

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013223
ARTICLE
Establishment of Rhodiola quadrifida Hairy Roots and Callus Culture to Produce Bioactive Compounds
1Group of Specialized Root Metabolism, К.А. Timiryazev Institute of Plant Physiology RAS, Moscow, 127276, Russia
2Bioanalytical laboratory, OOO “NVC Agrovetzaschita”, Moscow, 129329, Russia
*Corresponding Author: Anna Stepanova. Email: step_ann@mail.ru
Received: 28 July 2020; Accepted: 23 October 2020
Abstract: Rhodiola quadrifida is a rare mountain medicinal plant whose root extracts are used in traditional Chinese medicine as a hemostatic, antitussive, and tonic in the treatment of gynecological diseases. The aim of the study was to obtain R. quadrifida cultures at different degrees of differentiation in vitro and compare their growth characteristics and the content of salidroside and rosavin. Hairy roots were obtained by incubating cotyledons and hypocotyls in a suspension of Agrobacterium rhizogenes strain A4. The presence of the rolB and rolC genes was proven by polymerase chain reaction. The obtained roots were cultivated in Murashige-Skoog medium (MS). Calluses were obtained from the hairy roots in MS medium with the addition of hormones: 3 mg/L 2,4 D and 0.5 mg/L BAP. The presence of the main secondary metabolites of R. quadrifida, salidroside and rosavin, in calluses and salidroside in hairy roots by HPLC/MS was confirmed. The content of salidroside in callus culture was significantly higher than in hairy roots, 0.158 and 0.047%, respectively. The content of rosavin in callus culture was 0.07%. The content of rosavin and salidroside in callus culture was close to the level of these substances in the rhizomes of R. quadrifida plants growing in vivo, making this culture promising for its possible biotechnological use.
Keywords: Rhodiola quadrifida; hairy roots; callus culture; HPLC; salidroside; rosavin
Rhodiola quadrifida belongs to the Rhodiola genus, which includes about 90 species [1]. The area of its growth is limited to the highlands of the Altai Mountains in Mongolia and China. R. quadrifida is listed in the Red Books of many constituent entities of the Russian Federation and needs protection [2]. This species reproduces both vegetatively and generatively in nature. The seed productivity and survival rate of young R. quadrifida plants are low. Root extracts of R. quadrifida have a wide range of pharmacological effects. They are used in traditional Chinese medicine as a hemostatic, antitussive, and tonic in the treatment of gynecological diseases [3–5]. R. quadrifida increases physical endurance, improves memory, learning ability, and treats burns and bruises [6].
The medicinal properties of plants of the Rhodiola genus, including R. quadrifida, are due to the presence of a number of biologically active substances that have been identified by numerous in vitro and in vivo studies. The main ones are phenylethanoids (tyrosol and salidroside) and phenylpropanoids (cinnamyl alcohol glycosides-rosin, rosavin, rosarin). They are products of the shikimate pathway. However, their synthesis diverges at the level of aromatic amino acids: Tyrosine for phenylethanoids, and phenylalanine for phenylpropanoids. Glycosylation of tyrosol leads to the formation of salidroside, and glycosylation of cinnamon alcohol leads to the formation of rosin—its glycoside, from which rosavin and rosarin are formed by combining with arabinopyranose and arabinofuranose, respectively [7]. The levels of salidroside and rosavin content are used as one of the criteria for assessing the quality of raw materials [8,9].
R. quadrifida is classified as a low-potential species for introduction due to its narrow adaptability to specific habitat conditions [2]. Preserving R. quadrifida in nature and finding alternative methods for obtaining plant materials is necessary for the needs of traditional medicine and pharmacology. The solution to this problem is to use biotechnological approaches. Plant biomass can be grown in vitro in the form of undifferentiated cultures: suspensions, callus cultures, differentiated structures, including hairy roots. There is only one study that has described the preparation of a callus culture of R. quadrifida and also determined the content of salidroside, one of the most valuable secondary metabolites [10]. There are no publications devoted to the production and study of the hairy roots of this plant species. The level of synthesis of biologically active substances can significantly vary in differentiated and undifferentiated in vitro cultures. At the moment, there are no published data that compare the characteristics of callus culture and root cultures of R. quadrifida or other representatives of this genus. That comparison is needed to find the most promising system for biotechnological use and to study the mechanisms of the formation of phenolic compounds in plants of the Rhodiola genus. The purpose of this study was to obtain hairy roots and callus culture of R. quadrifida, analyze their growth characteristics, determine and compare the level of the main physiologically active secondary metabolites, salidroside and rosavin.
Seeds were used as start material for the in vitro preparation of Rhodiola quadrifida (Pall.) cultures. The seeds of Rhodiola were sterilized with a 0,1% diocide solution (composition: Ethylmercuric chloride, cetylpyridinium chloride) for 10 min to obtain an aseptic material. Then they were repeatedly washed with sterile distilled water and transferred for germination in Petri dishes in Murashige-Skoog (MS) medium [11].
The wild strain of Agrobacterium rhizogenes ATCC 15834 was used to obtain the hairy roots culture of R. quadrifida. The Agrobacterium suspension culture was grown in YEB liquid medium [12] for 24 h at 23°C on a circular shaker (amplitude 5−10 cm, rotation speed 90 rpm) before the transformation of the plant material.
The hairy roots of R. quadrifida were obtained as it was described previously [13]. The explants (cotyledons and hypocotyls) were incubated in Agrobacterium suspension for 12 h, then they were transplanted into fresh MS medium with the addition of 500 mg/L cefotaxime (Claforan, Great Britain). The explants were transplanted every three days until the complete elimination of the Agrobacterium. The appearance of primary roots was observed 14–28 days after transformation. They were separated and transplanted onto agar MS medium with 250 mg/L cefotaxime. The obtained roots were placed in liquid MS medium (the ratio of the volume of the flask and the medium was 100:20) with 250 mg/L cefotaxime after two passages on the MS medium with agar addition (Fig. 1). After four weeks of cultivation, hairy roots were transferred to medium without antibiotics. The cultivation cycle was four weeks. The plant material was cultured in darkness at the temperature of 23°C in the shaker at a rate of 90 rpm. The callus culture was obtained from roots stably growing for 12 months (Fig. 1). For this purpose, the roots were placed in MS medium with the addition of 3 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D, Merck, Germany) and 0.5 mg/L 6-benzylaminopurine (BAP, Merck, Germany). Nutrient media with the same hormone content have previously been successfully used to build up the callus cultures of other Rhodiolaspecies [14].
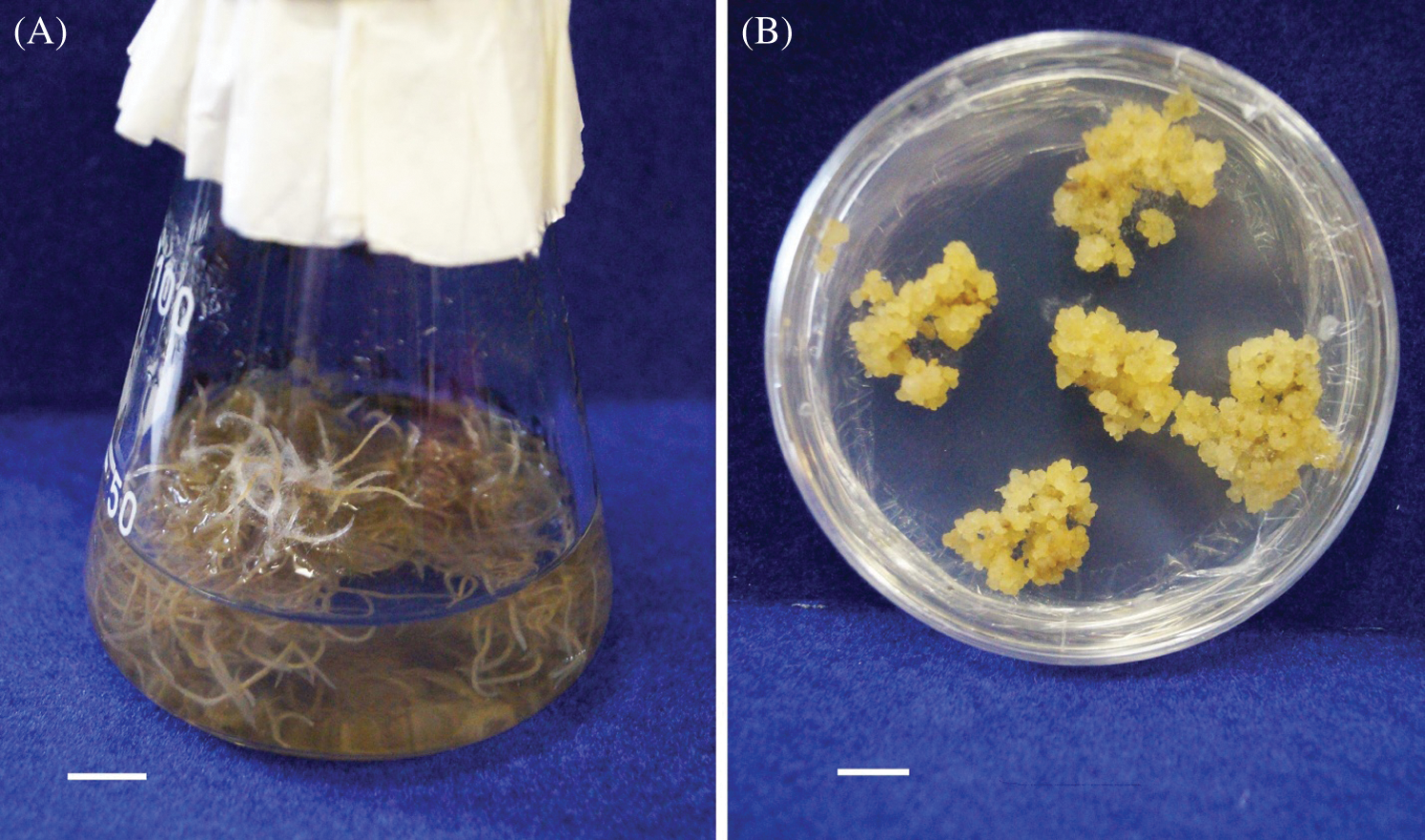
Figure 1: Hairy roots (A) and callus culture (B) of R. quadrifida. Scale bar = 1 cm
The callus culture was formed on the 14th day of cultivation. They were separated and transplanted to a fresh nutrient medium. Callus culture cultivation was carried out in a medium of the same composition. The cultivation cycle was four weeks. The callus cultures were cultured in the dark at 23°C.
The obtained roots and callus culture were tested for the presence of the rolB, rolC, rolD, and VirD genes by polymerase chain reaction (PCR) using the specific primers with the following oligonucleotide sequences (Litekh, Russia) for PCR reactions: The rolB gene (D:GCTCTTGCAGTGCTAGATTT, R:GAAGGTGCAAGCTACCTCTC); the rolC gene (D:CTCCTGACATCAAACTCGTC, R:TGCTTCGAGTTATGGGTACA); the rolD gene (D:CATCTGCAACTGAGCGTGTG, R:TGTCTGATAGGGAGGAACGA); the VirD gene (D:ATGTCGCAAGGCAGTAAGCCC, R:GGAGTCTTTCAGCATGGAGCAA). The PCR amplification (25 µl) mixture included 40 ng template DNA, 70 mM Tris-HCl (pH 8.6), 16.6 mM (NH4)2SO4, 0.0125% Cresol Red, 6.25% glycerol, 0.25 mM of each primer, 2U Taq DNA-polymerase, 0.2 mM of each dNTP, and 2.5 mM MgCl2. The thermal cycling program consisted of an initial denaturing step of 94°C for 2 min, followed by five cycles: Denaturation of 94°C for 20 s, annealing of 60−65°C for 10 s, elongation of 72°C for 10 s; 35 cycles: Denaturation of 94°C for 5 s, annealing of 60−65°C for 5 s, elongation of 72°C for 5 s, and final elongation step of 72°C for 2 min. The PCR products were separated by electrophoresis in a 2% agarose gel electrophoresis with 0.01% ethidium bromide in 1X TBE buffer.
To characterize the growth of hairy roots and callus culture, we used the growth index (I), which was calculated using the following formula:
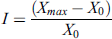
where Xmax is the highest raw weight level achieved by the culture (g), and X0 is the starting raw weight of the culture (g). The initial weight of the roots and calluses was 0.5 g. The selection was carried out every week.
Graphs and tables show the average arithmetic values of growth parameters from three to four biological repetitions for each variant.
2.4.1 Extraction and Preparation of Samples for HPLC-MS Analysis of Flavones
Sampling for the determination of salidroside and rosavin content was carried out at the end of the culture cycle (stationary growth phase). For HPLC analysis, lyophilized biomass samples were extracted with 75% ethanol (biomass: extractant ratio 1:100) according to a previously described method [15] in an FS14H ultrasonic bath (Fisher Scientific, United States) for 90 min, then 1 ml of the extract was taken and centrifuged for 15 min at 8000 rpm. After centrifugation, the 0.5 ml supernatant was transferred to a 1.5 ml Eppendorf tube and used for HPLC.
2.4.2 HPLC-MS Identification of Salidroside and Rosavin
The content of salidroside and rosavin in extracts of hairy roots and calluses of R. quadrifida was determined by high-performance liquid chromatography coupled with mass spectrometric detection. To avoid the broadening of chromatographic peaks, 150 μl of deionized water was added to 50 μl of the extract, and therefore, a dilution factor of 1:3 was further used in calculations. After dilution, 5 μl of the extract was injected into an Agilent 1200 HPLC (Agilent Technologies, USA), and a 150 * 2 mm Agilent Pursuit XRs C18 column (Agilent Technologies, USA) with a particle size of 5 μm was used for separation. The separation was carried out in a gradient mode; deionized water was used as phase A, and methanol (Applichem, Germany) was used as phase B. The signal for salidroside and rosavin was recorded using a 5500 QTRAP triple quadrupole mass spectrometer in the electrospray ionization mode (Sciex, USA), registration of negatively charged ions, multiple reaction monitoring (MRM).
For each substance, two MRM transitions were set: m/z 299.1 → 119.0, 299.0 → 89 for salidroside, m/z 427 → 293, 427 → 149 for rosavin. Linear calibration curves ranging from 1.56 to 50 ng/ml were constructed by analyzing salidroside (Sigma-Aldrich, Switzerland) and rosavin (Sigma-Aldrich, Switzerland) standard solutions in a water-methanol mixture (1:3).
Statistical data processing was performed using the Microsoft Excel program. Arithmetic average parameter values are outlined in the text. Bars on the diagram correspond to maximum values of the confidence intervals with a 95% probability level according to the Student t-criterion. All experiments were carried out in three replications.
The first works on in vitro culture establishment of representatives of the Rhodiola genus date back to the late twentieth and early twenty-first centuries. The Rhodiola genus is numerous, and most of its species are in demand in medicine and pharmacology. However, there are currently few publications on its in vitro culture establishment. According to the number of publications, species of the genus Rhodiola are distributed in the following order: R. rosea, R. crenulata, R. sachalinensis, R. kirilowii, and R. quadrifida [16]. Most of the above works to study callus cultures [16–19]. At the same time, the obtaining of in vitro cultures of the Rhodiola genus is still actual. The first study of Agrobacterium rhizogenes-mediated transformation of the Rhodiola genus (R. sachalinensis) was in 2007 and then was obtained hairy roots of R. kirilowii and R. rosea [20–23]. It was shown that the frequency of the hairy roots formation ranges from 9.0% to 94.7% because it depends on the type of explant (roots, stems, leaves, and cotyledons), Agrobacterium strain, illumination, species of the Rhodiola genus and region where the plants were collected [20–23]. Interestingly, the R. rosea plants from Russia were difficult to transform [20]. The hairy roots of R. quadrifida were obtained and characterized firstly in our work, as we known. The special features of our method for obtaining the hairy roots were long-term incubation of explants in Agrobacterium suspension (12 h) and their subsequent cultivation on MS agar medium with the addition of the antibiotic. In contrast, other authors used a short period of incubation of explants in Agrobacterium suspension (20–30 min), followed by its cultivation on agar medium without the addition of antibiotic for 48–72 h, then the explants were moved on medium with addition of antibiotic [20–22].
As a result of the transformation of 10 cotyledons and 15 hypocotyls, 4 explants with roots were obtained from hypocotyls. Hairy roots were not obtained from cotyledons. Thus, the method we used is quite effective and allows us to obtain hairy roots with a limited number of explants. The resulting culture of R. quadrifida hairy roots grew rapidly (Fig. 2). The growth index of hairy roots at the end of the cultivation cycle was 8.3. The callus culture initiated from the hairy roots of R. quadrifida on MS medium with the addition of 3 mg/L 2,4 D and 0.5 mg/L BAP grew slower (Fig. 2). Their growth index at the end of the cultivation cycle was 4.8.
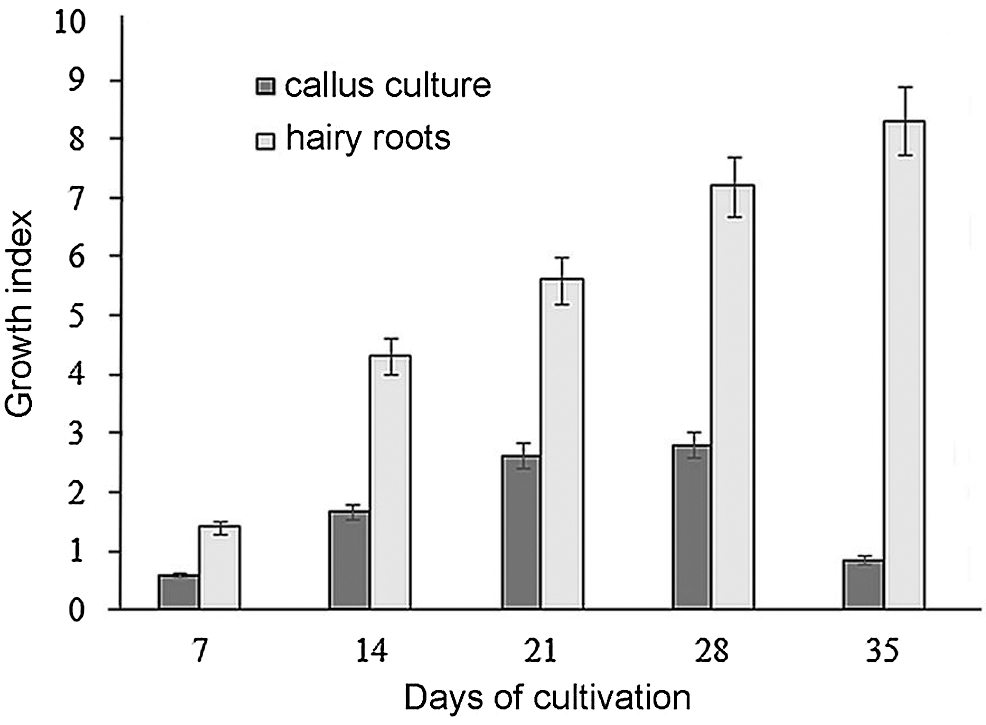
Figure 2: Growth index of hairy roots and callus culture of R. quadrifida that were cultured in vitro for five weeks. Hairy roots were cultured in liquid MS medium without hormones, and calluses were cultured on agar MS medium with 3 mg/L 2,4-D and 0.5 mg/L BAP. Bars represent means ± confidence intervals (n = 4) with a 95% probability level according to the Student t-criterion
Representatives of the Rhodiola genus grow rather slowly in natural conditions [24,25]. There is no published data on the growth rate of R. quadrifida, but it is known that the conditions for its growth are more extreme, and it grows more slowly than R. rosea. In our experiments, at the end of the culture cycle (4 weeks), the mass of hairy roots was 9.3 g/per flask which is equal to the growth of cultivated plants of Rhodiola rosea.
PCR analysis was performed to prove the transgenic nature and the absence of Agrobacterium contamination of hairy roots and callus culture initiated from them after several cycles of culturing them on an MS medium with cefotaxime. There was no contamination of the in vitro cultures with Agrobacterium since DNA isolated from the roots and callus cultures did not contain the VirD gene (Fig. 3). It was shown that the cultures obtained had PCR fragments of the expected length for rolC and rolD by PCR analysis with primers for Agrobacterium oncogenes (rolB, rolC, and rolD). This indicated the transgenic nature of the obtained hairy roots and callus cultures initiated from them.
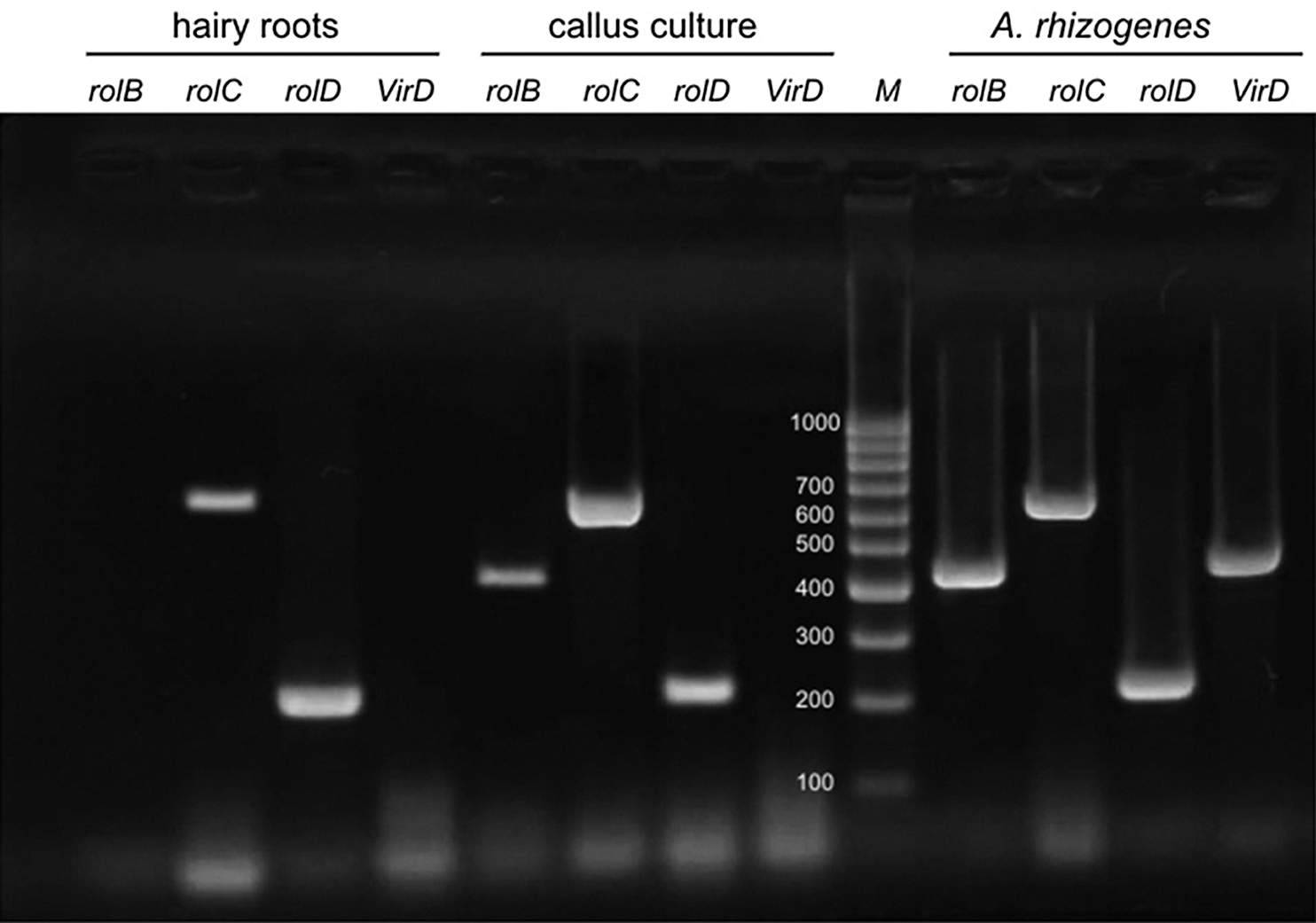
Figure 3: PCR products of the amplification of the rolB, rolC, rolD, and VirD primers in hairy roots and callus cultures of R. quadrifida and A. rhizogenes strain 15834. M: DNA marker (100 bp ladder). The presence of rolC and rolD genes and the absence of the VirD gene in callus cultures and hairy roots are shown. The presence of all four genes was indicated in Agrobacterium
The main biologically active substances of the Rhodiola genus are phenylethanoids (tyrosol and salidroside) and phenylpropanoids (rosin, rosavin, rosarin). The study of the content of salidroside and rosavin in different organs is mostly studied in the Rhodiola genus, R. rosea. It is shown that salidroside can be found both in the aerial part of the plant (shoots) and in the underground (rapidly growing roots and rhizomes), but the content is higher in plant storage organs (rhizomes) [25,26]. Rosavin was found exclusively in the underground part; small quantities were found in the roots and much more in the rhizomes [25]. It should be noted that in some studies, rosavin was not detected in the growing roots of R. rosea [26]. In other studies that compared two types of underground organs, R. rosea had a significantly lower content of rosavin (27 times) in well-growing roots than in storage rhizomes [27]. In our study, we confirmed the presence of the main secondary metabolites of R. quadrifida, salidroside and rosavin, in callus cultures and only salidroside in hairy roots by using HPLC/MS (Fig. 4). The presence of rosavin in the callus culture of R. quadrifida but not in the hairy roots is probably because its formation, unlike salidroside, occurs in tissues similar to the storage organs of plants, i.e., with slow growth and low level of differentiation. It should be noted that similar confinement of the synthesis of certain substances of secondary metabolism to unorganized growing tissue has been described for Dioscorea species and other cultures [28,29].
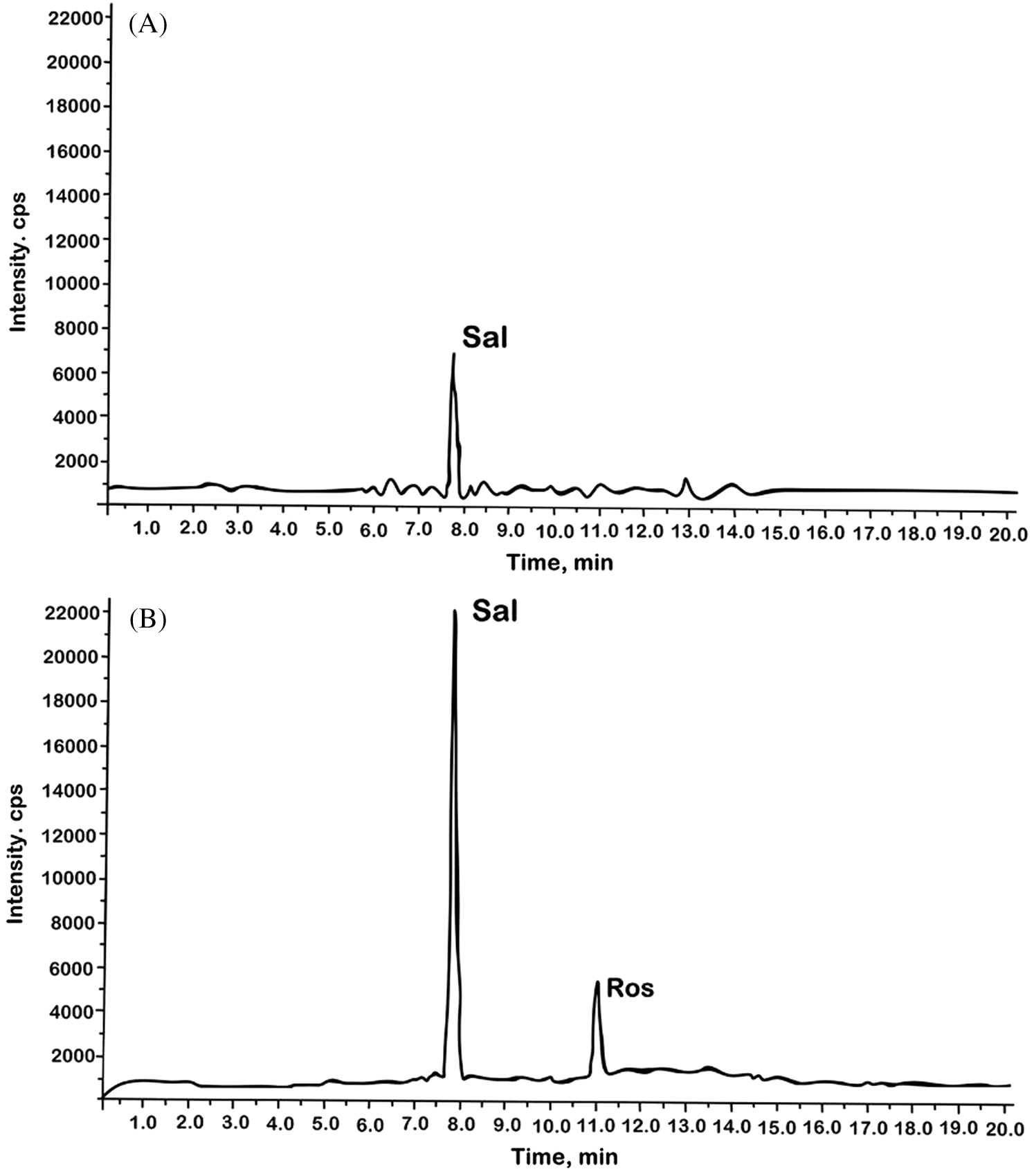
Figure 4: HPLC-MS chromatogram of 70% ethanol extract R. quadrifida hairy roots (A) and callus culture (B)
The content of salidroside and rosavin in the rhizomes of R. quadrifida сompared to R. rosea was significantly lower and amounted to 0.016−0.45% and 0.076−0.19% by dry weight, respectively [2]. In the work of Sheng et al. [10] the content of salidroside in callus tissue was also similar to its level in wild plants and amounted to 0.28%. In our study, the cultures obtained in vitro significantly differed in the content of the studied phenolic compounds. In callus cultures, the content of salidroside was 0.142−0.158% and rosavin was 0.041−0.047%. The content of salidroside in callus cultures resembled the level of these substances in the rhizomes of plants growing in natural conditions, which makes this culture promising for its biotechnological use. The salidroside content was significantly lower in hairy roots, 0.058−0.071%. The result may have several reasons. Firstly, growth inhibition is combined with slowing down protein synthesis, which promotes the use of free aromatic amino acids for secondary metabolism [30]. Therefore, in callus cultures, there was a higher salidroside content than in hairy roots. It may be associated with slower growth of callus culture. Secondly, the reason for the differences we noted in the synthetic ability of the obtained hairy roots and the callus culture initiated from them may be the influence of the hormones that were included in the callus cultivation medium to induct callus culture. Phytohormones can have a direct effect on the direction of biosynthesis pathways. For example, cell cultures of different types of Digitalis could form glycosides only in the presence of 2,4-D. It was found that a medium with 2,4-D contributed more than others to the formation of diosgenin when the culture of Dioscorea deltoidea cells had been analyzed [31,32]. When the object was another representative of the Rhodiola genus, R. sachalinensis, a significant increase in the content of salidroside with the addition of 2,4-D compared with other hormones (6-benzylaminopurine, indole-3-butyric acid) was seen [33].
However, it was pointed out in the same paper that an increase in salidroside content was possibly associated with slower growth of the culture in the medium with the addition of 2,4-D [34]. The level of expression of the phenylpropanoid pathway genes (phenylalanine ammonia-lyase, cinnamate 4-hydroxylase, caffeate/5-hydroxyferulate O-methyl-transferase) was studied, and an increase in their expression was shown when 2,4-D was added [34]. This proves that 2,4-D can affect the biosynthesis of phenolic compounds, not only indirectly through growth retardation, but also through a change in the expression of the most important genes for their synthesis.
Thus, in our work, the hairy roots of R. quadrifida were obtained and characterized for the first time. Moreover, its growth characteristics and ability to biosynthesize the main phenolic metabolites (salidroside and rosavin) of hairy roots and the callus culture initiated from them were compared. It was shown that the hairy roots of R. quadrifida have a growth index two times greater than the callus culture. However, the callus culture had a better biosynthetic ability than the hairy roots. In addition, rosavin was not registered in the hairy roots. The identified differences could be related to enzymes of the rosavin synthesis pathway only being in tissues similar to the storage organs of R. quadrifida plants and the presence of 2,4-D in the medium for obtaining and growing the callus culture. The presence of 2,4-D could indirectly affect the biosynthesis of phenolic compounds by slowing down tissue growth, and directly by changing the expression level of the phenylpropanoid pathway genes, which requires further study. The content of salidroside in the callus culture is close to its level in the rhizomes of R. quadrifida plants growing in natural conditions making this culture promising for its biotechnological use.
Funding Statement: This work was supported by the Ministry of Education and Science of the Russian Federation (Topic No. АААА-А19-119041890054-8).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Booker, A., Zhai, L., Gkouva, C., Li, S., Heinrich, M. (2016). From traditional resource to global commodities: A comparison of Rhodiola species using NMR spectroscopy-metabolomics and HPTLC. Frontiers in Pharmacology, 7(254), 1–11. DOI 10.3389/fphar.2016.00254. [Google Scholar] [CrossRef]
2. Altantsetseg, K., Przybyl, J., Węglarz, Z., Geszprych, A. (2007). Content of biologically active compounds in roseroot (Rhodiola sp.) raw material of different derivation. Herba Polonica, 53, 20–26. [Google Scholar]
3. Yoshikawa, M., Shimada, H., Shimoda, H., Matsuda, H., Yamahara, J. et al. (1995). Rhodiocyanosides A and B, new cyanoglycosides from chinese natural medicine Si Lie Hong Jing Tian, the underground part of Rhodiola quadrifida (Pall.) Fisch. Et Mey. Chemical Pharmaceutical Bulletin, 543(7), 1245–1247. DOI 10.1248/cpb.43.1245. [Google Scholar] [CrossRef]
4. Yoshikawa, M., Shimada, H., Shimoda, H., Murakami, N., Yamahara, J. et al. (1996). Bioactive constituents of chinese natural medicines, Chemical structures and antiallergic activity of rhodiocyanosides A and B from the underground part of Rhodiola quadrifida (Pall.) Fisch. et mey. (Crassulaceae). Chemical Pharmaceutical Bulletin, 44(11), 2086–2091. DOI 10.1248/cpb.44.2086. [Google Scholar] [CrossRef]
5. Wiedenfeld, H., Dumaa, M., Malinowski, M., Furmanowa, M., Narantuya, S. (2007). Phytochemical and analytical studies of extracts from Rhodiola rosea and Rhodiola quadrifida. Pharmazie, 62, 308–311. [Google Scholar]
6. Kędzia, B., Furmanowa, M., Krajewska-Patan, A., Hołderna-Kędzia, H., Mścisz, A. et al. (2006). Badania nadtoksycznością oraz działaniem adaptogennym i przeciwdrobnoustrojowym wyciągów otrzymanychz podziemnych części wybranych gatunków Rhodiola L. Herba Polonica, 52, 117–132. [Google Scholar]
7. Mirmazloum, I., Kiss, A., Ladányi, M., Györg, Z. (2019). Production of cinnamyl alcohol glycosides by biotransformation in roseroot callus cells. Plant Cell, Tissue and Organ Culture, 39, 129–137. [Google Scholar]
8. Kurkin, V. A., Zapesochnaya, G. G. (1986). Chemical composition and pharmacological properties of Rhodiola rosea L. Khimiko-Farmatsevticheskii Zhurnal, 20, 1231–1244. [Google Scholar]
9. Linh, P. T., Kim, Y. H., Hong, S. P., Jian, J. J., Kang, J. S. (2000). Quantitative determination of salidroside and tyrosol from the underground part of Rhodiola rosea by high performance liquid chromatography. Archives of Pharmacal Research, 23(4), 349–352. DOI 10.1007/BF02975446. [Google Scholar] [CrossRef]
10. Sheng, C. Z., Hu, T. Q., Bi, H., Yuan, Y. J., Jiang, Y. (2005). Effects of plant growth substances on induction and culture of callus from Rhodiola quadrifida. Zhongguo Zhong Yao Za Zhi, 30, 1237–1240. [Google Scholar]
11. Murashige, T., Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497. DOI 10.1111/j.1399-3054.1962.tb08052.x. [Google Scholar] [CrossRef]
12. Vervliet, G., Holsters, M., Teuchy, H., Van Montagu, M., Schell, J. (1975). Characterization of different plaque-forming and defective temperate phages in Agrobacterium strains. Journal of General Virolology, 26(1), 33–48. DOI 10.1099/0022-1317-26-1-33. [Google Scholar] [CrossRef]
13. Stepanova, A. Yu, Orlova, E. V., Teteshonok, D. V., Dolgikh, Yu, I. (2015). Obtaining transgenic alfalfa plants for improved phytoremediation of petroleum-contaminated soils. Russian Journal of Genetics: Applied Research, 13, 127–135. [Google Scholar]
14. Shi, L., Wang, C., Zhou, X., Zhang, Y., Liu, Y. et al. (2013). Production of salidroside and tyrosol in cell suspension cultures of Rhodiola crenulate. Plant Cell, Tissue and Organ Culture (PCTOC), 114(3), 295–303. DOI 10.1007/s11240-013-0325-z. [Google Scholar] [CrossRef]
15. Rodin, I. A., Stavrianidi, A. N., Braun, A. V., Shpigun, O. A., Popik, M. V. (2012). Simultaneous determination of salidroside, rosavin, and rosarin in extracts from Rhodiola rosea by high performance liquid chromatography with tandem mass spectrometry detection. Journal of Analytical Chemistry, 67(13), 1026–1030. DOI 10.1134/S1061934812130096. [Google Scholar] [CrossRef]
16. Grech-Baran, M. G., Sykłowska-Baranek, K., Pietrosiuk, A. (2015). Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp. in vitro cultures. Phytochemistry Reviews, 14(4), 657–674. DOI 10.1007/s11101-014-9368-y. [Google Scholar] [CrossRef]
17. Lütken, H., Meropi-Antypa, N., Kemp, O., Nymark Hegelund, J., Müller, R. (2017). Hairy root cultures of Rhodiola rosea to increase valuable bioactive compounds. Production of Plant Derived Natural Compounds through Hairy Root Culture. Switzerland, Cham: Springer. [Google Scholar]
18. Tasheva, K., Kosturkova, G. (2012). The role of biotechnology for conservation and biologically active substances production of Rhodiola rosea: Endangered medicinal species. Scientific World Journal, 2012(2429), 1–13. DOI 10.1100/2012/274942. [Google Scholar] [CrossRef]
19. Savin, P. S., Savina, T. A., Myasnikova, S. B. (2019). Physiological characteristics suspension culture of Rhodiola rosea L. at cultivation on the modified nutrient medium, Voprosy Biologicheskoi. Meditsinskoi i Farmatsevticheskoi Khimii, 22(5), 25–29. [Google Scholar]
20. Martínez, M. I., Barba-Espín, G., Favero, B. T., Lütken, H. (2020). Rhizobium rhizogenes-mediated transformation of Rhodiola rosea leaf explants. Bragantia, 79(2), 213–223. DOI 10.1590/1678-4499.20190428. [Google Scholar] [CrossRef]
21. Zhou, X., Wu, Y., Wang, X., Liu, B., Xu, H. (2007). Salidroside production by hairy roots of Rhodiola sachalinensis obtained after transformation with Agrobacterium rhizogenes. Biological & Pharmaceutical Bulletin, 30(3), 439–442. DOI 10.1248/bpb.30.439. [Google Scholar] [CrossRef]
22. Zych, M., Pietrosiuk, A., Karasiewicz, M., Bogacz, A., Kujawski, R. et al. (2008). Establishment of Rhodiola kirilowii hairy roots using Agrobacterium rhizogenes LBA 9402. Herba Polonica, 54(4), 7–16. [Google Scholar]
23. Himmelboe, M., Lauridsen, U. B., Hegelund, J. N., Müller, R., Lütken, H. (2015). Agrobacterium rhizogenes mediated transformation of Rhodiola sp.-An approach to enhance the level of bioactive compounds. Acta Horticulturae, 1098, 143–149. DOI 10.17660/ActaHortic.2015.1098.15. [Google Scholar] [CrossRef]
24. Platikanov, S., Evstatieva, L. (2008). Introduction of wild golden root (Rhodiola rosea L.) as a potential economic crop in Bulgaria. Economic Botany, 62(4), 62–627. DOI 10.1007/s12231-008-9051-6. [Google Scholar] [CrossRef]
25. Przybył, J., Węglarz, Z., Geszprych, A. (2008). Quality of roseroot (Rhodiola rosea L.) cultivated in Poland. Acta Horticulturae, 765, 143–150. [Google Scholar]
26. Tasheva, K., Kosturkova, G. (2010). Bulgarian golden root in vitro cultures for micropropagation and reintroduction. Central European Journal of Biology, 5(6), 853–863. [Google Scholar]
27. Zahozhi, I. G., Golovko, T. K. (2006). Content of cinnamyl alcohol glycosides and tyrosol in cultured Rhodiola rosea L. plants. Vestnik Insituta Biologii Komi NC UrO RAN, 5, 15–17. [Google Scholar]
28. Mehta, A. R., Staba, E. J. (1970). Presence of diosgenin in tissue cultures of Dioscorea composita Hemsl. and related species. Journal of Pharmaceutical Sciences, 59(6), 864–865. DOI 10.1002/jps.2600590635. [Google Scholar] [CrossRef]
29. Dornenburg, H., Knorr, D. (1995). Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme and Microbial Technology, 17(8), 674–684. DOI 10.1016/0141-0229(94)00108-4. [Google Scholar] [CrossRef]
30. Muthaiya, M. J., Nagella, P., Thiruvengadam, M., Mandal, A. K. A. (2013). Enhancement of the productivity of tea (Camellia sinensis) secondary metabolites in cell suspension cultures using pathway inducers. Journal of Crop Science and Biotechnology, 16(2), 143–149. DOI 10.1007/s12892-012-0124-9. [Google Scholar] [CrossRef]
31. Kaul, B., Staba, E. J. (1968). Production of cardioactive substances and isolation of diosgenin from Discorea deltoidea callus and suspension cells. Lloydia, 31(2), 171–179. [Google Scholar]
32. Marshall, J. G., Staba, E. J. (1976). Hormonal effects on diosgenin biosynthesis and growth in Dioscorea deltoidea tissue cultures. Phytochemistry, 15(1), 53–55. DOI 10.1016/S0031-9422(00)89052-4. [Google Scholar] [CrossRef]
33. Wu, S., Zu, Y., Wu, M. (2003). High yield production of salidroside in the suspension culture of Rhodiola sachalinensis. Journal of Biotechnology, 106(1), 33–43. DOI 10.1016/j.jbiotec.2003.07.009. [Google Scholar] [CrossRef]
34. Ma, Q., Ding, Y., Chang, J., Sun, X., Zhang, L. et al. (2014). Comprehensive insights on how 2,4-dichlorophenoxyacetic acid retards senescence in post-harvest citrus fruits using transcriptomic and proteomic approaches. Journal of Experimental Botany, 65(1), 61–74. DOI 10.1093/jxb/ert344. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |