

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013693
ARTICLE
An Efficient Plant Regeneration System of Hydrangea bretschneideri Dipp via Stem Segments as Explants
Institute of Forest Tree Genetic Breeding, Forestry College, Inner Mongolia Agricultural University, Hohhot, 010000, China
*Corresponding Author: Yu’e Bai. Email: baiyue@imau.edu.cn
Received: 17 August 2020; Accepted: 06 November 2020
#These authors contributed equally to this work
Abstract: Hydrangea bretschneideri Dipp is a highly popular ornamental plant for garden decoration. Genetic engineering technology has been successfully used in many plant species, but it is limited in Hydrangea. Here we established an efficient regeneration system by using stem segments as explants for the first time. In our study, the plant growth regulators (PGRs) were evaluated at the different regeneration processes, including axillary shoots regeneration and root induction. We found that the optimal concentration for axillary buds’ induction was 2.0 mgL−1 6-BA and 0.5 mgL−11 IAA, its highest induction rate was 70%. Moreover, the highest axillary shoots proliferation coefficient was 10.7 on the Murashige and Skoog (MS) medium with 2.0 mgL−1 6-benzyladenine (BA), 0.2 mgL−1 indole-3-butyric acid (IBA), and 1.0 mgL−1 gibberellin A3 (GA3). The highest frequency of root induction was 80.0 ± 0.06% by culturing the elongated shoots in 1/2 MS medium containing 0.1 mgL−1 IBA. In summary, our study will provide an effective technology for large-scale propagation and important pathway for promoting the popularization and application of Hydrangea bretschneideri Dipp.
Keywords: Hydrangea bretschneideri Dipp; stem segments; regeneration
Native from Southern and Eastern Asia, and North and South America, the Hydrangea genus contains about 75 species [1]. Members of the Hydrangea genus, which are highly popular as ornamental plants for garden decoration, are now commercially produced for the cutting of flower branches, due to the red, mauve, purple, violet, and blue colors of their sepals [2]. The Hydrangea bretschneideri Dipp species is a shrub about 1-3m tall, with small-toothed dark green leaves on stiff-erected stems and medium-size flower heads, distributing along riversides and forest edges. The strong adaptability and resistance of these plants, with flowering times ranging from early spring to late fall, makes them good prospects with wide applications and a promising economic development in China.
Breeding programs in Hydrangea usually have specific goals depending on how the specific species will be used. The flowering traits, stem strength and durability are always considered as a priority in Hydrangea breeding programs [3,4]. Hydrangea are vegetatively propagated crop; however, as each cultivar possesses a specific genotype, several seedlings exhibit variability in its regeneration process, and do not always produce the desired characteristics or morphology [5,6]. Therefore, the clonal reproduction method is the best way for the commercial propagation of Hydrangea members. In recent years, plant tissue culture techniques have been widely used as viable tools for mass propagation and germplasm conservation of various economic important plants [7], including several genotypes of H. heteromalla (“Snow Cap”) and H. petiolaris [8,9]. Many tissues like stem tips, stem segments, petioles and leaves have been used as explants for tissue culture [10–12].
Despite advances with many Hydrangea species, there is still a need for the optimization of tissue culture protocols in the Hydrangea genus. For example, a previous study indicated that thidiazuron (TDZ) plays an important role in the in vitro shoot proliferation of Hydrangea [13]; however, it is necessary to find cheaper PGR alternatives, considering the costs of TDZ.
With respect to H. bretschneideri Dipp, as far as it has been investigated, there is not an effective protocol reporting its efficient tissue culture propagation. Thus, the objective of the present study was to establish, for the first time, an efficient reproductive protocol for this important species, by modification of the culture media. As a result, an efficient and reproducible protocol for the regeneration of H. bretschneideri Dipp using in vitro tissue culture techniques was successfully established. This protocol constitutes the basis for the future optimization of the regeneration protocols, as well as for attempting the genetic manipulation of this species.
2.1 Plant Regeneration System of Hydrangea bretschneideri Dipp
To obtain an efficient plant regeneration system of Hydrangea bretschneideri Dipp, stem segments from one-year-old plants were used as explants, and different plant growth regulators (PGR) to induce the production of axillary buds, proliferation of axillary shoots and elongation of regenerated shoots, were evaluated. The regenerated shoots were induced to produce root, and the well-rooted plantlets were cultivated and acclimatized to environmental conditions to obtain the plants.
2.2 Plant Material and Explant Preparation
Healthy stem segments from one-year-old H. bretschneideri Dipp plants were collected from Daqing Mountain, Hohhot, Inner Mongolia, China. Stem segments were washed and put into clean glass bottles and added with two drops of Tween 2.0. The bottles were sealed with gauze and explants were rinsed with running water for 30–50 min. The rinsed stems were surface sterilized with 75% ethanol for 30 s, followed by 0.1% (w/v) aqueous solution of mercuric chloride for 8 min, and then washed with sterilized water 5–7 times under aseptic conditions. Finally, the sterilized explants were cut into 0.5–1.0 cm length stem segments with 1–2 axillary buds.
2.3 Induction of Axillary Buds
The disinfected stem segments were placed into culture bottles containing induction medium, consisting in MS basal medium supplemented with different concentrations of 6-BA (0, 1.5, 2.0, and 2.5 mgL−1) and IAA (0, 0.1, 0.5, and 1.0 mgL−1). The stem segments were then incubated under a light intensity of 60–70 µmol(m2s)−1 in a 16 h light/8 h dark photoperiod at 28 ± 2°C. The time for neonatal axillary buds’ initiation (days after planting), and the induction percentage after 30 days [(the number of plants generating axillary buds/number of inoculated plants) ×100], were measured. Each treatment consisted in 10 bottles inoculated with one explant each. All experiments were repeated at least three times.
2.4 Proliferation of Axillary Shoots
For proliferation of axillary shoots, the induced axillary buds were inoculated in MS, B5, 1/2 MS or 1/4 MS basic media, each supplemented with 6-BA (0, 2.0 and 2.5 mg mL−1), IBA (0, 0.1, 0.2 and 0.5 mgL−1), and GA3 (0, 0.5, 1.0 and 1.5 mgL−1). The initial time of bud proliferation and the proliferation coefficient (the number of new generated axillary bud plants/the number of inoculated plants containing one axillary bud) were recorded at 30 days after inoculation. The axillary buds were then incubated under a light intensity of 60–70 µmol(m2s)−1 in a 16 h light/8 h dark photoperiod at 28 ± 2°C. Each treatment consisted in 10 bottles inoculated with one explant each. All experiments were repeated at least three times.
2.5 Elongation of Regenerated Shoots
The growth of axillary shoots was evaluated by incubation of proliferated axillary buds (clusters of 4 or 5 axillary shoots) in MS basic medium supplemented with different combinations of 6-BA (0, 0.3 and 0. 5 mgL−1) and IBA (0, 0.1, 0.2 and 0.3 mgL−1), respectively. The proliferated axillary buds were then incubated under a light intensity of 60–70 µmol(m2s)−1 in a 16 h light/8 h dark photoperiod at 28 ± 2°C. Each treatment consisted in 10 bottles inoculated with one explant each. All experiments were repeated at least three times. After 30 days, the average seedling height and axillary shoots growth were calculated.
For root induction, the elongated shoots (5 cm length) were excised and transferred into 1/2 MS medium containing different concentrations of IBA (0, 0.1, 0.3 and 0.5 mgL−1) and NAA (0, 0.1, 0.3 and 0.5 mgL−1). All the plants were kept in a greenhouse with high relative humidity at (25 ± 2)°C under a 16h light/ 8h dark photoperiod for 4 weeks. Rooting rate [(the number of rooting plants/Number of inoculated plants) × 100%] and number of roots were recorded for each treatment.
2.7 Acclimatization and Transplanting
Well-rooted plantlets were transferred to plastic boxes containing sterile perlite. Glasses were covered with a transparent plastic to maintain a humidity of 90% for two weeks. Subsequently, the plastic boxes were opened occasionally to acclimatize the plantlets to lower humidity conditions. Plantlets were occasionally watered. Two weeks later, growing of acclimatized plantlets were continued under greenhouse conditions.
All the experiments in this study were repeated at least three times. The Spss software was used for variance analysis. The data were analyzed with one-way ANOVA to calculate statistical significance and the Duncan’s new multiple range test was used for comparison among treatment means
3.1 Induction of Axillary Bud Development
For a rapid in vitro clonal propagation of Hydrangea bretschneideri Dipp, the one-year-old young stem segments were collected as explants and cultured on the axillary bud induction MS medium (Fig. 1A–1C). Numerous axillary buds could be observed at the cut site after one week (Fig. 1B). But its induction efficiency was very low. To improve the induction efficiency, various combinations of cytokinin and auxin were supplemented. In the axillary bud induction, we evaluated the single factor of 6-BA and IAA respectively. Analysis results showed that the inducted axillary buds were green and growth well in 2.0 mgL−1 6-BA medium. When 6-BA was increased to 2.5 mgL−1, its induction frequency was conversely decreased at the medium IM7-IM10. The similar inhibitory effect also was observed in 6-BA less than the optimal concentrations 2.0 mgL−1 at IM2-IM4. Therefore, 2.0 mgL−1 6-BA was the best for the axillary bud induction. In addition, we found that the highest induction percentage was obtained when the 0.5 mgL−1 IAA was added. Lower (0.1 mgL−1) and higher (1.0 mgL−1) concentrations of IAA will lead to the abnormal leaves and slow growth of seedlings. Therefore, the intermediate concentration of 6-BA (2.0 mgL−1) and IAA (0.5 mgL−1) (IM6) was the best for axillary bud induction (Tab. 1). The highest induction rate reached up to 70.0 ± 0.58% (Tab. 1).
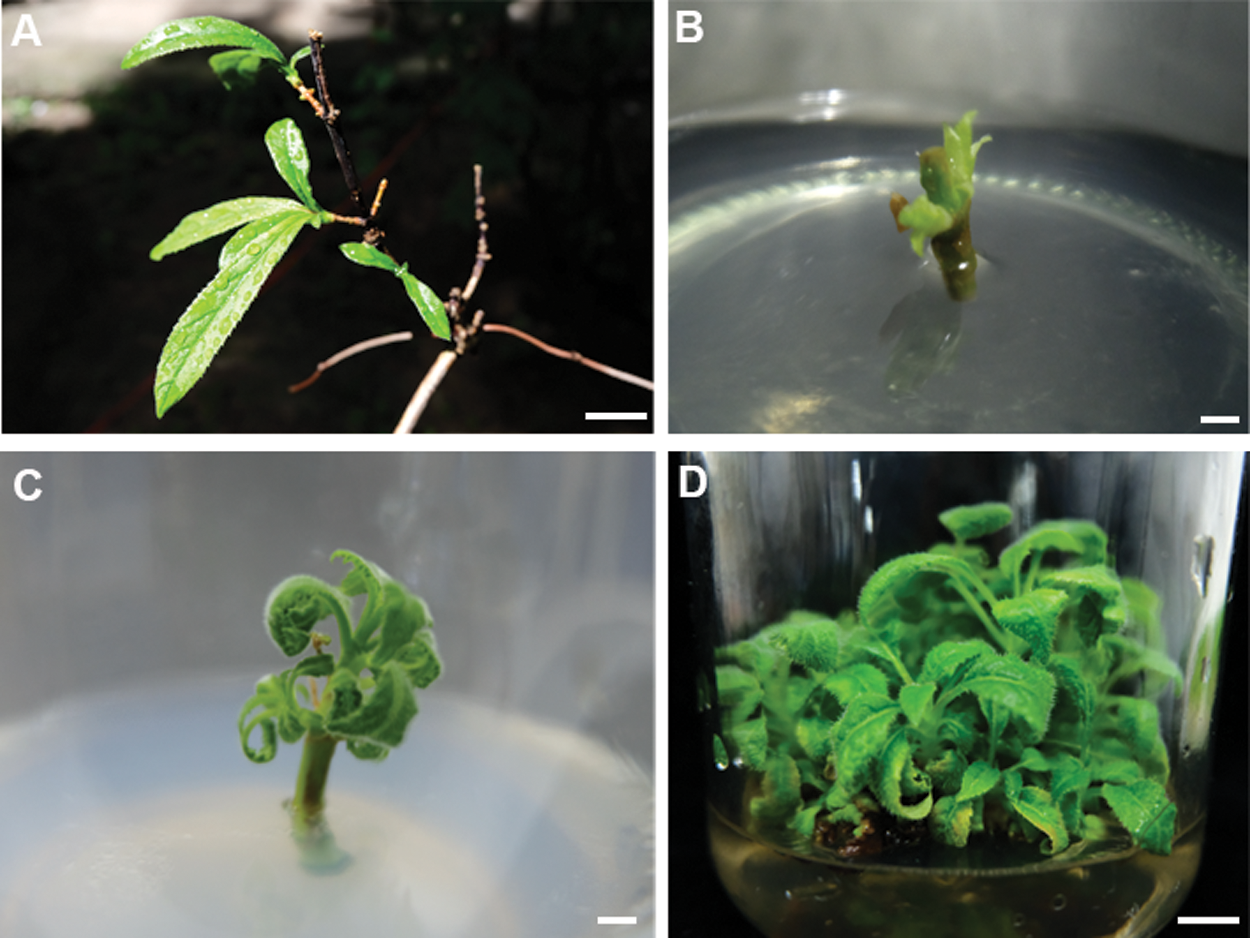
Figure 1: Plantlet regeneration from stem segment of Hydrangea bretschneideri Dipp. (A) a stem segment explants for adventitious bud induction. Bar: 0.1 cm; (B) adventitious buds induced from stem segment explants. Bar: 0.5 cm; (C) adventitious buds grew after 10 days. Bar: 0.3 cm; (D) adventitious bud proliferation at four weeks. Bar: 0.5 cm
Table 1: Effect of different plant growth regulators for induction of axillary buds
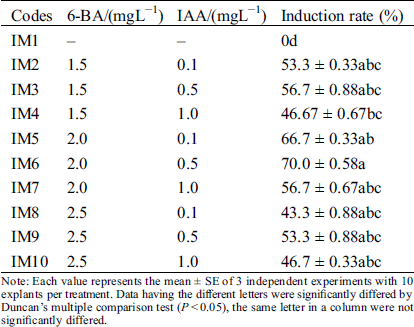
Note: Each value represents the mean ± SE of 3 independent experiments with 10 explants per treatment. Data having the different letters were significantly differed by Duncan’s multiple comparison test (P < 0.05), the same letter in a column were not significantly differed.
3.2 Proliferation of Axillary Shoots
3.2.1 Effects of Different 6-BA, IBA and GA3 on the Proliferation of Axillary Buds
The induced axillary shoots quickly proliferated at the shoot tips of explants after transferred to the proliferation medium with 6-BA and IBA (PM2-PM4) (Fig. 1D). But their proliferation coefficient was very low. The statistical results showed that the best group was PM3 that its proliferation coefficient was 3.9 ± 0.03 (Tab. 2). To improve the proliferation efficiency, GA3 was added into the PM3. We found that the optimal concentration of GA3 was 1.0 mg/L, its proliferation coefficient was 10.7 ± 0.45. But its concentration should not be higher than 1.0 mg/L. To further improve the proliferation efficiency, we supplemented the 6-BA and IBA on the basis of 1.0 mg/L GA3 (PM8-PM9). When only 6-BA was increased to 2.5 mg/L, the proliferation rate was decreased to 7.5 ± 0.20 with abnormal leaves bulging. Consistently, their proliferation coefficient was also decreased to 8.8 ± 0.33, when only changed the concentration of IBA. IBA was a larger positive effect on leaves morphology, most of leaves were green and normal morphology at 0.1~0.2 mgL−1 IBA, but excessive IBA (0.5 mgL−1) lead to most leaves’ morphology becoming abnormal with jagged edges. Combined with the above factors, our results confirmed that the positive effect PGRs combination was 6-BA 2.0 mgL−1, IBA 0.2 mgL−1, GA3 1.0 mgL−1 (PM6) in axillary shoots proliferation. The maximum proliferation coefficient was 10.7 ± 0.45.
Table 2: Effects of different 6-BA, IBA and GA3 on proliferation of axillary buds
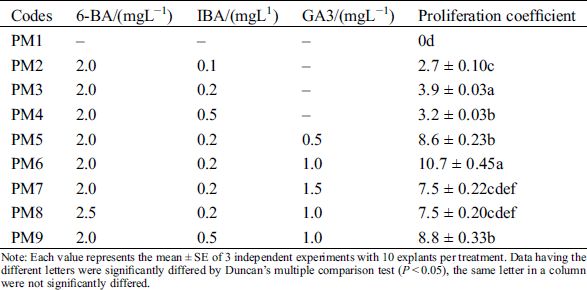
Note: Each value represents the mean ± SE of 3 independent experiments with 10 explants per treatment. Data having the different letters were significantly differed by Duncan’s multiple comparison test (P < 0.05), the same letter in a column were not significantly differed.
3.2.2 Effect of Different Basic Medium on Proliferation of Axillary Buds
To further improve the efficient of proliferation, we also tested several basal mediums, including MS, 1/2 MS, 1/4MS and B5 (Figs. 2A–2D). On MS medium, axillary shoots began to grow after five days and rapidly proliferated after three weeks (Figs. 2A–2D). Subsequently, they were rapidly developed to green leaves after 25 to 30 days (Fig. 3A). Their proliferation coefficient was 10.7. In the other medium, the axillary shoots grow slowly after two weeks. They also began to rapidly proliferate after three weeks, subsequently (Fig. 2B). But their leaves gradually become yellow-green with rooting in 1/4 MS and B5 medium (Figs. 2C, 2D). The growth condition of axillary shoots was unhealthy with partial death, and its multiplication coefficient was only 1.6 and 3.3, respectively (Tab. 3). Therefore, MS was the best basal culture medium for axillary shoots proliferation.
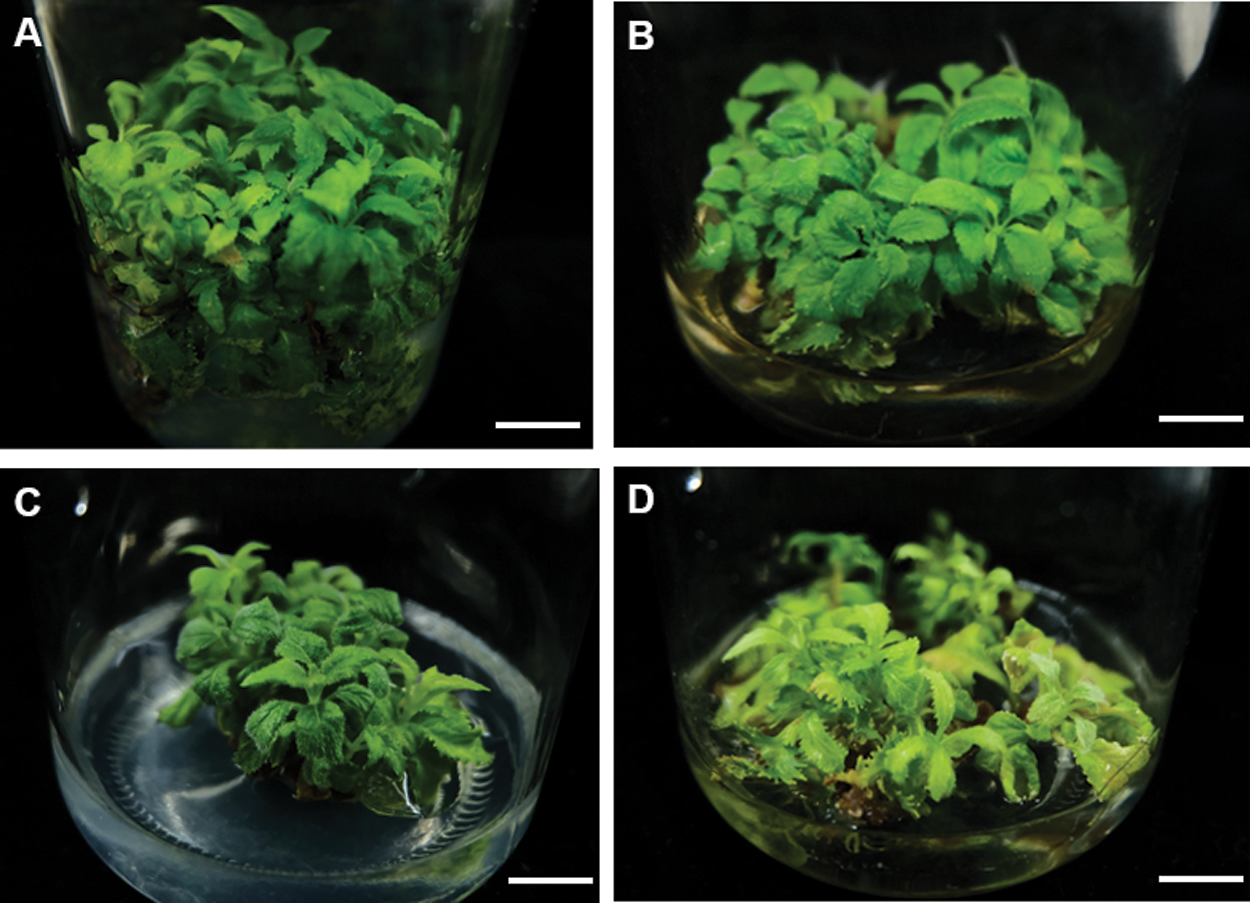
Figure 2: Effects of different basic culture medium on the adventitious bud proliferation. (A–D) the proliferation of adventitious bud in different basic culture medium MS (A), B5 (B), 1/2 MS (C), 1/4 MS (D). Bar: 1.0 cm

Figure 3: Rooting and plantlets acclimatization of Hydrangea bretschneideri Dipp. (A) Emerged root form shoots after two weeks; (B) Emerged root at 4 weeks. Bar: 0.5 cm; (C) regenerated plantlet in vitro at 4 weeks after rooting. Bar: 1.0 cm; (D) hardened plants. Bar: 1.5 cm
Table 3: Effect of different basic medium on proliferation of axillary buds
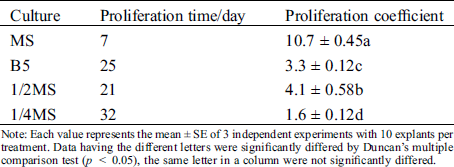
Note: Each value represents the mean ± SE of 3 independent experiments with 10 explants per treatment. Data having the different letters were significantly differed by Duncan’s multiple comparison test (p < 0.05), the same letter in a column were not significantly differed.
3.3 Elongation of Regenerated Shoots
Among the elongation of regenerated shoots, we tested several elongation mediums (Tab. 4). Statistical results showed that the proliferated adventitious shoots grew normally, but they were weak and shoot length was only 2.2 ± 0.02 cm without adding any plant growth regulators. Then, we combined the different concentrations of 6-BA and IBA for promoting their elongation (Tab. 4). We found that the regenerated shoot’s length was positively correlated with the concentration of 6-BA, the shoots grew taller with green color. When different concentrations of IBA was supplemented with 0.3 mgL−11 6-BA at the same time, the maximum shoot length was 3.2 ± 0.09 at EM5 with 0.2 mgL−1 IBA. Lower and higher concentrations of IBA both showed inhibitory effects, their shoot length was 3.0 ± 0.07 and 2.9 ± 0.06, respectively. When 6-BA was up to 0.5 mgL−1, although shoot length was between 2.7 and 3.0 cm with increasing of IBA from 0.1 to 0.3 mgL−1, the overall seedlings were short than other groups. Taken all these factors into consideration, the optimal shoots elongation PGRs combination was 0.3 mgL−1–1 6-BA and 0.2 mgL−1 IBA (EM5). The seedlings elongation length was 3. 2 ± 0.09 cm.
Table 4: Effect in different 6-BA and IBA on elongation of shoots
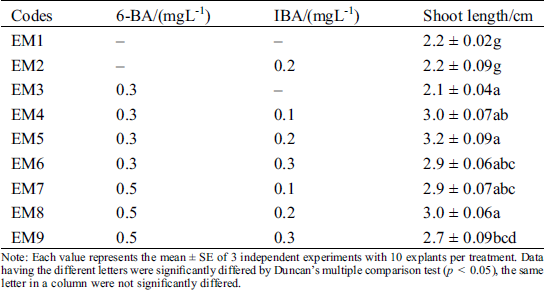
Note: Each value represents the mean ± SE of 3 independent experiments with 10 explants per treatment. Data having the different letters were significantly differed by Duncan’s multiple comparison test (p < 0.05), the same letter in a column were not significantly differed.
3.4 Root Induction of Regenerated Plantlets
For root induction and its development in regenerated shoots, 1/2 MS media supplemented with various concentrations of NAA and IBA were evaluated. Both PGRs (NAA and IBA) could induce the root produce (Figs. 3A–3C), but all the rooting initial time of IBA was earlier than NAA (Tab. 5). The highest percentage of rooting was 80% and the longest roots length were 4.1 cm at the RIM2 with 0.1 mgL−1 IBA (Tab. 5). On the contrary, the initial time of rooting was delayed; the roots gradually become shorter and growth slower; the number of lateral roots were decreased with increasing of IBA. Similar to IBA, each concentration of NAA also could induce plant rooting. The highest percentage of rooting was 63% and the longest roots was 3.2 cm. However, the callus was generated with the increasing of NAA. Formed callus could induce the roots generation and inhibit the root growth. Its roots were short and thick; a few of the lateral roots were induced. Therefore, IBA was better than NAA for root induction.
Table 5: Effect of difference plant growth regulators combinations on root induction
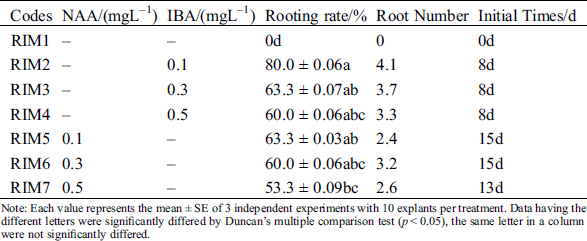
Note: Each value represents the mean ± SE of 3 independent experiments with 10 explants per treatment. Data having the different letters were significantly differed by Duncan’s multiple comparison test (p < 0.05), the same letter in a column were not significantly differed.
3.5 Plantlets Hardening and Acclimatization
Rooted plantlets were transferred into a soilless mixture containing sterile perlite and irrigated regularly with 1/2 MS salt-solution and tap water. They were successfully acclimatized in greenhouse after growing for a period of time. The acclimatized plants did not show any visible variations from the mother plants (Fig. 3D). However, its survival rate was only 66.7%. We suspected that Hydrangea bretschneideri Dipp plants perhaps need some special conditions for acclimatization. It is necessary to improve the cultivation environment for increasing the survival rate in further studies.
4.1 Shoot Regeneration and Development from Stem Segments Explants
It is known that the balance between cytokinin and auxin determines the fate of regenerating tissues and organs: lower auxin/cytokinin ratio triggers shoot regeneration, but higher ratio results in root regeneration [14]. In our study, we found that 2.0 mgL−1 6-BA is significantly positive on axillary bud induction. It is likely that 6-BA directly interacts with endogenous auxin to promote the axillary shoots regeneration [15]. In addition, exogenous supplement of 0.5 mgL−1 IAA also improves the induction rate; the shoots are green and growth healthy. However, excessive addition of IAA will greatly decrease shoots regeneration. The reason perhaps that high concentration of auxin directly inhibits shoot organogenesis but triggers somatic embryogenesis [16].
In the early stage of proliferation, although high concentrations of cytokinin improves the proliferation coefficient, the proliferated buds are small and weak, which is not conducive to the production of roots and reduced the survival rate in later plantlets’ hardening and acclimatization. We used lower concentrations of 6-BA and IBA combination. Moreover, we found that exogenous supplements of GA3 in MS medium with 6-BA and IBA is beneficial for shoots’ proliferation. Therefore, the optimal concentration of PGRs (2.0 mgL−1 6-BA, 0.2 mgL−1 IBA, and 1.0 mgL−1 GA3) in MS medium play an important role in proliferation of Hydrangea bretschneideri Dipp. In addition, 6-BA (0.3 mgL−1) and (IBA 0.1 mgL−1) reduces the costs and shorten cultivation times at the elongation of regenerated shoots. They are beneficial for promoting the application and industrialization of Hydrangea bretschneideri Dipp.
4.2 Rooting of the Regenerated Shoots and Plantlets Acclimatization
Auxin also plays a pivotal role in de novo root formation [17,18]. Previous studies indicated that the YUC4, an auxin biosynthesis gene, was expressed within 4 h after excision in mesophyll cells distant from wound sites. The elevated expression of YUC will lead to the maximum auxin response in the same region within the next two days [17]. In addition, WOX transcription factor family promotes a process known as first-step cell fate transition during root regeneration [18,19]. For example, WOX11/12 activate WOX5/WOX7 that results in converting the root founder cells into root primordia [18,20]. In turn, WOX11 directly upregulates the expression of LATERAL ORGAN BOUNDARIES DOMAIN 16 (LBD16) to regulate root meristem initiation, root regeneration and lateral root formation near wound sites [19]. Exogenous application of auxin (IBA and NAA) enhances regenerative responses of root in vitro via the above signal transduction process. In our study, IBA is better than NAA in root induction. With the increase of IBA, the root’s initial time is earlier and the rooting rate is higher. Afterwards, roots gradually grow faster and better overall. But exogenous application of NAA could promote more callus at the base of explants, the roots are short with fewer lateral roots. Therefore, the best rooting medium is 1/2 MS with 0.1 mgL−1 IBA. However, the reason why IBA rather than NAA promotes root formation remains unknown. Its molecular mechanisms still need to be further studied in Hydrangea bretschneideri Dipp issue culture system.
Author Contribution Statement: Yan Jia wrote the manuscript, organized data and designed researches. Wang Si ran and Ha bur conducted experiments. Bai Yu’e conceived and designed research. All authors read and approved the manuscript.
Funding Statement: This work is supported by the Grassland Talent Project: The Innovation Team of New Varieties Breeding at the Economic and Ecological Shrub, and the Evaluation of the Economic and Ecological Shrub Resources and New Variety Breeding in Inner Mongolia (No. 201702077).
Conflicts of Interest: No conflict of interest exists in the submission of this manuscript.
1. Mcclintock, E. (1957). A monograph of the genus Hydrangea. Proceedings of the California Academy of Sciences, 29, 147–256. [Google Scholar]
2. Warner, O. O., Hirsch, G. N., Wetzstein, H. Y. (2005). Genotypic variation in flower induction and development in Hydrangea macrophylla. American Society for Horticultural Science, 40(6), 1695–1698. [Google Scholar]
3. Uemachi, T., Okumura, A. (2012). The inheritance of inflorescence types in Hydrangea macrophylla. Japanese Society for Horticultural Science, 81(3), 263–268. DOI 10.2503/jjshs1.81.263. [Google Scholar] [CrossRef]
4. Hempel, P., Hohe, A., Tränkner, C. (2018). Molecular reconstruction of an old pedigree of diploid and triploid Hydrangea macrophylla genotypes. Frontiers in Plant Science, 9, 221. DOI 10.3389/fpls.2018.00429. [Google Scholar] [CrossRef]
5. Sacco, E., Savona, M., Antonetti, M., Grassotti, A., Pasqualetto, P. L. et al. (2012). In vitro propagation and regeneration of several Hydrangea genotypes. Acta Horticulturae, 937, 68. [Google Scholar]
6. Ruffoni, B., Sacco, E., Savona, M. (2013). In vitro propagation of Hydrangea spp. Methods in Molecular Biology, 11013, 231–244. [Google Scholar]
7. Victor, M., Loyola, V., Neftalí, O. A. (2018). An introduction to plant tissue culture: Advances and perspectives. Methods in Molecular Biology (Clifton, N.J.), 1815, 3–13. [Google Scholar]
8. Sebastian, T. K., Heurser, C. W. (1987). In vitro propagation of Hydrangea quercifolia Bartr. Scientia Horticulturae, 31(3–4), 303–309. DOI 10.1016/0304-4238(87)90056-2. [Google Scholar] [CrossRef]
9. Ledbetter, D. I., Preece, J. E. (2004). Thidiazuron stimulates adventitious shoot production from Hydrangea quercifolia Bartr. leaf explants. Scientia Horticulturae, 101(1–2), 121–126. DOI 10.1016/j.scienta.2003.09.014. [Google Scholar] [CrossRef]
10. Barbara, R., Ermanno, S., Marco, S. (2012). In vitro propagation of Hydrangea macrophylla Thunb. Protocols for Micropropagation of Selected Economically-Important Horticultural Plants, 994, 231–244. [Google Scholar]
11. Boccon-Gibod, J., Billard, C., Maltete, S. (2000). In vitro regeneration system of Hydrangea macrophylla plantlets from leaves and internodes. Acta Horticulturae, 508(508), 229–232. DOI 10.17660/ActaHortic.2000.508.32. [Google Scholar] [CrossRef]
12. Liu, F., Li, H. L., Li, Y. L., Reinhoud, P., Jongsma, M. A. et al. (2011). Shoot organogenesis in leaf explants of Hydrangea macrophylla Hyd1 and assessing genetic stability of regenerants using ISSR markers. Plant Cell, Tissue Organ Culture, 104(1), 111–117. DOI 10.1007/s11240-010-9797-2. [Google Scholar] [CrossRef]
13. Doil, A., Zhang, R., Schum, A., Serek, M., Wilkelmann, T. (2008). In vitro regeneration and propagation of Hydrangea macrophylla Thunb. “Nachtigall”. Propagation of Ornamental Plants, 8, 151–153. [Google Scholar]
14. Momoko, I., David, S. F., Yuki, S., Akira, I., Duncan, C. et al. (2019). Molecular mechanisms of plant regeneration. Annual Review of Plant Biology, 70(1), 377–406. DOI 10.1146/annurev-arplant-050718-100434. [Google Scholar] [CrossRef]
15. Hu, W., Fagundez, S., Katin-Grazzini, L., Li, Y. Li, W. et al. (2017). Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Horticulture Research, 4(1), 471. DOI 10.1038/hortres.2017.71. [Google Scholar] [CrossRef]
16. Shukla, P. S., Das, A. K., Jha, B., Agarwal, P. K. (2014). High-frequency in vitro shoot regeneration in cucumis sativus by inhibition of endogenous auxin. In Vitro Cellular Developmental Biology Plant, 50, 729–737. [Google Scholar]
17. Chen, L., Tong, J., Xiao, L., Ruan, Y., Liu, J. et al. (2016). YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. Journal of Experimental Botany, 67(14), 4273–4284. DOI 10.1093/jxb/erw213. [Google Scholar] [CrossRef]
18. Liu, J., Sheng, L., Xu, Y., Li, J., Yang, Z. et al. (2014). WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell, 26(3), 1081–1093. DOI 10.1105/tpc.114.122887. [Google Scholar] [CrossRef]
19. Sheng, L., Hu, X., Duan, Y., Zhang, G., Huang, H. et al. (2017). Non-canonical WOX11-mediated root branching contributes to plasticity in Arabidopsis root system architecture. Company of Biologists, 144(17), 3126–3133. [Google Scholar]
20. Hu, X. M., Xu, L. (2016). Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiology, 172(4), 2363–2373. DOI 10.1104/pp.16.01067. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |