

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.012359
ARTICLE
Ameliorative Role of Pre-Sowing Proline Treatment in Coriandrum sativum L. Seedlings under Mercury Toxicity
1Department of Botany, School of Bioengineering and Biosciences, Lovely Professional University, Phagwara, 144411, India
2Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, 143005, India
3Department of Agriculture, Food and Environment, University of Pisa, Pisa, 56124, Italy
4State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou, 311300, China
*Corresponding Authors: Anket Sharma. Email: anketsharma@gmail.com; Marco Landi. Email: marco.landi@unipi.it
Received: 27 June 2020; Accepted: 30 September 2020
Abstract: Heavy metal toxicity is one of the major ecosystem concerns globally in present time and is also responsible for significant threat to agronomic crops. The current work was conducted to investigate the possible ameliorative role of proline in Coriandrum sativum L. seedlings treated with mercury (Hg). The seedlings were exposed to different concentrations of Hg (0, 0.1, 0.3 and 0.5 mM) for 20 days. The effects of pre-sowing treatment with proline were studied on C. sativum seedlings in terms of pigment (chlorophylls, carotenoids and anthocyanins), malondialdehyde (MDA), antioxidant compound (glutathione, total phenolic compounds, ascorbic acid) and osmolytes (proline, glycine betaine). Additionally, activities of antioxidant enzymes, namely catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX) and dehydroascorbate reductase (DHAR) were also studied. A strong decline of photosynthetic pigment concentrations was observed in leaves of C. sativum under Hg toxicity. Treatment of seeds with proline reduced the loss of photosynthetic pigments, counteract Hg-triggered oxidative stress, likely preserving the functionality of antioxidant apparatus under Hg stress. The increment of total polyphenols and glycine betaine also contributed in ameliorating Hg toxicity, suggesting the use of exogenous proline as a potential method to enhance the plant tolerance against heavy metal stress.
Keywords: Anthocyanins; Hg stress; metal toxicity; polyphenols; proline pre-treatment
Global industrialization has progressively increased the release of various environmental contaminants such as heavy metals, which affect the quality of soil and water, and disturb the ecological balance of the environment compartments [1,2]. Heavy metals enter into our food chain which becomes carcinogenic and display fatal effects on human health [3] as well as in other biological systems, for examples aquatic ecosystems [4]. Environmental enrichment of heavy metals is principally attributable to anthropogenic sources including medical, domestic, agriculture, technological and industrial fields [5]. Though several heavy metals are found in small amount, a slight increase in their concentration can be toxic and hazardous plants [6]. Therefore, these are considered as major pollutants affecting agricultural soils [7].
Reactive oxygen species (ROS) homeostasis is finely modulated in biological systems, including plants and ROS has to be maintained at physiological level to avoid cellular damages due to ROS spread. ROS generation increases dramatically when plants are exposed to different biotic and abiotic stresses [8], including heavy metals. One has also to consider that some heavy metals, such as copper (Cu), nickel (Ni), zinc (Zn), iron (Fe), molybdenum (Mo), and manganese (Mn) are micronutrients and act as cofactor which are crucial for certain enzyme activities and other essential role beneficial to plants [9]. Nevertheless, their excess also seriously impairs plant performance [10]. However, when the metal ions break through the primary defense wall, plants activate other physiological and biochemical strategies aimed at counteracting the hazardous effect of metal ions to plant metabolism [11]. Among them, a powerful antioxidant system, both enzymatic and non-enzymatic compounds represent the first biochemical barrier against environmental cues, including metal-triggered toxicity [12].
Hg is released naturally as well as by human activities, for example by fertilizers, fungicides, manures, agricultural sludge and seed coat dressing which prevent seed from fungal attack [13]. Hg is found in nature in different chemical form, which can be elemental Hg (HgO), inorganic Hg (Hg2+) and organic Hg (CH3-Hg) [14] and the most abundant form in soil is Hg2+ [15]. When Hg is released into the soil, it remains in the solid phase by adsorption on sulphides, organic matters and clay particles [5]. Hg2+ can also be easily accumulated in aquatic plants [16]. The effect of Hg consists in the interruption of the activity of the mitochondria and induction of oxidative stress, which eventually leads to disruption of lipid membrane and metabolism of plant cell [17]. Hg is also known to cause seed injuries by disrupting the stability of the cell membrane leading to reduced viability, affect seed germination, reduced elongation of the primary roots, decrease rate of photosynthesis, transpiration rate, chlorophyll synthesis and water uptake [18].
Proline is an amino acid which is required for primary metabolism and often it increases upon plant exposure to various stresses [19]. The ameliorative role of proline was firstly demonstrated in bacteria against biotic factors, where it demonstrated its osmo-protective function [20]. However, a rise in proline level is also a common response of plants against abiotic stress, as shown in Arabidopsis thaliana exposed to salinity and drought, or in rice varieties in which the level of drought tolerance is related to the proline content in leaves [20]. Therefore, high proline accumulation is beneficial to plants under unfavorable conditions, including edaphic heavy metal accumulation [21]. In condition of metal toxicity, proline can act as a heavy metal chelator, osmo-protectant and ROS quencher, all these roles ameliorating plant performances [22].
Coriandrum sativum L. is an aromatic herb which is considered as an economically important crop, widely used as a spice and appreciated for their medicinal prerogatives [23]. Extracts and essential oils obtained from C. sativum have promising biological activities such as antifungal, antibacterial, and antioxidant properties and also used as flavoring agent, preservatives, pharmaceutical products and in perfumes [24–26]. People consumes coriander plant as a whole, but mostly fresh leaf and dried powdered seeds are preferred however, essential oils are present in all the parts of the plant such as leaf, flower, stem, seeds, though the composition of essential oil in each plant parts are not the same [23].
The current work was designed to investigate the possible ameliorative effects of proline on Hg toxicity in C. sativum plants in order to possibly increase the resilience of this species in condition of Hg excess. Edaphic Hg accumulation was indeed reported in the soil of various regions in Punjab [27] where coriander is largely cultivated.
Seeds of C. sativum L. seeds were purchased from the local market of Hargobind Nagar in Phagwara, Punjab (India). The soil for the experiment was taken from herbal garden, Ayushya Vatika of Lovely Professional University, Punjab (India). The soil was mixed with manure (3:1, soil:manure) and added into 24 pots (2 L each). Seeds were given a pre-sowing treatment of 50 mM proline and soaked over-night. Hg is applied on seeds by dipping them in the Hg solution at distinct concentrations, i.e., at 0.1 mM, 0.3 mM and 0.5 mM. Treatment of Hg was given in the form of mercuric acetate and then exposed to a day/night temperature of 25°C and 21 ± 2.5°C, respectively with a photoperiod of 16 h. Seedlings (4–5 cm in height) were harvested after 20 days of sowing for further analysis.
Membrane damage caused by Hg stress was assessed in terms of malondialdehyde (MDA) content by following the method of Heath et al. [28]. 0.5 g of leaves were extracted in 5 ml of 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 5,000 g for 10 min a 4°C. 1 ml of supernatant was mixed with 6 ml of 20% (w/v) TCA containing 0.5% (w/v) of thio barbituric acid, then this mixture was heated at 95°C for 30 min and immediately cooled on ice. The absorbance of the supernatant was taken at 532 nm. Correction of non-specific absorbance was done by subtraction of absorbance taken at 600 nm.
The total content of chlorophyll was measured using Arnon [29] method. One g of fresh leaves were crushed in mortar and pestle using 3 ml of 80% acetone. Then, the homogenized material was centrifuged at 10,000 g for 20 min at 4°C (Eltek cooling centrifuge, Elektrocraft Pvt. Ltd., India). The supernatant absorbance was collected at 645 nm and 663 nm and pigments were quantified according to Arnon [29] by using an UV-visible, double beam spectrophotometer (Systronics 2202, Systronics India Ltd., Ahmedabad, India).
The total carotenoid content was measured using Maclachlan et al. [30] method. Fresh leaves (1 g) were homogenized in mortar and pestle using 4 ml of 80% acetone. Then, the homogenized material was centrifuged at 10,000 g for 20 min at 4°C. The supernatant’s absorbance was measured at both 480 nm and 510 nm.
Mancinelli [31] method was followed to estimate total anthocyanin content. Fresh leaves (1 g) were homogenized in chilled pestle and mortar with 3 ml of extraction mixture. The extraction mixture was acidified in methanol (methanol:water:HCl, 79:20:1). The crushed material was then centrifuged for 20 minutes at 5,000 g in Eltek cooling centrifuge at a temperature of 4°C. The absorbance of the supernatant was taken at 530 nm and 657 nm.
Proline was estimated by Bates et al. [32] method. 0.5 g of fresh leaves was homogenized in mortar and pestle using sulfosalicylic acid (3% v/v), and centrifuged for 10 min at 10,000 g for 10 min at 4°C. 2 ml of ninhydrin and glacial acetic acid was added to 2 ml of supernatant and the mixture was incubated in a boiling water bath for 1 h. Absorbance was taken at 520 nm. A graph of absorbance vs. concentration was plotted for the standard solutions of L-proline and the amount of proline in the sample was calculated using a linear correlation equation.
Glycine-betaine (GB) content was estimated by following the method of Grieve et al. [33]. Fresh leaves (1 g) were homogenized in mortar and pestle using 10 ml of distilled water. After filtration, 1 ml of 2 M HCl was added in 1 ml of supernatant. Then, 0.5 ml of this mixture were taken, and 0.2 ml of potassium tri-iodide solution was added. After cooling and shaking, 2 ml of chilled distilled water and 20 ml of 1–2 dichloromethane were added to it. Two layers were formed in the mixture. While the tubes were still in the ice bath (4°C) a continuous stream of air was passed for 2 min to mix the layers in the mixture. The upper layer was discarded, and the absorbance of the organic layer was taken at 365 nm. The concentrations of the betaine were calculated against the standard curve.
2.4 Protein Content and Activities of Antioxidative Enzymes
2.4.1 Activities of Antioxidative Enzymes
The determination of Catalase (CAT) activity is done by using method of Aebi [34]. Fresh leaves (1 g) were extracted in 3 ml phosphate buffer and subjected to centrifugation at 7,000 g for 20 min. Cuvette contained phosphate buffer 1.5 ml (50 mM, pH 7.0), hydrogen peroxide 1.2 ml (15 mM) and 300 μl of plant sample and decrease in absorbance was measured at 240 nm.
Superoxide dismutase (SOD) was estimated according to method given by Kono [35]. Fresh leaves (1 g) were extracted in 3 ml sodium carbonate buffer. Cuvette contained 1.3 ml sodium carbonate buffer (50 mM, pH 10.2), 500 μl NBT (24 mM) and 100 μl Triton X-100 (0.03% v/v) was taken. Then 100 μl hydroxylamine hydrochloride was added. After 2 min, 70 μl of plant sample was added. The absorbance was measured at 540 nm.
Ascorbate peroxidase (APX) was estimated by Nakano et al. [36] method. Fresh leaves (1 g) were extracted in 3 ml of phosphate buffer and centrifuged at 7,000 g for 20 min. Cuvette contained 1.5 ml phosphate buffer (50 mM, pH 7.0), 300 µl ascorbate (0.05 mM), 600 µl H2O2 (1 mM) and 600 µl plant sample and reduction in absorbance was noticed at 290 nm.
Dehydroascorbate reductase (DHAR) activity was estimated by Dalton et al. [37] method. Fresh leaves (1 g) were extracted in 3 ml phosphate buffer and separated at 7,000 g for 20 min. Cuvette followed by 50 mM phosphate buffer, 0.1 mM EDTA, 1.5 mM glutathione reduced, 0.2 mM dehydroascorbate and 400 μl plant sample. Absorbance was collected at 265 nm.
2.4.2 Total Protein Determination
Protein content was determined following the method of Lowry et al. [38]. The 0.25 g plant samples was extracted in 3 ml phosphate buffer and then separated at 12,000 g for 10 min. 0.1 ml of the sample and standard were pipetted into a series of test tubes. 1 ml was made up in all test tubes with distilled water. 5 ml of reagent C was added to each tube. Then 0.5 ml of reagent D was added, mixed well and incubated at room temperature in dark for 30 min. Blue color was developed and readings were taken at 660 nm.
2.5 Antioxidant Compounds and Total Phenolic Content
The amount of ascorbic acid (AsA) was calculated using the Roe et al. [39] method. Addition of 0.5 ml of 50% TCA and 100 mg charcoal were done in 0.5 ml of plant extract. After mixing and filtration, 0.4 ml of 2,4-dinitro phenyl hydrazine was added and then mixture was incubated for 3 h at 37°C, and cooled immediately. 1.6 ml of 65% H2SO4 was mixed and kept at room temperature for 30 min. Absorbance was measured at 520 nm.
The content of glutathione (GSH) was determined using the Sedlak et al. [40] method. 1 ml of Tris-HCl buffer, 4 ml of absolute methanol and 50 ml of 5’-dithiobis-2-nitrobenzoic acid were added in 100 ml of plant extract. Incubation of mixture was done for 50 min at room temperature and the absorbance of the supernatant was measured at 412 nm.
Total phenolic content was determined as described by Singleton et al. [41]. 0.4 g of dried plant extract was added to 40 ml of pure ethanol. After filtration and further dilution, 2.5 ml from the plant sample diluted with 25 ml of distilled water. 2 ml of the sample was combined with a 10 ml Folin-Ciocalteau (FC) reagent and 2 ml of 7.5% sodium carbonate after 5 min. The absorbance was taken at 765 nm.
All the experiments were carried out in triplicate. Data is expressed as the mean ± SE of replicated samples. Bartlett’s test was used to test homoscedasticity of data. To test the statistically significant difference between the treatments, one way-analysis of variance (ANOVA) was carried out, followed by Tukey’s test (P = 0.05) using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Hg toxicity increased progressively the level of MDA in C. sativum leaves (Fig. 1A). Proline pre-sowing treatment lead to reduced level of MDA at any dose of Hg and even in control plants when compared to proline-untreated samples. MDA level in Hg alone and in combination with proline pretreated plants increased in a dose dependent manner where highest MDA level was observed at 0.5 mM Hg alone treated plants (1.243 mg g–1 fw).
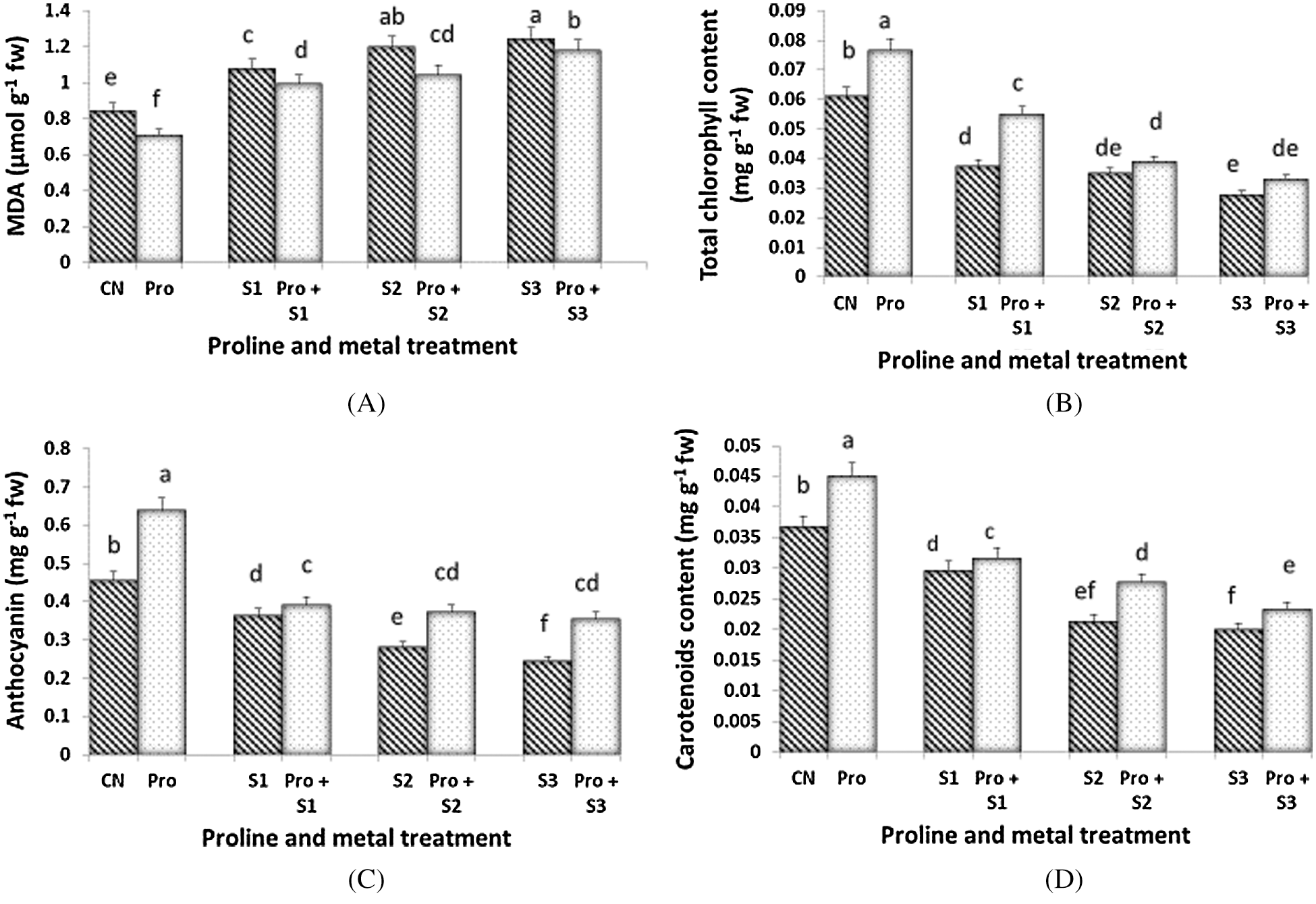
Figure 1: Effect of proline on MDA (A), Total chlorophyll (B), Total anthocyanin (C) and Total carotenoid content (D) of 20 days old seedlings of Coriandrum sativum under Hg stress (CN = Control, Pro = Proline, S1 = 0.1 mM Hg, S2 = 0.3 mM Hg, S3 = 0.5 mM Hg); fw = fresh weight
Chlorophyll content declined following the severity of Hg stress and the lowest concentration was observed at 0.5 mM Hg (0.028 mg g–1 fw) (Fig. 1B). Proline treatment reduced the loss of total chlorophylls, especially at the lowest dose of 0.1 mM Hg (0.054 mg g–1 fw). Maximum chlorophyll content (0.076 mg g–1 fw) was observed in proline alone pretreated plants followed by proline untreated control plants (0.061 mg g–1 fw). Maximum anthocyanin content was found in the seedlings treated with proline alone (0.639 mg g–1 fw; Fig. 1C). As similar as per chlorophylls, the anthocyanin content declined along with Hg severity and reached the lowest value in seedlings treated with 0.5 mM Hg (0.243 mg g–1 fw). When proline was utilized in pre-sowing treatment, C. sativum leaves maintained a higher level of anthocyanins with respect to their relative Hg-treated counterparts. The proline treatment was however not affected by Hg doses and either 0.1, 0.3, or 0.5 mM Hg-treated plants exhibited the similar levels of total anthocyanins. The concentration of total carotenoids was reduced by Hg imposition and the most severe doses of Hg (0.3 and 0.5 mM) induced a similar loss of total carotenoids (Fig. 1D). Proline treatment ameliorate the decline observed in total carotenoid levels. Content of total carotenoids in Hg alone treated plants decreased with increasing the concentration of Hg.
Glycine betaine (GB) content increased with increasing the Hg level and it was found to be more increased when Hg treatment was applied in proline-pretreated plants (Fig. 2A). Therefore, the maximum content was found to be 8.7 mg g–1 fw when proline-pretreated seedlings were given 0.5 mM Hg. Treatment with proline significantly improved the GB content to ameliorate the toxicity induced by Hg in C. sativum. Proline content increased with Hg application (except for 0.3 mM Hg; Fig. 2B). Proline-pretreated plants showed higher levels of proline than untreated counterparts. When the seedlings were treated with the lowest metal concentration (0.1 mM Hg), the proline content was at its maximum (6.602 mg g–1 fw). Lowest proline content among Hg alone treated and in Hg plus proline treated plants was 5.247 mg g–1 fw and 5.652 mg g–1 fw respectively, at 0.3 mM Hg.
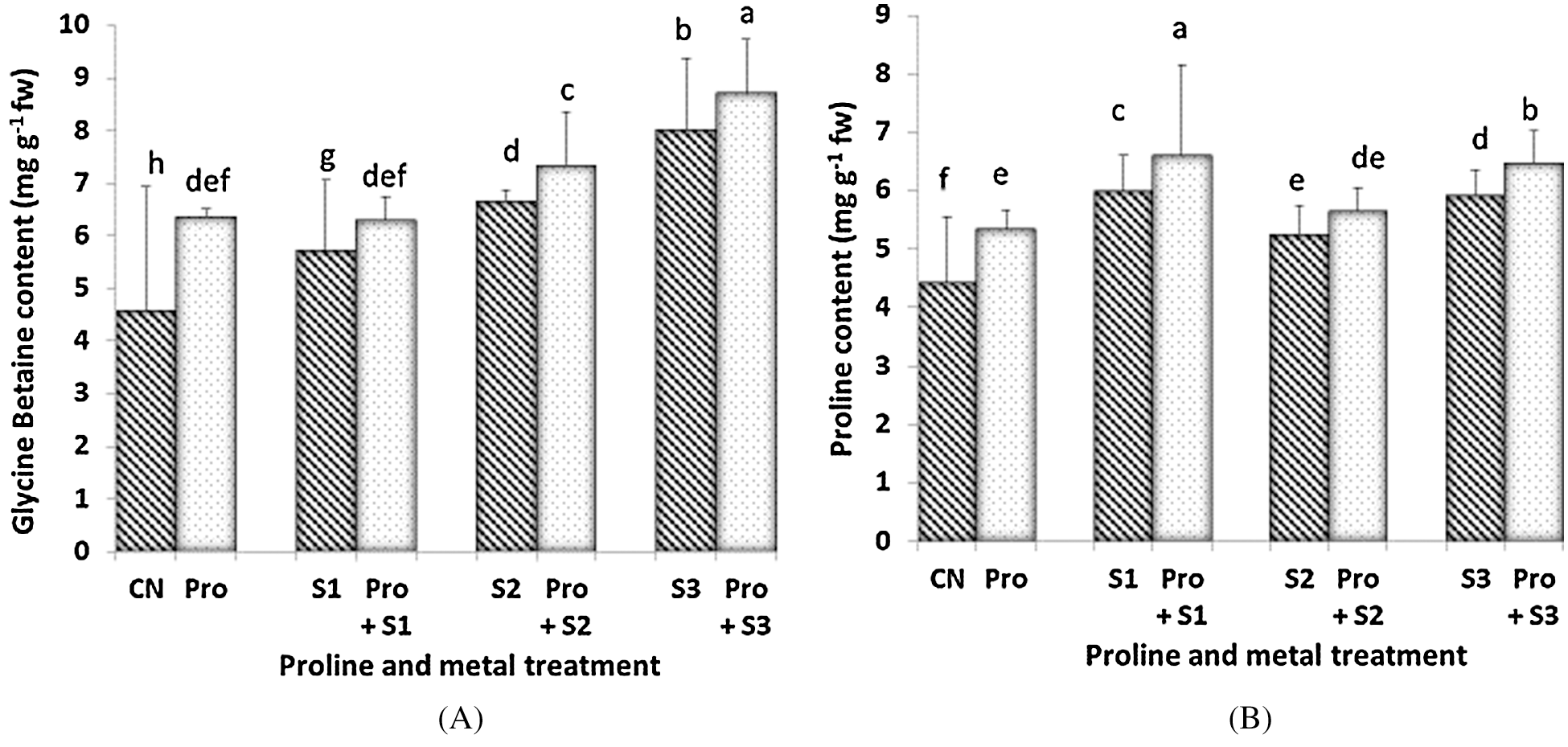
Figure 2: Effect of proline on Glycine Betaine (A) and endogenous Proline content (B) of 20 days old seedlings of Coriandrum sativum under Hg stress (CN = Control, Pro = Proline, S1 = 0.1 mM Hg, S2 = 0.3 mM Hg, S3 = 0.5 mM Hg); fw = fresh weight
3.4 Activities of Antioxidative Enzymes
The activity of CAT was stimulated by Hg stress in a dose-dependent manner (Fig. 3A). The activity of CAT increased in control plants treated with proline, whereas CAT activity remained unchanged in both Hg-treated and Hg-treated + proline plants at each dose of Hg. Maximum (6.628 µmol UA mg protein–1) and minimum (2.177 µmol UA mg protein–1) CAT activity was observed at 0.5 mM Hg and in untreated control plants respectively. The enzymatic activity of SOD was highest when the seedlings were treated with proline alone (0.59 µmol UA mg protein–1; Fig. 3B). Under Hg stress, the activity of SOD was found to decrease as compared to control plants. However, the SOD activity decreased to a lower extent when the seedlings were pretreated with proline, at each Hg dose. Hg imposition induced a general increment of DHAR activity (Fig. 3C). The Hg stressed seedlings pretreated with proline showed higher level of DHAR activity than the untreated ones. The highest value of DHAR activity was found in 0.1 mM Hg treated plants (0.054 µmol UA mg protein–1) subjected to proline pre-sowing treatment. DHAR activity was decreased with increasing the concentration of Hg. The activity of APX was generally inhibited by Hg stress (Fig. 3D). Maximum APX activity (0.487 µmol UA mg protein–1) was measured in seedlings pretreated with proline alone. Pretreatment with proline only preserve APX activity to be inhibited at 0.3 mM Hg. APX activity decreased as concentration of Hg increased in both Hg alone and in Hg + proline treated plants being lowest at 0.5 mM Hg concentration.
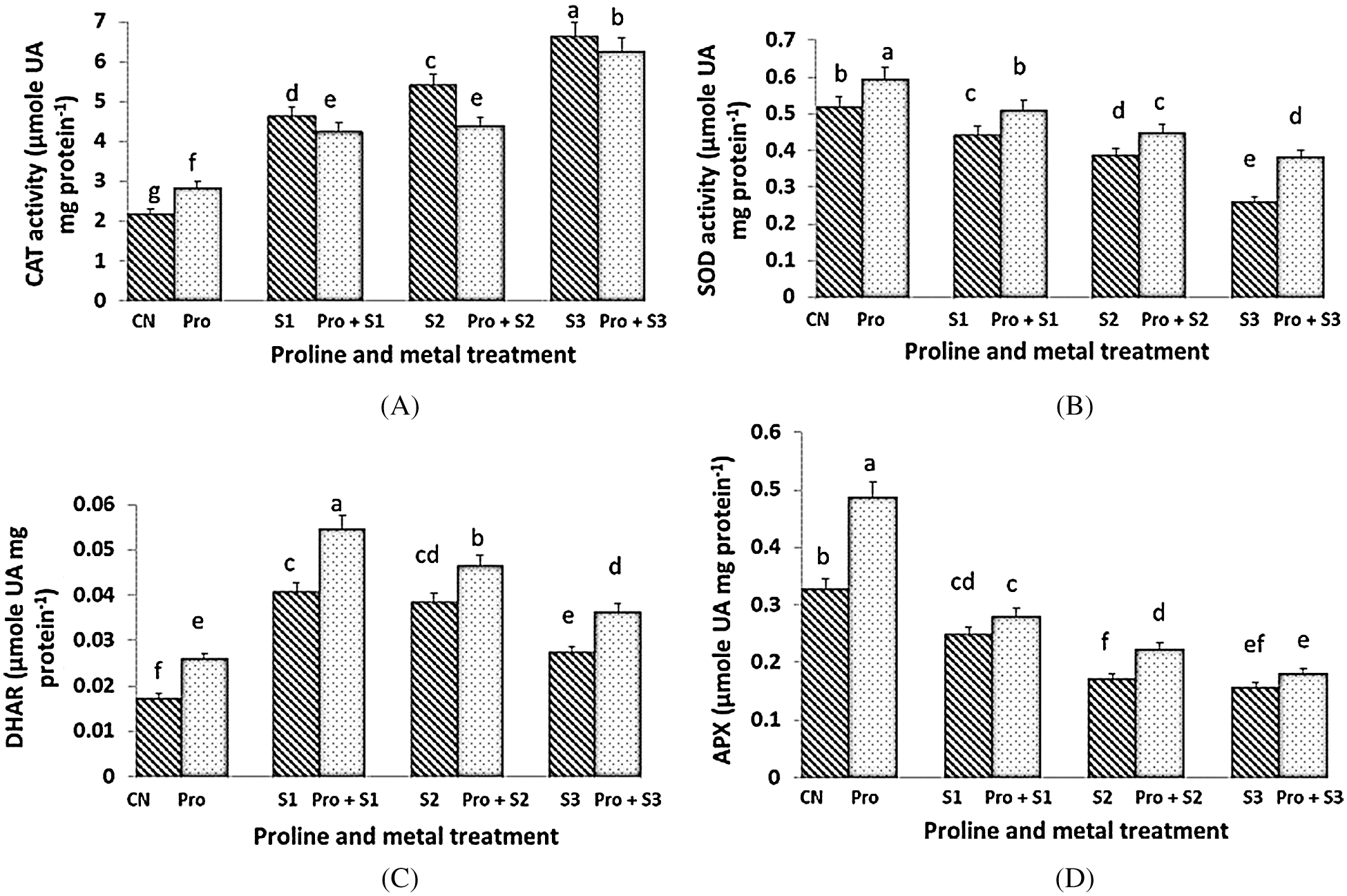
Figure 3: Effect of proline on activities of Catalase (CAT) (A), Superoxide Dismutase (SOD) (B), Dehydroascorbate Reductase (DHAR) (C) and Ascorbate Peroxidase (APX) (D) enzymes of 20 days old seedlings of Coriandrum sativum under Hg stress (CN = Control, Pro = Proline, S1 = 0.1 mM Hg, S2 = 0.3 mM Hg, S3 = 0.5 mM Hg); fw = fresh weight
3.5 Antioxidant Compounds, Total Phenolic and Protein Content
GSH content was found to be maximum (5.985 mg g–1 fw) at proline alone treated plants (Fig. 4A). Hg stress (0.1 and 0.3 mM) induced a decline of GSH levels, but when 0.5 mM Hg were given, an increment of this compound was observed. Proline only induced an increment of GSH in 0.5 mM Hg treated plants. The AsA content was more depressed by the highest dose of Hg (Fig. 4B). Proline pre-sowing treatment enhanced the level of AsA only in Hg-untreated plants and in seedlings subjected to the highest dose of Hg. Maximum AsA content was found in proline alone treated plants (7.252 mg g–1 fw) and minimum was at 0.5 mM Hg (3.582 mg g–1 fw). The highest phenolic content (6.5 mg g–1 fw; Fig. 4C) was measured when the seedlings were treated with proline alone. When the seedlings were exposed to Hg, the phenolic content decreased in a dose-dependent manner. The lowest phenolic content (0.31 mg g–1 fw) was found when seedlings were treated with 0.5 mM Hg. Proline pre-treated seedlings showed higher values of total polyphenols when compared to the untreated counterparts at each Hg level. Protein content was affected by Hg and both the highest two Hg doses induced similar decline of this parameter (Fig. 4D). Proline treatment enhanced the level of protein in proline alone treated plants (which accounted for the highest level of protein content 5.016 mg g–1 fw) as well as in Hg-treated ones. Protein content decreased both in Hg alone and in with proline treated plants as the concentration of Hg increased.
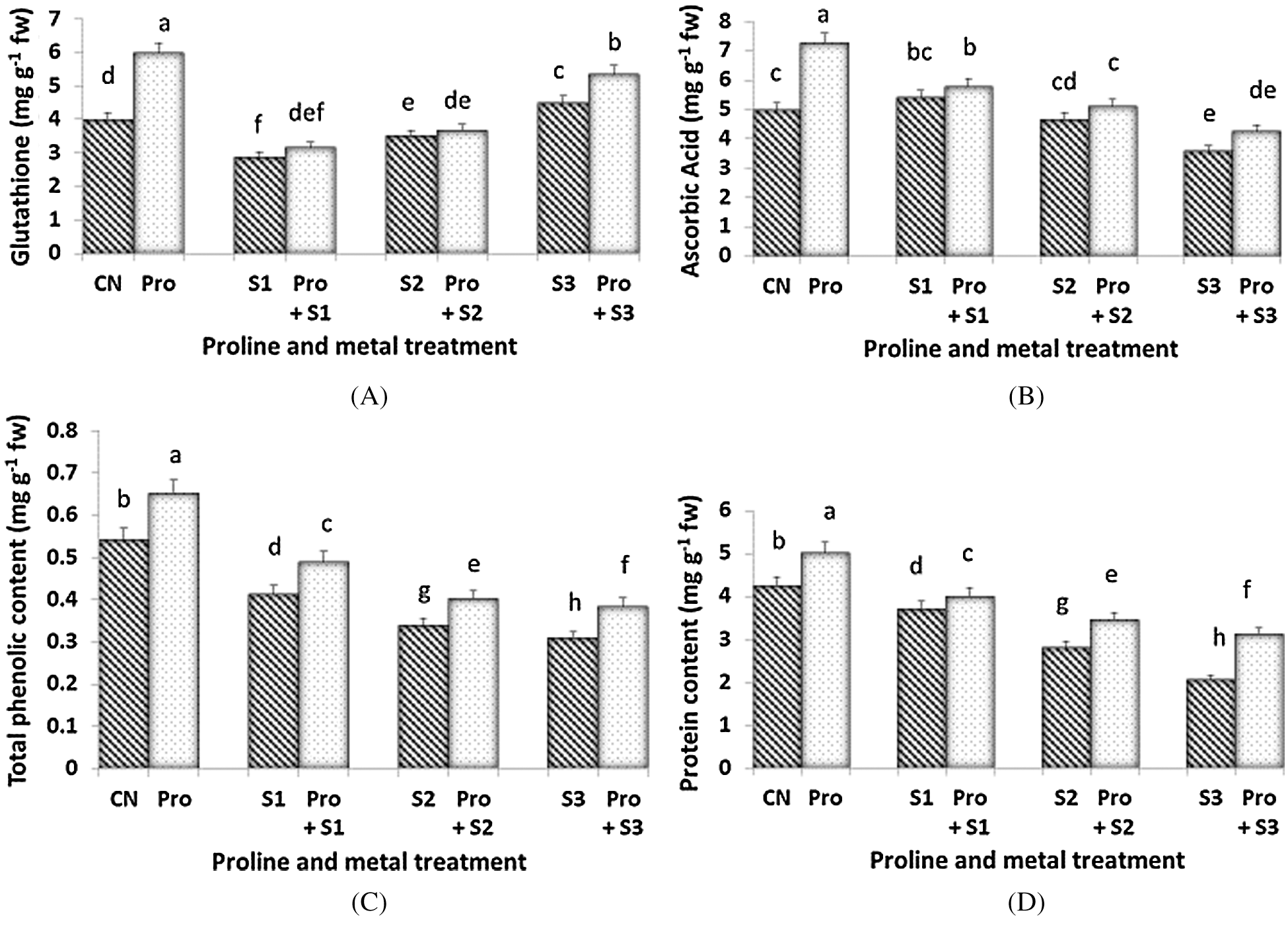
Figure 4: Effect of proline on Glutathione (A), Ascorbic Acid (B), Total phenolic (C) and Protein content (D) of 20 days old seedlings of Coriandrum sativum under Hg stress (CN = Control, Pro = Proline, S1 = 0.1 mM Hg, S2 = 0.3 mM Hg, S3 = 0.5 mM Hg); fw = fresh weight
The existing work was set to study the role and activity of proline in ameliorating the stress condition caused by the toxicity of Hg in C. sativum L. plants. The plant belongs to Umbelliferae family and is commonly called as coriander or Dhania. The plant has high economic value and has many pharmaceutical values. They are aromatic plants and mostly used as spice and flavoring agent [42].
In the present study, MDA level, which is a marker for heavy metal stress being the end product of lipid peroxidation [43], decreased when the Hg-treated coriander plants were pretreated with 10 mM proline. This indicates that proline pre-sowing treatment reduced the oxidative stress in seedlings of C. sativum. Reduced oxidative stress may be due to the antioxidant properties of proline which are able to enhance the scavenging of reactive oxygen species [44,45].
Compared with control seedlings, when treated with proline the photosynthetic pigment content in C. sativum plants were greatly enhanced, highlighting a positive role exerted by proline. The pigment level was decreased when the seedlings were subjected to Hg stress and the lowest concentration was observed when Hg was given at a concentration of 0.5 mM. But when the Hg-treated seedlings were pretreated with proline, the Hg impact to photosynthetic pigments was remarkably lower. This might be attributable to the positive role exerted by proline in stabilizing the cell membrane and mitochondrial electron transport and defend the plant during stress [46]. Moreover, proline can help to protect the photosynthetic apparatus in plants under abiotic stress [47,48].
In the present study, proline pretreatment increased the amount of GB in comparison to alone Hg treated plants. Glycine betaine is an important osmoprotectant and has antioxidant properties, which can be helpful in amelioration of heavy metal toxicity [49]. Similarly, the protein content was at its maximum when the seedlings were treated with proline alone than control seedlings. Protein content was found to decrease when exposed to Hg treatment but when they are treated in combination with Hg and proline, the protein content was enhanced. Similar results were found in Cicer arietinum where application of proline significantly increased the protein content under Cd stress [50]. Besides, antioxidant enzyme activity (SOD, APX, CAT, and DHAR) was less impaired when the Hg-stressed seedlings were treated with proline. It has long been known that antioxidant enzymes (Asada-Halliwell pathway) play a crucial role in scavenging and maintaining ROS homeostasis in condition of abiotic stress including heavy metals [51]. Proline application alleviated the harmful effects of heavy metals by decreasing the oxidative damage triggered via improving the antioxidative enzymes activities [52,53].
Levels of antioxidant compounds, including GSH, AsA, and polyphenols were also stimulated by proline application. These non-enzymatic antioxidant molecules are very important for plants to counter negative impacts of abiotic stresses [54] which further support the outcomes of our studies. In our study, proline pretreatment improved the content of phenolic under Hg stress in C. sativum plants. Phenolics are very well known to protect plants from abiotic stresses including heavy metals [55]. Application of proline alleviated the Cu toxicity in wheat plants by declining the overaccumulation of ROS and increasing the accumulation of endogenous proline and protein content [56]. Our dataset highlights an ameliorative role exerted by proline pre-treatment in Hg-treated seedlings in C. sativum. Principally, proline exerts a positive effect in maintaining the level of photosynthetic pigments which are essential for a proper functioning of the photosynthetic apparatus. Proline also preserve the activity of antioxidant enzymes (e.g., SOD activity) and in some cases promote (e.g., DHAR, CAT) their activity, thereby helping in counteracting Hg-trigged ROS production. As a final evidence, the lower level of MDA, which testimony the capability of proline-pretreated plants to counteract better the Hg-promoted oxidative stress.
Advances in industrialization and the use of modern technologies have increased the release into the world of various heavy metals. Heavy metals, including Hg, represent a hazardous factor for plants when the concentration of these elements exceed plant detoxifying capacity. Results of the present experiment highlight that proline pre-treatment ameliorates the performance of Coriandrum sativum plants when subsequently subjected to Hg stress. We offer the evidence that proline help in maintaining the functionality of antioxidant apparatus, thereby reducing the level of oxidative stress in plant tissue. As an applicative result, proline application in pre-sowing represent a useful tool to enhance the performance of plants of C. sativum when those plants grown in Hg-contaminated soil. Further studies are however necessary to understand the fate of Hg in plant tissue, this in order to understand whether or not proline pre-sowing also result in lowering the accumulation of Hg in plant tissue, which would result a further benefit exerted by this amino acid.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Verma, N., Sharma, R. (2017). Bioremediation of toxic heavy metals: A patent review. Recent Patents on Biotechnology, 11(3), 171–187. DOI 10.2174/1872208311666170111111631. [Google Scholar] [CrossRef]
2. Kumar, V., Parihar, R. D., Sharma, A., Bakshi, P., Sidhu, G. P. S. et al. (2019). Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere, 236, 124364. DOI 10.1016/j.chemosphere.2019.124364. [Google Scholar] [CrossRef]
3. Saraswat, S. (2014). Patent analysis on bioremediation of environmental pollutants. Journal of Bioremediation and Biodegradation, 5(6), 1–6. DOI 10.4172/2155-6199.1000251. [Google Scholar] [CrossRef]
4. Gawali Ashruta, A., Nanoty, V., Bhalekar, U. (2014). Biosorption of heavy metals from aqueous solution using bacterial EPS. International Journal Life Sciences, 2, 373–377. [Google Scholar]
5. Verma, N., Kaur, G. (2016). Trends on biosensing systems for heavy metal detection. Comprehensive Analytical Chemistry. Amsterdam: Elsevier, pp. 33–71. [Google Scholar]
6. Olaniran, A. O., Balgobind, A., Pillay, B. (2013). Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. International Journal of Molecular Sciences, 14(5), 10197–10228. DOI 10.3390/ijms140510197. [Google Scholar] [CrossRef]
7. Kumar, V., Sharma, A., Kaur, P., Sidhu, G. P. S., Bali, A. S. et al. (2019). Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere, 216, 449–462. DOI 10.1016/j.chemosphere.2018.10.066. [Google Scholar] [CrossRef]
8. Hasanuzzaman, M., Bhuyan, M. H. M., Zulfiqar, F., Raza, A., Mohsin, S. M. et al. (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants, 9(8), 681. DOI 10.3390/antiox9080681. [Google Scholar] [CrossRef]
9. Sanghera, G. S., Kashyap, P. L., Singh, G., da Silva, J. A. T. (2011). Transgenics: Fast track to plant stress amelioration. Transgenic Plant Journal, 5(1), 1–26. [Google Scholar]
10. Abbas, G., Murtaza, B., Bibi, I., Shahid, M., Niazi, N. et al. (2018). Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. International Journal of Environmental Research and Public Health, 15(1), 59. DOI 10.3390/ijerph15010059. [Google Scholar] [CrossRef]
11. Sharma, S. S., Dietz, K. J. (2006). The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. Journal of Experimental Botany, 57(4), 711–726. DOI 10.1093/jxb/erj073. [Google Scholar] [CrossRef]
12. Sharma, A., Sidhu, G. P. S., Araniti, F., Bali, A. S., Shahzad, B. et al. (2020). The role of salicylic acid in plants exposed to heavy metals. Molecules, 25(3), 540. DOI 10.3390/molecules25030540. [Google Scholar] [CrossRef]
13. Patra, M., Sharma, A. (2000). Mercury toxicity in plants. Botanical Review, 66(3), 379–422. DOI 10.1007/BF02868923. [Google Scholar] [CrossRef]
14. Nagajyoti, P. C., Lee, K. D., Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: A review. Environmental Chemistry Letters, 8(3), 199–216. DOI 10.1007/s10311-010-0297-8. [Google Scholar] [CrossRef]
15. Han, S., Zhou, X., Tang, Y., He, M., Zhang, X. et al. (2016). Practical, highly sensitive, and regenerable evanescent-wave biosensor for detection of Hg2+ and Pb2+ in water. Biosensors and Bioelectronics, 80, 265–272. DOI 10.1016/j.bios.2016.01.070. [Google Scholar] [CrossRef]
16. Zhou, Z. S., Guo, K., Elbaz, A. A., Yang, Z. M. (2009). Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environmental and Experimental Botany, 65(1), 27–34. DOI 10.1016/j.envexpbot.2008.06.001. [Google Scholar] [CrossRef]
17. Cargnelutti, D., Tabaldi, L. A., Spanevello, R. M., de Oliveira Jucoski, G. Battisti, V. et al. (2006). Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere, 65(6), 999–1006. DOI 10.1016/j.chemosphere.2006.03.037. [Google Scholar] [CrossRef]
18. Chen, C. W., Chen, C. F., Dong, C. D. (2012). Distribution and accumulation of mercury in sediments of Kaohsiung River Mouth, Taiwan. APCBEE Procedia, 1, 153–158. DOI 10.1016/j.apcbee.2012.03.025. [Google Scholar] [CrossRef]
19. Sharma, A., Shahzad, B., Kumar, V., Kohli, S. K., Sidhu, G. P. S. et al. (2019). Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules, 9(7), 285. DOI 10.3390/biom9070285. [Google Scholar] [CrossRef]
20. Szabados, L., Savoure, A. (2010). Proline: A multifunctional amino acid. Trends in Plant Science, 15(2), 89–97. DOI 10.1016/j.tplants.2009.11.009. [Google Scholar] [CrossRef]
21. Aslam, M., Saeed, M. S., Sattar, S., Sajad, S., Sajjad, M. et al. (2017). Specific role of proline against heavy metals toxicity in plants. International Journal of Pure Applied Biosciences, 5(6), 27–34. DOI 10.18782/2320-7051.6032. [Google Scholar] [CrossRef]
22. Handa, N., Kohli, S. K., Sharma, A., Thukral, A. K., Bhardwaj, R. et al. (2018). Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. South African Journal of Botany, 119, 1–10. DOI 10.1016/j.sajb.2018.08.003. [Google Scholar] [CrossRef]
23. Mandal, S., Mandal, M. (2015). Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine, 5(6), 421–428. DOI 10.1016/j.apjtb.2015.04.001. [Google Scholar] [CrossRef]
24. Sahib, N. G., Anwar, F., Gilani, A. H., Hamid, A. A., Saari, N. et al. (2013). Coriander (Coriandrum sativum L.A potential source of high-value components for functional foods and nutraceuticals—A review. Phytotherapy Research, 27(10), 1439–1456. DOI 10.1002/ptr.4897. [Google Scholar] [CrossRef]
25. Al-Snafi, A. E. (2016). A review on chemical constituents and pharmacological activities of Coriandrum sativum. IOSR Journal of Pharmacy, 6(7), 17–42. [Google Scholar]
26. Saxena, S. N., Rathore, S. S., Saxena, R., Kakani, R. K. (2013). Physiological parameters and their relation to seed yield in coriander (Coriandrum sativum L.). International Journal of Seed Spices, 3(2), 85–88. [Google Scholar]
27. Rajendiran, S., Dotaniya, M. L., Coumar, M. V., Panwar, N. R., Saha, J. K. (2015). Heavy metal polluted soils in India: Status and counter measures. JNKVV Research Journal, 49(3), 320–337. [Google Scholar]
28. Heath, R. L., Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198. DOI 10.1016/0003-9861(68)90654-1. [Google Scholar] [CrossRef]
29. Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), 1–15. DOI 10.1104/pp.24.1.1. [Google Scholar] [CrossRef]
30. Maclachlan, S., Zalik, S. (1963). Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Canadian Journal of Botany, 41(7), 1053–1062. DOI 10.1139/b63-088. [Google Scholar] [CrossRef]
31. Mancinelli, A. L. (1984). Photoregulation of anthocyanin synthesis: Effect of light pretreatments. Plant Physiology, 75(2), 447–453. DOI 10.1104/pp.75.2.447. [Google Scholar] [CrossRef]
32. Bates, L. S., Waldren, R. P., Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
33. Grieve, C. M., Grattan, S. R. (1983). Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant and Soil, 70(2), 303–307. DOI 10.1007/BF02374789. [Google Scholar] [CrossRef]
34. Aebi, H. (1983). Catalase. In: Bergmeyer, H. U. (ed.) Methods of enzymatic analysis. New York and London: Academic Press, pp. 673–684. [Google Scholar]
35. Kono, Y. (1978). Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Archives of Biochemistry and Biophysics, 186(1), 189–195. DOI 10.1016/0003-9861(78)90479-4. [Google Scholar] [CrossRef]
36. Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22(5), 867–880. [Google Scholar]
37. Dalton, D. A., Russell, S. A., Hanus, F. J., Pascoe, G. A., Evans, H. J. (1986). Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proceedings of the National Academy of Sciences, 83(11), 3811–3815. DOI 10.1073/pnas.83.11.3811. [Google Scholar] [CrossRef]
38. Lowry, O. H. (1951). Measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275. [Google Scholar]
39. Roe, J. H., Kuether, C. A. (1943). The determination of ascorbic acid in whole blood and urine through the 2, 4-dinitrophenylhydrazine derivative of dehydroascorbic acid. Journal of Biological Chemistry, 147, 399–407. [Google Scholar]
40. Sedlak, J., Lindsay, R. H. (1968). Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochemistry, 25, 192–205. DOI 10.1016/0003-2697(68)90092-4. [Google Scholar] [CrossRef]
41. Singleton, V. L., Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phospho tungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158. [Google Scholar]
42. Shashidhar, M. D., Ravi, P., Sharatbabu, A. G., Geeta, B. P., Arif, A. et al. (2017). Cultivation of coriander (Coriandrum sativum L.A review article. Indian Journal of Pure & Applied Biosciences, 5, 796–802. [Google Scholar]
43. Singh, R., Kesavan, A. K., Landi, M., Kaur, S., Thakur, S. et al. (2020). 5-aminolevulinic acid regulates Krebs cycle, antioxidative system and gene expression in Brassica juncea L. to confer tolerance against lead toxicity. Journal of Biotechnology, 323, 283–292. DOI 10.1016/j.jbiotec.2020.09.004. [Google Scholar] [CrossRef]
44. Yadav, P., Kaur, R., Kanwar, M. K., Sharma, A., Verma, V. et al. (2018). Castasterone confers copper stress tolerance by regulating antioxidant enzyme responses, antioxidants, and amino acid balance in B. juncea seedlings. Ecotoxicology and Environmental Safety, 147, 725–734. DOI 10.1016/j.ecoenv.2017.09.035. [Google Scholar] [CrossRef]
45. Wang, J., Chen, J., Sharma, A., Tao, S., Zheng, B. et al. (2019). Melatonin stimulates activities and expression level of antioxidant enzymes and preserves functionality of photosynthetic apparatus in hickory plants (Carya cathayensis Sarg.) under peg-promoted drought. Agronomy, 9(11), 702. DOI 10.3390/agronomy9110702. [Google Scholar] [CrossRef]
46. Ben Ahmed, C., Ben Rouina, B., Sensoy, S., Boukhriss, M., Ben Abdullah, F. (2010). Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. Journal of Agricultural and Food Chemistry, 58(7), 4216–4222. DOI 10.1021/jf9041479. [Google Scholar] [CrossRef]
47. Tonhati, R., Mello, S. C., Momesso, P., Pedroso, R. M. (2020). L-proline alleviates heat stress of tomato plants grown under protected environment. Scientia Horticulturae, 268, 109370. DOI 10.1016/j.scienta.2020.109370. [Google Scholar] [CrossRef]
48. Altuntaş, C., Demiralay, M., Muslu, A. S., Terzi, R. (2020). Proline-stimulated signaling primarily targets the chlorophyll degradation pathway and photosynthesis associated processes to cope with short-term water deficit in maize. Photosynthesis Research, 144(1), 35–48. DOI 10.1007/s11120-020-00727-w. [Google Scholar] [CrossRef]
49. Ali, S., Abbas, Z., Seleiman, M. F., Rizwan, M., YavaŞ, İ. et al. (2020). Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants, 9(7), 896. DOI 10.3390/plants9070896. [Google Scholar] [CrossRef]
50. Hayat, S., Hayat, Q., Alyemeni, M. N., Ahmad, A. (2013). Proline enhances antioxidative enzyme activity, photosynthesis and yield of Cicer arietinum L. exposed to cadmium stress. Acta Botanica Croatica, 72(2), 323–335. DOI 10.2478/v10184-012-0019-3. [Google Scholar] [CrossRef]
51. Mittler, R. (2017). ROS are good. Trends in Plant Science, 22(1), 11–19. DOI 10.1016/j.tplants.2016.08.002. [Google Scholar] [CrossRef]
52. Zouari, M., Ahmed, C. B., Zorrig, W., Elloumi, N., Rabhi, M. et al. (2016). Exogenous proline mediates alleviation of cadmium stress by promoting photosynthetic activity, water status and antioxidative enzymes activities of young date palm (Phoenix dactylifera L.). Ecotoxicology and Environmental Safety, 128, 100–108. DOI 10.1016/j.ecoenv.2016.02.015. [Google Scholar] [CrossRef]
53. Yaqoob, H., Akram, N. A., Iftikhar, S., Ashraf, M., Khalid, N. et al. (2019). Seed pretreatment and foliar application of proline regulate morphological, physio-biochemical processes and activity of antioxidant enzymes in plants of two cultivars of quinoa (Chenopodium quinoa Willd.). Plants, 8(12), 588. DOI 10.3390/plants8120588. [Google Scholar] [CrossRef]
54. Sharma, A., Kumar, V., Thukral, A. K., Bhardwaj, R. (2016). Epibrassinolide-imidacloprid interaction enhances non-enzymatic antioxidants in Brassica juncea L. Indian Journal of Plant Physiology, 21(1), 70–75. DOI 10.1007/s40502-016-0203-x. [Google Scholar] [CrossRef]
55. Sharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M. et al. (2019). Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules, 24(13), 2452. DOI 10.3390/molecules24132452. [Google Scholar] [CrossRef]
56. Noreen, S., Akhter, M. S., Yaamin, T., Arfan, M. (2018). The ameliorative effects of exogenously applied proline on physiological and biochemical parameters of wheat (Triticum aestivum L.) crop under copper stress condition. Journal of Plant Interactions, 13(1), 221–230. DOI 10.1080/17429145.2018.1437480. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |