

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013657
ARTICLE
Exogenous Selenium Mitigates Salt Stress in Soybean by Improving Growth, Physiology, Glutathione Homeostasis and Antioxidant Defense
1Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
2Department of Agronomy, Sher-e-Bangla Agricultural University, Dhaka, 1207, Bangladesh
*Corresponding Author: Mirza Hasanuzzaman. Email: mhzsauag@yahoo.com
Received: 15 August 2020; Accepted: 16 October 2020
Abstract: The mechanism of selenium (Se)-induced salt tolerance was studied in moderately sensitive soybean (Glycine max L.) plants. To execute this view, soybean plants were imposed with salt stress (EC 6 dS m−1) applying NaCl. In other treatments, Se (0, 25, 50 and 75 µM Na2SeO4) was sprayed as co-application with that level of salt stress. Plant height, stem diameter, leaf area, SPAD value decreased noticeably under salt stress. Altered proline (Pro) level, together with decreased leaf relative water content (RWC) was observed in salt-affected plants. Salt stress resulted in brutal oxidative damage and increased the content of H2O2, MDA level and electrolyte leakage. Exogenous Se spray alleviated oxidative damage through boosting up the antioxidant defense system by increasing the activity of antioxidant enzymes such as catalase (CAT), peroxidase (POD) and glutathione reductase (GR), as well as by improving non-enzymatic antioxidants like glutathione (GSH) and GSH/glutathione disulfide (GSSG). The upregulated antioxidant defense system, restored Pro and leaf RWC, higher SPAD value conferred better growth and development in Se-sprayed salt-affected soybean plants which altogether put forth for the progressive yield contributing parameters and finally, seed yield. Among different doses of Se, soybean plants sprayed with 50 µM Na2SeO4 showed better salt tolerance.
Keywords: Trace elements; selenium; antioxidant system; oxidative stress; salinity; oilseed crop
Global climate change is negatively affecting plants’ habitat and productivity. Anthropogenic activities are also major reasons for altering the micro or macro-environment for growing plants. Melting glaciers, sea-level rise, cyclone, typhoon, tsunami, and modified regimes of precipitation increase salt-affected areas [1,2]. Groundwater lifting for irrigation or other purposes may increase salinity. Poor drainage facilities and irrigation systems also increase salinity [3]. According to FAO [4], more than one-third of the irrigated lands in this planet are affected by salinity which is increasing gradually. Salt stress imposing the osmotic and ionic stress in plant cell/tissue causes anomaly in physiological and biochemical processes of plants [5–7]. Protein synthesis and many other primary metabolism pathways are inhibited by salt stress. Water and nutrient uptake and transport in plants is also hampered significantly when the plants exposed to salt stress. Photosynthesis, mitochondrial and cytosolic reactions, assimilate translocation are adversely affected under salt stress [8–10]. Salinity stress restrains plant growth and development, can cause drastic yield reduction. Severe salt stress can cause plant death [5]. Salt stress also causes oxidative damage as this stress generates excessive reactive oxygen species (ROS) such as superoxide anion (O2•−), hydroxyl radical (•OH), hydrogen peroxide (H2O2) and singlet oxygen (1O2) [11]. Scavenging ROS antioxidant system protects plants from oxidative damage. Non-enzymatic antioxidants (ascorbic acid, AsA; glutathione, GSH; phenolic compounds, alkaloids, non-protein amino acids and α-tocopherols) and antioxidant enzymes (superoxide dismutase, SOD; catalase, CAT; ascorbate peroxidase, APX; glutathione reductase, GR; monodehydroascorbate reductase, MDHAR; dehydroascorbate reductase, DHAR; glutathione peroxidase, GPX; and glutathione-S-transferase, GST) are the major components of the antioxidant defense system of plants. Enhanced antioxidant defense mechanism is considered as one of the criteria to improve salt stress tolerance [11].
Selenium (Se) is not considered as essential nutrients but its beneficial roles have been proved to improve plant growth and developmental processes. Selenium improves root and shoot growth, reproductive development and yield performance of crops [5,12–14]. Selenium improves different physiological processes of plants, enhance membrane stability and increase root growth [15–17]. Selenium induces accumulation of osmoprotective molecules [15,18] which help in higher uptake of water from soil or plant growing media. Selenium up-regulates the uptake of macro and micro nutrients [19] in plants. Selenium supplementation enhances abiotic stress tolerance in plants includes drought, salt stress, extreme temperature stresses and heavy metal stresses [14,20–22]. Exogenous Se application restricts the uptake of Na+ and Cl− and maintains better K+/Na+ homeostasis and maintains nutrient homeostasis in salt-affected plants which enhance salt stress tolerance [5,12]. Role of Se as a regulator of antioxidant defense system has been also reported in some plants [12,16,17,23].
Soybean (Glycine max L.) is one of one of the most versatile plants cultivated mainly for protein and oil consumption in both temperate and tropical environments. Being a moderate salt-susceptible crop soybean often faces salt stress under field growing condition, which results in damaging effects in terms of growth, reproductive development and yield performance [24,25]. So, enhancing salt stress tolerance in such a widely adapted crop species is significant. The present study demonstrates the effect of salt stress on physiology, growth and developmental attributes as well as on yield of soybean plants. This study also elucidates the role of different doses of Se to alleviate the salt-induced damages on those studied parameters in soybean plants.
2.1 Experimental Materials and Treatments
Soybean (Glycine max L.) seeds were grown in an experimental shed at the greenhouse. Empty plastic pots with 18-inch depth and 14-inch diameter were used for the experiments. Twelve kilograms sun-dried soils along with organic manures and fertilizers (according to recommended dose) were applied in each pot. After that, pots were prepared for seed sowing. Five seedlings were kept in each pot and there were two sets of pots – one for morpho-physiological investigation and one for yield traits measurement. The soil of the experiment was non-calcareous dark grey. The pH value of the soil was 5.6. Salinity (6 dS m−1) was imposed to plants by adding NaCl gradually with irrigation water starting from 30 days after sowing (DAS) and a respective control was maintained adding fresh water. Both control and salt-stressed plants were supplemented with four levels of Se (0, 25, 50 and 75 µM Na2SeO4). Selenium was applied as a foliar spray at 1-week interval up to 75 DAS. The experiment was laid out in a randomized complete block design (RCBD).
The height of the plants was recorded after the duration of treatment was completed, at 15 days interval starting from 45 DAS. It was measured beginning from the ground level up to the tip of the leaf. The average height of five plants was considered as the height of the plant for each pot. Stem diameters were measured with a slide calliper. For leaf area measurement, first leaf images were taken by a digital camera and the area was calculated by using Image-J software.
2.3 Determination of Relative Water Content
Leaf was weighed and considered as fresh weight (FW). After that leaf was immersed in DH2O in Petri dish between two layers of filter paper and placed in the dark for a few hours. After removing water from the leaf surface the leaf was weighed once more. This weight was recorded as turgid weight (TW). After oven drying the leaf was weight again and recorded as dry weight (DW). Then, RWC was calculated as follows [26]:
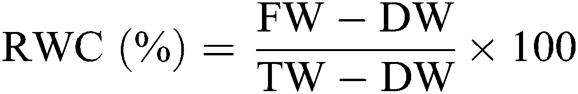
2.4 Determination of Proline Content
To estimate proline (Pro) Bates et al. [27] young leaf samples (0.5 g) was homogenized in sulfo-salicylic acid (3%) and centrifuged at 11,500 × g. The leaf extract, acid ninhydrin, and glacial acetic acid were mixed in 1:1:1 ratio, and then heated in a hot water bath. The solution was cooled in ice for the termination of reaction. Toluene was mixed thoroughly and the upper toluene chromophore with Pro was used for spectrophotometric measurement at 520 nm. A standard curve used to determine Pro concentrations.
Five leaves were randomly selected from each pot. Three places of each leaflet were measured by SPAD meter (atLEAF, FT Green LLC, USA) as SPAD.
Plant sample (0.5 g leaf) was collected from each pot and then entered into the 15 ml empty falcon tube and then filled up with distilled water. After this falcon tubes were put into water bath at 40°C for 60 min. After that electrical conductivity (EC1) was taken by using the EC meter. Then falcon tubes were put into an autoclave. After autoclaving falcon tubes were cooled in room temperature then EC2 was taken. Electrolyte leakage (EL) was determined by using the following formula: EL = (EC1/EC2) × 100.
2.7 Estimation of H2O2 Content
Potassium-phosphate buffer (pH 6.5) was used to homogenize leaf sample (0.5 g). Then it was centrifuged (11,500 × g) for 15 min. Leaf extract was mixed with 0.1% TiCl4 in 20% H2SO4 (v/v). The supernatant was read for its absorbance at 410 nm in spectrophotometer. The H2O2 content was calculated using an extinction coefficient of 0.28 μM−1 cm−1 and expressed in nmol g−1 FW [28].
2.8 Determination of Lipid Peroxidation
Membrane lipid peroxidation had been estimated based on malondialdehyde (MDA) content [29]. Leaf tissue (0.5 g) was homogenized with trichloroacetic acid (TCA), and centrifuged at 11,500 × g for 12 min. Using thiobarbituric acid, MDA was determined in the UV-visible spectrophotometer at 532 nm, and corrected at 600 nm for non-specific absorbance.
The extraction buffer was 5% meta-phosphoric acid amended with ethylenediaminetetraacetic acid (EDTA). Leaf sample (0.5 g) was homogenized and centrifuged (11,500 × g) to collect supernatant, which was neutralized by K-P buffer (pH 7.0).
Glutathione was measured following the method of Hasanuzzaman et al. [29]. Reduced glutathione (GSH) was oxidized by 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB) and reduced with NADPH in the presence of GR. The absorption changes of 2-nitro-5 thiobenzoic acid (NTB) produced from the reduction of DTNB was measured. We used UV-visible spectrophotometer and absorbance was recorded at 412 nm.
Oxidized glutathione (GSSG) was determined removing GSH by 2-vinylpyridine. Total GSH and GSSG were measured by using standard curves with known concentrations of GSH and GSSG. Then, the GSH has been calculated subtracting GSSG from total glutathione.
2.10 Antioxidant Enzymes’ Activity Assay
Leaf (0.5 g) was homogenized in K-P buffer (pH 7.0) containing AsA, KCl, β-mercaptoethanol, and 10% glycerol (w/v) and the homogenate was centrifuged at 11,500 × g for 15 min.
Protein was detected [30] using bovine serum albumin (BSA) as protein standard.
CAT (EC: 1.11.1.6) activity was assayed considering reduction in optical absorbance recorded for 1 min at 240 nm caused by the degradation of H2O2 in reaction mixture comprised of K-P buffer (pH 7.0), H2O2, and enzyme solution. During calculating CAT activity extinction coefficient of 39.4 M−1 cm−1 was used [29].
DHAR (EC: 1.8.5.1) activity was determined using K-P buffer (pH 7.0), GSH, EDTA, and DHA were mixed to form the reaction buffer solution. The DHAR activity was estimated by measuring the absorbance at 265 nm for 1 min and 14 mM−1 cm−1 was the extinction coefficient [31].
GR (EC: 1.6.4.2) activity was determined using a reaction buffer solution of K-P buffer (pH 7), EDTA, GSSG, NADPH, and enzyme. Reduced absorbance at 340 nm for 1 min was recorded. The GR activity then was estimated using 6.2 mM−1 cm−1 as the extinction coefficient [29].
Peroxidase (POD, EC: 1.11.1.7) activity was estimated according to Nakano et al. [31].
2.11 Yield Attributes and Yields
The total number of pod plant-1 was counted from selected ten sample plants and then averaged. Ten pods from each plant were selected, and seeds were counted from each individual pod and then averaged. One hundred clean sun-dried grains were counted from the seed stock obtained from the sample plants and weighed by using an electronic balance. The seeds were separated manually and then sun-dried and weighed.
The obtained data were subjected to one-way analysis of variance (ANOVA) and mean means were separated following Tukey’s honestly significant difference (HSD) test at p ≤ 0.05.
Plant height was observed at 45, 60 and 75 DAS. Plant height increased due to the addition of Se, compared to control treatment (under non-stressed condition). Increase of Se dose gradually increased the plant height except for 75 µM Se. Rather this dose decreased the plant height. Salt stress caused a reduction of plant height by 31%, compared to control whereas Se supplementation in salt growing media restored the plant height except the 75 µM Se. This trend of the plant height was noticed at 45, 60 and 75 DAS (Figs. 1A–1C).
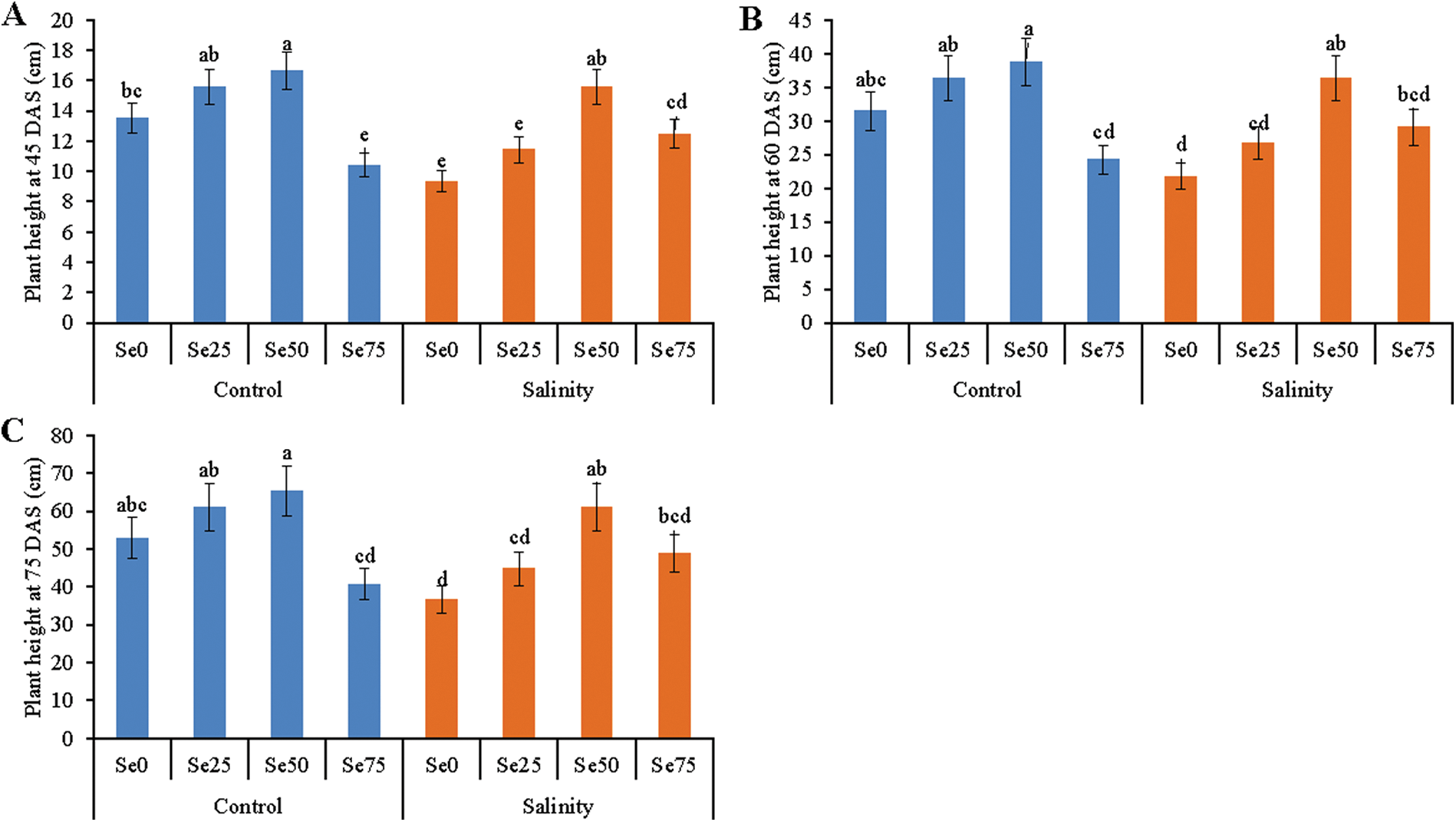
Figure 1: Effect of Se (0, 25, 50 and 75 µM Na2SeO4) on plant height at 45 days after sowing (DAS) (A), 60 DAS (B) and 75 DAS (C) in soybean plants grown under non-saline and salt-stress (6 dS m−1, induced by NaCl) condition. Data presented are the means ± SD of three replicates and data followed by the same letters are not significantly different at p < 0.05 as per Tukey’s HSD test
3.2 Leaf Area and Stem Diameter
The leaf area showed a slight increase in Se sprayed treatments than the control treatment. The imposition of salt stress decreased the leaf area of soybean plants, compared to control. Selenium sprayed soybean plants exposed to salt stress retrieved the leaf area of salt-affected plants except for the addition of 75 µM Se (Fig. 2A).
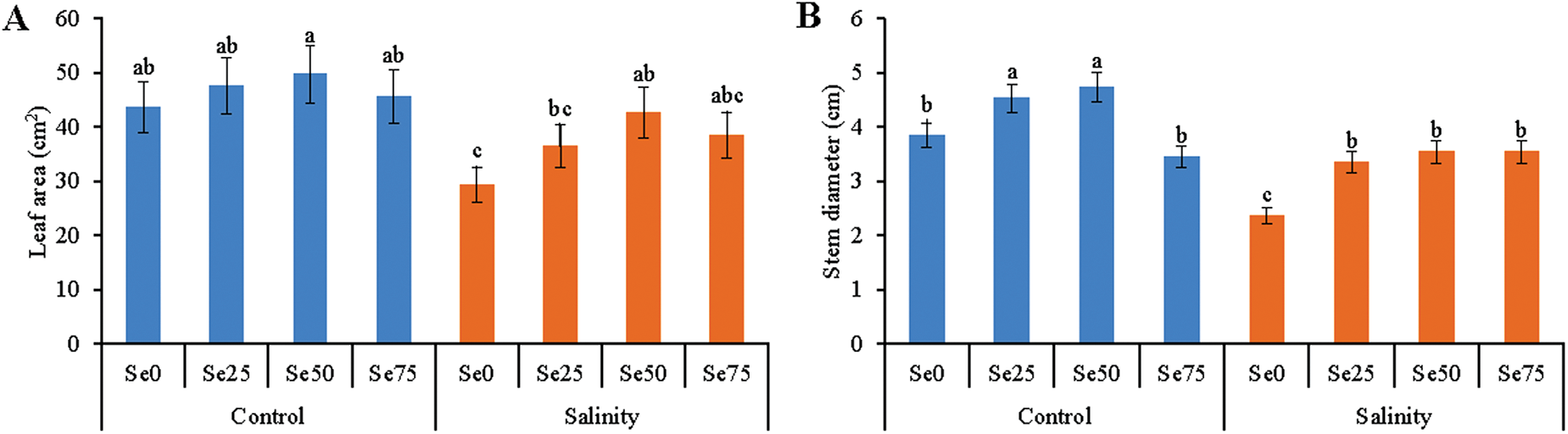
Figure 2: Effect of Se (0, 25, 50 and 75 µM Na2SeO4) on leaf area (A) and stem diameter (B) in soybean plants grown under non-saline and salt-stress (6 dS m−1, induced by NaCl) condition. Data presented are the means ± SD of three replicates and data followed by the same letters are not significantly different at p < 0.05 as per Tukey’s HSD test
Selenium (25 and 50 µM) increased stem diameter of soybean plants than the control treatment. Salt stress reduced the diameter, compared to control. When Se was applied the salt-affected plants reduced the damaging effects of salt stress and the stem diameter was increased, compared to salt-affected plants. The highest dose of Se failed to increase the stem diameter of salt-stressed plants (Fig. 2B).
Reduction of leaf RWC under salt stress, compared to the control treatment had been noticed in soybean plants. Selenium spray at the rate of 25 and 50 µM with the salt added soil increased the RWC by 24% and 38%, respectively. No change was observed for 75 µM Se addition under salt stress (Fig. 3A).
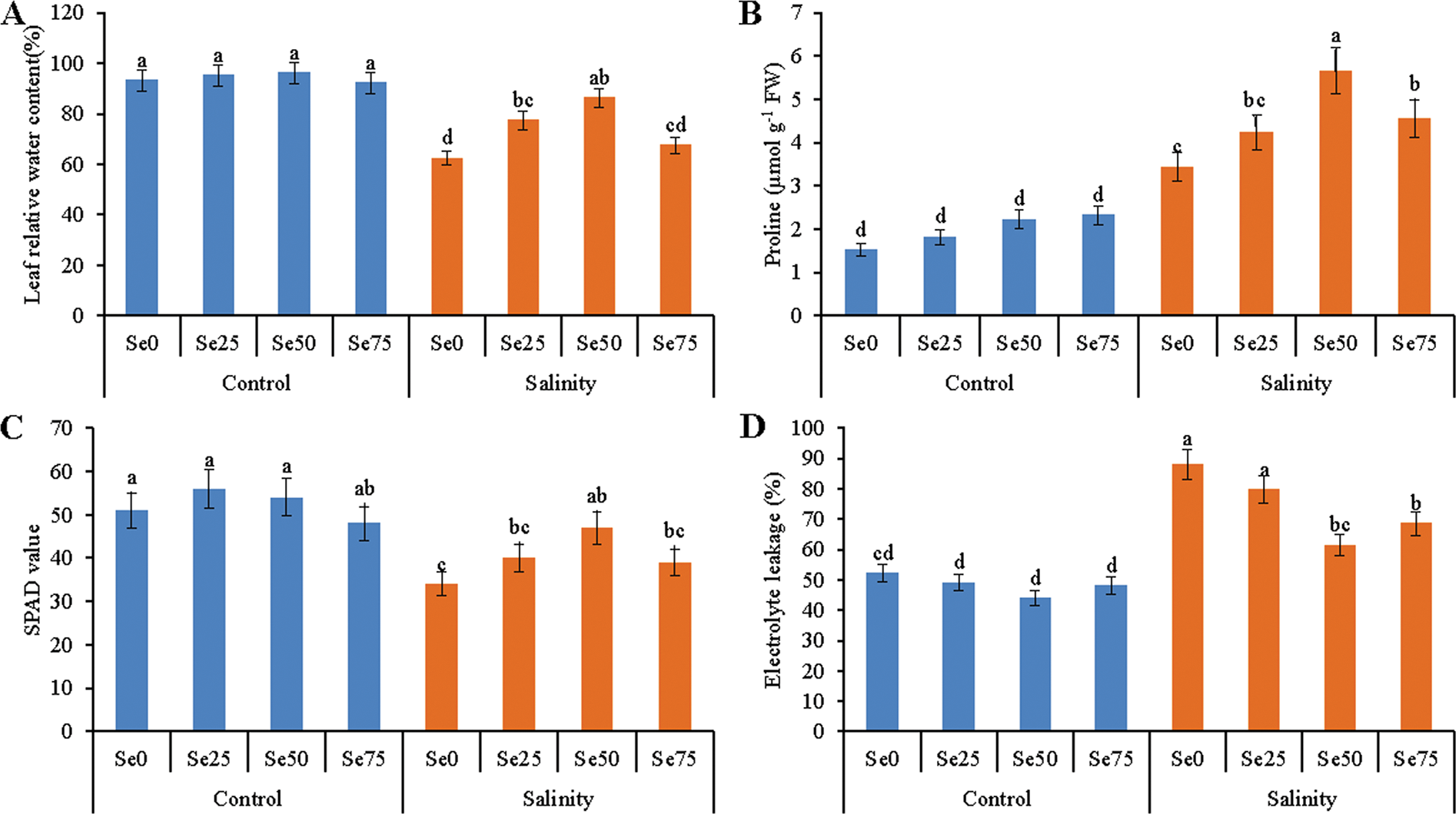
Figure 3: Effect of Se (0, 25, 50 and 75 µM Na2SeO4) on leaf relative water content (RWC) (A), proline (Pro) content (B), SPAD value (C) and electrolyte leakage (D) in soybean plants grown under non-saline and salt-stress (6 dS m−1, induced by NaCl) condition. Data presented are the means ± SD of three replicates and data followed by the same letters are not significantly different at p < 0.05 as per Tukey’s HSD test
Salt stress increased the Pro content of soybean plants by 56%, compared to control. Further increase of Pro level by 24%, 63% and 31%, were recorded in 25, 50 and 75 µM Se added soybean plants under salt stress, compared to only salt-stressed soybean plants (Fig. 3B).
The SPAD values of leaves reduced drastically due to salt toxicity. Salt affected plants showed 33% reduction in SPAD value when compared with control. Alleviating the damaging effect of salt stress to some extent 50 µM Se co-treatment improved the SPAD value by 38% than the salt-affected soybean plants without Se addition. Neither the 25 µM nor the 75 µM Se addition was able to improve the SPAD value of salt-affected plants (Fig. 3C).
Salt stress caused electrolyte leakage as evidenced by 69% increase than the control. Selenium supplementation with salt stress decreased electrolyte leakage. In case of 25 µM Se addition with salinity the change of electrolyte leakage was not significant while 50 and 75 µM Se decreased electrolyte leakage by 30% and 22%, compared to that of salt distressed soybean plants (Fig. 3D).
While looking at the non-stress condition, i.e., growing media without salinity exogenous Se addition slightly increased H2O2 level (compared to control) except 25 µM Se. Salt stress caused higher generation of H2O2 by 75%, compared to control. Co-treatment of Se of 25, 50 and 75 µM as foliar spray reduced H2O2 level by 21%, 36% and 36%, respectively, compared to salt stress alone (Fig. 4A).
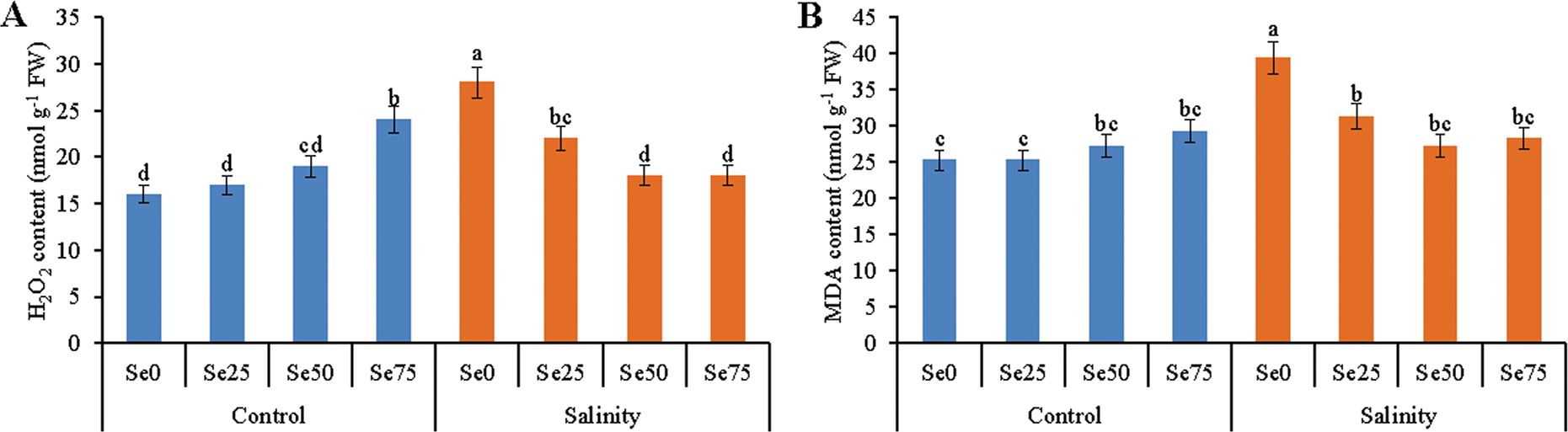
Figure 4: Effect of Se (0, 25, 50 and 75 µM Na2SeO4) on the content of H2O2 (A) and MDA (B) in soybean plants grown under non-saline and salt-stress (6 dS m−1, induced by NaCl) condition. Data presented are the means ± SD of three replicates and data followed by the same letters are not significantly different at p < 0.05 as per Tukey’s HSD test
There was no difference among the MDA levels of non-stressed treatments. Increase of MDA level is one of the salt stress-induced oxidative stress indicators. The imposition of salt stress increased MDA by 56%, compared to control. This increase of MDA level reversed when salt-imposed soybean plants were supplemented with Se. Soybean plants sprayed with 25, 50 and 75 µM Se diminished the MDA level by 21%, 31% and 28%, respectively, compared to the MDA level of salt-affected soybean plants (Fig. 4B).
Soybean plants showed differential responses for glutathione metabolism. Salt stress did not alter GSH level but highly raised the GSSG level (by 56%), compared to control treatment (Figs. 5A and 5B). As a result, a drastic reduction of GSH/GSSG by 43% was confirmed, compared to that ratio of control plants (Fig. 5C). Adding of 25 and 50 µM Se increased GSH level by 14% and 34%, respectively in salt-affected soybean plants, compared to salt stress only (Fig. 5A). Foliar spray of 25, 50 and 75 µM Se with salt treatment lessened the GSSG level by 28%, 31% and 30%, respectively, compared salt treated plants alone (Fig. 5B). As a result, higher GSH/GSSG of 43%, 94% and 54%, respectively were observed in 25, 50 and 74 µM Se treated salt-affected plants (compared to salt-affected plants without Se spray) (Fig. 5C).
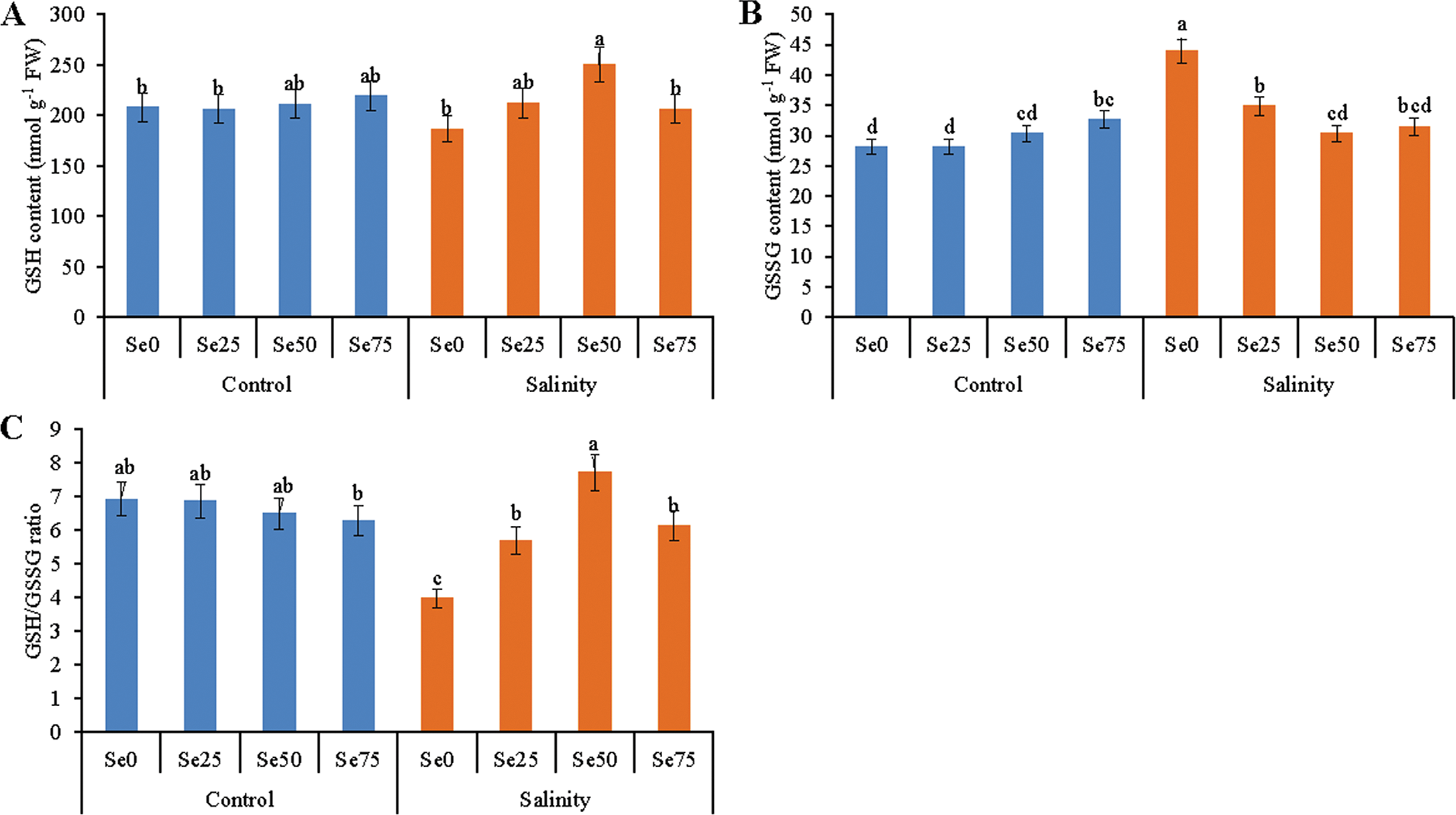
Figure 5: Effect of Se (0, 25, 50 and 75 µM Na2SeO4) on the content of GSH (A) and GSSG (B); GSH/GSSG (C) in soybean plants grown under non-saline and salt-stress (6 dS m−1, induced by NaCl) condition. Data presented are the means ± SD of three replicates and data followed by the same letters are not significantly different at p < 0.05 as per Tukey’s HSD test
3.6 Activities of Antioxidant Enzymes
Salt stress was the reason for the reduction of GR activity by 32% in contrast to control. Its activity was restored by Se addition. An increase in GR activity by 22% was noticed for 25 µM Se addition with salt treatment, compared to salt treatment alone. Adding 50 and 75 µM Se with salt treatment upregulated GR activity by 58% and 74%, respectively compared to salt stress treatment (Fig. 6A).
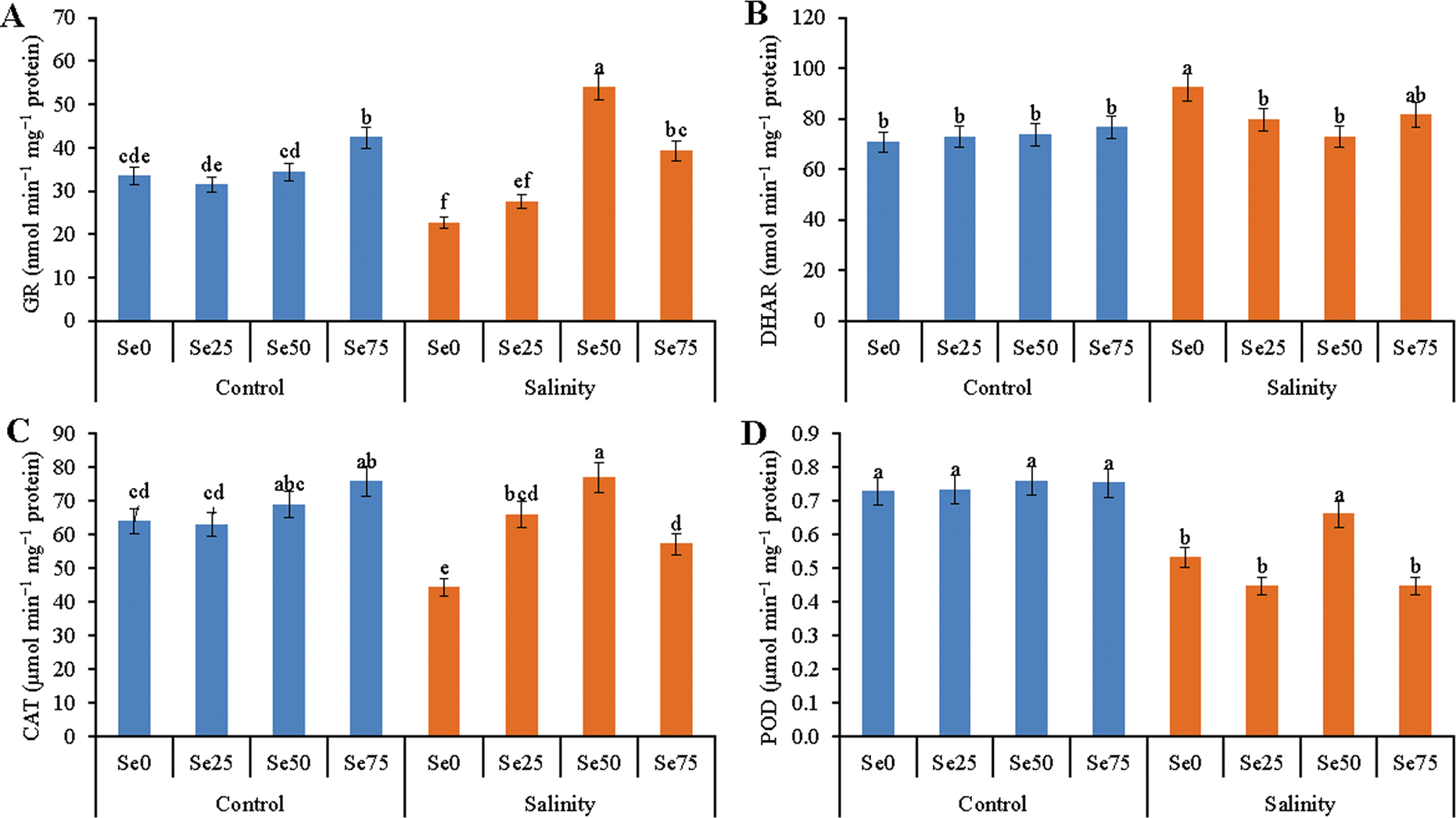
Figure 6: Effect of Se (0, 25, 50 and 75 µM Na2SeO4) on the activity of GR (A), DHAR (B), CAT (C) and POD (D) in soybean plants under non-saline and salt-stress (6 dS m−1, induced by NaCl) condition. Data presented are the means ± SD of three replicates and data followed by the same letters are not significantly different at p < 0.05 as per Tukey’s HSD test
The activity of DHAR increased under salt stress by 31% when compared to control. Slight decrease in DHAR activity had been noticed in Se added salt treated plants irrespective of dose of Se (Fig. 6B).
The activity of CAT highly decreased due to salt stress by 31%, compared to control. Exogenous Se addition restored its activity in salt-affected soybean plants. The highest increase of CAT activity (by 73%, compared to control) was recorded for 50 µM Se under salt stress, compared to salt treatment alone. Without this, when 25 and 75 µM Se were applied as a foliar spray, the CAT activity increased by 49% and 29% in salt treated plants, compared to salt treatment alone (Fig. 6C).
Drastic reduction in POD activity was induced by salt stress. Its activity reduced by 27%, compared to control. In case of 25 and 75 µM Se added salt treatment the POD activity didn’t increase but the addition of 50 µM Se increased POD activity increased by 24% under salt stress, compared to salt stress alone (Fig. 6D).
Salt toxicity adversely affected different physiological attributes and growth parameters. As a result, salt-affected plants showed a reduction in number of pods plant−1. Salt stress resulted in 43% reduction in number of pods plant−1 than the control plants without salt stress. While adding Se, this parameter showed better performance showing the putative effect due to application of 50 µM Se. Foliar spray of 50 µM Se with salinity resulted in 41% higher number of pods plant−1 than the salt treatment without Se (Fig. 7A).
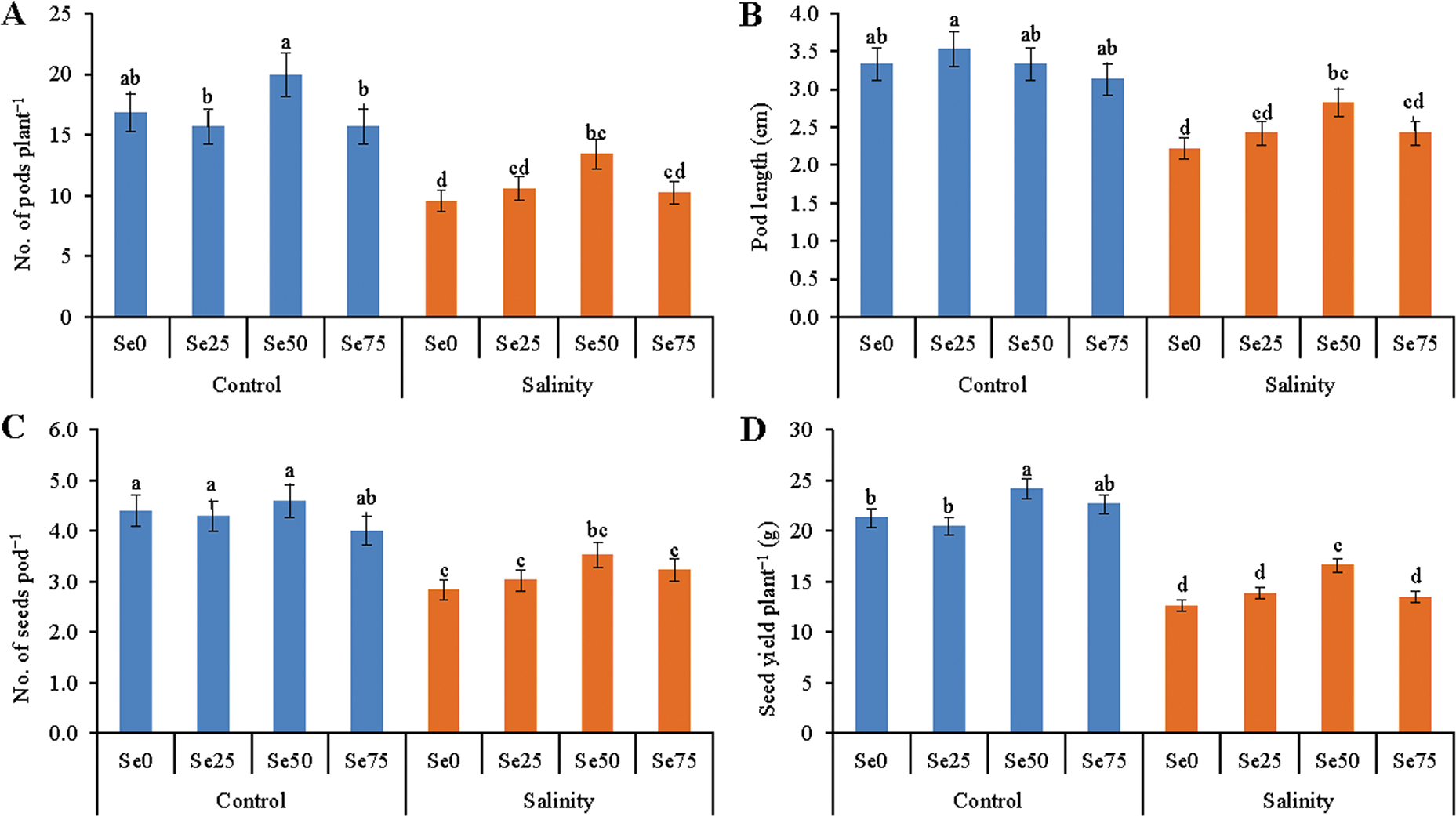
Figure 7: Effect of Se (0, 25, 50 and 75 µM Na2SeO4) on No. of pods plant−1 (A), pod length (B), No. of seeds pod−1 (C) and seed yield plant−1 (D) in soybean plants grown under non-saline and salt-stress (6 dS m−1, induced by NaCl) condition. Data presented are the means ± SD of three replicates and data followed by the same letters are not significantly different at p < 0.05 as per Tukey’s HSD test
Selenium application could not improve the pod length significantly under non-stress condition, compared to control. As a result of salt stress pod length decreased by 50%, compared to control. Selenium addition increased the pod length of salt-affected plants with the highest (by 27%, compared salt treatment alone) increase in 50 µM Se addition with salt stress (Fig. 7B).
In this study, no. of seeds pod−1 negatively affected by salt stress showing a reduction by 36%, compared to control. In case of Se added salt treatment increase of no. of seeds pod−1 was noticed for 50 µM Se addition. Addition of 50 µM Se with salt stress caused 24% increase of no. of seeds pod−1, compared to salt treatment alone (Fig. 7C).
Salt stress significantly decreased the seed yield of soybean. Soybean plants suppressed with 6 dS m−1 NaCl resulted 41% reduction of seed yield plant−1, compared to control plants without salt stress. The results again demonstrated that 50 µM Se was more proficient in alleviating injurious effect of salt stress; in this case, Se supplementation increased seed yield plant−1 by 32%, compared to salt treatment alone (Fig. 7D).
Being a moderate salt-susceptible crop soybean cannot tolerate high saline condition [24,25], and this plant may face salt stress in their growing period. In this study, we investigated the effect of salt stress on soybean and of the protective role of exogenous Se.
Salt-induced primary stress is water stress caused by altered osmotic potential differences between the outer side of the root and inner cytosol of the root. This reduced water uptake adversely affects growth processes. Salt stress causes distorted cytosolic metabolism, reduction of assimilates production and mobilization of photosynthetic products. These can hamper vegetative as well as reproductive growth and development [32,33]. Reduction of photosynthetic pigment content, decreased photosynthesis rate also affect growth under salt stress. In the present study plant height, stem diameter and leaf area examined as growth parameters also decreased under salt stress, but Se-sprayed soybean plants reversed those negative effects on growth parameters and improved growth parameters had noticed, compared to salt stress alone. Several previous reports proved that Se put forth beneficial effects on growth parameters and improved different growth parameters such as dry matter, plant height, leaf area, etc., in salt-affected plants [12,34].
Due to alteration of osmotic potential in the root zone of a plant grown in the salt imposed growing media plants suffer from osmotic stress that reduces the water uptake, compared to the normal growing condition without any stress. So, salt-affected plants show decreased water content in the plant [6,7]. The leaf RWC decreased in salt stressed soybean plants in our study, compared to control. Again, when Se was applied as foliar spray plants recovered from that stress condition to some extent and improved leaf RWC was noticed, compared to salt-affected plants without Se supplementation [6,7]. Higher accumulation of endogenous Pro by exogenous Se addition in the present studied soybean plants and the consequent osmoprotective function is supposed to work in improving the leaf RWC [6,7]. Some other research findings not only observed higher Pro accumulation but also observed higher accumulation of other osmoprotectants like soluble protein, sugar [15] in Se treated salt-affected plants which can improve plant water uptake. Selenium-induced improved root growth [15,16] and membrane structure of plants [6,7,11] improve water uptake by root and transport it to other parts of the plants, which increase the water content.
As an osmolyte, Pro has been proved itself as a regulator of osmotic potential and water potential. Up-regulation of Pro is a common phenomenon of any kind of reduced water stress induced by either direct drought stress, or physiological drought stress occurred as a secondary effect of other stresses like salt stress, metal toxicity stress, etc. [29]. Increase of Pro has been documented in broad-spectrum of cases for salt stressed plants as plants have their own stress adaptation mechanism [15,18]. Salt affected plants of this present experiment accumulated higher Pro, compared to control. In this study, compared to control, the addition of Se as foliar spray resulted in a slight increase in Pro level (compared to control) indicating its role in Pro biosynthesis or regulating metabolism or degradation [15,18,35]. Moreover, compared to the only salt treated plants, Se prayed salt treated plants showed significant higher Pro accumulation demonstrating the role of Se as a regulator of Pro accumulation. In previous studies, other plants showed an acceleration of Pro accumulation by Se addition under salt stress [15,18] and this evidence indicates the regulatory role Se for Pro biosynthesis or degradation. In their study, Sattar et al. [15] proved that Se is not the only enhancer of Pro accumulation but also enhancer for soluble protein, sugar accumulation in wheat plants under salt stress which acted as vital osmoregulators for enhancing salt tolerance.
The decreased SPAD value indicating the greenness of leaf contributed by decreased chlorophyll (chl) content under salt stress (compared to control) whereas Se sprayed salt-affected plants showed higher chl level (compared to salt stress alone). Salt stress can increase the ROS production, which has damaging effects on the structural integrity of the membrane of the cell as well as the membrane of its ultrastructural organs. So, disintegration of the chloroplast membrane can decrease the Chl or greenness of leaves [12,36]. Again salt-induced reduction in magnesium (Mg) content can be correlated to the reduction of Chl content as it is the vital structural component of Chl. In the case of Se supplemented salt-affected soybean plants, the Mg content as well as the Chl content increased significantly (compared to salt stress alone) [37]. Supplementation of Se in salt-affected Solanum melongena L. cv. Baladi increased the chl content (compared to only salt stress treatment), which was indicated by higher SPAD value [36]. These findings support the finding of the present study that foliar spray of Se increased SPAD value of salt-affected soybean plants.
Like other abiotic stresses, salt stress provokes oxidative stress in different ways. Modified stomatal conductance, disrupted photosystem activity, altered enzymatic activities of cytosol, chloroplast, mitochondrial or other ultrastructural organs are the common reasons for salt-induced oxidative stress [38,39]. Salt stress highly increased the content of H2O2 and MDA as well as the electrolyte leakage in the soybean plants in the present study, which are clear indicators of oxidative stress. Selenium spray decreased the H2O2 and MDA as well as the electrolyte leakage, compared to salt stress alone and in this case, the lowest reduction of these oxidative stress markers has been documented for 50 µM Se. Selenium has been proved to up-regulate the content of different non-enzymatic antioxidants in addition to the activity of enzymes of the antioxidant defense system under stressed conditions which alleviate oxidative stress induced by different abiotic stress [23,40]. Similar to the findings of our study, previous studies also reported the decreased H2O2 and MDA under salt stress [11,16] and other abiotic stresses [23,41,42] due to Se supplementation.
Glutathione is one of the strongest ROS scavengers which decreased due to salt stress but increased in Se supplemented salt-affected plants. Increase of H2O2 and MDA as well as the electrolyte leakage in the soybean plants partly due to this reduction of GSH level, this finding is supported by previous other reports [11,43]. The GSH is oxidized to GSSG during scavenging ROS. So, a high level of GSSG is a clue that the plant is in stress, and the similar rise of GSSG in salt-affected soybean plants of the present study indicate that the salt stress effected on those plants. However, when the exogenous Se was sprayed on salt-affected soybean plants, the GSH level increased (with the higher increase in 50 µM Se) and GSSG level declined significantly which are the mark for stress relaxation. So, it can be assumed that Se can reduce the salt stress effects to some degree [11]. Not only the content of GSH but also the GSH/GSSG plays a vital role to maintain cellular redox homeostasis. The role of GSH/GSSG in intercellular signaling system is vital for the adaptive response of plants [44–46]. Drastic reduction of GSH/GSSG was the result of salt stress, and this detrimental effect was alleviated to a great extent when exogenous Se was applied as a foliar spray, and an upregulated GSH/GSSG was noticed (compared to salt stress alone). This up-regulated GSH/GSSG is suggested to play a crucial role in maintaining cellular redox homeostasis and adaptive response for salt stress tolerance in soybean plants of the present study [11,44–46].
Catalase is one of the most capable and active enzymes for scavenging H2O2. Severe reduction of the CAT activity of salt distressed soybean plants showed a high level of H2O2. The activity of POD also decreased in salt added treatment (compared to control). The DHAR is one of the major enzymes in ascorbate-glutathione (AsA-GSH) cycle and liable for recycling of ascorbate, which is a very strong ROS scavenger. Noteworthy decrease of DHAR activity was observed in salt-affected soybean plants. In AsA-GSH cycle the oxidized GSSG is recycled back to the reduced form GSH by the activity of GR and continuously support the antioxidant defense system doing the work of ROS scavenging. Salt stress decreased the GR activity at such level that GSH level decreased, GSSG and GSH/GSSG altered to cause a significant increase of ROS and subsequent oxidative damage. Then, in Se sprayed salt-affected soybean plants, the activity of GR increased in such point that GSH level increased, GSSG level decreased, and GSH/GSSG improved to cause significant down-regulation of ROS generation and the consequent oxidative injury. Foliar spray of Se with salt stress also up-regulated the activity of CAT and POD, compared to salt stress alone. These higher enzymatic activities also contributed to the reduction of ROS and oxidative damage. The altered activity of enzymatic antioxidants as affected by salt stress (compared to control) of the present study and then their up-regulation in Se added salt treatment (compared to the salt stress alone) are in the same line with the findings of several previous studies [11,12,16,17]. There can be assorted elucidation for Se persuaded improved antioxidant defense system. Selenium priming boosted the expression of mitogen-activated protein kinase and calcium-dependent protein kinase genes which act as a signal to improve the antioxidant defense system components [12]. Exogenous Se addition improved the translocation of minerals like iron, zinc, and manganese towards the rice shoot [19] and as these minerals are biochemical, structural components of antioxidant enzymes which improved the SOD, POD, and CAT activities [47]. These reviews suggest and support the findings of the present study, where it is evident that Se improves the antioxidant defense system and decrease oxidative stress. There might have other cross-talk between and among Se, ROS, antioxidant components and other elements or compounds of soybean plants which demands further investigation.
Seed yield is one of the ultimate goals of soybean plant production as the seed is its edible part. Salinity reasons a 20%–40% reduction in seed yield of soybean [48,49]. Liu et al. [5] reported a 30%–58% reduction of seed weight under salt stress. Salt stress can decrease the seed yield of the soybean plants due to various reasons. Salt stress caused a reduction of the total number of flower, decreased pollen size and deformed its shape, reduced pollen wall thickness, pollen aperture size, pollen viability, pollen germination rate and pollen tube length [50]. These kinds of reproductive development anomalies are a major reason to reduce pod set, pod size, seed set and thus reduce soybean yield. In the present study, the plants were imposed with salt stress from the beginning of their growth stage which unfavorably affected the vegetative growth and reproductive development. As a consequence, decreased no. of pods plant−1, pod length, no. of seeds pod−1 were recorded which were then contributed in the reduction of seed yield plant−1 and these damaging effects of salt stress are similar to previous other studies [5,48,49,51,52]. Higher Na+ and the Cl− concentrations in seeds and pod walls hampered pod development, caused reduction in pod number plant−1, seed number plant−1, seed weight plant−1 and 100-seed weight [5]. However, foliar spray of Se alleviated the damaging effect of salt stress, improved the yield contributing characters and the final yield. This result is supported by previous research findings that Se addition increases yield in soybean or other crops [53,54].
The present study investigates the beneficial role exogenous Se in improving salt tolerance in soybean plants. Selenium played a pivotal function for alleviating oxidative damage through regulating the antioxidant defense system, which includes both the non-enzymatic and enzymatic components. Selenium successfully restored the greenness of leaves from chlorosis induced by salt toxicity. Thus, Se spray in salt affected soybean plants improved the biochemical together with the physiological attributes which played a constructive role to recover soybean plants from damage due to salinity. Application of Se as foliar spray improved growth parameters, yield contributing characters and the targeted seed yield. However, keeping the regulatory role of Se in mind, that was obtained from the present study and from the previous other studies, further research can be considered in various aspects. Dose-dependent experiment with different growth stages, the function of Se in different biosynthesis, metabolic or catabolic pathways, role of Se in different other physiological processes can be executed under different types of salt stress. There might have cross-talk between and among Se, ROS, antioxidant components, other elements or compounds of soybean plants which demands further investigation.
Funding Statement: This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under Grant No. (RG-20-130-40). The authors, therefore, acknowledge with thanks to DSR technical and financial support.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
1. Nicholls, R. J., Cazenave, A. (2010). Sea-level rise and its impact on coastal zones. Science, 328(5985), 1517–1520. DOI 10.1126/science.1185782. [Google Scholar] [CrossRef]
2. IPCC. (2014). (Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: IPCC. [Google Scholar]
3. Rengasamy, P. (2006). World salinization with emphasis on Australia. Journal of Experimental Botany, 57(5), 1017–1023. DOI 10.1093/jxb/erj108. [Google Scholar] [CrossRef]
4. FAO. (2011). The state of the world’s land and water resources for food and agriculture (SOLAW)—Managing systems at risk (Food and Agriculture Organization of the United Nations and Earthscan). http://www.fao.org/3/a-i1688e.pdf. [Google Scholar]
5. Liu, Y., Yu, L., Qu, Y., Chen, J. Liu, X. et al. (2016). GmSALT3, which confers improved soybean salt tolerance in the field, increases leaf Cl− exclusion prior to Na+ exclusion but does not improve early vigor under salinity. Frontiers in Plant Science, 7(25), 697. DOI 10.3389/fpls.2016.01485. [Google Scholar] [CrossRef]
6. Mozafariyan, M., Kamelmanesh, M. M., Hawrylak-Nowak, B. (2016). Ameliorative effect of selenium on tomato plants grown under salinity stress. Archives of Agronomy and Soil Science, 62(10), 1368–1380. DOI 10.1080/03650340.2016.1149816. [Google Scholar] [CrossRef]
7. Astaneh, R. K., Bolandnazar, S., Nahandi, F. Z., Oustan, S. (2018). The effects of selenium on some physiological traits and K, Na concentration of garlic (Allium sativum L.) under NaCl stress. Information Processing in Agriculture, 5(1), 156–161. DOI 10.1016/j.inpa.2017.09.003. [Google Scholar] [CrossRef]
8. Chartzoulakis, K., Klapaki, G. (2000). Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Scientia Horticulturae, 86(3), 247–260. DOI 10.1016/S0304-4238(00)00151-5. [Google Scholar] [CrossRef]
9. Tanveer, M., Shahzad, B., Sharma, A., Biju, S., Bhardwaj, R. (2018). 24-epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiology & Biochemistry, 130, 69–79. [Google Scholar]
10. Rattan, A., Kapoor, D., Kapoor, N., Bhardwaj, R., Sharma, A. (2020). Brassinosteroids regulate functional components of antioxidative defense system in salt stressed maize seedlings. Journal of Plant Growth Regulation, 7, 276. DOI 10.1007/s00344-020-10097-1. [Google Scholar] [CrossRef]
11. Hasanuzzaman, M., Hossain, M. A., Fujita, M. (2011). Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biological Trace Element Research, 143(3), 1704–1721. DOI 10.1007/s12011-011-8958-4. [Google Scholar] [CrossRef]
12. Jiang, C., Zu, C., Lu, D., Zheng, Q., Shen, J. et al. (2017). Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Scientific Reports, 7(1), 441. DOI 10.1038/srep42039. [Google Scholar] [CrossRef]
13. Hawrylak-Nowak, B., Matraszek, R., Pogorzelec, M. (2015). The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiologiae Plantarum, 37(2), 169. DOI 10.1007/s11738-015-1788-9. [Google Scholar] [CrossRef]
14. Hawrylak-Nowak, B., Dresler, S., Rubinowska, K., Matraszek-Gawron, R., Woch, W. et al. (2018). Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiology and Biochemistry, 127, 446–456. DOI 10.1016/j.plaphy.2018.04.018. [Google Scholar] [CrossRef]
15. Sattar, A., Cheema, M. A., Abbas, T., Sher, A., Ijaz, M. et al. (2017). Separate and combined effects of silicon and selenium on salt tolerance of wheat plants. Russian Journal of Plant Physiology, 64(3), 341–348. DOI 10.1134/S1021443717030141. [Google Scholar] [CrossRef]
16. Astaneh, R. K., Bolandnazar, S., Nahandi, F. Z., Oustan, S. (2019). Effects of selenium on enzymatic changes and productivity of garlic under salinity stress. South African Journal of Botany, 121, 447–455. DOI 10.1016/j.sajb.2018.10.037. [Google Scholar] [CrossRef]
17. Subramanyam, K., Du Laing, G., Van Damme, E. J. M. (2019). Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Frontiers in Plant Science, 10, 61. DOI 10.3389/fpls.2019.00116. [Google Scholar] [CrossRef]
18. Hawrylak-Nowak, B. (2009). Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biological Trace Element Research, 132(1–3), 259–269. DOI 10.1007/s12011-009-8402-1. [Google Scholar] [CrossRef]
19. Moulick, D., Santra, S. C., Ghosh, D. (2018). Seed priming with Se mitigates As-induced phytotoxicity in rice seedlings by enhancing essential micronutrient uptake and translocation and reducing As translocation. Environmental Science and Pollution Research, 25(27), 26978–26991. DOI 10.1007/s11356-018-2711-x. [Google Scholar] [CrossRef]
20. Hawrylak-Nowak, B., Matraszek, R., Szymańska, M. (2010). Selenium modifies the effect of short-term chilling stress on cucumber plants. Biological Trace Element Research, 138(1–3), 307–315. DOI 10.1007/s12011-010-8613-5. [Google Scholar] [CrossRef]
21. Hawrylak-Nowak, B., Dresler, S., Wójcik, M. (2014). Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Scientia Horticulturae, 172, 10–18. DOI 10.1016/j.scienta.2014.03.040. [Google Scholar] [CrossRef]
22. Hawrylak-Nowak, B., Hasanuzzaman, M., Matraszek-Gawron, R. (2018). Mechanisms of selenium-induced enhancement of abiotic stress tolerance in plants. In: Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B. (eds.Plant nutrients and abiotic stress tolerance, pp. 269–295. Singapore: Springer. [Google Scholar]
23. Hasanuzzaman, M., Nahar, K., Alam, M. M., Fujita, M. (2014). Modulation of antioxidant machinery and the methylglyoxal detoxification system in selenium supplemented Brassica napus seedlings confers tolerance to high temperature stress. Biological Trace Element Research, 161(3), 297–307. DOI 10.1007/s12011-014-0120-7. [Google Scholar] [CrossRef]
24. Katerji, N., van. Hoorn, J. W., Hamdy, A., Mastrorilli, M. (2000). Salt tolerance classification of crops according to soil salinity and to water stress day index. Agricultural Water Management, 43(1), 99–109. DOI 10.1016/S0378-3774(99)00048-7. [Google Scholar] [CrossRef]
25. Tuyen, D. D., Lal, S. K., Xu, D. H. (2010). Identification of a major QTL allele from wild soybean (Glycine soja Sieb. & Zucc.) for increasing alkaline salt tolerance in soybean. Theoretical and Applied Genetics, 121(2), 229–236. DOI 10.1007/s00122-010-1304-y. [Google Scholar] [CrossRef]
26. Barrs, H. D., Weatherley, P. E. (1962). A re-examination of the relative turgidity technique for estimating water deficits in leaves. Australian Journal of Biological Sciences, 15(3), 413–428. DOI 10.1071/BI9620413. [Google Scholar] [CrossRef]
27. Bates, L. S., Waldren, R. P., Teari, D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 39(1), 205– 207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
28. Yu, C. W., Murphy, T. M., Lin, C. H. (2003). Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Functional Plant Biology, 30(9), 955–963. DOI 10.1071/FP03091. [Google Scholar] [CrossRef]
29. Hasanuzzaman, M., Nahar, K., Anee, T. I., Fujita, M. (2017). Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Frontiers in Plant Science, 8, 395. DOI 10.3389/fpls.2017.01061. [Google Scholar] [CrossRef]
30. Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
31. Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880. [Google Scholar]
32. Purwaningrahayu, D. R., Sebayang, T. H., Syekhfani, S., Aini, N. (2015). Resistance level of some soybean (Glycine max L. Merrill) genotypes towards salinity stress. Journal of Biological Researches, 20(2), 7–14. DOI 10.23869/bphjbr.20.2.20152. [Google Scholar] [CrossRef]
33. Jacob, O. O., Francis, N. I. (2015). Effect of different levels of NaCl and Na2SO4 salinity on dry matter and ionic content of cowpea (Vigna unguiculata L.). African Journal of Agricultural Research, 10, 1239–1243. [Google Scholar]
34. Hawrylak-Nowak, B., Rubinowska, K., Molas, J., Woch, W., Matraszek-Gawron, R. et al. (2019). Selenium-induced improvements in the ornamental value and salt stress resistance of Plectranthus scutellarioides (L.) R. Br. Folia Horticulturae, 31(1), 213–221. DOI 10.2478/fhort-2019-0016. [Google Scholar] [CrossRef]
35. Sharma, A., Kapoor, D., Wang, J., landi, M., Zheng, B. et al. (2020). Nitric oxide mediated mechanisms adopted by plants to cope with salinity. Biologia Plantarum, 64(II), 512–518. DOI 10.32615/bp.2020.070. [Google Scholar] [CrossRef]
36. Abul-Soud, M. A., Abd-Elrahman, S. H. (2016). Foliar selenium application to improve the tolerance of eggplant grown under salt stress conditions. International Journal of Plant & Soil Science, 9(1), 1–10. DOI 10.9734/IJPSS/2016/19992. [Google Scholar] [CrossRef]
37. Ardebili, N. O., Saadatmand, S., Niknam, V., Khavari-Nejad, R. A. (2014). The alleviating effects of selenium and salicylic acid in salinity exposed soybean. Acta Physiologiae Plantarum, 36(12), 3199–3205. DOI 10.1007/s11738-014-1686-6. [Google Scholar] [CrossRef]
38. Hasanuzzaman, M., Nahar, K., Fujita, M. (2013). Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad, P., Azooz, M. M., Prasad, M. N. V. (eds.Ecophysiology and responses of plants under salt stress, pp. 25–87. New York: Springer. [Google Scholar]
39. Hasanuzzaman, M., Oku, H., Nahar, K., Bhuyan, M. H. M. B., Mahmud, J. A. et al. (2018). Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnology Reports, 12(2), 77–92. DOI 10.1007/s11816-018-0480-0. [Google Scholar] [CrossRef]
40. Rady, M. M., Belal, H. E. E., Gadallah, F. M., Semida, W. M. (2020). Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Scientia Horticulturae, 266, 109290. DOI 10.1016/j.scienta.2020.109290. [Google Scholar] [CrossRef]
41. Hasanuzzaman, M., Fujita, M. (2011). Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biological Trace Element Research, 143(3), 1758–1776. DOI 10.1007/s12011-011-8998-9. [Google Scholar] [CrossRef]
42. Hasanuzzaman, M., Hossain, M. A., Fujita, M. (2012). Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating the antioxidant defense and methylglyoxal detoxification systems. Biological Trace Element Research, 149(2), 248–261. DOI 10.1007/s12011-012-9419-4. [Google Scholar] [CrossRef]
43. Hasanuzzaman, M., Nahar, K., Rohman, M. M., Anee, T. I., Huang, Y. et al. (2018). Exogenous silicon protects Brassica napus plants from salinity-induced oxidative stress through the modulation of AsA-GSH pathway, thiol-dependent antioxidant enzymes and glyoxalase systems. Gesunde Pflanzen, 70(4), 185–194. DOI 10.1007/s10343-018-0430-3. [Google Scholar] [CrossRef]
44. Szalai, G., KellÅs, T., Galiba, G., Kocsy, G. (2009). Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. Plant Growth Regulation, 28(1), 66–80. DOI 10.1007/s00344-008-9075-2. [Google Scholar] [CrossRef]
45. Kumar, B., Singla-Pareek, S. L., Sopory, S. K. (2010). Glutathione homeostasis: Crucial for abiotic stress tolerance in plants. In: Pareek, A., Sopory, S. K., Bohnert, J. H., Govindjee, A. (eds.Abiotic stress adaptation in plants: physiological, molecular and genomic foundation, pp. 263–282. New York: Springer. [Google Scholar]
46. Nahar, K., Hasanuzzaman, M., Fujita, M. (2016). Physiological roles of glutathione in conferring abiotic stress tolerance to plants. In: Gill, S. S., Tuteja, N. (eds.Abiotic stress response in plants, pp. 151–179. Weinheim: Wiley. [Google Scholar]
47. Nouet, C. C., Motte, P., Hanikenne, M. (2011). Chloroplastic and mitochondrial metal homeostasis. Trends in Plant Science, 16(7), 395–404. DOI 10.1016/j.tplants.2011.03.005. [Google Scholar] [CrossRef]
48. Chinnusamy, V., Jagendorf, A., Zhu, J. K. (2005). Understanding and improving salt tolerance in plants. Crop Science, 45(2), 437–448. DOI 10.2135/cropsci2005.0437. [Google Scholar] [CrossRef]
49. Papiernik, S. K., Grieve, C. M., Lesch, S. M., Yates, S. R. (2005). Effects of salinity, imazethapyr, and chlorimuron application on soybean growth and yield. Communications in Soil Science and Plant Analysis, 36(7–8), 951–967. DOI 10.1081/CSS-200050280. [Google Scholar] [CrossRef]
50. Kilic, S., Seker, A. (2018). Potential impact of salt stress on male reproductive development of Glycine max (L.) Merr. (Soybean). International Journal of Environment, Agriculture and Biotechnology, 3(1), 132–139. DOI 10.22161/ijeab/3.1.17. [Google Scholar] [CrossRef]
51. Yasuta, Y., Kokubun, M. (2015). Salinity tolerance of super-nodulating soybean genotype. Plant Production Science, 17(1), 32–40. DOI 10.1626/pps.17.32. [Google Scholar] [CrossRef]
52. EL Sabagh, A., Barutçular, C., Saneoka, H. (2015). Assessment of drought tolerance maize hybrids at grain growth stage in Mediterranean area. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering, 9, 962–965. [Google Scholar]
53. Agarwal, N., Singh, A., Ashok, K. (2015). Salinity effects on growth and productivity of two soybean genotypes (Glycine max L.). Indian Journal of Scientific Research, 6, 59–69. [Google Scholar]
54. Zahedi, S. M., Abdelrahman, M., Hosseini, M. S., Hoveizeh, N. F., Tran, L. S. P. (2019). Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environmental Pollution, 253, 246–258. DOI 10.1016/j.envpol.2019.04.078. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |