

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.011685
REVIEW
Applications of Decision Support System: A Case Study of Solanaceous Vegetables
Department of Horticulture, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, 60800, Pakistan
*Corresponding Author: Muhammad Akbar Anjum. Email: akbaranjum@bzu.edu.pk
Received: 23 May 2020; Accepted: 23 July 2020
Abstract: Crop simulation models constitute the major proportion in decision support systems. A large number of crop models have been developed for potato and few for tomato and peppers. In the literature, thirty three crop models have been reported to simulate potato, nine for tomato and six for peppers. Some of these models dealt with the climate change scenario and others with the crop management practices such as sowing time, irrigation, nitrogen, and insect-pests management. The most evaluated and applied models for potato include; SUBSTOR, and LINTUL-Potato, whereas CROPGRO-tomato model is the most tested and applied for tomato. The AQUACROP is the most widely used model to simulate the water dynamics. The CROPGRO model has been tested for elevated temperatures and CO2 under greenhouse conditions for tomato. In tomato and peppers, almost similar models have been applied for field conditions as well as under greenhouse environments with some modifications. Nitrogen dynamics has been widely tested by employing the EU-Rotate-N model for tomato and peppers. Simulation studies dealing with changing climate conditions are rare in potato and are not found for tomato and peppers. To modify potato, tomato and peppers models for climate impact studies, it is required that they are (a) calibrated and evaluated with new cultivars under various agro-environmental conditions and (b) assessed under varying field conditions under changing climates and crop management practices, including temperature increases, water and nutrient management and their interactions. These comprehensive model studies and modifications need a collaborative international effort and a multi-year, large scale field research studies on potato, tomato and peppers.
Keywords: Crop growth models; management issues; simulation modeling; Solanaceae
Plant, soil and environment interactions are intricate, with several factors affecting any desired outcome. However, developments using computer equipments have made possible to study the effects of mutual influences of various factors for different interactions. Therefore, it is likely to study quantitative relationships among plants, soils and climatic conditions to enhance the accuracy in predicting crop productivity. Thus, increased use of economical and efficient computer techniques and application of new technologies in agriculture are emerging [1,2].
This integrated approach utilizes the simulation models for crop modeling of individual crops and cropping systems. Crop models are fed with recent data of soil, crop genetics and management practices in a process-oriented manner. The models are then evaluated utilizing the input data, and then they may be applied for prediction of crop yield under varying management options and changing climates [3–6].
Many mathematical equations have been combined in an integrated manner to develop a model relating plants, soils and the atmosphere [5,7]. These models simulate the performance of the study crop, by accurately predicting its growth and developmental morphology stages of its parts, such as stem, roots, leaves and fruits. So, the model simulates and predicts dry matter production or biomass by utilizing information regarding the processes occurring in the growth and development of a plant [2,3].
Crop models offer a theoretical outline for organizing research studies [8,9]. Models are usually justified by their application as well as for their precision in crop management. Generally, crop models provide quantitative data from which decisions can be taken at the field level regarding sowing time, irrigation, fertilization and crop protection. At the regional level, policies can be formulated from potential yield estimations, irrigation requirements, fertilizer needs and other aspects [10–12].
The use of crop simulation models in agriculture started about 60 years ago by the development of the first crop model in Wageningen University, Netherlands [13,14]. Since then, many research groups in Netherlands, USA, Australia and China have successfully developed more than 200 crop models [2,13,15,16]. During 1960–1980, many famous crop models such as CERES and CROPGRO series models were developed by the researchers from the USA [17–19]. Some models were employed to predict climate change effects on agricultural productivity [20–22]. A few studies indicated application of simulation models for estimating productivity at a regional scale employing specific crop cultivars, sowing dates and representative soil(s) [23–25]. The environmental conditions vary in each region; therefore, crop simulation models are used to give suitable decisions for selecting cultivars not only at a regional but also at a global scale. Crop simulation models help in decision making at various levels (Fig. 1).
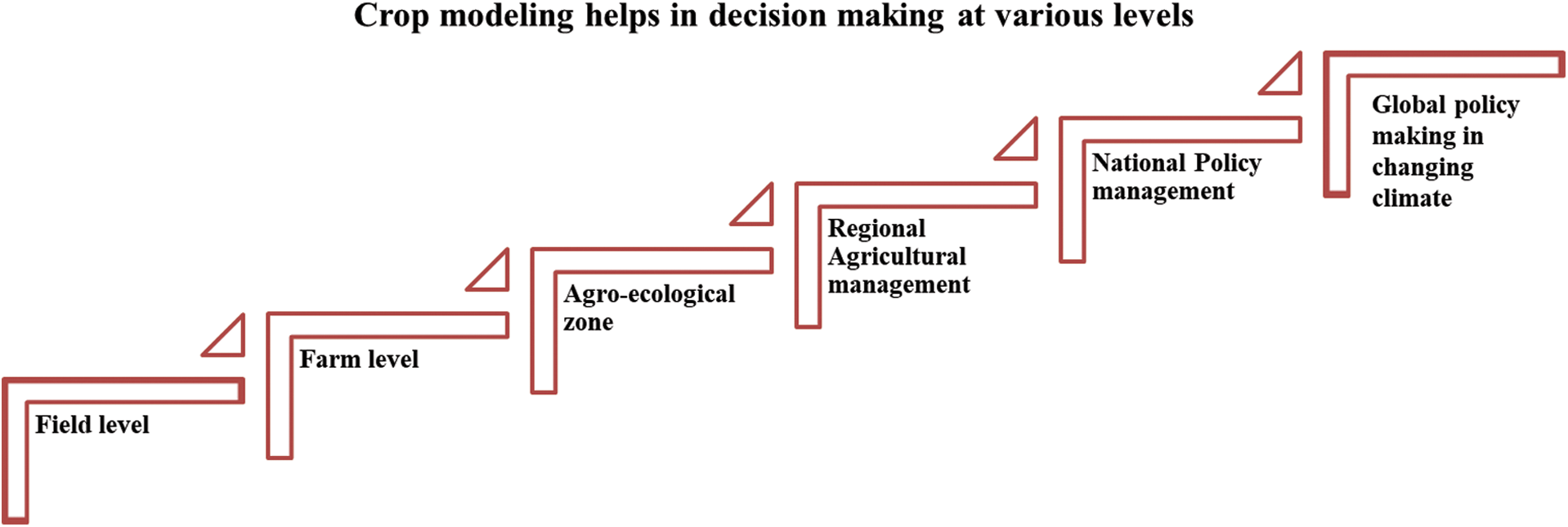
Figure 1: Schematic representation of different levels of decision making by using crop growth simulation models
2 Need of Crop Modeling in Horticulture
Horticulture needs crop models for extensive applications, comprising yield predictions, policy making and crop management. Yield prediction and harvest timing are the most important factors for producers to fit into the market demands as well as industrial requirements. The production timing of field vegetable crops is very critical. Crop growth models enabled growers to organize planting schedules, e.g., in tomato [26], lettuce [27,28] and cauliflower [29,30]. Likewise, the market price of cut flowers is higher and their demand increases during very specific and short times of the year, i.e., Mothers’ day, Valentine’s day, as well as Christmas. Moreover, the market also enforces specific quality standards. Therefore, crop development models have been successfully applied to simulate timings of productivity, plant height, along many other characteristics to enhance decision-making for fetching high prices [31–34].
There is a competition for production of horticultural commodities among different regions of a country as well as in the world. Therefore, it is important to know about the production limits of various crops in different regions. So far, crop simulation models are widely used in many field crops but not very common in horticultural crops. Change in climate is a worldwide phenomenon which has hampered agronomic as well as horticultural crop yields during the previous decades, and it can also have an influence during the forthcoming decades [22,25,35–38].
3 Types of Decision Problems in Horticulture
In horticultural crops, crop management issues are diverse in nature but they are certainly similar to those faced by agronomic crops, like irrigation and fertilizer applications [39,40], climate effects and timing of crop growth [41–44]. Others are more specific, for example, for fruit set and other physiological problems including source to sink ratios in many vegetables, including sweet pepper. Greenhouse production systems offer a high level of resource use and environmental control for crop growth [10,45,46].
Planting time is an important factor which largely affects the successful growth and yield traits of different vegetables including sweet pepper [47,48], tomato [26] and potato [49]. Optimum sowing time brings about proper growth and development of plants resulting in maximum yield of the crop and economic use of the land [48]. Planting of a crop at different times in various regions influences its yield due to the change in agro-environmental conditions at the different crop developmental morphology stages [50].
Generally, past studies were conducted on determining optimum planting date of any crop at a particular locality using multi-year field experiments, but planting date responses vary among years and locations. However, crop simulation models well calibrated against field experimental data hold the promise for inferring short duration field experimental results to other locations and years by using long-term weather and soil data [51].
Nutrient management in vegetable crops is a very important issue. Proper application of nutrients at the right time and with the correct methodology ensures an effective growth and high yield of vegetables. Intensive vegetable production systems normally require frequent irrigation and nutrient applications, [mainly nitrogen (N)] to attain high yields [52].
Generally water and nutrients are interactively managed in vegetables. Crop nutrient requirements are largely influenced by the irrigation practices. Interactive effects of nutrients and drought stress on water relations (besides crop nutrition) have been documented earlier [53–56]. Water deficit can reduce the capacity of plant roots to absorb maximum nitrogen levels, while a reduction in nitrogen supply can reduce water-use-efficiency. Optimization and application of site-specific strategies for obtaining an appropriate timing and requirements of water and nitrogen necessitate extensive and expensive field experimentation [55].
As it is not possible to test altogether interactions among nutrient and water requirements during different cropping seasons, application of simulation models can significantly assist in the assessment of different production processes and/or environments, and thus increase the efficiency of decision-making [57]. It is necessary to use this advanced technical tool for optimal irrigation and N management to facilitate growers in the decision-making process [52]. Simulation models can help in developing crop water and nitrogen management plans that ponder these issues [52,58].
Irrigation planning is one of the most important tools in developing best management options for irrigated areas [59–61]. Appropriate irrigation management is of vital importance to conserve water resources, quantitatively and qualitatively, and to enhance food production with the available water [60]. Improper water management not only causes resource wastage but also reduces crop yield [62]. Thus, it is imperative to improve the water management methods for attaining optimum decisions. Decision Support Systems (DSS) are valuable solutions to facilitate farmers to optimize their water and land resource utilization. Many models have been used in the decision-making process for water management [63]. These models vary in their functions, structures, description of involved processes and time scales (i.e., daily or seasonal) [61].
CropSyst [64,65] is a dynamic model, developed for multi-crops, multi-year experiments and for simulating daily crop water and nutrient dynamics. It simulates crop growth by integrating soil water and climatic conditions for productivity and environment sustainability [61]. AquaCrop is another crop simulation model which was developed for the optimization of water management options. Rinaldi et al. [66] found this model suitable for the optimization of irrigation applications under water deficit conditions in Mediterranean climates in order to save water resources and obtaining a greater water use efficiency. Simulation models embedded in different Decision Support Systems have the ability to simulate water management options including irrigation scheduling, quality of water, water drainage and method of application. Therefore, models should be incorporated in decision-making policy of crops [67].
3.4 Insect Pest and Disease Management
A number of insect-pests and diseases negatively affect vegetable production either in open fields or greenhouses. The major diseases and insect-pests affecting different vegetable crops include early plus late blight in potato, Phytophthora root-rot, viruses in Capsicum species, early blight, viruses, fruit borer, blossom end rot in tomato, and shoot and fruit borer in brinjal crop. Thrips are major insects in onions, whereas plant roots are mostly affected by aphids. There are various chemical, physical and biological methods which are applied to overcome the issues of diseases and insects. These are either applied individually or on an integrated manner. Efficacy of any control measure depends on the decision involving time, amount, method and frequency of application. The DSS facilitate these decision-making process by combining field data with complex models and geographical positioning system, and thus results in the development of predictive models which give site specific recommendations for integrated pest management (IPM) [68,69].
A model usually utilizes various processes and functions to make predictions about future planning from a set of initial conditions [70,71]. In horticulture, models are currently in use to predict pest populations and forecast probable damage to crops [71–73]. ENDURE network conducted a survey in Europe during 2007–2008 to identify the uses of DSS in pest management and reported that seven DSS were used for pest management in horticultural crops. These systems mostly have the ability to integrate with each other [74].
Gebauer et al. [75] applied a simulation model to demonstrate that the rise in temperature would not affect the asynchrony among the populations of cabbage aphid pests and their parasitoids; instead they may appear earlier than their parasitoids. This example enlightens the importance of modeling in future pest control programs. Moreover, modeling can contribute in a better way to compare the cost/benefit ratio of pest control [70]. The models also facilitate the farmers in identifying the correct timing of pest control measures [76–79] and can help to redesign the IPM program.
While discussing the advantages of decision support system/simulation models, these also have some limitations [68]. For example, DSS need to be continuously updated because of the changing climatic and field conditions, and continuous funding is required to keep the systems and databases up-to-date [69,80,81].
Optimum plant spacing guarantees proper growth and development of plants, resulting in maximum crop yields and a best land use. Yield of bell pepper has been reported to be dependent on the number of plants per unit area [50]. Plant growth and the resultant yield are enhanced by increasing the number of plants per unit area up to a limit, where they start to decline. Plant density is regarded as an important aspect affecting fruit setting percentage, production as well as fruit quality parameters. Mehla et al. [82] found that yield characteristics in tomato are largely influenced by plant spacing. Various studies indicated the application of DSS for optimizing plant density and/or plant spacing in different agronomic crops such as rice [39,40], maize [83,84] and soybean [85,86]. However, in the case of vegetable crops, especially for those solanaceous, no reference has been found showing the use of crop simulation models in optimizing plant density. Planting density is an important factor affecting the yield as well as quality of vegetables; therefore, it should be included in future studies involving DSS.
4 Growth, Development and Yield Processes
The simulation of crop growth, biomass and yield is possible through the evaluation of the different growth/development phases of crops. Plants undergo a series of growth and development phases which are controlled by several processes. Some important processes include photosynthesis, growth and maintenance respiration, growth conversion efficiency, assimilate partitioning, pod/seed addition, protein mobilization, leaf senescence, and maturity. Carbon balance is basic to and dry matter production is the outcome of these processes. The rate of change in crop dry matter (g/m2) can be described as in Eq. (1):

where, W* is daily new growth, and SL, SS, and SR are senesced parts of leaves, stems and roots, respectively.
In turn, W* can be calculated using the following equation:

where E is conversion efficiency, Pg is photosynthesis, and Rm is maintenance respiration
Photosynthesis is the key process in dry matter production, and daily crop photosynthesis can be described as
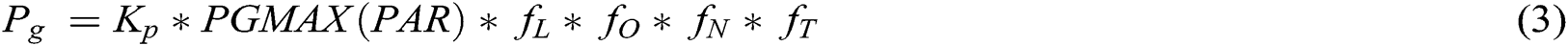
where, Kp = adjustment (soil fertility) = SLPF; PGMAX(PAR) is response to Photosynthetic Active Radiation; and fL, f0, fN, and fT are responses (0–1) to leaf area index, water supply, leaf nitrogen concentration and day temperature, respectively [8,87,88].
Respiration may be either maintenance or growth respiration. Maintenance respiration (MR) is highly affected by the atmospheric temperature, crop photosynthetic rate and crop biomass produced. The simulation model CROPGRO utilizes the following relationship to determine maintenance respiration [8].
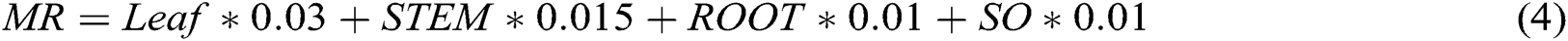
where leaf, stem, root and SO represent biomasses for leaves, stems, roots and storage organs (SO), respectively.
It is obvious from the above equation that leaves and stems need more maintenance than roots and storage organs. The model calculates growth respiration by using the input values of biochemical composition of the existing tissues. It can be summarized through following relationship [88]:
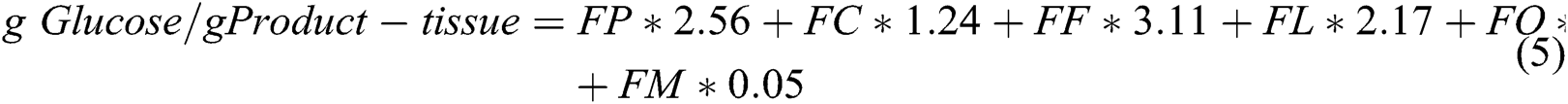
where FP, FC, FF, FL, FO and FM represent the fractions of protein, cellulose-starch, fat, lignin, organic acids and minerals, respectively. It is important to know that sum of all these fractions should be equal to 1.
The rate of assimilate partitioning to sink organs depends upon the plant growth stage, and the water and nitrogen deficits. Crop models normally use two approaches in assimilate partitioning processes. These approaches include the either computation of sink strength of all organs or partitioning calendar as a function of thermal time. In vegetable models, the partitioning calendar approach is mostly used. Models utilize the priority in partitioning of assimilates to plant organs. At the early vegetative stage, assimilates are partitioned to vegetative tissues. At the early pod formation stage, the greater partitioning is towards the pods. Assimilates are partitioned first to the seeds, then to shells and afterward towards leaves, stems and roots [8,88].
4.4 Mobilization of Protein (N) and Carbohydrate (C)
Mobilization of proteins and carbohydrates occurs during the vegetative and reproductive phases of the plant. In the vegetative phase, it occurs at a slow rate when compounds have to mobilize from old to new, vegetative tissues. However, during the seed formation and filling periods, this mobilization happens up to twice as fast. Seed growth increases through mobilization of proteins, and in turn leaf N decreases, photosynthesis and maintenance respiration are reduced, and leaves start to abscise at a slow rate [8,88].
Senescence is a gradual process and it continues as seed grow and move towards maturity. Mobilization of proteins from the leaves reduce their N content, leading to less photosynthesis, and leaf abscission starts initially at a slow rate. Senescence is enhanced by water stress. Leaf abscission reduces seed growth and it stops in individual cohorts, when the ratio of seed/(seed + shell) exceeds a limit called as THRESH. Seed growth continues from physiological to harvest maturity until all cohorts reach the THRESH limit. THRESH is a cultivar dependent trait which identify the ratio of seed to pod mass, where pod mass is equal to seed + shell mass [8].
5 Growth and Yield Mechanisms in Solanaceous Vegetables
Potato is a tuber crop and its dry matter production is characterized by its tuber growth and size. Most of the potato growth models define five growth phases: (a) pre-planting (b) planting to sprout germination (c) sprout germination to emergence (d) emergence to tuber initiation and (e) tuber initiation to maturity. Temperature has a great influence on all these growth stages. During the pre-emergence period, growth occurs by utilizing the stored carbohydrates in the seed potato. Tuber initiation is affected by day length, temperature, nitrogen status and water stress. The effect of day length and temperature is cultivar specific, and tuber initiation is less affected by high temperatures in early than late cultivars. Potato models simulate this effect as the cultivar-specific coefficient for critical temperature (TC). Tuber initiation is inhibited above the critical temperature. The relationship is described in the following equation.

where TEMP is the daily mean temperature, and RTFTI varies from 0 to 1.
Tuber bulking is the most important simulation process in potato modeling. Once tuber bulking starts, assimilates are partitioned to tubers as a first priority. Vegetative growth is reduced. Tuber growth rate is affected by temperature. It is also affected by nitrogen and water stress, and sink strength [89].
Tomato and pepper possess almost the same processes as discussed above in the general growth section. However, there are a few differences in understanding the growth mechanisms and simulating these processes in most of the horticultural crops, including tomato and pepper. The major difference is that tomato and pepper are indeterminate (most cultivars) in growth habit, and their growth continues until the last day of simulation. The CROPGRO model simulated well these mechanisms in tomato [90].
Another factor to be considered is that fruit and seed yields are often reported on a fresh rather than a dry basis, as it occurs in most cereal crops. Typically, all growth models emphasize the dry weight of pods or seeds. However, the following relationship can be used to calculate the fruit fresh mass using dry matter concentration and dry mass on the tomato fruit:

where, Fw = fresh mass per fruit, Dw = dry mass per fruit, and DMP = Dry matter concentration.
Dry matter concentration is a function of thermal time, and it may be affected by water deficit, season, cultivar and soil nutrition status [90].
6 Decision Support Systems Developed for Horticulture
Starting from 1965, a large number of decision support programs have been developed for horticultural crops either by research scientists or commercial firms. It is not possible to list all these DSS’s but their usage in different programs and approaches can be classified [6,87]. Horticultural crop production is mainly focused on two approaches (i.e., producing crops under open field conditions or inside greenhouses where almost all environmental factors can be controlled). Decision support systems also deal separately with these two approaches. A large number of crop models have been developed for potato (Tab. 1), and only a few for tomato (Tab. 2) and peppers (Tab. 3).
Table 1: Potato crop models used under field conditions with their applications

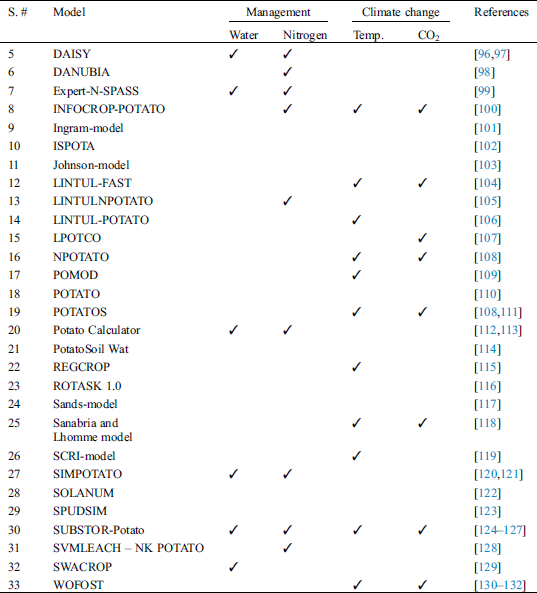
Table 2: Tomato crop models used under field conditions with their applications
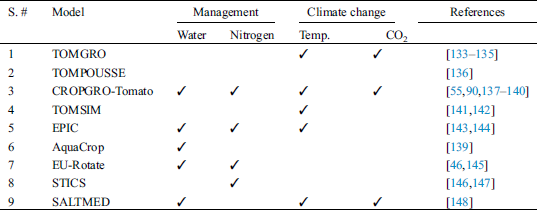
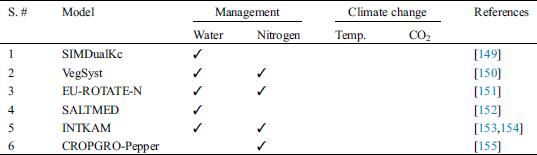
Table 3: Pepper crop models used under field conditions with their applications
Potato is the most important solanaceous vegetable, and its importance can be evaluated by the development of a great number of crop models for this crop. Most of these models have been applied to study the effects of climate change as well as to optimize management practices for this crop [5]. The most cited models in the literature are LINTUL-POTATO [106] and SUBSTOR-Potato [125,156]. The SUBSTOR-Potato model has been used widely to assess the impact of water [157,158], weather [124,159,160] and nitrogen dynamics [161,162]. This model has also been used to simulate potato growth and yields with varying management options and across wide geographic regions. Silva et al. [163] evaluated this model’s performance in 19 countries with 87 field experiments involving 204 treatments. Experiments varied in irrigation management, soil nitrogen levels and properties, cultivar selection, planting time and atmospheric conditions. Tuber growth and yields were accurately simulated with such model under diverse environmental conditions with different cultivars and management practices. Similarly, Arora et al. [89] reported that SUBSTOR-Potato model simulated well tuber yield at varying nitrogen and irrigation treatments. The results determined that yield was more responsive to irrigation in the presence than in the absence of nitrogen. Higher initial soil water content require less nitrogen and water than lower initial water content for an equivalent tuber yield. Gayler et al. [99] integrated a modeling program Expert-N with a crop simulation model Soil-Plant-Atmosphere System Simulation (SPASS) and were able to simulate carbon and nitrogen balances in potato. An adequate modelling predicted the optimum fertilizer requirement of two potato cultivars and they suggested that this model can be used to predict the growth, nitrogen uptake and yield in potato under different environmental conditions and fertilizer management scenarios. Alva et al. [95] studied two growth models (CROPSYSTVB and CSPOTATO) in an integrated way to simulate the fate of nitrogen under field conditions. Simulated yield was in accordance with the actual yield. It was concluded that these models can be used as a valuable tool in predicting potato yield and nitrogen demand under different crop rotation systems.
Under field conditions, tomato crop has been simulated by using different crop models such as EPIC [143,144], CROPGRO or TOMGRO [45,55,133,137–140,164–168] and TOMSIM [141,142]. CROPGRO-Tomato model is the most cited in literature which has been widely used to simulate tomato growth functions and yield parameters under open field conditions. Rinaldi et al. [55] evaluated the CROPGRO model in processing tomato to assess various irrigation and nitrogen management situations by utilizing 53 years of local weather data. The results confirmed that the model was a useful tool as a DSS for optimal management of tomato crop. Some modifications were made by Boote et al. [45] in the CROPGRO model in order to accurately simulate the crop phenology, growth parameters and yield in response to various temperatures. Models well calibrated with changing weather conditions can be applied to simulate and predict tomato fruit set, biomass, water use efficiency and yield. AquaCrop is another crop simulation model which was developed for optimization of water management options. Rinaldi et al. [66] found this model suitable for optimization of irrigation applications under water deficit conditions in Mediterranean climates in order to save water resources and obtain greater water use efficiency.
Research on development of growth models for pepper crop remains scarce, when compared to other vegetable crops such as tomatoes. As a result, few crop simulation models such as VegSyst [150] SALTMED [152], INTKAM [153], SIMDualKc [149], FARQUHAR [169], EU-ROTATE-N [151] are cited in literature.
In pepper crop, the important crop management issues are excessive nitrogen applications and irrigations. Therefore, most models were evaluated for optimizing water and nitrogen applications [150]. Therefore, researchers have focused on increasing water and nitrogen use efficiency. Mathematical simulation is the current solution to improve water and fertilizer management at the field for achieving maximum yields and minimum nitrate leaching in soil, and drainage losses under water deficit conditions. Similarly, another study [151] indicated application of simulation models for nitrogen management in pepper crop. The EU-ROTATE-N model was applied for optimizing water and nitrogen usage in pepper. Simulation results revealed that this model has the capacity to estimate soil moisture and nitrate percentage under varying fertilizer management options for Isfahan climatic situations, and models can be applied in decision-making processes regarding fertilizer and water management at the farm level. This model can be used to optimize nitrogen fertilization in various vegetables without requiring much calibration and can be used under a wide range of growing environments [151]. Moreover, there are many crop growth models which simulate crop growth and yield characteristics of sweet pepper, as a function of time dealing with phenological stages, fruit set and harvest index in various levels of detail [169–171].
7 Model Applications in Greenhouse Environments
The raising of vegetables in greenhouses has become popular in recent decades. In the current scenario, with increasing demands of vegetables and shrinking of them in arable lands, the technique of growing vegetables in greenhouses is gaining popularity. Greenhouses facilitate in providing a suitable environment for plant growth and developmental processes. The microclimate in greenhouses is the integration of various physical phenomenon which are controlled by environmental factors, crop species, growth stages and greenhouse structures. In protected cultivation, the main factors affecting crop development are temperature, relative humidity and solar radiation. Many crop simulation models have been developed which facilitate the management of microclimate in various greenhouses as well as the growth of crops [172].
In addition to climate change, nutrient and water management are other important aspects in greenhouse management. In greenhouses, vegetable production is more intensive than in open fields. Nutrient and water applications need more precision and accuracy as these are applied frequently in protected horticulture. The term “spoon feeding” is used in greenhouses for nitrogen and water application to the plants [173,174]. However, this intensive and accurate nutrient and water management is not being managed using fixed fertilization programs [173]. Crop water and nutrient demands should match when suppling them, and they would enhance the resource use efficiency [175]. In order to improve the efficiency of this “spoon feeding” phenomenon, daily crop nutrient and water uptake must be calculated, and simulation models should estimate daily crop water and nitrogen demands. For commercial applications, these models are required to be integrated with user-friendly DSS [146].
In greenhouses, simulation studies were performed by using the same models which were used under field conditions such as EPIC [144], CROPGRO-Tomato [45,55,138–140,167,168] and TOMSIM [141,142]. These models were modified according to the structure and type of greenhouse as well as the crop condition. For example, SALTMED model, which has been widely applied to simulate crop growth in various crops under field conditions, was modified. Silva et al. [148] applied this model to simulate tomato growth under unheated greenhouse conditions; results revealed that the model accurately simulated tomato growth, and it can be used as a Decision-Support tool for optimizing crop cultivar, planting time, irrigation needs and predicting yields. In another study, Dimokas et al. [134] used the TOMGRO model to simulate the crop development and climate in plastic greenhouses in Greece. The aim was to shorten the life span of tomato plants, of indeterminate growth habit, without affecting their dry matter and fruit production. That model worked as a useful tool in simulating those measurements in plastic greenhouses, and it can be used in decision making processes to optimize functioning of the greenhouses for obtaining a better growth and yield. EU-ROTATE-N is another simulation model which has been applied to field grown vegetable crops [145,176]. Soto et al. [46] used this model to optimize various fertilization treatments in 4-year experiments. Results concluded that EU-ROTATE-N can assess the effects of crop management in relation to water drainage, nitrate leaching and soil nitrogen dynamics on tomato raised under greenhouse conditions.
A few studies indicated the development and application of crop growth models for pepper under greenhouse conditions [153]. The plant-nutrient relationship is an important part when greenhouse systems are used. Marcelis et al. [154] used a simulation model developed for cucumber by Marcelis [“INTKAM”; 177] to simulate dry matter production in sweet pepper as an example. Data from two different climatic zones (i.e., southern Spain and northern France) were used for evaluation of the model. The model simulates water uptake, and different nutrients and growth of plant organs in relation with plant-nutrient processes. Likewise, Marcelis et al. [169] developed another crop model “FARQUHAR” for simulation of sweet pepper crop under a greenhouse climate. Six crops were grown at two locations viz. Netherlands and France for collection of the data. Radiation interception was measured for a uniform canopy, and canopy area was predicted using the model functions and considering the temperature data at planting time. Valdés-Gómeza et al. [146] evaluated the VegSyst model for optimizing nitrogen and water management of sweet pepper under greenhouse conditions. They found that daily nitrogen uptake, evapotranspiration rate and dry matter production were well simulated by the model.
Salinity problems emerge under greenhouse conditions due to excessive application of fertilizers, resulting in a constraint of plant growth. Model applications also played a role in meeting this challenge. The SALTMED is the widely used model in this context, and has been used by several workers [163,178–180] in different experiments. Rameshwaran et al. [152] applied this model on a sweet pepper crop irrigated with saline water under greenhouse conditions. Results revealed that measured data were in good agreement with the simulated results in predicting crop growth and yield under saline conditions. The crop evapotranspiration (ETc) is the critical process in a greenhouse system for an effective irrigation management. The SIMDualKc is a model developed for simulation of crop (ETc). Qiu et al. [149] evaluated this model to simulate the ETc of chili pepper grown in Northwest China under greenhouse conditions. The model was found to be appropriate in calculating ETc, and thus increasing the efficiency of irrigation application to pepper crop in greenhouses.
It is evident from the available literature and the current discussion that only a few studies revealed the development, testing and application of DSS in solanaceous vegetables as compared with agronomic or cereal crops. Researchers focused more on potato than tomato and peppers. Since tomato and peppers are also important fruit vegetables in horticulture, there is a need to develop more crop models for these crops. These models should be tested in contrasting growing conditions worldwide, with modern cultivars and varying management options. Planting time, water, nutrients, planting geometry, disease management, and soil-plant-climate interactions should be focused in this regard. Regarding greenhouse conditions, these models should be calibrated and tested according to the varying environmental conditions for tomato and especially for peppers in different greenhouses. International collaboration and research programs are needed to develop and apply crop models worldwide under changing climates and contrasting growing conditions.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Jones, J. W., Antle, J. M., Basso, B., Boote, K. J., Conant, R. T. et al. (2017). Brief history of agricultural systems modeling. Agricultural Systems, 155, 240–254. DOI 10.1016/j.agsy.2016.05.014. [Google Scholar] [CrossRef]
2. Hoogenboom, G., White, J. W., Messina, C. D. (2004). From genome to crop: integration through simulation modelling. Field Crops Research, 90(1), 145–163. DOI 10.1016/j.fcr.2004.07.014. [Google Scholar] [CrossRef]
3. Jame, Y. W., Cutforth, H. W. (1996). Crop growth-models for decision-support systems. Canadian Journal of Plant Science, 76(1), 9–19. DOI 10.4141/cjps96-003. [Google Scholar] [CrossRef]
4. Dai, X. Q., Huo, Z. L., Wang, H. M. (2011). Simulation for response of crop yield to soil moisture and salinity with artificial neural network. Field Crops Research, 12(3), 441–449. DOI 10.1016/j.fcr.2011.01.016. [Google Scholar] [CrossRef]
5. Raymundo, R., Asseng, S., Cammarano, D., Quiroz, R. (2014). Potato, sweet potato, and yam models for climate change: a review. Field Crops Research, 166, 173–185. DOI 10.1016/j.fcr.2014.06.017. [Google Scholar] [CrossRef]
6. Holzworth, D. P., Snow, V., Janssen, S., Athanasiadis, I. N., Donatelli, M. et al. (2015). Agricultural production systems modelling and software: current status and future prospects. Environmental Modeling and Software, 7, 276–286. DOI 10.1016/j.envsoft.2014.12.013. [Google Scholar] [CrossRef]
7. Badenko, V. L., Topaj, A. G., Yakushev, V. V., Mirschel, W., Nendel, C. (2017). Crop models as research and interpretative tools. Agricultural Biology, 52, 437–445. [Google Scholar]
8. Boote, K., Jones, J., Hoogenboom, G., Pickering, N. (2013). The CROPGRO model for grain legumes. In: Tsuji, G., Hoogenboom, G., Thornton, P. (eds.Understanding options for agricultural production, pp. 99–128. Dordrecht: Springer. [Google Scholar]
9. Boote, K., Jones, J., Hoogenboom, G., White, J. (2010). The role of crop systems simulation in agriculture and environment. In: Petraq, P., Pinet, F. (eds.New Technologies for constructing complex agricultural and environmental systems, pp. 41–54. USA: IGI Global. [Google Scholar]
10. Gary, C., Jones, J. W., Tchamitchian, M. (1998). Crop models in horticulture: state of the art. Scientia Horticulturae, 74(1–2), 3–20. DOI 10.1016/S0304-4238(98)00080-6. [Google Scholar] [CrossRef]
11. Molahleli, L., Steyn, J. M., Haverkort, A. J. (2013). Potato crop response to genotype and environment in a subtropical high land Agro-ecology. Potato Research, 56(3), 237–258. DOI 10.1007/s11540-013-9241-1. [Google Scholar] [CrossRef]
12. Svubure, O., Struik, P. C., Hsverkort, A. J., Steyn, J. M. (2015). Yield gap analysis and resource footprints of Irish potato production systems in Zimbabwe. Field Crops Research, 178, 77–90. DOI 10.1016/j.fcr.2015.04.002. [Google Scholar] [CrossRef]
13. de Wit, C. T. (1958). Transpiration and crop yields. Scientific report No. 64. Wageneingen University, The Netherlands. [Google Scholar]
14. de Wit, C. T. (1965). Photosynthesis of leaf canopies. agricultural research report 663. Wageningen University, The Netherlands. [Google Scholar]
15. Holzworth, D. P., Huth, N. I., deVoil, P. G., Zurcher, E. J., Herrmann, N. I. et al. (2014). APSIM-evolution towards a new generation of agricultural systems simulation. Environmental Modelling & Software, 62, 327–350. DOI 10.1016/j.envsoft.2014.07.009. [Google Scholar] [CrossRef]
16. Wenjia, W., Hao, F. (2012). The progress and problems in the development of foreign crop models. Water Saving Irrigation, 8, 63–68. [Google Scholar]
17. Ritchie, J. (1995). International consortium for agricultural systems applications (ICASAestablishment and purpose. Agricultural Systems, 49(4), 329–335. DOI 10.1016/0308-521X(95)00028-4. [Google Scholar] [CrossRef]
18. Liu, H., Zhu, Y., Li, S., Yang, J., Bai, Y. (2011). Development and application of DSSAT cropping system model. Agriculture Network Information, 11, 003. [Google Scholar]
19. Zhang, Y., Li, C., Zhou, X., Moore, B., III. (2002). A simulation model linking crop growth and soil biogeochemistry for sustainable agriculture. Ecological Modelling, 151(1), 75–108. DOI 10.1016/S0304-3800(01)00527-0. [Google Scholar] [CrossRef]
20. Žalud, Z., Dubrovský, M. (2002). Modelling climate change impacts on maize growth and development in the Czech Republic. Theoretical and Applied Climatology, 72(1–2), 85–102. DOI 10.1007/s007040200015. [Google Scholar] [CrossRef]
21. Bannayan, M., Crout, N., Hoogenboom, G. (2003). Application of the CERES-Wheat model for within-season prediction of winter wheat yield in the United Kingdom. Agronomy Journal, 95(1), 114–125. DOI 10.2134/agronj2003.0114. [Google Scholar] [CrossRef]
22. White, J. W., Hoogenboom, G., Kimball, B. A., Wall, G. W. (2011). Methodologies for simulating impact of climate change on crop production. Field Crops Research, 124(3), 357–368. DOI 10.1016/j.fcr.2011.07.001. [Google Scholar] [CrossRef]
23. Therond, O., Hengsdijk, H., Casellas, E., Wallach, D., Adam, M. et al. (2011). Using a cropping system model at regional scale: low-data approaches for crop management information and model calibration. Agriculture, Ecosystems & Environment, 142(1–2), 85–94. DOI 10.1016/j.agee.2010.05.007. [Google Scholar] [CrossRef]
24. Xiong, W., Holman, I., Conway, D., Lin, E., Li, Y. (2008). A crop model cross calibration for use in regional climate impacts studies. Ecological Modelling, 213(3–4), 365–380. DOI 10.1016/j.ecolmodel.2008.01.005. [Google Scholar] [CrossRef]
25. Challinor, A. J., Ewert, F., Arnold, S., Simelton, E., Fraser, E. (2009). Crops and climate change: progress, trends, and challenges in simulating impacts and informing adaptation. Journal of Experimental Botany, 60(10), 2775–2789. DOI 10.1093/jxb/erp062. [Google Scholar] [CrossRef]
26. Ventrella, D., Charfeddine, M., Giglio, L., Castellini, M. (2012). Application of DSSAT models for an agronomic adaptation strategy under climate change in Southern Italy: optimum sowing and transplanting time for winter durum wheat and tomato. Italian Journal of Agronomy, 7, 109–115. DOI 10.4081/ija.2012.e16. [Google Scholar] [CrossRef]
27. Wurr, D. C. E., Fellows, J. R., Suckling, R. F. (1988). Crop continuity and prediction of maturity in the crisp lettuce variety Saladin. Journal of Agricultural Science, 111(3), 481–486. DOI 10.1017/S0021859600083672. [Google Scholar] [CrossRef]
28. Navarrete, M., Bail, M. L. (2007). SALADPLAN: a model of the decision-making process in lettuce and endive cropping. Agronomy for Sustainable Development, 27(3), 209–221. DOI 10.1051/agro:2007009. [Google Scholar] [CrossRef]
29. Wurr, D. C. E., Fellows, J. R., Fuller, M. P. (2004). Simulated effects of climate change on the production pattern of winter cauliflower in the UK. Scientia Horticulturae, 101(4), 359–372. DOI 10.1016/j.scienta.2003.11.011. [Google Scholar] [CrossRef]
30. Wurr, D. C. E., Fellows, J. R., Phelps, K. (2002). Crop scheduling and prediction: principles and opportunities with field vegetables. Advances in Agronomy, 76, 201–234. DOI 10.1016/S0065-2113(02)76006-9. [Google Scholar] [CrossRef]
31. Munir, M., Hadley, P., Carew, J., Adams, S., Pearson, S. (2017). Modification of photo-thermal model by accommodating light integrals using antirrhinum flowering and leaf number data from restricted range of environmental conditions. Pakistan Journal of Botany, 49(1), 181–186. [Google Scholar]
32. Fisher, P. R., Heins, R. D. (1996). The greenhouse care system: a decision-support system for height control and scheduling of potted flower plants. Acta Horticulturae, 417, 41–46. DOI 10.17660/ActaHortic.1996.417.4. [Google Scholar] [CrossRef]
33. Yeo, K. H., Cho, Y. Y., Lee, Y. B. (2011). Estimation of growth and yield for single-stemmed rose ‘vital’ in a single stem system. Horticulture, Environment and Biotechnology, 52(5), 455–465. DOI 10.1007/s13580-011-0146-0. [Google Scholar] [CrossRef]
34. Ansar, H., Seetharamu, G. K., Shwetha, K. B., Kumar, S. A. (2014). Effect of planting geometry and nutrient levels on flowering, yield and quality of rose cv. charisma. Madras Agricultural Journal, 101, 280–283. [Google Scholar]
35. Darbyshire, R., Webb, L., Goodwin, I., Barlow, E. W. R. (2014). Challenges in predicting climate change impacts on pome fruit phenology. International Journal of Biometeorology, 58(6), 1119–1133. DOI 10.1007/s00484-013-0705-4. [Google Scholar] [CrossRef]
36. Bisbis, M. B., Gruda, N., Blanke, M. (2018). Potential impacts of climate change on vegetable production and product quality—A review. Journal of Cleaner Production, 170, 1602–1620. DOI 10.1016/j.jclepro.2017.09.224. [Google Scholar] [CrossRef]
37. Legave, J. M., Farrera, I., Almeras, T., Calleja, M. (2008). Selecting models of apple flowering time and understanding how global warming has had an impact on this trait. Journal of Horticultural Science and Biotechnology, 83(1), 76–84. DOI 10.1080/14620316.2008.11512350. [Google Scholar] [CrossRef]
38. Legave, J. M., Guédon, Y., Malagi, G., El Yaacoubi, A., Bonhomme, M. (2015). Differentiated responses of apple tree floral phenology to global warming in contrasting climatic regions. Frontiers in Plant Science, 6, 1–13. DOI 10.3389/fpls.2015.01054. [Google Scholar] [CrossRef]
39. Ahmad, S., Ahmad, A., Soler, C. M. T., Ali, H., Zia-Ul-Haq, M. et al. (2012). Application of the CSM-CERES-Rice model for evaluation of plant density and nitrogen management of fine transplanted rice for an irrigated semiarid environment. Precision Agriculture, 13(2), 200–218. DOI 10.1007/s11119-011-9238-1. [Google Scholar] [CrossRef]
40. Ahmad, S., Ahmad, A., Ali, H., Hussain, A., Garcia, A. et al. (2013). Application of the CSM-CERES-Rice model for evaluation of plant density and irrigation management of transplanted rice for an irrigated semiarid environment. Irrigation Science, 31(3), 491–506. DOI 10.1007/s00271-012-0324-6. [Google Scholar] [CrossRef]
41. Ahmad, S., Abbas, G., Fatima, Z., Khan, R. J., Anjum, M. A. et al. (2017). Quantification of the impacts of climate warming and crop management on canola phenology in Punjab, Pakistan. Crop Science, 203(5), 442–452. DOI 10.1111/jac.12206. [Google Scholar] [CrossRef]
42. Ahmad, S., Nadeem, M., Abbas, G., Fatima, Z., Khan, R. J. et al. (2017). Quantification of the effects of climate warming and crop management on sugarcane phenology. Climate Research, 71(1), 47–61. DOI 10.3354/cr01419. [Google Scholar] [CrossRef]
43. Ahmad, S., Abbas, Q., Abbas, G., Fatima, Z., Atique-ur-Rehman et al. (2017). Quantification of climate warming and crop management impacts on cotton phenology. Plants, 7(1), 1–16. DOI 10.3390/plants7010001. [Google Scholar] [CrossRef]
44. Abbas, G., Ahmad, S., Ahmad, A., Nasim, W., Fatima, Z. et al. (2017). Quantification the impacts of climate change and crop management on phenology of maize-based cropping system in Punjab, Pakistan. Agricultural and Forest Meteorology, 247, 42–55. DOI 10.1016/j.agrformet.2017.07.012. [Google Scholar] [CrossRef]
45. Boote, K. J., Rybak, M. R., Scholberg, J. M. S., Jones, J. W. (2012). Improving the CROPGRO-tomato model for predicting growth and yield response to temperature. HortScience, 47(8), 1038–1049. DOI 10.21273/HORTSCI.47.8.1038. [Google Scholar] [CrossRef]
46. Soto, F., Gallardo, M., Giménez, C., Peña-Fleitas, T., Thompson, R. B. (2014). Simulation of tomato growth, water and N dynamics using the EU-Rotate-N model in Mediterranean greenhouses with drip irrigation and fertigation. Agricultural Water Management, 132, 46–59. DOI 10.1016/j.agwat.2013.10.002. [Google Scholar] [CrossRef]
47. Russo, V. (1996). Planting date, fertilizer rate, and harvest timing affect yield of Jalapeno and Banana peppers. HortScience, 31(7), 1124–1125. DOI 10.21273/HORTSCI.31.7.1124. [Google Scholar] [CrossRef]
48. Islam, M., Saha, S., Akand, H., Rahim, A. (2010). Effect of sowing date on the growth and yield of seeet pepper (Capsicum annuum L.). Agronomski Glasnik, 72, 3–14. [Google Scholar]
49. Fleisher, D. H., Condori, B., Quiroz, R., Alva, A., Asseng, S. et al. (2016). Effects of geography, weather variability, and climate change on potato model uncertainty. Proceedings of the Korean Society of Agricultural and Forest Meteorology Conference. pp. 41–43. Agricultural Research Center for Climate Change, Jeju, Korea. [Google Scholar]
50. Kamboj, N. K., Sharma, H. D. (2015). Effect of planting time and spacing on maturity, growth and fruit yield of bell pepper, Capsicum annuum L. International Journal of Farm Sciences, 5, 17–23. [Google Scholar]
51. Matthews, R., Stephens, W., Hess, T., Middleton, T., Graves, A. (2002). Applications of crop/soil simulation models in tropical agricultural systems. Advances in Agronomy, 76, 31–124. DOI 10.1016/S0065-2113(02)76003-3. [Google Scholar] [CrossRef]
52. Gallardo, M., Giménez, C., Martínez-Gaitán, C., Stöckle, C. O., Thompson, R. B. et al. (2011). Evaluation of the VegSyst model with muskmelon to simulate crop growth, nitrogen uptake and evapotranspiration. Agricultural Water Management, 101(1), 107–111. DOI 10.1016/j.agwat.2011.09.008. [Google Scholar] [CrossRef]
53. Nangia, V., Turral, H., Molden, D. (2008). Increasing water productivity with improved N fertilizer management. Irrigation and Drainage Systems, 22(4), 193–207. DOI 10.1007/s10795-008-9051-9. [Google Scholar] [CrossRef]
54. Shangguan, Z. P., Shao, M. A., Dyckmans, J. (2000). Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environmental and Experimental Botany, 44(2), 141–149. DOI 10.1016/S0098-8472(00)00064-2. [Google Scholar] [CrossRef]
55. Rinaldi, M., Ventrella, D., Gagliano, C. (2007). Comparison of nitrogen and irrigation strategies in tomato using CROPGRO model. A case study from Southern Italy. Agricultural Water Management, 87(1), 91–105. DOI 10.1016/j.agwat.2006.06.006. [Google Scholar] [CrossRef]
56. Elia, A., Conversa, G. (2015). A decision support system (GesCoN) for managing fertigation in open field vegetable crops. Part I-methodological approach and description of the software. Frontiers in Plant Science, 6, 1–18. DOI 10.3389/fpls.2015.00319. [Google Scholar] [CrossRef]
57. Rinaldi, M., Castrignanò, A., Mastrorilli, M., Rana, G., Ventrella, D. et al. (2006). Decision support systems to manage water resources at irrigation district level in Southern Italy using remote sensing information. An Integrated Project (AQUATER). AIP Conference Proceedings American Institute of Physics, 852(1), 107–114. DOI 10.1063/1.2349334. [Google Scholar] [CrossRef]
58. Hoogenboom, G., Porter, C. H., Shelia, V., Boote, K. J., Singh, U. et al. (2017). Decision Support System for Agrotechnology Transfer (DSSAT). Version 4.7 (http://dssat.net). DSSAT Foundation, Gainesville, Florida, USA. [Google Scholar]
59. Al-Jamal, M. S., Sammis, T. W., Ball, S., Smeal, D. (1999). Yield-based, irrigated onion crop coefficients. Applied Engineering in Agriculture, 15(6), 659–668. DOI 10.13031/2013.5835. [Google Scholar] [CrossRef]
60. Mermoud, A., Tamini, T. D., Yacouba, H. (2005). Impacts of different irrigation schedules on the water balance components of an onion crop in a semi-arid zone. Agricultural Water Management, 77(1–3), 282–295. DOI 10.1016/j.agwat.2004.09.033. [Google Scholar] [CrossRef]
61. Ragab, R., Battilani, A., Matovic, G., Stikic, R., Psarras, G. et al. (2015). SALTMED model as an integrated management tool for water, crop, soil and N-fertilizer water management strategies and productivity: Field and simulation study. Irrigation and Drainage, 64(1), 13–28. DOI 10.1002/ird.1898. [Google Scholar] [CrossRef]
62. Imtiyaz, M., Mgadla, N. P., Chepete, B., Manase, S. K. (2000). Response of six vegetable crops to irrigation schedules. Agricultural Water Management, 45(3), 331–342. DOI 10.1016/S0378-3774(99)00105-5. [Google Scholar] [CrossRef]
63. Garcia-Villa, M., Fereres, E. (2012). Combining the simulation crop model AquaCrop with an economic model for the optimisation of irrigation management at farm level. European Journal of Agronomy, 36(1), 21–31. DOI 10.1016/j.eja.2011.08.003. [Google Scholar] [CrossRef]
64. Stöckle, C. O., Donatelli, M., Nelson, R. (2003). CropSyst, a cropping systems simulation model. European Journal of Agronomy, 18(3–4), 289–307. DOI 10.1016/S1161-0301(02)00109-0. [Google Scholar] [CrossRef]
65. Stöckle, C. O., Nelson, R. (2005). Cropsyst user’s manual (Version 3.04.08). Biological Systems Engineering Department, Washington State University, Pullman, WA, USA. [Google Scholar]
66. Rinaldi, M., Garofalo, P., Rubino, P., Steduto, P. (2011). Processing tomatoes under different irrigation regimes in Southern Italy: agronomic and economic assessments in a simulation case study. Italian Journal of Agrometeorology, 3, 39–56. [Google Scholar]
67. Cancela, J. J., Cuesta, T. J., Neira, X. X., Pereira, L. S. (2006). Modelling for improved irrigation water management in a temperate region of Northern Spain. Biosystems Engineering, 94(1), 151–163. DOI 10.1016/j.biosystemseng.2006.02.010. [Google Scholar] [CrossRef]
68. Rossi, V., Caffi, T., Salinari, F. (2012). Helping farmers face the increasing complexity of decision-making for crop protection. Phytopathologia Mediterranea, 51, 457–479. [Google Scholar]
69. Shtienberg, D. (2013). Will decision-support systems be widely used for the management of plant diseases? Annual Review of Phytopathology, 51(1), 1–16. DOI 10.1146/annurev-phyto-082712-102244. [Google Scholar] [CrossRef]
70. Tang, S., Cheke, R. A. (2008). Models for integrated pest control and their biological implications. Mathematical Biosciences, 215(1), 115–125. DOI 10.1016/j.mbs.2008.06.008. [Google Scholar] [CrossRef]
71. Peck, S. L., Bouyer, J. (2012). Mathematical modeling, spatial complexity, and critical decisions in tsetse control. Journal of Economic Entomology, 105(5), 1477–1486. DOI 10.1603/EC12067. [Google Scholar] [CrossRef]
72. Damos, P. T., Savopoulou-Soultani, M. (2010). Development and statistical evaluation of models in forecasting moth phenology of major lepidopterous peach pest complex for Integrated Pest Management programs. Crop Protection, 29(10), 1190–1199. DOI 10.1016/j.cropro.2010.06.022. [Google Scholar] [CrossRef]
73. Vinatier, F., Lescourret, F., Duyck, P. F., Tixier, P. (2012). From IBM to IPM: Using individual-based models to design the spatial arrangement of traps and crops in integrated pest management strategies. Agriculture, Ecosystems and Environment, 146(1), 52–59. DOI 10.1016/j.agee.2011.10.005. [Google Scholar] [CrossRef]
74. Been, T., Berti, A., Evans, N., Gouache, D., Gutsche, V. et al. (2009). Review of new technologies critical to effective implementation of Decision Support Systems (DSS’s) and farm management systems (FMS’s). Report from the ENDURE Network. Aarhus University, Aarhus, Denmark. [Google Scholar]
75. Gebauer, K., Hemerik, L., Meyhofer, R. (2015). Effects of climate change on pest-parasitoid dynamics: development of a simulation model and first results. Journal of Plant Diseases and Protection, 122(1), 28–35. DOI 10.1007/BF03356527. [Google Scholar] [CrossRef]
76. Thomas, M. B. (1999). Ecological approaches and the development of ‘truly integrated’ pest management. Proceedings of the National Academy of Sciences of the United States of America, 96(11), 5944–5951. DOI 10.1073/pnas.96.11.5944. [Google Scholar] [CrossRef]
77. Michaud, O. D., Boivin, G., Stewart, R. K. (1989). Economic threshold for tarnished plant bug (Hemiptera: Miridae) in apple orchards. Journal of Economic Entomology, 82(6), 1722–1728. DOI 10.1093/jee/82.6.1722. [Google Scholar] [CrossRef]
78. Mitchell, P. D., Hutchison, W. D. (2009). Decision making and economic risk in IPM. In: Radcliffe, E. B., Hutchison, W. D., Cancelado, R. E. (eds.Integrated pest management. concepts, tactics, strategies and case studies, pp. 33–50. Cambridge, England: Cambridge University Press. [Google Scholar]
79. Linder, C., Baroffio, C., Mittaz, C. (2012). New method for monitoring the damages of Anthonomus rubi on raspberry. Revue Suisse de Viticulture, Arboriculture et Horticulture, 44, 162–166. [Google Scholar]
80. Willett, M. J., Andrews, P. K. (1996). Knowledge-based systems for fruit and vegetable production management. AI Applications, 10, 75–92. [Google Scholar]
81. Rossi, V., Salinari, F., Poni, S., Caffi, T., Bettati, T. (2014). Addressing the implementation problem in agricultural decision support systems: the example of vite.net. Computers and Electronics in Agriculture, 100, 88–99. DOI 10.1016/j.compag.2013.10.011. [Google Scholar] [CrossRef]
82. Mehla, C. P., Srivastava, V. K., Jage, S., Mangat, R., Singh, J. et al. (2000). Response of tomato varities to N and P fertilization and spacing. Indian Journal of Agricultural Research, 34, 182–184. [Google Scholar]
83. Salmerón, M., Urrego, Y. F., Isla, R., Cavero, J. (2012). Effect of non-uniform sprinkler irrigation and plant density on simulated maize yield. Agricultural Water Management, 113, 1–9. DOI 10.1016/j.agwat.2012.06.007. [Google Scholar] [CrossRef]
84. Nyakudya, I. W., Stroosnijder, L. (2014). Effect of rooting depth, plant density and planting date on maize (Zea mays L.) yield and water use efficiency in semi-arid Zimbabwe: modelling with AquaCrop. Agricultural Water Management, 146, 280–296. DOI 10.1016/j.agwat.2014.08.024. [Google Scholar] [CrossRef]
85. Battisti, R., Sentelhas, P. C., Parker, P. S., Nendel, C., Gil, M. D. S. et al. (2018). Assessment of crop-management strategies to improve soybean resilience to climate change in Southern Brazil. Crop and Pasture Science, 69(2), 154–162. DOI 10.1071/CP17293. [Google Scholar] [CrossRef]
86. Calviño, P. A., Sadras, V. O., Andrade, F. H. (2003). Quantification of environmental and management effects on the yield of late-sown soybean. Field Crops Research, 83(1), 67–77. DOI 10.1016/S0378-4290(03)00062-5. [Google Scholar] [CrossRef]
87. Lentz, W. (1998). Model applications in horticulture: a review. Scientia Horticulturae, 74(1–2), 151–174. DOI 10.1016/S0304-4238(98)00085-5. [Google Scholar] [CrossRef]
88. Jones, J. W., Hoogenboom, G., Porter, C. H., Boote, K. J., Batchelor, W. D. et al. (2003). The DSSAT cropping system model. European Journal of Agronomy, 18(3–4), 235–265. DOI 10.1016/S1161-0301(02)00107-7. [Google Scholar] [CrossRef]
89. Singh, U., Matthews, R. B., Griffin, T. S., Ritchie, J. T., Hunt, L. A. et al. (1998). Modeling growth and development of root and tuber crops. In: Tsuji, G. Y., Hoogenboom, G., Thornton, P. K. (eds.Understanding options for agricultural production, pp. 129–156. Dordrecht: Springer. [Google Scholar]
90. Boote, K. J., Scholberg, J. M. S. (2006). Developing, parameterizing and testing of dynamic crop growth models for horticultural crops. Acta Horticulturae, 718, 23–34. DOI 10.17660/ActaHortic.2006.718.1. [Google Scholar] [CrossRef]
91. Tang, J., Wang, J., Fang, Q., Dayananda, B., Yu, Q. et al. (2019). Identifying agronomic options for better potato production and conserving water resources in the agro-pastoral ecotone in North China. Agricultural and Forest Meteorology, 272, 91–101. DOI 10.1016/j.agrformet.2019.04.001. [Google Scholar] [CrossRef]
92. Lisson, S. N., Cotching, W. E. (2011). Modelling the fate of water and nitrogen in the mixed vegetable farming systems of northern Tasmania, Australia. Agricultural Systems, 104(8), 600–608. DOI 10.1016/j.agsy.2011.06.002. [Google Scholar] [CrossRef]
93. Takács, S., Rácz, I., Csengeri, E., Bíró, T. (2019). Biomass production estimation of processing tomato using AquaCrop under different irrigation treatments. Acta Agraria Debreceniensis, 2(2), 131–136. [Google Scholar]
94. Peralta, J. M., Stockle, V. (2002). Dynamics of nitrate leaching under irrigated potato rotation in Washington State: a long-term simulation study. Agriculture, Ecosystems and Environment, 88(1), 23–34. DOI 10.1016/S0167-8809(01)00157-8. [Google Scholar] [CrossRef]
95. Alva, A. K., Marcos, J., Stocle, C., Reddy, V. R., Timlim, D. (2010). A crop simulation model for predicting yield and fate of nitrogen in irrigated potato rotation cropping system. Journal of Crop Improvement, 24(2), 142–152. DOI 10.1080/15427520903581239. [Google Scholar] [CrossRef]
96. Hansen, S., Abrahamsen, P., Petersen, C. T., Styczen, M. (2012). Daisy: model use, calibration, and validation. Transactions of the American Society of Agricultural and Biological Engineers, 55(4), 1317–1333. [Google Scholar]
97. Dolezal, F., Zumr, D., Vacek, J., Zavadil, J., Battilani, A. et al. (2007). Dual permeability soil water dynamics and water uptake by roots in irrigated potato fields. Biologia, 62(5), 552–556. DOI 10.2478/s11756-007-0109-1. [Google Scholar] [CrossRef]
98. Lenz-Wiedemann, V. I. S., Klar, C. W., Schneider, K. (2010). Development and test of a crop growth model for application within a global change decision support system. Ecological Modelling, 221(2), 314–329. DOI 10.1016/j.ecolmodel.2009.10.014. [Google Scholar] [CrossRef]
99. Gayler, S., Wang, E., Priesack, E., Schaaf, T., Maidl, F. X. (2002). Modeling biomass growth, N-uptake andphenological development of potato crop. Geoderma, 105(3–4), 367–383. DOI 10.1016/S0016-7061(01)00113-6. [Google Scholar] [CrossRef]
100. Singh, J. P., Govindakrishnan, P. M., Lal, S. S., Aggarwal, P. K. (2005). Increasing the efficiency of agronomy experiments in potato using INFOCROP-POTATO model. Potato Research, 48(3–4), 131–152. DOI 10.1007/BF02742372. [Google Scholar] [CrossRef]
101. Ingram, K. T., McCloud, D. E. (1984). Simulation of potato crop growth and development. Crop Science, 24(1), 21–27. DOI 10.2135/cropsci1984.0011183X002400010006x. [Google Scholar] [CrossRef]
102. Fishman, S., Talpaz, H., Dinar, M., Levy, M., Arazi, Y. et al. (1984). A phenomenological model of dry-matter partitioning among plant organs for simulation of potato growth. Agricultural Systems, 14(3), 159–179. DOI 10.1016/0308-521X(84)90003-9. [Google Scholar] [CrossRef]
103. Johnson, K. B., Johnson, S. B., Teng, P. S. (1986). Development of a simple potato growth-model for use in crop-pest management. Agricultural Systems, 19(3), 189–209. DOI 10.1016/0308-521X(86)90052-1. [Google Scholar] [CrossRef]
104. Angulo, C., Rotter, R., Lock, R., Enders, A., Fronzek, S. et al. (2013). Implication of crop model calibration strategies for assessing regional impacts of climate change in Europe. Agricultural and Forest Meteorology, 170, 32–46. DOI 10.1016/j.agrformet.2012.11.017. [Google Scholar] [CrossRef]
105. Van Delden, A., Schroder, J. J., Kropff, M. J., Grashoff, C., Booij, R. (2003). Simulated potato yield, and crop and soil nitrogen dynamics under different organic nitrogen management strategies in The Netherlands. Agriculture, Ecosystem and Environment, 96(1–3), 77–95. DOI 10.1016/S0167-8809(03)00012-4. [Google Scholar] [CrossRef]
106. Machakaire, A. T., Steyn, J. M., Caldiz, D. O., Haverkort, A. J. (2016). Forecasting yield and tuber size of processing potatoes in South Africa using the LINTUL-potato-DSS model. Potato Research, 59(3), 195–206. DOI 10.1007/s11540-016-9321-0. [Google Scholar] [CrossRef]
107. Wolf, J., Van Oijen, M. (2003). Model simulation of effects of changes in climate and atmospheric CO2 and O3 on tuber yield potential of potato (cv. Bintje) in the European Union. Agriculture, Ecosystem and Environment, 94(2), 141–157. DOI 10.1016/S0167-8809(02)00029-4. [Google Scholar] [CrossRef]
108. Wolf, J. (2002). Comparison of two potato simulation models under climate change. I. Model calibration and sensitivity analyses. Climate Research, 21, 173–186. DOI 10.3354/cr021173. [Google Scholar] [CrossRef]
109. Kadaja, J., Tooming, H. (2004). Potato production model based on principle of maximum plant productivity. Agricultural and Forest Meteorology, 127(1–2), 17–33. DOI 10.1016/j.agrformet.2004.08.003. [Google Scholar] [CrossRef]
110. Ng, E., Loomis, R. S. (1984). Simulation of growth and yield of the potato crop. Wageningen, The Netherlands: Centre for Agricultural Publishing and Documentation. [Google Scholar]
111. Wolf, J. (2002). Comparison of two potato simulation models under climate change. II. Application of climate change scenarios. Climate Research, 21, 187–198. [Google Scholar]
112. Jamieson, P. D., Zyskowski, R. F., Sinton, S. M., Brown, H. E., Butler, R. C. (2006). The potato calculator: a tool for scheduling nitrogen fertilizer applications. Agronomy New Zealand, 36, 49–53. [Google Scholar]
113. Jamieson, P. D., Zyskowski, R. F., Li, F. Y., Semenov, M. A. (2009). Water and nitrogen uptake and responses in models of wheat, potatoes, and maize. In: Ma, L., Ahuja, L. R., Bruulsema, T. (eds.Quantifying and understanding plant nitrogen uptake for systems modeling, pp. 127–145. Boca Raton, Florida, USA: CRC Press. [Google Scholar]
114. Roth, O., Derron, J., Fischlin, A., Nemecek, T., Ulrich, M. (1990). Implementation, parameter adaptation, and integration of a potato crop and a soil water simulation model. Proceedings of the 1st International Workshop on Potato Modelling, pp. 1–30. International Agricultural Centre Wageningen, Wageningen, The Netherlands. Pudoc, Wageningen. [Google Scholar]
115. Gobin, A. (2010). Modelling climate impacts on crop yields in Belgium. Climate Research, 44(1), 55–68. DOI 10.3354/cr00925. [Google Scholar] [CrossRef]
116. Jongschaap, R. E. E. (2006). Run-time calibration of simulation models by integrating remote sensing estimates of leaf area index and canopy nitrogen. European Journal of Agronomy, 24(4), 316–324. DOI 10.1016/j.eja.2005.10.009. [Google Scholar] [CrossRef]
117. Sands, P. J., Hackett, C., Nix, H. A. (1979). A model of the development and bulking of potatoes (Solanum tuberosum L.). 1. Derivation from well-managed field crops. Field Crops Research, 2, 309–331. DOI 10.1016/0378-4290(79)90031-5. [Google Scholar] [CrossRef]
118. Sanabria, J., Lhomme, J. (2013). Climate change and potato cropping in the Peruvian Altiplano. Theoretical and Applied Climatology, 112(3–4), 683–695. DOI 10.1007/s00704-012-0764-1. [Google Scholar] [CrossRef]
119. Mackerron, D. K. L., Waister, P. D. (1985). A simple-model of potato growth and yield. Part 1. Model development and sensitivity analysis. Agricultural and Forest Meteorology, 34(2–3), 241–252. DOI 10.1016/0168-1923(85)90024-3. [Google Scholar] [CrossRef]
120. Hodges, T., Johnson, S. L., Johnson, B. S. (1992). A modular structure for crop simulation models: implemented in the SIMPOTATO model. Agronomy Journal, 84(5), 911–915. DOI 10.2134/agronj1992.00021962008400050027x. [Google Scholar] [CrossRef]
121. Han, S., Evans, R. G., Hodges, T., Rawlins, S. L. (1995). Linking a geographic information system with a potato simulation model for site-specific crop management. Journal of Environmental Quality, 24(4), 772–777. DOI 10.2134/jeq1995.00472425002400040031x. [Google Scholar] [CrossRef]
122. Condori, B., Hijmans, R. J., Quiroz, R., Ledent, J. F. (2010). Quantifying the expression of potato genetic diversity in the high Andes through growth analysis and modeling. Field Crops Research, 119(1), 135–144. DOI 10.1016/j.fcr.2010.07.003. [Google Scholar] [CrossRef]
123. Fleisher, D. H., Timlin, D. J., Yang, Y., Reddy, V. R. (2010). Simulation of potato gas exchange rates using SPUDSIM. Agricultural and Forest Meteorology, 150(3), 432–442. DOI 10.1016/j.agrformet.2010.01.005. [Google Scholar] [CrossRef]
124. Griffin, T. S., Bradley, S. J., Ritchie, J. T. (1993). Simulation model for potato growth and development: SUBSTOR-Potato version 2.0. Department of Agronomy and Soil Science, College of Tropical Agriculture and Human Resources. University of Hawai, Honolulu. [Google Scholar]
125. Raymundo, R., Asseng, S., Prassad, R., Kleinwechter, U., Concha, J. et al. (2017). Performance of the SUBSTOR-potato model across contrasting growing conditions. Field Crops Research, 202, 57–76. DOI 10.1016/j.fcr.2016.04.012. [Google Scholar] [CrossRef]
126. Balpande, R., Paliwal, H. B., Kumar, A., Kumar, P., Kumar, V. (2019). Effect of sowing date on the growth and yield of different verities of potato by using DSSAT model. Journal of Pharmacognosy and Phytochemistry, 8(3), 4737–4739. [Google Scholar]
127. Vashisht, B. B., Nigon, T., Mulla, D. J., Rosen, C., Xu, H. et al. (2015). Adaptation of water and nitrogen management to future climates for sustaining potato yield in Minnesota: field and simulation study. Agricultural Water Management, 152, 198–206. DOI 10.1016/j.agwat.2015.01.011. [Google Scholar] [CrossRef]
128. Fortin, J. G., Morais, A., Anctil, F., Parent, L. E. (2015). SVMLEACH – NK POTATO: A simple software tool to simulate nitrate and potassium co-leaching under potato crop. Computers and Electronics in Agriculture, 110, 259–266. DOI 10.1016/j.compag.2014.11.025. [Google Scholar] [CrossRef]
129. Van den Broek, B. J., Kabat, P. (1995). SWACROP: dynamic simulation model of soil water and crop yield applied to potatoes. In: Kabat, P., Marshall, B., van den Broek, B. J.,Vos, J., van Keulen, H.(eds.Modelling and parameterization of the soil-plant-atmosphere system: a comparison of potato growth models, pp. 299–333. Wageningen, The Netherlands: Wageningen Press. [Google Scholar]
130. Boogaard, H., Kroes, J. (1998). Leaching of nitrogen and phosphorus from rural areas to surface waters in the Netherlands. Nutrient Cycling in Agroecosystems, 50(1/3), 321–324. DOI 10.1023/A:1009773202654. [Google Scholar] [CrossRef]
131. Supit, I., van Diepen, C., de Wit, A., Wolf, J., Kabat, P. et al. (2012). Assessing climate change effects on European crop yields using the crop growth monitoring system and a weather generator. Agricultural and Forest Meteorology, 164, 96–111. DOI 10.1016/j.agrformet.2012.05.005. [Google Scholar] [CrossRef]
132. van Walsum, P. E. V., Supit, I. (2012). Influence of ecohydrologic feedbacks from simulated crop growth on integrated regional hydrologic simulations under climate scenarios. Hydrology and Earth System Sciences, 16(6), 1577–1593. DOI 10.5194/hess-16-1577-2012. [Google Scholar] [CrossRef]
133. Jones, J. W., Dayan, E., Allen, L. H., Van Keulen, H., Challa, H. (1991). A dynamic tomato growth and yield model (TOMGRO). Transactions of the American Society of Agricultural and Biological Engineers, 34(2), 663–672. DOI 10.13031/2013.31715. [Google Scholar] [CrossRef]
134. Dimokas, G., Tchamitchian, M., Kittas, C. (2010). Calibration and validation of a biological model to simulate the development and production of tomatoes in Mediterranean greenhouses during winter period. Biosystems Engineering, 103(2), 217–227. DOI 10.1016/j.biosystemseng.2009.01.004. [Google Scholar] [CrossRef]
135. Giuliani, M. M., Gatta, G., Cappelli, G., Gagliardi, A., Donatelli, M. et al. (2019). Identifying the most promising agronomic adaptation strategies for the tomato growing systems in Southern Italy via simulation modeling. European Journal of Agronomy, 111, 125937. DOI 10.1016/j.eja.2019.125937. [Google Scholar] [CrossRef]
136. Gary, C., Baille, A., Navarrete, M., Epanet, R. (1997). TOMPOUSSE, un modele simplifie de prevision du rendement et du caliber de la tomate. In: Baille, A. (ed.Actes du seminaire de I’AIP intersectorielle “Serres”, pp. 100–109. Avignon: INRA. [Google Scholar]
137. Scholberg, J. M. S., Boote, K. J., Jones, J. W., McNeal, B. L. (1997). Adaptation of the CROPGRO model to simulate field-grown tomato. In: Kropff, M. J., Teng, P. S., Aggarwal, P. K., Bouma, J., Bouman, B. A. M. et al. (eds.Application of systems approaches at the field level. systems approaches for sustainable agricultural development, pp. 135–151. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
138. Messina, C. D., Jones, J. W., Hansen, J. W. (2001). Understanding ENSO effects on tomato yields in Florida: A modelling approach. Proceedings of the Second International Symposium on Modelling Cropping Systems, pp. 155–156. Florence, Italy. [Google Scholar]
139. Koo, J. (2002). Modeling the impacts of climate variability on tomato disease management and production (Ph.D. Thesis). University of Florida, USA. [Google Scholar]
140. Ramirez, A., Rodriguez, F., Berenguel, M., Heuvelink, E. (2004). Calibration and validation of complex and simplified tomato growth models for control purposes in the southeast of Spain. Acta Horticulturae, 654, 147–154. DOI 10.17660/ActaHortic.2004.654.15. [Google Scholar] [CrossRef]
141. Heuvelink, E., Bertin, N. (1994). Dry matter partitioning in a tomato crop: comparison of two simulation models. Journal of Horticultural Science, 69(5), 885–903. DOI 10.1080/14620316.1994.11516525. [Google Scholar] [CrossRef]
142. Heuvelink, E. (1996). Dry matter partitioning in tomato: Validation of a dynamic simulation model. Annuals of Botany, 77(1), 71–80. DOI 10.1006/anbo.1996.0009. [Google Scholar] [CrossRef]
143. Cavero, J., Plant, R. E., Shennan, C., Friedman, D. B., Williams, J. R. et al. (1998). Application of EPIC model to nitrogen cycling in irrigated processing tomatoes under different management systems. Agricultural Systems, 56(4), 391–414. DOI 10.1016/S0308-521X(96)00100-X. [Google Scholar] [CrossRef]
144. Rinaldi, M., Di Paolo, E., Colucci, R., Di Lena, B. (2001). Validation of EPIC model in simulating tomato field crop in Italian environments. Proceedings of the Second International Symposium on Modelling Cropping Systems, pp. 167–168. Florence, Italy. [Google Scholar]
145. Doltra, J., Munoz, P. (2010). Simulation of nitrogen leaching from a fertigated crop rotation in a Mediterranean climate using the EU-Rotate_N and Hydrus-2D models. Agricultural Water Management, 97(2), 277–285. DOI 10.1016/j.agwat.2009.09.019. [Google Scholar] [CrossRef]
146. Valdés-Gómeza, H., Gary, C., Brisson, N., Matus, F. (2014). Modelling indeterminate development, dry matter partitioning and the effect of nitrogen supply in tomato with the generic STICS crop-soil model. Scientia Horticulturae, 175, 44–56. DOI 10.1016/j.scienta.2014.05.030. [Google Scholar] [CrossRef]
147. Brisson, N., Mary, B., Ripoche, D., Jeuffroy, M. H., Ruget, F. et al. (1998). STICS: a generic model for the simulation of crops and their water and nitrogen balances. I. Theory and parameterization applied to wheat and corn. Agronomie, 18, 311–346. [Google Scholar]
148. Silva, L. L., Baptista, F. J., Meneses, J. F., Ragab, R. (2017). Evaluation of the SALTMED model for tomato crop production in unheated greenhouses. Acta Horticulturae, 1170, 441–446. DOI 10.17660/ActaHortic.2017.1170.54. [Google Scholar] [CrossRef]
149. Qiu, V., Du, T., Kang, S., Chen, R., Wu, L. (2015). Assessing the SIMDualKc model for estimating evapotranspiration of hot pepper grown in a solar greenhouse in Northwest China. Agricultural Systems, 138, 1–9. DOI 10.1016/j.agsy.2015.05.001. [Google Scholar] [CrossRef]
150. Giménez, C., Gallardo, M., Martínez-Gaitán, C., Stöckle, C. O., Thompson, R. B. et al. (2013). VegSyst, a simulation model of daily crop growth, nitrogen uptake and evapotranspiration for pepper crops for use in an on-farm decision support system. Irrigation Science, 31(3), 465–477. DOI 10.1007/s00271-011-0312-2. [Google Scholar] [CrossRef]
151. Fazel, F., Khorramdel, N. G., Gheysari, M. (2017). Simulation of soil water content and nitrate under different fertigation strategies for sweet pepper in Isfahan by EU-ROTATE-N model. Journal of Water and Soil, 31, 263–276. [Google Scholar]
152. Rameshwaran, P., Tepe, A., Yazar, A., Ragab, R. (2015). The effect of saline irrigation water on the yield of pepper: experimental and modelling study. Irrigation and Drainage, 64(1), 41–49. DOI 10.1002/ird.1867. [Google Scholar] [CrossRef]
153. Sánchez-Molina, J. A., Pérez, N., Rodríguez, F., Guzmán, J. L., López, J. C. (2015). Support system for decision making in the management of the greenhouse environmental based on growth model for sweet pepper. Agricultural Systems, 139, 144–152. DOI 10.1016/j.agsy.2015.06.009. [Google Scholar] [CrossRef]
154. Marcelis, L. F. M., Brajeul, E., Elings, A., Garate, A., Heuvelink, E. (2005). Modelling nutrient uptake of sweet pepper. Acta Horticulturae, 691, 285–292. DOI 10.17660/ActaHortic.2005.691.33. [Google Scholar] [CrossRef]
155. Reddy, A. R. G., Tiwari, K. N. (2018). Assessment of CROPGRO-Bell pepper model under different nitrogen levels through fertigation. International Journal of Agricultural Engineering, 11(1), 101–107. DOI 10.15740/HAS/IJAE/11.1/101-107. [Google Scholar] [CrossRef]
156. Arora, V. K., Nath, J. C., Singh, C. B. (2013). Analyzing potato response to irrigation and nitrogen regimes in a sub-tropical environment using SUBSTOR-Potato model. Agricultural Water Management, 124, 69–76. DOI 10.1016/j.agwat.2013.03.021. [Google Scholar] [CrossRef]
157. Abdrabbo, M. A. A., Khalil, A. A., Hassanien, M. K. K., Abou-Hadid, A. F. (2010). Sensitivity of potato yield to climate change. Journal of Applied Science Research, 6, 751–755. [Google Scholar]
158. Woli, P., Hoogenboom, G., Alva, A. (2016). Simulation of potato yield, nitrate leaching, and profit margins as influenced by irrigation and nitrogen management in different soils and production regions. Agricultural Water Management, 171, 120–130. DOI 10.1016/j.agwat.2016.04.003. [Google Scholar] [CrossRef]
159. Griffin, T. S., Johnson, B. S., Ritchie, J. T. (1992). SUBSTOR-Potato version 2.0: A simulation model for potato growth and development. Michigan State University, Michigan, USA. [Google Scholar]
160. Travasso, M. I., Caldiz, D. O., Saluzzo, J. A. (1996). Yield prediction using the SUBSTOR-Potato model under Argentinean conditions. Potato Research, 39(2), 305–312. DOI 10.1007/BF02360922. [Google Scholar] [CrossRef]
161. Snapp, S. S., Fortuna, A. M. (2003). Predicting nitrogen availability in irrigated potato systems. HortTechnology, 13(4), 598–604. DOI 10.21273/HORTTECH.13.4.0598. [Google Scholar] [CrossRef]
162. Prasad, R., Hochmuth, G. J., Boote, K. J. (2015). Estimation of nitrogen pools in irrigated potato production on sandy soil using the model SUBSTOR. PLoS One, 10(1), e0117891. DOI 10.1371/journal.pone.0117891. [Google Scholar] [CrossRef]
163. Silva, L. L., Ragab, R., Duarte, I., Lourenco, E., Simoes, N. et al. (2013). Calibration and validation of SALTMED model under dry and wet year conditions using chickpea field data from southern Portugal. Irrigation Science, 31(4), 651–659. DOI 10.1007/s00271-012-0341-5. [Google Scholar] [CrossRef]
164. Bertin, N., Gary, C. (1993). Tomato fruit-set: a case study for validation of the model TOMGRO. Acta Horticulturae, 328, 185–194. DOI 10.17660/ActaHortic.1993.328.17. [Google Scholar] [CrossRef]
165. Scholberg, J. M. S. (1996). Adaptive use of crop growth models to simulate the growth of field-grown tomato (Doctoral Dissertation). University of Florida, Gainesville, Florida. [Google Scholar]
166. Pien, H., Lemeur, R., De Cordt, W., Baets, W. (1999). The use of TOMGRO as a simplified diagnostic tool for growers. Acta Horticulturae, 507, 285–292. DOI 10.17660/ActaHortic.1999.507.33. [Google Scholar] [CrossRef]
167. Cooman, A., Schrevens, E. (2004). The uncertainty on TOMGRO predictions caused by variations on model parameters. Acta Horticulturae, 654, 155–162. DOI 10.17660/ActaHortic.2004.654.16. [Google Scholar] [CrossRef]
168. Cooman, A., Schrevens, E. (2004). Sensitivity analyses of TOMGRO output variables to variations in climate conditions. Acta Horticulturae, 654, 317–324. DOI 10.17660/ActaHortic.2004.654.37. [Google Scholar] [CrossRef]
169. Marcelis, L. F. M., Elings, A., Bakker, M. J., Brajeul, E., Dieleman, J. A. et al. (2006). Modelling dry matter production and partitioning in sweet pepper. Acta Horticulturae, 718, 121–128. DOI 10.17660/ActaHortic.2006.718.13. [Google Scholar] [CrossRef]
170. Schepers, H., Kromdijk, W., Van Kooten, O. (2006). The conveyor belt model for fruit bearing vegetables: application to sweet pepper yield oscillations. Acta Horticulturae, 718, 43–50. DOI 10.17660/ActaHortic.2006.718.3. [Google Scholar] [CrossRef]
171. Buwalda, F., Van Henten, E. J., De Gelder, A., Bontsema, J., Hemming, J. (2006). Toward an optimal control strategy for sweet pepper cultivation. A dynamic crop model. Acta Horticulturae, 718, 367–374. DOI 10.17660/ActaHortic.2006.718.42. [Google Scholar] [CrossRef]
172. Singh, M. C., Yousuf, A., Singh, J. P. (2016). Greenhouse microclimate modeling under cropped conditions-A review. Research in Environment and Life Sciences, 9, 1552–1557. [Google Scholar]
173. Thompson, R. B., Martinez-Gaitan, C., Gallardo, M., Gimenez, C., Fernandez, M. D. (2007). Identification of irrigation and N management practices that contribute to nitrate leaching loss from an intensive vegetable production system by use of a comprehensive survey. Agricultural Water Management, 89(3), 261–274. DOI 10.1016/j.agwat.2007.01.013. [Google Scholar] [CrossRef]
174. Cespedes, A. J., Garcia, M. C., Perez-Parra, J. J., Cuadrado, I. M. (2009). Characterization of protected horticultural exploitation of almeria. Foundation for Agrarian Research in the Province of Almeria. Almeria, Spain (In Spanish). [Google Scholar]
175. Murphy, D. V., Stockdale, E. A., Hoyle, F. C., Smith, J. U., Fillery, I. R. P. et al. (2004). Matching supply with demand. In: Hatch, D. J., Chadwick, D. R., Jarvis, S. C., Roker, J. A. (eds.Controlling nitrogen flows and losses, pp. 101–112. The Netherlands: Wageningen Academic Publishers. [Google Scholar]
176. Nendel, C., Venezia, A., Piro, F., Ren, T., Lillywhite, R. D. et al. (2013). The performance of the EU-Rotate_N model in predicting the growth and nitrogen uptake of rotations of field vegetable crops in a Mediterranean environment. Journal of Agricultural Science, 151(4), 538–555. DOI 10.1017/S0021859612000688. [Google Scholar] [CrossRef]
177. Marcelis, L. F. M. (1994). A simulation model for dry matter partitioning in cucumber. Annals of Botany, 74(1), 43–52. DOI 10.1093/aob/74.1.43. [Google Scholar] [CrossRef]
178. Hirich, A., Choukr-Allah, R., Ragab, R., Jacobsen, S. E., El Youssfi, L. et al. (2012). The SALTMED model calibration and validation using field data from Morocco. Journal of Materials and Environmental Science, 3, 342–359. [Google Scholar]
179. Hirich, A., Ragab, R., Choukr-Allah, R., Rami, A. (2013). The effect of deficit irrigation with treated wastewater on sweet corn: Experimental and modelling study using SALTMED model. Irrigation Science, 32(3), 205–219. DOI 10.1007/s00271-013-0422-0. [Google Scholar] [CrossRef]
180. Pulvento, C., Riccardi, M., Lavini, A., D’andria, R., Ragab, R. (2013). SALTMED model to simulate yield and dry matter for quinoa crop and soil moisture content under different irrigation strategies in south Italy. Irrigation and Drainage, 62(2), 229–238. DOI 10.1002/ird.1727. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |