growth and nutrient acquisition capacity. Earthworm activities redistribute mycorrhizal fungi spores and give diversified effects on root mycorrhizal fungal colonization. Dual inoculation with both earthworms and AMF strongly magnifies the response on plant growth through increased soil enzyme activities and changes in soil nutrient availability, collectively mitigating the negative effects of heavy metal pollution in plants and soils. This thus enhances phytoremediation and plant disease resistance. This review simply outlines the effects of earthworms and AMF on the soil-plant relationship. The effects of earthworms on root AMF colonization and activities are also analyzed. This paper also summarizes the interaction between earthworms and AMF on plants along with suggested future research.
Keywords: Earthworm; nutrient; mycorrhiza; soil enzyme; stress; symbiosis
Earthworms are terrestrial invertebrates of the subclass Oligochaete of the phylum Annelidae. In general, earthworms are an important part of soil ecosystems, playing the role of consumers, disintegrators and regulators. The activities of earthworms promote the formation of soil aggregates by modifying the aggregate structures, thus modulating soil porosity and structure [1,2]. The excretion of earthworms has certain visible impacts on the soil carbon (C) cycle, nutrient content, and organic matter, and (2) microbial load, biomass and activity [3,4]. Earthworms affect the physical, chemical, and biological properties of soil by complimenting soil fertility with respect to plant growth. They also improve the bioavailability of essential nutrients in the soil, indirectly affecting plant growth [5].
Arbuscular mycorrhizal fungi (AMF) are a type of obligate symbiotic soil microorganisms widely existing in various ecosystems, which can establish mutualistic arbuscular mycorrhiza (AM) symbioses with more than 80% of land’s plants [6]. AMF facilitate the absorption area of the host plant roots, and promote their absorption capacity of water and nutrients, thus improving plant growth and enhancing the host plant resistance toabiotic and biotic stresses [7–10]. Studies on the interaction between AMF and earthworms are expected to be limited, especially the effects on plant growth and the rhizosphere environment under synergy conditions. The present review summarizes the effects of earthworms on soil and AMF, and highlights the dual roles of both earthworms and AMF on plant growth and biotic stress resistance. The direction of possible future research is also addressed.
2 Effects of Earthworms on Soil Properties
Soil aggregates are an important component of the soil functionality and the basic unit of soil structure. Good soil structure contributes to the improvement of the soil water retention capacity and soil fertility, thus reducing soil and water loss [11]. Earthworms, as a representative of large soil animals in the terrestrial ecosystem, mainly affect the formation of soil aggregates through their activities such as turning and burrowing of soil masses. Earthworm activities help to break down soil organic matter and stimulate the process of soil aggregation to improve soil porosity and structure. This in turn increases the soil water-stable aggregates, that eventually create favorable conditions for enhanced soil microbial activities. At the same time, the coprolites from earthworm intestines have an effect on cementing soil particles, because they are able to feed on the soil and produce aggregates with different particle sizes through excretion. As the size of earthworms’ increases, the aggregates are also be increased, thus producing a positive effect on soil aggregates [12,13]. Earlier studies showed that earthworms have a stimulating effect on soil aggregate formation and then stability, dependent on the earthworm species [14–16].
Earthworms play a decisive role in soil fertility through feeding, digestion, excretion (earthworm coprolites), secretion (mucous) and burrowing, thus collectively contributing towards the soil nutrient cycling [2]. Earthworm activities increase the soil-atmosphere C cycle, conducive to transformation of immobile nutrients (e.g., N, P, K and C) into plant available forms [16]. On the other hand, earthworm manure and their secretions and residues are used as the bio-organic fertilizers to elevate soil enzyme activities and the diversity of soil microbial communities, enhancing soil fertility, and expanding the sink capacity for plant nutrient absorption [17]. The addition of earthworms into turfy and matrix soils exhibited a considerable increase in the total N, total K, total P, alkali-hydrolyzed N, available P and available K of soil [17]. In other words, earthworm application improves soil physical and chemical properties along with soil fertility, partly reducing the amount of inorganic fertilizers required for optimum crop performance [18]. Earthworms influence the soil C cycle via their life activities and interactions with other organisms in the soil [19]. The physical disturbance of earthworms accelerates the regeneration rate of organic carbon in the soil [19].
Soil enzymes are derived from the secretions and decomposition products of animals, plants and microbial cells in the soil, and microorganisms are the main source of soil enzymes. Soil enzymes are an indicator of soil fertility. The type and activity of soil enzymes reflect the supply state of soil nutrients [3]. There are numerous enzymes in earthworms, which play an important role in the decomposition of organic matter. The organic matter entering the earthworm is broken down and transformed further by these soil enzymes [20]. Earthworms inoculated in soil contaminated with heavy metals increased the activity of sucrase, urease and phosphatase in soil, and alleviated the potential adverse effect of heavy metal pollution on soil enzyme activities [21]. Earthworms produce the coprolites through feeding and excreting, and the coprolites contain a large number of microorganisms, which improve the activity of soil enzymes. The activity of sucrase was the strongest in the coprolites [22], thus facilitating crop growth performance [23].
Soil microorganisms are one of the important components of the soil ecosystem, and a strong storehouse in earthworms [24]. A large number of studies have shown that the combined action of earthworms and microorganisms play an important role in the decomposition of organic matter and the release of mineral nutrients [25]. The activities of earthworms affect the number of microbial populations, and the microorganisms promote the release of minerals, increase fertility, and promote plant growth [26,27]. Earthworms treatment in heavy metal contaminated soils significantly increased the number and activity of microorganisms in the soil [28,29], thus, suggesting the pollutant cleaning ability of earthworms in contaminated soils.
There are a large number of different kinds of microorganisms in the soil, among which AMF play an important role in plant growth, nutrient acquisition, and stress tolerance [30,31].
It is well known that AMF colonize plant roots and form mycorrhizal symbiosis, beneficial for plant growth [7]. After forming a symbiotic relationship with plants, mycorrhizal mycelia colonize into the root cortical cells to form a dichotomously branched structure, the arbuscule. AMF inoculation improves plant growth, including citrus [7], tea [32], walnut [9] and other many plant species. However, the growth improvement is dependent on the combination of the AMF and host species. Liu et al. [33] found that Funneliformis mosseae significantly promoted growth of trifoliate orange seedlings, while Glomus etunicatum did not promote plant growth, and even appeared to have an inhibitory effect. The mycorrhiza-induced improvement of plant growth is attributable to the increase of nutrient acquisition, root system architecture, and hormone balance [34].
3.2 Improvement of Nutrient Acquisition
After forming a symbiotic relationship with plants, AMF establish a rich mycelial network around the root, and the huge extraradical mycelium can act as an extension of the root system, increasing the contact area for soil nutrients, thus capturing more nutrients for the plant. Numerous studies have confirmed that AMF considerably promoted the uptake of soil nutrients, especially phosphorus, by the host plant [35]. AMF improve the mineral absorption capacity of plants, play a key role in improving plant growth and promoting nutrient absorption, besides being considered as one of the most promising strategies for optimizing plant performance to cope with nutrient deficiency stress [36,37]. The results of studies on trifoliate orange showed that inoculation with F. mosseae improved N, P, Mg, and B contents [7]. Hereinto, AMF-accelerated P acquisition is attributed to the release of a soil acid and neutral phosphatase, the improvement of root morphology, and the up-regulation of root phosphate transporter gene expression [31].
3.3 Enhancement of Stress Tolerance
Mycorrhizal hyphae absorb soil mineral elements [36] for the host plant, regulate the plant hormone balance, and affect plant development, thereby alleviating the impact of environmental stress in a coordinated manner [38–45]. In addition, AMF also release a special glycoprotein, glomalin, into the rhizosphere, which can contribute to the soil organic C and N, stabilize soil aggregation, and improve soil structure, thus improving plant growth and enhancing stress tolerance [46,47]. Studies have shown that AMF enhanced the stress tolerance in many plants, and the potential mechanisms mainly include: (1) Mycorrhizal extraradical hyphae are directly involved in soil water and nutrient uptake, which are promptly transported to the cortical cells contained in the arbuscule and released into the host; (2) Levels of organic and inorganic osmotic substances are increased by mycorrhizas to improve osmoregulation of the host; (3) The composition and content of some metabolic substances such as polyamines, fatty acids, and calmodulin are optimized, thus activating the antioxidant defense system; (4) The expression level of many stress-responsive genes is induced by mycorrhization in response to stresses [8,10,31,38–45].
3.4 Effects of Earthworms on AMF Colonization and Activities
AMF are widely distributed in terrestrial ecosystems and can establish symbiotic relationships with terrestrial plants [48,49]. Mycorrhizal extraradical hyphae can simultaneously colonize other neighboring plants in the same ecosystem, thus forming a common mycorrhizal network among plants and transferring signals between them [50]. Because AMF cannot form aboveground fungal bodies, the sporophytes used for propagation of AMF mainly reside in the soil after AMF separate from the host plant. Some soil animals, such as earthworms, spread the spores of AMF to areas beyond their ability through earthworm activities in the soil [51]. This expands the area of mycorrhizal fungi colonization and enrichs the diversity of soil microorganisms. However, the movement of earthworms in soil and their selective feeding can significantly affect the development of mycorrhizas and the establishment of common mycorrhizal networks.
Earthworms selectively feed on mycorrhizal extraradical hyphae, AMF-colonized roots, or soil containing mycorrhizal spores. Under the action of chitinase, fungi are self-dissolved, thus reducing the rate of mycorrhizal colonization and the density of soil mycorrhizal hyphae [51,52]. The earthworms’ activities and feeding in the soil damage the structure of underground common mycelium networks, thus reducing mycorrhizal formation and disturbing common mycorrhizal networks [53]. Previous studies revealed that when exogenous organic substances were added, earthworms preferred to utilize these substances as a food source, which would effectively reduce the negative effects of earthworm activities on mycorrhizal development [54,55]. In another study conducted by Dobson et al. [56], five forest plants in northeastern North America, including Actaea pachypoda, Aquilegia canadensis, Cornus racemosa, Quercus rubra, and Prenanthes alba, were used to analyze the response to earthworms. These authors found that root AMF colonization in Q. rubra was reduced by earthworms, but AMF in other forest species remained unaffected. In soils contaminated with heavy metals like Cd and As, earthworms significantly increased root mycorrhizal fungal colonization [28,57], and the positive effects depended on the earthworm number and timing of earthworm inoculation. Recently, Yang et al. [58] reported that application of earthworms could increase AMF dominance in wheat roots, stimulating mycorrhizal N uptake only under no-till and straw removal practices conditions.
Natural selection gaves AMF a unique self-protective mechanism after a long period of evolution to avoid serious depletion of soil animals and other microorganisms, especially earthworms [59]. Earthworms can provide more C sources for the development of mycorrhizal hyphae by increasing the biomass of host plants and activating the soil C, thus promoting the development of hypha [60,61]. After earthworms are fed with AMF, mycorrhizal fungal spores survived in large number in earthworms, and thus ensured a high spore density and facilitated fungal colonization [62]; chitinases in earthworms have an inhibitory effect on the development of extracellular hyphae. Other studies have reported that the activity of earthworms in the rhizosphere did not inhibit the development of hyphae, but promoted its growth, but did not transmit the AMF spores over long distances [52]. It was also reported that the activity of earthworms had no effect on AMF [63]. These results indicated the complex relationship between earthworms and AMF (Fig. 1), which needs to be disentangle through in-depth studies from various angles.
4 Interaction of Earthworms and AMF on Plants
4.1 Promotion of Plant Growth and Nutrient Acquisition
Plants absorb nutrients from the soil in two paths: One is directly absorbed by the root epidermis and root hairs, and the other is mainly absorbed by mycorrhizas [64]. Unlike other types of fungi, AMF do not have an overground fungi body, but earthworms spread mycorrhizal fungal propagules to other regions beyond the periphery of mycorrhizal colonized areas [65]. Although earthworms have a direct effect on soil, they also help plants indirectly by regulating the soil nutrients reserve and microorganisms. The relevant results of earlier studies conducted on the interaction effects of earthworms and AMF on plant growth are summarized as follows (Tab. 1). Millaret et al. [66] studied the effects of earthworms, AMF, and their interaction on growth and nutrient absorption of Allium porrum, and found that the earthworm plus AMF treatment significantly promoted the plant root biomass. Other studies [67,68] reported that both AMF and earthworm inoculation significantly increased AMF colonization of maize roots, besides promoting the shoot and root biomasses. Li et al. [54] also confirmed that the interaction between earthworms and AMF improved the shoot and root biomasses in maize plants by promoting P acquisition. In sweet potato plants, earthworms regulated the activities of the soil urease and alkaline phosphatase to increase the efficiency of N and P in the soil, and the AMF stimulated the soil phosphatase activities and increased root phosphorus absorption [69]. These results suggest that the interaction of earthworms and mycorrhiza fungi is mutually beneficial to both participating partners.
4.2 Enhancement of Stress Tolerance
When plants are exposed to pathogen infection, saline-alkali environment and heavy metal pollution, they often face diversified threats. Earthworms act as consumers, decomposers and regulators in soil ecosystems, while AMF are a ubiquitous rhizosphere microorganism, that can be used as biofertilizers, bioreactants and bioprotectants. Earthworm-AMF interactions have different dimension effects on plant resistance to salt and alkali, heavy metals, chemical pollution and diseases.
In a few studies, dual inoculations of AMF and earthworms had positive effects on salt tolerance of plants (Tab. 1). Zhang et al. [70] discussed the effects of earthworm and AMF interaction on salinity tolerance and root biomass of maize. They found that double earthworm and AMF inoculation improved the salinity tolerance of maize by increasing soil macro-aggregates and soil bacterial diversity, collectively promoting root mineral absorption and photosynthesis. Therefore, growth improvement of plants under salinity stress via the interaction of AMF and earthworms is associated with the change in the chemical, physical and biological properties of soil and their relevant plant ecophysiological implications.
4.2.2 Heavy Metal Pollution and Phytoremediation
Numerous studies have been conducted on the effects of earthworms and AMF on alleviating soil heavy metal pollution and promoting plant remediation (Tab. 1). Earthworms play an important role in the terrestrial ecosystem through regulating the migration and transformation of heavy metals in the soil-plant system by their activities such as feeding, metabolism and excretion [71]. Mycorrhizal fungi affect the uptake, accumulation, and detoxification of heavy metals by plants through a variety of ways under heavy metal-polluted soils [72]. Fu et al. [71] found that in maidenhair (Tagetes patula) plants, single earthworm or AMF addition alleviated the toxicity of Cu contamination of soil and plants, and the combination of AMF and earthworms significantly improved plant biomass, Cu accumulation, and soil enzyme activities, which increased the remediation efficiency of maidenhair in a Cu-polluted soil. Similarly, in Cd-stressed Solanum nigrum, co-inoculation with earthworms and AMF activated an antioxidant defense system that improved soil quality parameters (e.g., soil enzyme activity), thus enhancing metal tolerance of plants and improving the quality of polluted soil [73,74]. In polychlorinated biphenyl (PCB)-contaminated paddy soil, the interaction between AMF and earthworms could significantly improve the ability of ryegrass to repair the PCB-contaminated soil; the removal rate of PCB was 61.05%, which was the best effect among treatments (Tab. 1) [75,76]. The results further confirmed the effectiveness of both AMF and earthworms in the phytoremediation of contaminated soils. Cheng et al. [28] also observed that earthworms improved root mycorrhizal fungal colonization and increased the root Cd accumulation on ryegrass, and mycorrhizal fungi promoted the transport of Cd from roots to shoots. As a result, dual inoculation of AMF and earthworms promoted the transport of Cd to shoots. In the Pb and Cd-contaminated soil, dual inoculation of AMF and earthworms increased shoot biomass of plants and the accumulation of Pb and Cd in shoots [77]. Therefore, the combination of earthworms and AMF is of profound significance for the phytoremediation of heavy metal contaminated soils.
The interaction between earthworms and AMF displays a positive influence on the disease resistance of plants. In the roots of strawberry plants infected with Fusarium oxysporum, earthworms and AMF significantly reduced the soil pH value (Tab. 1). This was due to the production of humic and fulvic acids by the intestinal microorganisms of earthworms, and the release of organic acid secretions by AMF. Additionally, earthworms influence the fungal pathogens by actively feeding and inhibiting the spread of fungal spores, which enhanced the disease resistance of plants. For example, earthworms inhibited the F. oxysporum infestation on strawberry roots indirectly (by increasing soil organic matter content) and directly (by selective feeding), thus reducing the plant wilt disease index. AMF significantly increased the soil organic matter concentration by releasing carbohydrate-rich exudates from the host plant roots and glomalin from the mycorrhizal fungi. This resulted in a strong possibility of bacterial infection, thus increasing bacterial diversity, but a decrease on the intensity of F. oxysporum on the plant roots. Therefore, the dual inoculation provided a synergistic effect on enhancing the plant resistance to disease. Therefore, it is pertinent to state that such joint strategy, involving both earthworms and AMF, is a potential alternative biological control approach to mitigate disease bursts [78].
The effect of single either earthworms or AMF on soil properties and plant growth has been studied, while studies on the interacting effects between earthworms and AMF are only a few. Although earthworms and AMF belong to different nutrition classes, earthworms affect mycorrhizal functions directly and indirectly. Earthworms are large soil animals whose biomass accounts for 60% of the total biomass of soil organisms. It is estimated that the annual excretion volume of earthworms reached 18.7-40.3 t/hm2, equivalent to an annual excretion of a soil layer of 5 cm depth [79]. When earthworms interact with soil microorganisms, they produce hormonal substances, which stimulate the root colonization of mycorrhizal fungi and, thus play an important role in plant metabolism for promoting plant biomass and crop quality [80,81]. Previous studies have been carried out on the synergistic effect of earthworm-mycorrhizal fungi on plants (Tab. 1). Based on these previous studies, we propose a conceptual model of earthworms-AMF-plants interaction (Fig. 1). Even so, the interaction between earthworms and AMF is still worth exploring through the following areas of future research:
1. It is still unknown whether earthworm excrement affects AMF colonization and activities, plant growth, and nutrient acquisition. The internal mechanism of their interaction has yet to be studied.
2. It is possible that earthworms might inhale soil spores of AMF when feeding on soil. Future work could analyze the diversity of endophytic mycorrhizal fungi in earthworms, and also analyze the function of the fungi within earthworms and the difference between the in vitro and in vivo synergistic effects of the interaction of the two organisms in plants.
3. Early studies conducted on the effect of soil sterilization on the dynamics of earthworms and AMs failed to provide any valid clue, which needs to be supplemented through in-vitro, at the plant and field scales.
4. Determination of the internal mechanism of earthworms’ regulation and its effect on the diversity of microorganisms and plants using molecular biology and genetic engineering techniques.
5. According to the invasion and evolution of various earthworm and allien species, and different AMF types, molecular ecological techniques could be applied to reveal the interaction between mycorrhizal fungi and earthworms along with its feedback effects on plants.
6. Most studies have confirmed that the dual application of earthworms and AMF stimulated growth of host plants in heavy metal-contaminated soils, but the underlying mechanism/s is/are still unclear (e.g., do earthworms or AMF play a dominant role?).
7. Recent studies have shown that the earthworm Pontoscolex corethrurus could alleviate the inhibitory effect of mycorrhizal fungi (e.g., Rhizophagus intraradices) on soil bacterial abundance [82]. However, the change in the soil bacterial community because of the interaction between AMF and earthworms needs to be further studied.
Table 1: Interaction between earthworms and AMF and their effects on plants
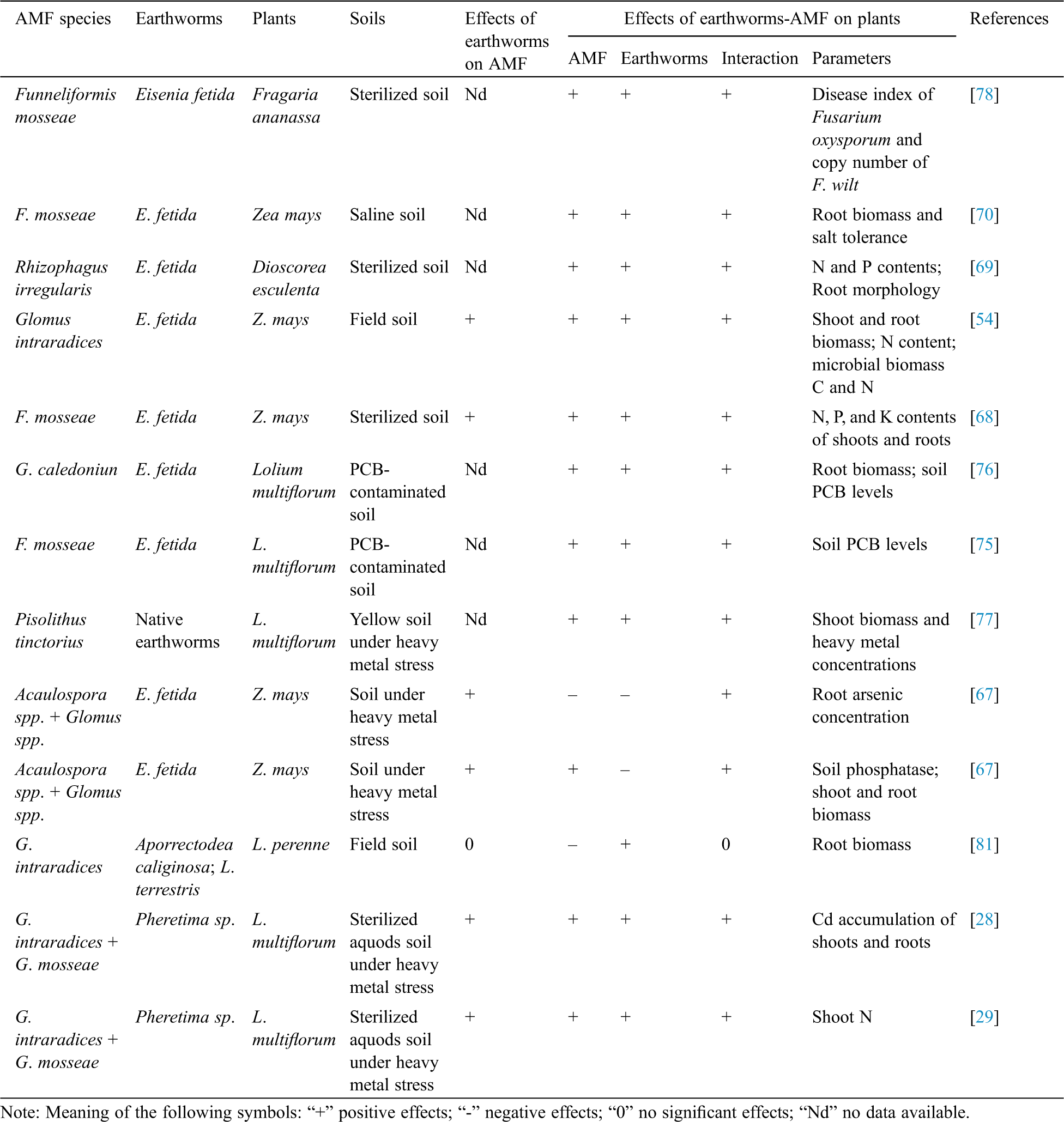
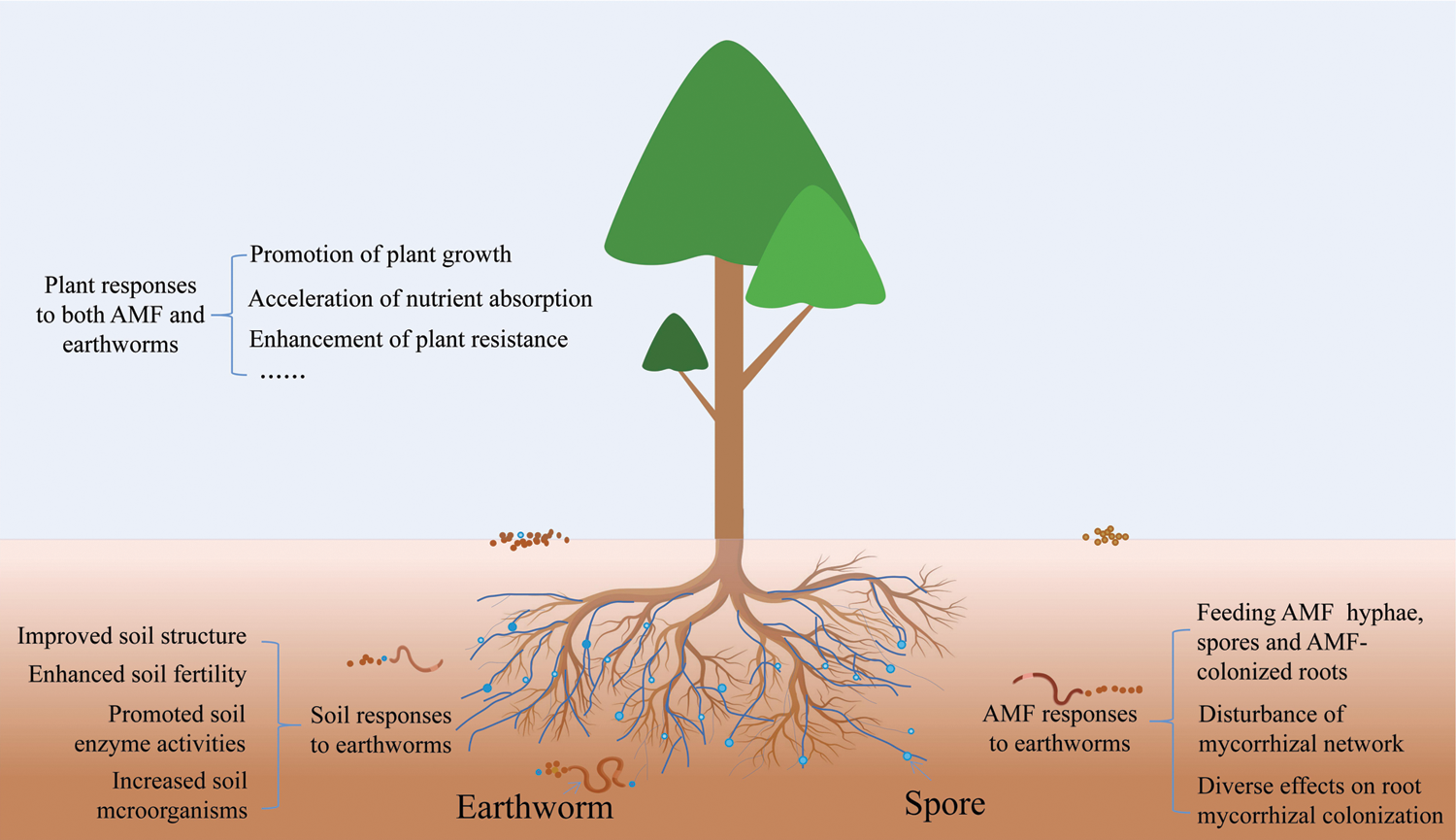
Figure 1: A conceptual model of earthworms-AMF-plants interaction. Earthworms strongly affect soil properties and AMF behavior and activity. AMF have been widely demonstrated to improve water and nutrient acquisition of the host and enhance host resistance to abiotic and biotic stresses. In addition, in the association of earthworms-AMF-plants, earthworms feed on mycorrhizal hyphae, spores, and mycorrhizal colonized roots and also disturb common mycorrhizal networks. Two different trophic levels of organisms work together to further promote plant growth, accelerate nutrient absorption, improve soil properties, enhance plant resistance, etc.
Funding Statement: This work was supported by the Plan in Scientific and Technological Innovation Team of Outstanding Young Scientists, Hubei Provincial Department of Education (T201604), the Hubei Agricultural Science and Technology Innovation Action Project (Hubei Nongfa [2018] No. 1), and the UHK Project VT2019-2021.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Zangerlé, A., Pando, A., Lavelle, P. (2011). Do earthworms and roots cooperate to build soil macroaggregates? A microcosm experiment. Geoderma, 167–168(8), 303–309. DOI 10.1016/j.geoderma.2011.09.004. [Google Scholar] [CrossRef]
2. Cui, Y. Y., Wu, J. L., Zhang, C., Zhou, B., Ren, Z. L. et al. (2020). Impacts of different ecological types of earthworm on aggregate distribution and stability in typical latosolic red and red soils. Journal of South China Agricultural University, 41(1), 83–90. [Google Scholar]
3. Chen, P., Zhao, B., Yang, L., Zhao, X. H., Zhang, C. Y. et al. (2018). Effects of earthworm and litter application on soil nutrients and soil microbial biomass and activities in Pinus tabuliformis plantation. Journal of Beijing Forestry University, 40(6), 63–71. [Google Scholar]
4. Chen, Y. Q., Zhang, H., Zhou, C. F. (2019). Research process in the ecological functions of earthworms. Inner Mongolia Forestry Investigation and Design, 42(6), 107–110. [Google Scholar]
5. Fonte, S. J., Quintero, D. C., Velásquez, E., Lavelle, P. (2012). Interactive effects of plants and earthworms on the physical stabilization of soil organic matter in aggregates. Plant and Soil, 359(1–2), 205–214. DOI 10.1007/s11104-012-1199-2. [Google Scholar] [CrossRef]
6. Yu, J. G., Feng, H., Li, H. X., Wang, Q. J., Wang, T. (2010). Effects of earthworms on soil aggregates formation, stability and soil organic carbon distribution. Journal of Soil and Water Conservation, 81(9), 1121–1130. [Google Scholar]
7. Yang, L., Zou, Y. N., Tian, Z. H., Wu, Q. S., Kuča, K. (2021). Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Scientia Horticulturae, 277, 109815. DOI 10.1016/j.scienta.2020.109815. [Google Scholar] [CrossRef]
8. Zhang, F., Zou, Y. N., Wu, Q. S. (2018). Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Scientia Horticulturae, 229, 132–136. DOI 10.1016/j.scienta.2017.10.038. [Google Scholar] [CrossRef]
9. Huang, G. M., Zou, Y. N., Wu, Q. S., Xu, Y. J., Kuča, K. (2020). Mycorrhizal roles in plant growth, gas exchange, root morphology, and nutrient uptake of walnuts. Plant, Soil and Environment, 66(6), 295–302. DOI 10.17221/240/2020-PSE. [Google Scholar] [CrossRef]
10. Xie, M. M., Chen, S. M., Zou, Y. N., Srivastava, A. K., Rahman, M. M. et al. (2021). Effects of Rhizophagus intraradices and Rhizobium trifolii on growth and N assimilation of white clover. Plant Growth Regulation. DOI 10.1007/s10725-020-00689-y. [Google Scholar] [CrossRef]
11. Debeljak, M., van Elteren, J. T., Spruk, A., Andrei, I., Frank, V. et al. (2018). The role of arbuscular mycorrhiza in mercury and mineral nutrient uptake in maize. Chemosphere, 212(241), 1076–1084. DOI 10.1016/j.chemosphere.2018.08.147. [Google Scholar] [CrossRef]
12. Yuan, X. Y., Jiao, J. G., Zhu, L., Liu, M. Q., Li, H. X. et al. (2011). Effects of earthworm activity on soil aggregates’ stability and organic carbon distribution under different manipulations of corn straw. Soil, 43(6), 968–974. [Google Scholar]
13. Wu, J. T., Li, H. Q., Zhang, W. X., Li, F., Huang, J. et al. (2017). Contrasting impacts of two subtropical earthworm species on leaf litter carbon sequestration into soil aggregates. Journal of Soils and Sediments, 17(6), 1672–1681. DOI 10.1007/s11368-017-1657-9. [Google Scholar] [CrossRef]
14. Lin, X. F. (2017). Nitrogen and earthworm impacts on clover and maize growth and promoting mechanism (Ph.D. Thesis). Nanjing Agricultural University, Nanjing. [Google Scholar]
15. Yu, J. G., Hu, F., Li, H. X., Wang, Q. J., Wang, T. (2010). Effects of earthworm inoculation on distribution, stability and organic carbon storage of soil aggregate. Journal of Soil and Water Conservation, 24(3), 175–179, 184. [Google Scholar]
16. Lu, M. Z., Lv, X. G., Guan, Q., Wu, H. T. (2015). Effects of earthworm on greenhouse gas emission from soil and its mechanism. Acta Pedologica Sinica, 52(6), 1209–1225. [Google Scholar]
17. Zhang, X. C., Wang, Y. L. (2017). Effect of Eisenia foetida on soil nutrient elements. Gansu Science and Technology, 33(7), 44–48. [Google Scholar]
18. Na, L. P., Li, Y. T., He, J. Q., Li, J. F., Wu, Y. P. (2020). Effects of earthworms on nitrogen uptake by lettuce and microbial nitrogen fixation after nitrogen application. Journal of Agro-Environment Science, 39(2), 343–350. [Google Scholar]
19. Guo, X. R., Wei, M., Yuan, F., Zhi, L. et al. (2019). Effects of microbial activities (earthworm) on corn yield. Agricultural Technology and Equipment, 358(10), 95–96. [Google Scholar]
20. Xu, X. Y., He, Y. S., Gan, X. J., Long, X. Y. (2011). Effects of earthworm activities on soil urease activities. Journal of Anhui Agriculture, 39(16), 9693–9694. [Google Scholar]
21. Wang, D. D., Li, H. X., Hu, F., Wang, X. (2006). Effects of earthworm activities on microbial community structure and enzyme activities in zinc contaminated soil. Ecology and Environment, 15(3), 538–542. [Google Scholar]
22. Zeng, L. T., Wang, D. S., Wang, Z. W., Wang, S. Q., Sheng, X. J. (2016). Effects of earthworm compost combined with probiotics on soil fertility and microbial characteristics. Soils, 48(6), 1100–1107. [Google Scholar]
23. Groffman, P. M., McDowell, W. H., Myers, J. C., Memam, J. L. (2001). Soil microbial biomass and activity in tropical riparian forests. Soil Biology and Biochemistry, 33(10), 1339–1348. DOI 10.1016/S0038-0717(01)00039-6. [Google Scholar] [CrossRef]
24. Yang, G. G., Xin, M. Y., Ma, X. J., Wang, Y. (2017). The microbial characteristics in earthworm-microorganism symbiotic system. Environmental Engineering, 35(1), 124–128. [Google Scholar]
25. Gao, L., Ling, S., Zhan, X. P., Lin, Z. F., Zhang, W. et al. (2017). Interaction effects and mechanism of Pb pollution and soil microorganism in the presence of earthworm. Chemosphere, 173, 227–234. DOI 10.1016/j.chemosphere.2017.01.022. [Google Scholar] [CrossRef]
26. Sun, Y. J., Diao, X. P., Shen, J. Z. (2005). Effects of avermectin B1a on soil microorganism and earthworm (Eisenia fetida). Chinese Journal of Applied Ecology, 16(11), 2140–2143. [Google Scholar]
27. Yong, Y. X., Zhang, Y., Cao, Y. E. (2018). Effect of earthworm fermentation liquor on nutrients and microorganism in medlar soil. Chinese Agricultural Science Bulletin, 34(6), 91–96. [Google Scholar]
28. Cheng, J. M., Yu, X. Z., Huang, M. H. (2007). Effect of earthworm-mycorrhiza interaction on transformation of Cd from soil to plant. Acta Scientiae Circum Stantiae, 27(2), 228–234. [Google Scholar]
29. Cheng, J. M., Yu, X. Z., Huang, M. H. (2006). Effect of earthworm-mycorrhiza interaction on available nutrients and ryegrass growth in Cd contaminated soil. Journal of Agro-Environment Science, 25(3), 685–689. [Google Scholar]
30. Wang, F. Y., Lin, X. G., Zhou, J. M. (2004). Latest advances in the classification of arbuscular mycorrhizal fungi. Journal of Microbiology, 25(3), 41–45. [Google Scholar]
31. Wu, Q. S., Srivastava, A. K., Zou, Y. N. (2013). AMF-induced tolerance to drought stress in citrus: A review. Scientia Horticulturae, 164, 77–87. DOI 10.1016/j.scienta.2013.09.010. [Google Scholar] [CrossRef]
32. Shao, Y. D., Hu, X. C., Wu, Q. S., Yang, T. Y., Srivastava, A. K. et al. (2021). Mycorrhizas promote P acquisition of tea plants through changes in root morphology and P transporter gene expression. South African Journal of Botany, 137, 455–462. DOI 10.1016/j.sajb.2020.11.028. [Google Scholar] [CrossRef]
33. Liu, C. Y., Srivastava, A. K., Wu, Q. S. (2017). Mycorrhizal fungi regulate root responses and leaf physiological activities in trifoliate orange. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 45(1), 17–21. DOI 10.15835/nbha45110658. [Google Scholar] [CrossRef]
34. Lü, L. H., Zou, Y. N., Wu, Q. S. (2018). Relationship between arbuscular mycorrhizas and plant growth: Improvement or depression? In: Giri, B., Prasad, R., Varma, A. (eds.Root biology. Soil biology. vol. 52, pp. 451–464. Cham: Springer. [Google Scholar]
35. Wu, Q. S., Srivastava, A. K., Zou, Y. N., Malhotra, S. K. (2017). Mycorrhizas in citrus: Beyond soil fertility and plant nutrition. Indian Journal of Agricultural Sciences, 87, 427–443. [Google Scholar]
36. Wu, Q. S., Gao, W. Q., Srivastava, A. K., Zhang, F., Zou, Y. N. (2020). Nutrient acquisition and fruit quality of Ponkan mandarin in response to AMF inoculation. Indian Journal of Agricultural Sciences, 90, 1563–1567. [Google Scholar]
37. Xie, M. M., Zou, Y. N., Wu, Q. S., Zhang, Z. Z., Kuča, K. (2020). Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant, Soil and Environment, 66(6), 287–294. DOI 10.17221/234/2020-PSE. [Google Scholar] [CrossRef]
38. Wu, Q. S., He, J. D., Srivastava, A. K., Zou, Y. N., Kuča, K. (2019). Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiology, 39(7), 1149–1158. DOI 10.1093/treephys/tpz039. [Google Scholar] [CrossRef]
39. Zhang, F., Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environmental and Experimental Botany, 171, 103962. DOI 10.1016/j.envexpbot.2019.103962. [Google Scholar] [CrossRef]
40. Zhang, F., Wang, P., Zou, Y. N., Wu, Q. S., Kuča, K. (2019). Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Archives of Agronomy and Soil Science, 65(9), 1316–1330. DOI 10.1080/03650340.2018.1563780. [Google Scholar] [CrossRef]
41. Cheng, H. Q., Giri, B., Wu, Q. S., Zou, Y. N., Kuča, K. (2021). Arbuscular mycorrhizal fungi mitigate drought stress in citrus by modulating root microenvironment. Archives of Agronomy and Soil Science, 57, 1–12. DOI 10.1080/03650340.2021.1878497. [Google Scholar] [CrossRef]
42. He, J. D., Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Scientia Horticulturae, 262, 108745. DOI 10.1016/j.scienta.2019.108745. [Google Scholar] [CrossRef]
43. Zou, Y. N., Wu, H. H., Giri, B., Wu, Q. S., Kuča, K. (2019). Mycorrhizal symbiosis down-regulates or does not change root aquaporin expression in trifoliate orange under drought stress. Plant Physiology and Biochemistry, 144, 292–299. DOI 10.1016/j.plaphy.2019.10.001. [Google Scholar] [CrossRef]
44. Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biology, 82, 1227. DOI 10.1111/plb.13161. [Google Scholar] [CrossRef]
45. Zou, Y. N., Zhang, F., Srivastava, A. K., Wu, Q. S., Kuča, K. (2021). Arbuscular mycorrhizal fungi regulate polyamine homeostasis in roots of trifoliate orange for improved adaptation to soil moisture deficit stress. Frontiers in Plant Science, 11, 600792. DOI 10.3389/fpls.2020.600792. [Google Scholar] [CrossRef]
46. He, J. D., Chi, G. G., Zou, Y. N., Shu, B., Wu, Q. S. et al. (2020). Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Applied Soil Ecology, 154, 103592. DOI 10.1016/j.apsoil.2020.103592. [Google Scholar] [CrossRef]
47. Meng, L. L., He, J. D., Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total nitrogen in trifoliate orange. Plant, Soil and Environment, 66(4), 183–189. DOI 10.17221/100/2020-PSE. [Google Scholar] [CrossRef]
48. Liu, C. Y., Wang, P., Zhang, D. J., Zou, Y. N., Kuča, K. et al. (2018). Mycorrhiza-induced change in root hair growth is associated with IAA accumulation and expression of EXPs in trifoliate orange under two P levels. Scientia Horticulturae, 234, 227–235. DOI 10.1016/j.scienta.2018.02.052. [Google Scholar] [CrossRef]
49. Xie, M. M., Wang, Y., Li, Q. S., Kuča, K., Wu, Q. S. (2020). A friendly-environmental strategy: Application of arbuscular mycorrhizal fungi to ornamental plants for plant growth and garden landscape. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 48(3), 1100–1115. DOI 10.15835/nbha48312055. [Google Scholar] [CrossRef]
50. Zhang, Y. C., Zou, Y. N., Liu, L. P., Wu, Q. S. (2019). Common mycorrhizal networks activate salicylic acid defense responses of trifoliate orange (Poncirus trifoliata). Journal of Integrative Plant Biology, 61(10), 1099–1111. DOI 10.1111/jipb.12743. [Google Scholar] [CrossRef]
51. Klironomos, J. N., Moutoglis, P. (1999). Colonization of nonmycorrhizal plants by mycorrhizal neighbours as influenced by the collembolan, Folsomia candida. Biology and Fertility of Soils, 29(3), 277–281. DOI 10.1007/s003740050553. [Google Scholar] [CrossRef]
52. Tuffen, F., Eason, W. R., Scullion, J. (2002). The effect of earthworms and arbuscular mycorrhizal fungi on growth of and 32P transfer between Allium porrum plants. Soil Biology and Biochemistry, 34(7), 1027–1036. DOI 10.1016/S0038-0717(02)00036-6. [Google Scholar] [CrossRef]
53. Pattinson, G. S., Smith, S. E., Doube, B. M. (1997). Earthworm Aporrectodea trapezoides had no effect on the dispersal of a vesicular-arbuscular mycorrhizal fungi, Glomus intraradices. Soil Biology and Biochemistry, 29(7), 1079–1088. DOI 10.1016/S0038-0717(97)00005-9. [Google Scholar] [CrossRef]
54. Li, H., Wang, C., Wang, S. Y. (2015). Interaction of earthworms and amfungi on maize growth, and nutrogen and phosphorus uptake. Journal of Plant Nutrition and Fertilizer, 21(4), 920–926. [Google Scholar]
55. Ortiz-Ceballos, A. I., Pea-Cabriales, J. J., Fragoso, C. (2007). Mycorrhizal colonization and nitrogen uptake by maize: Combined effect of tropical earthworms and velvetbean mulch. Biology and Fertility of Soils, 44(1), 181–186. DOI 10.1007/s00374-007-0193-y. [Google Scholar] [CrossRef]
56. Dobson, A., Richardson, J., Blossey, B. (2020). Effects of earthworms and white-tailed deer on roots, arbuscular mycorrhizae, and forest seedling performance. Ecology, 101(1), e02903. DOI 10.1002/ecy.2903. [Google Scholar] [CrossRef]
57. Gange, A. C. (1993). Translocation of mycorrhizal fungi by earthworms during early succession. Soil Biology and Biochemistry, 25(8), 1021–1026. DOI 10.1016/0038-0717(93)90149-6. [Google Scholar] [CrossRef]
58. Yang, H. S., Zhou, J. J., Weih, M., Li, Y. F., Zhai, S. L. et al. (2020). Mycorrhizal nitrogen uptake of wheat is increased by earthworm activity only under no-till and straw removal conditions. Applied Soil Ecology, 155, 103672. DOI 10.1016/j.apsoil.2020.103672. [Google Scholar] [CrossRef]
59. Brown, G. G. (1995). How do earthworms affect microfloral and faunal community diversity? Plant and Soil, 170(1), 209–231. DOI 10.1007/BF02183068. [Google Scholar] [CrossRef]
60. Gormsen, D., Olsson, P. A., Hedlend, K. (2004). The influence of collembolans and earthworms on AM fungal mycelium. Applied Soil Ecology, 27(3), 211–220. DOI 10.1016/j.apsoil.2004.06.001. [Google Scholar] [CrossRef]
61. Johansen, A., Jakobsen, I., Jensen, E. S. (1994). Hyphal N transport by a vesicular-arbuscular mycorrhizal fungus associated with cucumber grown at three nitrogen levels. Plant and Soil, 160(1), 1–9. DOI 10.1007/BF00150340. [Google Scholar] [CrossRef]
62. Li, H., Li, X. L., Zhang, J. L., Gai, J. P., Wang, C. et al. (2011). Interaction between earthworm and arbuscular mycorrhizal fungi and their effects on plants. Acta Pedologica Sinica, 48(4), 847–855. [Google Scholar]
63. Zhang, W. X., Chen, D. M., Zhao, C. C. (2007). Functions of earthworm in ecosystem. Biodiversity Science, 15(2), 142–153. DOI 10.1360/biodiv.060294. [Google Scholar] [CrossRef]
64. Yuan, F. Y., Wang, F., Niu, Z. R., Wang, F. (2004). Earthworm community structure of representative agrotypes and its indicator function for heavy metals contamination in North China. Research of Environmental Sciences, 17(6), 70–72. [Google Scholar]
65. Wurst, S., Dugassa-Gobena, D., Langel, R., Michael, B., Ftesan, S. (2004). Combined effects of earthworms and vesicular-arbuscular mycorrhizas on plant and aphid performance. New Phytologist, 163(1), 169–176. DOI 10.1111/j.1469-8137.2004.01106.x. [Google Scholar] [CrossRef]
66. Milleret, R., Bayon, R. C. L., Gobat, J. M. (2009). Root, mycorrhiza and earthworm interactions: Their effects on soil structuring processes, plant and soil nutrient concentration and plant biomass. Plant and Soil, 1(2), 1–12. DOI 10.1007/s11104-008-9753-7. [Google Scholar] [CrossRef]
67. Xiao, Y. P., Shao, Y. F., Sheng, S. Y., Yi, Y., Ling, X. G. et al. (2010). Effect of arbuscular mycorrhizal fungi and earthworms on photo remediation of As-contaminated soils using maize. Journal of Ecology and Rural Environment, 26(3), 235–240. [Google Scholar]
68. Cao, J., Wang, C., Huang, Y. (2015). Interactive impacts of earthworms (Eisenia fetida) and arbuscular mycorrhizal fungi (Funneliformis mosseae) on the bioavailability of calcium phosphates. Plant and Soil, 396(1–2), 45–57. DOI 10.1007/s11104-015-2588-0. [Google Scholar] [CrossRef]
69. Li, H., Du, Z. Y., Liu, Q., Shi, Y. X. (2016). Effect of earthworm-mycorrhiza interaction on soil enzyme activities. Journal of Plant Nutrition and Fertilizer, 22(1), 209–215. [Google Scholar]
70. Zhang, W. W., Wang, C., Lu, T. Y., Zheng, Y. J. (2018). Cooperation between arbuscular mycorrhizal fungi and earthworms promotes the physiological adaptation of maize under a high salt stress. Plant and Soil, 423(1–2), 125–140. DOI 10.1007/s11104-017-3481-9. [Google Scholar] [CrossRef]
71. Gao, Y., Luo, Y. M. (2005). The indicative effect of earthworm on soil pollution and its potential for enhanced remediation. Acta Pedologica Sinica, 42(1), 140–148. [Google Scholar]
72. Ning, C. H., Li, W. B., Xu, Q. K., Li, M., Guo, S. X. (2019). Arbuscular mycorrhizal fungi enhance cadmium uptake of wetland plants in contaminated water. Chinese Journal of Applied Ecology, 30(6), 2063–2071. [Google Scholar]
73. Fu, L. (2016). Remediation of Cu contaminated soil by French marigold, earthworm and arbuscular mycorrhizal fungi (Ph.D. Thesis). Nanjing Agricultural University, Nanjing. [Google Scholar]
74. Wang, G., Wang, L., Ma, F., Yang, D. G., You, Y. Q. (2021). Earthworm and arbuscular mycorrhiza interactions: Strategies to motivate antioxidant responses and improve soil functionality. Environmental Pollution, 272, 115980. DOI 10.1016/j.envpol.2020.115980. [Google Scholar] [CrossRef]
75. Zhuo, S., Su, J. X., Li, H. S., Lin, Y. M., He, H. Z. et al. (2011). Combined remediation effects of ryegrass mycorrhizal fungus-earthworms on PCB contaminated soils. Acta Scientiae Circumstantiae, 31(1), 150–157. [Google Scholar]
76. Lu, Y. F., Lu, M., Peng, F., Wan, Y., Liao, M. H. (2014). Remediation of polychlorinated biphenyl-contaminated soil by using a combination of ryegrass, arbuscular mycorrhizal fungi and earthworms. Chemosphere, 106, 44–50. DOI 10.1016/j.chemosphere.2013.12.089. [Google Scholar] [CrossRef]
77. Yang, L., Li, G. Z., Tong, Q. Q., He, T. B. (2010). Effect of earthworm, mycorrhizal fungi and combined action on phytoremediation under Pb2+ and Cd2+ Stress. Guizhou Agricultural Sciences, 38(11), 156–158. [Google Scholar]
78. Li, N., Wang, C., Li, X. L., Liu, M. L. (2019). Effects of earthworms and arbuscular mycorrhizal fungi on preventing Fusarium oxysporum infection in the strawberry plant. Plant and Soil, 443(1–2), 139–153. DOI 10.1007/s11104-019-04224-5. [Google Scholar] [CrossRef]
79. Yuan, F. Y., Wang, F., Niu, Z. R., Wang, F. (2004). Earthworm community structure of representative agrotypes and its indicator function for heavy metals contamination in north China. Research of Environmental Sciences, 17(6), 70–72. [Google Scholar]
80. Hu, P., Liu, D. H., Hu, F., Shen, Q. R. (2002). Plant hormones in earthworm casts and their promotion on ad- ventitious root formation of mung bean cutting. Acta Ecologica Sinica, 22(8), 1211–1214. [Google Scholar]
81. Eisenhauer, N., König, S., Sabais, A. C., Renker, C., Buscot, F. et al. (2009). Impacts of earthworms and arbuscular mycorrhizal fungi (Glomus intraradices) on plant performance are not interrelated. Soil Biology and Biochemistry, 41(3), 561–567. DOI 10.1016/j.soilbio.2008.12.017. [Google Scholar] [CrossRef]
82. He, X. X., Li, X. B., Liu, T., Yang, X. J., Cao, J. B. et al. (2020). Earthworms negate the adverse effect of arbuscular mycorrhizae on living bacterial biomass and bacterial necromass accumulation in a subtropical soil. Soil Biology and Biochemistry, 151(6), 108052. DOI 10.1016/j.soilbio.2020.108052. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |