

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013912
ARTICLE
Optimization of Factors Influencing Adventitious Rooting in Hybrid Larch
1State Key Laboratory of Tree Genetics and Breeding, Key Laboratory of Tree Breeding and Cultivation of State Forestry and Grassland Administration, Research Institute of Forestry, Chinese Academy of Forestry, Beijing, 100091, China
2Guangxi Key Laboratory of Superior Timber Trees Resource Cultivation & Key Laboratory of Central South Fast-Growing Timber Cultivation of Forestry Ministry of China, Guangxi Forestry Research Institute, Nanning, 530002, China
*Corresponding Author: Xiaomei Sun. Email: xmsun@caf.ac.cn
Received: 26 August 2020; Accepted: 21 September 2020
Abstract: Optimization of in vitro adventitious root induction contributes to the development of a large-scale production system of hybrid clone seedlings of larch (Larix spp.). We used orthogonal testing to investigate the factors that affect the rooting of hybrid larch shoots–activated carbon, vitamin B1, sucrose, glycine, glutamic acid (Glu), cysteine, pH, and induction time in the dark and indole-3-butyric acid (IBA). Variance analysis showed that the effects of sucrose concentration on rooting rate, survival rate, and average root number, pH on the average number of roots; and Glu on survival rate were all significant (p < 0.05) when compared to the mock treatments. Among all of the 45 treatments, the highest rooting rate was 90%, the highest survival rate was 100%, and the highest average root number was 11.8. The theoretically optimal procedure of in vitro root induction of hybrid larch shoots is shoot induction on L9 modified medium (pH 6.7) containing 2 mg.L–1 naphthalene acetic acid, 0.25 mg.L–1 IBA, 1/3 macroelements, 7.5 g.L–1 sucrose, and 5 mg.L–1 Glu for 8 days, and then root induction on L9 modified medium without auxin.
Keywords: Hybrid larch; adventitious root; orthogonal test; variance analysis
Vegetative propagation is extensively used in breeding and the commercial propagation of forest trees [1]. Compared with the conventional vegetative propagation methods such as cutting and grafting, tissue culture has the advantage of large-scale production, providing plantlets whenever needed. In vitro micropropagation of larch is processed via organogenesis from different species [2,3]. The formation of adventitious roots is a vital step in establishing in vitro cultures. The rooting of larch is performed using a two-step procedure [4]. The first step is the induction phase, in which shoots are cultured on medium supplemented with auxin, and the second step is the root development phase, in which shoots are cultured on hormone-free medium. This two-step rooting method is suitable for the rooting of larch [5,6].
Heterosis (hybrid vigor) is a well-known phenomenon whereby hybrid offspring of diverse varieties of a species or crosses between species exhibit greater vigor than both parents [7]. The hybrid larch which combines the superior characteristics of its parents has clear heterosis in biomass, stress resistance, and speed of development [8]. Vegetative propagation is an important means of fixing the genotype of hybrids that effectively provides homogenous phenotypes of plants. With fast juvenile growth, adaptability to various environmental conditions, and exceptional wood properties, larch is one of the most important coniferous tree species in China [9]. An efficient micropropagation system allows genetic fixation and exploitation of heterosis in hybrid larch.
Organogenesis is strongly influenced by genotype, and a capacity for adventitious root generation is genotype-dependent [10–12]. The optimal conditions for morphogenesis need to be determined for each plant species and even for different genotypes of the same species. Besides auxins, vitamins, amino-acids, sucrose, pH, activated carbon (AC), and induction time in the dark affect rooting [13–18]. In this study, we explored the factors that affect the in vitro rooting of hybrid larch shoots, and the rooting conditions of tissue culture were optimized using an orthogonal experimental design to provide technical support for the construction of an efficient and stable tissue culture regeneration system for hybrid larch and the large-scale production of hybrid larch seedlings.
2.1 Plant Materials and Indoor Cultivation
Liaofeng1, a selected elite clone with fast growth and strong rooting ability, was bred from the hybrids Larix kaempferi × L. olgensis at the Chinese Academy of Forestry. Cut seedlings of this clone were collected from the Da GuJia National Larch Breeding Centre, Liaoning Province, China (long. 124°47’, lat. 42°22’N). A two-year old clone of hybrid larch was cultured in a climate chamber as the stock plant. This chamber was equipped with an LED lamp as the light source; the photoperiod of each day was 16 h, the illumination intensity was 2000–3000 lx, the temperature was 23°C, and the humidity was 50%.
2.2 Sterilization of Explants and Initial Cultures
Shoots of ~ 5 cm in length were cut from the stock hybrid larch, and the leaves within 2 cm of the base of each shoot were cut off. After being shaken and soaked for 20 min in washing solution, they were washed in water for 30 min. Sodium hypochlorite (15% v/v) was used as the sterilizing agent. Under aseptic conditions on a super-clean platform, after treatment with sterilizing agent for 10 min the shoots were washed 3 times in aseptic water, then inoculated on B1 medium for initial culture for 4 weeks [2]. The medium was made up with ultrapure water. HCl and NaOH were used to adjust the pH. The reagents were from Sangon Biotech (Shanghai) Co., Ltd. LED lamps were used as the light source, the photoperiod was 16 h, the illumination intensity 2000–3000 lx, and the temperature 23°C.
2.3 Orthogonal Tests of Rooting
After four weeks of cultivation on B1, shoots were subcultured for root induction on L9 medium of one third strength supplemented with 2 mg.L–1 naphthalene acetic acid (NAA) [2], and then in the same medium without auxin for root development. L18 (37) orthogonal testing was conducted using AC (0.2, 0.5, and 0.8 g.L−1), vitamin B1 (VB1; 0.2, 0.4, and 0.6 mg.L−1), sucrose (2.5, 5, 7.5, 10, 12.5, 15, and 25 g.L−1), glycine (Gly; 5, 25, and 50 mg.L−1), glutamic acid (Glu; 5, 25, and 50 mg.L−1), and cysteine (Cys; 5, 25, and 50 mg.L−1), at pH 5.2, 5.7, 6.2, 6.7, 7.0, and 7.3 before sterilization of the medium, indole-3-butyric acid (IBA; 0.25, 1.00, and 1.75 mg.L−1), auxin induction periods of 3, 6, 8, 9, 11, and 14 days, and dark culture periods of 2, 4, and 6 days. Each treatment was inoculated with 20 shoots. LED lamps were the light source, photoperiod was 16 h, illumination intensity 2000–3000 lx, and temperature 23°C.
The rooting rate, average root number, and survival rate of plantlets were evaluated 8 weeks after root induction on L9 medium with auxin. A blank column was added to the orthogonal test. The percentage data were transformed into arcsine before analysis with ANOVA. Intuitive analysis and ANOVA analysis were performed using the statistical package IBM SPSS 15.0.
3.1 Orthogonal Tests of the Effects of Dark Culture, AC, VB1, sucrose, pH, and Induction Time on Rooting
The orthogonal test and results are shown in Tab. 1. The rooting rate of 2 treatments (#1 and #6) were the highest, reaching 60%. Both the rooting rate and the average rooting number of 6 treatments (#8, #9, #10, #12, #13, and #17) were 0%. The survival rate of 4 combinations (#6, #11, #16, and #18) was 100%. The lowest survival rate (#17) was 30.0%. The average number of roots of 2 combinations (#5 and #7) was >4 (4.2 and 4.1, respectively).
Table 1: The experimental design based on L18(37) orthogonal array and effects of dark culture, AC, VB1, sucrose, pH and induce time on rooting
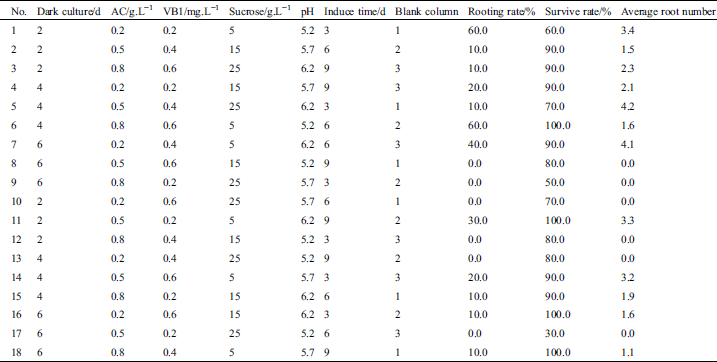
3.2 Intuitive Analysis of the Effects of Dark Culture, AC, VB1, sucrose, pH, and Induction Time on Rooting
Range analysis of the orthogonal test is shown in Tab. 2. sucrose had the greatest effect on rooting rate (range 33.30) and survival rate (range 25.00). In the three sucrose concentrations of 5, 15, and 25 g.L−1, the k values of rooting rate and survival rate decreased with increasing concentration. The k values of rooting rate and survival rate with 5 g.L−1 sucrose were the highest (36.67 and 90.00, respectively). The rooting rate ranges of VB1 and induction time, and the survival rate range of AC were the lowest, at 8.33. pH had the greatest effect on the average rooting number (range 2.07). With increasing pH (5.2, 5.7, and 6.2), the k values of the average rooting number showed an upward trend, and the highest k value was 2.9 at pH 6.2. The average rooting number range of VB1 was the lowest, at 0.37.
Table 2: Intuitive analysis of effects of dark culture, AC, VB1, sucrose, pH and induce time on rooting
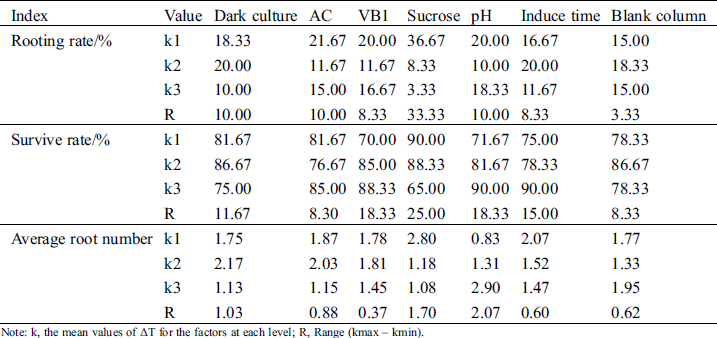
Note: k, the mean values of ΔT for the factors at each level; R, Range (kmax – kmin).
3.3 Variance Analysis of the Effects of Dark Culture, Ac, Vb1, sucrose, pH, and Induction Time on Rooting
Sucrose significantly influenced the rooting rate and survival rate (p < 0.05), while other factors had no significant effect on rooting. The F-values of the 6 influential factors for rooting rate revealed the following order: sucrose > dark culture > pH > AC > VB1 > induction time (Tab. 3). The F-values of survival rate were in the following order: sucrose > pH > VB > induction time > AC > dark culture. pH significantly influenced the average root number (p < 0.05). The F-values of average root number were in the order: pH > sucrose > dark culture > AC > induction time > VB1.
Table 3: Variance analysis of effects of dark culture, AC, VB1, sucrose, pH and induce time on rooting
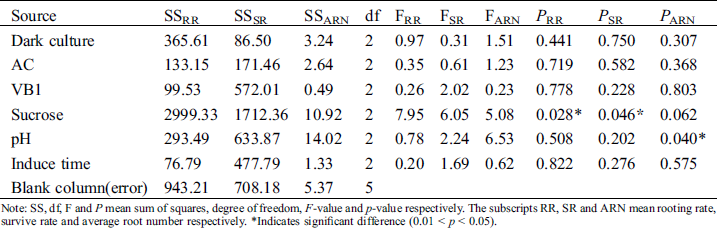
Note: SS, df, F and P mean sum of squares, degree of freedom, F-value and p-value respectively. The subscripts RR, SR and ARN mean rooting rate, survive rate and average root number respectively. *Indicates significant difference (0.01 < p < 0.05).
3.4 Orthogonal Test of the Effects of Glu, Cys, Gly, sucrose, Induction Period, and pH on Rooting
The influence of Glu, Cys, Gly, sucrose, induction period, and pH on rooting was analyzed using orthogonal tests. The results of 18 treatments are shown in Tab. 4. The rooting rate of 2 treatments (#17 and #18) reached 70%, while the rooting rate and average rooting number of 2 treatments (#6 and #15) were 0%. The survival rate of 3 treatments (#1, #2, and #3) was 100%, and that for 1 combination (#15) was the lowest, at 10.0%. The average number of roots of 3 treatments (#14, #17, and #18) exceeded 7 (7.5, 7.9, and 7.1, respectively).
Table 4: The experimental design based on L18(37) orthogonal array and effects of Glu, Cys, Gly, sucrose, induce period and pH on rooting
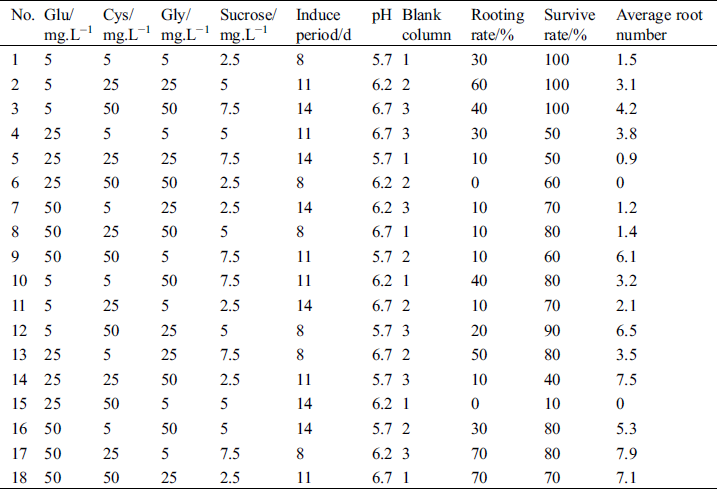
3.5 Intuitive Analysis of the Effects of Glu, Cys, Gly, sucrose, Induction Period, and pH on Rooting
The range analysis of orthogonal test results is shown in Tab. 5. The strongest effect on rooting rate (range 20.00) and average number of roots (range 2.85) was induction period. In three induction periods (8, 11, and 14 days), the rooting rate and average root number k values increased first and then decreased. The rooting rate and average root number k values were both the highest in the induction period of 11 days (36.67 and 5.13, respectively). Cys and sucrose had the least effect on rooting rate (range 8.33) and survival rate (range 6.67). Glu had the greatest effect on survival rate (range 41.67). In the three Glu concentrations of 5, 25, and 50 mg.L−1, the survival rate k values decreased first and then increased with increasing Glu concentration. The survival rate with 5 g.L−1 Glu was highest (k value 90.00). Gly had the least effect on the average number of roots (range 0.15).
Table 5: Intuitive analysis of effects of Glu, Cys, Gly, sucrose, induce period and pH on rooting
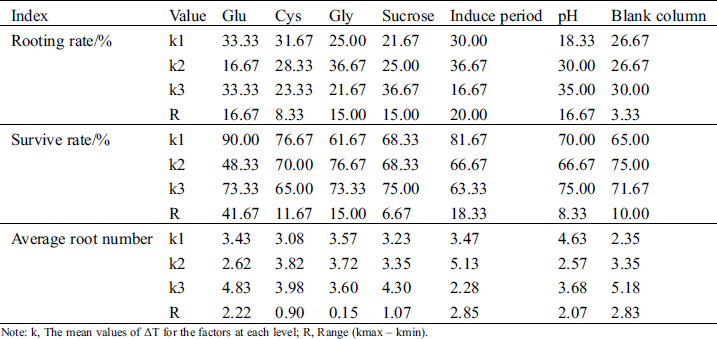
Note: k, The mean values of ΔT for the factors at each level; R, Range (kmax – kmin).
3.6 Variance Analysis of the Effects of Glu, Cys, Gly, sucrose, Induction Period, and pH on Rooting
Glu significantly influenced survival rate (p < 0.05) (Tab. 6). Other factors had no significant effect on rooting. Different from the effects of 5, 15, and 25 g.L−1 (Tab. 3), 2.5, 5, and 7.5 g.L−1 sucrose had no significant effect on rooting. pH of 5.7, 6.2, and 6.7 had a similar rooting effect. The F-values of the 6 influential factors for rooting rate revealed the following order: Glu > induction period > sucrose > Gly > pH > Cys. The F-values for survival rate were in the following order: Glu > induction period > Gly > Cys > pH > sucrose, and those for average root number were in the order: induction period > Glu > pH > sucrose > Cys > Gly.
Table 6: Variance analysis of effects of Glu, Cys, Gly, sucrose, induce period and pH on rooting
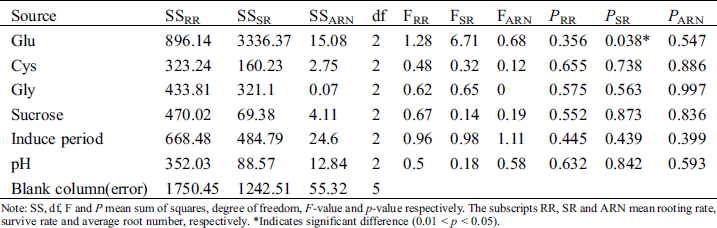
Note: SS, df, F and P mean sum of squares, degree of freedom, F-value and p-value respectively. The subscripts RR, SR and ARN mean rooting rate, survive rate and average root number, respectively. *Indicates significant difference (0.01 < p < 0.05).
3.7 Orthogonal Test of the Effects of IBA, sucrose, and pH on Rooting
The orthogonal test and the results of 9 treatments are shown in Tab. 7. The medium of all 9 treatments contained 5 mg.L−1 Glu. The rooting rate of 2 treatments (#1 and #5) were the highest, reaching 90%, and the lowest (#6) was 10%. The survival rate of 6 treatments (#1, #2, #4, #5, #7, and #8) was 100% and that of 1 combination (#6) was the lowest at 50.0%. The average root number of 1 combination (#1) was > 11 and a rooted shoot in vitro is shown in Fig. 1. The lowest average number of roots of 2 treatments (#3 and #9) was 2.5.
Table 7: The experimental design based on L18(34) orthogonal array and effects of IBA, sucrose and pH on rooting
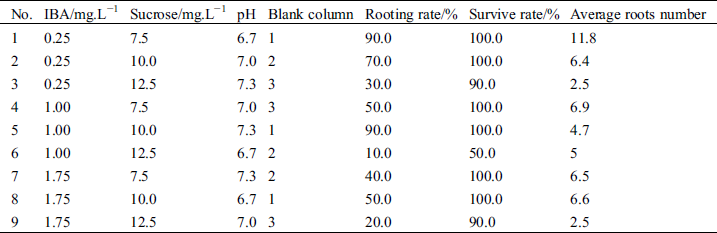

Figure 1: Root formation of hybrid larch in vitro. (a) Root growth of shoots on rooting medium without auxin for 7 weeks. (b) Rooted plantlets removed from culture vessel
3.8 Intuitive Analysis of the Effects of IBA, sucrose, and pH on Rooting
The range analysis of the orthogonal test is shown in Tab. 8. Sucrose had the greatest effect on the three indexes. The ranges of rooting rate, survival rate, and average root number were highest (50.00, 23.33, and 5.07, respectively). The k value of rooting rate in 10 g.L−1 sucrose was highest (70.0%), and that for survival rate in both 7.5 g.L−1 and 10 g.L−1 reached 100%. The k value of the average rooting number showed a downward trend with increasing sucrose, and the highest value was 8.4.
Table 8: Intuitive analysis of effects of IBA, sucrose and pH on rooting
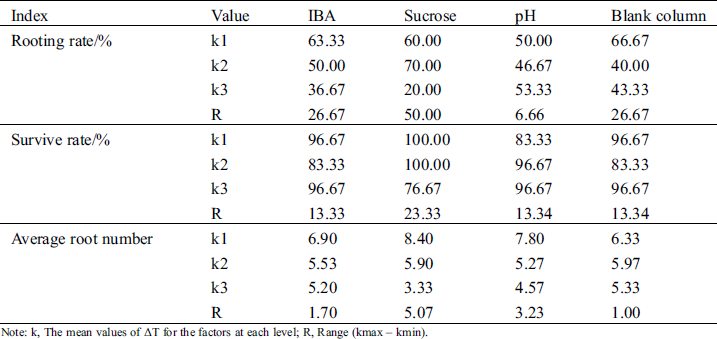
Note: k, The mean values of ΔT for the factors at each level; R, Range (kmax – kmin).
3.9 Variance Analysis of the Effects of IBA, sucrose, and pH on Rooting
Sucrose significantly influenced the average root number (p < 0.05) (Tab. 9). The F-values of the 3 influential factors on rooting rate revealed the following order: sucrose > IBA > pH; that for survival rate was in the order: sucrose > IBA > pH; and that of average root number was in the order: sucrose > pH > IBA. The variance analysis was in agreement with the results of range analysis; sucrose had the greatest effect on rooting.
Table 9: Variance analysis of effects of IBA, sucrose and pH on rooting
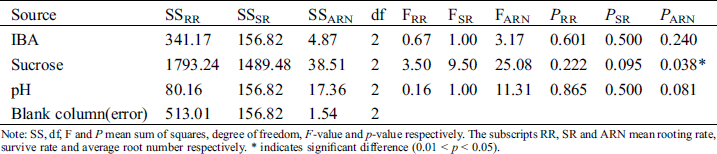
Note: SS, df, F and P mean sum of squares, degree of freedom, F-value and p-value respectively. The subscripts RR, SR and ARN mean rooting rate, survive rate and average root number respectively. * indicates significant difference (0.01 < p < 0.05).
The capacity for adventitious root formation is an important index of the economic value of woody plant varieties. A high rooting rate and large root number are standards for good rooting capacity [19]. Exogenous auxins have positive effects on rooting. IBA, NAA, and indole-3-acetic acid have different effects on root induction [20]. A higher rooting percentage and root number can be induced by NAA combined with IBA [21]. In this study, when 2.0 mg/L NAA was added to the medium, the IBA concentration did not significantly influence the root induction index. A higher rooting rate, survival rate, and average root number was obtained with medium containing 2.0 mg/L NAA and 0.25 mg/L IBA. Adventitious root formation is affected by light, AC, VB1, and induction time of rooting [13,17–18]. In this experiment, dark culture period, AC, VB1, and induction time had no significant effect on rooting. The optimal induction period with auxin is 8 days and the root primordium is formed on the eighth day after rooting induction [22,23].
Glutathione (GSH) plays multiple roles in the plant life cycle, including the regulation of root architecture and the detoxification of reactive oxygen species, heavy metals, and xenobiotics. GSH is composed of the amino-acids Glu, Cys, and Gly [24]. In our study, Gly and Cys had no significant effect on rooting capacity, but Glu had a significant effect on the survival rate of rooting shoots. Glu is an amino-N donor for the synthesis of other amino-acids and occupies a critical position in plant N metabolism [25]. GSH and Glu significantly alters root architecture [14], and GSH affects root growth by alterations in auxin homeostasis. Glu is associated with adventitious root initiation as a precursor of indole-3-acetic acid [26,27]. The addition of Glu had no significant effect on the rooting rate and number of hybrid shoots, which may be due to the Glu stored in shoots which meet the requirement of adventitious root formation. In our study, adding 5 mg.L−1 Glu to the culture medium had the best effect on rooting, and the survival rate of shoots reached 90%.
Sucrose provides the energy for cell growth, regulates osmotic pressure, and is the main carbohydrate source in tissue culture [15]. Sucrose contributes to the synthesis of cell constituents as a substrate of metabolites [28,29]. The sucrose concentration affects the growth of newly-generated roots. High concentrations inhibit the induction of adventitious roots, possibly because the higher osmotic pressure caused by excessive sucrose is deleterious to root primordium induction [15,28]. In our research, sucrose concentrations of 5, 15, and 25 g.L−1 had a significant effect on rooting rate and survival rate and each index showed a downward trend with increasing sucrose concentration. However, the sucrose concentrations 2.5, 5, and 7.5 g.L−1 did not significantly influence root induction. The concentrations 7.5, 10, and 12.5 g.L−1 had a significant effect on the average root number and 7.5 g.L−1 sucrose produced the highest average root number. Among the concentrations 2.5, 5, 7.5, 10, 12.5, 15, and 25 g.L−1, 7.5 g.L−1 sucrose had best effect, with a rooting rate of 60.0%, a survival rate of 100%, and an average root number of 8.4.
The pH of the medium influences the growth and development of explants by enhancing the activity of growth regulators and enzymes [30,31]. It is associated with the amount of callus formed [16], changes with NH4+ and NO3− uptake, and affects auxin uptake [32,33]. Cell differentiation and organ regeneration usually require a suitable pH. In most plants, the medium to induce adventitious rooting is slightly acidic. In our study, the pH values 5.2, 5.7 and 6.2 had a significant effect on the average root number, while the pH values among 5.7, 6.2 and 6.7 and among 6.7, 7.0 and 7.2 did not significantly influence root induction. The rooting effect was best when the pH of the medium was 6.7: the rooting rate was 50.00%, the average number of roots was 7.80, and the survival rate was 83.33%.
Funding Statement: This research was funded by The National Key Research and Development Program of China (Project No. 2017YFD0600401).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Hartmann, H. T., Kester, D. E., Davies, J. R., Geneve, R. L. (2002). Plant propagation: Principles and practices, pp. 1–10. Upper Saddle River: Prentice Hall. [Google Scholar]
2. Ewald, D. (2007). Micropropagation of Larix specials via organogenesis. In: Jain, S.M., Häggman, H., Protocols for micropropagation of woody trees and fruits, pp. 125–136. Dordrecht: Springer. [Google Scholar]
3. Lin, X. F., Zhang, W. B., Takano, H., Takio, S., Ono, K. (2004). Efficient plant regeneration and micropropagation from callus derived from mature zygotic embryo of Larix gmelinii. Plant Biotechnology, 21(2), 159–163. DOI 10.5511/plantbiotechnology.21.159. [Google Scholar] [CrossRef]
4. Ragonezi, C., Klimaszewska, K., Castro, M. R., Lima, M. Oliveira, P. et al. (2010). Adventitious rooting of conifers: Influence of physical and chemical factors. Trees, 24(6), 975–992. DOI 10.1007/s00468-010-0488-8. [Google Scholar] [CrossRef]
5. Chalupa, V. (2004). In vitro propagation of European larch (Larix decidua mill.). Journal of Forest Science, 50(12), 553–558. DOI 10.17221/4656-JFS. [Google Scholar] [CrossRef]
6. Ewald, D., Kretzschmar, U., Chen, Y. (1997). Continuous micropropagation of juvenile larch from different species via adventitious bud formation. Biologia Plantarum, 39(3), 321–329. DOI 10.1023/A:1000959621891. [Google Scholar] [CrossRef]
7. Birchler, J. A., Yao, H., Chudalayandi, S., Vaiman, D., Veitia, R. A. (2010). Heterosis. Plant Cell, 22(7), 2105–2112. DOI 10.1105/tpc.110.076133. [Google Scholar] [CrossRef]
8. Sun, X. M., Zhang, S. G., Zhou, D. Y., Wang, X. D., Ding, B. et al. (2008). Phenological variation of Larix species and their intra-species and inter-species hybrid families and early selection. Scientia Silvae Sinicae, 44(8), 77–84. DOI 10.1016/S1872-2040(08)60061-4. [Google Scholar] [CrossRef]
9. Chen, X. B., Sun, X. M., Dong, L. M., Zhang, S. G. (2018). Mating patterns and pollen dispersal in a Japanese larch (Larix kaempferi) clonal seed orchard: A case study. Science China-Life Sciences, 61(9), 1011–1023. DOI 10.1007/s11427-018-9305-7. [Google Scholar] [CrossRef]
10. Ishag, S., Osman, M., Khalafalla, M. (2009). Effects of growth regulators and genotype on shoot r regeneration in tomato. International Journal Sustainable Crop Production, 4(6), 7–13. [Google Scholar]
11. Bergmann, B. A., Stomp, A. (1994). Effect of genotype on rooting of hypocotyls and in vitro-produced shoots of Pinus radiata. Plant Cell, Tissue and Organ Culture, 39(3), 195–202. DOI 10.1007/BF00035970. [Google Scholar] [CrossRef]
12. Kim, S. G., Chang, J. R., Cha, H. C., Lee, K. W. (1988). Callus growth and plant regeneration in diverse cultivars of cucumber (Cucumis sativus L.). Plant Cell, Tissue and Organ Culture, 12(1), 67–84. DOI 10.1007/BF00043109. [Google Scholar] [CrossRef]
13. Fett-Neto, A. G., Fett, J. P., Veira-Goulart, L. W., Pasquali, G., Termignoni, R. R. et al. (2001). Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiology, 21(7), 457–464. DOI 10.1093/treephys/21.7.457. [Google Scholar] [CrossRef]
14. Walch-Liu, P., Liu, L. H., Remans, T., Tester, M., Forde, B. G. (2006). Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant and Cell Physiology, 47(8), 1045–1057. DOI 10.1093/pcp/pcj075. [Google Scholar] [CrossRef]
15. Cui, X. H., Murthy, H. N., Wu, C. H., Paek, K. Y. (2010). Sucrose-induced osmotic stress affects biomass, metabolite, and antioxidant levels in root suspension cultures of Hypericum perforatum L. Plant Cell, Tissue and Organ Culture (PCTOC), 103(1), 7–14. DOI 10.1007/s11240-010-9747-z. [Google Scholar] [CrossRef]
16. Leifert, C., Pryce, S., Lumsden, P. J., Waites, W. M. (1992). Effect of medium acidity on growth and rooting of different plant species growing in vitro. Plant Cell, Tissue and Organ Culture, 30(3), 171–179. DOI 10.1007/BF00040019. [Google Scholar] [CrossRef]
17. Yang, L., Xu, C. J., Hu, G. B., Chen, K. S. (2006). Direct shoot organogenesis and plant regeneration in Fortunella crassifolia. Biologia Plantarum, 50(4), 729–732. DOI 10.1007/s10535-006-0117-y. [Google Scholar] [CrossRef]
18. Rodríguez, R., Valledor, L., Sánchez, P., Fraga, M. F., Berdasco, M. et al. (2007). Propagation of selected pinus genotypes regardless of age. In: Jain, S.M., Häggman, H., Protocols for micropropagation of woody trees and fruits, pp. 137–146. Dordrecht: Springer. [Google Scholar]
19. Klerk, G. J. D., Brugge, J. T., Marinova, S. (1997). Effectiveness of indoleacetic acid, indolebutyric acid and naphthaleneacetic acid during adventitious root formation in vitro in malus ‘jork 9’. Plant Cell, Tissue and Organ Culture, 49(1), 39–44. DOI 10.1023/A:1005850222973. [Google Scholar] [CrossRef]
20. Gianguzzi, V., Barone, E., Sottile, A. F. (2020). In vitro rooting of Capparis spinosa L. as affected by genotype and by the proliferation method adopted during the multiplication phase. Plants, 9(3), 398–409. DOI 10.3390/plants9030398. [Google Scholar] [CrossRef]
21. Huang, H., Li, J. C., Ouyang, K. X., Zhao, X. H., Li, P. et al. (2014). Direct adventitious shoot organogenesis and plant regeneration from cotyledon explants in Neolamarckia cadamba. Plant Biotechnology, 31(2), 115–121. DOI 10.5511/plantbiotechnology.14.0125a. [Google Scholar] [CrossRef]
22. Li, K. P., Sun, X. M., Han, H., Zhang, S. G. (2014). Isolation, characterization and expression analysis of the baby boom (BBM) gene from Larix kaempferi × L. olgensis during adventitious rooting. Gene, 551(2), 111–118. DOI 10.1016/j.gene.2014.08.023. [Google Scholar] [CrossRef]
23. Wang, H. M., Li, K. P., Sun, X. M., Xie, Y. H., Han, X. M. et al. (2019). Isolation and characterization of larch BABY BOOM2 and its regulation of adventitious root development. Gene, 690, 90–98. DOI 10.1016/j.gene.2018.12.049. [Google Scholar] [CrossRef]
24. Rouhier, N., Lemaire, S. D., Jacquot, J. P. (2008). The role of glutathione in photosynthetic organisms: Emerging functions for glutaredoxins and glutathionylation. Annual Review of Plant Biology, 59(1), 143–166. DOI 10.1146/annurev.arplant.59.032607.092811. [Google Scholar] [CrossRef]
25. Stitt, M., Müller, C., Matt, P., Gibon, Y., Petronia, C. et al. (2002). Steps towards an integrated view of nitrogen metabolism. Journal of Experimental Botany, 53(370), 959–970. DOI 10.1093/jexbot/53.370.959. [Google Scholar] [CrossRef]
26. Blakesley, D., Weston, G. D., Hall, J. F. (1991). The role of endogenous auxin in root initiation. Plant Growth Regulation, 10(4), 341–353. DOI 10.1007/BF00024593. [Google Scholar] [CrossRef]
27. Cohen, J. D., Bialek, K. (1984). The biosynthesis and metabolism of plant hormones, pp. 165–182. Cambridge: Cambridge University Press. [Google Scholar]
28. Baque, M. A., Elgirban, A., Lee, E. J., Paek, K. Y. (2012). Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiologiae Plantarum, 34(2), 405–415. DOI 10.1007/s11738-011-0837-2. [Google Scholar] [CrossRef]
29. Zhang, J., Gao, W. Y., Wang, J., Li, X. L. (2012). Effects of sucrose concentration and exogenous hormones on growth and periplocin accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiologiae Plantarum, 34(4), 1345–1351. DOI 10.1007/s11738-012-0931-0. [Google Scholar] [CrossRef]
30. George, E. F., Hall, M. A., Hall, M. A., De Klerk, G. J. (2008). Plant propagation by tissue culture, pp. 115–174. Dordrecht: Springer. [Google Scholar]
31. Van Winkle, S. C., Johnson, S., Pullman, G. S. (2003). The impact of gelrite and activated carbon on the elemental composition of two conifer embryogenic tissue initiation media. Plant Cell Reports, 21(12), 1175–1182. DOI 10.1007/s00299-003-0637-2. [Google Scholar] [CrossRef]
32. De Klerk, G., Hanecakova, J., Jasik, J. (2008). Effect of medium-pH and MES on adventitious root formation from stem disks of apple. Plant Cell, Tissue and Organ Culture, 95(3), 285–292. DOI 10.1007/s11240-008-9442-5. [Google Scholar] [CrossRef]
33. Schmitz, U., Lörz, H. (1990). Nutrient uptake in suspension cultures of gramineae. II. Suspension cultures of rice (Oryza sativa L.). Plant Science, 66(1), 95–111. DOI 10.1016/0168-9452(90)90174-M. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |