

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013259
ARTICLE
Potassium-Induced Regulation of Cellular Antioxidant Defense and Improvement of Physiological Processes in Wheat under Water Deficit Condition
1Department of Agronomy, Sher-e-Bangla Agricultural University, Dhaka, 1207, Bangladesh
2Citrus Research Center, Bangladesh Agricultural Research Institute, Jaintapur, 3156, Bangladesh
3Department of Agroforestry and Environmental Science, Sher-e-Bangla Agricultural University, Dhaka, 1207, Bangladesh
4Department of Agricultural Botany, Sher-e-Bangla Agricultural University, Dhaka, 1207, Bangladesh
5Laboratory of Plant Stress Responses, Department of Applied Biological Sciences, Kagawa University, Kagawa, 761-0795, Japan
*Corresponding Author: Mirza Hasanuzzaman. Email: mhzsauag@yahoo.com
Received: 30 July 2020; Accepted: 07 October 2020
Abstract: Drought is the most common form of abiotic stress that reduces plant growth and productivity. It causes plant injuries through elevated production of reactive oxygen species (ROS). Potassium (K) is a vital plant nutrient that notably ameliorates the detrimental effect of drought stress in the plant. A pot experiment was conducted at the Laboratory of Plant Stress Responses, Faculty of Agriculture, Kagawa University, Japan, under controlled environment of green house to explore the role of K in mitigating drought severity in wheat (Triticum asevitum L.) seedlings. Three days after germination, seedlings were exposed to three water regimes viz., 100, 50, and 20% field capacity (FC) for 21 days. Potassium was adjusted in Hoagland nutrient solution at 0, 6 and 12 mM concentration and applied to pot instead of normal water. Results show that, water deficit stress notably reduced plant growth, biomass accumulation, leaf relative water content (RWC) along with reduced photosynthetic pigments. Increased amount of biochemical stress markers viz., malondialdehyde (MDA), hydrogen peroxide (H2O2), methylglyoxal (MG), proline (Pro) as well as an impaired antioxidant defense system were observed in drought affected wheat plants. On the contrary, K supplementation resulted in improvement of biochemical and physiological parameters that worked behind in improving growth and development of the wheat plants. In addition, enzymes of ascorbate-glutathione (AsA-GSH) cycle were also enhanced by supplemented K that accelerated the ROS detoxification process in plant. Although glyoxalse system did not performed well till MG was detoxified might following another short stepped pathways. Our results revealed that drought stressed plants showed better performances in terms of biochemical and physiological attributes, antioxidant defense and glyoxalase system, as well as ROS detoxification due to K supplementation with better performance at 12 mM K added in 50% FC growing condition.
Keywords: Abiotic stress; drought; plant nutrient; relative water content; reactive oxygen species; antioxidant; H2O2; methylglyoxal
Climate change is constantly happening due to numerous anthropogenic activities in every corner of the world. Scientists predict that, by 2050 world will be the habitat of approximately 10.9 billion people which may be a big challenge to feed this ever-increasing population along with different environmental stresses [1]. Both biotic and abiotic factors are responsible for reducing crop productivity where abiotic factors are the most damaging that causes more than 50% reduction of world crop productivity [2]. Deficit water stress or drought stress cumulatively affects all plant tissues through impairing the morphological, physiological along with biochemical traits and eventually decreases the yield attributes [3]. Drought drastically reduces the respiration and stomatal conductance, photosynthetic efficiency, carboxylation and water-use efficiency (WUE), carbon dioxide (CO2) diffusion and transpiration rate, electron transport system (ETS) and enzymatic activities [4]. In stress conditions, different types of ROS viz., singlet oxygen (1O2), superoxide anion (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (OH•) are produced in plant those adversely affect the entire plant physiology [5]. Excessive production of H2O2 is a vital indicator of oxidative stress in plant. Drought can alone produce around 70% of H2O2 through the photorespiration in mitochondria, and chloroplasts [6]. In stressed condition, H2O2 act as a signaling molecule when it is generated in small amount but over production of H2O2 beyond the detoxification capacity causes cell damage, photosynthesis retardation and other metabolic abnormalities in plants [7]. Drought cumulatively imparts oxidative damage to essential biomolecules and alters the antioxidant defense system. Lipid peroxidation and cell membrane damage, as well as disabling nucleic acids and proteins, are a few common phenomena [3]. Therefore, to defend against the oxidative damage, an antioxidant defense system comprises of antioxidant enzymes (GPX; glutathione peroxidase, CAT; catalase, APX; ascorbate peroxidase, DHAR; dehydroascorbate reductase, MDHAR; monodehydroascorbate reductase, GST; glutathione-S-transferase, SOD; superoxide dismutase) and non-enzymatic antioxidants (non-protein amino acids; alkaloids; ascorbic acid, AsA; glutathione, GSH; phenolic compounds; α-tocopherol) protect plants during stress periods [8]. Methylglyoxal (MG) is the byproduct of the plant respiration system, which is cytotoxic and tends to increased many folds under particular abiotic stress conditions. Furthermore, Glyoxalase I and II (Gly I and Gly II) are two vital enzymes that protect plants from the deleterious effect of MG in the presence of GSH [3]. In previous findings on mustard, it also observed that a slight adjustment in the glyoxalase system could increase plant tolerance against various abiotic stresses to a great extent [9]. However, plant tolerant to drought is entirely dependent on the cumulative action of both antioxidant defense and glyoxalase system, which simply determine the amount of overproduced ROS scavenged by the plant [10].
Potassium—as an essential macronutrient, takes part in most of the biochemical reactions in plants. Stomatal regulation, osmotic adjustment, protein synthesis, carbohydrate metabolism, and enzyme activation are few vital plant processes where K regulates either the rate of reaction or the amount of produces [11]. In addition, the gaseous exchange rate, turgor pressure, and ionic conductivity get increased as hydraulic conductivity increases while plants are supplemented with K [12]. For long-distance transport of both inorganic and organic anions through the vascular bundle, K is the dominant cation in neutralizing the anions. Therefore, uninterrupted supply and accumulation of K is regarded as a vital force for osmotic cell expansion [13]. Also, the supplementation of K enhances the grain yield and crop quality through increasing nutrient translocation and photosynthetic CO2 fixation [14]. Drought limits nutrient uptake by root due to moisture scarcity in the soil, which declines the nutrient diffusion rate in the soil to the root surface. As a result, crop growth and productivity get limited while K diffusion in rhizosphere soil and dissolution of K from organic or inorganic matter by microorganisms is hampered [15]. On the contrary, mineral supplementation promotes root growth under drought stress that accelerates the water and major ions transportation capacity from deep soil.
Wheat is a vital cereal crop that is consumed as staple food by 21% of world’s population. Compared to other major cereals, wheat provides a substantial amount of essential proteins in human diet [16]. Although wheat consumption is increasing worldwide, it has yet not occupied the satisfactory level of production due to detrimental effect of environmental stresses. K is a vital mineral nutrient for wheat production where about 67% of the wheat fields still encounter K deficiency [17]. Therefore, Low K reduces photosynthates and biomass production under drought stress while maintaining high K in tissues gives plant better protection against the drought condition [18,19]. In wheat, high K application rate increased spike length, grain size and grain yield by improving photosynthesis and photosynthates translocation from source to sink [20]. Only a few studies have been conducted to investigate the underlying mechanism and role of K during drought stress. Hardly any study focused the role K in modulating antioxidant defense system, glyoxalase pathway as well as nutrient homeostasis under drought stress. Addressing these facts, the present study was conducted to explore the K-induced improvement of physiological processes and modulation of antioxidant defense system under deficit water stress in wheat seedlings.
2.1 Seed Germination and Stress Treatments
At the beginning, Wagner pots (25 cm diameter) were filled with 5 kg salt-free sundried sand. Later on, water was added up to when water started to accumulate over the soil surface and this condition was considered as the over saturation point. The pot left for 24 h to remove water by gravitational force. This condition of water status was counted as saturation point where ½ amount of water required for saturation point was considered as Well-watered or 100% FC. Water levels for field capacities (100%, 50% and 20%) were controlled by balancing different amount of water to each pot every three alter days and were continued till 21 days. Healthy and mechanical injury-free wheat seeds were sorted manually and dipped in distilled water (DH2O) for 4 h immediately after surface-sterilizing by 70% ethanol for 8–10 mins. Twenty-five seeds were sown in each pot, maintaining homogenous distance. Finally, after germination, ten uniform seedlings were kept pot-1. The entire experiment was performed under a source of natural sunlight and ambient photoperiodic condition was facilitated along with controlled airflow. Three days after germination, seedlings were watered with regulated K doses at three different concentrations viz., 0, 6, and 12 mM in Hoagland’s nutrient solution. Plants were allowed to grow in these water regimes (100%, 50% and 20% FC) for 21 days. Later on, a continuous drought stress was imposed by the withdrawal of water for 9 days. Data were studied for different morpho-physiological and biochemical parameters from harvested leaves after 9 days stress period. Following the completely randomized design (CRD) with a combination of total 9 treatments, the entire experiment was repeated three times (3 replications).
2.2 Plant Height and Biomass Production
Average of 10 randomly selected plants from ground-level to the tip of the leaf at two intervals (15 and 30 DAS) was considered to calculate the plant height. After completion of stress period, average of 10 whole plants were taken for fresh weight (FW) and dry weight (DW) plant-1. After washing the plants, water was soaked by paper towel and FW was balanced. Then the samples were dried in an electric dryer (ADVANTEK, SP-450, and Japan) at 80°C for 48 h. The estimated weight after drying was finally averaged to derive the plant dry weight (DW).
2.3 Estimation of Relative Water Content (RWC) and Proline (Pro) Content
To measure the RWC, method described by Barrs et al. [21] was followed. According to the method, leaf laminas of 5 leaves were weighed as FW and then immersed in distilled water in a Petri dish between two layers of filter paper in a dark place for 12 h. After removing excess surface water by a paper towel, leaf turgid weight (TW) was balanced. Leaves DW was measured after drying at 80°C for 48 h. Finally, RWC was calculated using the following formula:
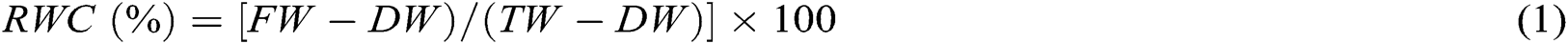
For estimation of Pro content, fresh leaves were homogenated with 3% sulfosalicylic acid at 11500× g followed by the mixture of supernatant, acid ninhydrin solution (ninhydrin in glacial acetic acid mixed with 6 M phosphoric acid), glacial acetic acid at 1:1:1 ratio. The entire mixture was incubated for 1 h at 100°C. At 520 nm, toluene mixed chromophore was observed under the spectrophotometer. Finally, the amount of Pro in the plant sample was derived from the standard curve using laboratory-grade Pro [22].
2.4 Determination of Photosynthetic Pigments content
To measure the photosynthetic pigments content according to Arnon [23], 0.5 g fresh leaf tissue was grinded with 10 mL acetone (80%) by a pre-cooled mortar and pestle. The homogenate was then centrifuged for 10 min at 2000×g. The supernatant was transferred in to test tubes, and after required dilution, the absorbance was measured at 663, 645, and 470 nm for chlorophyll (chl) a, b, and carotenoid contents, respectively, with the help of a UV-visible spectrophotometer. chl (a + b) is the summation values of chl a, and chl b.
2.5 Determination of Oxidative Stress Indicators
To estimate MDA content, 3 mL 5% (w/v) trichloroacetic acid (TCA) was used to homogenate 0.5 g leaf sample and then centrifuged at 11500×g for 12 min. Centrifuged supernatant and thiobarbituric acid (TBA) reagent (0.5% of TBA in 20% TCA) was then mixed at 4:1 ratio. The mixture was cooled for 20 min soon after 30 min heating at 95°C. The colored supernatant was observed spectrophotometrically at 532 nm and at 600 nm for non-specific absorbance. The final MDA content was estimated by using the extinction coefficient 155 mM−1cm−1 expressed as nmol g−1 FW [24].
To estimate H2O2, K-phosphate (K-P) buffer (pH 6.5) was used for leaf extraction. Later the supernatant and reaction mixture (0.1% TiCl4 in 20% H2SO4) was mixed at 1:3 ratio and left at room temperature for 8–10 minutes. Prior to optically observe the absorbance of the colored supernatant at 410 nm, the mixture was again centrifuged at 11,500×g for 12 min. Finally, the amount of H2O2 content was determined using the extinction coefficient 0.28 μM−1cm−1 and expressed as nmol g–1 FW [25].
2.6 Determination of Mineral Contents (K, Ca, Mg)
Mineral contents were estimated according to the method of Rahman et al. [26]. At the beginning, plant samples (both root and shoot) were oven-dried at 80°C for 48 h. Later on, 0.1 g dried sample was digested with 5 mL acid mixture [HNO3:HClO4 (5:1 v/v)] at 70°C in a digestion chamber for 48 h. After that, 200 µL digested sample was volume up to 5 mL by 10% HNO3. Finally, the absorbance was observed in the atomic absorption spectrophotometer by the flame method and compare with the respective series of standards.
2.7 Measurement of Non-Enzymatic Antioxidant
Three mL extraction buffer containing 1 mM ethylenediaminetetraacetic acid (EDTA) in 5% meta-phosphoric acid was used to extract 0.5 g fresh wheat leaves using a pre-cooled mortar and pestle to assay the AsA and GSH pools. The homogenate was centrifuged at 11,500×g for 12 min at 4°C. Aliquots (0.2 mL) of supernatant were neutralized with 0.3 mL K-P buffer (0.5 M, pH 7.0) followed by reduction of the oxidized fraction with 0.1 M dithiothreitol. The reduced and total AsA was assayed spectrophotometrically for 1 min at 265 nm. A standard curve of known AsA sample was prepared and used for the quantification of AsA, whereas the oxidized fraction—DHA was enumerated after subtracting reduced AsA from total AsA. The method of Hossain et al. [27] was used to determine AsA content.
For assaying GSH, 0.2 mL supernatant was neutralized as described for AsA. Later on, proper oxidation and reduction of GSH was confirmed in the presence of GR that was observed for 1 min at 412 nm to determine total GSH content. Total GSH and oxidized glutathione (GSSG) content were calculated from the standard curve plotted against known concentrations of GSH and GSSG. To measure GSSG, 2-vinyl pyridine was used to remove GSH. Finally, the reduced GSH content was derived by subtracting GSSG from total GSH. The method of Parvin et al. [28] was used throughout the process.
2.8 Determination of Protein and Enzyme Activity Assays
Leaf tissue was homogenized with 1 ml of extraction buffer containing 50 mM K-P buffer (pH 7.0), 100 mM KCl, 1 mM AsA, 5 mM β-mercaptoethanol and 10% (w/v) glycerol followed by centrifugation at 11,500×g for 15 min. The clear supernatant was shifted in new Eppendorf tubes and preserved at 4°C for assaying soluble protein and enzymatic activity of the respective enzymes. The protein determination process required Bradford reagent that contains coomassie brilliant blue (CBB G-250) and was observed at 595 nm. After that, the amount of protein from each sample was calculated by using Bovine Serum Albumin (BSA) as standard [29].
Ascorbate peroxidase (APX, EC: 1.11.1.11) activity was assayed following the procedure of Nakano et al. [30]. Reaction mixture contained K-P buffer (pH 7.0), AsA, H2O2, and EDTA 50, 0.5, 0.1 and 0.1 mM, respectively. Final volume (700 μl; reaction mixture+enzyme extract) was observed at 290 nm for 1 min. A decrease in absorbance was observed as soon as the reaction was started by adding H2O2. APX activity was computed finally with the extinction coefficient of 2.8 mM−1cm−1.
Catalase (CAT, EC: 1.11.1.6) activity was recorded by the method of Hasanuzzaman et al. [31]. The decrease in absorbance was recorded for 1 min at 240 nm in 700 μl volume of mixture containing 70 µl 50 mM K-P buffer (50 mM, pH 7.0), 10 µl H2O2, and 8 µl enzyme solution. Activity was calculated by using the extinction coefficient of 39.4 M−1cm−1.
Monodehydroascorbate reductase (MDHAR, EC: 1.6.5.4) activity was estimated by following the method of Noctor et al. [32]. The reaction buffer contained Tris-HCl buffer (pH 7.5), NADPH, AsA, AO (50, 0.2, 2.5 mM, 0.5 units, respectively) and enzyme solution. The addition of AO initiated the reaction and changes were recorded at 340 nm for 1 min. Finally, the activity was calculated considering the extinction coefficient of 6.2 mM– 1cm–1.
Dehydroascorbatereductase (DHAR, EC: 1.8.5.1) activity was determined according to the methodology of Nahar et al. [33]. Buffer solution contained K-P buffer (50 mM, pH 7.0), GSH (2.5 mM), EDTA (0.1 mM) and dehydroascorbate (DHA) (0.1 mM). The reaction was initiated when the buffer solution was mixed with enzyme extract. At 265 nm for 1 min, the activity was observed and the amount calculated by using the extinction coefficient 14 mM–1cm–1.
Glutathione reductase (GR, EC: 1.6.4.2) activity was estimated following the procedure of Hasanuzzaman et al. [31]. The total volume of 1 ml reaction buffer and enzyme extract was observed for 1 min at 340 nm wavelength. Buffer solution contained K-P buffer (0.1 M, pH 7.0), 1 mM EDTA, 1mM GSSG and 0.2 mM NADPH. The reaction started with the addition of GSSG, and the absorbance showed a decreasing trend. The activity of GR was quantified by using the extinction coefficient of 6.2 mM–1cm–1.
Glutathione peroxidase (GPX, EC: 1.11.1.9) activity was assayed by observing the method proposed by Elia et al. [34]. K-P buffer (100 mM, pH 7.0), EDTA (1 mM), sodium azide (NaN3) (1 mM), NADPH (0.12 mM), GSH (2 mM), GR (1 unit) and H2O2 (0.6 mM) were required to prepare the reaction buffer. Oxidation of NADPH initiated the reaction and the absorbance was recorded at 340 nm for 1 min. The final calculation was done by the extinction coefficient of 6.62 mM−1cm−1.
2.9 Methylglyoxal Content and Glyoxalase Activity
Leaf sample (0.25 g) was crushed in 2.5 ml of 5% perchloric Acid (PCA) and centrifuged at 11000×g at 4°C for 10 min. Activated charcoal was used to decolorize the supernatant and immediately neutralized by Na2CO3/K2CO3 at room temperature. The neutralized extract was further mixed with sodium dihydrogen phosphate and N-Acetyl-L-cysteine. Response was then observed spectrophotometrically at 288 nm. A standard curve of known concentration of MG was considered to calculate the MG content and expressed as μmol g-1 FW. Method of Wild et al. [35] was followed for the overall quantification.
Glyoxalase I (Gly I, EC: 4.4.1.5) activity was estimated by the procedure of Mahmud et al. [9]. K-P buffer (100 mM, pH 7.0), magnesium sulfate (MgSO4, 5 mM) GSH (1.7 mM), and MG (3.5 mM) was mixed together and finally volume to 700 μL by adding enzyme extract. The increase in absorbance was started by adding MG, which was observed for 1 min at 240 nm. The extinction coefficient for calculating the glyoxalase GlyI activity was 3.37 mM–1cm–1.
Glyoxalase II (Gly II, EC: 3.1.2.6) activity was determined by following Principato et al. [36]. The generation of GSH initiated the enzyme activity which was observed at 412 nm for 1 min. The reaction mixture consists of 100 mM Tris-HCl buffer (pH 7.2), 0.2 mM DTNB and 1 mM S-D-lactaylglutathione (SLG). Final volume (1 mL) was made by mixing the enzyme sample with the reaction mixture. SLG is the reaction starter in this mixture. An extinction coefficient of 13.6 mM–1cm–1 was used for final calculation.
Mean separation was compared by Fisher’s Least Significant Difference (LSD) test at 5% level of significance. Analysis of variance (ANOVA) was done for all the measured data. Correlation analysis was done using XLSTAT V. 2018 [37].
3.1 Supplemented K Enhanced Plant Growth Parameters under Water Deficit Conditions
A significant reduction of plant height and plant biomass was observed in wheat seedlings due to imposition of drought at different levels (Fig. 1). However, K treatment increased the plant height under drought stress except the reduction observed at 20% FC with 12 mM K. Specifically, 6 mM K application increased plant height by 24%, 9%, and 6% under Well-watered, 50% and 20% FC, respectively.

Figure 1: Effect of potassium (0, 6 and 12 mM) on plant growth under (A) Well-watered, (B) 50% FC and (C) 20% FC condition in wheat seedlings
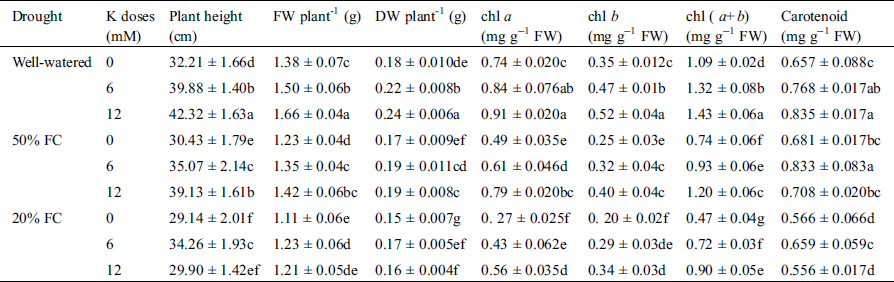
On the contrary, supplementation of 12 mM K application increased plant height by 31% and 21% at Well-watered and 50% FC, respectively while at 20% FC, 7% plant height was reduced compared to Well-watered condition (Tab. 1). A sharp decrease in FW and DW was also observed at both water deficit regimes, which was dramatically increased with supplemented K. Highest increase of FW and DW was 3% and 10% at 50% FC with 12 mM K supply compared to Well-watered condition where at 20% FC supplementation of K had no effect. That is how both FW and DW reduced under drought condition (Tab. 1).
Table 1: Effect of K on plant height, fresh weight, dry weight and chl content under three different water regimes (Well-watered, 50% FC, 20% FC)
3.2 K Conserved chl Content under Drought Condition
At 50% FC, chl a and chl b content were decreased by 34% and 63% while at 20% FC, chl a and chl b content were decreased by 30%, 44%, respectively in wheat seedlings compared to Well-watered condition. As a consequence, the chl (a + b) content also gets decreased. In case of carotenoid, the highest reduction14% was observed at 20% FC. However, stressed seedlings treated with K showed increased chl a, chl b, chl (a + b), and carotenoid content (Tab. 1). The highest increase of chl (a + b) content 9% was observed at 50% FC treated with 12 mM K, while the highest increase in carotenoid content by 27% was monitored at 50% FC with 6 mM K (Tab. 1).
3.3 K-Fed Wheat Showed Lesser Damages by Oxidative Stress Markers (MDA, H2O2)
An increase in MDA content due to lipid peroxidation causes severe cell membrane damage under drought stress. Compared to Well-watered condition, 17% and 36% of increased MDA content was observed at 50% and 20% FC, respectively (Fig. 2A). On the other hand, H2O2 content was increased by 30% and 59% at 50 and 20% FC compared to the Well-watered condition (Fig. 2B). Conversely, K application reduced the H2O2 level, which ultimately minimized the lipid peroxidation under drought stress. Both at 50% and 20% FC, 13% MDA content was decreased due to application of 6 mM K whereas, the highest MDA content reduced by 31% with 12 mM K at 50% FC in comparison to Well-watered condition (Fig. 2A).

Figure 2: Effect of K on MDA (A), and H2O2 (B) content while seedlings exposed to three different water regimes (Well-watered, 50% FC, 20% FC)
3.4 K Improves Osmotic Balance under Water-Stressed Conditions
Drought stress significantly decreased relative water content of wheat seedlings compared to Well-watered condition, whereas, supplemented K increased plant RWC in a significant manner. At 50% FC, both 6 mM and 12mM K increased leaf RWC by 9% and 8%, respectively compared to Well-watered condition. However, the highest 33% of RWC reduction was observed at 20% FC where no K was supplemented (Fig. 3A).

Figure 3: Effect of K on osmotic stress marker, leaf RWC (A) and Pro content (B) while seedlings exposed to three different water regimes (well-watered, 50% FC, 20% FC)
Compared to Well-watered condition, Pro content was notably enhanced by 35 and 57% in wheat seedlings, while exposed to 50% and 20% FC respectively. On the contrary, plants treated with K under 50% and 20% FC showed a significant reduction in Pro content. The maximum amount of Pro was reduced by 34% at 50% FC with 12 mM K application where at 20% FC with 12 mM K increased Pro content by 25% only in comparison to Well-watered condition (Fig. 3B).
3.5 K Modulated Antioxidant Defense System under Deficit Water Condition
Ascorbate content was decreased under drought stress. At 50% FC, AsA content reduced by 13% where at 20% FC it reduced by 46% (Fig. 4A). Under stress condition, supplemented K helped in reducing the DHA content, which increased AsA content by 4% and 10% at 50% FC with 6 mM and 12 mM K, respectively (Fig. 4A). This mechanism finally uplifted the AsA/DHA ratio, where the highest 4% was observed at 50% FC supplemented with 12 mM K compared to Well-watered condition (Fig. 4E). In contrast, under both water deficit regimes (50% and 20% FC) GSH content reduced by 19% and 37% compared to Well-watered condition (Fig. 4B). K helps in increasing the GSH and GSH/GSSG ratio by reducing the GSSG content (Fig. 4D). Compared to Well-watered condition, the highest GSH/GSSG ratio was observed at 50% FC with 12 mM supplemented K (Fig. 4F).

Figure 4: Effect of K on AsA (A), GSH (B), DHA (C), and GSSG (D) contents and AsA/DHA (E) and GSH/GSSG (F) ratio of while seedlings exposed to three different water regimes (Well-watered, 50% FC, 20% FC)
Deficit water condition significantly impaired the enzymatic antioxidant system. Compared to Well-watered condition, 50% and 20% FC resulted in 58% and 138% increase in APX activity, respectively (Fig. 5B) whereas, 24% and 51% CAT activity were reduced under 50% and 20% FC, respectively (Fig. 5A). However, addition of K further increased the APX activity where the maximum APX activity (150%) was observed at 20% FC with 12 mM K treatment at Well-watered condition (Fig. 5B) and the highest increased CAT activity (18%) was observed at 50% FC with 6 mM K (Fig. 5A). In addition, 14% and 23% MDHAR and DHAR activity were reduced at 50% FC whereas, 34% and 42% MDHAR and DHAR was reduced at 20% FC, compared to Well-watered condition respectively (Figs. 5C, 5D). Supplemented K increased substantial amount of MDHAR and DHAR (15% and 13% respectively) compared to Well-watered condition with 12 mM K (Figs. 5C, 5D). In case of GR and GPX, a decreasing trend of activity was also observed (Figs. 5E, 5F) with a highest reduction (56%) in GPX at 20% FC compared to Well-watered condition (Fig. 5F). Conversely, a highest 11% increment in GR activity was observed at 20% FC with 6 mM supplemented K compared to Well-watered condition.

Figure 5: Effect of K on enzymatic antioxidants, CAT (A), APX (B), MDHAR (C), DHAR (D), GR (E) and GPX (F) activities of wheat seedlings exposed to three different water regimes (Well-watered, 50% FC, 20% FC)
3.6 Glyoxalase System and Methylglyoxal (MG) Detoxification
Two major contributors of glyoxalase system are Gly I and Gly II enzymes. Under the drought stress condition their activity were remarkably distorted, and as a result, the detoxification process of MG was also influenced. Gly I and Gly II activities tend to decrease with increasing drought severity (Figs. 6B, 6C), which later on up-regulated in Gly I with the addition of exogenous K (Fig. 6C). At stressed condition (50 and 20% FC) Gly I was reduced by 29 and 54%, whereas 33 and 56% reduction of Gly II activity was observed accordingly, compared to Well-watered condition (Figs. 6B, 6C).
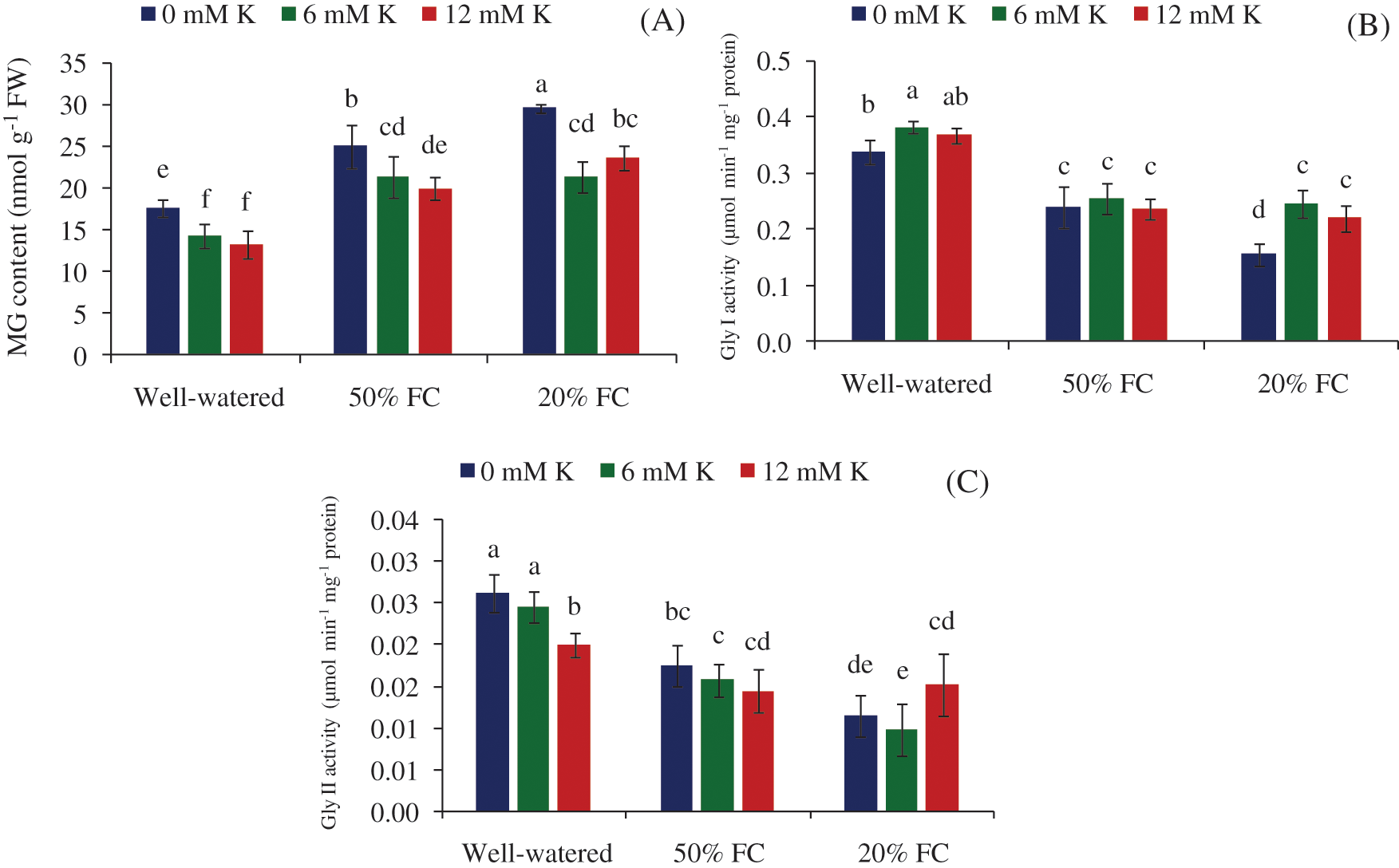
Figure 6: Effect of K on glyoxalase system, MG content (A), Gly I (B) and Gly II (C) activity of wheat seedlings exposed to three different water regimes (Well-watered, 50% FC, 20% FC)
Under drought stress condition enhanced level of MG content was observed (Fig. 6A). As a consequence, compared to Well-watered condition, 42 and 68% MG content was increased under 50 and 20% FC, respectively. Although the enzymes of glyoxalase system are significantly down-regulated under stressed condition, till the MG detoxification process was accelerated due to application of exogenous K. Supplemented K reduced MG content by 21% and 29% at 6 and 12 mM under 50% FC, respectively, while a sharp decrease (47%) was observed at 20% FC with 6 mM K supply (Fig. 6A).
3.7 K Regulated K, Ca and Mg Content in Shoot
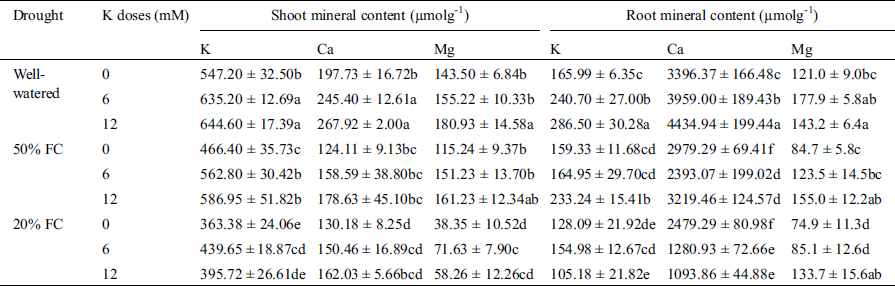
Shoot mineral content also significantly decreased under drought condition. Among K, Ca and Mg, the highest (52%) reduction was observed in Mg content under 20% FC compared to Well-watered condition. Application of K helped in K and Mg enrichment in shoot except the Ca content. Although supplemented K increased the Ca content under water deficit condition, the increment was not as significant as compared to the Well-watered condition. Highest K and Mg content (7% and 12%) increased in shoot in 50% FC while 12 mM K was applied (Tab. 2).
Table 2: Effect of K on K, Ca, Mg content both in root and shoot of wheat seedlings exposed to three different water regimes (Well-watered, 50% FC, 20% FC)
3.8 K Enhanced K, Ca and Mg Content in Root
Compared to Well-watered condition, increasing drought severity notably reduced the root mineral contents (K, Ca, and Mg). Root K content was decreased by 4% and 23% at 50% and 20% FC respectively compared to Well-watered condition. At 50% FC with 6 mM supplemented K, plant showed better amount of K content in root. However, at 50% FC with 12 mM K, highest (41%) amount of increased K in root was observed. Though Ca content increased in root under drought stress, Mg content notably reduced. At 20% FC, a highest (38%) reduction of Mg content was observed. Conversely, addition of 12 mM K at 50% FC showed the highest (28%) increase of Mg content in root. Interestingly, K application at any concentration did not uplift the Ca content in root under drought stress (Tab. 2).
Potassium—is the precursor of different metabolites in the cell and it is the most abundant inorganic cation that builds up 10% of plant’s dry weight [38]. Large amount of K is located in newly emerging tissues and reproductive parts of plants demonstrating its necessity in plant growth and cell metabolism [13]. Water is a fundamental constituent for proper cell expansion and development. Deficiency of water provokes a number of plants physiological and biochemical processes considering the severity and duration of the stress period [39]. Plant height reduction (Fig. 1) and loss of seedling FW and DW (Tab. 1) under drought stress were observed in our study. Same findings as seedlings growth retardation under drought were also observed by Al Hassan et al. [40] in three wild monocots under different environmental conditions. Conversely, we observed a positive effect on above-mentioned parameters while wheat seedlings were treated with supplemented K under drought stress. In tobacco (Nicotiana rustica L.) plants, Bahrami-Rad et al. [41] found the active role of K in improving the plant height under drought stress. Reduced RWC, leaf water potential, net photosynthesis rate, stomatal conductance and leaf transpiration rate with a parallel increase in leaf surface temperature is a very common phenomenon under deficit water stress [42]. In our study, RWC reduced with increasing drought stress which might be due to reduced leaf water potential and increased transpiration activities under drought stress. However, application of K notably increased the RWC in our study. Similar parameters like water potential, osmotic potential, turgor pressure as well as chl contents were increased while 2% K was supplemented in drought stressed canola seedlings [43]. Martineau et al. [44] also studied the pivotal role of K in optimizing the yield of maize (Zea mays) by improving the growth parameters like leaf area, plant height, water potential, photosynthetic pigments and the grain filling.
Proline is an organic osmolytes that acts as an osmoprotectant under drought stress and helps in osmotic adjustment. Higher accumulation of Pro under stressed condition is because of the expression of the gene encoding pyrroline-5-carboxylate synthase which might adjust the dehydration tolerance in plant [2]. Therefore in our study, the higher Pro accumulation due to drought stress might be because of the increase in Pro biosynthesis, together with a decrease in its oxidation [45]. Multiple defensive roles of Pro under different abiotic stress condition were reported in past experiments, which further gave protection to stress-induced dehydration and oxidative damages [1,46,47]. Drought-induced Brassica Seedlings resulted in high accumulation of Pro content helped in the growth and development of mustard plant was observed by Khan et al. [39]. Furthermore, in the different transgenic method, it has been suggested that overproduced Pro has a useful, active defending role in stress mitigation [48]. A substantial increase in Pro content due to K deficiency was observed in four mustard (Brassica juncea L.) varieties by Ahmad et al. [49]. The reason behind Pro increased under K starvation period is yet not been explored, but it could be due to role of K in amino acids metabolism or particular effect of K nutrition on Pro metabolic pathway [41]. However, K treatment in drought-stressed plant showed a reduced amount of Pro was observed in our study which might be associated with the relative influence of K and NO3− (as KNO3 applied) in reducing soluble sugars that could be essential to energy balancing in the wheat plant under drought stress. This result supports the findings of Nounjan et al. [50].
Drought directly affects on photosynthesis process by reducing the plant leaf area. Sudden closure of stomata severely hampered the transpiration rate that in turn impede on the photosynthesis process by limiting the entry of CO2 [51,52]. Drought reduces the photosynthetic pigments by enlarging or distorting the lamellae vesiculation, destroying the chloroplast membranes and in case forming lipid droplets [53]. During water deficit condition we observed reduced photosynthetic pigments which happened while higher produced ROS destroyed the components of photosynthetic transport system, as a result the photosynthesis process was notably hampered [50]. Moreover, previous findings suggest that destruction of the chloroplast membranes, lamellae distortion, and vesiculation due to oxidative stress might be another reason for decreased chl content under prolonged drought condition [54]. In our study, leaf chl contents were significantly increased by supplemented K under drought stress. This might be happened due to K role in higher biosynthesis of chl pigments [14]. In wheat, Photosynthetic pigments were significantly increased while 10 mM K2O was applied along with 15% PEG induced drought stress [55]. The application of 6 mM KCl through foliar and root application enhanced photosynthesis rate considerably under drought stress in tobacco supports our findings that K elevates leaf photosynthesis rates [41].
Over production of ROS is very common incident under abiotic stress. Cell membrane disintegration happens due to lipid peroxidation while polyunsaturated materials of the cell membrane react with ROS [56]. Our findings suggest that stressed plants showed a higher accumulation of H2O2 and caused more significant lipid peroxidation as indicated by higher MDA production. Similar to our result, Nxele et al. [57] also reported a significant increase in H2O2 content under drought stress. However, K treatment reduced the generation of H2O2, which is well agreed with the earlier experiment of Ahanger et al. [58]. In addition to that, Fayez et al. [59] reported that, cell membrane repairing process get accelerated by K supplementation that reduces MDA content from the cell and also strengthens the antioxidant defense system thus guard against the stress-induced damages in barley seedlings (Hordeum vulgare).
APX, MDHAR, DHAR, GR are the major enzymatic antioxidants of AsA-GSH cycle which along with other non-enzymatic antioxidants like AsA and GSH acts together to reduce the deleterious effect of oxidative stresses [11]. Among the free radicals, H2O2 is the most persistent in nature which converted to H2O by APX through AsA-GSH cycle, at the same time monodehydroascorbate (MDHA) formed by oxidation of AsA which immediately converted to AsA again by MDHAR. Meanwhile, MDHA is further oxidized to DHA that requires DHAR and GSH to be reduced to AsA [60]. Like APX, CAT also scavenges H2O2 from the cell. Besides, the AsA/DHA ratio plays a significant role in cell redox balancing under oxidative stress condition [61]. However, major enzymes of antioxidant defense system and GPX were down-regulated except the APX, which was up-regulated in our study due to imposed water deficit stress. But the addition of external K remarkably increased the activity of these vital enzymatic antioxidants to guard against drought-induced oxidative stress, which is parallel to an earlier result of Islam et al. [62]. Supplemented K substantially increased the activity of APX and GR enzymes (Figs. 5B, 5E) through increased generation of AsA and GSH (Figs. 4A, 4B). In our study AsA content reduced in the water-stressed plant while APX activity was increased, similar results reported by Rizwan et al. [63] and Khan et al. [39]. Hence, the protective role of K, as found in this study regarding antioxidant enzymes, can mitigate the stress-induced dysfunctions in plant cells through rebuilding redox balance and strengthening cell components.
Both AsA and GSH play a vital role against multiple stresses in plant, like AsA accelerates photosynthesis rate and electron transport chain by scavenging extra amount of ROS [64] whereas, GSH helps in redox homeostasis by regenerating AsA via AsA-GSH cycle [65]. Under drought condition, increased production of GSH helps in establishing optimal balance in other components of AsA-GSH cycle [66]. Here, in our study we report increased total AsA and GSH content in K-treated wheat seedlings (Fig. 4A), which suggests that K plays an inevitable role in the biosynthesis of GSH and AsA. In addition, reduced level of H2O2 and O2•− due to supplemented K in our study also responsible for the elevated level of AsA and GSH that emphasizes the role of AsA, GSH and K in ROS detoxification. Furthermore, in stress signaling and adjustment of redox potential, GSH/GSSG ratio diversely put an impactful role [60]. In this study, GSH content, as well as GSH/GSSG ratio, was reduced while increasing the GSSG content was visual under drought stress (Figs. 4A, 4D, 4F). It is assumed that, due to the higher generation of GSSG the ratio of GSH/GSSG might have decreased [67]. However, the application of K notably uplifts the GSH/GSSG ratio while reducing the GSSG content in deficit watered plants (Figs. 4F, 4D). Besides this, K treated plant showed an increased level of GSH by up-regulating the GPX activity which assists in scavenging oxidative free radicals earlier investigated by Elloumi et al. [68] in loquat trees.
Under abiotic stress condition, another highly reactive cytotoxic compound MG production aggravated, which enhances the severity of oxidative stress. In Arabidopsis, MG impedes on the regular morpho-physiological activities of plant, such as retarded growth, decreased RWC and reduced chl pigments etc. [69].Two vital enzymes of glyoxalase system viz., Gly I and Gly II are responsible for MG detoxification. Their up-regulation significantly detoxifies MG level in plants under drought stress [31]. In the present study we observed increased amount of MG content under drought stress which was finally reduced due to application of exogenous K although the glyoxalase system was not activated under stress. This might happen through many other MG detoxification pathways than glyoxalase system. Aldose/aldehyde reductase (ALR) and aldo-keto reductase (AKR) are the reductase family of enzymes those are getting more emphasis in recent days as they convert MG to acetol and lactaldehyde using NADPH. This ALR gene in tobacco plant exhibited defending role against abiotic stresses (low temperature, cadmium and drought stress) [70]. An experiment on Thai jasmine rice revealed that an NADPH-related reductase enzyme named Aldo–keto reductase notably reduces MG content in subsequent alcohol [71]. Similarly, irreversible oxidation of MG to related carboxylic acids is performed by the activity of aldehyde dehydrogenase enzyme [72]. Furthermore, Gly III enzyme is the recent addition to MG detoxification and has been classified as a GSH-independent glyoxalases which provide a shorter route for MG detoxification [69,73]. Presence of Gly III was first reported in bacteria where it helps in MG detoxification by single step without GSH. Now the presence of Gly III-like proteins has been well identified in monocots, dicots, lycopods, gymnosperm and bryophytes [73]. Gly III enzyme irreversibly converts MG to D-lactate without the help of GSH and which do not include generation or breakdown of S-D-lactoylglutathione [74].
Nutrient availability, partitioning, and transport systems are greatly hampered under deficit water conditions. Different cultivars of mungbean (Vigna mungo L.) showed down-regulation of all the mineral nutrients except the iron under drought condition [75]. Drought restricts the water and nutrient supply from root to shoot. As a result, all three growth stages of wheat plant comprising spike length, grain yield and nutrient uptake was adversely affected [76]. In our study, mineral nutrients like Ca, Mg, and K content were significantly reduced under deficit water conditions. In mungbean, under PEG-induced drought stress, Ali et al. [75] observed the decrease in shoot K and Ca irrespective of their genotypic background. Likewise, Shah et al. [76] documented that drought considerably influenced N, P, K, Ca and Na uptake by two varieties of wheat while foliar application of K application under drought enhanced most of these attributes. Under drought condition, K mobility in soil decrease rapidly, as a consequence K ion cannot uptake by the root from soil and plant exhibits a shortage of K in intercellular regions [41]. Therefore, compared to Well-watered condition, the insufficient root–shoot K content was observed in water-stressed wheat seedlings in our study too. Experiment conducted by Zain et al. [77] on barley also found the same results that increasing drought severity creates scarcity of available K in the plant. Reversely, our result demonstrates that K supplementation under drought notably increased the amount of Ca, P, and K in the plant, which corroborates the earlier findings of Ibrahim et al. [78].
The harmful effect of drought is exerted in terms of different physiological or morphological changes in the plant. In the present study, drought stress significantly reduced the plant height, FW and DW, Leaf RWC and chl contents, which was restored due to the application of K. At drought condition plants suffered due to significant increase in MDA, H2O2 and Pro contents that was decreased ultimately while K was supplemented. The role of K in reducing stress marker elements signifies its mitigating role during drought stress. Besides alteration in AsA-GSH content, drought negatively modulates the antioxidant defense system and therefore higher amount of ROS produced in plants. However, K treatment enhanced the antioxidant defense system by up-regulating both the enzymatic and non-enzymatic antioxidants. In living organelles, glyoxalase system is the vital pathway of MG detoxification. Beside this, glyoxalase system recycles GSH and maintains glutathione homeostasis in cell. Therefore, the mechanism of glyoxalase system needs to reinvestigate along with exploring several other pathways of MG detoxification. In sum, our findings serve the knowledge that increasing K concentration to a particular dose helps in detoxifying the excess production of ROS. At 50% FC with 12 mM K the best physiological and biochemical performances were observed in the stressed plants. However, K-mediated defense mechanism and K-signaling different pathways is yet to be discovered to find the association of K with other elements in mitigating drought as well as other stresses.
Acknowledgement: The author ACCM expresses his humble gratitude to ministry of science and technology (MoST), Bangladesh and Japan Student Services Organization (JASSO) to facilitate fund and Bangladesh Agricultural Research Institute (BARI), for providing quality seed to conduct this experiment. The author also wants to acknowledge Md. Shahadat Hossen, Mahmodul Hasan Sohag and Moumita, Laboratory of Plant Stress Responses, Faculty of Agriculture, Kagawa University, Japan, for their unconditional help during the entire research period.
Funding Statement: Ministry of Science and Technology (MoST), Bangladesh and Japan Student Services Organization (JASSO).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Bhuyan, M. H. M., Hasanuzzaman, M., Mahmud, J. A., Hossain, M., Bhuiyan, T. F. (2019). Unraveling morphophysiological and biochemical responses of Triticum aestivum L. to extreme pH: Coordinated actions of antioxidant defense and glyoxalase systems. Plants, 8(1), 24. DOI 10.3390/plants8010024. [Google Scholar] [CrossRef]
2. Yadav, S., Modi, P., Dave, A., Vijapura, A., Patel, D. et al. (2020). Effect of abiotic stress on crops. In: Hasanuzzaman, M., Carvalho, M., Filho, M. T., Fujita, M., Nogueira, T. A. R. et al. (eds.Sustainable Crop Production, London: IntechOpen, 3–24. [Google Scholar]
3. Hasanuzzaman, M., Nahar, K., Hossain, M., Mahmud, J. A., Rahman, A. et al. (2017). Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. International Journal of Molecular Sciences, 18(1), 200. DOI 10.3390/ijms18010200. [Google Scholar] [CrossRef]
4. Khan, W. U., Ahmad, S. R., Yasin, N. A., Ali, A., Ahmad, A. et al. (2017). Application of Bacillus megaterium MCR-8 improved phytoextraction and stress alleviation of nickel in Vinca rosea. International Journal of Phytoremediation, 19(9), 813–824. DOI 10.1080/15226514.2017.1290580. [Google Scholar] [CrossRef]
5. Mittler, R. (2017). ROS are good. Trends in Plant Science, 22(1), 11–19. DOI 10.1016/j.tplants.2016.08.002. [Google Scholar] [CrossRef]
6. Coudhury, F. K., Rivero, R. M., Blumwald, E., Mittler, R. (2017). Reactive oxygen species, abiotic stress and stress combination. Plant Journal, 90(5), 856–867. DOI 10.1111/tpj.13299. [Google Scholar] [CrossRef]
7. Hussain, S., Rao, M. J., Anjum, M. A., Ejaz, S., Zakir, I. et al. (2019). Oxidative stress and antioxidant defense in plants under drought conditions. In: Hasanuzzaman, M., Hakeem, K. R., Nahar, K., Alharby, H. F. (eds.Plant Abiotic stree tolerance, Cham: Springer, pp. 207–219. [Google Scholar]
8. Hasanuzzaman, M., Nahar, K., Gill, S. S., Alharby, H. F., Razafindrabe, B. H. et al. (2017). Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Frontiers in Plant Science, 8, 115. DOI 10.3389/fpls.2017.00115. [Google Scholar] [CrossRef]
9. Mahmud, J. A., Hasanuzzaman, M., Nahar, K., Rahman, A., Hossain, M. S. et al. (2017). Maleic acid assisted improvement of metal chelation and antioxidant metabolism confers chromium tolerance in Brassica juncea L. Ecotoxicology and Environmental Safety, 144, 216–226. DOI 10.1016/j.ecoenv.2017.06.010. [Google Scholar] [CrossRef]
10. Li, C., Han, Y., Hao, J., Qin, X., Liu, C. et al. (2020). Effects of exogenous spermidine on antioxidants and glyoxalase system of lettuce seedlings under high temperature. Plant Signaling & Behavior, 18, 1824697. DOI 10.1080/15592324.2020.1824697. [Google Scholar] [CrossRef]
11. Hasanuzzaman, M., Bhuyan, M., Nahar, K., Hossain, M., Mahmud, J. et al. (2018). Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy, 8(3), 31. DOI 10.3390/agronomy8030031. [Google Scholar] [CrossRef]
12. Oddo, E., Abbate, L., Inzerillo, S., Carimi, F., Motisi, A. et al. (2020). Water relations of two Sicilian grapevine cultivars in response to potassium availability and drought stress. Plant Physiology and Biochemistry, 148, 282–290. DOI 10.1016/j.plaphy.2020.01.025. [Google Scholar] [CrossRef]
13. Ahanger, M. A., Agarwal, R. M., Tomar, N. S., Shrivastava, M. (2015). Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L cultivar Kent). Journal of Plant Interactions, 10(1), 211–223. DOI 10.1080/17429145.2015.1056260. [Google Scholar] [CrossRef]
14. Lu, Z., Lu, J., Pan, Y., Lu, P., Li, X. et al. (2016). Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant, Cell & Environment, 39(11), 2428–2439. DOI 10.1111/pce.12795. [Google Scholar] [CrossRef]
15. Wang, X., Mohamed, I., Ali, M., Abbas, M. H., Shah, G. M. et al. (2019). Potassium distribution in root and non-root zones of two cotton genotypes and its accumulation in their organs as affected by drought and potassium stress conditions. Journal of Plant Nutrition and Soil Science, 182(1), 72–81. DOI 10.1002/jpln.201800026. [Google Scholar] [CrossRef]
16. Waraich, E. A., Ahmad, Z., Ahmad, R., Saifullah, Ashraf, M. Y. (2015). Foliar applied phosphorous enhanced growth, chlorophyll contents, gas exchange attributes and PUE in wheat (Triticum aestivum L.). Journal of Plant Nutrition, 38(12), 1929–1943. DOI 10.1080/01904167.2015.1043377. [Google Scholar] [CrossRef]
17. Iftikhar, H., Ghori, Z., Ali, S. H., Sheikh, S., Gul, A. (2019). Wheat responses to stress and biotechnological approaches for improvement. In: Hasanuzzaman, M., Nahar, K., Hossain, M. A. (eds.Wheat production in changing environments, Singapore: Springer, 343–392. [Google Scholar]
18. Asif, M., Yilmaz, O., Ozturk, L. (2017). Potassium deficiency impedes elevated carbon dioxide-induced biomass enhancement in well watered or drought-stressed bread wheat. Journal of Plant Nutrition and Soil Science, 180(4), 474–481. DOI 10.1002/jpln.201600616. [Google Scholar] [CrossRef]
19. Zhang, X., Wu, H., Chen, L., Wang, N., Wei, C. et al. (2019). Mesophyll cells’ ability to maintain potassium is correlated with drought tolerance in tea (Camellia sinensis). Plant Physiology and Biochemistry, 136, 196–203. DOI 10.1016/j.plaphy.2019.01.020. [Google Scholar] [CrossRef]
20. Wang, Y., Zhang, Z., Liang, Y., Han, Y., Han, Y. et al. (2020). High potassium application rate increased grain yield of shading-stressed winter wheat by improving photosynthesis and photosynthate translocation. Frontiers in Plant Science, 11, 134. DOI 10.3389/fpls.2020.00134. [Google Scholar] [CrossRef]
21. Barrs, H. D., Weatherley, P. E. (1962). A re-examination of the relative turgidity technique for estimating water deficits in leaves. Australian Journal of Biological Sciences, 15(3), 413–428. DOI 10.1071/BI9620413. [Google Scholar] [CrossRef]
22. Bates, L. S., Waldren, R. P., Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
23. Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiology, 24(1), 1–15. DOI 10.1104/pp.24.1.1. [Google Scholar] [CrossRef]
24. Heath, R. L., Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198. DOI 10.1016/0003-9861(68)90654-1. [Google Scholar] [CrossRef]
25. Yu, C. W., Murphy, T. M., Lin, C. H. (2003). Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Functional Plant Biology, 30(9), 955–963. DOI 10.1071/FP03091. [Google Scholar] [CrossRef]
26. Rahman, A., Nahar, K., Hasanuzzaman, M., Fujita, M. (2016). Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt–stressed rice seedlings. Frontiers in Plant Science, 7, 609. DOI 10.3389/fpls.2016.00609. [Google Scholar] [CrossRef]
27. Hossain, M. S., Hasanuzzaman, M., Sohag, M. M. H., Bhuyan, M. B., Fujita, M. (2019). Acetate-induced modulation of ascorbate: Glutathione cycle and restriction of sodium accumulation in shoot confer salt tolerance in Lens culinaris Medik. Physiology and Molecular Biology of Plants, 25(2), 443–455. DOI 10.1007/s12298-018-00640-6. [Google Scholar] [CrossRef]
28. Parvin, K., Hasanuzzaman, M., Bhuyan, M. H. M., Mohsin, S. M., Fujita, M. (2019). Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants, 8(8), 247. DOI 10.3390/plants8080247. [Google Scholar] [CrossRef]
29. Bradford, M. M. (1976). Analytical biochemistry. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
30. Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880. [Google Scholar]
31. Hasanuzzaman, M., Nahar, K., Anee, T. I., Khan, M. I. R., Fujita, M. (2018). Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. South African Journal of Botany, 115, 50–57. DOI 10.1016/j.sajb.2017.12.006. [Google Scholar] [CrossRef]
32. Noctor, G., Mhamdi, A., Foyer, C. H. (2016). Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant, Cell & Environment, 39, 1140–1160. [Google Scholar]
33. Nahar, K., Hasanuzzaman, M., Rahman, A., Alam, M. M., Mahmud, J. A. et al. (2016). Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Frontiers in Plant Science, 7, 1104. DOI 10.3389/fpls.2016.01104. [Google Scholar] [CrossRef]
34. Elia, A. C., Galarini, R., Taticchi, M. I., Dörr, A. J. M., Mantilacci, L. (2003). Antioxidant responses and bioaccumulation in Lctalurus melas under mercury exposure. Ecotoxicology and Environmental Safety, 55(2), 162–167. DOI 10.1016/S0147-6513(02)00123-9. [Google Scholar] [CrossRef]
35. Wild, R., Ooi, L., Srikanth, V., Münch, G. (2012). A quick, convenient and economicalmethod for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Analytical and Bioanalytical Chemistry, 403(9), 2577–2581. DOI 10.1007/s00216-012-6086-4. [Google Scholar] [CrossRef]
36. Principato, G. B., Rosi, G., Talesa, V., Giovanni, E., Uotila, L. (1987). Purification and characterization of two forms of glyoxalase II from the liver and brain of wistar rats. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 911(3), 349–355. DOI 10.1016/0167-4838(87)90076-8. [Google Scholar] [CrossRef]
37. Addinsoft. (2016). XLSTAT v. 2016: Data analysis and statistics software for Microsoft Excel. Paris, France: Addinsoft. [Google Scholar]
38. Zhu, B., Xu, Q., Zou, Y., Ma, S., Zhang, X. et al. (2020). Effect of potassium deficiency on growth, antioxidants, ionome and metabolism in rapeseed under drought stress. Plant Growth Regulation, 90(3), 455–466. DOI 10.1007/s10725-019-00545-8. [Google Scholar] [CrossRef]
39. Khan, A., Anwar, Y., Hasan, M., Iqbal, A., Ali, M. et al. (2017). Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants, 6(4), 20. DOI 10.3390/plants6020020. [Google Scholar] [CrossRef]
40. Al Hassan, M., Chaura, J., Donat-Torres, M. P., Boscaiu, M., Vicente, O. (2017). Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB PLANTS, 9(2), 369. DOI 10.1093/aobpla/plx009. [Google Scholar] [CrossRef]
41. Bahrami-Rad, S., Hajiboland, R. (2017). Effect of potassium application in drought-stressed tobacco (Nicotiana rustica L.) plants: Comparison of root with foliar application. Annals of Agricultural Sciences, 62(2), 121–130. DOI 10.1016/j.aoas.2017.08.001. [Google Scholar] [CrossRef]
42. Maghsoudi, K., Emam, Y., Pessarakli, M. (2016). Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. Journal of Plant Nutrition, 39(7), 1001–1015. DOI 10.1080/01904167.2015.1109108. [Google Scholar] [CrossRef]
43. Waraich, E. A., Rashid, F., Ahmad, Z., Ahmad, R., Ahmad, M. (2020). Foliar applied potassium stimulate drought tolerance in canola under water deficit conditions. Journal of Plant Nutrition, 43(13), 1923–1934. DOI 10.1080/01904167.2020.1758132. [Google Scholar] [CrossRef]
44. Martineau, E., Domec, J. C., Bosc, A., Denoroy, P., Fandino, V. A. et al. (2017). The effects of potassium nutrition on water use in field-grown maize (Zea mays L.). Environmental and Experimental Botany, 134, 62–71. DOI 10.1016/j.envexpbot.2016.11.004. [Google Scholar] [CrossRef]
45. Amist, N., Singh, N. B. (2017). Responses of enzymes involved in proline biosynthesis and degradation in wheat seedlings under stress. Allelopathy Journal, 42(2), 197–208. DOI 10.26651/allelo.j./2017-42-2-1116. [Google Scholar] [CrossRef]
46. Nahar, K., Hasanuzzaman, M., Suzuki, T., Fujita, M. (2017). Polyamines-induced aluminium tolerance in mung bean: A study on antioxidant defense and methylglyoxal detoxification systems. Ecotoxicology, 26(1), 58–73. DOI 10.1007/s10646-016-1740-9. [Google Scholar] [CrossRef]
47. Hasanuzzaman, M., Nahar, K., Hossain, M. S., Anee, T. I. Parvin, K. et al. (2017). Nitric oxide pretreatment enhances antioxidant defense and glyoxalase systems to confer PEG-induced oxidative stress in rapeseed. Journal of Plant Interactions, 12(1), 323–331. DOI 10.1080/17429145.2017.1362052. [Google Scholar] [CrossRef]
48. Mahawar, L., Shekhawat, G. S. (2019). EsHO 1 mediated mitigation of NaCl induced oxidative stress and correlation between ROS, antioxidants and HO 1 in seedlings of Eruca sativa: Underutilized oil yielding crop of arid region. Physiology and Molecular Biology of Plants, 25(4), 895–904. DOI 10.1007/s12298-019-00663-7. [Google Scholar] [CrossRef]
49. Ahmad, P., Ashraf, M., Hakeem, K. R., Azooz, M. M., Rasool, S. et al. (2014). Potassium starvation-induced oxidative stress and antioxidant defense responses in Brassica juncea. Journal of Plant Interactions, 9(1), 1–9. DOI 10.1080/17429145.2012.747629. [Google Scholar] [CrossRef]
50. Nounjan, N., Chansongkrow, P., Charoensawan, V., Siangliw, J. L., Toojinda, T. et al. (2018). High performance of photosynthesis and osmotic adjustment are associated with salt tolerance ability in rice carrying drought tolerance QTL: Physiological and co-expression network analysis. Frontiers in Plant Science, 9, 1135. DOI 10.3389/fpls.2018.01135. [Google Scholar] [CrossRef]
51. Basu, S., Ramegowda, V., Kumar, A., Pereira, A. (2016). Plant adaptation to drought stress. F1000 Research, 5, F1000 Faculty Rev-1554. DOI 10.12688/f1000research.7678.1. [Google Scholar] [CrossRef]
52. Talaat, N. B. (2019). Abiotic stresses-induced physiological alteration in wheat. In: Hasanuzzaman, M., Nahar, K., Hossain, M. A. (eds.Wheat production in changing environments, pp. 1–30. Singapore: Springer. [Google Scholar]
53. Ahanger, M. A., Tomar, N. S., Tittal, M., Argal, S., Agarwal, R. (2017). Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiology and Molecular Biology of Plants, 23(4), 731– 744. DOI 10.1007/s12298-017-0462-7. [Google Scholar] [CrossRef]
54. Hasanuzzaman, M., Nahar, K., Rahman, A., Inafuku, M., Oku, H. et al. (2018). Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiology and Molecular Biology of Plants, 24(6), 993–1004. DOI 10.1007/s12298-018-0531-6. [Google Scholar] [CrossRef]
55. Jatav, K. S., Agarwal, R. M., Singh, R. P., Shrivastava, M. (2012). Growth and yield responses of wheat (Triticum aestivum L.) to suboptimal water supply and different potassium doses. Journal of Functional and Environmental Botany, 2(1), 39–51. DOI 10.5958/j.2231-1742.2.1.005. [Google Scholar] [CrossRef]
56. Gill, S. S., Anjum, N. A., Gill, R., Yadav, S., Hasanuzzaman, M. et al. (2015). Superoxide dismutase mentor of abiotic stress tolerance in crop plants. Environmental Science and Pollution Research, 22(14), 10375–10394. DOI 10.1007/s11356-015-4532-5. [Google Scholar] [CrossRef]
57. Nxele, X., Klein, A., Ndimba, B. K. (2017). Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. South African Journal of Botany, 108, 261–266. DOI 10.1016/j.sajb.2016.11.003. [Google Scholar] [CrossRef]
58. Ahanger, M. A., Agarwal, R. M. (2017). Potassium upregulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivumL). Protoplasma, 254(4), 1471–1486. DOI 10.1007/s00709-016-1037-0. [Google Scholar] [CrossRef]
59. Fayez, K. A., Bazaid, S. A. (2014). Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. Journal of the Saudi Society of Agricultural Sciences, 13(1), 45–55. DOI 10.1016/j.jssas.2013.01.001. [Google Scholar] [CrossRef]
60. Hasanuzzaman, M., Alam, M. M., Nahar, K., Mohsin, S. M., Bhuyan, M. H. M. B. et al. (2019). Silicon-induced antioxidant defense and methylglyoxal detoxification works co-ordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology, 28(3), 261–276. DOI 10.1007/s10646-019-02019-z. [Google Scholar] [CrossRef]
61. Mahmud, J. A., Hasanuzzaman, M., Nahar, K., Rahman, A., Fujita, M. (2019). EDTA reduces cadmium toxicity in mustard (Brassica juncea L.) by enhancing metal chelation, antioxidant defense and glyoxalase systems. Acta Agrobotanica, 72(2), 1722. DOI 10.5586/aa.1772. [Google Scholar] [CrossRef]
62. Islam, F., Yasmeen, T., Ali, S., Ali, B., Farooq, M. A. et al. (2015). Priming-induced antioxidative responses in two wheat cultivars under saline stress. Acta Physiologiae Plantarum, 37(8), 1–12. DOI 10.1007/s11738-015-1897-5. [Google Scholar] [CrossRef]
63. Rizwan, M., Mostofa, M. G., Ahmad, M. Z., Imtiaz, M., Mehmood, S. et al. (2018). Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere, 191, 23–35. DOI 10.1016/j.chemosphere.2017.09.068. [Google Scholar] [CrossRef]
64. Miyaji, T., Kuromori, T., Takeuchi, Y., Yamaji, N., Yokosho, K. et al. (2015). AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nature Communications, 6(1), 365. DOI 10.1038/ncomms6928. [Google Scholar] [CrossRef]
65. Anjum, N. A., Khan, N. A., Sofo, A., Baier, M., Kizek, R. (2016). Redox homeostasis managers in plants under environmental stresses. Frontiers in Environmental Science, 4, 35. DOI 10.3389/fenvs.2016.00035. [Google Scholar] [CrossRef]
66. Cui, G., Zhao, X., Liu, S., Sun, F., Zhang, C. et al. (2017). Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiology and Biochemistry, 118, 138–149. DOI 10.1016/j.plaphy.2017.06.014. [Google Scholar] [CrossRef]
67. Gill, S. S., Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930. DOI 10.1016/j.plaphy.2010.08.016. [Google Scholar] [CrossRef]
68. Elloumi, N., Zouari, M., Mezghani, I., Abdallah, F. B., Woodward, S. et al. (2017). Adaptive biochemical and physiological responses of Eriobotrya japonica to fluoride air pollution. Ecotoxicology, 26(7), 991–1001. DOI 10.1007/s10646-017-1827-y. [Google Scholar] [CrossRef]
69. Kaur, C., Kushwaha, H. R., Mustafiz, A., Pareek, A., Sopory, S. K. et al. (2015). Analysis of global gene expression profile of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Frontiers in Plant Science, 6, 682. DOI 10.3389/fpls.2015.00682. [Google Scholar] [CrossRef]
70. Hossain, M. A., Hasanuzzaman, M., Fujita, M. (2011). Coordinate induction of antioxidant defense and glyoxalase system by exogenous proline and glycinebetaine is correlated with salt tolerance in mung bean. Frontiers of Agriculture in China, 5(1), 1–14. DOI 10.1007/s11703-010-1070-2. [Google Scholar] [CrossRef]
71. Narawongsanont, R., Kabinpong, S., Auiyawong, B., Tantitadapitak, C. (2012). Cloning and characterization of AKR4C14, a rice aldo-keto reductase, from Thai Jasmine rice. Protein Journal, 31(1), 35–42. DOI 10.1007/s10930-011-9371-8. [Google Scholar] [CrossRef]
72. Kirch, H. H., Schlingensiepen, S., Kotchoni, S., Sunkar, R., Bartels, D. (2005). Detailed expression analysis of selected genes of the aldehyde dehydrogenase (ALDH) gene superfamily in Arabidopsis thaliana. Plant Molecular Biology, 57(3), 315–332. DOI 10.1007/s11103-004-7796-6. [Google Scholar] [CrossRef]
73. Ghosh, A., Kushwaha, H. R., Hasan, M. R., Pareek, A., Sopory, S. K. et al. (2016). Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Scientific Reports, 6, 1–15. DOI 10.1038/s41598-016-0001-8. [Google Scholar] [CrossRef]
74. Hoque, T. S., Hossain, M. A., Mostofa, M. G., Burritt, D. J., Fujita, M. et al. (2016). Methylglyoxal: An emerging signaling molecule in plant abiotic stress responses and tolerance. Frontiers in Plant Science, 7(827951), 161. DOI 10.3389/fpls.2016.01341. [Google Scholar] [CrossRef]
75. Ali, Q., Haider, M. Z., Iftikhar, W., Jamil, S., Tariq Javed, M. et al. (2016). Drought tolerance potential of Vigna mungo L. lines as deciphered by modulated growth, antioxidant defense, and nutrient acquisition patterns. Brazilian Journal of Botany, 39(3), 801–812. DOI 10.1007/s40415-016-0282-y. [Google Scholar] [CrossRef]
76. Shah, T., Khan, A. Z., Numan, M., Ahmad, W., Zahoor, M. et al. (2017). Nutrient uptake and yield of wheat varieties as influenced by foliar potassium under drought condition. Cercetari Agronomice in Moldova, 50(2), 5–20. DOI 10.1515/cerce-2017-0011. [Google Scholar] [CrossRef]
77. Zain, N. A. M., Ismail, M. R., Puteh, A., Mahmood, M., Islam, M. R. (2014). Drought tolerance and ion accumulation of rice following application of additional potassium fertilizer. Communications in Soil Science and Plant Analysis, 45(19), 2502–2514. DOI 10.1080/00103624.2014.932374. [Google Scholar] [CrossRef]
78. Ibrahim, M. F., El-Samad, A., Ashour, H., El-Sawy, A. M., Hikal, M. et al. (2020). Regulation of agronomic traits, nutrient uptake, osmolytes and antioxidants of maize as influenced by exogenous potassium silicate under deficit irrigation and semiarid conditions. Agronomy, 10(8), 1212. DOI 10.3390/agronomy10081212. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |