

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013166
ARTICLE
Evaluation of Ocimum basilicum L. with Different Concentrations of K+ as an Inhibitor of Pathogenic Bacterial Strains
1Universidad Politécnica de Gómez Palacio, Gómez Palacio, 35120, México
2Universidad Autónoma Chapingo, Centro Regional Universitario Centro-Norte, Zacatecas, Zac., Universidad Autónoma de Zacatecas, Facultad de Matemáticas, Zacatecas, 98053, México
3Departamento de Investigación y Posgrado en Alimentos, Universidad de Sonora, Hermosillo, 83000, México
4Facultad de Agricultura y Zootecnia, Universidad Juárez del Estado de Durango, Gómez Palacio, 34000, México
*Corresponding Author: José Luis García-Hernández. Email: luis_garher@hotmail.com
Received: 28 July 2020; Accepted: 15 October 2020
Abstract: The extraction of bioactive compounds has become one of the most interesting areas of modern chemistry. For therapeutic reasons, it´s important to obtain antimicrobial agents from natural origin. The objective of the present study was to determine the inhibitory effect of ethanolic extract of basil (Ocimum basilicum L. var. Red Rubin) subjected to different concentrations of potassium (K+) on the activity of three bacterial strains that are pathogens in humans. Susceptibility was evaluated by inhibition surface and these results were compared to two antibiotics: Gentamicin (GE) and Ciprofloxacin (CPF) for their efficacy against each bacterial strain. Analyzed variables presented significant difference (p ≤ 0.05). According to the results, basil extract evaluated showed positive antibacterial activity against the three strains of Pseudomonas aeruginosa, Listeria monocytogenes and Staphylococcus aureus on Mueller Hinton agar. It was observed highest inhibition areas of different diameters (15.3 mm, 21.3 mm and 21.6 mm respectively) when the extract used was obtained from the plants with the highest concentration of potassium (13 mmol L–1). These values were even superior to the highest values showed on the treatments with the antibiotic gentamicin.
Keywords: Basil; inhibition; potassium; secondary metabolites; antibiotics
Humans have evolved with plants for several years [1]. Plants are a huge source of chemical compounds that have been extraordinarily useful for human health [2]. They permanently produce bioactive molecules; many of these as response to different stressing factor of the environment [3].
Plants are the highest reservoir of compound resources; they have been used in traditional and modern systems of medicine, food supplements, pharmacognosy, and others [4]. Due to above mentioned, there is a great interest in the denominated medicinal plants; because they are of mayor relevance for human health [5].
Basil (Ocimum basilicum L.) is an aromatic medicinal species native to India, Africa and South Asia [6]. Currently, it is cultivated in many countries around the world [7,8]. This aromatic species [9] is extensively used in traditional medicine [10]. Basil extracts have showed antimicrobial and antifungal properties [11]. These properties are attributed to high levels of phenolic compounds, including phenolic acids and flavonoids. These components contribute to the antioxidant capacity of the plant [11,12].
In the other hand, the concentration of minerals existent in plants have a critical role in the resistance of plants to environmental stress factors [13]. Mineral composition in vegetative tissue is essential for many physiological processes, such as photosynthesis, translocation of photosynthates, enzyme activation, and reduction of excess ion uptake, in addition to osmoregulation, nutrient flow, and distribution of primary metabolites. In this respect, K+ contributes to plant survival [14].
The antibacterial activity of basil has been proven in different bacteria; Escherichia coli has been most susceptible bacterium [15]. Such susceptibility may be measured by the diameters of inhibition areas to compare them with diameters observed when an antibiotic known to be effective against this bacterium is used [16]. Antimicrobial natural agents such as basil constituents are gaining importance in food packaging as they present a lower risk to consumers [17]. More recently, literature has emerged that offers findings about correlations between the content of antioxidant compounds and antimicrobial activity have been reported [18].
The issue raised in importance in recent years due to some studies have been realized on some species of Ocimum genus, including O. basilicum L. var. cinammom, O. anisatum, O. purpurescens, O. album, O. thyrsiflorum and O. gratissimum. It has been demonstrated that they present chemical compounds such as oxygenated monoterpenes and phenylpropanoids [19]. According to previous studies, these compounds present antibacterial and antifungal biological activity. For that reason, these plants are positive candidates in the search of secondary metabolites. This, in order to develop an economic and environmentally viable alternative for medicinal use and in the food industry [20].
The aim of this study was to determine the inhibitory effect of ethanolic extract of basil (var. Red Rubin) on three bacterial strains (Pseudomonas aeruginosa, Listeria monocytogenes and Staphylococcus aureus) by modifying the potassium (K+) concentration in crop irrigation.
This investigation was carried out at the Universidad Politécnica de Gómez Palacio (UPGP). The university is located in the community named Vergel, in the city of Gómez Palacio, Durango, Mexico; in the coordinates: 25°38’19.83’’N–103°31’52.12’’W. The crop of basil was established in the experimental field of this university. Microbiological analyses were realized in the Microbiology Laboratory of the same university.
The basil variety used in this study was Red Rubin. The sowing was done in 250-cavity polystyrene germination trays and Peat Moss Pro-Mix® (Premier Tech, Ltd., Quebec, Canada) was used as a substrate. After seedlings presented three to four true leaves and a height of 10 cm to 15 cm, they were transplanted into one-gallon black plastic pots, which contained a mixture of sand:perlite (80:20). These pots were maintained in a semi-automatic greenhouse. Temperature and relative humidity values oscillated between 25°C–30°C and 70%–80%, respectively. Plants were irrigated with Stainer solution [21], which was modified with different concentrations of K+ respect to the treatments applied. The irrigation volume was 9.8 L per day. Irrigation was performed since sowing, every hour for 5 min (automated) using pressure sprayer from 12:00 to 16:00 h until harvest. The management of pests and diseases was executed in a preventive way, adding garlic extract in irrigation, according to Eagan et al. [22] the hazards of pests in basil are commonly presented 50 days after sowing. Harvest was performed 45 days after transplanting.
2.3 Potassium (K+) Treatments on Basil Plants
Basil plants were treated with four concentrations of K+. In this respect, reagent KOH was used (Jalmek, Mexico). Four solutions were prepared at concentrations of 7, 9, 11 and 13 mmol L–1 of K+.
2.4 Preparation of the Extract
In the aftermath of 45 days of transplanting the plants were harvested. Complete plants were cut using only the leaves and stem to obtain the extract. The rest of the plant was discarded.
Collected basil tissues were first washed with abundant clean water. Subsequently, leaves were separated from the stem and placed on blotting paper (both parts) and left to dry at room temperature (25 ± 2°C) for 15 days. When samples were dry, they were ground in a blender (Hamilton Beach) and stored at 5°C. Then extracts were performed. A solid-liquid extraction proceeds with 1.0 g of the sample in 10 mL of ethanol. The substance was maintained in stir for 24 h at room temperature (25 ± 2°C) on a “Stuart” shaker. After 24 h, the sample extract was filtered and the ethanol evaporated using rotatory vacuum evaporator (Buchí® Modelo-210, USA) and a water bath (RIOSA® Mexico) at 35°C. The extracts obtained were stored in small bottles at –20°C.
The antibacterial effectiveness of basil extract was evaluated using three bacterial strains. One strain Gram negative (P. aeruginosa) and two Gram positive (L. monocytogenes and S. aureus) bacteria. Strains were obtained from the collection of the Biotechnology Laboratory of the UPGP. Viability test of each isolate was developed by each cultured organism in nutrient broth and incubated at 37°C for 24 h.
2.6 Antibacterial Activity of Basil Extract
The sensitivity of the bacteria to the ethanolic extract of basil was evaluated using the disk diffusion technique described by Sahalie et al. [23] such technique is recognized by the National Committee for Clinical Laboratory Standard. Twenty-seven Petri dishes were set up for each bacterium on Mueller Hinton agar. Such agar was prepared using the Bauer-Kirby technique [24]. The agar was sterilized and incubated for each microorganism to be evaluated. Sterile filter paper discs (5 mm in diameter and 0.6 mm thick) added with plant extract were placed on the top of Mueller-Hilton agar plates. Each disc was loaded with 50 mL of extract; then agar plates were incubated at 37°C for 24 h [16]. To compare the diameter of the inhibition zone of the extract, same method was performed with standardized antibiotics (Tab. 1). The antibiotics used for comparison were Gentamicin (GE) and Ciprofloxacin (CPF).
Table 1: Inhibition zones (mm) for each antibiotic by the National Committee for Clinical Laboratory Standard
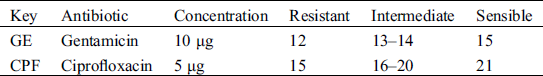
Variance analysis (ANOVA) of bacterial inhibition was performed using the Statistical Analysis Software [25]. For those variables that showed significant difference among treatments by the ANOVA, a mean comparison test was carried out using the LSD with significance value of p ≤ 0.05 with the same statistical package.
Results revealed that basil plant extract was potentially effective in suppressing microbial growth for the three strains evaluated (P. aeruginosa, L. monocytogenes and S. aureus). Evaluation of antibacterial activity of this plant extract was recorded in Tab. 2.
Table 2: Antimicrobial screening test of ethanolic basil plant extract at different concentration of K+ against bacterial strains measure by inhibition zones (mm)
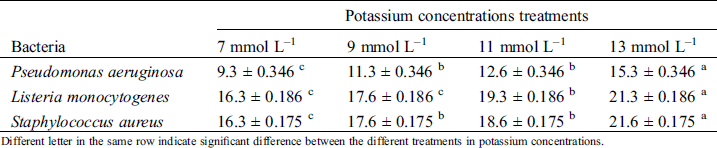
Different letter in the same row indicate significant difference between the different treatments in potassium concentrations.
There were observed inhibition zones for L. monocytogenes, S. aureus and P. aeruginosa (Tab. 2). Most effective extract retarding microbial growth of all tested pathogenic bacteria was at concentration of 13 mmol L–1 of K+. These diameters indicate the sensitivity of bacteria in basil extract presence.
P. aeruginosa sensitivity was only observed in presence of highest K+ concentration extract when it was compared to GE (Tab. 3) but not for CPF (Tab. 4). L. monocytogenes revealed sensitivity to the four different K+ concentrations extracts compared to GE. However, only the extract with the highest concentration of K+ acquired the diameter size to be compared to CPF. Similar results were reported in the behavior of S. aureus, where the inhibition zones demonstrated sensitive with the four potassium concentrations extract were added; in contrast to the inhibition diameters when are compared with CPF. Thus, only treatment of 13 mmol L–1 of K+ demonstrated sensitivity (Tabs. 3 and 4) for such a species.
Table 3: Bacteria sensitivity in presence of basil extract to different K+ concentration compared to gentamicin

(–): Resistant; (+): Intermediate; (++): Sensible.
Table 4: Bacteria sensitivity in presence of basil extract to different K+ concentration compared to ciprofloxacin

(–): Resistant; (+): Intermediate; (++): Sensible
The extract with the treatment of 13 mmol L–1 of K+ revealed sensitivity when it was compared to both antibiotics. Effects on P. aeruginosa (Gram negative) showed inhibition of 100 and 72.8 % compared with GE and CPF respectively. Gram positive strains (L. monocytogenes and S. aureus) obtained 100% inhibition compared with both antibiotics (Fig. 1).
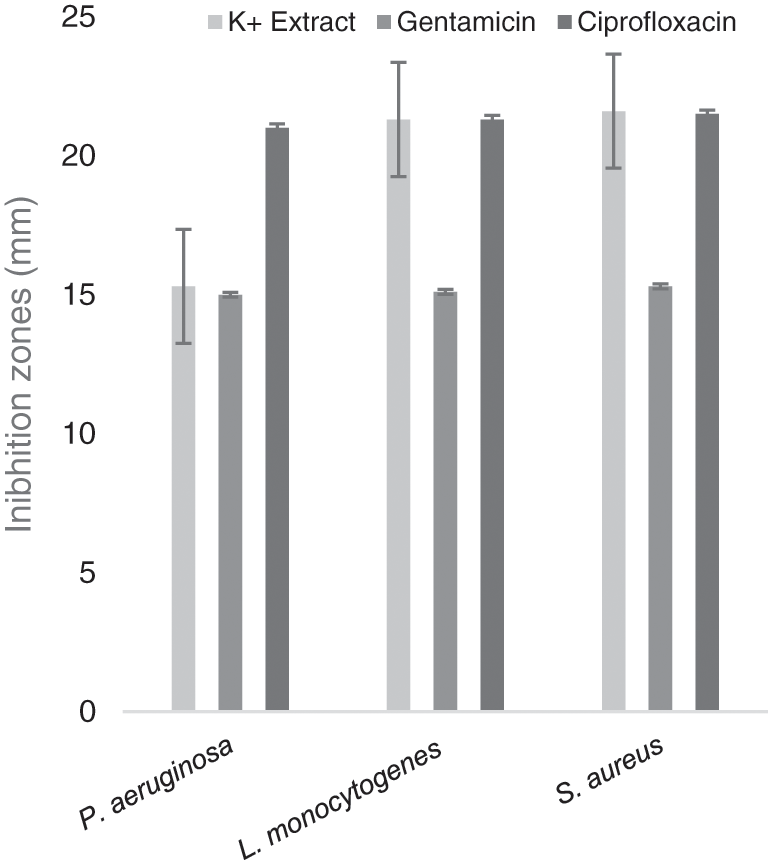
Figure 1: Gentamicin and ciprofloxacin compared with basil extract (13 mmol L–1 of K+)
Plants have always been used as traditional medicine to control and cure many bacterial infections [26]. Since ancient times, medicinal plants have been considered indispensable to treat several diseases [27]. Infectious diseases are the principal cause of morbidity and mortality around the world [28]. Therefore, methodologies have been developed to known and study the behavior of microorganisms in presence of extracts obtained from plants recognized by their beneficial properties [29]. Disc diffusion methods is extensively used to investigate the antibacterial activity of plant extracts [30].
Results of this study indicate that the antibacterial activity of O. basilicum extract was similar for both Gram positive (L. monocytogenes and S. aureus) and Gram negative (P. aeruginosa) strains. This study produced results which corroborate the findings of a great deal from prior works in this field. These results are in accordance with those of Geberikon et al. [31] who reported inhibition zones of 24.3 and 14 mm for S. aureus and P. aeruginosa bacteria respectively. These results are consistent with the current study results when the extract used was from the highest concentration of K+.
Adam et al. [32] conducted a study with extracts of basil leaves of Rehan variety where they showed antimicrobial activity for the different strains they used. Comparing S. aureus (13.9 mm diameter) and P. aeruginosa (13.9 mm diameter) strains obtained in the study above mentioned demonstrated lower inhibition zones than those obtained in the present study. It is possible that these results are due to a direct response to different concentrations of K+ in the nutrient solutions used in irrigation of the plants in their growth stage [33]. Inhibition diameter was higher in presence of highest K+ concentration extract. However, the inhibited area observed was similar with results reported by Adam et al. [32] when concentration of the macro element used in the plant was lower. Recently, Marcelino et al. [34] developed a similar study, the results observed in that evaluation were not optimal (<10 mm) when S. aureus bacteria was exposed to O. basilicum extract. The diameters obtained from extract demonstrated no inhibition compared with control parameters, in contrast to the results obtained in this work. A possible explanation for this might be that the sample amounts used by the researchers to prepare extracts were less than those reported in this study.
Listeria species (Gram positive bacteria in stick form) are common in the human environment and grow in several food products. Therefore, consumption of food contaminated with L. monocytogenes can constitute a serious health risk [35]. Dimic et al. [36] reported inhibition areas of 10 mm when the discs in the agar contained 20 mL of O. basilicum extract in presence of L. monocytogenes. These results differ from obtained behavior of L. monocytogenes in the current study where bacterium developed higher inhibition zones, it is worth mentioning the extract quantity on discs was 50 mL in this study.
Samir et al. [37] determined the antimicrobial activity of different alcoholic basil extracts against pathogenic bacteria such as P. aeruginosa. In that study, inhibition areas ranged from 12 mm to 30 mm in presence of basil, those diameters are similar to those obtained in this study with the extracts of different K+ concentrations. Similar results were reported in the study by Muniandy et al. [38] coincided to inhibition zones obtained in the present work. Researchers observed diameters of 20.62 and 17.25 mm in the inhibition of L. monocytogenes and S. aureus respectively when both strains were exposed to raw extracts of blue ginger (Dichorisandra thyrsiflora). This plant is recognized for antibacterial and antifungal activity due to the secondary metabolites present in it [39].
Some studies indicate that K+ promote amide and protein synthesis and is an enzymatic activator. Those characteristics has been associated to phytonutrient production therefore, has implications for the biochemical synthesis of secondary plant metabolism products [40]. Such information demonstrates that control of potassium in nutrient solution can be viable to improve nutrition aspect referring to total phenolic compounds and antioxidant capacity without affecting basil yield and the inhibitory capacity against different pathogenic strains [41]. Studies confirm that the flavonoid content destroys the cell wall of bacteria, resulting in cell death and inhibition of the protein synthesis process similar to the mechanism of action of antibiotics [42]. Major inhibition is expressed in basil extract with higher potassium concentration; according to previous study was demonstrated that higher concentration of K+ available for the plant contributes to increase secondary metabolites [43].
Due to differences in evaluation methodologies for antimicrobial properties and differences in plant compositions of different geographical regions, it is difficult to compare published studies on the antimicrobial activity of O. basilicum. However, established parameters for basil var. Red Rubin developed positive results in the current study. Basil extracts treated with distinct concentrations of K+ proved a viable alternative for the inhibition of bacterial growth of S. aureus, L. monocytogenes and P. aeruginosa. Extract with the highest dose of K+ (13 mmol L–1) used in the crop indicated superior inhibitory effect on the three strains used. Future research should include the minimum inhibitory concentration (MIC) of basil extract using the same K+ concentrations modifying the quantity loaded in discs, the antimicrobial activity of extracts varying the plant concentrations, the use of other solvents and extraction techniques to observe bacteria behavior modifying variables mentioned before.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Lu, D. Y., Lu, T. R. (2019). Herbal medicine in new era. Hospice and Palliative Medicine International Journal, 3(4), 125–130. DOI 10.15406/hpmij.2019.03.00165. [Google Scholar] [CrossRef]
2. Bernstein, N., Akram, M., Daniyal, M., Koltai, H., Fridlender, M. et al. (2018). Anti-inflammatory potential of medicinal plants: A source for therapeutic secondary metabolites. Advances in Agronomy, 150, 131–183. [Google Scholar]
3. Nduche, M. U., Otaka, C. L. (2019). Phytochemical screening and antimicrobial activity of Talinium triangulare (JACQ) willd, Ocimum gratissimum L. Chromoleana odorata L., and Aloe vera (L.) Burm. F. International Journal of Research in Pharmacy and Biosciences, 6(2), 1–12. [Google Scholar]
4. Srivastava, A. K. (2018). Chapter 1—Significance of medicinal plants in human life. Switzerland: Springer. [Google Scholar]
5. Jamshidi-Kia, F., Lorigooini, Z., Amini-Khoei, H. (2018). Medicinal plants: Past history and future perspective. Journal of Herbmed Pharmacology, 7(1), 1–7. DOI 10.15171/jhp.2018.01. [Google Scholar] [CrossRef]
6. Piras, A., Gonçalves, M. J., Alves, J., Falconieri, D., Porcedda, S. et al. (2018). Ocimum tenuiflorum L. and Ocimum basilicum L., two spices of Lamiaceae family with bioactive essential oils. Industrial Crops and Products, 113, 89–97. DOI 10.1016/j.indcrop.2018.01.024. [Google Scholar] [CrossRef]
7. Naik, S., Shyam, P., Marx, K. P., Baskari, S., Devi, C. V. R. (2015). Antimicrobial activity and phytochemical analysis of Ocimum tenuiflorum leaf extract. International Journal of PharmTech Research, 8(1), 88–95. [Google Scholar]
8. Nurzyńska-Wierdak, R., Borowski, B., Dzida, K., Zawiślak, G., Kowalski, R. (2013). Essential oil composition of sweet basil cultivars as affected by nitrogen and potassium fertilization. Turkish Journal of Agriculture and Forestry, 37, 427–436. [Google Scholar]
9. Mostafavi, S., Asadi-Gharneh, H. A., Miransari, M. (2019). The phytochemical variability of fatty acids in basil seeds (Ocimum basilicum L.) affected by genotype and geographical differences. Food Chemistry, 276, 700–706. DOI 10.1016/j.foodchem.2018.10.027. [Google Scholar] [CrossRef]
10. Mannan, A., Hossain, S., Nipa, N. N., Khatun, A., Amin, R. et al. (2019). Macro and micro nutrients in Holy basil (TulsiA possible supplement for natural medicine. International Journal of Chemistry Studies, 3(4), 43–47. [Google Scholar]
11. Nguyen, P. M., Kwee, E. M., Niemeyer, E. D. (2010). Potassium rate alters the antioxidant capacity and phenolic concentration of basil (Ocimum basilicum L.) leaves. Food Chemistry, 123(4), 1235–1241. DOI 10.1016/j.foodchem.2010.05.092. [Google Scholar] [CrossRef]
12. Lee, J., Scagel, C. F. (2009). Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chemistry, 115(2), 650–656. DOI 10.1016/j.foodchem.2008.12.075. [Google Scholar] [CrossRef]
13. Yadav, B. K., Sidhu, A. S. (2016). Dynamics of potassium and their bioavailability for plant nutrition, pp. 187–201. New Delhi: Springer India. [Google Scholar]
14. Amtmann, A., Troufflard, S., Armengaud, P. (2008). The effect of potassium nutrition on pest and disease resistance in plants. Physiologia Plantarum, 133(4), 682–691. DOI 10.1111/j.1399-3054.2008.01075.x. [Google Scholar] [CrossRef]
15. Upadhyay, R. K., Dwivedi, P. R., Ahmad, S. (2010). Screening of antibacterial activity of six plant essential oils against pathogenic bacterial strains. Journal of Pharmacy Research, 4(4), 1153–1156. [Google Scholar]
16. Romulo, A., Zuhud, E. A. M., Rondevaldova, J., Kokoska, L. (2018). Screening of in vitro antimicrobial activity of plants used in traditional Indonesian medicine. Pharmaceutical Biology, 56(1), 287–293. DOI 10.1080/13880209.2018.1462834. [Google Scholar] [CrossRef]
17. Zengin, G., Ferrante, C., Gnapi, D. E., Sinan, K. I., Orlando, G. et al. (2019). Comprehensive approaches on the chemical constituents and pharmacological properties of flowers and leaves of American basil (Ocimum americanum L). Food Research International, 125, 108610. DOI 10.1016/j.foodres.2019.108610. [Google Scholar] [CrossRef]
18. Mekinić, G. I., Skroza, D., Ljubenkov, I., Katalinić, V., Šimat, V. (2019). Antioxidant and antimicrobial potential of phenolic metabolites from traditionally used mediterranean herbs and spices. Foods, 8(11), 579. DOI 10.3390/foods8110579. [Google Scholar] [CrossRef]
19. Singletary, K. W. (2018). Basil: A brief summary of potential health benefits. Nutrition Today, 53(2), 92–97. DOI 10.1097/NT.0000000000000267. [Google Scholar] [CrossRef]
20. Fernandes, F., Pereira, E., Círić, A., Soković, M., Calhelha, R. C. et al. (2019). Ocimum basilicum var. purpurascens leaves (red rubin basilA source of bioactive compounds and natural pigments for the food industry. Food & Function, 10(6), 3161–3171. DOI 10.1039/C9FO00578A. [Google Scholar] [CrossRef]
21. Steiner, A. A. (1961). A universal method for preparing nutrient solutions of a certain desired composition. Plant and Soil, 15(2), 134–154. DOI 10.1007/BF01347224. [Google Scholar] [CrossRef]
22. Eagan, S., Dhandayuthapani, U. N. (2018). Organic agriculture: Techniques to improve crop production. Eco-friendly agro-biological techniques for enhancing crop productivity, pp. 1–24. Singapore: Springer Nature. [Google Scholar]
23. Sahalie, N. A., Abrha, L. H., Tolesa, L. D. (2018). Chemical composition and antimicrobial activity of leave extract of Ocimum lamiifolium (Damakese) as a treatment for urinary tract infection. Cogent Chemistry, 4(1), 1466. DOI 10.1080/23312009.2018.1440894. [Google Scholar] [CrossRef]
24. Bauer, A. W., Kirby, W. M. M., Sherris, J. C., Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, 1(45), 493–496. DOI 10.1093/ajcp/45.4_ts.493. [Google Scholar] [CrossRef]
25. SAS. (2004). Statistical analysis system institute. Cary, NC: SAS Institute. [Google Scholar]
26. Godswill, C. (2019). Medicinal plants: The medical, food, and nutritional biochemistry and uses. International Journal of Advanced Academic Research, 5(11), 220–241. [Google Scholar]
27. Petrovska, B. B. (2012). Historical review of medicinal plants’ usage. Pharmacognosy Reviews, 6(11), 1–5. DOI 10.4103/0973-7847.95849. [Google Scholar] [CrossRef]
28. Lindahl, J. F., Grace, D. (2015). The consequences of human actions on risks for infectious diseases: A review. Infection Ecology & Epidemiology, 5(1), 30048. DOI 10.3402/iee.v5.30048. [Google Scholar] [CrossRef]
29. Abkhoo, J., Jahani, S. (2017). Antibacterial effects of aqueous and ethanolic extracts of medicinal plants against pathogenic strains. International Journal of Infection, 4(2), 20. DOI 10.5812/iji.42624. [Google Scholar] [CrossRef]
30. Mostafa, A. A., Al-Askar, A. A., Almaary, K. S., Dawoud, T. M., Sholkamy, E. N. et al. (2018). Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi Journal of Biological Sciences, 25(2), 361–366. DOI 10.1016/j.sjbs.2017.02.004. [Google Scholar] [CrossRef]
31. Geberikon, G. M., Daboo, A. D., Agbo, E. B. (2018). Phytochemical and antibacterial activities of combined leaves and flower extracts of English camphor basil (Ocimum canum) on some selected bacteria associated with skin infections. International Journal of Contemporary Research and Review, 9(6), 20246–20253. DOI 10.15520/ijcrr/2018/9/06/534. [Google Scholar] [CrossRef]
32. Adam, Z. A., Omer, A. A. (2015). Antibacterial activity of Ocimum basilicum (Rehan) leaf extract against bacterial pathogens in Sudan. American Journal of Research Communication, 3(8), 94–99. [Google Scholar]
33. Hellal, F., El-Sayed, S., Hady, M. A. (2020). Barley responses to potassium fertilization under water stress condition. Plant Archives, 20(1), 3140–3147. [Google Scholar]
34. Marcelino, N., Clara, E. C., Fauhan, K. I., Ramadhana, A. S., Lister, I. N. E. (2020). Comparison of effectiveness of basil leaves ethanol extract with garlic on Staphylococcus aureus bacteria. Biospecies, 13(1), 8–14. DOI 10.22437/biospecies.v13i1.8387. [Google Scholar] [CrossRef]
35. Jordan, K., McAuliffe, O. (2018). Listeria monocytogenes in foods. Food and Nutrition Research, 86, 181–213. [Google Scholar]
36. Dimic, G., Kocic-Tanackov, S., Jovanov, O., Cvetkovic, D., Markov, S. et al. (2012). Antibacterial activity of lemon, caraway and basil extracts on Listeria spp. Acta Periódica Tecnológica, 43(1), 239–246. [Google Scholar]
37. Samir, W., Haggag, M., Abo El-Nasr, A. A. (2015). Biological activity of extracts from olive and basil leaves against pathogenic microbial isolates. Egyptian Journal of Medical Microbiology, 24(2), 1–9. DOI 10.12816/0026081. [Google Scholar] [CrossRef]
38. Muniandy, P., Paramasivam, M., Chear, N. J. Y., Singh, D., Kernain, D. (2019). A study of antibacterial efficacy of Alpinia galangal extracts against Staphylococcus aureus, Staphylococcus epidermidis and Listeria monocytogenes. Journal of Pharmaceutical Sciences and Research, 11(8), 3061–3066. [Google Scholar]
39. Chouni, A., Paul, S. (2017). A review on phytochemical and pharmacological potential of Alpinia galanga. Pharmacognosy Journal, 10(1), 9–15. DOI 10.5530/pj.2018.1.2. [Google Scholar] [CrossRef]
40. Inthichack, P., Nishimura, Y., Fukumoto, Y. (2012). Effect of potassium sources and rates on plant growth, mineral absorption, and the incidence of tip burn in cabbage, celery, and lettuce. Horticulture, Environment, and Biotechnology, 53(2), 135–142. DOI 10.1007/s13580-012-0126-z. [Google Scholar] [CrossRef]
41. Mardanluo, S., Souri, M. K., Ahmadi, M. (2018). Plant growth and fruit quality of two pepper cultivars under different potassium levels of nutrient solutions. Journal of Plant Nutrition, 41(12), 1604–1614. DOI 10.1080/01904167.2018.1463383. [Google Scholar] [CrossRef]
42. Threenesia, A., Ramadhian, M. R. (2019). Perbandingan efek pemberian ekstrak etanol daun kemangi (Ocimum sanctum L.) terhadap daya hambat pertumbuhan Staphylococcus aureus dan Salmonella typhi secara in vitro. Journal Agromedicine, 6(1), 120–124. [Google Scholar]
43. Salas-Pérez, L., Fornari-Reale, T., Preciado-Rangel, P., García-Hernández, J., Sánchez-Chávez, E. et al. (2018). Cultivar variety and added potassium influence the nutraceutical and antioxidant content in hydroponically grown basil (Ocimum basilicum L.). Agronomy, 8(2), 13. DOI 10.3390/agronomy8020013. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |