

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014376
ARTICLE
Alleviation of Cadmium Toxicity to Medicago Truncatula by AMF Involves the Changes of Cd Speciation in Rhizosphere Soil and Subcellular Distribution
1School of Life Science and Engineering, Southwest University of Science and Technology, Mianyang, 621010, China
2State Key Laboratory of Environmentally Friendly Energy Materials, National Collaborative Innovation Center for Nuclear Waste and Environmental Safety, School of National Defence, Sichuan Civil-Military Integration Institute, Southwest University of Science and Technology, Mianyang, 621010, China
*Corresponding Authors: Ke Chen. Email: Michael_chenke@163.com; Wenkun Zhu. Email: zhuwenkun@swust.edu.cn
Received: 22 September 2020; Accepted: 27 October 2020
#These authors contributed equally
Abstract: Arbuscular mycorrhizal fungi (AMF) can improve plant tolerance to several abiotic stresses, including heavy metals, drought or salinity exposure. However, the role of AMF in alleviation of soil cadmium (Cd)-induced toxicity to plants is still largely unknown. In this study, Cd speciation in soil and subcellular distribution of Cd were used to characterize the roles of application AM fungi in the alleviation of Cd toxicity in alfalfa plants. Our results showed that the addition of Glomus mosseae in Cd contaminated soil (10 mg/Kg) significantly increased soil pH, cation exchange capacity (CEC) and organic matter in rhizosphere soil with Medicago truncatula L., and then account for significantly decreased contents of exchangeable and carbonate-bounded Cd speciation in rhizosphere soil, indicating alleviation of plant toxicity by reduction of bioavailable fractions of Cd. Although there is no significant difference found in Cd accumulation by roots and shoots respectively between Cd and AM-Cd treatments, more portion of Cd was recorded compartmentalization in cell wall fraction of both root and shoot in treatment of Cd with AM application, indicating alleviation of Cd toxicity to plant cell. Herein, application of AM fungi in Cd treatments performed to inhibit the appearance of Cd toxicity symptoms, including the improvement of leaf electrolyte leakage, root elongation, seedling growth and biomass. This information provides a clearer understanding of detoxification strategy of AM fungi on Cd behavior with development and stabilization of soil structure and subcellular distribution of plant.
Keywords: AM fungi; cadmium toxicity; Cd form; subcellular compartmentalization; phytoremediation
In consideration of a closely combination on public food safety issues, agricultural soil contaminated with cadmium is of globally serious ecological concern [1–3]. Among the global soil resources, the cadmium content in France is the highest (16.7 mg/Kg), followed by Belgium (7.61 mg/Kg) and China (7.43 mg/Kg). In China, Cd over-standard rate of paddy field was 33.2% and low-level pollution accounted for 74.1% [4]. Currently, to address this widespread crisis on soil ecological environment, many researchers have figured out various combined phytoremediation approaches (such as chemic-biological, microbial-associated and etc.), as sustainable and environment-friendly remedy technologies, and made great progress on the limitations like small biomass and low accumulation of traditional single phytoremediation [5–8]. Although these combined phytoremediation technologies have contributed to the imitations on biomass, metal toxicity and effective removal rate of traditional remediation approaches, a further comprehension of the mechanisms in these methods is essential to overcoming the remaining limitations.
Arbuscular mycorrhizal symbioses have been widely utilized into phytoremediation-associated with the contribution to plant stability and development as well as improvement of soil environment status [9]. The investigations of the application and mechanisms of AM fungi on heavy metal pollution were increasingly and widely conducted in past decade due to a ubiquitous occurrence on more than 80% land plant species with AM fungi in ecosystems and contribution to plant development [10,11]. The remediation mechanisms by AM fungi mainly focused on physio-biochemical variations in fungi (containing colonization rate, spore density, some specific secretions and induced gene expression), plant (growth and development, bio-physiological responses, related gene expression, metal distribution and accumulation) and soil (residual metal and changes on form) [12,13]. There are two universally accepted putative detoxification mechanisms of AM fungi recognized by most researchers: avoidance and compartmentalization strategies. Avoidance mechanism principally includes metabolic binding, immobilization in hype and extra-radical mycelium, chelation of specific secretion to immobilize metal in soil. Besides, compartmentalization mechanism mainly devotes on adsorption and segregation in non-essential organelles, chelate production with organic acids, amino acids, phytochelatins (PCs) and metallothioneins (MTs) to weaken the toxicity on the symbiotic plants [14–16]. However, the detoxification mechanism of AM fungi in polluted soil ecologically remediation still needs to be expounded.
Generally, a low-concentration heavy metal contaminated-soil was widespread occupied among the deleterious heavy metal pollution with varying degrees in global, while the toxicity of the low-level Cd elements largely depends on its speciation in soils. Heavy metal speciation determines its toxicity via bio-mobility and chemical complexation performing in the accumulation, bio-modification and compartmentalization inside the organisms [17]. According to Tessier et al. [18], the Cd speciation was divided into five forms: exchangeable, carbonate-bounded, iron and manganese oxides-bounded, organic-bounded and residual forms. Among them the exchangeable and carbonate speciation closely related to soil pH and cation exchange capacity are regarded as the most deleterious forms due to their larger specific surface area with prominent capacity and easily absorbed both by plants and soil components [19]. Additionally, the organic and oxidized forms are relatively stable and low-toxicity with close correlation of soil organic matters and carbon, because their powerful combination performance of soil organic matters. Residual form is considered as the most stable in soils with few poison since united into secondary mineral and not easily assimilated by plants [19–21]. Besides, heavy metal speciation closely related to their bioavailability is complexly influenced by a large range of characteristics of the soil matrix such as hydrous oxides, clay minerals, organic matter and some physio-chemical conditions, such as pH and cation exchange capacity [22,23]. As far as we concerned, few articles have determined the relationship between phytoremediation combined with AM fungi and Cd speciation. Meanwhile, the information on uptake and tolerance of remediation plant species with AM symbiosis affected by heavy metals speciation and their content in soils is less known. There have been a lot of data expounded the Cd remission mechanisms with AM fungi-combined phytoremediation currently, but a deeper comprehension of AM fungi symbiosis between Cd behavior and soil characteristics as well as its effect on plant cell distribution is still far off at low-level heavy metal stresses. This work could contribute to a small gap between remediation principle and heavy metal behavior to predict a reliable detoxification for AM fungi heavy metal restoration application, therefore be regarded as work for further comprehension on the AM fungi symbiotic function in relationship of variation on Cd behavior and soil properties.
Five representative Cd forms contents and a series of soil basic parameters (Organic matter, pH and cation exchange capacity) were used to illustrate the relation between rhizosphere soil and Cd forms with AM fungi symbiosis. Furthermore, plant growth, development and leaf cell permeability parameter as well as Cd subcellular distribution were applied to clarify this detoxification strategy and the performance of AM fungi under Cd stress. To meet these objectives, we set up four treatments pot cultures: control (only plants Medicago truncatula without Cd), AM fungi (Glomus ortuosum + M. truncatula without Cd), Cd (M. truncatula with 10 mg/Kg Cd), Cd + AM fungi (G. ortuosum + M. truncatula with 10 mg/Kg Cd). The experiment was conducted from June 17th to August 18th, 2019 in a sterile shed built outdoors in Southwest University of Science and Technology. The climate was mild and wet with average temperature of 2°C and the mean rainfall of 179 mm during this period (source: http://weatherspark.com). Each treatment was in triplicates with 15 plants.
2.2 Soil Collection, Characterization and Processing
The experimental soil was collected from top layer (0–20 cm) in a farmland area without any pollution history, Mianyang Sichuan Province China (N31°32′2″, E104°41′41″). The collected soil was screened through a 2 mm sieve and sterilized in the high-capacity autoclave sterilizer (TOMY SX-700, USA) at 121°C lasting for 20 min. A subsample of the experimental soil was characterized for physico-chemical characteristics including: 1.32 cmol/Kg CEC, pH 5.03, 11.04 g/Kg Organic matter, 6.38 g/Kg Organic carbon, 43 mg/Kg Cr, 42 mg/Kg Cu, 7.7 mg/Kg Pb, 35.3 mg/Kg Mn, 0.17 mg/Kg Hg and 0.16 mg/Kg Cd.
Two weeks before sowing, all the sterilized soils were artificially added with cadmium at 10 mg/Kg and thoroughly mixed to provide a homogenous soil. Cadmium was prepared as appropriate aliquots of aqueous solutions by dissolving cadmium chloride in ultra-pure deionized water (Sartorius, arium pro). The same deionized water was used to prepare all the regents in this study.
2.3 Preparation of AM Fungal Inoculum and Growing Conditions
In this study, Glomus mosseae (BGC HUBO1A) was selected as fungal inoculum and provided by School of Life Science and Engineering of Southwest University of Science and Technology (Mianyang, Sichuan Province, China). Prior to the pot culture experiment, M. truncatula was cultivated in a soil-sand substrate mixture (volume ratio: 7:1) to propagate the restorative fungi inoculum for 2 months in the greenhouse, with light 12 h/d and temperature 25 ± 3°C. Subsequently, all the soil and the belowground parts of M. truncatula were collected, consisting of mixed rhizosphere soil samples from M. truncatula cultures containing 68 spores per gram of soil adhering to the method of Bi et al. [24], and of each fungal hyphe as well as strongly infected root fractions with many internal spores. The harvested AM fungi inoculums (G. mosseae) were mixed thoroughly in a sterile basin with shovels and stored in the disposable plastic bags then settled in a drafty and air-dried environment two months for further investigation.
Screened plump and uniform M. truncatula seeds were surface-disinfected for 10 min in 10% H2O2, followed by rinsing with distilled-water. Five grams sterilized seeds were sown evenly in alcohol-disinfected plastic pots (15 cm diameter, 30 cm depth) with tweezers filled with artificially well-mixed 1 Kg sterilized soil and 20 g fungal inoculums. In control pots, the same weight fungal inoculums were disinfected in the autoclave sterilizer at 121°C for 20 min to exclude possible AM fungal propagules and pathogens as well as to block AM colonization in the control (non-AM fungi) pots. After the M. truncatula seedlings emerged, the plants were thinned into fifteen in each pot. 30 ml of Hoagland nutrient solution was supplied to each pot weekly almost at the same time. All the plants in pots were irrigated by de-ionized distilled water every 2 days. The pot moisture content was monitored by the weight variation of an electronic balance to keep around 60–70%.
2.4 Soil Characteristics and Cd Speciation Analysis
By the end of the experiment, the soil samples were collected from surface (0–10 cm) of the experiment pot in the rhizosphere. The samples were stored in plastic bags, mixed artificially to obtain a homogeneous substance, and then were analyzed the following characteristics: Cation exchange capacity (CEC), pH and the content of organic matter. CEC was determined according to a simple barium chloride method adhering to Hendershot et al. [25]. Soil pH was measured in 0.01 M CaCl2 (ASTMD2974-14, USA). The measurement of organic matter was determined by potassium dichromate (K2Cr2O7)-sulfuric acid mixture based on Walkley-Black (WB) titration method [26]. To evaluate the differences in speciation of Cd in the sediments, the metal speciation of Cadmium including exchangeable, carbonate, Fe-Mn oxide, organic and residual speciation was followed with Tessier et al. [18] and determined by atomic absorption spectrometry (EWAI AA-7003, China).
2.5 Cd Accumulation and Subcellular Distribution in Plant
After 60-day treatment, the plant samples were destructively harvested. All the plants were removed from the pots gently, and rinsed with distilled water until washed away the soil particles. Three grams of M. truncatula plants were collected to determine cadmium subcellular distribution as described previously to evaluate the cadmium content in cell wall, solutions and cell soluble fraction [15]. The fresh roots and shoots were separated and homogenized in 50 mmol L−1 Tris–HCl solution (pH = 7.5), then obtained the components by centrifugation at different speeds: 3000 r min−1 for 15 min, the centrifuged deposit represented to the cell wall fraction; 15000 r min−1 for 30 min for the preceding centrifugal supernatant. The obtained deposit and supernatant referred the organelle and cell soluble fractions and then digested the two deposits with a mixture of 5 ml HNO3 and 3 ml H2O2 using a graphite digestion instrument (KDNX-20) and determined the Cd content by atomic absorption spectrometer (EWAI AA-7003, China).
2.6 Plant Growth and Leaf Percentage of Electrolyte Leakage Analysis
After 60-day treatment, three M. truncatula plants were taken from each pot and their shoot and root length, fresh biomass was measured by Vernier calipers and electronic analytical balance (Sartorius BSA 224S-CW, India). The leaf percentage of electrolyte leakage was assessed (CyberScan CON 510, Singapore) followed by Zhong et al. [27]. Briefly, two grams of fresh M. truncatula leaves were collected and darken at room temperature for 1 h, then soaked in 20 ml deionized water for 2 h and measured the initial electrical conductivity EL1. The contents then were heated in a water bath at 100°C for 20 min and the final electrical conductivity EL2 was determined. The leaf percentage of electrolyte leakage was defined as follows: EL1/EL2 × 100%.
All the experimental data were analysed by Statistic Package (SPSS 22.0) with one-way analysis of variance (ANOVA) to evaluate the growth performance and metal behaviours in both Medicago and soils. Duncan test was applied to analyse the statistical significance among treatments at a probability level of P < 0.05. Furthermore, all the figures were designed by GraphPad Prism 8.
3.1 Growth Improvement of M. truncatula by AM Symbiosis under Cd Stress
After 2-month incubation following sowing, the total height and fresh biomass were determined in each treatment as shown in Fig. 1. Compared with other groups, the plant height and biomass of M. truncatula in Cd treatment decreased significantly (P < 0.05), indicating the toxic effects of Cd on alfalfa growth. Normally, the presence of Cd, a non-essential element of the plant, is toxic to plants particularly excessively exits. It has been explained that Cd can alter the uptake of minerals by plants through its effects on the availability of minerals from the soil, or through a reduction in the population of soil microbes [28], and the impact of Cd on plant metabolism and development processes was closely related to its concentration and speciation in soils [29]. Accordingly, our results showed the application of G. mosseae in soil contaminated with Cd could increase the growth of M. truncatula including biomass, root and shoot length significantly as compared with Cd treatment, but not significant with the control, indicating the alleviation effect of Cd-toxicity to alfalfa plant by AM fungi. As described in previous study, AM fungi could effectively promote plant growth via establishing a vast connection between the roots of plants and nutrient elements such as phosphorus in soils [30]. In consideration of our results described above, it was seemed that the deduction of bioavailable portion of Cd speciation in rhizosphere soil and the increase of Cd distribution in cell wall fraction resulting from application of AM fungi under Cd stress were of benefit to the alleviation of Cd toxic effects on plants.

Figure 1: Height and fresh biomass of Medicago under different treatments. Different shape bars represent standard ± errors in treatment means and * represents the difference at 0.05 levels of significance with control
3.2 Cd Uptake and Subcellular Distribution in M. truncatula
The total uptake of Cd in root and shoot of M. truncatula and the subcellular distribution in M. truncatula cell including cell wall, organelles and soluble fraction were shown in Fig. 2.
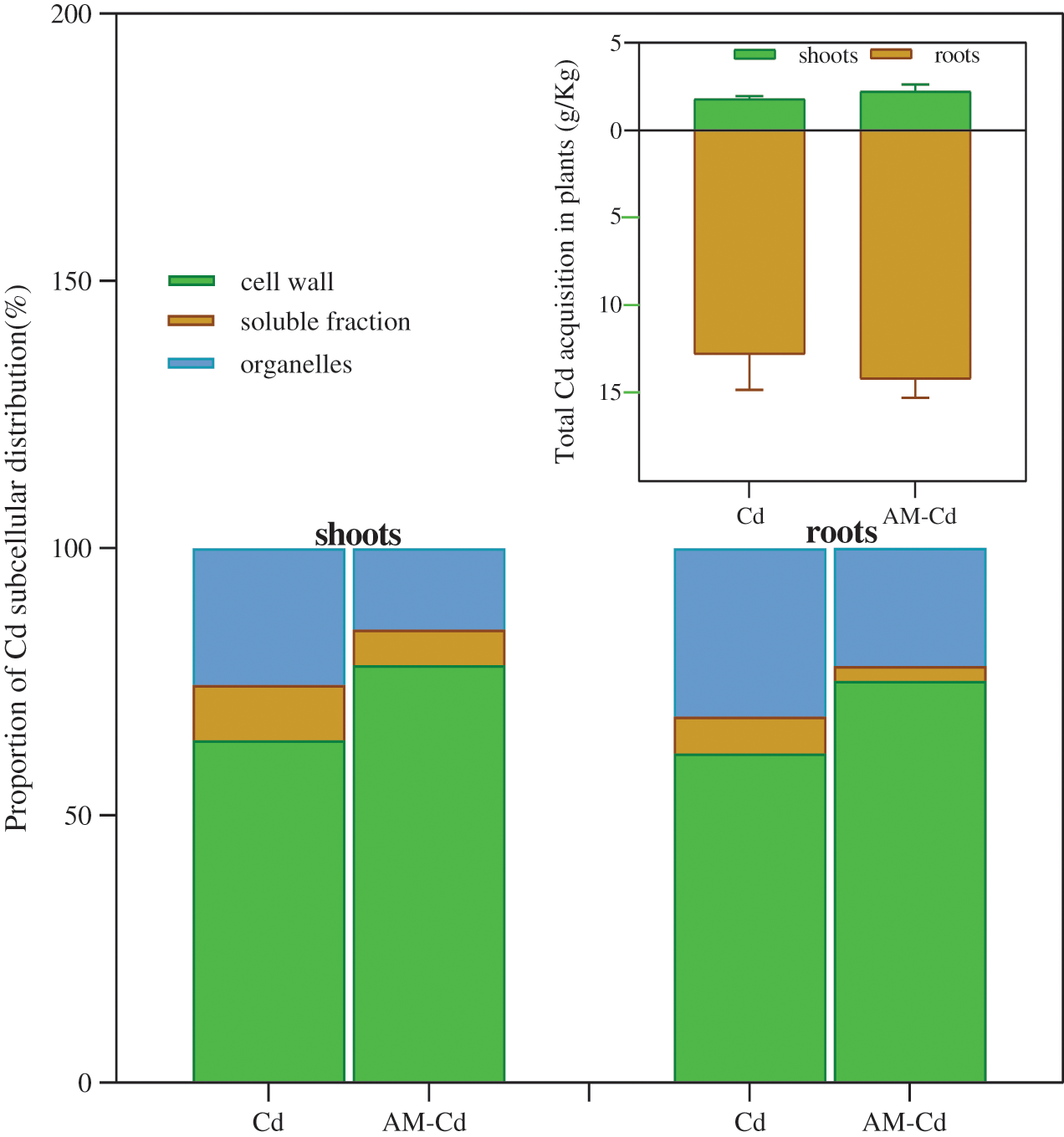
Figure 2: Cd behavior in M. truncatula seedlings cells changed by AM fungi: The above graph represents the total Cd acquistion in plant cells of root and shoots parts. The below graph corresponds to the subcellular distribution of Cd in M. truncatula seedlings cells. Different shape bars represent ± standard errors in treatment means
In our work, the total uptake of Cd in M. truncatula was enhanced by AM fungi symbiosis. Some previous studies have shown that plant uptake of heavy metals could be limited by symbiosis of AM fungi [31,32], but there was a similar increase tendency presented in the Huang et al. [33] and Audet et al. [34]. They explained whether AM fungi could provide the positive effects to improve the uptake and transport of Cd to above-ground part depends on whether cadmium is at a relative low concentration in soil (20 mg/Kg Cd). Besides, slight increases of the total cadmium content in root and shoot cells were observed in AM-Cd groups. Combined with the variations of soil characteristics and leaf electrolyte leakage between Cd and AM-Cd treatments (Figs. 3 and 4), we deduced two possible reasons on this phenomenon: the cell membrane permeability on cation could be enhanced with the joint action of AM symbiosis and Cd stress. Meanwhile the entry of heavy metals to M. truncatula cells could occur through the same transport systems applied for performing the uptake of macro and micronutrients. So this means AM fungi may regard Cd at low-level in plants as alternative elements via trans-membrane carriers involved in the uptake of magnesium (Mg), calcium (Ca), iron (Fe), zinc (Zn) and copper (Cu) and sequentially promoted the uptake and transfer of low-level Cd [15,35]. Furthermore, the cell wall is regarded as the first defense barrier in cells contains a wide range of additional compounds such as cellulose, xylan and lignin, which is prone to combine with cationic charge [36]. In our work, the biggest amount of Cd collected in the cell wall fraction, with less in organelle fraction and the least in soluble fraction, both for the shoot and root cells. For shoot cells, the cell wall portion of Cd was increased by 14.02% and the proportion of soluble and organelle fractions were decreased by 3.69% and 4.16% respectively. For root cells, the cell wall fraction was increased by 13.57%, and the soluble and organelle fractions were decreased by 10.33% and 9.41%. The concentration of Cd in the cell wall fraction was increased more than in the shoot part, indicating that AM fungi mainly play a moderating Cd toxicity role in the root cells. In fact, there are three procedures occurred in AM fungi symbiont for metal elements transportation to plant cell, including an energy-dependent process from soil to fungal cytoplasm, then translocation to root cells via cytoplasmic streaming, and transferring in xylem from roots to shoots [37,38].

Figure 3: Leaf electrolyte leakage of M. truncatula seedlings in each treatment. The same lowercases are not significantly different at 0.05 levels of significance by the Duncan’s test

Figure 4: Changes in soil of pH, CEC and organic matter as influenced by different treatments. Different shape bars represent ± standard errors in treatment means and same lowercases are not significantly different at 0.05 levels of probability
3.3 Increase of Leaf Percentage of Electrolyte Leakage by AM Symbiosis under Cd Stress
This index was used to assess cell membrane permeability in relation to Cd stress [39]. In this work, the leaf percentage of electrolyte leakage was decreased significantly both in Cd and AM treatments compared to the control and AM-Cd groups. The reduction of the leaf percentage of electrolyte leakage in AM groups was similar with the investigation of Arthikala et al. [40], who presumed AM fungi could down-regulate plant cell death via decreasing electrolyte leakage in plant cells. Besides, the analogous observation was also found in Arthikala et al. [40] that the decrease of electrolyte leakage in AM treatments indicated successful colonization and more ions such as phosphate ion uptake and store in the AM fungi-symbiont. The decrease of leaf percentage of electrolyte leakage was also shown in Cd groups indicated that M. truncatula leaf cell membrane permeability was inhibited (Fig. 3). With occurrence of Cd, the ionic homeostasis, nitrogen and carbon metabolism of plant could be stunted even causing chlorosis, thus lower absorption of nutrients ions by plant tissues [41]. Moreover, Cd have strong affinity for nitrogen, sulfur ligands and proteins and competed preferentially compared with other nutrient ions such as manganese [42,43].
Combined with AM fungi, the increase of M. truncatula leaf cell membrane permeability may be due to AM fungi induce Cd ions efflux through cell membrane-associated Ca2+ transporter and displacement of Ca2+ pooled in cell wall fraction [36,44], indicating that AM fungi could improve membrane stability and tolerance of symbiotic plants under Cd stress. Results revealed that the decrease by the percentage of electrolyte leakage of the M. truncatula leaves in Cd groups could be a self-protection mechanism of plants to prevent nutrition ions efflux and the flow of toxicity ions [1].
3.4 Changes of Cd Speciation Portion in Rhizosphere Soil
The toxicity of soil contaminated with heavy metals is primarily determined by its metal speciation not only its concentration. In our study, five-part sequential extraction Tessier’s method was used to evaluate the influence of AM fungi on the transformation among five forms of cadmium. Adhering to this approach, the metals are partitioned into five fractions and the mobility and bio-toxicity of metals decreased in the order of exchangeable, carbonate, Fe-Mn oxide, organic and residual [45].
The concentration of Cd in each fraction was presented in Tab. 1. The proportion of former four speciation of Cd decreased in AM fungi treatment compared to Cd groups, significantly in exchangeable and carbonates-bounded speciation (P < 0.05). Heavy metal speciation is influenced by a series of basic properties in soil and one of the most important factors considered to manage soil heavy metal toxicity is soil pH [46]. The acid soluble fraction (including exchangeable and carbonate phases) was significantly decreased by 28.98% and 11.83% respectively under AM-Cd treatment compared to Cd treatment. As described by Sungur et al. [47], the acid soluble fraction of metal ions has a strongly positive correlation on heavy metal extractability and bio-availability. Meanwhile, the soil pH value decreased significantly in Cd-added groups whilst increased in AM-Cd treatments (Fig. 4a), which could be an assuasive strategy of AM fungi on Cd-polluted soil acidification. By contrast, the residual fraction of Cd speciation consisting of chemically stable and bio-inactive metals has a negative correlation with heavy metal bioavailability, affecting the extraction rate of heavy metals from plants.
Table 1: Cd speciation portion in rhizosphere soil under Cd and AM-Cd treatments

Note: Values are means of standard error, where the * represents significant difference in data
In this study, the proportion of residual form of Cd speciation was increased by 20.29%, indicating AM fungi could alleviate the toxicity of Cd speciation as well as the environmental risk of Cd. Furthermore, both the Fe and Mn oxides-bounded and organic forms of Cd speciation were decreased by 4.61% and 5.95% respectively with comparison Cd groups. It is worth noting that AM-Cd increased the content of reducible speciation compared with Cd groups, indicating that AM fungi could reduce the Cd movability and alleviate Cd stress on themselves and symbiotic plants via metabolism. We presumed that AM fungal exudates such as glomalin contribute to the bioavailability of Cd, appearing to play a role in heavy metal immobilization [48].
3.5 Changes of Soil Properties Contributing to Detoxification
The availability of cadmium is influenced by a series of soil characteristics, including pH, CEC, and organic matter content and et al., and among them pH is the foremost [49]. To evaluate the mobilization potential of cadmium, these parameters were measured and presented in Fig. 4, respectively. The soil characteristics in four groups were alleviated by AM fungi symbiosis under Cd stress. Cd stress affected the soil original properties condition significantly in a negative way meanwhile organic matter and CEC decreased whilst AM fungi symbiosis mitigated this impact in comparison with control.
In Fig. 4a, there was no statistically difference on pH value between control and AM treatments (P > 0.05), whilst the pH value was decreased significantly and the soil acidification was intensified under Cd treatment (P < 0.05), which is consistent with the findings of Zhu et al. [50] that cadmium bio-availability increased following pH value decreased and there is a negative correlation between pH and metal sorption in soil proved by Elbana et al. [41]. To minimize the toxicity of cadmium, plants developed varieties of cellular and molecular strategies for their detoxification, including secretion humic acid for more capture of bio-availability cadmium stored in subcellular sequestration, which belongs to one of the possible explanations for soil acidification under Cd stress [51]. It is worth noting that the pH value in AM-Cd treatments had a significant increase compared to Cd groups (P < 0.05), which were possibly due to a secretion from AM fungi called glomalin with monomer structure. Although haven’t been fully established, the structure of this secretion could reduce cadmium mobility in soil via hydrophobic interaction combined with H+ as well as cation metal ions [15].
The variation tendency of soil CEC was consistent with that of pH (Fig. 4b). The CEC content in Cd groups was decreased significantly compared to all treatments (P < 0.05). A similar observation was confirmed by Honma et al. [21] that the soil CEC is strongly negatively correlated with exchangeable cadmium and positively with inorganically bound cadmium. This was largely depended by variation on soil of total C, clay, and amorphous iron and aluminum oxides closely related to active Cd forms [52]. Since inner-sphere complexes, one of the chemical binding means in reactive sites of soil inorganic constituents, can form firm metal hydroxides [53]. The soil CEC of AM treatment was lower than in the control group and higher than the Cd stress with AM application group but without statistical difference (P > 0.05). The results were similar with the results of Fang et al. [54]. Here, we outlined two potential mechanisms between AM symbiosis and soil CEC as followed: Firstly, AM fungi prefer utilizing nutrient cation such as NH4+ in soil generally that we observed CEC had a slight decrease as compared to the control [54]; Secondly, under cadmium stress, CEC increased with AM fungi inoculation, enabling the soil to absorb more cation potentially (such as N, Ca, S, K, Zn), which may be a dilution protective mechanism to reduce cadmium toxicity on plant symbiont [55].
Cation exchange capacity is capable of holding nutrients in soils and has a positive correlation with metal ions absorbability [21], therefore the organic matter in soils was increased significantly with AM treatments with comparison to control and Cd groups (Fig. 4b). There are other possible evidences leading this increased phenomenon that AM fungi, as well as called “soil fertilizer”, have high affinity with total phosphorus, soil microorganisms, organic carbon and matter due to their strong extra-radicul mycelium and big surface area in hype [56]. In Fig. 4c, AM fungi increased total soil organic matter compared to control (P < 0.05), which occurrence supported by the speculation of Daynes et al. [57]. However, the content of organic matter was dramatically decreased in Cd treatments, whilst this inhabitation effect was alleviated in AM-Cd treatments compared to Cd groups, though AM-Cd treatments had a slight decrease with comparison to control.
In this metal-behavior survey, we have focused on the relationship between soil properties and Cd speciation with AM fungi symbiosis. AM fungi as a soil buffer among phytoremediation could reduce the mobility and bioavailability of Cd in soils via alleviating the acidification of soil as well as increasing the content of CEC by dilution action in soil under the toxic effect of Cd. In addition, the content of organic matter in soil were also promoted due to the fertilizer function of AM fungi. We also recognized the role of AM fungi played at the subcellular level of Medicago sp. that more Cd was accumulated in the cell wall fraction to increase the plant tolerance to heavy metal with AMF application under Cd stress. Herein, application of AM fungi in Cd treatments performed to inhibit the appearance of Cd toxicity symptoms, including the improvement of leaf electrolyte leakage, root elongation, seedling growth and biomass. However, since this work was conducted under lab scale condition, further researches applied into natural ecosystems in comprehension of metal behaviors affected by AM fungi symbiont are necessary to utilize efficient bio-inoculants as well as considerable the Cd concentration levels and host plant species.
Author Contributions: Conceptualization, Ke Chen; Formal analysis, Renhua Huang; Funding acquisition, Wenkun Zhu; Investigation, Yuying Jiang; Project administration, Lei Jiang; Resources, Renhua Huang; Supervision, Ke Chen; Validation, Lei Jiang; Writing—original draft, Yuying Jiang; Writing—review & editing, Wenkun Zhu and Ke Chen.
Funding Statement: This work was financially supported by Grants of Science and Technology Department of Sichuan Province (2019YFS0469, 2020YFS0344) and Educational Department of Sichuan province (17ZB0438).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Liu, L. W., Wei, L., Song, W. P., Guo, M. X. (2018). Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Science of the Total Environment, 633, 206–219. DOI 10.1016/j.scitotenv.2018.03.161. [Google Scholar] [CrossRef]
2. Liu, X. J., Tian, G. J., Dong, J., Zhang, C., Kong, L. Q. (2016). Cadmium (cd) distribution and contamination in Chinese paddy soils on national scale. Environmental Science and Pollution Research, 23(18), 17941–17952. DOI 10.1007/s11356-016-6968-7. [Google Scholar] [CrossRef]
3. Rizwan, M., Shafaqat, A., Muhammad, A., Muhammad, I. Daniel, C. W. et al. (2017). A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere, 182, 90–105. DOI 10.1016/j.chemosphere.2017.05.013. [Google Scholar] [CrossRef]
4. Liu, Y., Liu, K., Li, Y., Yang, W., Wu, F. et al. (2016). Cadmium contamination of soil and crops is affected by intercropping and rotation systems in the lower reaches of the Minjiang river in South-Western China. Environmental Geochemistry & Health, 38(3), 811–820. DOI 10.1007/s10653-015-9762-4. [Google Scholar] [CrossRef]
5. Ashraf, S., Qasim, A., Zahir, A. Z., Sobia, A., Hafiz, N. A. (2019). Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicology and Environmental Safety, 174, 714–727. DOI 10.1016/j.ecoenv.2019.02.068. [Google Scholar] [CrossRef]
6. Mahar, A., Ping, W., Amjad, A., Mukesh, K. A., Altaf, H. L. et al. (2016). Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicology and Environmental Safety, 126, 111–121. DOI 10.1016/j.ecoenv.2015.12.023. [Google Scholar] [CrossRef]
7. Davide, R., Roberto, B., Sergio, F. (2015). Electrokinetic remediation of soils polluted by heavy metals (mercury in particular). Chemical Engineering Journal, 264, 16–23. DOI 10.1016/j.cej.2014.11.074. [Google Scholar] [CrossRef]
8. Vocciante, M., Caretta, A., Letizia, B., Roberto, B., Elisabetta, F. et al. (2019). Enhancements in phytoremediation technology: Environmental assessment including different options of biomass disposal and comparison with a consolidated approach. Journal of Environmental Management, 237, 560–568. DOI 10.1016/j.jenvman.2019.02.104. [Google Scholar] [CrossRef]
9. Garg, N., Bhandari, P. (2014). Cadmium toxicity in crop plants and its alleviation by Arbuscular Mycorrhizal (AM) fungi: An overview. Plant Biosystems, 148(4), 609–621. DOI 10.1080/11263504.2013.788096. [Google Scholar] [CrossRef]
10. Das, M., Vijay, S. J., Alok, A. (2018). Role of mycorrhiza in phytoremediation processes: A review. Springer International Publishing, 271–286. DOI 10.1007/978-3-319-68867-1_14. [Google Scholar] [CrossRef]
11. Rasouli-Sadaghiani, M. H., Barin, M., Khodaverdiloo, H., Siavash, S. M., Damalas, C. A. et al. (2019). Arbuscular mycorrhizal fungi and rhizobacteria promote growth of Russian Knapweed (Acroptilon Repens L.) in a Cd-contaminated soil. Journal of Plant Growth Regulation, 38(1), 113–424. DOI 10.1007/s00344-018-9815-x. [Google Scholar] [CrossRef]
12. Ferrol, N., Elisabeth, T., Paola, V. (2016). The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. Journal of Experimental Botany, 67(22), 6253–6565. DOI 10.1093/jxb/erw403. [Google Scholar] [CrossRef]
13. Muthukrishnan, G., Sabarinathan, K. G., Pandiyarajan, P. (2018). Prospects of arbuscular mycorrhizal fungi for heavy metal-polluted soil management. In: Microorganisms for Green Revolution. pp. 91–113, Singapore: Springer. [Google Scholar]
14. Deng, Z. J., Li, X. C. (2017). Fungal endophytes and their interactions with plants in phytoremediation: A review. Chemosphere, 168, 1100–1106. DOI 10.1016/j.chemosphere.2016.10.097. [Google Scholar] [CrossRef]
15. Gong, X., Tian, D. Q. (2019). Study on the effect mechanism of arbuscular mycorrhiza on the absorption of heavy metal elements in soil by plants. IOP Conference Series: Earth and Environmental Science, 267(5), 1–7. DOI 10.1088/1755-1315/267/5/052064. [Google Scholar] [CrossRef]
16. Pawlowska, T. E., Charvat, I. (2004). Heavy-metal stress and developmental patterns of arbuscular mycorrhizal fungi. Applied and Environmental Microbiology, 70(11), 6643–6649. DOI 10.1128/AEM.70.11.6643-6649.2004. [Google Scholar] [CrossRef]
17. Beiyuan, J. Z., Daniel, C. W., Tsang, N. S., Bolan, K. B., Xiang, D. L. (2018). Interactions of food waste compost with metals and metal-chelant complexes during soil remediation. Journal of Cleaner Production, 192(4), 225–230. DOI 10.1016/j.jclepro.2018.04.239. [Google Scholar] [CrossRef]
18. Tessier, A., Campbell, P. G. C., Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851. DOI 10.1021/ac50043a017. [Google Scholar] [CrossRef]
19. Han, S., Li, X., Amombo, E., Fu, J., Xie, Y. (2018). Cadmium tolerance of perennial ryegrass induced by Aspergillus aculeatus. Frontiers in Microbiology, 9, 1579. DOI 10.3389/fmicb.2018.01579. [Google Scholar] [CrossRef]
20. Fu, J. T., Yu, D. M., Chen, X., Su, Y., Li, C. H. et al. (2019). Recent research progress in geochemical properties and restoration of heavy metals in contaminated soil by phytoremediation. Journal of Mountain Science, 16(9), 2079–2095. DOI 10.1007/s11629-017-4752-x. [Google Scholar] [CrossRef]
21. Honma, T., Hirotomo, O., Tomoyuki, M., Takuji, O. (2015). Relationship between cadmium fractions obtained by sequential extraction of soil and the soil properties in contaminated and uncontaminated paddy soils. Journal of Chemistry, 2015, 1–9. DOI 10.1155/2015/714680. [Google Scholar] [CrossRef]
22. He, Z. L., Xu, H. P., Zhu, Y. M., Yang, X. E., Chen, G. C. (2005). Adsorption-desorption characteristics of cadmium in variable charge soils. Environmental Letters, 40(4), 805–822. DOI 10.1081/ESE-200048273. [Google Scholar] [CrossRef]
23. Kubier, A., Wilkin, R. T., Pichler, T. (2019). Cadmium in soils and groundwater: A review. Applied Geochemistry, 108(104388), 1–9. DOI 10.1016/j.apgeochem.2019.104388. [Google Scholar] [CrossRef]
24. Bi, Y. L., Wang, W. U. (2007). Dyeing method-a kind of the method for mycorrhizal spore density quick improved measurement. Energy Environmental Protection, 2, 10–12. DOI 10.3969/j.issn.1006-8759.2007.02.003. [Google Scholar] [CrossRef]
25. Hendershot, W. H., Duquette, M. (1986). A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Science Society of America Journal, 50(3), 605–608. DOI 10.2136/sssaj1986.03615995005000030013x. [Google Scholar] [CrossRef]
26. Walkley, A. (1947). A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Science, 63(4), 251–264. DOI 10.1097/00010694-194704000-00001. [Google Scholar] [CrossRef]
27. Zhong, Q., Meng, K., Guo, M., Wang, X. H. (2011). Leaf frost sensitivity and percentage of electrolyte leakage of the evergreen woody species in Tiantong region, Zhejiang Province. Journal of East China Normal University, 185(1), 62–67. DOI 10.1016/j.tvjl.2010.04.015. [Google Scholar] [CrossRef]
28. Benavides, M. P., Gallego, S. M., Tomaro, M. L. (2005). Cadmium toxicity in plants. Brazilian Journal of Plant Physiology, 17(1), 21–34. DOI 10.1590/S1677-04202005000100003. [Google Scholar] [CrossRef]
29. Ismael, M. A., Elyamine, A. M., Moussa, M. G., Cai, M. M., Zhao, X. H. et al. (2019). Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics, 11(2), 255–277. DOI 10.1039/C8MT00247A. [Google Scholar] [CrossRef]
30. Graf, F., Bast, A., Holger, G., Yildiz, A. (2019). “Effects of mycorrhizal fungi on slope stabilisation functions of plants,” pp. 55–77, Springer International Publishing. DOI 10.1007/978-3-319-89671-7_6. [Google Scholar] [CrossRef]
31. Antoniadis, V., Levizou, E., Shaheen, S. M., Yong, S. O., Sebastian, A. et al. (2017). Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth-Science Reviews, 171, 621–645. DOI 10.1016/j.earscirev.2017.06.005. [Google Scholar] [CrossRef]
32. Wang, Y. P., Huang, J., Gao, Y. Z. (2012). Arbuscular mycorrhizal colonization alters subcellular distribution and chemical forms of cadmium in Medicago sativa L. and resists cadmium toxicity. PLoS One, 7(11), 1–7. DOI 10.1371/journal.pone.0048669. [Google Scholar] [CrossRef]
33. Huang, X., Wang, L., Zhu, S. (2018). Unraveling the effects of arbuscular mycorrhizal fungus on uptake, translocation, and distribution of cadmium in Phragmites australis (Cav.) Trin. ex Steud. Ecotoxicology and Environmental Safety, 149, 43–50. DOI 10.1016/j.ecoenv.2017.11.011. [Google Scholar] [CrossRef]
34. Audet, P., Charest, C. (2007). Heavy metal phytoremediation from a meta-analytical perspective. Environmental Pollution, 147(1), 231–237. DOI 10.1016/j.envpol.2006.08.011. [Google Scholar] [CrossRef]
35. Webster, E. A., Geoffrey, M. G. (1996). Cadmium replaces calcium in the cell wall of Ulva Lactuca. Biometals, 9(3), 241–244. DOI 10.1007/BF00817922. [Google Scholar] [CrossRef]
36. Weng, B., Xie, X., Weiss, D. J., Liu, J., Lu, H. et al. (2012). Kandelia Obovata (S., L.) Yong tolerance mechanisms to cadmium: Subcellular distribution, chemical forms and thiol pools. Marine Pollution Bulletin, 64(11), 2453–2460. DOI 10.1016/j.marpolbul.2012.07.047. [Google Scholar] [CrossRef]
37. Cooper, K. M., Tinker, P. B. (2006). Translocation and transfer of nutrients in vesicular-arbuscular Mycorrhizas II. Uptake and translocation of phosphorus, zinc and sulphur. New Phytologist, 81(1), 43–52. DOI 10.1111/j.1469-8137.1978.tb01602.x. [Google Scholar] [CrossRef]
38. Haselwandter, C., Leyval, K., Turnau, K. (1997). Effect of heavy metal pollution on mycorrhizal colonization and function: Physiological, ecological and applied aspects. Mycorrhiza, 7(3), 139–153. DOI 10.1007/s005720050174. [Google Scholar] [CrossRef]
39. Jodeh, S., Raed, A., Hamed, R., Samhan, S. (2015). The study of electrolyte leakage from barley (Hordeum Vulgare L.) and pearlmillet using plant growth promotion (PGPR) and reverse osmosis. Journal of Food and Nutrition Research, 3(7), 422–429. DOI 10.12691/jfnr-3-7-3. [Google Scholar] [CrossRef]
40. Arthikala, M. K., Nava, N., Quinto, C. (2015). Effect of rhizobium and arbuscular mycorrhizal fungi inoculation on electrolyte leakage in Phaseolus vulgaris roots overexpressing RbohB. Plant Signaling and Behavior, 10(4), 1–4. DOI 10.1080/15592324.2014.1000167. [Google Scholar] [CrossRef]
41. Elbana, T. A., Magdi, S., Nazanin, A., April, N. Shaheen, S. M. et al. (2018). Freundlich sorption parameters for cadmium, copper, nickel, lead, and zinc for different soils: Influence of kinetics. Geoderma, 324, 80–88. DOI 10.1016/j.geoderma.2018.03.019. [Google Scholar] [CrossRef]
42. Gonalves, J. F., Becker, A. G., Cargnelutti, D., Tabaldi, L. A., Schetinger, M. R. C. (2007). Cadmium toxicity causes oxidative stress and induces response of the antioxidant system in cucumber seedlings. Brazilian Journal of Plant Physiology, 19(3), 119–123. DOI 10.1590/S1677-04202007000200004. [Google Scholar] [CrossRef]
43. Javed, M. T., Greger, M. (2010). Cadmium triggers elodea canadensis to change the surrounding water pH and thereby cd uptake. International Journal of Phytoremediation, 13(1), 95–106. DOI 10.1080/15226511003753987. [Google Scholar] [CrossRef]
44. Ma, Y., Rajkumar, M., Oliveira, R. S., Zhang, C., Helena, F. (2019). Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. Journal of Hazardous Materials, 379, 1–9. DOI 10.1016/j.jhazmat.2019.120813. [Google Scholar] [CrossRef]
45. Dagne, B. B., Teju, E., Tesfahun, K., Negash, D. (2019). Levels of some toxic heavy metals (Cr, Cd and Pb) in selected vegetables and soil around Eastern Industry Zone, Central Ethiopia. African Journal of Agricultural Research, 14(2), 92–101. DOI 10.5897/AJAR2018.13324. [Google Scholar] [CrossRef]
46. Ardestani, M. M., Cornelis, A. M., Gestel, A. M. (2013). Using a toxicokinetics approach to explain the effect of soil pH on cadmium bioavailability to Folsomia candida. Environmental Pollution, 180, 122–130. DOI 10.1016/j.envpol.2013.05.024. [Google Scholar] [CrossRef]
47. Sungur, A., Soylak, M., Ozcan, H. (2014). Investigation of heavy metal mobility and availability by the BCR sequential extraction procedure: Relationship between soil properties and heavy metals availability. Chemical Speciation and Bioavailability, 26(4), 219–230. DOI 10.3184/095422914X14147781158674. [Google Scholar] [CrossRef]
48. Wright, S. F., Upadhyaya, A. (1996). Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Science, 161(9), 575–586. DOI 10.1097/00010694-199609000-00003. [Google Scholar] [CrossRef]
49. Kirkham, M. B. (2006). Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma, 137(1–2), 19–32. DOI 10.1016/j.geoderma.2006.08.024. [Google Scholar] [CrossRef]
50. Zhu, H. H., Chen, C., Chao, X., Zhu, Q. H., Huang, D. Y. (2016). Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of Central Subtropical China. Environmental Pollution, 219, 99–106. DOI 10.1016/j.envpol.2016.10.043. [Google Scholar] [CrossRef]
51. Mahajan, P., Jyotsna, K. (2018). Role of phytoremediation in reducing cadmium toxicity in soil and water. Journal of Toxicology, 2018(3), 48643–48665. DOI 10.1155/2018/4864365. [Google Scholar] [CrossRef]
52. Gérard, F. (2016). Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils–A myth revisited. Geoderma: An International Journal of Soil Science, 262, 213–226. DOI 10.1016/j.geoderma.2015.08.036. [Google Scholar] [CrossRef]
53. Sparks, D. L. (2005). Toxic metals in the environment: The role of surfaces. Elements, 1(4), 193–197. DOI 10.2113/gselements.1.4.193. [Google Scholar] [CrossRef]
54. Fang, F. R., Wang, C. Y., Fei, W., Ming, T., Russell, D. (2020). Arbuscular mycorrhizal fungi mitigate nitrogen leaching under poplar seedlings. Forests, 11(3), 1–17. DOI 10.3390/f11030325. [Google Scholar] [CrossRef]
55. Zhang, X. H., Zhu, Y. G., Chen, B. D., Lin, A. J., Smith, S. E. et al. (2005). Arbuscular mycorrhizal fungi contribute to resistance of upland rice to combined metal contamination of soil. Journal of Plant Nutrition, 28(12), 2065–2077. DOI 10.1080/01904160500320871. [Google Scholar] [CrossRef]
56. Syibli, M. A., Muhibuddi, A., Djauhari, S. (2013). Arbuscular mycorrhiza fungi as an indicator of soil fertility. Agrivita, 35(1), 44–53. DOI 10.17503/Agrivita-2013-35-1-p44-53. [Google Scholar] [CrossRef]
57. Daynes, C. N., Field, D. J., Saleeba, J. A., Cole, M. A., McGee, P. A. (2013). Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biology and Biochemistry, 57, 683–694. DOI 10.1016/j.soilbio.2012.09.020. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |