DOI:10.32604/phyton.2021.012968

| Phyton-International Journal of Experimental Botany DOI:10.32604/phyton.2021.012968 |  |
| Article |
Species Diversity of Arbuscular Mycorrhizal Fungi in the Rhizosphere of Hevea brasiliensis in Hainan Island, China
1Guizhou Tea Research Institute, Guizhou Province Academy of Agricultural Science, Guiyang, 550006, China
2Agricultural Technology Research and Extension Center of Qiongzhong Li and Miao Autonomous County, Qiongzhong, 572900, China
3The Provincial Key Laboratory for Agricultural Pest Management in Mountainous Region, Institute of Plant Protection, College of Agriculture, Guizhou University, Guiyang, 550025, China
4College of Environment and Plant Protection, Hainan University, Haikou, 570228, China
*Corresponding Author: Zengping Li. Email: lzping301155@126.com
Received: 20 July 2020; Accepted: 31 July 2020
Abstract: Hevea brasiliensis is one of the important economic trees with a great economic value for natural rubber production. Symbiosis between roots of H. brasiliensis and arbuscular mycorrhizal fungi (AMF) is widely recognized, and can provide a range of benefits for both of them. Hainan Island harbors is one of the largest plantations of H. brasiliensis in China, whereas the information regarding the diversity of AMF in the rhizosphere of H. brasiliensis on this island is scarce. The diversity of AMF species in the rhizosphere of rubber tree plantations in Hainan was investigated in this study. A total of 72 soil samples from the rhizosphere of H. brasiliensis RY7-33-97 were collected. These included 48 samples from plantations in 11 cities or counties that had been planted for 15–25 years, and 24 samples from a demonstrating plantation site of the China National Rubber Tree Germplasm Repository representing plantations with tree plantation ages from one to 40 year-old. Collectively, a total of 68 morphotypes of AMF, belonging to the genera of Archaeospora (1), Glomus (43), Acaulospora (18), Entrophospora (3), Scutellospora (2), and Gigaspora (1) were isolated and identified, as per morphological characteristics of spores presented in the collected soil samples. Glomus (Frequency, F = 100%) and Acaulospora (F = 100%) were the predominant genera, and A. mellea (F = 63.9%) and A. scrobiculata (F = 63.9%) were the predominant species. AMF species differed significantly among collected sites in spore density (SD, 290.7–2,186.7 spores per 100 g dry soil), species richness (SR, 4.3–12.3), and Shannon-Weiner index of diversity (H, 1.24–2.24). SD was negatively correlated with available phosphorus level in the soil; SR was positively correlated with soil total phosphorus content; and H was positively correlated with levels of soil organic matter and total phosphorus. Similarly, SD, SR, and H were also correlated with H. brasiliensis plantation age, and an increasing trend was observed up to 40 years. These results suggest that the AMF community was complex and ubiquitous in the island plantation ecosystems of H. brasiliensis, with high species abundance and diversity. Soil factors and plantation age dramatically affected AMF diversity at species level.
Keywords: Arbuscular mycorrhizal fungi; rubber tree; species diversity; influence factors; plantation age
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
Arbuscular mycorrhizal fungi (AMF) are widely distributed across various ecosystems [1–4]. AMF can establish symbiotic association with roots of host plants to stimulate water and nutrient acquisition of host plants [5–8]. AMF species composition varies with different environment conditions [9] and host plants [10,11]. Although AMF diversity has been extensively studied in grasses [12], bushes [13,14], and dwarf trees [15,16], the information regarding AMF species diversity associated with perennial deciduous megaphanerophytes in an island plantation ecosystem is scarce.
Hevea brasiliensis (Willd. ex A.Juss.) Müll. Arg. is a perennial deciduous megaphanerophyte tree species that is native to the Amazon River Basin of South America. It is planted around the world for its production of natural rubber, which constitutes one of the four major industrial raw materials and is particularly important in the military and traffic industries. Earlier studies have shown that H. brasiliensis formed arbuscular mycorrhizal (AM) structures in roots [17]. Jayaratne [18] and Ikram et al. [19] found that the extent of root AMF colonization ranged from 0 to 50%, and Glomus and Acaulospora were the most common AMF genera in Sri Lanka and Malaysia, respectively. Herrmann et al. [20] found that roots of H. brasiliensis were highly colonized by AMF, whereas the colonization level was not affected by either soil nutrient levels or tree ages. Previous studies mainly focused on AM morphological structures and AMF populations associated with the roots of H. brasiliensis in a natural ecosystem, less is reported for island plantation ecosystems in Southeast Asia.
With more than 5,000,000 hectares of H. brasiliensis plantations, Hainan Island is one of the largest plantation areas for H. brasiliensis in China. The closed and independent plantation ecosystems of H. brasiliensis in Hainan Island represent a unique example of island biogeography. In addition, less aggressive cultivation practices have been applied in the plantations in Hainan Island, compared to plantation ecosystems of other species, such as tea or coffee, which are also perennials with abundant AMF populations [13,21]. Considering that cultivation practices in plantations are known to adversely affect AMF colonization and non-Glomus AMF spore populations [22], the present work hypothesized that soil factors or plantation age affected AMF species diversity in the rhizosphere of H. brasiliensis, and high AMF abundance and species diversity were expected due to low degree of cultivation disturbance in the plantations. Understanding of AMF diversity in H. brasiliensis is essential for improving land management, sustainability, and productivity of plantation ecosystems, particularly the ones with highly diverse AMF communities [23–25].
Topographically, Hainan Island (N 18°10–20°10, E 108°37–111°03) is dominated by hills and low mountains with 500–800 meters above sea level. This region has a typical tropical oceanic monsoon climate, with long summer, short winter, high solar radiation, and a narrow temperature range. The annual average rainfall ranges from 1,607 to 2,000 mm and annual average temperature ranges from 20.5 to 28.5°C. In the whole Hainan Island, eleven cities or counties with the largest H. brasiliensis plantation areas was selected as sampling sites (Fig. 1), and RY7-33-97, the most common H. brasiliensis planted in these plantations, as the sampling cultivar.
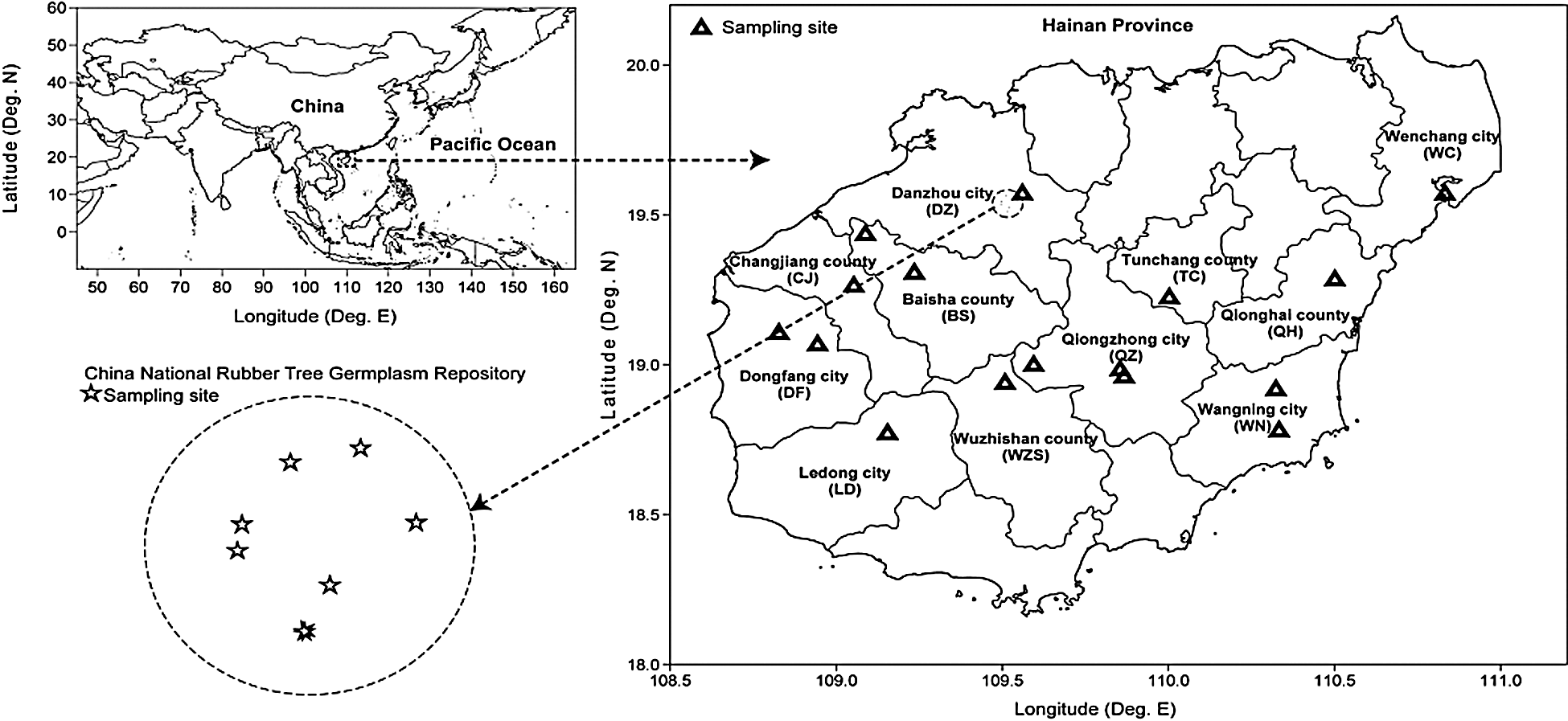
Figure 1: Location of the sampling site in this study. Samples from 11 cities or counties were collected from different plantations planted at 15–25 years. Samples from China National Rubber Tree Germplasm Repository were collected from 1–40 planting years. Three samples were collected in each sampling site
The China National Rubber Tree Germplasm Repository, possessing the oldest and largest rubber tree plantation, is located in Danzhou, northwest of Hainan Island. This repository belongs to the test farm of the Chinese Academy of Tropical Agricultural Sciences and has a tropical zone and monsoon climate with distinct dry and wet seasons. The annual average temperature in this region ranges from 20.5 to 28.5°C. Presently, this repository is the largest germplasm resource garden in China, with 6,075 rubber trees belonging to six genera.
2.2 Collection of Soil Samples
Soil samples were randomly collected at the base of five trees, in an area of 500 m2 at each site with a drill with their intact root systems up to 40 cm soil depth taking up to 3 kg of soil per sample. All the 72 soil samples were collected from the rhizosphere of H. brasiliensis RY7-33-97. Of these, 48 soil samples were collected from the rhizosphere of H. brasiliensis during the most productive phase (between 15 and 25 years) across 11 cities or counties on Hainan Island in July 2008, while 24 soil samples were collected from trees cultivated for 1–40 years from the China National Rubber Tree Germplasm Repository in August 2009 (Fig. 1). Five soil samples (0.5 kg each) located at the top layer of soil (40 cm in depth) were randomly collected from each tree at the same plantation site, and thoroughly mixed and stored in plastic bags after being air-dried.
2.3 Counts and Identification of AMF Spores
Spores or sporocarps were isolated from 20–100 g of soil sample according to the protocol described by Gerdemann et al. [26], followed by sucrose centrifugation [27]. The spores were counted three times under an Olympus-BX51 microscope (Japan). In addition, spores of AMF were placed on glass slides in polyvinyl lactoglycerol (PVLG) and Melzer’s reagent (v:v = 1:1) and identified based on spore morphological characteristics under microscope, such as spore size, spore color, spore wall thickness and so on, following the descriptions published on INVAM (http://invam.caf.wvu.edu) and the taxonomic criteria of Morton et al. [28].
2.4 Determination of Soil Chemical Properties and Plantation Age
Soil pH, total phosphorus (Pt), available phosphorus (Pa), available K (Ka), and organic matter (OM), were measured according to Bao [29]. The plantation age was estimated by measuring the length of the tapped bark on the trunk of an H. brasiliensis tree following the local conventional practice in harvesting latex using an annual tapping system. Rubber trees are tapped five years after being transplanted in the field, following which latex saps are harvested about 50 times per year by means of a half spiral downward cut in the bark. The tapped barks differ visibly across the years, even when the same bark was tapped twice or more. The length of the tapped bark segments was approximately 5 cm per year, with each cut being about 1 mm. Cuts were made about 50 times per year. According to the tapping length, the selected trees were placed in different plantation age groups. Untapped H. brasiliensis plants were assigned to the 0–5-year-plantation group, and trees with tapped bark ≤25 cm in length were assigned to the 5–10-year-plantation group. Similar increments up to 40 years were made.
Species richness (SR), spore density (SD), and AMF species frequency (F) were expressed as follows: SR = number of AMF species found in the total number of sample sites; SD = number of AM fungal spores in 100 g dry soil; F = number of samples in which the genus or species was observed/Total number of samples × 100%. Based on the frequency of occurrence, the AMF genera and species with a frequency greater than 50% (F > 50%) were classified as “predominant” according to Zhang et al. [30]. Species diversity was assessed using the Shannon–Wiener index (H) as follows: H = −Sum (Pi ln[Pi]), where Pi = ni/N, ni = Number of individuals in species I, and N is the total number of individuals in all species. The mapping software employed in the study was Surfer (R) (Version 8.0, Golden Software, Inc.). All data were analyzed by using SPSS (Version 20.0, IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) was used to test the significance of the overall variations in all measured indexes among the sample sites. Statistical analyses were performed for cases with no missing data for any variable. When appropriate, the means were compared using Tukey’s test. Correlations between soil edaphic factors and SD, SR, and H were examined.
A total of 68 AMF morphotypes were identified from the rhizosphere of H. brasiliensis (Tab. 1). Among them, 59 morphotypes were identified to the species level, whereas nine morphotypes were identified to the genus level and were designated as sp. The 68 morphotypes belonged to six genera. Among these morphotypes, one belonged to Archaeospora (species-level), 43 to Glomus (39 species-level + 4 genus-level), 18 to Acaulospora (16 species-level + 2 genus-level), three to Entrophospora (1 species-level + 2 genus-level), two to Scutellospora (species level), and one to Gigaspora (genus level). Overall, Glomus was the most abundant genus (63.2% of total total morphotypes) among all samples, followed by Acaulospora (26.5%), and Entrophospora (8.9%). These results indicated that AMF were abundant in the rhizosphere of H. brasiliensis.
Table 1: Frequency of AMF genera/species in the rhizosphere of H. brasiliensis at different sampling sites on Hainan Island
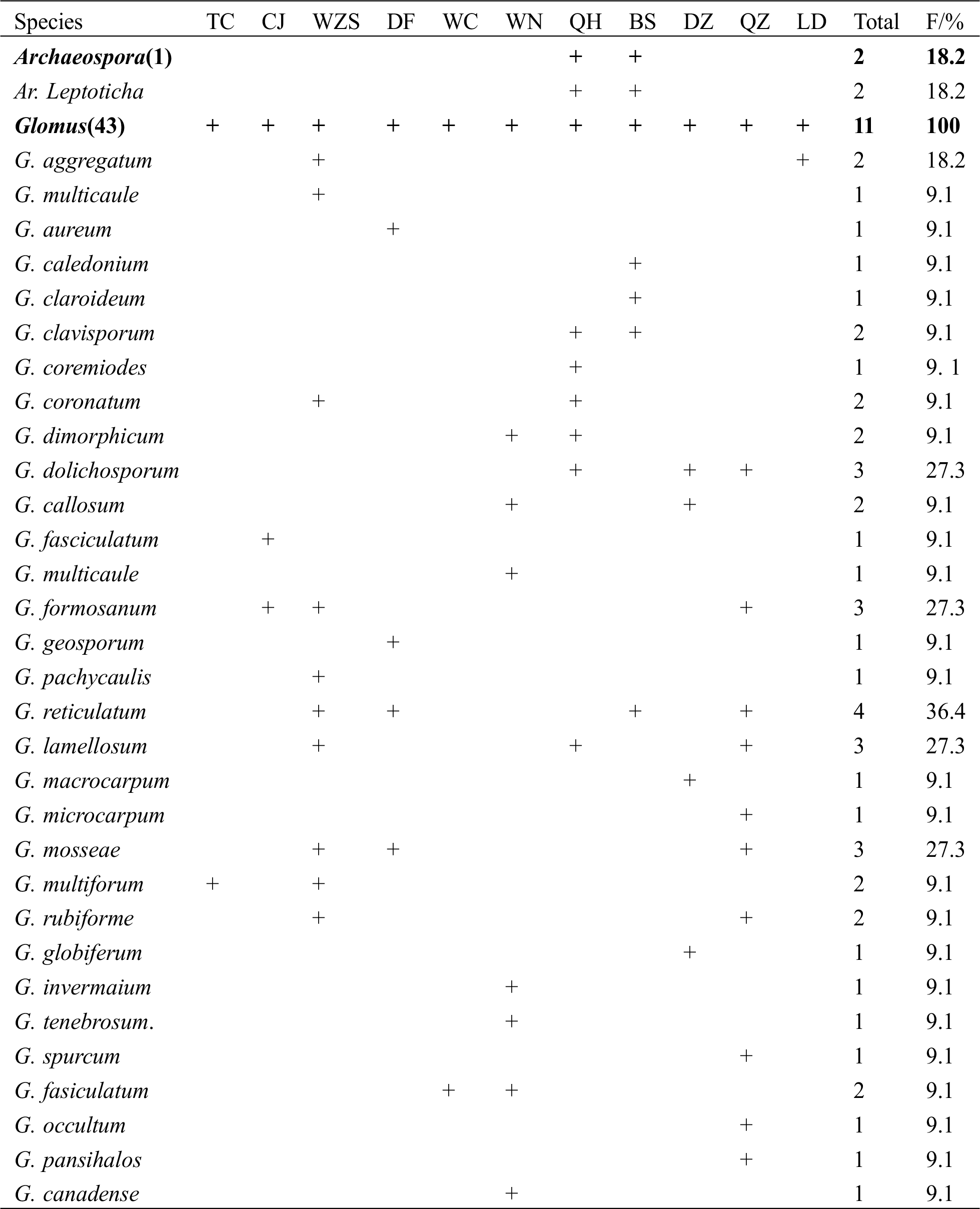
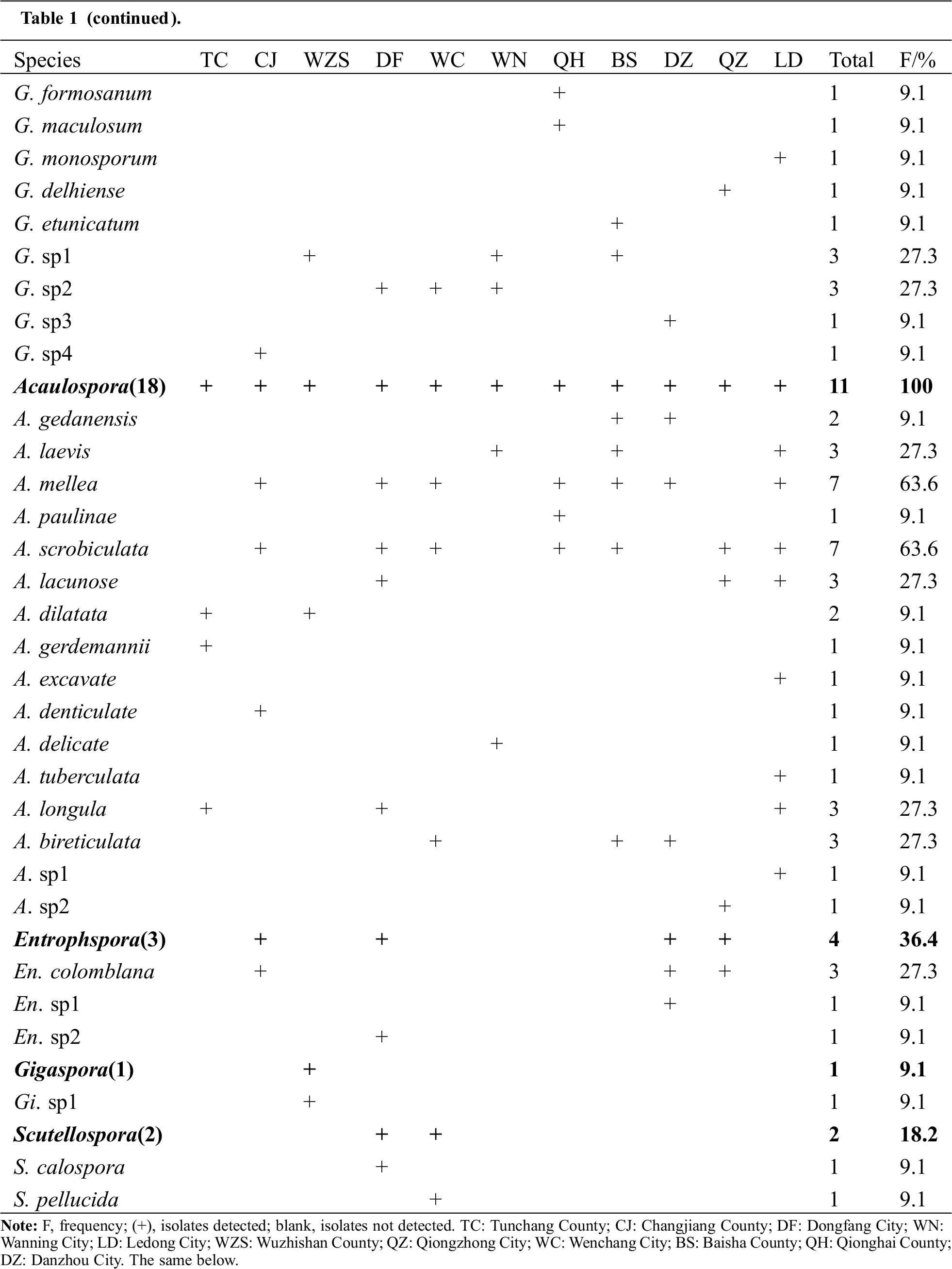
3.2 Isolation Frequency of AMF Species
The frequencies of AMF morphotypes representing various genera and species were presented in Tab. 1. Morphotypes belonging to Glomus and Acaulospora were isolated from all 11 sampling sites, with a frequency of 100%, indicating that they were the predominant genera in the rhizosphere of H. brasiliensis. Specifically, two members of the genus Acaulospora, A. mellea (F = 63.9%) and A. scrobiculata (F = 63.9%), were the predominant AMF species.
3.3 AMF Spore Density and Species Richness
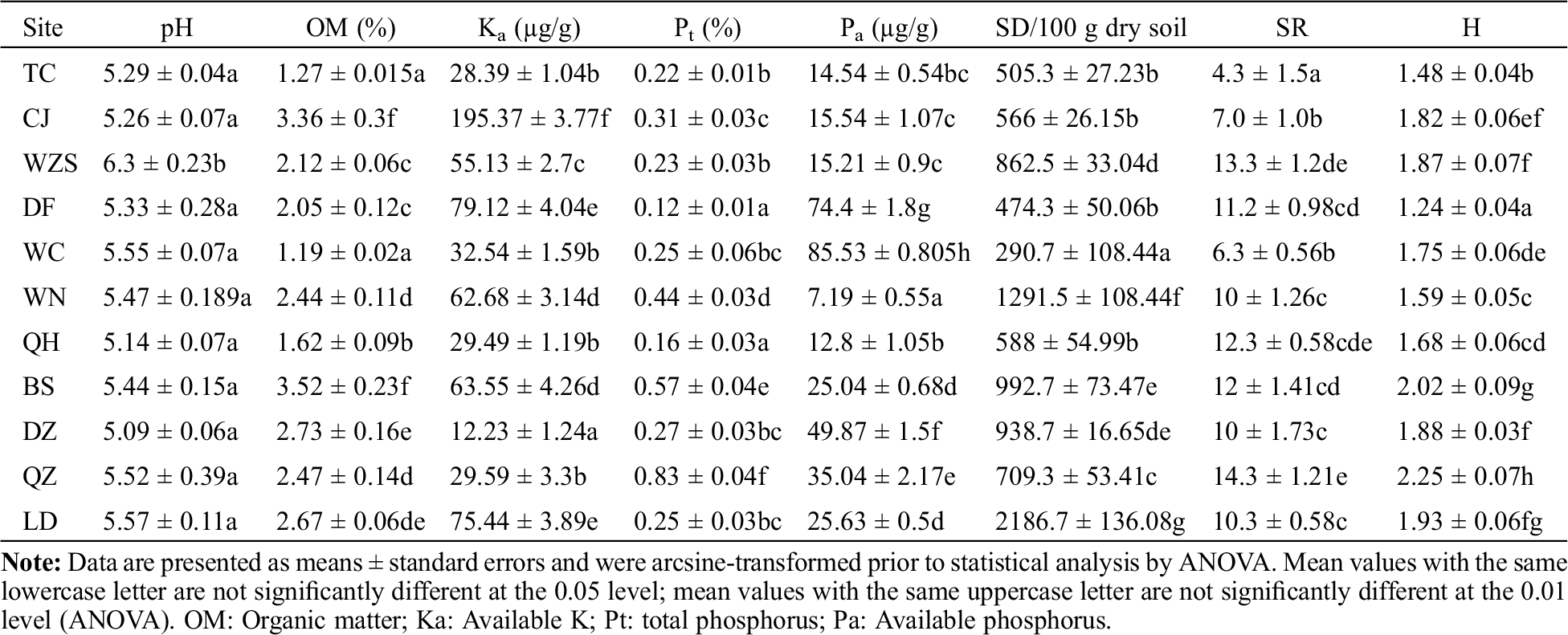
The AMF SD and SR varied among sampling sites (Tab. 2). The highest SD value was 2,186.7 spores per 100 g dry soil, whereas the lowest SD was only 290.7 spores per 100 g dry soil. The SR value ranged from 4.3 to 12.3. However, high SR did not necessarily correlate to high SD. The highest SR values, 12.0 and 12.3, were observed in Qionghai county (QZ) and Baisha county (BS), respectively, but the corresponding SD values were relatively low, with only 588 and 992.7 spores per 100 g dry soil, respectively. The highest SD value (2,186.7 spores per 100 g dry soil) was observed in Ledong City (LD), with an SR value of 10.3 (Tab. 2).
Table 2: Soil factors, spore density (SD), species richness (SR), and species diversity (H) of AMF from H. brasiliensis of different sampling sites
3.4 AMF Species Diversity and Correlations Among Parameters
The averaged AMF Shannon–Weiner index (H) value in the rhizosphere of H. brasiliensis in Hainan Island was found to be 1.77. The highest H value (2.25) was found in the samples collected from Qiongzhong city (QZ), whereas the lowest (1.24) was observed in those from Dongfang City (DF) (Fig. 2).
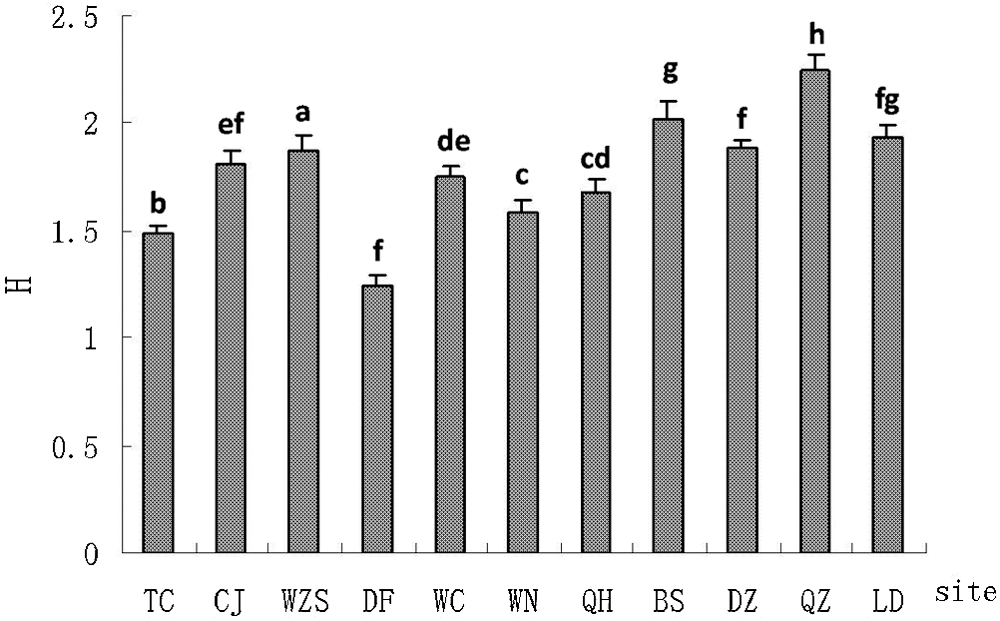
Figure 2: Species diversity of AMF in the rhizosphere of H. brasiliensis at different sampling sites. Error bars are indicated as standard errors, and different lowercase letters above the bar indicate significant difference at the 0.05 level
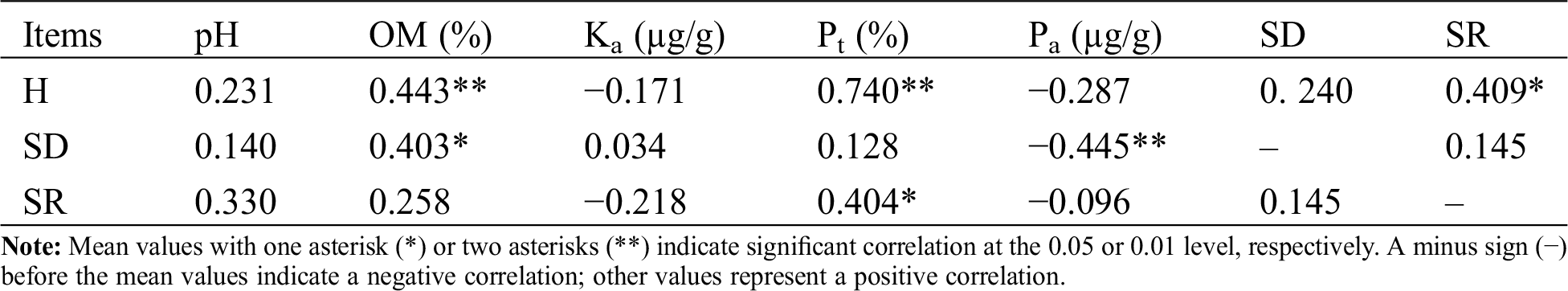
SD was significant positively correlated with OM (at the 5% level) and negatively correlated with Pa (at the 1% level). SR had a negative correlation with Pt (at the 5% level). Across all the sample sites, H value had a significant positive correlation with SR (at the 5% level) and soil factors, such as OM (at 1% level) and Pt (at the 1% level) (Tab. 3).
Table 3: Correlation between soil factors and species diversity (H), spore density (SD), species richness (SR) of AMF species
There was no significant difference in the first 15 years of plantation, SD (Fig. 3a) and H value (Fig. 3c) significantly increased after 15 years. Similarly, SR (Fig. 3b) significantly increased after 10 years of plantation. These results showed that SD, SR, and H value in the rhizosphere of H. brasiliensis were influenced by the plantation age.
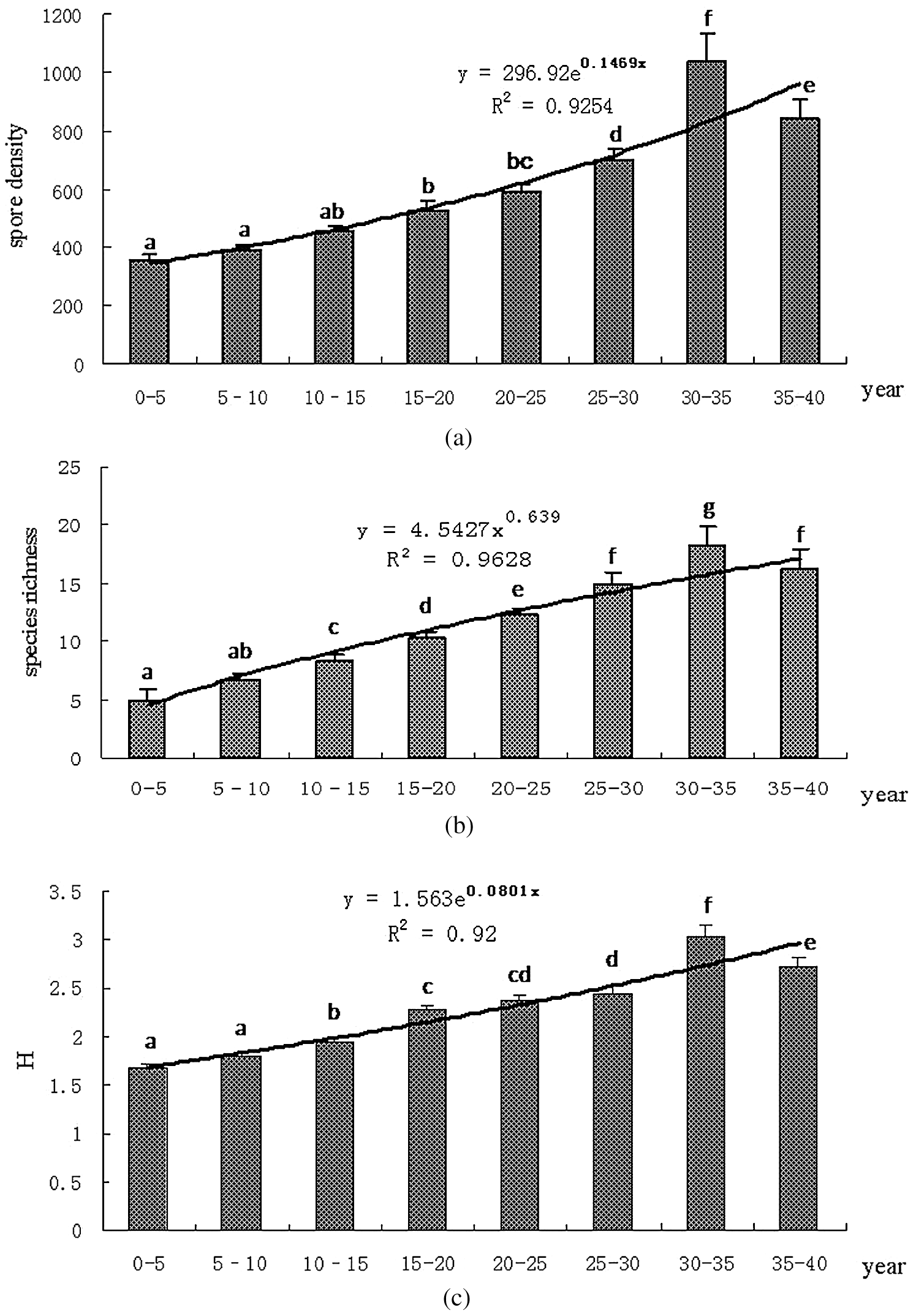
Figure 3: Effects of plantation age of H. brasiliensis on SD (Fig. 3a), SR (Fig. 3b), and H (Fig. 3c) of AMF species. The curve shows the variation trend, and R is the correlation coefficient. Different letters indicate significant difference at the 0.05 level
Earlier studies reported no more than 33 AMF species identified from the Hainan Island, China [31–35]. The present study isolated and identified 68 morphotypes of AMF from the rhizosphere of H. brasiliensis, with 59 morphotypes identified to the species level and nine morphotypes to the genus level. The 68 AMF morphotypes represented a relatively high number, compared with those that have been reported to be associated with other plant species in Hainan Island [31–35] and other foreign islands [36–41]. Thus, the present results indicated a relatively high abundance of AMF species in the rhizosphere of H. brasiliensis in Hainan Island. All the identified 59 AMF species had been reported in other plants in China. Among these species, G. fasciulatum, G. macrocarpum, G. microcarpum, G. mosseae, G. multiforum, G. monosporum, A. scrobiculata, and S. calospora have also been reported in rhizosphere of H. brasiliensis in Sri Lanka [18].
Long-term interactions with unique plant species help shape the dominant species in AMF communities. Glomus and Acaulospora were the predominant AMF genera in this study, consistent with findings from previous reports [2,9,12,32,41,42]. In addition, the predominant species associated with H. brasiliensis were A. mellea and A. scrobiculata, which were rarely isolated from other plants and few reported as the predominant AMF species. Nevertheless, Glomus mosseae was always reported as the predominant AMF species in other studies [43], whose frequency was found to be only 27.3% in the present study.
The H value of H. brasiliensis AMF (1.77) in the present study was similar to that of Camellia sinensis (1.93) [13]. Although both H. brasiliensis and C. sinensis are perennial cultivated plants, their AMF H values are relatively low compared to desert ephemeral plants in the Junnggar Basin, where AMF H values ranged from 2.16 to 2.86 in four desert plant community types [44]. Moreover, The AMF H value of H. brasiliensis was also lower than that of pine trees in the forest ecosystem, in which H values were recored as high as 2.75 [45]. Generally, H values of AMF tend to be lower in plantation ecosystems due to the indiscriminate use of fertilizers [46] and pesticides [47,48]. Furthermore, continuous farming may exert negative effects on AMF colonization and non-Glomus AMF spore populations [49].
In a given host plant rhizosphere and climatic condition, it is widely accepted that edaphic factors, such as soil pH, Pt, Pa, and OM, substantially influence the abundance and diversity of AMF [9,50,51]. The present study indicated that soil P levels and OM influenced AMF SD, SR, and H: SR was found to be positively correlated with Pt, and H was positively correlated with soil OM and Pt. Furthermore, SD, SR, and H of AMF increased with the plantation age of H. brasiliensis up to 40 years. Plantation age represents the number of years for which the trees have been planted in a plantation, and provides an indication of the symbiotic relationship that may have been established. Therefore, plantation age is considered to be one of the most important characteristics for plantations, especially for tall perennial trees. The present work indicated that within 40 years of plantation, the older H. brasiliensis trees appeared to have higher AMF SD, SR, and diversity than the younger ones. This result is consistent with the findings in Liu et al. [52] and Singh et al. [13], which reported that older plants tended to have more extensive AMF colonization and more AMF species within a certain range of years. Interestingly, Cheng et al. [53] and Schreiner et al. [54] collectively asserted that AMF declined as plantation age increases, and AMF abundance negatively correlated with plantation age.
One of the reasons that causes H. brasiliensis AMF SD, SR, and H values to increase with plantation age may be that root activity and its spatial distribution, as well as soil organic carbon, increases with plantation age [55–57]. These factors significantly regulate the colonization and development of AMF. A second explanation could be that seasonal changes in AMF lead to annual variations in AMF distribution. Studies had indicated that AMF spore production and root colonization with numerous plants represented seasonal variation [58–62]. Lutgen et al. examined AMF extraradical hyphae and their exuded products, such as glomalin, which was directly related to ecosystem processes. It was found that these factors varied significantly with seasons [63], because of variations in seasonal tillage practices, fertilization, rainfall, fluctuation, and phenology of plants [64–67]. As a result, these seasonal changes may be associated with the annual variations in SD, SR, and H values of AMF. A third explanation may be disturbance, including current annual cultivation practices, in combination with carbon drain experienced in older plants, which potentially resulted in the loss of some large-spore of AMF, such as Scutellospora and Gigaspora [13]. In contrast, small-spore AMF, such as those belonging to the genus Glomus, were enriched.
Currently, the mechanism underlying the increase of SD, SR, and H values of AMF with the plantation age of H. brasiliensis remains poorly understood. Further study on this aspect will help us better understand the life history of AMF, as well as their basic biology in a plantation ecosystem. Importantly, the plantation age of H. brasiliensis, which was estimated based on the length of tapped bark, is only an approximation of the actual tree age. In some cases, H. brasiliensis might not be tapped for up to several months or a whole year due to diseases or extreme weather conditions. Also, prior to being transplanted in the field, H. brasiliensis seedlings with 6–7 loose leaves are generally grafted and grown for 2–4 months.
In summary, abundant and diverse AMF species were detected in the rhizosphere of H. brasiliensis grown in the Hainan Island. Soil factors (organic matter and total phosphorus are the most important soil factors) and cultivation age considerably affected AMF diversity at the species level. In the future, the mechanism regarding the influence of cultivation age on AMF diversity should be explored to guide field cultivation of H. brasiliensis.
Acknowledgement: The authors would like to thank Dr. Haiying Liang, Associate Professor of Clemson University, South Carolina, United States, for helping review and edit the article.
Funding Statement: This work was supported by the National Natural Science Foundation of China (31960616), the Construction of Modern Agriculture (tea) Industry Technology System (CARS-19) (China), the Key Agricultural Science and Technology Foundation of Guizhou Province ([2016]2570) (China), the Science and Technology Foundation of Guizhou Province (2013[2155]) (China), and the High-value Patent Cultivation Project of Guizhou Province Academy of Agricultural Sciences ([2018]02) (China).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Estrada, B., Beltrán-Hermoso, M. D., Palenzuela, J., Iwase, K., Ruiz-Lozano, J. M. et al. (2013). Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Asteriscus maritimus (L.) Less., a representative plant species in arid and saline Mediterranean ecosystems. Journal of Arid Environments, 97(10), 170–175. DOI 10.1016/j.jaridenv.2013.05.019.
2. Liu, R. J., Tian, M., Liu, N., Li, Q. (2014). Advances in the study of dual symbionts formed on plant roots. Journal of Fungal Research, 12(1), 1–6 (in Chinese). [Google Scholar]
3. Bonfim, J. A., Vasconcellos, R. L. F., Gumiere, T., Mescolotti, D. D. L. C., Oehl, F. et al. (2016). Diversity of arbuscular mycorrhizal fungi in a Brazilian Atlantic forest toposequence. Microbial Ecology, 71(1), 164–177. DOI 10.1007/s00248-015-0661-0.
4. Yu, J., Xue, Z., He, X., Liu, C., Steinberger, Y. (2017). Shifts in composition and diversity of arbuscular mycorrhizal fungi and glomalin contents during revegetation of desertified semiarid grassland. Applied Soil Ecology, 115, 60–67. DOI 10.1016/j.apsoil.2017.03.015. [Google Scholar] [CrossRef]
5. He, J. D., Chi, G. G., Zou, Y. N., Shu, B., Wu, Q. S. et al. (2020). Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Applied Soil Ecology, 154, 103592. DOI 10.1016/j.apsoil.2020.103592. [Google Scholar] [CrossRef]
6. Meng, L. L., He, J. D., Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total nitrogen in trifoliate orange. Plant, Soil and Environment, 66(4), 183–189. DOI 10.17221/100/2020-PSE.
7. Rausch, C., Daram, P., Brunner, S., Jansa, J., Lalol, M. et al. (2001). Phosphate transporter expressed in arbuscule-containing cells in potato. Nature, 414(6862), 462–465. DOI 10.1038/35106601.
8. Zhang, F., Zou, Y. N., Wu, Q. S., Kuča, K. (2020). Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environmental and Experimental Botany, 171, 103962. DOI 10.1016/j.envexpbot.2019.103962. [Google Scholar] [CrossRef]
9. Silva, I. R. D., Mello, C. M. A. D., Neto, R. A. F., Silva, D. K. A. D., Mello, A. L. D. et al. (2014). Diversity of arbuscular mycorrhizal fungi along an environmental gradient in the Brazilian semiarid. Applied Soil Ecology, 84, 166–175. DOI 10.1016/j.apsoil.2014.07.008. [Google Scholar] [CrossRef]
10. Kennedy, L. J., Tiller, R. L., Stutz, J. C. (2002). Associations between arbuscular mycorrhizal fungi and Sporobolus wrightii in riparian habitats in arid southwestern North America. Journal of Arid Environments, 50(3), 459–475. DOI 10.1006/jare.2001.0899. [Google Scholar] [CrossRef]
11. Chaurasia, B., Pandey, A., Palni, L. M. S. (2005). Distribution, colonization and diversity of arbuscular mycorrhizal fungi in rhododendrons of central Himalayan region of India. Forest Ecology and Management, 207(3), 315–324. DOI 10.1016/j.foreco.2004.10.014. [Google Scholar] [CrossRef]
12. Lugo, M. A., Ferrero, M., Menoyo, E., Estěvez, M. C., Siňeriz, F. et al. (2008). Arbuscular mycorrhizal fungi and rhizospheric bacteria diversity along an altitudinal gradient in south American Puna grassland. Microbial Ecology, 55(4), 705–713. DOI 10.1007/s00248-007-9313-3. [Google Scholar] [CrossRef]
13. Singh, S., Pandey, A., Chaurasia, B., Palni, L. M. S. (2008). Diversity of arbuscular mycorrhizal fungi associated with the rhizosphere of tea growing in ‘natural’ and ‘cultivated’ ecosites. Biology and Fertility of Soils, 44(3), 491–500. DOI 10.1007/s00374-007-0231-9. [Google Scholar] [CrossRef]
14. Liu, S., Wang, J. G., Ci, Z. L., Bai, S. L., Shao, D. H. (2012). AMF diversity in Caragana in different habitats of Oerhtossu plateau. Journal of Inner Mongolia Agricultural University, 33(4), 74–80 (in Chinese). [Google Scholar]
15. Wu, Q. S., Shao, Y. D., Gao, X. B., Xia, T. J., Kuca, K. (2019). Characterization of AMF-diversity of endosphere versus rhizosphere of tea (Camellia sinensis) crops. Indian Journal of Agricultural Sciences, 89(2), 348–352. [Google Scholar]
16. Tarafdar, J. C., Rao, A. V. (1990). Survey of Indian arid zone tree species for the occurrence of VAM infection. Proceedings of the National Conference on Mycorrhizae. Hisar, India, Haryana Agricultural University, 47–49. [Google Scholar]
17. Angremond, D. A., Hell, V. W. F. (1939). Mycorrhiza van Hevea brasiliensis Müll.Arg. Reprinted from Versl. Ver. Proefst-Personeel, Medan, 1–16. [Google Scholar]
18. Jayaratne, A. H. R. (1982). Endomycorrhizas of rubber growing soils of Sri Lanka. Journal of Rubber Research Institute of Malaysia, 60, 47–85. [Google Scholar]
19. Ikram, A., Mahmud, A. W. (1984). Endomycorrhizal fungi in soils under rubber. Journal of Rubber Research Institute of Malaysia, 32(2), 198–206. [Google Scholar]
20. Herrmann, L., Bräu, L., Robin, A., Robain, H., Wiriyakitnateekul, W. et al. (2016). High colonization by native arbuscular mycorrhizal fungi (AMF) of rubber trees in small-holders plantations on low fertility soils in north east Thailand. Archives of Agronomy and Soil Science, 62(7), 1041–1048. DOI 10.1080/03650340.2015.1110238. [Google Scholar] [CrossRef]
21. Muleta, D., Assefa, F., Nemomissa, S., Granhall, U. (2008). Distribution of arbuscular mycorrhizal fungi spores in soils of smallholder agroforestry and monocultural coffee systems in southwestern Ethiopia. Biology and Fertility of Soils, 44(4), 653–659. DOI 10.1007/s00374-007-0261-3. [Google Scholar] [CrossRef]
22. Jansa, J., Mozafar, A., Anken, T., Ruh, R., Sanders, I. R. et al. (2002). Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza, 12(5), 225–234. DOI 10.1007/s00572-002-0163-z. [Google Scholar] [CrossRef]
23. Bever, J., Morton, J. B., Antonovics, J., Schultz, P. A. (1996). Host-dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland. Journal of Ecology, 84(1), 71–82. DOI 10.2307/2261701. [Google Scholar] [CrossRef]
24. Klironomos, J. N., McCune, J., Hart, M., Neville, J. (2000). The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecology Letters, 3(2), 137–141. DOI 10.1046/j.1461-0248.2000.00131.x.
25. Kowalczyk, S., Blaszkowski, J. (2011). Arbuscular mycorrhizal fungi (Glomeromycota) associated with roots of plants of the Lubuskie province. Acta Mycologica, 46(1), 3–18. DOI 10.5586/am.2011.001. [Google Scholar] [CrossRef]
26. Gerdemann, J. W., Nicolson, T. H. (1963). Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society, 46(2), 235–244. DOI 10.1016/S0007-1536(63)80079-0. [Google Scholar] [CrossRef]
27. Jenkins, W. R. (1964). A rapid centrifugal-flotation technique for separating nematodesfrom soil. Plant Disease Reporter, 48, 692. [Google Scholar]
28. Morton, J. B., Redecker, D. (2001). Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia, 93(1), 181–195. DOI 10.1080/00275514.2001.12063147. [Google Scholar] [CrossRef]
29. Bao, S. D. (2000). Soil and agricultural chemistry analysis. Beijing: Agriculture of China Press (in Chinese). [Google Scholar]
30. Zhang, Y., Liu, R. J. (2004). Survey of arbuscular mycorrhizal fungi in deforested and natural forest land in the subtropical region of Dujianyan, southwest China. Plant and Soil, 261(1/2), 257–263. DOI 10.1023/B:PLSO.0000035572.15098.f6. [Google Scholar] [CrossRef]
31. Shi, Z. Y., Chen, Y., Liu, R. J. (2003). Arbuscular mycorrrhizal fungi of dipterocarpaceae in Jianfengling mountain, Hainan province. Mycosystema, 22(2), 211–215 (in Chinese). [Google Scholar]
32. Shi, Z. Y., Chen, Y. L., Feng, G., Christie, P., Li, X. L. (2006). Arbuscular mycorrhizal fungi associated with the Meliaceae on Hainan Island, China. Mycorrhiza, 16(2), 81–87. DOI 10.1007/s00572-005-0017-6. [Google Scholar] [CrossRef]
33. Liang, C. C., Zhao, S. Y., Liu, L., Huang, J. S. (2010). Arbuscular mycorrhizal fungi associated with common trees species in a tropical rainforest in Bwangling of Hainan Island, China. Chinese Journal of Ecology, 29(2), 269–273 (in Chinese). [Google Scholar]
34. Zhang, W., Chen, Y., Li, Z. P. (2014). Species diversity of arbuscular mycorrhizal fungus associated with mangrove forests in Bamenbay in Hainan Province. Chinese Journal of Tropical Crops, 35(3), 583–589 (in Chinese). [Google Scholar]
35. Zhang, J. L., Huang, Z. R., Wang, Q., Feng, C. Y., Long, Y. Y. et al. (2016). The arbuscular mycorrrhizal fungi associated with the rhizosphere of wild Saccharum spontaneum in Hainan province. Abstract collection of papers of 2016 annual meeting of mycological society of China. Fuzhou, Mycological Society of China Press (in Chinese). [Google Scholar]
36. Mangan, S. A., Eom, A. H., Adler, G. H., Yavitt, J. B., Herre, E. A. (2004). Diversity of arbuscular mycorrhizal fungi across a fragmented forest in Panama: insular spore communities differ from mainland communities. Oecologia, 141(4), 687–700. DOI 10.1007/s00442-004-1684-2. [Google Scholar] [CrossRef]
37. Stover, H. J., Thorn, R. G., Bowles, J. M., Bernards, M. A., Jacobs, C. R. (2012). Arbuscular mycorrhizal fungi and vascular plant species abundance and community structure in tallgrass prairies with varying agricultural disturbance histories. Applied Soil Ecology, 60, 61–70. DOI 10.1016/j.apsoil.2012.02.016.
38. Melo, C. D., Walker, C., Rodríguez-Echeverría, S., Borges, P. A. V., Freitas, H. (2014). Species composition of arbuscular mycorrhizal fungi differ in semi-natural and intensively managed pastures in an isolated oceanic island (Terceira, Azores). Symbiosis, 64(2), 73–85. DOI 10.1007/s13199-014-0303-1.
39. Melo, C. D., Luna, S., Krüger, C., Walker, C., Mendonça, D. et al. (2017). Arbuscular mycorrhizal fungal community composition associated with Juniperus brevifolia in native Azorean forest. Acta Oecologica, 79, 48–61. DOI 10.1016/j.actao.2016.12.006.
40. Johansen, R. B., Vestberg, M., Burns, B. R., Park, D., Hooker, J. E. et al. (2015). A coastal sand dune in New Zealand reveals high arbuscular mycorrhizal fungal diversity. Symbiosis, 66(3), 111–121. DOI 10.1007/s13199-015-0355-x.
41. Yang, C., Hamel, C., Schellenberg, M. P., Perez, J. C., Berbara, R. L. (2010). Diversity and functionality of arbuscular mycorrhizal fungi in three plant communities in semiarid grasslands national park, Canada. Microbial Ecology, 59(4), 724–733. DOI 10.1007/s00248-009-9629-2. [Google Scholar] [CrossRef]
42. Pagano, M. C., Zandavalli, R. B., Araújo, F. S. (2013). Biodiversity of arbuscular mycorrhizas in three vegetational types from the semiarid of Ceará State, Brazil. Applied Soil Ecology, 67, 37–46. DOI 10.1016/j.apsoil.2013.02.007. [Google Scholar] [CrossRef]
43. Liu, R. J., Jiao, H., Li, Y., Li, M., Zhu, X. C. (2009). Research advances in species diversity of arbuscular mycorrhizal fungi. Chinese Journal of Applied Ecology, 20(9), 2301–2307 (in Chinese). [Google Scholar]
44. Shi, Z. Y., Zhang, L. Y., Li, X. L., Feng, G., Tian, C. Y. et al. (2007). Diversity of arbuscular mycorrhizal fungi associated with desert ephemerals in plant communities of Junggar Basin, northwest China. Applied Soil Ecology, 35(1), 10–20. DOI 10.1016/j.apsoil.2006.06.002. [Google Scholar] [CrossRef]
45. Wagg, C., Pautler, M., Massicotte, H. B., Peterson, R. L. (2008). The co-occurrence of ectomycorrhizal, arbuscular mycorrhizal, and dark septate fungi in seedlings of four members of the Pinaceae. Mycorrhiza, 18(2), 103–110. DOI 10.1007/s00572-007-0157-y. [Google Scholar] [CrossRef]
46. Muthukumar, T., Udaiyan, K. (2000). Influence of organic manures on arbuscular mycorrhizal fungi associated with Vignaun guiculata (L.) Walp. in relation to tissue nutrients and soluble carbohydrate in roots under field conditions. Biology and Fertility of Soils, 31(2), 114–120. DOI 10.1007/s003740050633. [Google Scholar] [CrossRef]
47. Schweiger, P. F., Jakobsen, I. (1998). Dose–response relationships between four pesticides and phosphorus uptake by hyphae of arbuscular mycorrhizas. Soil Biology and Biochemistry, 30(10–11), 1415–1422. DOI 10.1016/S0038-0717(97)00259-9. [Google Scholar] [CrossRef]
48. Connor, O. P. J., Smith, S. E., Smith, F. A. (2002). Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytologist, 154(1), 209–218. DOI 10.1046/j.1469-8137.2002.00364.x. [Google Scholar] [CrossRef]
49. Jansa, J., Mozafar, A., Anken, T., Ruh, R., Sanders, I. R. et al. (2002). Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza, 12(5), 225–234. DOI 10.1007/s00572-002-0163-z. [Google Scholar] [CrossRef]
50. Abbott, K. L., Robson, A. D. (1999). Factors influencing the occurrence of vesicular arbuscular mycorrhizas. Agriculture, Ecosystems & Environment, 35(2–3), 121–150. DOI 10.1016/0167-8809(91)90048-3. [Google Scholar] [CrossRef]
51. Ji, B., Bentivenga, S. P., Casper, B. B. (2012). Comparisons of AM fungal spore communities with the same hosts but different soil chemistries over local and geographic scales. Oecologia, 168(1), 187–197. DOI 10.1007/s00442-011-2067-0. [Google Scholar] [CrossRef]
52. Liu, Z. K., Tian, S., Tang, M. (2013). Spatial distribution of arbuscular mycorrhizal fungi and its relationship with soil factors in the rhizosphere of Robinia pseudoacacia at different ages. Scientia Silvae Sinicae, 49(8), 89–95 (in Chinese). [Google Scholar]
53. Cheng, S., Widden, P., Messeier, C. (2005). Light and tree size influence belowground development in yellow birch and sugar maple. Plant and Soil, 270(1), 321–330. DOI 10.1007/s11104-004-1726-x. [Google Scholar] [CrossRef]
54. Schreiner, R. P., Mihara, K. L. (2009). The diversity of arbuscular mycorrhizal fungi amplified from grapevine roots (Vitisvinifera L.) in Oregon vineyards is seasonally stable and influenced by soil and vine age. Mycologia, 101(5), 599–611. DOI 10.3852/08-169. [Google Scholar] [CrossRef]
55. Liu, C. Q., Song, X. K. (1986). Distribution of root activity of young rubber trees. Chinese Journal of Tropical Crops, 7(1), 19–24 (in Chinese). [Google Scholar]
56. Jessy, M. D., Thomas, V., Vijayakumar, K. R. (2005). Fine rootproduction of rubber trees (Hevea brasiliensis) in relation to precipitation. International Natural Rubber Conference, pp. 156–163, Cochin, India.
57. Guan, L. M., Wu, Z. X., Yang, C., Xie, G. S., Zhou, Z. D. (2012). Soil organic carbon pool and its influencing factors in rubber planted forest ecosystem at different ages in west Hainan province. Agricultural Science &Technology, 13(10), 2163–2168 (in Chinese). [Google Scholar]
58. Anderson, R. C., Liberta, A. E., Dickman, L. A. (1984). Interaction of vascular plants and vesicular-arbuscular mycorrhizal fungi across a soil moisture-nutrient gradient. Oecologia, 64(1), 111–117. DOI 10.1007/BF00377552. [Google Scholar] [CrossRef]
59. Gay, P. E., Grubb, P. J., Hudson, H. J. (1982). Seasonal changes in the concentrations of nitrogen, phosphorus, and potassium, and in the density of mycorrhiza, in bienniel and matrix-forming perennial species of closed chalkland turf. Journal of Ecology, 70(2), 571–593. DOI 10.2307/2259924.
60. Johnson, N. C., Zak, D. R., Tilman, D., Pfleger, F. L. (1991). Dynamics of vesicular-arbuscular mycorrhizae during old field succession. Oecologia, 86(3), 349–358. DOI 10.1007/BF00317600.
61. Sanders, F. E., Fitter, A. H. (1992). The ecology and functioning of vesicular-arbuscular mycorrhizas in co-existing grassland species. I. Seasonal patterns of mycorrhizal occurrence and morphology. New Phytologist, 120(4), 517–524. DOI 10.1111/j.1469-8137.1992.tb01801.x.
62. Mullen, R. B., Schmidt, S. K. (1993). Mycorrhizal infection, phosphorus uptake, and phenology in Ranunculus adoneus: implications for the functioning of mycorrhizae in alpine systems. Oecologia, 94(2), 229–234. DOI 10.1007/BF00341321. [Google Scholar] [CrossRef]
63. Lutgen, E. R., Muir-Clairmont, D., Graham, J., Rillig, M. C. (2003). Seasonality of arbuscular mycorrhizal hyphae and glomalin in a western Montana grassland. Plant and Soil, 257(1), 71–83. DOI 10.1023/A:1026224209597. [Google Scholar] [CrossRef]
64. Sigüenza, C., Espejel, I., Allen, E. B. (1996). Seasonality of mycorrhizae in coastal sand dunes of Baja California. Mycorrhiza, 6(2), 151–157. DOI 10.1007/s005720050120. [Google Scholar] [CrossRef]
65. Kabir, Z., Halloran, O. I. P., Fyles, J. W., Hamel, C. (1997). Seasonal changes of arbuscular mycorrhizal fungi as affected by tillage practices and fertilization: hyphal density and mycorrhizal root colonization. Plant and Soil, 192(2), 285–293. DOI 10.1023/A:1004205828485.
66. Bever, J. D., Schultz, P. A., Pringle, A., Morton, J. B. (2001). Arbuscular mycorrhizal fungi: more diverse than meets the eye, and the ecological tale of why. BioScience, 51(11), 923–931. DOI 10.1641/0006-3568(2001)051[0923:AMFMDT]2.0.CO;2.
67. Lara-Pérez, L. A., Noa-Carrazana, J. C., Hernández-González, S., Alarcón-Gutiérrez, E., Sánchez-Velásquez, L. R. et al. (2014). Diversity and colonization of arbuscular mycorrhizal fungi in the tree fern Alsophila firma in rainy and dry season. Symbiosis, 62(3), 143–150. DOI 10.1007/s13199-014-0279-x. [Google Scholar] [CrossRef]