

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013247
ARTICLE
Character Identification of an Early Flowering Mutant
1College of Life Sciences, Jilin Agricultural University, Changchun, 130118, China
2College of Agronomy, Jilin Agricultural University, Changchun, 130118, China
*Corresponding Authors: Shuyan Guan. Email: 18722126836@189.cn; Yiyong Ma. Email: m18404319202_1@126.com
Received: 30 July 2020; Accepted: 19 November 2020
Abstract: The concept of gene-function-genetic trait was introduced to explore the effects of early flowering on the growth and development of maize at the jointing stage and to obtain early flowering mutants using ethyl methanesulfonate mutagenesis. First, we studied gene expression, phytohormones, and lignin content to explore the physiological peculiarities of the early flowering mutant. Then we analyzed the genetic features of the mutants during the jointing stage by measuring physiological and biochemical indices of drought tolerance. The results showed that the photosynthetic rate of the mutant was significantly higher than that of the control and the rate of accumulation of dry matter was rapid. In addition, the lignin content increased while drought resistance diminished. Therefore, we concluded that early flowering leads to faster overall growth and development.
Keywords: Maize; jointing stage; genetic analysis; resistance
Maize (Zea mays L.) is one of the most versatile emerging crops with crucial strategic resources [1]. It was first grown in Central and South America and then widely distributed throughout the United States, China, Brazil, and other countries.
Studies on the period of maize flowering have attracted attention in research. Sunshine duration during the maize flowering period has been reported by several studies to affect its growth, development, and yield. In recent years, with the development and integration of genomics and genetics, biologists have conducted preliminary studies on the regulatory mechanisms involved in maize flowering and have cloned and studied multiple maize flowering regulatory genes. Some of these genes, CONZ1 (CON-STANS 1), ZCN8, and ZmCCT, are mainly related to the pathways of floral organ development, photoperiod response, hormone synthesis and transmission, florigen regulation, and abiotic stress. Maize yields are severely affected by sunshine duration in northeast China, and it is important and urgent to cultivate high-quality maize varieties that are less affected by sunshine duration. For example, mutation breeding in maize shows great advantages over conventional breeding because the breeding period is short, resulting in fast and stable inheritance. Studies on mutants may further clarify the effects of changes in flowering time and other characteristics on the development and growth of maize. Sunshine duration has been reported to affect the growth and development, dry matter accumulation, water content, and seed formation of maize [2].
In this study, early flowering mutants were produced using the ethyl methanesulfonate (EMS) mutagenesis method. We analyzed the photosynthesis and respiration rates, drought resistance, and other genetic traits to explore the effects of changes in maize flowering periods on growth and development. At the same time, the mutants provide new plant material for the development of maize germplasm.
2.1 Materials and Field Design
Materials
D12164 was based on S121 × 9h164 in 2007. The plant height was approximately 193 cm, and the primary ear position was at 72 cm. The whole plant had 19 leaves; the ear was 18 cm long and 4.2 cm thick. It required 128 days from emergence to maturity (Tab. 1).
Table 1: Phenotypic differences

Equipment: Grains analyzer (NIRS DS2500), Photosynthesis meter
The M12164 mutant was obtained from D12164 induced by EMS. The plant height was approximately 188 cm, and the ear position was 64 cm. The whole plant had 15 leaves; the ear was 14 cm long and 3.2 cm thick. It required 108 days from emergence to maturity. This experimental M12164 mutant is the M4 generation.
The maize seeds for the early flower mutant M12164 (M) and the wild type D12164 (CK) were provided by the Center for Biotechnology Research of the Jilin Agricultural University.
Field design
The D12164 and M12164 seeds were sown 30 cm apart in a 5 m long drought resistant shed. The drought resistance treatments were administered when the plants grew to the V2 stage (June 15). On June 15, 18, 21, and 24, samples of D12164 and M12164 leaves were simultaneously taken every 3 days as materials for subsequent experiments.
2.2.1 Investigation of Gene Expression Using RT-PCR
During the jointing period, on June 22 and 27 and July 2, 7, 12, and 17, samples were taken from the eighth leaf from the ground in the M12164 and D12164 plants for quantitative fluorescence analysis. First, maize RNA was extracted using the TRIzol method, and the integrity of the RNA was detected by 1% agarose gel electrophoresis. The concentration and purity of the RNA were detected using a spectrophotometer through which high-quality RNA was selected and stored in a refrigerator at −40°C along with the cDNA obtained by reverse transcription. Then, we used Primer 5.0 (Primer design software) to design primers (Tab. 2). Finally, the amplification reactions were performed using a fluorescence quantitative PCR instrument. The experiments were performed with three biological replicates.

2.2.2 Determination of Photosynthetic Rate
The photosynthesis rate of the M12164 and D12164 plants was measured in samples from the eighth leaf from the ground on June 22, July 2, and July 12. The temperature of the maize leaves and the photosynthetic system (LI-6400;-LI-COR Biosciences, USA) were set at 25°C, and measurements were taken at 8:00, 10:00, 12:00, 14:00, and 16:00 h. the measurements were performed in triplicates.
2.2.3 Determination of Dry Matter Accumulation Rate
The dry matter accumulation rate was measured daily on June 22−25, July 1−4, and July 10−13. Samples were taken from three plants at the same growth stage, and the plant tissue was subjected to heating in an oven for 30 min at 110°C and drying at a constant temperature of 70°C. Finally, the dry matter content was measured, and the accumulation rates were calculated. There were three biological replicates.
2.2.4 Measurement of Hormone Content
On June 22, July 2, and July 12, M12164 and D12164 leaves were sampled to determine the content of three hormones, IAA, GA3, and ABA.
The hormones were extracted using a solvent mixture of methanol, ethyl acetate, and formic acid (50:50:1). The ELISA method was used for hormone determination, following the manufacturer’s instructions for the IAA, GA3, and ABA kits. The samples were labeled and placed in the instrument for hormone content determination.
2.2.5 Determination of Lignin Content
The Klason method described by Fan et al. [3] was used to assess the lignin content of the corn stems on June 22, July 2, and July 12. The measurements were performed in triplicates.
2.2.6 Biochemical and Physiological Indices
The biochemical and physiological indices of the eighth leaf from the ground were measured on June 15, 18, 21, and 24. The soluble protein content (SP) was calculated using the Coomassie brilliant blue method as described by Bradford [4], the malondialdehyde (MDA) content was determined using the thiobarbituric acid method, and the proline (Pro) content was estimated with the sulfosalicylic acid extraction-pentanone method as described by Bates et al. [5]. The relative conductivity was determined using the immersion method. The measurements were performed in triplicates.
For measurements of relative conductivity, leaves of the same size were sampled and washed with tap water, rinsed thrice with distilled water, and dried with filter paper. Three fresh samples of leaf tissue (aside from the midrib) were cut into strips of appropriate length and immediately weighed. One-gram samples were placed in 10 mL graduated test tubes containing deionized water, covered with a glass stopper, and soaked for 12 h at room temperature. The conductivity (R1) of the extracts was measured with a conductivity meter. The extracts were heated in a boiling water bath for 30 min, cooled to room temperature, shaken well, and the conductivity (R2) was measured again. Relative conductivity = 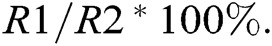
SPSS 13 was used for data processing, ANOVA was used for statistical analysis, and the heat map was constructed using Origin (2019b 32bit).
3.1 Clustering Analysis of Related Genes in Jointing Period
Based on field observations, the growth status of the M12164 mutants and CKs were the same at the seedling stage, whereas the growth rate of the mutants was higher than that of CKs after the jointing stage. For gene fluorescence quantitative analysis, samples from 8−9 mutant leaves were taken at the jointing stage every 5 days (Fig. 1). The genes analyzed included the photosynthesis-related genes Zmpck1, NADP-ME, and PPDK; sugar transport-related genes ZmGPT2 and ZmSPS1; cell division-related genes Zmrr3, Zmpp2c3, ZmMPK7, and ZAP1; and lignin synthesis promotion-related genes ZmCOMT1, ZmCCOAOMT2, and ZmCCR. Fig. 1 shows that each gene was not continuously highly expressed.
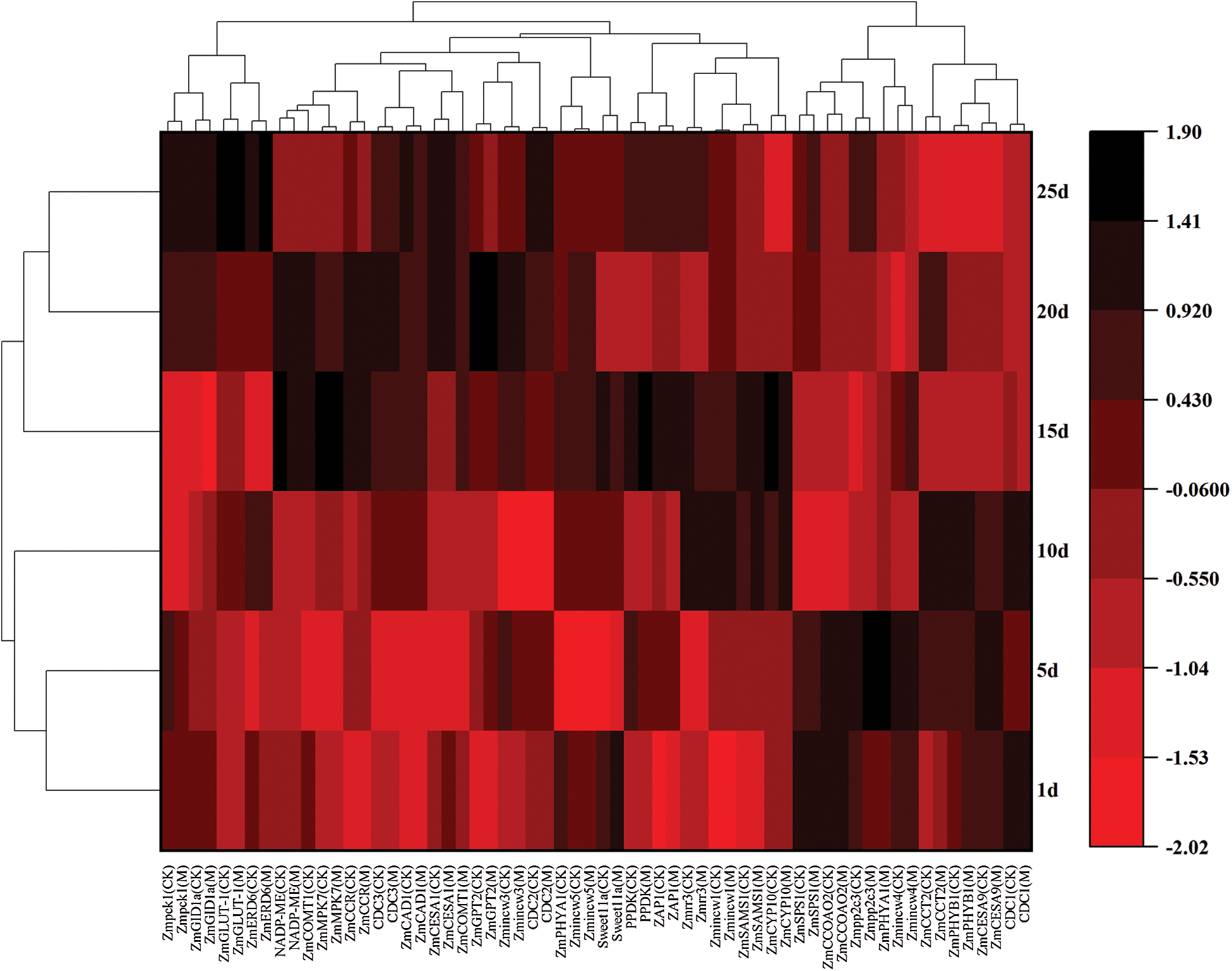
Figure 1: Heatmap of gene cluster analysis
The expression of Sweet11a, ZmSPS1, ZmCCOAOMT2, Zmpp2c3, ZmPHYA1, Zmincw4, ZmCCT2, ZmPHYB1, ZmCESA9, and CDC1 genes were increased at 1−10 days and that of Zmpck1, NADP-ME, ZmMPK7, PPDK, ZmGPT2, Zmrr3, ZAP1, Zmincw1, Zmincw3, and Zmincw5 at 10−20 days. The expression of ZmGLUT-1, ZmERD6, CDC2, CDC3, ZmGID1a, ZmCESA1, ZmCOMT1, ZmCCR, ZmCAD1, ZmSAMS1, and ZmCYP10 increased at different periods. When the maize plants were in the vigorous growth stage, the expression of genes analyzed at later periods decreased at the upper-middle level.
Fig. 2 shows the growth status of CK and M12164 on July 20. It can be seen that M12164 has grown tassels, and both corn stalks have grown rapidly

Figure 2: Developmental differences between M12164 and D12164
3.2 Determination of Major Genetic Traits
Fig. 3 shows that through gene-function-genetic trait heterogeneity analysis, differentially expressed gene functions are involved in CO2 enrichment, catalytic CO2 and lignin syntheses, plant development and defense, catalytic PEP production, cell division, sugar signaling and flower development, sucrose transport, glucose transport, and plant stress tolerance. The corresponding genetic traits are photosynthesis, respiration, lignin content, plant resistance, plant hormone content, and dry matter accumulation. The genetic features are analyzed in the following section.
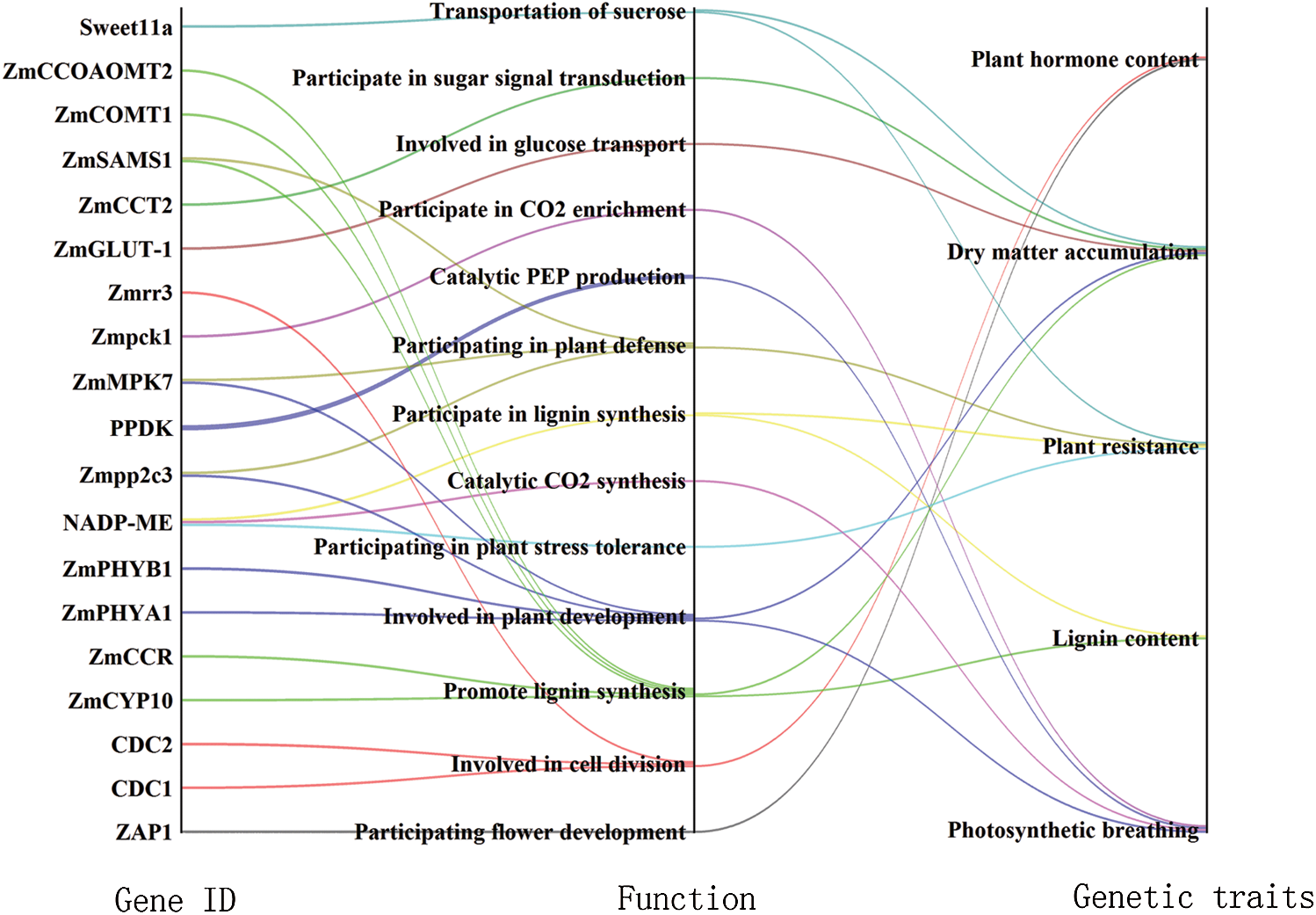
Figure 3: Gene-function-genetic trait network diagram
3.3 Physiological and Biochemical Index Analyses
3.3.1 Determination of Photosynthetic Rate and Dry Matter Accumulation Rate of Maize
Fig. 4 shows that the photosynthetic rates of maize leaves vary with the growth period. The results indicated that the M12164 respiration rate was close to that of CK, where both peaks appear. The respiration rate of both reached its peak on July 2, whereas the photosynthetic rate of M12164 was higher than that of CK throughout the jointing stage.

Figure 4: Photosynthetic rate at three dates Note: *, ** represent significant difference at 5% and 1% levels, respectively
As shown in Fig. 5, the dry matter accumulation rate between M and CK showed significant differences between June 22−25 and July 10−13. There was a considerable change in the dry matter accumulation rate during July 1–4. As per the correlation analyses, there was no substantial difference between photosynthetic rate and dry matter accumulation rate, which was 0.828.
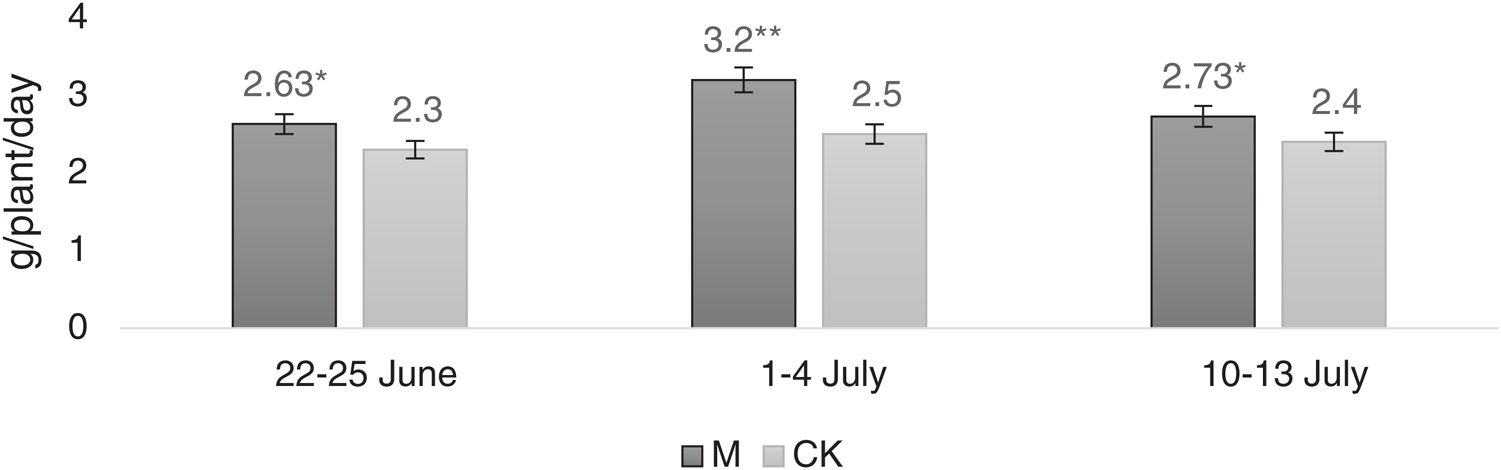
Figure 5: Dry matter accumulation rate at three periodsNote: *, ** represent significant difference at 5% and 1% levels, respectively
Fig. 6 shows the content of the three hormones. GA3 was 7−10 times higher than IAA and ABA. Changes in the three hormones were significant for the entire duration of the jointing stage. The highest contents for IAA was 0.87 µg/g FW on June 22, for GA3 was 6.7 µg/g FW on July 12, and for ABA was 0.33 µg/g FW on July 2. Overall, the hormone was higher than that of CK.
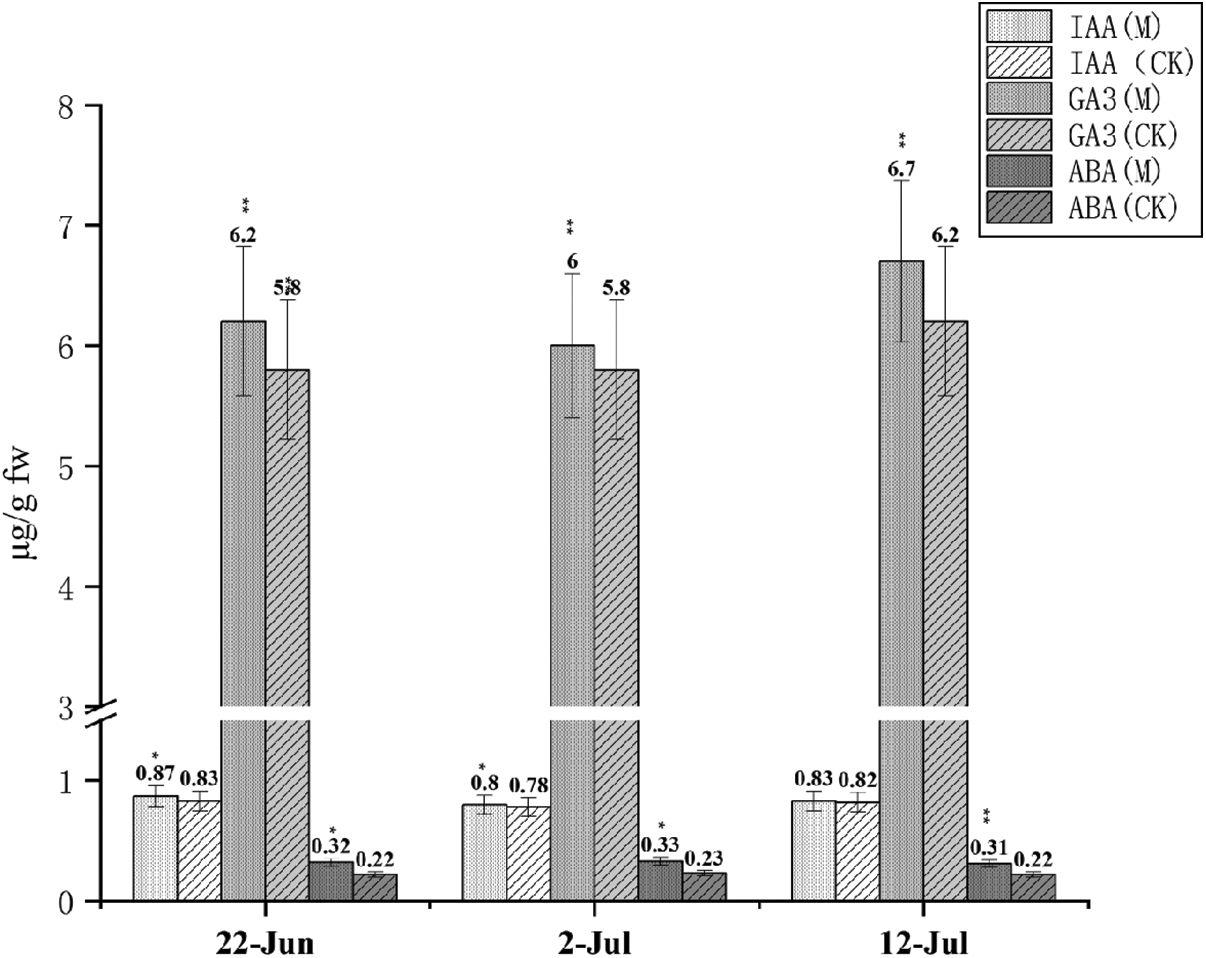
Figure 6: IAA, GA3 and ABA content at three dates Note: *, ** Represents significant difference at 5% and 1% levels, respectively
3.3.3 Determination of Lignin Content
As shown in Fig. 7, the lignin content of M and CK were measured at different times during the jointing stage. The lignin content of M increased at varying degrees relative to CK. The lignin content of CK increased by 1.52% and 16.63% on June 22, July 2, and July 12, whereas that of M12164 was 6.1% and 20.67%, respectively. Therefore, M12164 accelerates the synthesis of lignin to ensure more vigorous growth during the jointing stage.
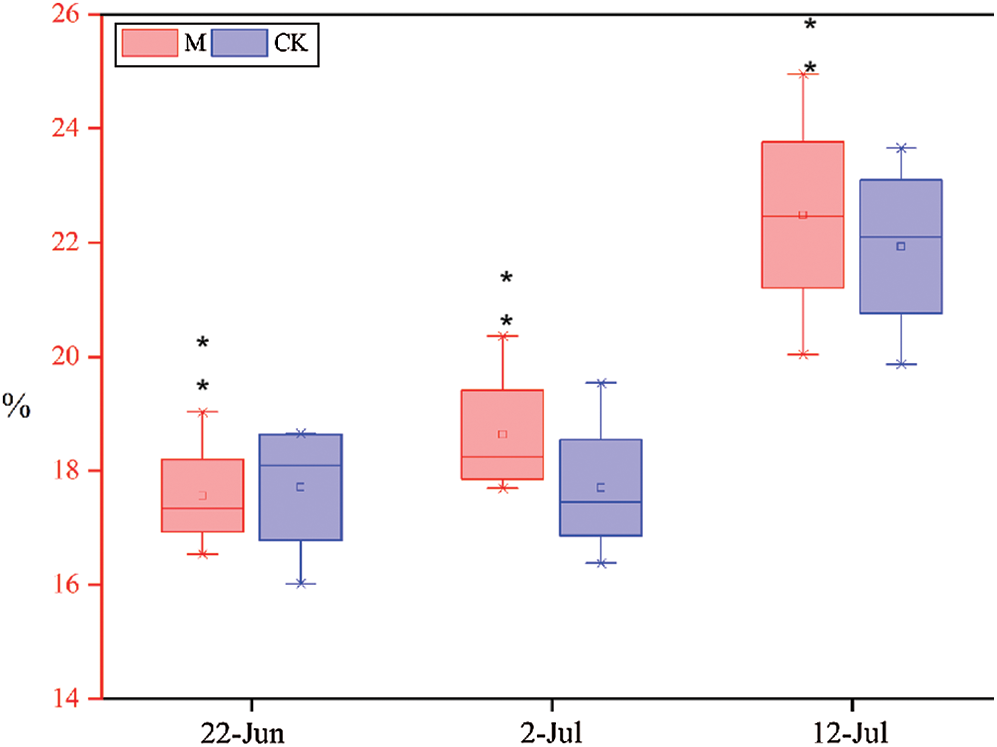
Figure 7: Lignin content at three dates Note: *, ** represents significant difference at 5% and 1% levels, respectively
3.3.4 Determination of Physiological and Biochemical Indicators of Droughts
The effect of drought on maize flowering has been confirmed. The drought-escape response accelerates flowering in response to drought stress, allowing maize to adaptively shorten its life cycle to ensure seed production. Because M12164 is an early flowering mutant, it is assumed that its drought resistance decreases owing to the advancement of the flowering period. The drought resistance of M12164 was tested to verify this hypothesis.
Proline and SP Contents
As shown in Fig. 8, the proline and SP content trends were similar in M and CK. The highest values were noted on day 21, which were 105.63 and 113.75 µg/g FW for M and CK, respectively, followed by which the values began to decrease but were generally at the upper-middle level. The changes in contents for M12164 were always lower than those of CK.
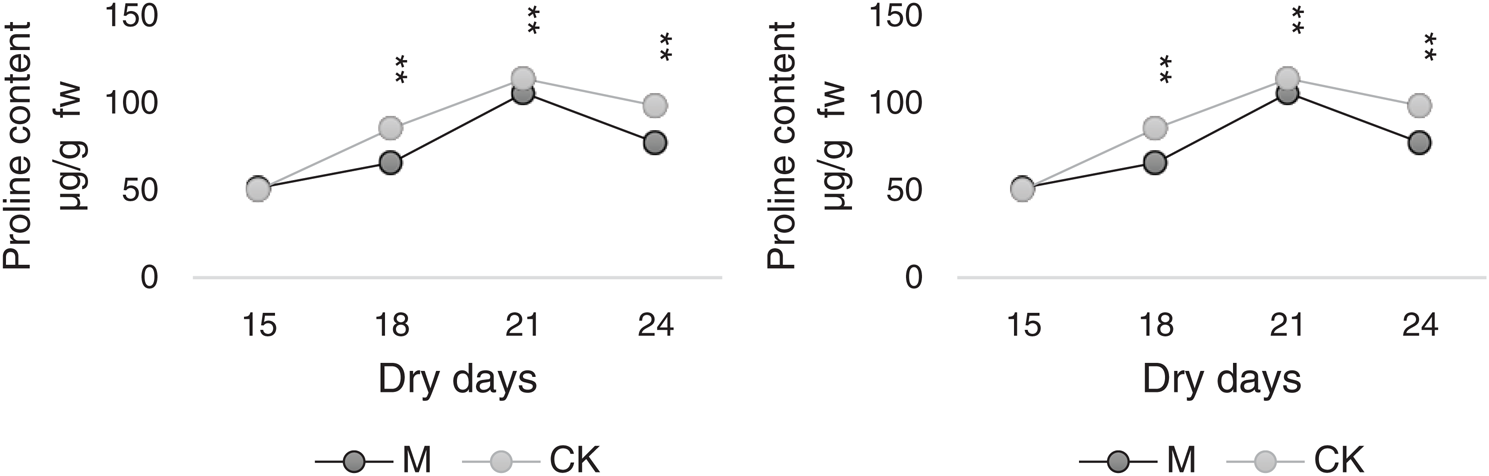
Figure 8: Dynamic diagrams for proline and soluble protein content Note: *, ** Represents significant difference at 5% and 1% levels, respectively
SPs are relevant osmotic regulators and nutrients that can improve the water-retaining capacity of cells and play a protective role in cell life and biofilms. Fig. 8 shows that the changing trends of proline and SP content in M and CK were similar. The variations were not significant between M and CK but were slightly higher in CK than in M12164.
Determination of MDA and Relative Conductivity of MDA and Leaves
Fig. 9 shows that the relative conductivity of leaves in M12164 and CK maize seedlings increased with drought time, with the highest values appearing on days 24 and 21, respectively. It also indicates that the MDA content increases, resulting in minor changes in its level at later stages; however, the degree of damage to the cells did not increase.
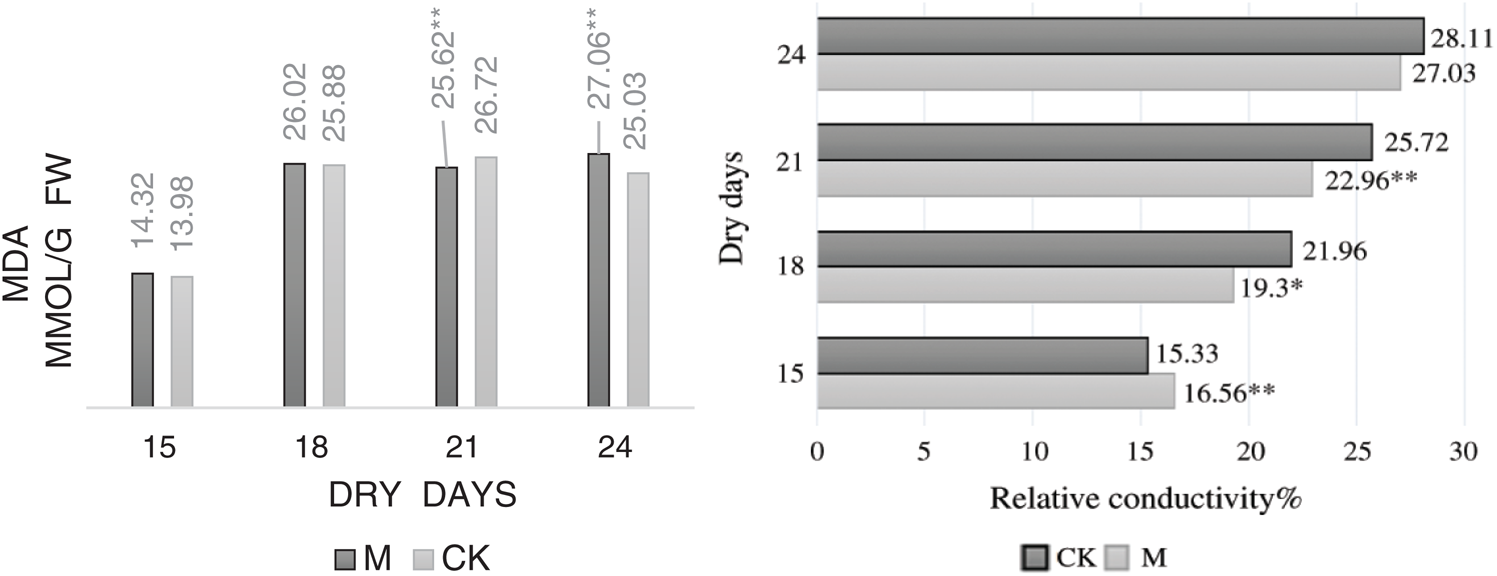
Figure 9: Dynamics malondialdehyde content and relative conductivity Note: *, ** Represents a significant difference at 5% and 1% levels, respectively
The growth stages of maize are primarily divided into vegetative and reproductive, and the flowering stage plays a watershed role [6]. However, the accumulation of dry matter in maize is closely related to and largely determined by photosynthesis, and therefore, scarcity of light severely restricts maize production [7]. Quantitative gene analysis showed that at the jointing stage, the Zmpck1 genes are controlled by light [8]. The PPDK genes in cytoplasm and chloroplasts catalyze the ATP production of PEP [9]. Additionally, the NADP-ME genes are closely related to NADPH and CO2 [10]; both play an important role in photosynthesis. The ZmSPS1 genes are essential for the synthesis of sucrose [11], whereas the transposable Zmrr3 genes significantly affect the development of the leaf sequence [12]. ZmMPK7 participates in ABA transduction pathways to facilitate the distribution of dry matter and stimulate plant growth [13]. According to gene clustering analyses, these genes were highly expressed at the jointing stage, during which the maize plants exhibited vigorous growth. Compared with the increased photosynthesis rate in mutants at different periods, the above two results suggested that earlier flowering led to faster growth and development at the jointing stage. Du et al. confirmed that ABA plays an important role in regulating flowering in rice [14]. Under drought stress, ABA regulates flowering-related genes to promote flowering, thus producing feedback effects on light perception and the biological clock [15].
The drought-escape response accelerates flowering in response to drought stress, allowing plants to adaptively shorten their life cycle. Proline can be accumulated in many plants under stress conditions such as droughts and salinity [16]. The accumulated proline serves as an osmotic regulator in plant cytoplasm and plays a vital role in cellular regulation by stabilizing biological macromolecule structures, reducing cell acidity, eliminating ammonia toxicity, and redox, thus acting as an energy reservoir [17]. Determination the level of soluble proteins in plants can be used to measure plant resistance; they can increase under external environmental stress, thereby making plants adaptable [18]. Hwang et al. [19] have confirmed that abf3 and abf4 promote flowering in Arabidopsis under drought stress. By comparing relative physiological and biochemical indices to identify drought resistance, Guo et al. [20] and He et al. [21] have reported that drought resistance in mutants is reduced, suggesting that mutants are sensitive to drought, especially at the seedling stage.
Current experimental studies have demonstrated that the early flowering traits in mutants produced by EMS can be stably passed on to their offspring. Genetic analyses showed that early flowering at the jointing stage resulted in high expression of genes associated with photosynthesis, cell division, sugar transport, and other functions in maize. Furthermore, increased photosynthesis enhanced the accumulation of dry matter. However, the drought resistance in mutant seedlings is reduced, whereas the mutants derived from EMS could enrich maize germplasm resources.
Acknowledgement: We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author Contribution: Zhenzhong Jiang are responsible for compiling the paper. Peng Jiao, Zhuo Qi and He Zhao visualization of the data. Jing Qu and Piwu Wang provide the experimental site. Siyan Liu and DanYao are responsible for technical support and drug ordering. Yiyong Ma and Shuyan Guan field phenotype survey.
Funding Statement: The research was awarded the Jilin Provincial Natural Science Foundation Project [20190201168JC], Jilin Province Science and Technology Development Plan Project [20170204005NY], Jilin Province Key Technology R&D Project [20180201029NY] and Jilin Province Support for the Science and Technology Development Program [20190802012ZG].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Zhao, T. C., Zhao, X. D., Shu, S. L., Guo, X. S. Hu, L. et al. (2016). Creation of new species of maize with gene cry1Ab13. Journal of South China Agricultural University, 37(5), 31–37. [Google Scholar]
2. Ze, H. W., Bai, C. R., Bin, Z., Peng, L., Ji, W. Z. (2019). Changes in grain dehydration characteristics and hormone content of summer maize varieties at different ripening stages. Acta Agronomic Sinica, 45(9), 1446–1453. [Google Scholar]
3. Fan, P. C., Tian, J., Huang, J. J., Lie, W. M., Qiu, H. D. (2008). Determination method of cellulose and lignin content in peanut shell. Journal of Chongqing University of Science and Technology, 67(5), 64–65. [Google Scholar]
4. Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utities of protein utilizing the principle of protein-dyebinding. Analytical Biochemistry, 72(1), 248–254. [Google Scholar]
5. Bates, L., Waldren, R., Teare, I. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
6. Liu, H., Zhou, X., Li, Q., Wang, L., Xing, Y. (2020). CCT domain-containing genes in cereal crops: Flowering time and beyond. Theoretical and Applied Genetics, 133(5), 1385–1396. DOI 10.1007/s00122-020-03554-8. [Google Scholar] [CrossRef]
7. Lin, L. Z., Shi, J. S., Chen, Z. J., Hao, J., Xu, D. Z. et al. (2018). Effects of different colored plastic film mulching and planting density on dry matter accumulation and yield of spring maize. Journal of Applied Ecology, 29(1), 116–127. [Google Scholar]
8. Su, S. (2018). Function alanalysis of ZmPCK1 gene in transgenic maize on photosynthesis. China: Journal of Jilin Agricultural University. [Google Scholar]
9. Zhang, H. F., Xu, W. G., Wang, H. W., Hu, L., Li, Y. et al. (2014). Pyramiding expression of maize genes encoding phosphoenolpyruvate carboxylase (PEPC) and pyruvate orthophosphate dikinase (PPDK) synergistically improve the photosynthetic characteristics of transgenic wheat. Protoplasma, 251(5), 1163–1173. DOI 10.1007/s00709-014-0624-1. [Google Scholar] [CrossRef]
10. Petra, S., Per, H., Nils, S., Heli, N., Tuomas, H. et al. (2013). Polymorphisms in oxidative stress-related genes and mortality in breast cancer patients–Potential differential effects by radiotherapy. Breast, 22(5), 817–835. [Google Scholar]
11. Huang, D., Li, S. X., Liao, Q., Qin, C. X., Li, L. et al. (2012). Research progress of plant sucrose phosphate synthase. Chinese Journal of Biological Engineering, 32(6), 109–119. [Google Scholar]
12. Qi, L. L., Ma, Q. (2008). Cloning of maize cytokinin Zmrr3 and construction of RNAi vector. Agricultural Disaster Research, 8(3), 3–5. [Google Scholar]
13. Xiao, J. Z., Da, P. L., Lin, K. G., De, Q. L., Li, X. L. et al. (2008). Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta, 229(3), 485–495. [Google Scholar]
14. Du, H., Huang, F., Wu, N., Li, X., Hu, H. et al. (2018). Integrative regulation of drought escape through ABA-dependent and -independent pathways in Rice. Molecular Plant, 11(4), 584–597. DOI 10.1016/j.molp.2018.01.004. [Google Scholar] [CrossRef]
15. Qayyum, S., Majid, S. A., Bibi, A., Ulfat, A., Khanum, K. et al. (2018). Effect of seed priming with hormonal combinations on morphological and biochemical attributes of maize seedlings. Phyton-International Journal of Experimental Botany, 87(1), 191–197. [Google Scholar]
16. Tsvetova, M., Elkonin, L., Italianskaya, Y. (2019). Pseudogamous apomixis in maize and sorghum in diploid-tetraploid crosses. Phyton-International Journal of Experimental Botany, 88(1), 389–401. [Google Scholar]
17. Furlan, A. L., Bianucci, E., Giordano, W., Castro, S., Becker, D. F. (2020). Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiology and Biochemistry, 151, 566–578. [Google Scholar]
18. Zafar-ul-Hye, M., Nasir, A., Aon, M., Hussain, S., Ahmad, M. et al. (2018). Seed inoculation with Pseudomonas fluorescens and Pseudomonas syringae enhanced maize growth in a compacted saline-sodic soil. Phyton-International Journal of Experimental Botany, 87(1), 25–31. [Google Scholar]
19. Hwang, K., Susila, H., Nasim, Z., Jung, J. Y., Ahn, J. H. (2019). Arabidopsis ABF3 and ABF4 transcription factors act with the NF-YC complex to regulate SOC1 expression and mediate drought-accelerated flowering. Molecular Plant, 12(4), 489–505. [Google Scholar]
20. Guo, J. S., Cao, L. R., Zhang, X., Zhang, J. J., Wei, X. et al. (2020). Effects of drought on leaf physiological characteristics and drought resistance of different maize varieties during elongation period. Chinese Agricultural Science Bulletin, 36(9), 14–18. [Google Scholar]
21. He, Z., Yong, Q. F., Mo, Z., Qi, W., Nan, J. et al. (2020). Identification and analysis of drought and saline-alkali resistance in seedling stage of maize inbred lines with transferred badh gene. Molecular Plant Breeding, 18(11), 3579–3586. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |