DOI:10.32604/phyton.2020.013668

| Phyton-International Journal of Experimental Botany DOI:10.32604/phyton.2020.013668 |  |
| Article |
College of Horticulture and Gardening, Yangtze University, Jingzhou, 434025, China
*Corresponding Author: Hongna Mu. Email: hongnamu@yangtzeu.edu.cn
Received: 26 August 2020; Accepted: 14 September 2020
Abstract: Pinus elliottii is an exotic afforestation pine extensively distributed in southern parts of China. In order to understand whether endophytic fungi can affect seedling growth of P. elliottii, Piriformospora indica (Pi), Funnelifcrmis mosseae (Fm), and Diversispora tortuosa (Dt) were inoculated respectively, and the non-inoculated group was set as control. The growth indexes, the contents of soluble sugar and soluble protein, and plant endogenous hormone levels in the leaves of P. elliottii, were analyzed. The results showed that Fm, Dt and Pi colonized the P. elliottii roots to form mycorrhizal structure and chlamydospores arranged in beads respectively. Three fungal inoculants exhibited the stimulated growth responses, whilst Dt illustrated the most positive effect on plant height, single fresh weight, trunk diameter and root system structure, compared with the control. On the other hand, the soluble sugar and soluble protein contents were increased distinctively in mycorrhizal plants. The endogenous IAA, GA3, ZR contents were increased, while the ABA contents were reduced in mycorrhizal plants versus non-mycorrhizal plants. The fungi-induced endogenous hormone changes triggered plant growth improvement of P. elliottii seedlings. This research unraveled the positive effect of AM fungi and P. indica on growth of pine seedlings, while, more application of endophytic fungi to fields needs to be explored.
Keywords: AM fungi; Piriformospora indica; Pinus elliottii; colonization; growth enhancing; hormone content
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
Arbuscular mycorrhizal (AM) fungi belong to the fungal subphylum Glomeromycotiaza, establishing endosymbiosis with the vast majority of land plants [1]. This endosymbiosis provides a series of benefits to plants such as improved intake of nutrients (mainly P and N) and water from the soil, and enhanced resistance against various biotic and abiotic stresses [2–4]. The mycorrhizal symbiosis exerts a strong influence on plant growth and fitness [5]. AM fungi are thought to have a broad host range, establishing extensive underground networks and connecting different plant species [6], which implies AM fungi must have highly efficient mechanisms to colonize massive plants intracellularly based on the above extremely broad host range.
There was report about the host preferences [7], which is a common phenomenon observable in nature. The symbiotic efficiency might be controlled by AM secrete protein acting as effector [8]. However, there were opposite views that every AM fungi can colonize a plenty of host plants, which indicates lack of host specificity [5]. It was found that the mixed AM fungi displayed better functions than single [9]. Singular AM fungi colonizes large numbers of plants, meanwhile the same plant can be colonized by different AM fungi.
Pinus elliottii was an exotic plant and was planted for landscape application, soil and water conservation, and economic forests in the southern parts of China, which was introduced in the 1930s, and developed rapidly after 1970s, which has been increasingly emphasizing especially in afforestation field for its outstanding character [10–12]. Regarding the complex site conditions, enhancing seedling quality and adaptability appears to be more and more necessary for annual or biennial seedlings of P. elliottii.
It is well-known that P. elliottii established a symbiotic relationship with mycorrhizal fungi. Ectomycorrhiza (EM) was the recognized and more studied fungi for pine. Lodge [13] observed that high levels of EM fungi colonization were related to depression of arbuscular mycorrhizal colonization in root terminal compared with non-terminal. AM fungi can co-exist with EM even in different regions of the same root of 35 different Poplars [14,15]. Dual infection of AM fungi and EM present mutual promotion or competition, some dual infection treatment accelerated host growth with relative high infection rate, but others played negative effect, which was attributed to mycorrhizal types and strains, in addition soil texture and pH, plant age and so on [16]. Therefore, we proposed that AM fungi can colonize the roots of P. elliottii in consideration of EM co-existence with AM fungi in poplar and its ectomycorrhizal character. If the above hypothesis will be proved by experiment, it will broaden the understanding of AM fungi and offer new information about mycorrhizal fungi of P. elliottii, which may accelerate young seedling growth and shorten the growth time in the seedbed.
The seeds of P. elliottii were soaked 30 seconds in sulphuric acid, rinsed 1 min under tap-water, then washed 2–3 times with sterilized water. The treated seeds were sown in soil (121°C, 60 minutes, sterilized twice, the same below), finally placed in an artificial climate chamber for seedling growth.
The infection experiment started when its height was up to 10 cm on November 29th, 2018. Every pot was filled with 1.9 kg sterilized soil. Four seedlings were planted in every plot, and four replicates were set for each treatment. For fungi inoculation, 40 g AM fungi was added per kilogram soil, and 0.4 g P. indica was added per kg soil, 40g sterilized soil added in the control group.
The AM fungi applied in this experiment included Funneliformis mosseae (Fm) and Diversispora tortuosa (Dt), which were bought from Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Sciences. Piriformospora indica (DSM11827) was offered by Ralf Oelmüller, University of Jena.
The seedlings of P. elliottii were harvested for determination of biomass and physiological indexes four months after inoculation. Plant height was measured with a calibrated scale, total fresh weight and root weight were measured with an electronic balance. The chlorophyll content was measured using fresh leaves [17], soluble protein (SP) was measured using Blue G-250 method [18], and soluble sugars (SS) were measured using anthrone method [19]; the content of POD and SOD was determined [20,21]. The extraction of hormone of ABA, GA3, IAA and ZR was carried out according to the method of Bollmark [22], purification and ELISA detection had been finished according to Yang et al. [23], concentration calculation were conducted as instruction of Weiler et al. [24].
2.3 Data Statistics and Analysis
The experimental data were statistically analyzed using SPSS 25.0. ** indicates the significant difference at the 0.01 level and * indicates the significant difference at the 0.05 level. The graph was drawn with prism 8.0.
3.1 AM Fungi and P. indica Colonized P. elliottii and Increased Host Biomass
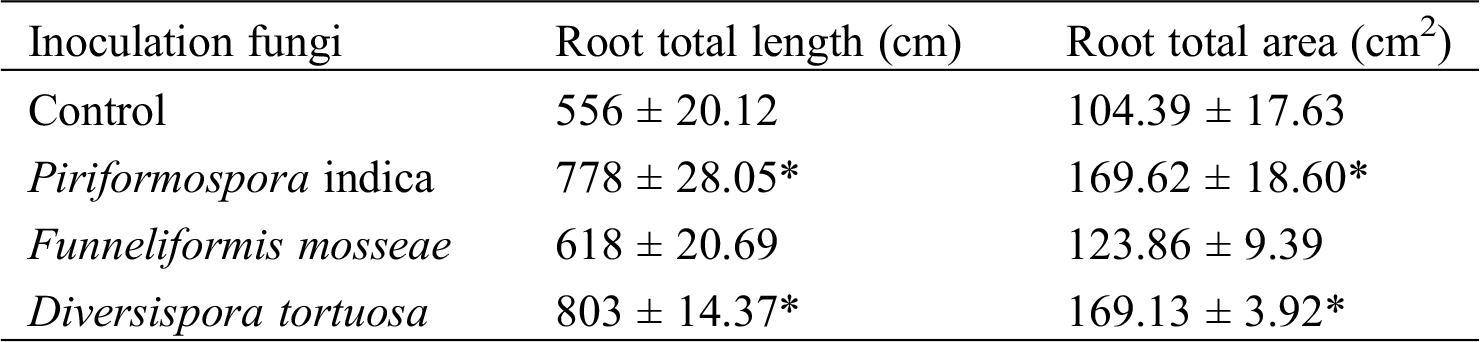
AM fungi colonized young root of P. elliottii one month after inoculation, the branching hypha can be distinguished obviously under optical microscope, which is regarded as a distinct feature of AM fungi (Fig. 1A). Meanwhile, the chlamydospores lined like strings of beads, which was the typical feature of P. indica (Fig. 1B). The inoculation mainly increased the total length and total area of root that was colonized by AM fungi and P. indica. The root length and tiny roots number were better in inoculation groups than control, the best inoculant was Dt, the second was Pi, which two present significant difference at 0.05 level (Fig. 2, Tab. 1). The root total length were 778 cm, 618 cm, and 803 cm and increased root total length by 39.93%, 11.15% and 44.42% respectively after inoculation of Pi, Fm and Dt four months, compared with control, the root total length was 556 cm (Tab. 1). P. elliottii mycorrhization illustrated similar effect on root total area, Pi and Dt showed the better influence, which increased root total area by 62.49% and 62.02% respectively. The above data indicated mycorrhization plays good effect on pine root, attributed to enlarged root absorption area.
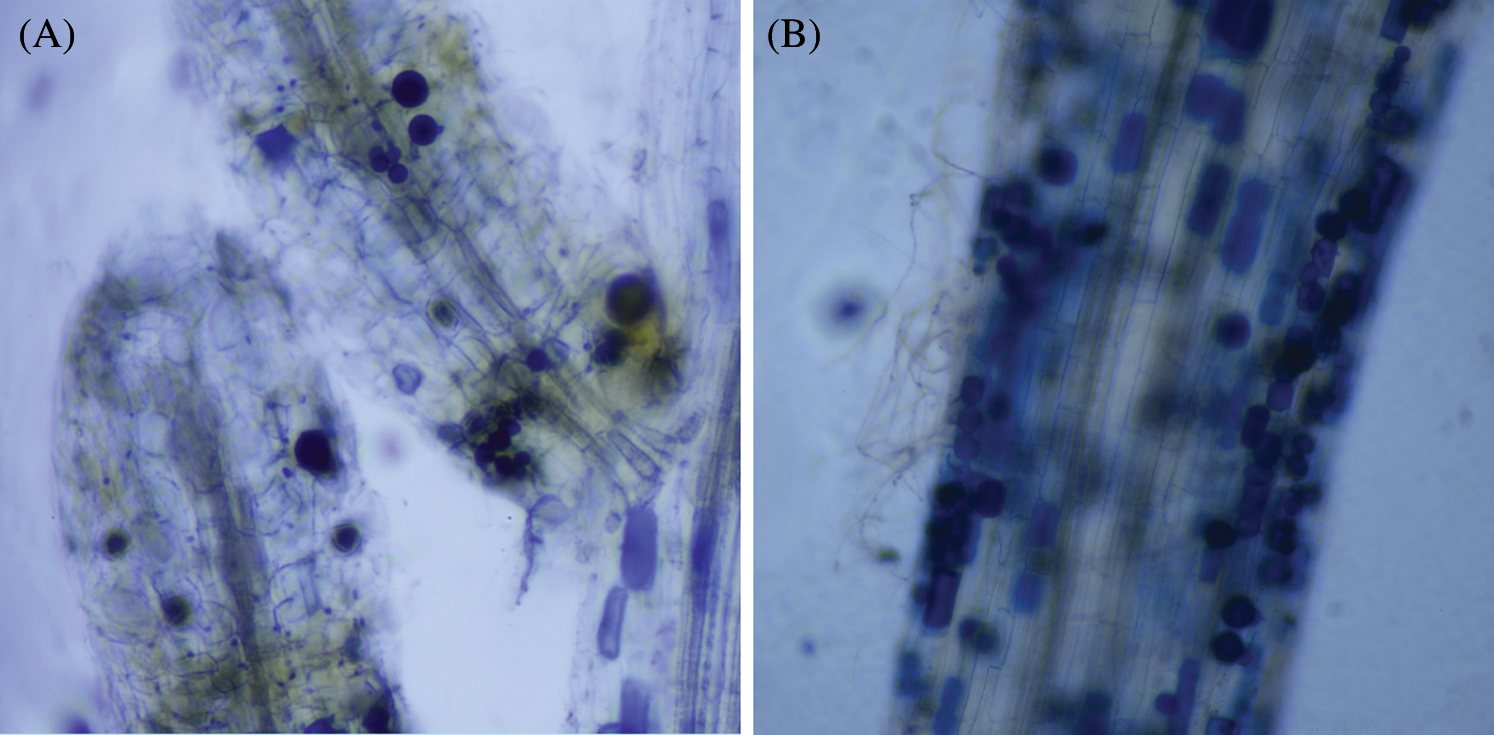
Figure 1: The colonization of AM fungi in roots of P. elliottii. A, the branching hypha in blue color, stained by trypan blue. B, chlamydospores stained by trypan blue lined in roots of P. elliottii
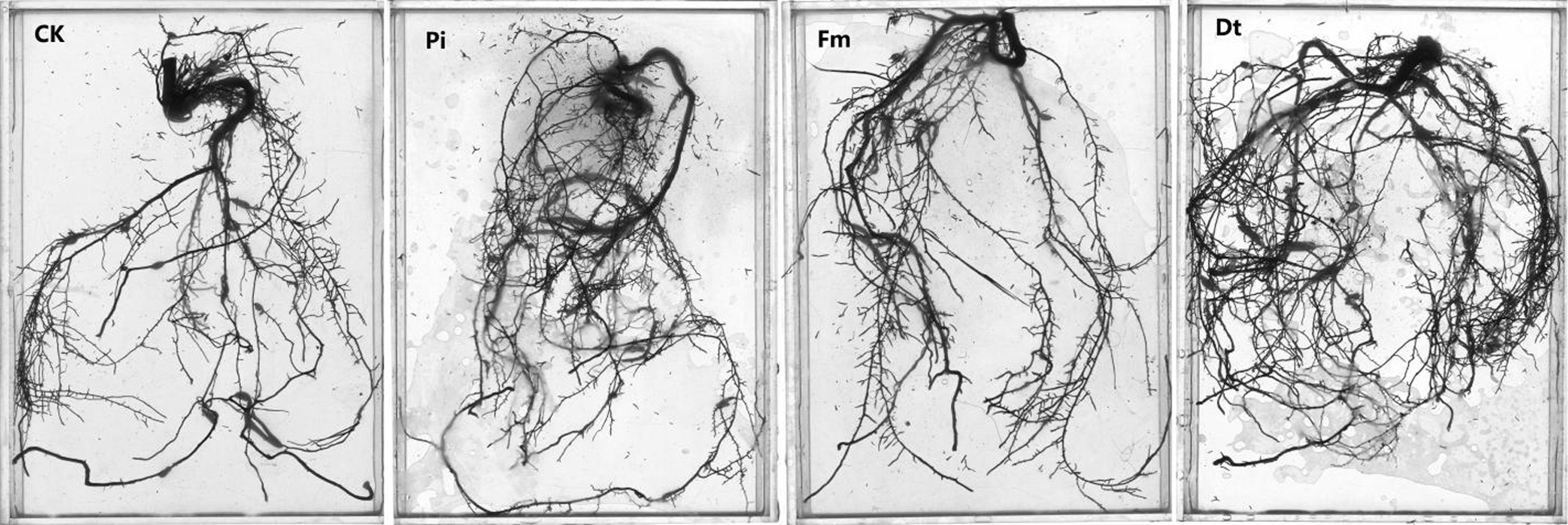
Figure 2: The colonization effects on root growth and development of P. elliottii. CK, Pi, Fm and Dt indicate non-inoculation control, P. indica, F. mosseae and D. tortuosa, respectively
Table 1: The inoculation effect on the root total length and total area by inoculated AM fungi and P. indica
AM fungi colonization had significant effect on plant height compared with control, Dt ranked the best with increasing one-fold (Fig. 3B). The fresh weight variation was similar to plant height (Fig. 3A). As for colonization effect on trunk diameter, Fm displayed best (Fig. 3C).
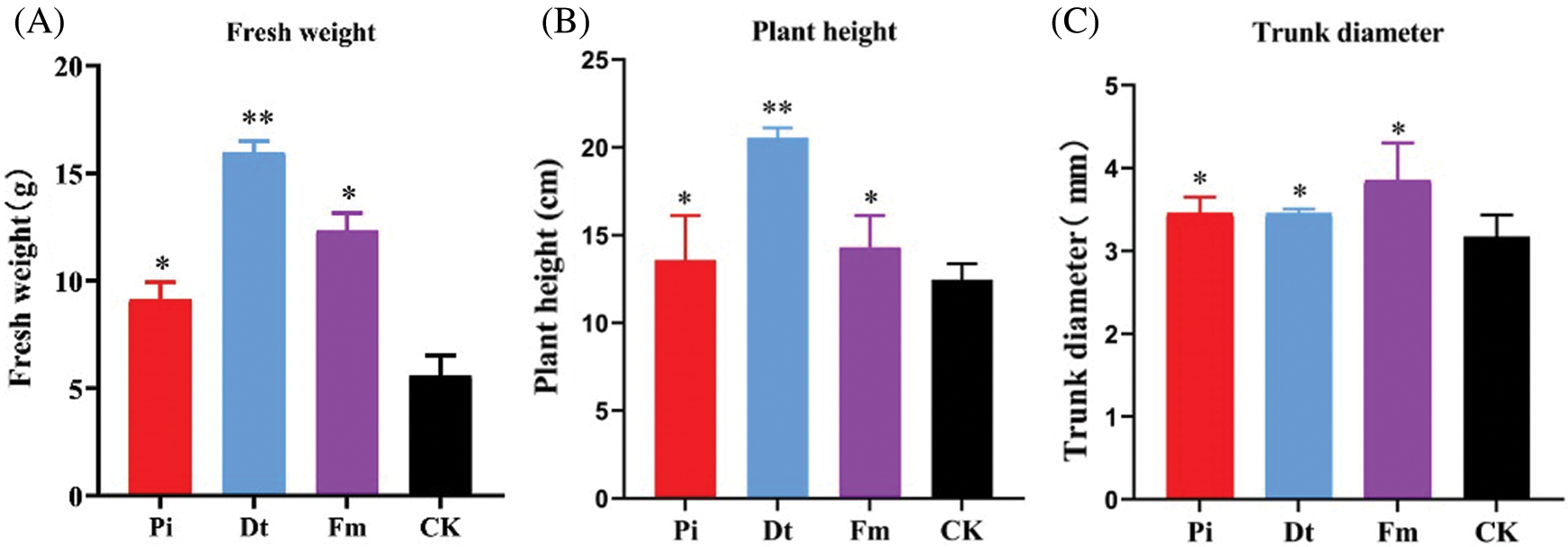
Figure 3: The excellent affection to the growth of P. elliottii after 4-month inoculation by AM fungi. A indicates the effect on fresh weight, in which * and ** represent that have significant differences at 0.05 level (P < 0.05) and 0.001 level (P < 0.001). B shows positive effect on single plant height, and C illustrates positive effect on single trunk diameter
3.2 AM Fungi and P. indica Altered Soluble Sugar and Soluble Protein Content
The colonization of endophytic fungi caused Soluble Sugar content change, the alter degree was decided by fungi type in this experiment. The best one was Dt, second Fm, Pi ranked third and the lowest was CK (Fig. 4A). As to Soluble Protein content, the three colonized groups were higher than control, distinctively different effects were found on Pi and Dt, with greater effort on Pi (Fig. 4B).
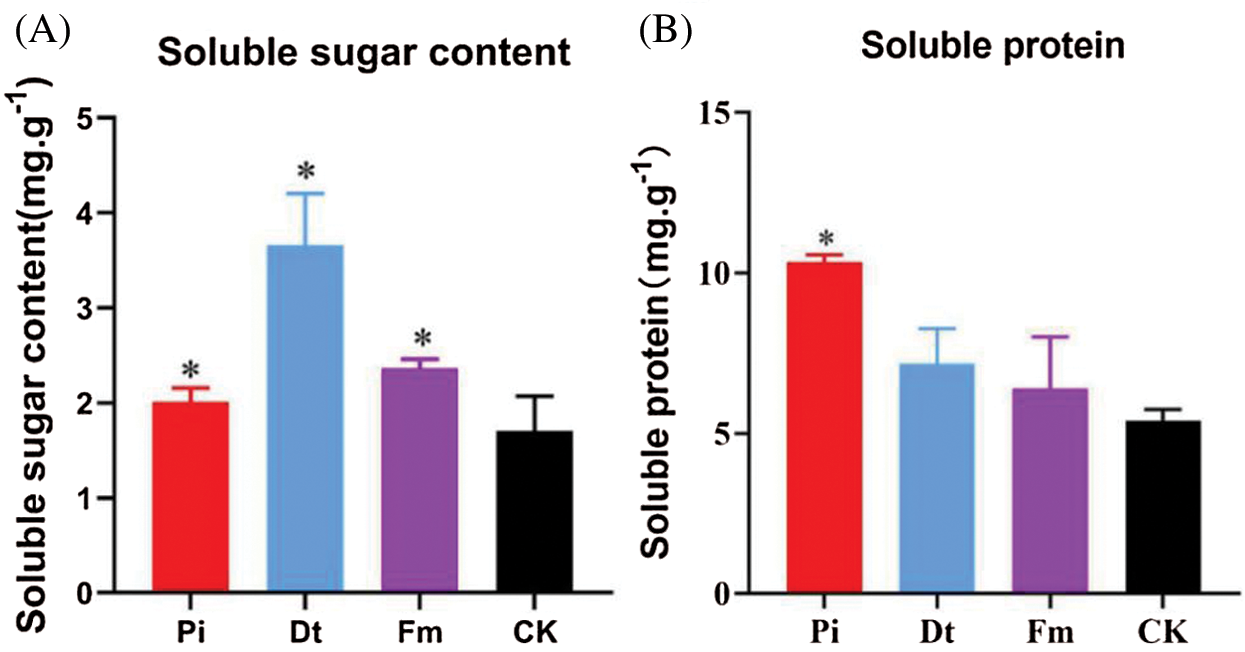
Figure 4: The application of endophytic fungi on the contents of soluble sugar and soluble protein in needles of P. elliottii
3.3 AM Fungi and P. indica Accelerated Growth via Regulating Hormone Fluctuation
The hormone concentration varied according to endophytic fungi types. GA3 concentration was affected mostly by Dt and Fm (P < 0.001), which increased nearly two-folds, and the effect of Pi presented significantly at 0.05 level (Fig. 5B). Other growth promoting hormones, IAA and ZR were all higher than control group (Figs. 5A and 5C), which indicated the positive effect of Dt and Fm was better than Pi. With regard to ZR, all three mycorrhizal groups showed significant effect compared to CK, the finest was Dt also (Fig. 5C). ABA content regulates plant growth negatively. As unraveled in Fig. 5D, lower ABA level was detected in mycorrhizal groups than CK, the lowest one was Dt. Therefore, the growth accelerating result may be attributed to higher content of GA3, IAA, ZR, and lower content of ABA in those mycorrhization plants.
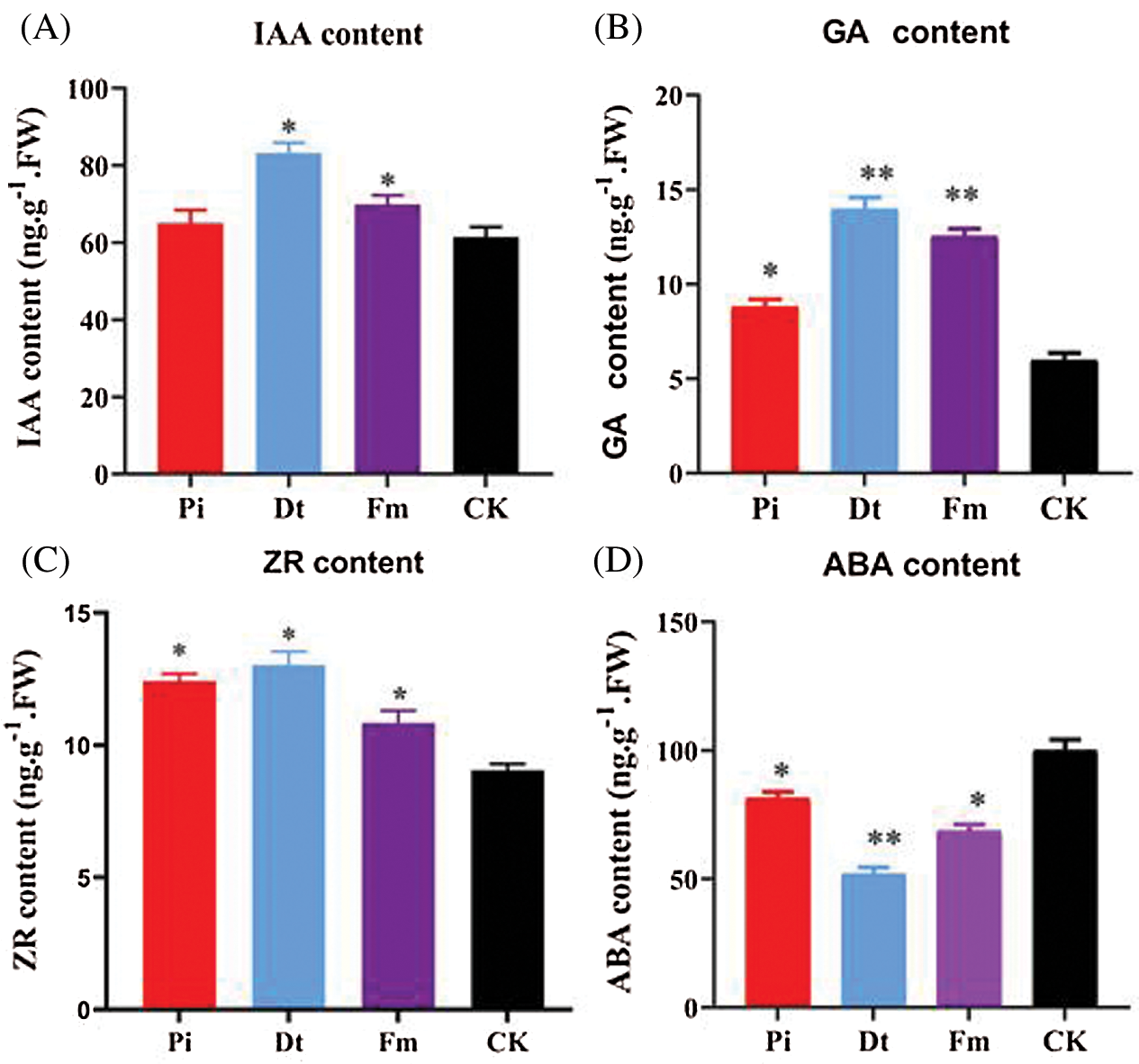
Figure 5: Levels [ng.g–1 of fresh weight (FW)] of total hormone isolated from needles of non-mycorrhizal (CK) and mycorrhizal (Pi, Dt, Fm) pine seedlings
In this experiment, AM fungi can colonize the roots of P. elliottii and increase its biomass. Pine had been regarded as lacking colonization with endophytic mycorrhiza. There were a plenty of documents illustrating EM had benefits to pine [25,26]. EM and AM were negatively associated with the same root of Populus delt [13], which was proved by Tang et al. [15]. Endophytic mycorrhiza had not been detected in earlier times, the possible causes may include too many disturbing substances (compounds) in its root to distinguish the typical feature, according to the theory “non endophytic mycorrhiza plant”. In this project, the feature “branching hypha” and strings of chlamydospore were uncovered in the following year when the experiment was redone (Fig. 1).
Plant growth is the successful consequence of a finely regulated network of hormone-controlled metabolic process [27]. AM fungi altered four examined hormones in this project, the content of IAA, GA3 and ZR was elevated significantly which formed strong comparison with the sharp decline of ABA. As for IAA, GA3 and ZR, alterations were similar to Song et al. [28]. However, the ABA decomposition changed obviously in needles of Populus delt, which conflicted with the report of Song et al. [28], but was in line with the report of Liu et al. [29]. On one hand, the possibe cause was that the tested AM fungi in our experiment were different from other researchers, in the light of different AM fungi functioning diversely. On the other hand, the infected host was distinct, which may trigger successive response between plants and AM fungi, leading to different result in ABA content.
The relative level of plant hormone was critical for plant growth and development. It was reported adjusting the level or ratio of plant hormone in the tissues of the plant can improve the growth and productivity of plant [30], in which plant growth responses were altered through the application of growth regulator (auxin, cytokinin). Furthermore, Cytokinin (CTK) had a stimulatory role in AM colonization for the increase of active CTK was paralleled with the increased AM colonization [31]. Exogenous factors or substances, such as N fertilizer, exogenous hormone, girdling and others can change the endogenous hormone level [32–35]. In this case, the relative level of GA3, IAA, ZR to ABA brought about remarkable promotion in growth. The regulation was triggered by the colonization of AM fungi, which was regarded as the most possible exogenous cause. The positive role of AM fungi had been reported by He et al. [4] and other researchers [36,37], for example AM colonization can control the ABA increase level during the replanting of Citrus maxima, which has a good effect on replanting in the document of Yang et al. [36]. Meanwhile, AM colonization increased the content of IAA, CTK with lower ABA content, which led to root development and increased shoot biomass by 34%, alleviating the damage caused by coal mining subsidence ground [37], In which IAA played an important role, IAA content has a direct and positive impact on leaves, the leaves mass decreases when IAA content declines [32]. Conversely, a high IAA level must accompany with more accumulation of shoots or leaves, which was proved in this experiment. The other affection of AM fungi to the inoculated seedlings declined the ABA content inside needles of P. elliottii, which was consistent with the documents reported by Liu et al. [29]. In conclusion, plant growth (roots and above-ground biomass) can be regulated by hormone level to a certain extent, Various factors can affect plant hormone content and relative level, although diversified experiment condition differ to the research result.
It was the report that P. elliottii was endophytic mycorrhizal plant also and illustrated the positive growth effect on pine seedlings. There were some details needed in advance of our experiment in the following study, such as limited hormone types were used to give an inadequate illustration of the underlying regulation mechanism, and the determination of photosynthetic parameters needs to be improved in view of the distinction between needles and broad leaves. Next, we will explore the underlying physiological causes extensively in the application of endophytic mycorrhizal fungi to seedling cultivation and pine afforestation.
Acknowledgement: We would like to thank Wu Chu, who offered funding in the seeds of P. elliottii and some suggestions on pine seeding and the spore of P. indica, which offered solid base for this experiment.
Funding Statement: We would like to thank the fund support of the Ideological and Political Course and Curriculum and Ideological and Political Demonstration Construction Project of Yangtze University (2020, No. 65). We would also thank the help provided by the Open Fund of Institute of Root Biology of Yangtze University, which supported the purchase of AM fungi strain from Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Sciences.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Spatafora, J. W., Chang, Y., Benny, G. L., Lazarus, K. Smith, M. E. et al. (2016). A phylum-level phylogenetic classification of zygomycete fungi based on gemone-scale data. Mycologia, 108(5), 1028–1046. DOI 10.3852/16-042.
2. Smith, S. E., Read, D. J. (2008). Mycorrhizal symbiosis. UK: Academic Press. [Google Scholar]
3. Zhang, F., Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environmental and Experimental Botany, 171, 103962. DOI 10.1016/j.envexpbot.2019.103962.
4. He, J. D., Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Scientia Horticulturae, 262, 108745. DOI 10.1016/j.scienta.2019.108745. [Google Scholar] [CrossRef]
5. Van der Heijden, M. G. A., Martin, F. M., Selosse, M. A., Sander Ian, R. (2015). Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytologist, 205(4), 1406–1423. [Google Scholar]
6. Helgason, T., Daniell, T. J., Husband, R., Fitter, A. H., Young, J. P. (1998). Ploughing up the wood-wide web? Nature, 394(6692), 431. DOI 10.1038/28764. [Google Scholar] [CrossRef]
7. Torrecillas, E., Alguacil, M. M., Roldan, A. (2012). Host preferences of arbuscular mycorrhizal fungi colonizing annual herbaceous plant species in semiarid Mediterranean prairies. Applied and Environmental Microbiology, 78(17), 6180–6186. DOI 10.1128/AEM.01287-12. [Google Scholar] [CrossRef]
8. Zeng, T., Holmer, R., Hontelez, J., Lintel-Hekkert, B. Marufu, L. et al. (2018). Host-and stage-dependent secretome of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Plant Journal, 94(3), 411–425. DOI 10.1111/tpj.13908. [Google Scholar] [CrossRef]
9. Datta, P., Kulkarni, M. (2014). Influence of two AM fungi in improvement of mineral profile in Arachis hypogaea L. under salinity stress. Legume Research, 37(3), 321–328. DOI 10.5958/j.0976-0571.37.3.049. [Google Scholar] [CrossRef]
10. Lopez-Zamora, I., Duryea, M. L., Wild, C. M. C., Comerford, N. B., Neary, D. G. (2001). Effect of pine needle removal and fertilization on tree growth and soil P availability in a Pinus elliottii Engelm var. elliottii stand. Forest Ecology and Management, 148(1), 125–134. DOI 10.1016/S0378-1127(00)00484-9. [Google Scholar] [CrossRef]
11. Osiecka, A., Minogue, P. J., Miwa, M., Lauer, D. K. (2020). Nutrient removals by pinestraw harvesting in slash pine plantations in Florida. Forest Science, 3(3), 314–325. DOI 10.1093/forsci/fxz080.
12. Wei, R. P. (1990). Introduction and management of Pinus elliottii over world and China. Word Forestry Research, 3, 56–62. [Google Scholar]
13. Lodge, D. J. (1990). Negative associations among mycorrhizal fungi and some ectomycorrhizal fungi inabiting the same root system. DIKOS, 57, 347–404. [Google Scholar]
14. Guo, X. Z., Zhao, T. C., Dou, Z. F., Guo, M. W. (1983). Preliminary study on mycorrhizal fungi in mixed forest of Populus canadensis and Robinia pseudoacacia. Forest Science and Technology, 7, 10–13. [Google Scholar]
15. Tang, M., Chen, H., Guo, J. L., Hou D. W. (1994). A study of the ectomycorrhiza of poplar. Scientia Silvae Sinicae, 30(5), 437–440. [Google Scholar]
16. Mu, Q. L. (2013). The influence of inoculation of arbuscular mycorrhizal fungi and ectomycorrhizal fungi on Populus cana (Master Thesis). University of Inner Mongolia, China. [Google Scholar]
17. Tan, G. Y., Zhou, B. C. (1987). Extraction and determination of dimethyl sulfoxide from chlorophyll of benthic algae. Ocean and Lake Marsh, 3, 295–300. [Google Scholar]
18. Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. [Google Scholar]
19. Wei, F., Zheng, Q. K., Luo, S. Q., Qiu, J., Yang, W. F. et al. (2014). A method for measuring soluble sugars and starch in bark and xylem of rubber tree. Chinese Journal of Tropical Agriculture, 4, 12–16. [Google Scholar]
20. Wang, X. K. (2012). Principles and techniques of plant physiology and biochemistry experiments. China: Higher Education Press. [Google Scholar]
21. Zou, Q. (2003). Plant physiology experiment guide. China: China Agriculture Press. [Google Scholar]
22. Bollmark, M., Kubat, B., Eliasson, L. (1988). Variations in endogenous cytokinin content during adventitious root formation in pea cutting. Journal of Plant Physiology, 132(3), 262–265. DOI 10.1016/S0176-1617(88)80102-0. [Google Scholar] [CrossRef]
23. Yang, J. C., Zhang, J. H., Wang, Z. Q., Zhu, Q. S., Wang, W. (2001). Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiology, 127(1), 315–323. DOI 10.1104/pp.127.1.315. [Google Scholar] [CrossRef]
24. Weiler, E. W., Jordan, P. S., Conrad, W. (1981). Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Plant, 153(6), 561–571. DOI 10.1007/BF00385542. [Google Scholar] [CrossRef]
25. Leyval, C., Berthelin, J. (1993). Rhizodeposition and net release of soluble organic compounds by pine and beech seedlings inoculated with rhizobacteria and ectomycorrhizal fungi. Biology & Fertility of Soils, 15(4), 259–267. DOI 10.1007/BF00337210. [Google Scholar] [CrossRef]
26. Duchesne, L. C., Peterson, R. L., Ellis, B. E. (2006). Pine root exudate stimulates antibiotic synthesis by the ectomycorrhizal fungus Paxillus involutus. New Phytologist, 108(4), 471–476. DOI 10.1111/j.1469-8137.1988.tb04188.x. [Google Scholar] [CrossRef]
27. Gómez-Cadenas, J. A., Kohlen, W., Charnikhova, T., Mulder, P. Undas, A. et al. (2010). Does abscisic acid affect strigolactone biosynthesis. New Phytologist, 187(2), 343–354. DOI 10.1111/j.1469-8137.2010.03291.x. [Google Scholar] [CrossRef]
28. Song, F., Kong, X. S., Dong, A. R., Liu, X. F. (2012). Impact of arbuscular mycorrhizal fungi on the growth and related physiological indexes of Amorpha fruticosa. Journal of Medicinal Plant Research, 6(20), 3648–3655. [Google Scholar]
29. Liu, R. J., Li, M., Meng, X. X., Liu, X., Li, X. L. (2000). Effects of AM fungi on endogenous hormones in corn and cotton plants. Mycosystema, 19(1), 91–96. [Google Scholar]
30. Stoller, J. H., Leclere, S., Liptay, A. (2006). Methods for improving growth and crop productivity of plants by adjusting plant hormone levels, ratios and/or co-factors. Patent No: US8207091B2. [Google Scholar]
31. Goh, D. M., Cosme, M., Kisiala, A. B., Mulholland, S., Said, Z. M. F. et al. (2019). A stimulatory role for cytokinin in the arbuscular mycorrhizal symbiosis of pea. Frontiers in Plant Science, 10, 1–18. DOI 10.3389/fpls.2019.00262. [Google Scholar] [CrossRef]
32. Cao, X. C., Zhu, C. Q., Zhong, C., Hussain, S. Zhu, L. F. et al. (2017). Mixed-nitrogen nutrition-mediated enhancement of drought tolerance of rice seedlings associated with photosynthesis, hormone balance and carbohydrate partitioning. Plant Growth Regulation, 12, 1–15. [Google Scholar]
33. Mwange, K., Hou, H. W., Cui, K. M. (2003). Relationship between endogenous indole-3-acetic acid and abscisic acid changes and bark recovery in Eucommia ulmoides Oliv. after girdling. Journal of Experimental Botany, 54(389), 1899–1907. DOI 10.1093/jxb/erg204.
34. Bialek, K., Meudt, W. J., Cohen, J. D. (1983). Indole-3-acetic Acid (IAA) and IAA conjugates applied to bean stem sections: IAA content and the growth response. Plant Physiology, 73(1), 130–134. DOI 10.1104/pp.73.1.130.
35. Zhang, W., Fan, J., Tan, Q., Zhao, M. (2016). Mechanisms underlying the regulation of root formation in Malus hupehensis stem cuttings by using exogenous hormones. Journal of Plant Growth Regulation, 9, 1–12. [Google Scholar]
36. Yang, R., Zheng, Q. Y., Xue, H. Q., Yang, X. H. (2009). Influences of AM fungi on growth and water ecophysiology of grouped Shatian pomelo during cultivating and training periods. Journal of Chongqing Normal University, 26(2), 115–119. [Google Scholar]
37. Bi, Y., Zhang, J., Song, Z., Wang, Z. G., Qiu, L. et al. (2019). Arbuscular mycorrhizal fungi alleviate root damage stress induced by simulated coal mining subsidence ground fissures. Science of the Total Environment, 652, 398–405. DOI 10.1016/j.scitotenv.2018.10.249. [Google Scholar] [CrossRef]