

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013557
ARTICLE
Doubled Haploid Production Using an Improved Anther Culture Protocol for Sorghum [Sorghum bicolor (L.) Moench]
1Cereal Research Non-profit Ltd., Alsó kikötő sor 9, Szeged, 6726, Hungary
2Doctoral School of Plant Science, Szent István University, Gödöllő H, 2103, Hungary
*Corresponding Author: János Pauk. Email: janos.pauk@gabonakutato.hu
Received: 11 August 2020; Accepted: 19 October 2020
Abstract: Sorghum [Sorghum bicolor (L.) Moench] can benefit from accelerated breeding and release of improved varieties through doubled haploid technology. The technology has been used in speeding up the breeding of other major cereals such as wheat, maize and rice, for which generally widely applied optimised protocols exist. A reproducible protocol for the crop, that can overcome genotype dependency and other species-specific challenges such as phenolic exudation is however lacking. This study aimed at sorghum doubled haploids production thereby contributing to the development of an improved protocol. From the 28 hybrid genotypes, both F1 registered- and experimental hybrids involved, this study successfully produced haploids from five genotypes and subsequently, four confirmed doubled-haploid lines on W14mf medium or its modification with 1.0 gl−1 L-proline, 1.0 gl−1 L-asparagine and 1.0 gl−1 KH2PO4. Medium 190-2Cu was used for regeneration and rooting, which occurred successfully, if the calli were transferred on to it less than 7 days after induction, and temperature was maintained at 25˚C under light condition. Genotype dependency was not wholly overcome; however, sorghum’s high tillering ability and abiotic stress tolerance were observed to contribute to attainment of haploid plantlets. Spontaneous diploids producing seeds at rates of upto 80.5% were obtained, therefore eliminating the need for colchicine duplication.
Keywords: In vitro androgenesis; doubled-haploid; sorghum; anther-culture; breeding
Sorghum [Sorghum bicolor (L.) Moench] belongs to the division Magnoliophyta in the class Liliopsida and the order cyperales and is a cultivated diploid (2n = 20), tropical cereal, C4 grass with a high photosynthetic efficiency and inherent high biomass [1]. It is one of the most important cereal crops globally, ranked fifth in cultivation area after wheat, maize, rice and barley [2]. According to some authors [3,4] the crop is a staple cereal grain for many of the world’s most food insecure people. The ability of the crop to tolerate low unpredictable rainfall, and to assure yields despite poor agronomic resource conditions [5] are the major reasons why it is heavily relied upon in resource poor areas.
In Europe, sorghum is not a routinely used crop, however, as is the case in the USA, cultivars have been bred as promising energy crops [6]. The crop is however being promoted in this region as its protein digests have been shown to be safe and tolerable to patients of celiac disease, which has high prevalence in Northern Europe [7]. Some of the countries producing sorghum in Europe include Spain, France, Italy, Hungary, Bulgaria, Ukraine and Russia.
While the area under sorghum in the major regions of its production for instance in Africa may increase, yields have remained unchanged. The region under sorghum cultivation in Africa increased by 7.23% from 22.69 million hectares in 2017/2018 planting season to 24.46 million hectares in 2018/2019, while average yield decreased by 4.1% from 1.68 metric tons ha−1 to 1.61 metric tons ha−1 [8]. Sorghum breeders have therefore had their breeding objectives oriented towards the yield reducing factors such as genetically low yielding landraces, drought, striga weed, pests and diseases [9].
Sorghum breeding has achieved significant milestones, however, with modern tools, improved varieties could be released at a quicker pace. It has been observed [10] that there had only been a release of 131 new varieties in the period 1970–2010, in five countries namely Burkina-Faso, Mali, Niger, Nigeria and Senegal. Doubled haploid technology may be tapped to speed up acquisition of these improved varieties [11].
One of the techniques through which doubled haploids are produced is androgenesis, which relies on the fact that if certain conditions are met, microspores which have a haploid chromosome complement are amenable to the shift from sporophytic to embryogenic pathway in many species [12]. These conditions include but are not limited to anther, inflorescence and or donor tiller pre-temperature shock, starvation, and or other stress treatment, media composition, and growth regulators. The abundance of the microspores in plants’ anthers thereby making it relatively easy to access and manipulate is a unique advantage to this technology [12].
The development in sorghum doubled haploid technology is evidently work in progress, with some authors rightly pointing out the unavailability of a reproducible protocol [13]. The aim of this study was to regenerate sorghum haploids via androgenesis from an established breeding program and diploidise them to achieve genetic and phenotypic homozygosity, thereby contributing to the improvement of a sorghum doubled haploid development protocol.
Over a period of 24 months, i.e., summer of 2018, winter of 2018/2019, summer of 2019 and winter of 2019/2020, twenty-eight Hungarian sorghum [Sorghum bicolor (L.) Moench] genotypes (Tab. 1), including F1 registered- and experimental hybrids were planted in the field and glasshouses in summers and winters respectively. All the sorghum genotypes were obtained from the sorghum breeding program of Cereal Research Non-Profit Ltd., Szeged, Hungary. Panicles were harvested while enclosed in the leaf sheath just before their lateral expansion caused the leaf sheath to split open, in the early vacuolated, uni-nucleated stage of microspores.
Table 1: Sorghum registered F1 hybrids and experimental ones used in the trial

2.2 Pre-Treatment and Sterilization
Following confirmation of the right stage of the microspores (early vacuolated, uni-nucleated stage) using an Olympus CK-2 inverted microscope (Olympus, Southern-on-Sea, UK) at ×40 magnification (Fig. 1), panicles from the summer of 2018 were placed at 4°C for 10 days while those from the winter of the same year were placed at 4°C, 8°C and 10°C each for 7, 10 and 15 days in lighted phytotron chamber at 85% relative humidity prior to sterilization. Following the results of year 2018, the panicles from the summer of 2019 and winter of 2019/2020 were held at 10°C for 15 days prior to sterilization in the lighted phytotrons at 85% relative humidity. After their respective pre-treatment periods, panicles were removed from the leaf sheaths and sterilized by agitation in 100 ml of 4% sodium hypochlorite containing two drops of ‘Tween 20’ for 30 minutes. This was followed by 3 washes with sterilized distilled water in a laminar air flow cabinet.
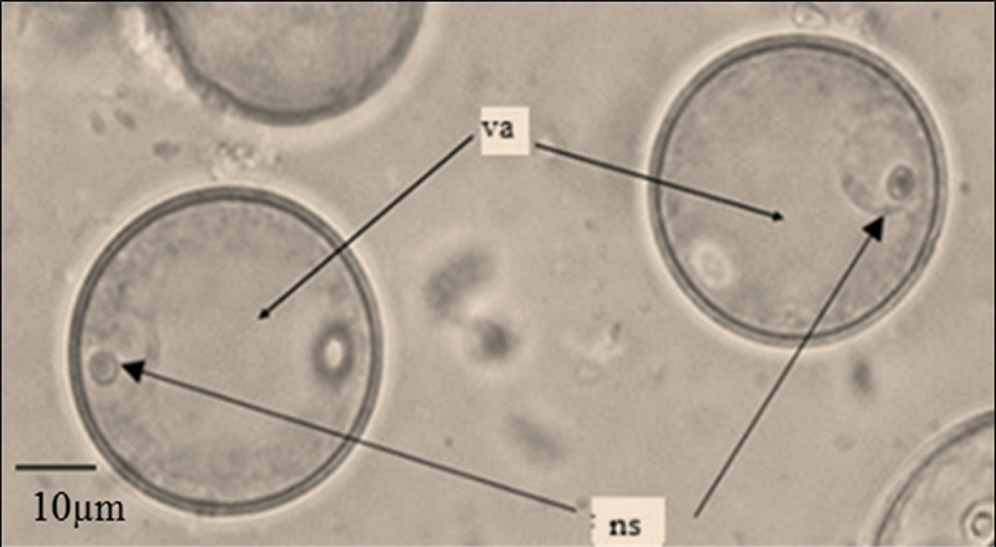
Figure 1: Sorghum bicolor (L.) Moench microspores at early vacuolated uni-nucleated stage: ns = nucleus, va = vacuole
For starvation pre-treatment trial, the isolated anthers from the summer of 2018, prior to incubation in 3 different induction media were placed in 3 replicates in 0.3 M mannitol while the controls were directly placed on the induction media, and both were placed at 32°C in a thermostat for 3 days. The anthers (33 anthers/Petri dish) in the 0.3M mannitol were then transferred to the induction media, following which all of them contained in 90 mm × 15 mm plastic Petri dishes (Sarstedt, Newton, MA, USA) were placed at 28°C in dark thermostat. Following the results of the starvation trial in year 1, anthers from the summer and winter of 2019/2020 were not placed in 0.3M mannitol, but were all placed at 32°C heat shock pre-treatment in a thermostat for 3 days before being transferred to the induction temperature of 28°C in another thermostat.
Three media (Tab. 2) including N6 medium [14], MS-t-z-2—A modified Murashige and Skoog medium [15]—and a Ficoll®400 supplemented W14mf medium [16], were used in the calli induction for the anthers from the summer of 2018. Following the results of the summer of 2018, the calli induction media used for the anthers from the winter of the same year and summer of 2019 were: W14mf (M1) as a control, W14mf supplemented with 1.0 gl−1 L-proline, 1.0 gl−1 l L-asparagine and 1.0 gl−1 KH2PO4 (M2); and w14mf supplemented with 1.0 gl−1 L-proline, 1.0 gl−1 L-asparagine but without KH2PO4 (M3). In the winter of 2019/2020, the induction media was M2, following results from the preceding summer’s work.
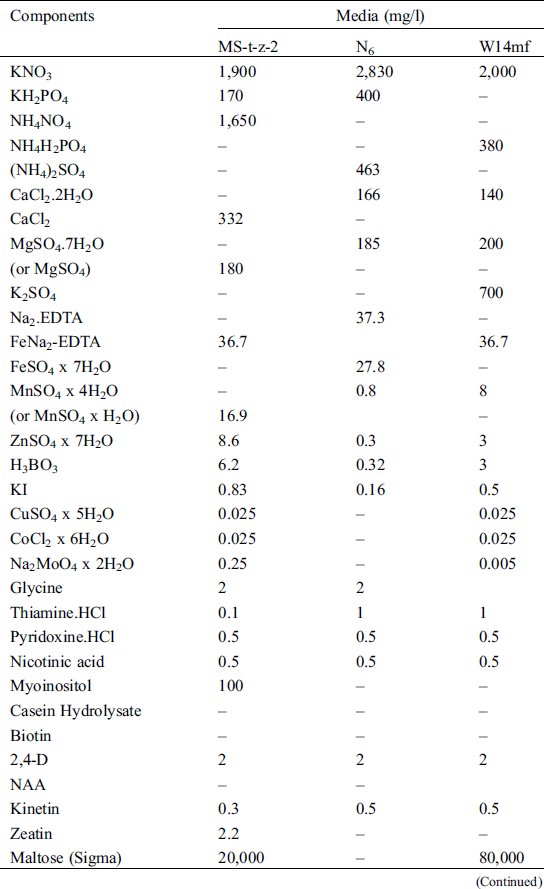
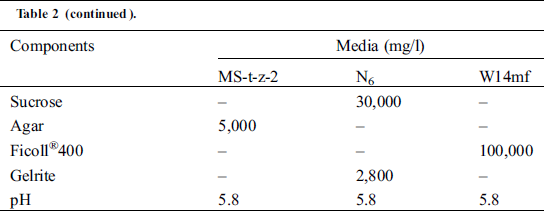
Table 2: Components of the three callus induction media in the sorghum anther culture experiment in the summer of 2018 and winter of 2018/2019
2.4 Regeneration and Hardening
The white to yellow embryogenic calli obtained from the anther culture on the induction media were regenerated on 190-2Cu medium [17] under light condition at 25°C for 20–30 days. The regenerants obtained were severally cloned and sub-cultured on the same regeneration medium. The plantlets were then transferred to a glasshouse at 4 leaf stage into sand and soil (1:1) soil mixture and put under a PVC cover for 4 days after the transfer.
2.5 Ploidy-Level Determination and Chromosome Doubling
On attainment of the 4-leaf stage, all the regenerants’ ploidy level was confirmed directly through flow cytometry. A confirmatory test involving DNA extraction and PCR with select SSR markers [18] was also conducted for some of the regenerants by determining allele sizes following Polyacrylamide Gel Electrophoresis (PAGE) on ALFExpress II DNA fragment analyser machine. Colchicine treatment was applied for two plants of ‘Róna 1’ regenerants from the induction work of the winter of 2018/2019.
The colchicine treated ‘Róna 1’ plants were then planted alongside the other regenerants of the same variety to check the level of occurrence of spontaneous diploidization after transplantation and its effectiveness in comparison with colchicine mediated diploidization in sorghum. This was done by comparison of the fertility of florets in the panicles.
Data on callus induction on the three modified W14mf media M1, M2 and M3 was analysed using Ri386 software version 3.6.2, where differences in the induction were analysed by a Kruskal-Wallis test. Histograms depicting the differences on the callus induction were drawn using Microsoft Office 365® Excel software.
Only four embryogenic calli were obtained from genotype ‘GK Zsófia’ on induction of the 33600anthers from the summer of 2018. The calli (Fig. 2) formed from anthers cultured on W14mf medium without 0.3M mannitol pre-treatment, after 18 days of incubation in darkness, the initial 3 days being at 32°C, while the rest were at 28 ± 1°C. The rest of the anthers discoloured and died on incubation for 30–35 days. All the four embryogenic calli recovered, regenerated on 190-2 Cu medium under light condition at 25 ± °C for 20–30 days and formed green plantlets.
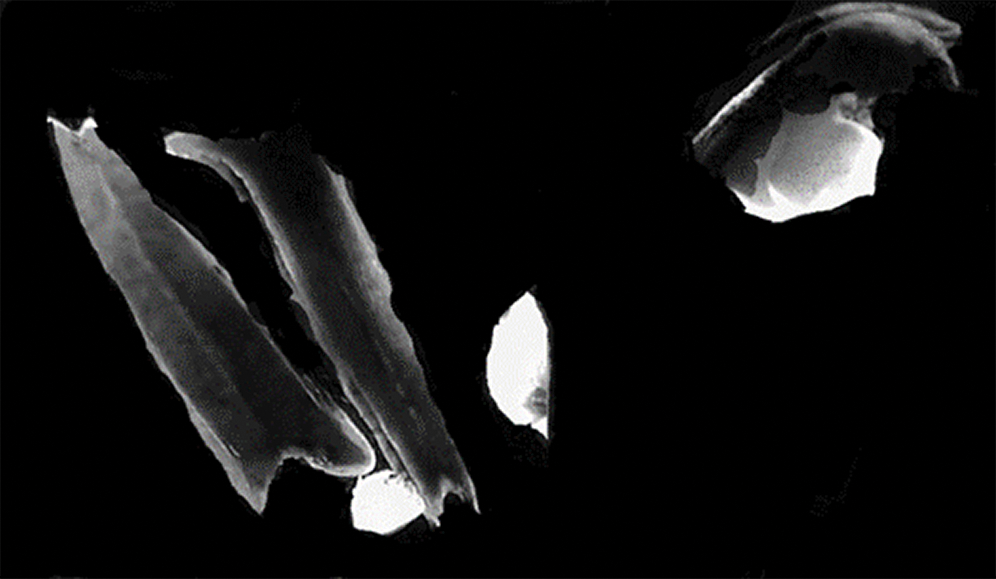
Figure 2: Embryogenic calli forming from the anther culture of sorghum in vitro
The anthers from the winter of year 1 produced five embryogenic calli from genotype ‘Róna 1’ on W14mf medium without 0.3M mannitol pre-treatment after 18 days of incubation in darkness, the initial 3 days being at 32°C, while the rest were at 28 ± 1°C. Similar to the results of the anthers incubated in the summer of the same year, the anthers that did not form calli discolored and died on incubation for 15 days. Only one of the 5 embryogenic calli from the induction work of the winter of the first year regenerated and proliferated on 190-2Cu medium under light condition at 25 ± 1°C for 20–30 days, producing 5 shoots that were subsequently cloned to produce 5 individual green plantlets. Like the regenerants from genotype ‘GK Zsófia’ earlier obtained, the regenerants from ‘Róna 1’ produced shoots first, then followed by roots on the regeneration medium.
In the callus induction work of the summer of 2019, a total of 57 calli from 9 genotypes–26 on induction medium M2, 17 on induction medium M3 and 14 on induction medium M1 were obtained (Fig. 3, Tab. 3). Out of these, 3 calli from 3 different genotypes (‘ARET×VSZ25KKD’; ‘AREL×SZE697/01’ and ‘AREL×ZSV04/30’) regenerated on 190-2Cu medium under light condition at 25 ± 1°C for 20–30 days, with the regenerant from variety ‘AREL×ZSV04/30’ being an albino. Among all the regenerants, shoots formed before the roots on the regeneration medium. All the three regenerants proliferated and produced more than five shoots each, which were cloned and transferred to fresh 190-2Cu regeneration medium on which they were developed as individual plantlets.
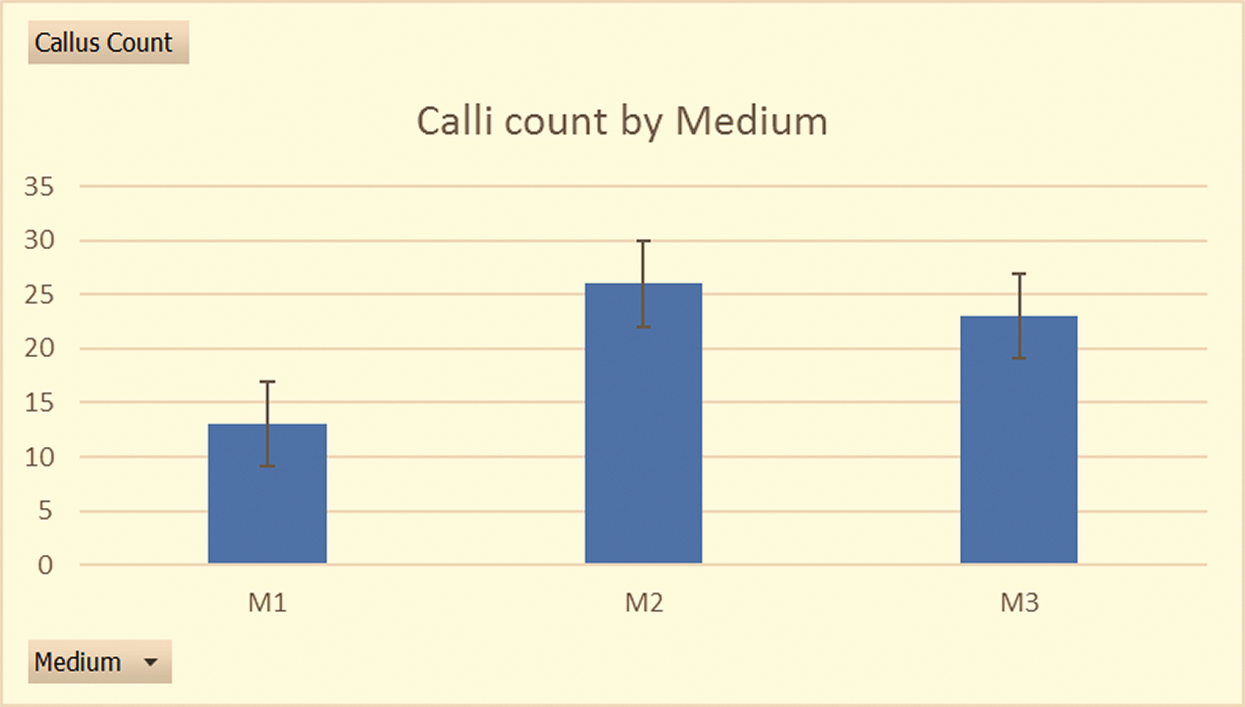
Figure 3: Differences in callus counts with different media (sample size of 100 anthers per treatment in 3 medium types across the 9 responsive genotypes): Histograms showing the cumulative callus counts across the 3 medium types in the summer of 2019
Table 3: Number of calli obtained on three anther culture media from sorghum F1 registered- and experimental hybrids in the summer and winter of 2018 and summer of 2019
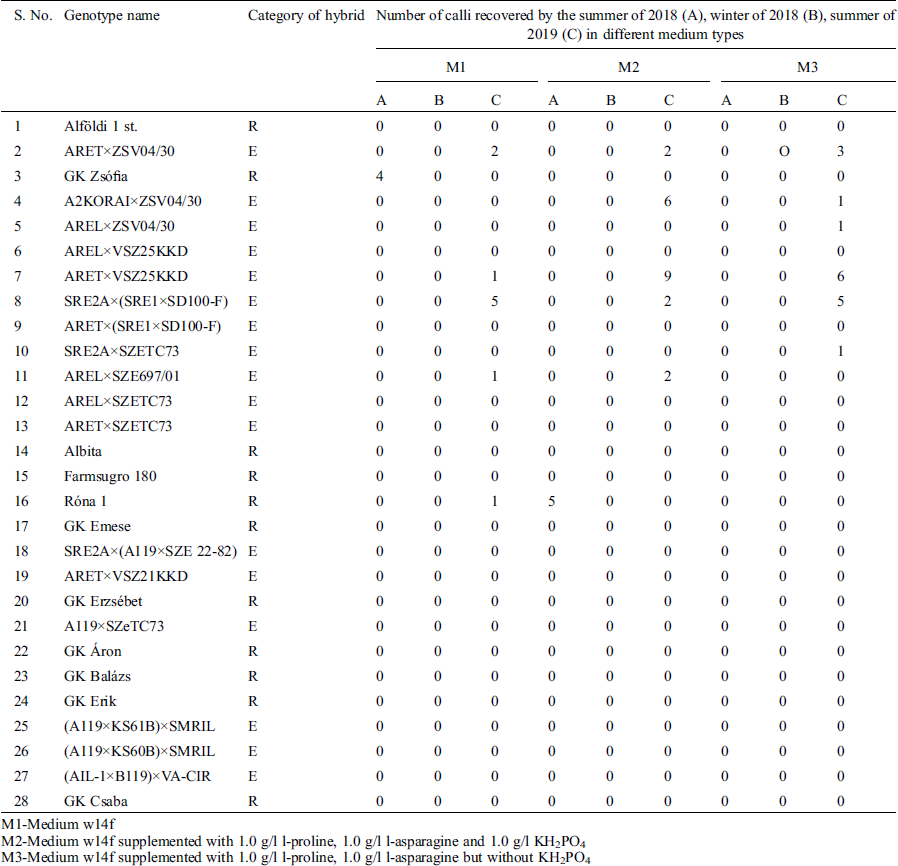
M1-Medium w14f
M2-Medium w14f supplemented with 1.0 g/l l-proline, 1.0 g/l l-asparagine and 1.0 g/l KH2PO4
M3-Medium w14f supplemented with 1.0 g/l l-proline, 1.0 g/l l-asparagine but without KH2PO4
Five embryogenic calli formed on W14mf medium as earlier explained in the induction work of the winter of 2018/2019, when anthers were pre-treated by holding them at 10°C for 15 days in a lighted phytotron chamber at 85% relative humidity prior to sterilization (Tab. 4). Subsequently, this pre-treatment temperature and duration was used during summer of 2019. In the winter of 2019/2020 however, having not obtained any major differences in the callus yield as a result of pre-treatment in the previous experiments, the anthers were induced directly upon collection from the plants.
Table 4: Evaluation of different pre-treatment temperatures in different durations for sorghum anther culture induction for registered hybrid ‘Róna 1’with a sample size of 100 anthers in 4 replications per treatment

The use of 0.3M mannitol to effect starvation pre-treatment in the three media used in the summer of 2018 did not result in any callus induction. Similarly, no callus was induced on media N6 and Mst-z-2, and therefore they were dropped in subsequent seasons, i.e., winter of 2018/2019 and both summer and winter of 2019/2020, in favour of W14mf or modified versions of W14mf (M1, M2 and M3) that gave callus yields. In the summer of 2018, W14mf resulted in the induction of 4 embryogenic calli that were obtained from genotype ‘GK Zsófia’ whose panicles were obtained from field grown plants. On medium M2, 5 embryogenic calli were induced from the anthers of ‘Róna 1’ in the winter of the same year, panicles being obtained from glasshouse grown plants. Noteworthy, in this season, callus induction from ‘GK Zsófia’ was not reproduced.
There was a significant increase in callus yield in the summer of 2019, where the induction media were M1, M2 and M3, and the anthers had been obtained from field grown plants, with a pre-treatment of 10˚C for 15 days in a lighted phytotron chamber at 85% relative humidity. Medium W14mf supplemented with 1.0 gl−1 L-proline, 1.0 gl−1 L-asparagine and 1.0 gl−1 KH2PO4 (M2) led to the highest induction (26 embryogenic calli) although a Kruskal-Wallis test conducted for the induced calli among media M1, M2 and M3 showed no significant differences (Chi square = 1.53, df = 2, P = 0.46). M1 and M3 led to the induction of calli in 6 of the 9 responsive genotypes while M2 had induction on only 5 of the 9 responsive genotypes but had the highest number of calli (9) for one individual genotype (F2 experimental hybrid ‘ARET×VSZ25KKD’). Following this outcome, a reproducibility test in the winter of 2019/2020 was done with M2 as the induction medium for only the 9 responsive genotypes. While all the 9 genotypes formed calli during this reproducibility test, only ‘AREL×ZSV04/30’ formed a green plantlet after its callus was transferred to the regeneration medium less than 7 days after induction, unlike in the previous season where the same genotypes yielded an albino plantlet.
The ploidy assessment using SSR markers for ‘Róna 1’ resulted in monomorphic alleles for all the loci tested in the regenerants and polymorphic alleles for the diploid control (Tab. 5, Fig. 4). The tests for the ploidy level of the mentioned genotypes and all other subsequent regenerants in this study was confirmed using the direct method of flow cytometry (Fig. 5).
Table 5: Polymorhic and monomrphic allele sizes as obtained from Poly Acrylamide Gel Electrophoresis (PAGE) analysis for registered hybrid ‘Róna 1’ with SSR markers for the diploid control and regenerants
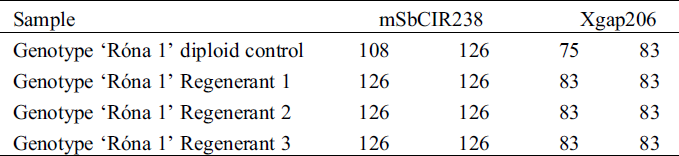

Figure 4: Gel images from molecular analysis of regenerants of genotype Róna 1 and its diploid control with SSR primer mSbCIR238: A) Results from ALFExpress II DNA fragment analyser machine showing Poly Acrylamide Gel Electrophoresis (PAGE) monomorphic alleles (126 bp) for the loci with the 3 regenerants tested; B) PAGE results showing polymorphic alleles (108 and 126 bp) for the loci with the diploid control; C) Examined various sorghum diploid genotypes (3 to 31) on ethidium bromide 2% NuSieveagarose gel with MM: Fermentas Gene Ruler 50 bp Ladder Plus. using primer mSbCIR238 with genotype Róna 1 (24) showing polymorphic alleles

Figure 5: Flow cytometry histograms of sorghum F1 experimental hybrid ‘ARET×VSZ25KKD’ haploids obtained (a) and their diploid control counterparts (b). P1 represents divided nuclear cells’ (cells at G2 phase of cell cycle) DNA complement and their corresponding coefficient of variation (CV); P2 represents ‘normal’ haploid/diploid cells’ (cells at G1 phase of cell cycle) DNA complement and their corresponding CV
Only one of the two ‘Róna 1’ regenerants that were treated with colchicine for chromosome duplication survived to heading, producing two additional tillers. The three non-colchicine treated regenerants produced at least 3 tillers each, subsequently producing panicles with varying quantities of seeds. The varying number of filled grains per panicle provided an opportunity to determine the fertility level of the individual plants: the non-colchicine treated plants grouped into 3 based on the visual observation of the seed quantity per panicle had 994, 146 and 24 as average number of grains per panicle, representing 80.5, 20.9 and 2.4% respectively as percentages of the total florets per panicle (Fig. 6). The colchicine treated plants had an average of 144 grains per panicle, representing 9.03% of the total number of florets per panicle.

Figure 6: Differential fertility levels recorded for genotype ‘Róna 1’ spontaneous di-haploid clones depicted by the number of seeds in a panicle, (a) panicle with seeds in all the florets, (b) panicle with seeds occupying partially the total number of florets, (c) panicle with very few or no seeds in the florets: ff = fertile floret; sf = sterile floret
Sorghum’s inherent tillering ability coupled with its high abiotic stress tolerance as was observed in this study, together with the appropriate induction and regeneration media, were found to be the greatest contributors to successful haploids production, if the culture conditions were set at optimum. Out of the 28 different genotypes in this study, haploids were successfully produced from five genotypes, four of which spontaneously diploidized. This was a significant improvement to earlier works [19–21] all of which reported the production of haploids from one genotype each. The success may be attributed to the W14mf basic medium that was used in the current study as opposed to the N6 and MS-t-z-2 media that had been used in the studies mentioned, but which did not yield any callus when tested in the current study.
When modified with components L-proline, L-asparagine and KH2PO4, the W14mf produced higher total number of calli compared to the control (non-modified W14mf medium), therefore agreeing with the findings [22–24] that addition of these components reduced production of phenolics and ld to higher callus induction rates in sorghum somatic tissue culture. It is however important to note that there were no significant differences observed in the callus induction among the control W14mf medium (M1) and the modified versions of it, i.e., M2 and M3. The results of the current study additionally agree with the observations [25] that the use of W14mf as an induction medium has the potential to yield a high number of embryogenic calli.
In the current study, and in agreement with an earlier finding [19], a long cool temperature stress pre-treatment—after collection of panicles—was not seen to have any effect to callus induction. Further, this study observed that collection of panicles when microspores were at mid to late uninucleate stage, as had been observed earlier [21], was more effective than the synchronization of microspores’ development effect realized by a long temperature pre-treatment. Additionally, 0.3M mannitol pre-treatment did not provide any advantage to callus induction and frequency in this work, unlike in the study [26] in androgenesis of barley, where this pre-treatment was reported as a causative for genotype independent induction-genotype dependency has been shown to inhibit efficient callus induction in sorghum [27].
Although embryogenic calli were induced for 9 genotypes in this study, genotype dependency was not entirely overcome as the several repeats of induction always resulted in induction of all or some of the nine responsive genotypes. Notably, the induction rates of the anthers from the summer season that were obtained from field grown crops were higher than those in the winter season obtained from crops grown in a glasshouse, therefore corroborating a study [28] that reported 6.4% and 3.7% sorghum callus induction for field anthers and glasshouse anthers respectively. When the calli were transferred on to the regeneration medium not more than 7 days after induction, there was more than 50% probability of regeneration. Further this study observed that the ideal temperature for sorghum regeneration under light condition was 25°C, like in an earlier finding [21]. In the reproducibility study in the winter of 2019/2020 of the current study, ‘AREL×ZSV04/30’ which had regenerated into an albino plantlet in the previous season formed a green plantlet when the phase from induction to regeneration was maintained at below 7 days. These findings were therefore in agreement with those of a research [20] that found the regenerations of albino plantlets or roots only in a medium to be greatly reduced with a reduced phase in the induction medium. In addition, the current study arrived at a similar conclusion to the aforementioned research [20], that calli forming roots before shoots had very dismal chances of developing into shoots and therefore regenerating into plantlets.
The haploids identified through flow cytometry were found to be monomorphic in the tested loci while diploids were polymorphic following the PCR confirmatory test with SSR markers. ‘Róna 1’ clones spontaneously diploidized and produced seeds at 80.5, 20.9 and 2.4%. The colchicine treated individual produced seeds at 9.03%, indicating that it was a partially fertile plant. This was indicative that enough fertility was obtained spontaneously by the di-haploids.
Acknowledgement: The authors are greatly indebted to the management and staff of Cereal Research Con., Ltd., Szeged, as well as the laboratory technical team at Szent István University, Gödöllő whose contribution to the actualization of this work was invaluable.
Funding Statement: This work was supported through the Stipendium Hungaricum Scholarship to the first author, János Bolyai Research Scholarship of the Hungarian Academy of Sciences, as well as research grants (TUDFO/51757/2019-ITM and K_16-K119835).
Conflicts of Interest: The authors declare that they have no conflict of interest to report regarding this present study.
1. Xu, H., Ding, A., Chen, S., Marowa, P., Wang, D. et al. (2018). Genome-wide analysis of sorghum GT47 family reveals functional divergences of MUR3-like genes. Frontiers in Plant Science, 9, 1775. DOI 10.3389/fpls.2018.01773. [Google Scholar] [CrossRef]
2. Druille, M., Williams, A. S., Torrecillas, M., Kim, S., Meki, N. et al. (2020). Modeling climate warming impacts on grain and forage sorghum yields in Argentina. Agronomy, 10(7), 964. DOI 10.3390/agronomy10070964. [Google Scholar] [CrossRef]
3. Lahouar, A., Marin, S., Crespo-Sempere, A., Saïd, S., Sanchis, V. (2016). Effects of temperature, water activity and incubation time on fungal growth and aflatoxin B1 production by toxinogenic Aspergillus flavus isolates on sorghum seeds. Revista Argentina de Microbiología, 48(1), 78–85. DOI 10.1016/j.ram.2015.10.001. [Google Scholar] [CrossRef]
4. Sannagoudar, M. S., Patil, R. H., Kumar, R. V., Singh, A. K., Ghosh, A. et al. (2020). Simulated impacts of rise in temperature on kharif sorghum genotypes in Northern Transitional Zone of Karnataka, India. Cereal Research Communications, 48(1), 113–120. DOI 10.1007/s42976-020-00011-6. [Google Scholar] [CrossRef]
5. Hadebe, S. T., Mabhaudhi, T., Modi, A. T. (2017). Water use of sorghum (Sorghum bicolor L. Moench) in response to varying planting dates evaluated under rainfed conditions. Water SA, 43(1), 91. DOI 10.4314/wsa.v43i1.12. [Google Scholar] [CrossRef]
6. Paschalidou, A., Tsatiris, M., Kitikidou, K. (2016). Energy crops for biofuel production or for food?—SWOT analysis (case study: Greece). Renewable Energy, 93, 636–647. DOI 10.1016/j.renene.2016.03.040. [Google Scholar] [CrossRef]
7. Singh, P., Arora, A., Strand, A., Leffler, A., Catassi, C. et al. (2018). Global prevalence of celiac disease: Systematic review and meta-analysis. Clinical Gastroenterology and Hepatology, 16(6), 823–836.e2. DOI 10.1016/j.cgh.2017.06.037. [Google Scholar] [CrossRef]
8. FAS/USDA–Foreign Agricultural Service/United States Department of Agriculture. (2020). Global Market Analysis. 2020. https://apps.fas.usda.gov/psdonline/circulars/production.pdf. [Google Scholar]
9. Tack, J., Lingenfelser, J., Jagadish, S. V. (2017). Disaggregating sorghum yield reductions under warming scenarios exposes narrow genetic diversity in US breeding programs. Proceedings of the National Academy of Sciences of the United States of America, 114(35), 9296–9301. DOI 10.1073/pnas.1706383114. [Google Scholar] [CrossRef]
10. Ndjeunga, J., Mausch, K., Simtowe, F. (2015). Assessing the effectiveness of agricultural R&D for groundnut, pearl millet, pigeonpea and sorghum in West and Central Africa and East and Southern Africa. International Crops Research Institute for the Semi-Arid Tropics, pp. 123–147. Niger. [Google Scholar]
11. Sharma, P., Chaudhary, H. K., Manoj, N. V., Kumar, P. (2019). New protocol for colchicine induced efficient doubled haploidy in haploid regenerants of tetraploid and hexaploid wheats at In vitro level. Cereal Research Communications, 47(2), 356–368. DOI 10.1556/0806.47.2019.09. [Google Scholar] [CrossRef]
12. Wędzony, M., Forster, B. P., Żur, I., Golemiec, E., Szechyńska-Hebda, M. et al. (2009). Progress in doubled haploid technology in higher plants. In: Touraev, A., Forster, P., Jain, M. (eds.Advances in haploid production in higher plants, pp. 1–33. Dordrecht: Springer. [Google Scholar]
13. Teingtham, K. (2017). Is doubled haploid production in sorghum impossible? King Mongkut’s University of Technology North Bangkok (KMUTNB). International Journal of Applied Science and Technology, 10, 247–256. [Google Scholar]
14. Chu, C., Wang, C., Sun, C., Chen, H., Yin, K. (1975). Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Scientia Sinica, 18, 659–668. [Google Scholar]
15. Murashige, T., Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497. DOI 10.1111/j.1399-3054.1962.tb08052.x. [Google Scholar] [CrossRef]
16. Puolimatka, M., Pauk, J. (2000). Effect of induction duration and medium composition on plant regeneration in wheat (Triticum aestivum L.) anther culture. Journal of Plant Physiology, 156(2), 197–203. DOI 10.1016/S0176-1617(00)80306-5. [Google Scholar] [CrossRef]
17. Pauk, J., Mihály, R., Monostori, T., Puolimatka, M. (2003). Protocol of triticale (x Triticosecale Wittmack) microspore culture. In: Maluszynski, M., Kasha, J., Forster, P., Szarejko, I. (eds.Doubled haploid production in crop plants, pp. 129–134. Dordrecht: Springer. [Google Scholar]
18. Billot, C., Ramu, P., Bouchet, S., Chantereau, J., Deu, M. et al. (2013). Massive sorghum collection genotyped with SSR markers to enhance use of global genetic resources. PLoS One, 8(4), e59714. DOI 10.1371/journal.pone.0059714. [Google Scholar] [CrossRef]
19. Rose, J., Dunwell, J., Sunderland, N. (1986). Anther culture of Sorghum bicolor (L.) Moench. Plant Cell, Tissue and Organ Culture, 6(1), 15–22. DOI 10.1007/BF00037754. [Google Scholar] [CrossRef]
20. Wen, F., Sorensen, E., Barnett, F., Liang, G. (1991). Callus induction and plant regeneration from anther and inflorescence culture of Sorghum. Euphytica, 52(3), 177–181. DOI 10.1007/BF00029394. [Google Scholar] [CrossRef]
21. Kumaravadivel, N., Sree-Rangasamy, S. (1994). Plant regeneration from sorghum anther cultures and field evaluation of progeny. Plant Cell Reports, 13(5), 286–290. DOI 10.1007/BF00233321. [Google Scholar] [CrossRef]
22. Chege, P., Palágyi, A., Lantos, C., Kiss, E., Pauk, J. (2020). Improved culture media for embryogenic callus generation in sorghum [Sorghum bicolor (L.) Moench]. Phyton-International Journal of Experimental Botany, 89, 111–119. [Google Scholar]
23. Liu, G., Gilding, E. K., Godwin, I. D. (2015). A robust tissue culture system for sorghum [Sorghum bicolor (L.) Moench]. South African Journal of Botany, 98, 157–160. DOI 10.1016/j.sajb.2015.03.179. [Google Scholar] [CrossRef]
24. Elkonin, L. A., Pakhomova, N. V. (2000). Influence of nitrogen and phosphorus on induction embryogenic callus of sorghum. Plant Cell, Tissue and Organ Culture, 61(2), 115–123. DOI 10.1023/A:1006472418218. [Google Scholar] [CrossRef]
25. Lantos, C., Pauk, J. (2016). Anther culture as an effective tool in winter wheat (Triticum aestivumL.) breeding. Генетика, 52, 910–918. [Google Scholar]
26. Kasha, K., Hu, T., Oro, R., Simion, E., Shim, Y. (2001). Nuclear fusion leads to chromosome doubling during mannitol pretreatment of barley (Hordeum vulgare L.) microspores. Journal of Experimental Botany, 52, 1227–1238. [Google Scholar]
27. Flinn, B., Dale, S., Disharoon, A., Kresovich, S. (2020). Comparative analysis of in vitro responses and regeneration between diverse bioenergy sorghum genotypes. Plants, 9(2), 248. DOI 10.3390/plants9020248. [Google Scholar] [CrossRef]
28. Can, N., Nakamura, S., Haryanto, T., Yoshida, T. (2015). Effects of physiological status of parent plants and culture medium composition on the anther culture of sorghum. Plant Production Science, 1(3), 211–215. DOI 10.1626/pps.1.211. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |