

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014191
ARTICLE
Efficient Evergreen Plant Regeneration of Cinnamomum japonicum Sieb. through in vitro Organogenesis
1Key Laboratory of Forest Genetics & Biotechnology of Ministry of Education, Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, 210037, China
2College of Biology and the Environment, Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, 210037, China
*Corresponding Author: Jinhui Chen. Email: chenjh@njfu.edu.cn
Received: 08 September 2020; Accepted: 29 September 2020
Abstract: Cinnamomum japonicum Sieb. is an excellent roadside tree and medicinal tree species with considerable ornamental and economic value. In this study, we successfully developed a large-scale micropropagation protocol for C. japonicum for the first time. Sterilized shoots were excised and used as explants for shoot induction on several basal media, supplemented with different concentrations of plant growth regulators (PGRs), such as Thidiazuron (TDZ), N6-Benzyladenine (6-benzylaminopurine) (BA), α-naphthaleneacetic acid (NAA) and Gibberellic acid (GA3). After comparison, the most efficient medium for shoot regeneration was 1/2 Murashige and Skoog (MS) medium containing 0.5 mg L–1 BA, 0.05 mg L–1 NAA and 0.2 mg L–1 GA3, which resulted in an average number of induced shoots per explant and shoot length of 5.2 and 1.62 cm at 28 d, respectively. Then, elongated adventitious shoots were transferred to induce roots. 86.7% of shoots was able to root on 1/2 MS medium supplemented with 0.5 mg L–1 NAA and 0.1 mg L–1 BA. The earliest rooting time observed was after 21 d and the average root length was up to 3.3 cm after 28 d. Our study shows that C. japonicum can be successfully regenerated through de novo organogenesis, which lays a foundation for future transformation research on this tree.
Keywords: Cinnamomum japonicum Sieb.; micropropagation; de novo organogenesis; shoot induction; rhizogenesis
Cinnamomum japonicum Sieb., a subtropical evergreen, broad-leaved, perennial tree species in the family Lauraceae, is distributed in the narrow range of the southeast coast of China, the southeast coast of Korea and certain areas of Japan, such as Shikoku, Kyushu and the Ryukyu Islands [1,2]. Adult C. japonicum is approximately 10–15 m tall, with slender, cylindrical, smooth, red or reddish-brown branches, and 30–40 cm in diameter at breast height (DBH); the leaves are subopposite ovate or oblong-lanceolate, leathery, bright and fragrant; the flowering period is from April to May and the fruiting period is from July to September [3]. C. japonicum is mainly used as a roadside tree and an ornamental garden tree. It has the characteristics of strong growth, lush leaf growth, a graceful tree shape, a pleasant fragrance; it has the capability of absorbing sulfur dioxide, carbon dioxide and other tail gasses; and it has a strong resistance to heat, cold, pest, diseases, wind and drought [1]. In addition, C. japonicum is not only a tree species used for greening and decoration, but also has significant economic benefits. C. japonicum is abundant in flavonoids [4], proanthocyanidins [5], cinnamic acid and cinnamaldehyde [6], as well as oil protecting against Aedes albopictus [7]. Furthermore, C. japonicum, has a very long history of usage as a medicinal species in China, Korea, Japan, as its extract, cinnamic acid and cinnamaldehyde, shows several physiological effects; such as anti-microbial activity, inhibition of dopachrome formation [8], an anti-inflammatory effect [6,9] and inhibition of angiogenesis in quail eggs [10].
The traditional cultivation methods of C. japonicum mainly include propagation via cuttings and seeds, which are time-consuming and cumbersome processes. Besides, inbreeding depression is commonly observed in plants [11], especially in species that have mixed mating systems (i.e., plants that both self and cross fertilize), which is common in flowering plants [12]. By contrast, large-scale micropropagation of plants through de novo organogenesis techniques, a morphogenetic route based on plant regeneration in which shoots or roots are formed in a direct or indirect way [13], could be a rapid way to produce uniform material in a short timeframe [14]. This regeneration technology mainly depends on the type of explant and the growth regulators used [13–16]. There are some advantages to de novo organogenesis, such as that it can rapidly reproduce excellent genotypes, avoids problems associated with variable seed production, maintains genetic diversity, helps expedite genetic improvement programs for forestry species and saves resources to prevent extinction [17,18]. De novo organogenesis has previously been successfully used to rapidly cultivate a large number of economic plants and thereby conserve endangered plant species, such as Cassia siamea Lam. [19], Genipa americana L. [20], Plumbago zeylanica L. [21], Haloxylon persicum (Bunge ex Boiss & Buhse) [22], Rhododendron wattii Cowan [23] and Quercus lusitanica Lam. [24]. Preliminary work on the tissue culture of related species like Cinnamomum zeylanicum Breyn. [25], Cinnamomum tamala Nees. [26] and Cinnamomum camphora [27,28] has been reported. In addition, exogenous hormones play an important role in the efficiency of in vitro regeneration, especially auxin and cytokinin, of which not only the combinations but also the concentrations are crucial for the regulation of organogenesis [29]. For example, recent research confirmed the effect of TDZ, which was recognized as a highly active cytokinin [30], in inducing various morphogenic responses [22]. Successful induction of organogenesis through TDZ has been reported in many species, such as Gymnocladus assamicus [31] and Bienertia sinuspersici [32].
Due to over-exploitation and environmental changes, the natural habitat of C. japonicum and its population structure have steadily declined. So, C. japonicum has been classified as an endangered species and is included in both the Chinese Endangered Plant Red Papers, considered as “Second class” and the IUCN Red List of Threatened Species, regarded as “near threatened” [1]. This classification indicates the urgency for developing an efficient system to propagate this species, which is currently fading away. In this study, we carried out extensive work to develop an effective protocol for direct organogenesis induction of C. japonicum, including adventitious shoots induction from explants, elongation and rooting of regenerated shoots. It was found that all explants, grown on 1/2 MS medium containing 0.5 mg L–1 BA, 0.05 mg L–1 NAA, 0.2 mg L–1 GA3, 3% (w/v) sucrose and 0.25% (w/v) Gelrite, were able to form the most and longest adventitious shoots. Then, more than 80% of elongated adventitious shoots, propagated on 1/2 MS medium supplemented with 5 mg L–1 VC, 0.5 mg L–1 NAA, 0.1 mg L–1 BA, 3% (w/v) sucrose, 0.1% (w/v) activated charcoal (AC) and 0.25% (w/v) Gelrite, could root efficiently. This study established a relatively efficient regeneration system, which could be used to rapidly propagate C. japonicum, an important and endangered economic tree resource, on a large scale. This study furthermore laid the foundation for future transformation research into this species.
2.1 Plant Material and Explant Surface Sterilization
Annual and healthy stems of C. japonicum, used as explants, were obtained from mature mother plants, then cut into segments with 1–2 axillary shoots or top shoots. These shoots were washed thoroughly with running tap water to remove soil debris, then sterilized with 70% ethanol (v/v) for 45 s, followed by treatment with 0.1% (v/v) HgCl2 with 2–3 drops of added Tween-20 for 6–9 min, at the end followed by 4–5 times rinsing in sterile water. After sterilization, the shoots were cultured in vitro on Murashige and Skoog (MS)-basal medium, with 3% (w/v) sucrose and 0.25% (w/v) Gelrite. The pH of the media was adjusted to 5.7–5.8 using 1 M KOH prior to adding 0.25% (w/v) Gelrite and then autoclaved at 121°C for 20 min.
2.2 Multiple Shoot Formation and Regeneration
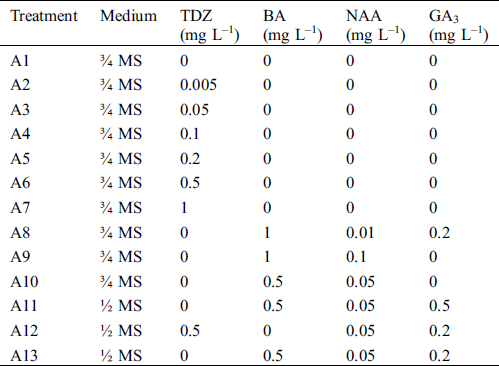
To explore the effect of various TDZ (a cytokinin-like plant growth regulator) concentrations on shoot regeneration, surface sterilized shoots were used as explants and cultured on 3/4 MS medium supplemented with various concentrations of TDZ (0, 0.005, 0.05, 0.1, 0.2, 0.5, 1 mg L–1), 5 mg L–1 VC, 3% (w/v) sucrose and 0.25% (w/v) Gelrite (Tab. 1, A1–A7). TDZ-free medium (A1) was used as a control. The rate of browning, the number of shoots per explant, and the shoot length were recorded after 28 d.
To compare the effect of TDZ alone and different PGRs combinations on shoot regeneration, no browning and sterilized shoots were used as explants and cultured on 1/2 or 3/4 MS medium containing 5 mg L–1 VC, 3% (w/v) sucrose and 0.25% (w/v) Gelrite supplemented with GA3 (0–0.5 mg L–1), BA (0–1 mg L–1) and NAA (0–0.1 mg L–1) for shoot induction assays (Tab. 1, A8–A13). The treatment with 0.5 mg L–1 TDZ alone (A6) which induced the most shoots was as a control. Shoot elongation parameters, such as the number and length of shoots, were also determined after 28 d.
Table 1: Different treatments with various plant growth regulators used for shoot induction and elongation assays
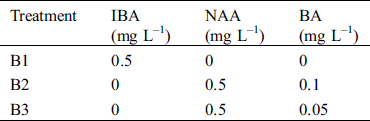
Elongated microshoots (1.5–2 cm in length) produced after shoot induction, were transferred to 1/2 MS medium supplemented with 5 mg L–1 VC, 3% (w/v) sucrose, 0.1% (w/v) activated charcoal (AC) and 0.25% (w/v) Gelrite for rooting. To explore the appropriate concentrations and combinations of auxins and cytokinins for rooting, indole-3-butyric acid (IBA) or NAA and BA, were added to 1/2 MS medium (Tab. 2). The earliest rooting time, rate of root formation and root length were determined after culturing on root induction medium for 28 d.
Table 2: Constitution of the culture medium used for rhizogenesis
All of the cultures were maintained at 25°C, under a photoperiod of 16-h light/8-h dark for 28 d, then subcultured every 28 d.
A completely randomized experimental design was adopted, using three replicates of 5 explants for each treatment. Statistical analysis was performed using SPSS 22.0.0.0. Data were analyzed by analysis of variance (ANOVA), followed by the least significant difference (LSD) test at P ≤ 0.05.
3.1 TDZ Promotes Cinnamomum japonicum Sieb. Shoot Induction
We cultivated sterilized shoots (1–1.5 cm in length) (Fig. 1e) on 3/4 MS medium with increasing concentrations of TDZ, which was previously reported to have the function of promoting more shoots and accelerating shoot elongation [31] (Tab. 1). When adding TDZ to 3/4 MS medium in increasing concentrations, we found that, to a certain extent, higher amounts of TDZ induced a higher number of regenerated shoots (Fig. 1b). However, there does seem to be an optimal TDZ concentration, as adding 1 mg L–1 (A7) was less effective than adding 0.5 mg L–1 (A6) (Fig. 1b). The shoot length showed a trend very similar to the number of regenerated shoots, although the decreased length at the highest concentration did not reach statistical significance (Figs. 1b and 1c). Interestingly, the shoot browning rate showed the opposite trend in which increasing concentrations of TDZ decreased the shoot browning rate, and the lowest browning rate was reached at a TDZ concentration of 0.5 mg L–1 (A6) (Fig. 1a). The extra shoots induced from the base of the shoots at 0.5 mg L–1 TDZ (A6) were almost 0.6 cm long on average (Fig. 1f). These findings illustrate that the TDZ hormone promotes shoot regeneration and decreases shoot browning in C. japonicum, yet should be added at an optimal concentration, as these effects could be reversed again by ever increasing concentrations.
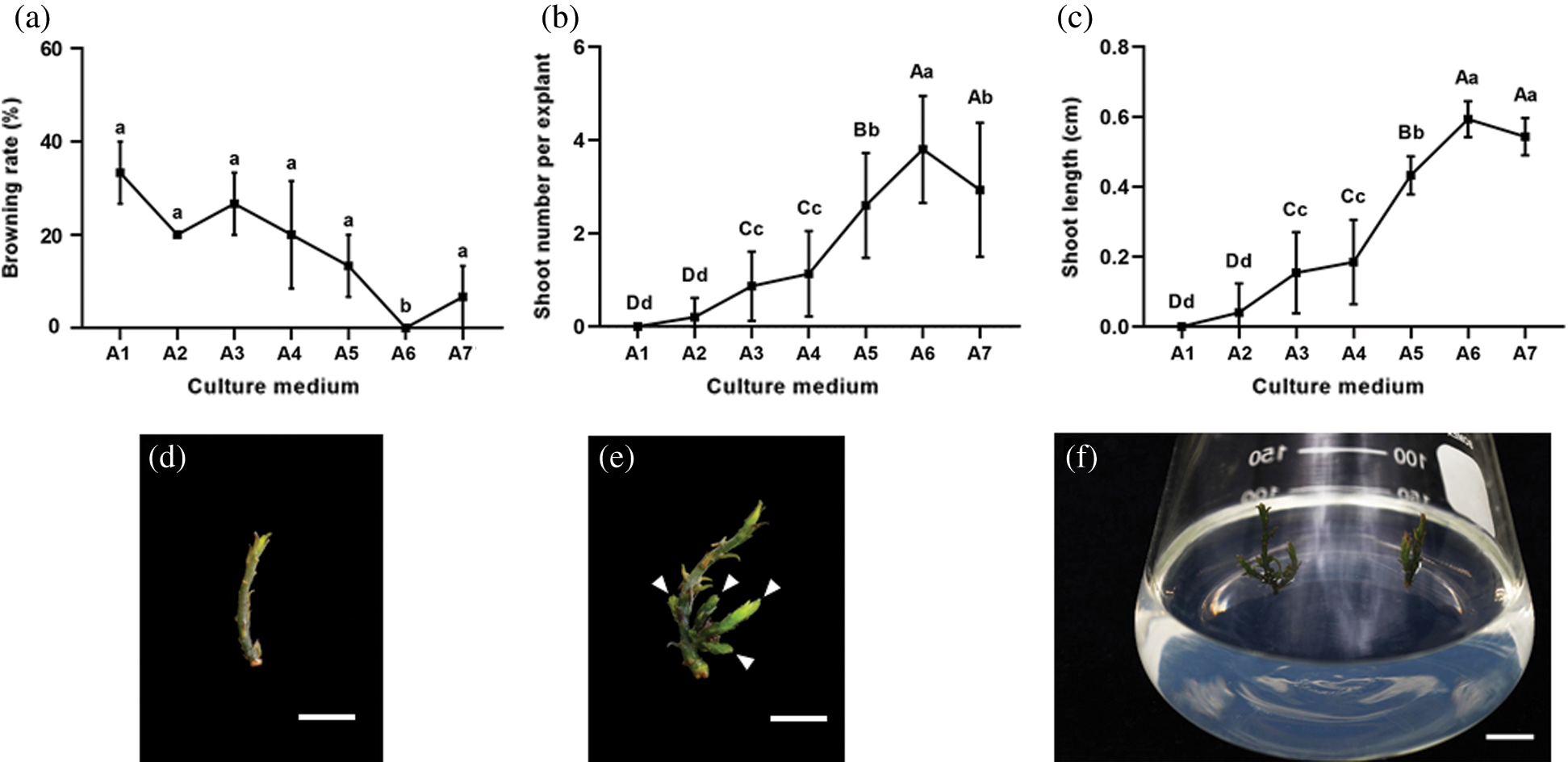
Figure 1: TDZ stimulates Cinnamomum japonicum Sieb. shoot regeneration and prevents tissue browning. (a) Browning rate. Data represents the mean ± SEM. N = 15. (b) Induced shoot number per explant. Data represents the mean ± SEM. N = 15. (c) Induced shoot length. Data represents the mean ± SD. N = 15. (d) Representative explant used to induce shoots. Bar = 0.5 cm. (e, f) Regenerated shoots (indicated by white arrows) induced from the explant on medium supplemented with 0.5 mg L–1 TDZ (A6). Statistics: different lowercase letters and uppercase letters are significantly different according to the least significant difference (LSD) test at P ≤ 0.05 and P ≤ 0.005, respectively. Bar: (e) 0.5 cm; (f) 1 cm
3.2 Multiple Hormones Cooperatively Promote Shoot Proliferation and Elongation
It is known that TDZ could promote the shoot regeneration and propagation as we mentioned before, but shoot inducing functions have also been reported for other plant hormones. Therefore, we treated shoots (1–1.5 cm in length) (Fig. 2c) with various combinations of multiple hormones including TDZ, BA, NAA and GA3 (Tab. 1) to see if we could obtain a more efficient shoot regeneration system. BA or TDZ combined with NAA was used to induce shoots and GA3 was used to improve shoot elongation. The number of shoots induced per explant and the induced shoot length reached their highest values on 1/2 MS medium containing 0.5 mg L–1 BA, 0.05 mg L–1 NAA and 0.2 mg L–1 GA3 (A13), being 5.2 and 1.62 cm, respectively. These values were close to 1.5 time and 3 times higher than those obtained on medium with 0.5 mg L–1 TDZ alone (Figs. 2a and 2b). Elongated shoots were induced from the explants base (Fig. 2d). Moreover, explants propagated on 1/2 MS medium could regenerate more shoots than those on 3/4 MS medium (Fig. 2a). In addition, shoot length was indeed mostly affected by GA3, as all media containing GA3 stimulated significantly longer shoots (Fig. 2b).
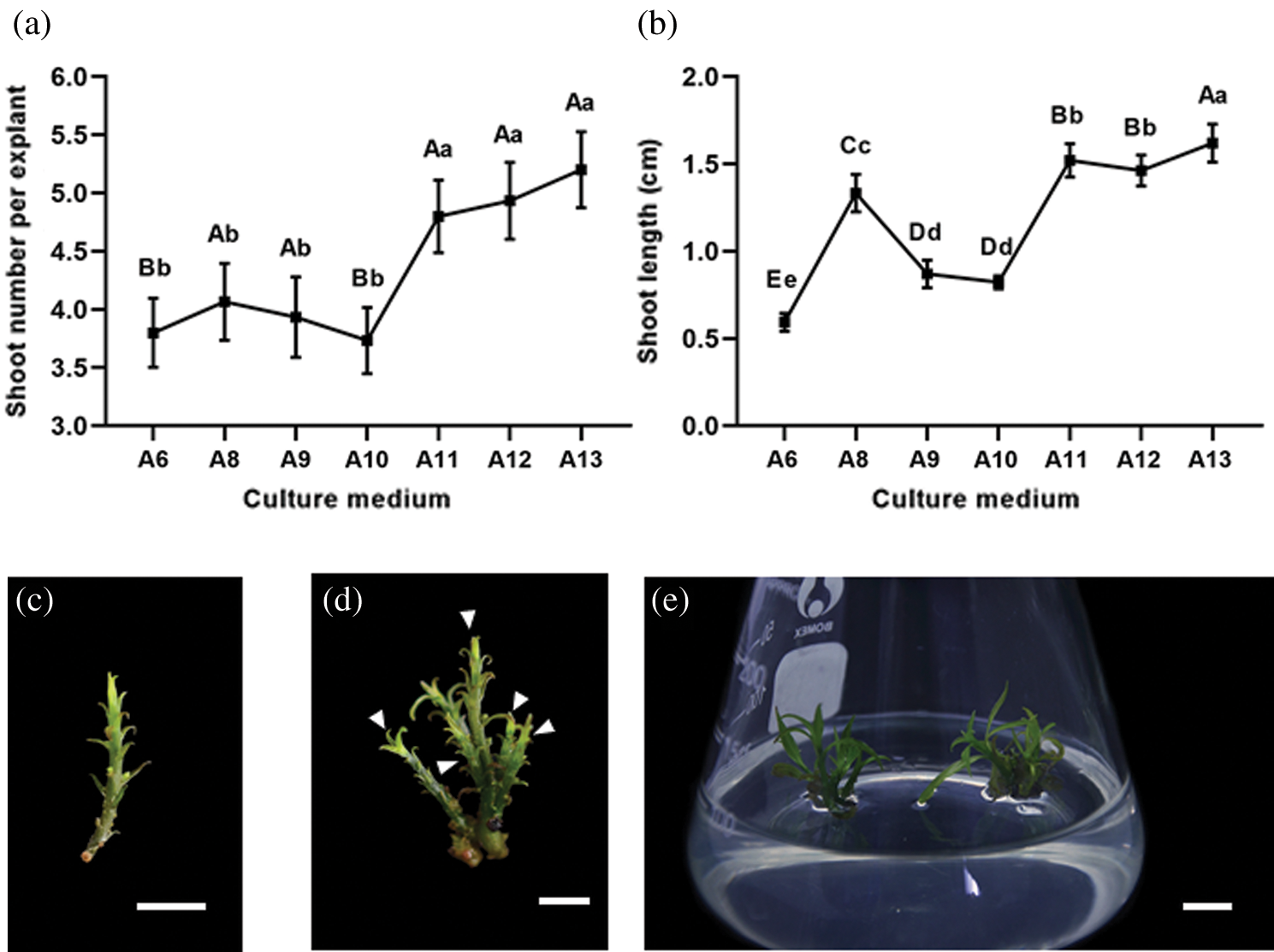
Figure 2: The effect of various combinations and concentrations of NAA, BA, GA and TDZ on Cinnamomum japonicum Sieb. shoot induction of. (a) Induced shoot number per explant. Data represents the mean ± SEM. N = 15. (b) Induced shoot length. Data represents the mean ± SD. N = 15. (c) Representative explant used to induce shoots. Bar = 0.5 cm. (d, e) Regenerated shoots (indicated by white arrows) induced from an explant on medium supplemented with 0.5 mg L–1 BA, 0.05 mg L–1 NAA and 0.2 mg L–1 GA3 (A13). Statistics: Different lowercase letters and uppercase letters are significantly different according to the least significant difference (LSD) test at P ≤ 0.05 and P ≤ 0.005, respectively. Bar: (d) 0.5 cm; (e) 1 cm
Root induction efficiency mainly depends on the type of auxin added to the medium and its concentration. We cultured regenerated shoots of around 1.5–2 cm in length (Fig. 3c) on auxin containing media to regenerate roots following shoot induction. We used different sets of auxin and cytokinin hormone combinations to study how they would affect root formation (Tab. 2). Off-white and thick roots regenerated from the shoot base on medium containing 0.5 mg L–1 NAA and 0.1 mg L–1 BA (B2) (Fig. 3d), with an average root length of 3.3 cm (slightly shorter than that induced on 0.5 mg L–1 IBA (B1)) (Fig. 3b). What’s more, the rooting rate and timing were the highest (86.7%) and earliest (21 d) at 0.5 mg L–1 NAA and 0.1 mg L–1 BA (B2) added (Fig. 3a). Therefore, NAA in combination with sufficient levels of BA was more effective at regenerating roots for C. japonicum than IBA alone. Following these experiments, we acclimatized the successfully rooted plantlets to ambient growth room conditions.
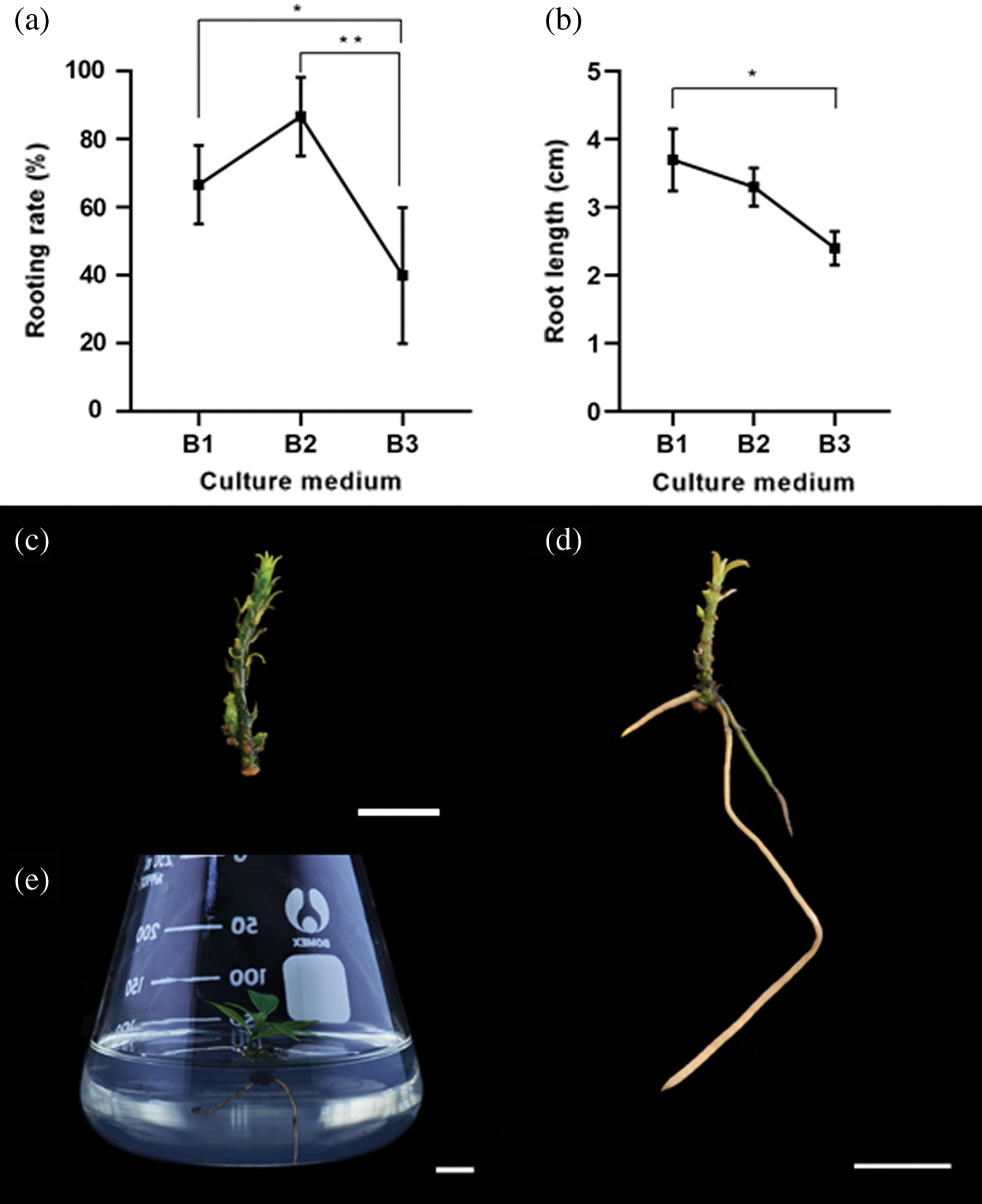
Figure 3: The effect of NAA, BA, and IBA on Cinnamomum japonicum Sieb. root induction. (a) Rooting rate. N = 15. (b) Induced root length. All data represent the mean ± SEM. N = 15. (c) Representative explant used for root induction. Bar = 0.5 cm. (d) Roots induced by 0.5 mg L–1 NAA and 0.1 mg L–1 BA. Bar = 1 cm. (e) A rooted seedling on ½ MS. Statistics: Asterisks indicate a statistically significant difference between every treatment according to the least significant difference (LSD) test at P ≤ 0.05 and P ≤ 0.005, respectively. Bar = 1 cm
Cinnamomum japonicum Sieb. is an endangered tree species with excellent medicinal and ornamental value that is so far understudied. To our knowledge, this is the first time an efficient regeneration protocol has been described for C. japonicum.
Medium composition is one of essential elements for plant cell and tissue culture. 1/2 MS [33], MMS (modified MS; ½-macro MS salts + full-strength micro MS salts + B5 vitamins) [34], WPM [35] and DKW [36] are now widely used for woody plants. 1/2 MS, MMS and WPM generally contain less total nitrogen and less ammonium than MS. However, DKM has a higher ammonium to nitrate ratio like MS, but less total nitrogen [34]. A micropropagation system for camphor tree (Cinnamomum camphora), another member of the Lauraceae family, was established on WPM, a medium with lower ammonium content that was formulated specifically for woody plants [37]. Therefore, medium with less total nitrogen and less ammonium could be more suitable for our Lauraceae plant, C. japonicum. In this study, 3/4 MS and 1/2 MS medium were chosen to use as basal media.
In addition, a high level of NH4+ is harmful to many plant species, causing poor root and shoot growth [38] and inducing ammonium toxicity [34]. In Aloe polyphylla, a higher concentration of NH4+ in the medium reduced the multiplication rate of the shoot and stimulated hyperhydricity, while lowering NH4+ to half of its value in MS medium eliminated hyperhydricity and improved the multiplication rate [39]. Previous studies also showed MS medium to be generally unsuitable for shoot cultures, easily stimulating hyperhydricity compared to low-salt media and MS dilution [40]. Similarly, media containing a lower concentration of NH4+, such as DKW and QL [41], were found to be superior to MS for shoot proliferation during the regeneration of some pear cultivars [42]. On the other hand, when the concentration of NH4+ was reduced or no NH4+ was added to the medium, the rooting rate and mean number of roots per shoot were increased in Eucalyptus globulus [43]. Previous studies have shown that media containing low NH4+ are suitable for rooting and widely used in many plants, such as Centaurium erythraea [44], Citrus limon [45] and Cinnamomum camphora [27]. Therefore, 1/2 MS basal medium with lower NH4+ may be more suitable for C. japonicum to culture in vitro.
Further developmental responses are stimulated by extra growth regulators such as auxin, cytokinin, gibberellins, ethylene and abscisic acid, when the basal nutrient needs of plant growth are met [34]. Some growth regulators, such as α-naphthaleneacetic acid (NAA), 2,4-Dichlorophenoxyacetic acid (2,4-D), Indole-3-butyric acid (IBA), Thidiazuron (TDZ), Zeatin (ZT), Kinetin (6-furfurylaminopurine) (KIN), N6-Benzyladenine (6-benzylaminopurine) (BA), 2-Isopentenyladenine [6-(γ, γ-dimethylallylamino)purine] (2-IP), Gibberellic acid (GA3), Abscisic acid (ABA) and Jasmonic acid (JA), have been studied for their role in plant regeneration. Therefore, we introduced a variety of growth regulators such as BA, NAA, GA3 and TDZ on C. japonicum shoot organogenesis, trying to find a simple, cost-effective tissue culture protocol.
TDZ is widely used for the induction of shoot proliferation in woody plants. In Saussurea involucrate, TDZ can induce shoot regeneration by changing the content of endogenous hormones and H2O2. Specifically, TDZ can promote the accumulation of endogenous hormones (IAA, ZT, GA3 and ABA) during early shoot organogenesis but a longer exposure to TDZ can inhibit their accumulation in plant tissues [46]. What’s more, the number of shoots per explant and the average shoot length with TDZ treatment were significantly higher compared to shoots treated with BA at the same concentration in Stevia rebaudiana Bertoni [47]. In this study, TDZ also effectively induced shoot regeneration and elongation, especially at its optimum concentration. However, we found that TDZ used alone seemed less efficient than a combination of BA and other PGRs, leading to reduced length of regenerating shoots (Fig. 2b). These findings are similar to results obtained in Gymnema sylvestre, where TDZ-induced adventitious shoots showed stunted growth and were relatively shorter when compared to those produced by BA-amended media [48]. Therefore, TDZ added alone in the medium to induce the shoot regeneration, was not as efficient as the combination of multiple plant growth regulators for C. japonicum, but TDZ maybe could replace BA when it was used with other hormones.
Gibberellic acid (GA3) always influenced seed development and may overcome dormancy [34]. For example, seeds soaked in GA3 solution for 12 h can break dormancy to improve their germination rate, such as Tilia miqueliana M. which changes the contents of soluble sugar, protein and starch [49,50] and flowering dogwood (Cornus florida L.) [51]. Furthermore, optimal media for shoot regeneration containing GA3 can promote shoot induction, shoot and root growth [52], mainly by stimulating mitotic division and cell elongation [53,54]. However, the addition of GA3 alone to the medium does generally not promote shoot elongation or axillary shoot formation [55]. In this study, the multiplication rate and shoot length of C. japonicum explants treated with a combination of BA and GA3 was significantly higher than that of explants treated without GA3. Previous studies performed on Citrus limon [45] and tree peony [56] showed that a combination of BA and GA3 dramatically increased the multiplication rate compared to treatment with BA alone. Furthermore, in Rotala rotundifolia [57], combined treatment with BA and GA3 had the same effect on the multiplication rate and shoot elongation was found for C. japonicum in this study. Therefore, combined treatment with BA and GA3 more strongly influences multiplication rate and shoot elongation compared to either BA or GA3 used alone during shoot organogenesis. Finally, in Magnolia sirindhorniae Noot. & Chalermglin, it has been shown that efficient micropropagation can be achieved by using 1/2 MS medium supplemented with 2.0 mg L–1 BA, 0.1 mg L–1 NAA, and 1.0 mg L–1 GA3 to effectively improve the time of shoot initiation and stimulate the growth of vigorous and green shoots [53]; the latter medium being very similar to that which we found to be optimal for C. japonicum micropropagation. Taken together, our study shows that the combination of BA, NAA and GA3 could efficiently improve shoot organogenesis and elongation in C. japonicum. Using the regeneration system of C. japonicum established in our lab, we could obtain a high number of regenerated seedlings with excellent growth characteristics and furthermore lay the foundation for future transformation research into this species.
Funding Statement: This research is supported by Key research and development plan of Jiangsu Province (BE2017376), Foundation of Jiangsu forestry bureau (LYKJ [2017]42), the Qinglan project of Jiangsu province and Priority Academic Program Development of Jiangsu Higher Education Institutions to J. H. Chen, and the Nature Science Foundation of China (31770715) to T. L. Cheng.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ren, X. L., Yang, C., Xin, G. L., Du, X. M., Jia, G. L. et al. (2019). Complete chloroplast genome of Cinnamomum japonicum (Laurales: Lauraceaean endangered tree species. Conservation Genetics Resources, 11(3), 267–269. DOI 10.1007/s12686-018-1004-5. [Google Scholar] [CrossRef]
2. Zhao, C. C., Yang, X. Y., Tian, H., Yang, L. (2018). An improved method to obtain essential oil, flavonols and proanthocyanidins from fresh Cinnamomum japonicum Sieb. leaves using solvent-free microwave-assisted distillation followed by homogenate extraction. Arabian Journal of Chemistry, 13(1), 2041–2052. DOI 10.1016/j.arabjc.2018.03.002. [Google Scholar] [CrossRef]
3. Zhou, D. Q., Xiong, Y. W., Lu, Z. G., Han, L. W., Wang, Z. Y. et al. (2013). A preliminary study on cold tolerance in Cinnamomum japonicum Sieb. at different ages. Agricultural Biotechnology, 2(6), 24–28. DOI 10.19759/j.cnki.2164-4993.2013.06.006. [Google Scholar] [CrossRef]
4. Ling, I. M., Li, W. H., Wang, L. H. (2009). In vitro skin permeation efficiency study on natural flavornoid extracts incorporated into nano-emulsions. Asian Journal of Chemistry, 21(8), 6237–6246. [Google Scholar]
5. Kin, R., Kato, S., Kaneto, N., Sakurai, H., Hayakawa, Y. et al. (2013). Procyanidin C1 from Cinnamomi Cortex inhibits TGF-β-induced epithelial-to-mesenchymal transition in the A549 lung cancer cell line. International Journal of Oncology, 43(6), 1901–1906. DOI 10.3892/ijo.2013.2139. [Google Scholar] [CrossRef]
6. Kim, M. S., Kim, J. Y. (2019). Cinnamon subcritical water extract attenuates intestinal inflammation and enhances intestinal tight junction in a Caco-2 and RAW264. 7 co-culture model. Food & Function, 10(7), 4350–4360. DOI 10.1039/C9FO00302A. [Google Scholar] [CrossRef]
7. Peng, Y. H., Jiang, L. J., Wang, L., Lu, Y. D., Yu, K. et al. (2013). Chemical composition and repellency of Cinnamomum japonicum leaf-derived essential oil against Aedes albopictus. Applied Mechanics and Materials, 295-298, 35–38. DOI 10.4028/www.scientific.net/AMM.295-298.35. [Google Scholar] [CrossRef]
8. Lin, C. C., Yang, C. H., Wu, P. S., Kwan, C. C., Chen, Y. S. (2011). Antimicrobial, anti-tyrosinase and antioxidant activities of aqueous aromatic extracts from forty-eight selected herbs. Journal of Medicinal Plants Research, 5(26), 6203–6209. DOI 10.5897/JMPR11.1134. [Google Scholar] [CrossRef]
9. Kim, M. S., Kim, J. Y. (2017). Intestinal anti-inflammatory effects of cinnamon extracts in a co-culture model of intestinal epithelial Caco-2 cells and RAW264. 7 macrophages. Applied Biological Chemistry, 60(5), 553–561. DOI 10.1007/s13765-017-0311-y. [Google Scholar] [CrossRef]
10. Seo, E. J., Kuete, V., Kadioglu, O., Krusche, B., Schröder, S. et al. (2013). Antiangiogenic activity and pharmacogenomics of medicinal plants from traditional Korean medicine. Evidence-Based Complementary and Alternative Medicine, 2013(4), 1–13. DOI 10.1155/2013/131306. [Google Scholar] [CrossRef]
11. Vergeer, P., Wagemaker, N. C., Ouborg, N. J. (2012). Evidence for an epigenetic role in inbreeding depression. Biology Letters, 8(5), 798–801. DOI 10.1098/rsbl.2012.0494. [Google Scholar] [CrossRef]
12. Goodwillie, C., Kalisz, S., Eckert, C. G. (2005). The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology Evolution & Systematics, 36(1), 47–79. DOI 10.1146/annurev.ecolsys.36.091704.175539. [Google Scholar] [CrossRef]
13. de Faria, R. B., de Carvalho, I. F., Rossi, A. A. B., de Matos, E. M. Rocha, D. I. et al. (2018). High responsiveness in de novo shoot organogenesis induction of Passiflora cristalina (Passifloraceaea wild Amazonian passion fruit species. In Vitro Cellular & Developmental Biology-Plant, 54(2), 166–174. DOI 10.1007/s11627-017-9881-y. [Google Scholar] [CrossRef]
14. Silvestri, C., Sabbatini, G., Marangelli, F., Rugini, E., Cristofori, V. (2018). Micropropagation and ex vitro rooting of wolfberry. HortScience, 53(10), 1494–1499. DOI 10.21273/HORTSCI13423-18. [Google Scholar] [CrossRef]
15. Rocha, D. I., Monte-Bello, C. C., Aizza, L. C. B., Dornelas, M. C. (2016). A passion fruit putative ortholog of the SOMATIC EMBRYOGENESIS RECEPTOR KINASE1 gene is expressed throughout the in vitro de novo shoot organogenesis developmental program. Plant Cell, Tissue and Organ Culture, 125(1), 107–117. DOI 10.1007/s11240-015-0933-x. [Google Scholar] [CrossRef]
16. Vieira, L. M., Rocha, D. I., Taquetti, M. F., da Silva, L. C., de Campos, J. M. S. et al. (2014). In vitro plant regeneration of Passiflora setacea D, (PassifloraceaeThe influence of explant type, growth regulators, and incubation conditions. Vitro Cellular & Developmental Biology-Plant, 50(6), 738–745. DOI 10.1007/s11627-014-9650-0. [Google Scholar] [CrossRef]
17. Merkle, S. A., Andrade, G. M., Nairn, C. J., Powell, W. A., Maynard, C. A. (2007). Restoration of threatened species: A noble cause for transgenic trees. Tree Genetics & Genomes, 3(2), 111–118. DOI 10.1007/s11295-006-0050-4. [Google Scholar] [CrossRef]
18. Stevens, M. E., Pijut, P. M. (2018). Rapid in vitro shoot multiplication of the recalcitrant species Juglans nigra L. In Vitro Cellular & Developmental Biology-Plant, 54(3), 309–317. DOI 10.1007/s11627-018-9892-3. [Google Scholar] [CrossRef]
19. Parveen, S., Shahzad, A., Saema, S. (2010). In vitro plant regeneration system for Cassia siamea Lam., a leguminous tree of economic importance. Agroforestry Systems, 80(1), 109–116. DOI 10.1007/s10457-010-9301-3. [Google Scholar] [CrossRef]
20. de Souza, R. R., de Oliveira Paiva, P. D., da Silva, R. R., da Silva, D. P. C., dos Reis, M. V. et al. (2019). Morphogenetic potential of different sources of explants for efficient in vitro regeneration of Genipa sp. Plant Cell, Tissue and Organ Culture, 136(1), 153–160. DOI 10.1007/s11240-018-1501-y. [Google Scholar] [CrossRef]
21. Sharma, U., Agrawal, V. (2018). In vitro shoot regeneration and enhanced synthesis of plumbagin in root callus of Plumbago zeylanica L.—An important medicinal herb. In Vitro Cellular & Developmental Biology-Plant, 54(4), 423–435. DOI 10.1007/s11627-018-9889-y. [Google Scholar] [CrossRef]
22. Kurup, S. S., Purayil, F. T., Alkhaili, M. M. S., Tawfik, N. H. Cheruth, A. J. et al. (2018). Thidiazuron (TDZ) induced organogenesis and clonal fidelity studies in Haloxylon persicum (Bunge ex Boiss & BuhseAn endangered desert tree species. Physiology and Molecular Biology of Plants, 24(4), 683–692. DOI 10.1007/s12298-018-0532-5. [Google Scholar] [CrossRef]
23. Mao, A. A., Vijayan, D., Nilasana Singha, R. K., Pradhan, S. (2018). In vitro propagation of Rhododendron wattii Cowan—A critically endangered and endemic plant from India. In Vitro Cellular & Developmental Biology-Plant, 54(1), 45–53. DOI 10.1007/s11627-017-9869-7. [Google Scholar] [CrossRef]
24. San José, M. C., Martínez, M. T., Cernadas, M. J., Montenegro, R., Mosteiro, F. et al. (2017). Biotechnological efforts for the propagation of Quercus lusitanica Lam., an endangered species. Trees, 31(5), 1571–1581. DOI 10.1007/s00468-017-1570-2. [Google Scholar] [CrossRef]
25. Ravi Shankar Rai, V., Jagadish Chandra, K. S. (1987). Clonal propagation of Cinnamomum zeylanicum Breyn. by tissue culture. Plant Cell, Tissue and Organ Culture, 9(1), 81–88. DOI 10.1007/BF00046082. [Google Scholar] [CrossRef]
26. Deb, C. R., Deb, M. S., Jamir, N. S. (2014). Effect of different factors on in vitro axillary shoot proliferation and plant regeneration of Cinnamomum tamala Nees.: A spice yielding plant. Indian Journal of Biotechnology, 13(4), 520–526. [Google Scholar]
27. Nirmal Babu, K., Sajina, A., Minoo, D., John, C. Z., Mini, P. M. et al. (2003). Micropropagation of camphor tree (Cinnamomum camphora). Plant Cell, Tissue and Organ Culture, 74(2), 179–183. DOI 10.1023/A:1023988110064. [Google Scholar] [CrossRef]
28. Du, L., Li, Y. P., Yao, Y., Zhang, L. W. (2015). An efficient protocol for plantlet regeneration via direct organogenesis by using nodal segments from embryo-cultured seedlings of Cinnamomum camphora L. PLoS One, 10(5), e0127215. DOI 10.1371/journal.pone.0127215. [Google Scholar] [CrossRef]
29. Cappelletti, R., Sabbadini, S., Mezzetti, B. (2016). The use of TDZ for the efficient in vitro regeneration and organogenesis of strawberry and blueberry cultivars. Scientia horticulturae, 207, 117–124. DOI 10.1016/j.scienta.2016.05.016. [Google Scholar] [CrossRef]
30. Murthy, B. N. S., Murch, S. J., Saxena, P. K. (1998). Thidiazuron: A potent regulator of in vitro plant morphogenesis. In Vitro Cellular & Developmental Biology-Plant, 34(4), 267–275. DOI 10.1007/BF02822732. [Google Scholar] [CrossRef]
31. Gupta, S., Mao, A. A., Sarma, S. (2020). Effects of Thidiazuron (tdz) on direct shoot organogenesis of Gymnocladus assamicus: A threatened and critically endangered species from Northeast India. National Academy Science Letters, 43(1), 85–91. DOI 10.1007/s40009-019-00801-5. [Google Scholar] [CrossRef]
32. Northmore, J. A., Sigurdson, D., Schoor, S., Rustum, A., Chuong, S. D. (2016). Thidiazuron induces high-frequency indirect shoot organogenesis of Bienertia sinuspersici: A single-cell C 4 species. Plant Cell, Tissue and Organ Culture, 126(1), 141–151. DOI 10.1007/s11240-016-0984-7. [Google Scholar] [CrossRef]
33. Murashige, T., Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497. DOI 10.1111/j.1399-3054.1962.tb08052.x. [Google Scholar] [CrossRef]
34. Phillips, G. C., Garda, M. (2019). Plant tissue culture media and practices: An overview. In Vitro Cellular & Developmental Biology-Plant, 55(3), 242–257. DOI 10.1007/s11627-019-09983-5. [Google Scholar] [CrossRef]
35. Llody, G., McCown, B. (1981). Commercially-feasible micropropagation of mountain laurel Kalmia latifolia, by use shoot-tip culture. Proceedings of the International Plant Propagators’ Society, 30, 421–427. [Google Scholar]
36. Driver, J. A., Kuniyuki, A. H. (1984). In vitro propagation of Paradox walnut rootstock. HortScience, 19(4), 507–509. [Google Scholar]
37. Alosaimi, A. A., Tripepi, R. R., Love, S. L. (2018). Micropropagation of Epilobium canum garretti (Firechalice) by axillary shoot culture. HortScience, 53(1), 62–66. DOI 10.21273/HORTSCI12396-17. [Google Scholar] [CrossRef]
38. Rogato, A., D’Apuzzo, E., Barbulova, A., Omrane, S., Parlati, A. et al. (2010). Characterization of a developmental root response caused by external ammonium supply in Lotus japonicus. Plant Physiology, 154(2), 784–795. DOI 10.1104/pp.110.160309. [Google Scholar] [CrossRef]
39. Ivanova, M., van Staden, J.,. (2008). Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell, Tissue and Organ Culture, 92(2), 227–231. DOI 10.1007/s11240-007-9311-7. [Google Scholar] [CrossRef]
40. Bosela, M. J., Michler, C. H. (2008). Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: Evaluation of multiple nutrient formulations and cytokinin types. In Vitro Cellular & Developmental Biology-Plant, 44(4), 316–329. DOI 10.1007/s11627-008-9114-5. [Google Scholar] [CrossRef]
41. Quoirin, M., Lepoivre, P. (1977). Etude de milieux adaptes aux cultures in vitro de Prunus. Acta Horticulturae, 78(78), 437–442. DOI 10.17660/ActaHortic.1977.78.54. [Google Scholar] [CrossRef]
42. Bell, R. L., Srinivasan, C., Lomberk, D. (2009). Effect of nutrient media on axillary shoot proliferation and preconditioning for adventitious shoot regeneration of pears. In Vitro Cellular & Developmental Biology-Plant, 45(6), 708–714. DOI 10.1007/s11627-009-9196-8. [Google Scholar] [CrossRef]
43. Bennett, I. J., McDavid, D. A. J., McComb, J. A. (2003). The influence of ammonium nitrate, pH and indole butyric acid on root induction and survival in soil of micropropagated Eucalyptus globulus. Biologia Plantarum, 47(3), 355–360. DOI 10.1023/B:BIOP.0000023877.21262.a5. [Google Scholar] [CrossRef]
44. Subotić, A., Jevremović, S., Grubišić, D. (2009). Influence of cytokinins on in vitro morphogenesis in root cultures of Centaurium erythraea—Valuable medicinal plant. Scientia Horticulturae, 120(3), 386–390. DOI 10.1016/j.scienta.2008.11.034. [Google Scholar] [CrossRef]
45. Pérez-Tornero, O., Tallón, C. I., Porras, I. (2010). An efficient protocol for micropropagation of lemon (Citrus limon) from mature nodal segments. Plant Cell, Tissue and Organ Culture, 100(3), 263–271. DOI 10.1007/s11240-009-9643-6. [Google Scholar] [CrossRef]
46. Guo, B., He, W., Zhao, Y., Wu, Y. D., Fu, Y. P. et al. (2017). Changes in endogenous hormones and H2O2 burst during shoot organogenesis in TDZ-treated Saussurea involucrate explants. Plant Cell, Tissue and Organ Culture (PCTOC), 128(1), 1–8. DOI 10.1007/s11240-016-1069-3. [Google Scholar] [CrossRef]
47. Lata, H., Chandra, S., Wang, Y. H., Raman, V., Khan, I. A. (2013). TDZ-induced high frequency plant regeneration through direct shoot organogenesis in Stevia rebaudiana Bertoni: An important medicinal plant and a natural sweetener. American Journal of Plant Sciences, 4(1), 117–128. DOI 10.4236/ajps.2013.41016. [Google Scholar] [CrossRef]
48. Isah, T. (2019). De novo in vitro shoot morphogenesis from shoot tip-induced callus cultures of Gymnema sylvestre (Retz.) (Retz.) R. Br. ex Sm. Biological Research, 52(1), 3. DOI 10.1186/s40659-019-0211-1. [Google Scholar] [CrossRef]
49. Yao, W. F., Shen, Y. B., Shi, F. H. (2015). Germination of Tilia miqueliana seeds following cold stratification and pretreatment with GA3 and magnetically-treated water. Seed Science and Technology, 43(3), 554–558. DOI 10.15258/sst.2015.43.3.21. [Google Scholar] [CrossRef]
50. Yao, W. F., Shen, Y. B. (2018). Effects of gibberellic acid and magnetically treated water on physiological characteristics of Tilia miqueliana seeds. Canadian Journal of Forest Research, 48(5), 554–558. DOI 10.1139/cjfr-2017-0289. [Google Scholar] [CrossRef]
51. Liu, H. L., Qian, C. M., Zhou, J., Zhang, X. Y., Ma, Q. Y. et al. (2015). Causes and breaking of seed dormancy in flowering dogwood (Cornus florida L.). HortScience, 50(7), 1041–1044. DOI 10.21273/HORTSCI.50.7.1041. [Google Scholar] [CrossRef]
52. Seon, K. M., Kim, D. H., Kang, K. W., Sivanesan, I. (2018). Highly competent in vitro propagation of Thrixspermum japonicum (Miq.) Rchb. f., a rare epiphytic orchid. Vitro Cellular & Developmental Biology-Plant, 54(3), 302–308. DOI 10.1007/s11627-018-9890-5. [Google Scholar] [CrossRef]
53. Cui, Y. Y., Deng, Y. W., Zheng, K. Y., Hu, X. M., Zhu, M. L. et al. (2019). An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Scientific Reports, 9(1), 9634. DOI 10.1038/s41598-019-46050-w. [Google Scholar] [CrossRef]
54. Ali, S., Khan, N., Nouroz, F., Erum, S., Nasim, W. et al. (2018). In vitro effects of GA3 on morphogenesis of CIP potato explants and acclimatization of plantlets in field. Vitro Cellular & Developmental Biology-Plant, 54(1), 104–111. DOI 10.1007/s11627-017-9874-x. [Google Scholar] [CrossRef]
55. Bouza, L., Jacques, M., Miginiac, E. (1994). In vitro propagation of Paeonia suffruticosa Andr. cv.‘Mme de Vatry’: Developmental effects of exogenous hormones during the multiplication phase. Scientia Horticulturae, 57(3), 241–251. DOI 10.1016/0304-4238(94)90144-9. [Google Scholar] [CrossRef]
56. Wen, S. S., Cheng, F. Y., Zhong, Y., Wang, X., Li, L. Z. et al. (2016). Efficient protocols for the micropropagation of tree peony (Paeonia suffruticosa ‘Jin Pao Hong’, P. suffruticosa ‘Wu Long Peng Sheng’, and P.× lemoinei ‘High Noon’) and application of arbuscular mycorrhizal fungi to improve plantlet establishment. Scientia Horticulturae, 201, 10–17. DOI 10.1016/j.scienta.2016.01.022. [Google Scholar] [CrossRef]
57. Karataş, M., Aasim, M., Çiftçioğlu, M. (2014). Adventitious shoot regeneration of roundleaf toothcup-Rotala rotundifolia [(Buch-Ham. ex Roxb) Koehne]. Journal of Animal & Plant Sciences, 24(3), 838–842. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |