DOI:10.32604/phyton.2020.013880

| Phyton-International Journal of Experimental Botany DOI:10.32604/phyton.2020.013880 |  |
| Article |
Cytogenetical Changes among Polyembryonic (PEm) and Non-PEm Maize Plants
1School of Chemistry, Universidad Autónoma de Coahuila. Blvd. V. Carranza e Ing. José Cárdenas V. s/n. Col. República Ote. C.P. 25280. Saltillo, Coahuila, México
2Plant Breeding Department, Universidad Autónoma Agraria Antonio Narro, Blvd. Antonio Narro 1923, Buenavista, Saltillo, México
*Corresponding Authors: F. Ramírez-Godina. Email: godramf@gmail.com; R. Rodríguez-Herrera. Email: raul.rodriguez@uadec.edu.mx
Received: 25 August 2020; Accepted: 21 October 2020
Abstract: Polyembryony in maize (PEm) contributes to improving the nutritional properties of the grain, as well as an increase in yield, since it generates multiple plants per seed, opening the possibility of developing new varieties. However, it is unknown whether polyembryony in maize is the product of chromosomal abnormalities. Based on the above, in this research a cytogenetic study was proposed to verify if chromosomal abnormalities are related to the maize polyembryony. For a meiotic study, maize genotypes with variable proportions of polyembryony (PEm), from the UA-IMM-BAP population and non-PEm (monoembryonic) maize were used, while for a mitosis analysis, 30 families of maternal half-siblings (MHS) from two different populations with high polyembryony, denominated as BHP (brachytic, high polyembryony) and NHP (normal height, high polyembryony) were used. All these genotypes were planted in the Buenavista Agricultural Experimental Station-UAAAN at Saltillo, Coahuila, Mexico. The frequency of PEm was estimated in all genotypes. It was found that the polyembryony occurs at frequencies from 34 to 66% in the D-02, BHP group, and from 13 to 21% in the segregating groups of the G3-0202 and G3-0201. The squash technique was used for both cytogenetic analyses. Different meiotic irregularities in maize chromosomes were detected, such as irregular association of 10% of chromosomes in metaphase 1, and agglomeration of chromatin in 100% of the cells. In addition, pollen viability was estimated by the staining technique with 1% acetocarmine dye, and it was found that the polyembryonic ones have pollen viability of 100%. In meiosis in prophase, I sub-phase diakinesis, ten pairs of bivalents were confirmed, confirming 2n = 20, both in non-PEm and PEm maize, corroborating mating, and balanced segregation, without the presence of univalent or multivalent. In the chromosomal count carried out in cells in mitosis, only in the BHP-200 polyembryonic family was registered two out of twenty triploid plants. This condition of ploidy is atypical in maize, so it is recommended to analyze more plants from this family.
Keywords: Zea mays; irregular meiosis; mitosis; chromosomes; chromatin; polyploidy
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
A genetic maize trait that could increase production is polyembryony (PEm), which can generate two to six complete plants per seed [1], the incidence of this characteristic has been observed under natural conditions, and when mutations were induced by X-ray applications [2]. In PEm maize, the concentration of oils and proteins is increased compared to monoembryonic corn, by 30 to 50% crude fat, 15 to 20% oleic acid and 20 to 50% lysine; conferring to this grain higher nutritional value [3]. While, it has been reported that fat and lysine content can be increased by increasing PEm levels in maize lines and hybrids [4]. In 1973, scientists from the Universidad Autonoma Agraria Antonio Narro (UAAAN) generated a population of maize, which presented polyembryonic seeds with a frequency of 1.5%, this population was genetically improved using recurrent selection until reaching polyembryonic frequencies of more than 60% [3]. In recent years, two to six plants from a single maize seed have been observed, which some occasions may have multiple roots [5] and in sequencing studies of ITS regions has been reported that plants generated from a single seed may have different nucleotide sequences [1]. Various agronomic, genetic and bromatological studies have been carried out on PE, and there are some attempts to associate different morphological traits to polyembryony. It has been mentioned that seed and seedling morphological traits in polyembryony citrus varieties were significantly different and were not correlated with polyembryony except for the clutch size and the number of branches [6]. In addition, it is still unknown whether the polyembryony in maize is the product of chromosomal abnormalities. Karyotype study describing the number and morphological features of all chromosomes is important in clinical, systematic, and evolutionary approaches [7]. In treated seeds with X-rays was observed that increasing doses of radiation enhanced the appearance of PEm (up to 18.3%) and plants that emerged from these seeds showed different plant height, suggesting that X-rays irradiation could produce haploid polyembryonic plants (2). Different chromosomal abnormalities have been observed in maize. It was reported various genes that mutate and affect meiosis, causing different aberrations that compromise plant productivity [8] and the presence of some knobs varied among maize inbred lines [9].
Chromosomal abnormalities can be divided into structural and numerical abnormalities, defining the former as changes in the arrangement of genes or chromosomal fragments, and numerical abnormalities as a change in the number of chromosomes due to aneuploidy or polyploidy [10]. Chromosomal abnormalities can occur in meiosis I and II, carrying out the non-disjunction of chromosomes or the non-disjunction of chromatids, respectively, causing an alteration in the number of chromosomes in the daughter cells [11]. The appearance of chromosomal abnormalities, such as aneuploidy, monoploidy, and other levels of ploidy, may be directly related to PEm in maize. The present study was carried out under the following objectives: 1. To identify changes in chromosome number in PEm and Non-PEm plants, 2. To determine mitotic and meiotic irregularities in the morphology of chromosomes of PEm and Non-PE plants and 3. To evaluate the viability of pollen in PEm and non-PEm maize plants.
Five genotypes of polyembryonic maize (PEm) and three non-PEm were tested, in addition to control (Tuxpeño HOC population) (Tab. 1). Polyembryony population: BHP is a brachytic, high frequency polyembryony population (more than 55%). Abbreviation = D, and Non-Polyembryony population: BBP is a brachytic, low frequency polyembryony population (lower than 1%). Abbreviation = B. while B-03 and B-07 are two half-sib families constituent from the BBP population. They have very low polyembryony frequency (lower than 1%). So that, they can be classified as Non-Polyembryony, and D-02 and D-05 are two half-sib families constituent from the BHP population. They show a high polyembryony frequency (over 55 %). They belong to the polyembryony class. The segregating G-group is constituted by G3-0201 and G3-0202 belong to a group of families that segregates the trait polyembryony. They are the third generation in the sequence F1’s, F2’s. Plants of this last kind were pollinated under an assortative mating (positive) system. It is why they are named G3 instead of F3. The numbers (0201 and 0202) correspond to the plots number where the mother plants were allocated into the field. The polyembryony frequency of these genotypes is about 18%, which can be considered as polyembryony class. These genotypes were sown on June 17, 2017, at the Buenavista Agricultural Experimental Station-Universidad Autonoma Agraria Antonio Narro (UAAAN) located 7.5 km South of Saltillo City, Coahuila, Mexico. The geographical coordinates and altitude of the site are 25° 23´ N, −101° 02´ W, and 1743 masl. During planting day, experimental plots were fertilized with 100 units of nitrogen (N) and 80 units of phosphorous (P), using Urea and Ammonium Monophosphate. Experimental plots were fertilized again, at 40 days after sowing with 60 units of N using Urea. When needed, water was supplied by means of drip tape. In addition, seeds from 30 maternal half-siblings (MHS) families from each of the maize high-polyembryony populations, called BHP (brachytic) and NHP (normal height) were used. Each family was represented by 26 seeds, taken at random, which were placed on paper towels and moistened for germination. All lab tasks were performed at the Cytogenetics Laboratory–Plant Breeding Department, Universidad Autonoma Agraria Antonio Narro (UAAAN).
Table 1: Polyembryonic (PEm) and monoembryonic (No-PEm) maize genotypes used in this study
2.2 Determination of Polyembryony (PEm) Percentage
To estimate the frequency of PEm in the tested maize genotypes, the total number of germinated seeds was counted, as well as, the single, double and triple seedlings produced from each seed of the tested genotypes. The number of seedlings from each seed per genotype is detailed in Tab. 2. The PEm frequency was calculated summing over the last two columns and divided by the numbers of plants and then multiplied by 100 [2].
Table 2: Number of tested plants per genotype, number of plants with individual (Non-PEm), double (PEm) and triple (PEm) seedlings
2.3 Cell Analysis during Meiosis
Harvesting immature spikes of 6 plants per maize genotype were performed 60 days after planting, in this case, spikes from twin and triple plants were including, then, the spikes were stored in Farmer fixative (1:3 glacial acetic acid/96% ethyl alcohol) for 24 hours, in order to instantly kill the tissue and fix the phases of cell division. For the cytological study of the cells in meiosis, the fixed spikes were placed in Petri dishes with distilled water to extract the anthers, which were placed on slides with a drop of acetocarmine. The anthers were then halved to release the microsporocytes. Subsequently, the residues were removed from the anther envelopes, a coverslip was placed on top of the microsporocytes, then, the sample was heated and the microsporocytes were gently pressed manually and without lateral movements on a filter paper, after that, the sample was observed under a microscope, if the microsporocytes are over-colored, a drop of propionic acid was added along the edges of the coverslip and it was pressed again and heated [12]. Finally, the sample was observed under a microscope on a 10x and 40x objective.
To perform a comparison between the meiotic division of PEm and non-PEm maize cells, all the meiosis phases were analyzed. In addition, in order to identify the number and morphology of chromosomes, the phases of diakinesis and metaphase 1 were observed. In this step, 409 photographs of PEm and 130 Non-PEm maize cells were analyzed in prophase I sub-phase diakinesis and in metaphase I of meiosis. Once the phase of interest in the preparations was found, bees wax was placed on the edges of the coverslip, in order to make a temporary preparation and thus proceed to take photographs with a 40x objective in a Carl Zeiss compound microscope (Axiostar plus) with Moticam 5.0 MP digital microscope camera, and Motic Image Plus 3.0 software.
2.4 Cell Analysis during Mitosis
The maternal half-sibling families were evaluated by groups of four families at a time, which was germinated every eight days in rolls of sterile germination absorbent paper. In this step, 26 grains were taken at random and arranged in two blocks of 13 seeds for better development of the seedlings. Germination was carried out in an incubation room with permanent white artificial light, at a temperature of 25°C ± 1, and irrigating the rolls with fresh water every third day. On the ninth day of planting, germination was quantified, having seedlings developed with a minimum of two leaves, thus identifying mono-embryonic and poly-embryonic cases; Root tips were cut (with the help of a scalpel and slides) of three seedlings between 9:00 and 9:20 am. The tips were placed in a 0.04% 8-Hydroxyquinoline pretreatment solution, for three hours. The apices were then changed to a Farmer fixative (3:1 ethanol: glacial acetic acid) for 24 hours, in order to instantly kill the tissue and fix the phases of mitotic cell division [12]. Subsequently, samples were changed to carmine-propionic dye, first removing the fixative and rinsing with distilled water, then, samples remained there resting for two hours, after that, it was proceeded to heat almost to a boil, placing samples directly in the fire with spaces of 10 to 12 seconds. At the end of this process, the apical meristems were washed with distilled water and underwent hydrolysis in an enzyme complex for 12 hours under refrigeration, this enzyme complex was obtained from the stomach liquid of the garden snail (Helix pomata). Finally, the enzyme complex was removed, the samples were rinsed, and carmine-propionic was added again. To make the preparations, the maceration method (squash technique) was used as the mounting medium for the coloring solution (carmine-propionic 45%), then direct heating to the fire and crushing of the sample in a uniform manner without lateral movements, propionic acid was used. In case the preparation was very colored for its analysis, the edges were sealed with hot paraffin to avoid the evaporation of the dye. Thus, the samples were ready for chromosomal evaluation. At least, three preparations were made for each seedling, and chromosomes from 10 to 15 metaphase cells at mitosis, were counted per case.
In order to observe if the chromosomal abnormalities were related to the viability of pollen, PEm maize spikelets were collected. Removal of anthers was carried out on a slide, ensuring that the distribution was uniform, a drop of acetocarmine dye (1%) was deposited on the sample, then, a coverslip was placed on top and after 5 to 10 seconds, the observation was carried out under a microscope. The rounded and red-colored pollen grains were considered viable and the constrained and unstained pollen grains were rated as non-viable. The pollen counts were carried out in two microscope fields per preparation, the number of viable and unviable pollen grains was counted, then, it was estimated the percentage of pollen viability [13,14]. All observations were made with 10 X and 40 X objectives on a Carl Zeiss compound microscope. The viability results were expressed as a percentage of stained pollen since the unstained ones were considered unviable.
3.1 Percentage of PEm in Different Maize Genotypes
After seed germination, the percentage of polyembryony per genotype was determined (Tab. 3). A frequency of 0% was observed in the control genotype, as well as, in the genotypes B-03 (3) and B-07 (3) classified as Non-PEm maize showed a frequency lower than 0.1% (3). The D-02 (3) and BHP genotypes showed a 66% of PEm frequency, these genotypes are classified as having high polyembryony, according to (3) and the D-05 (3), G3-0202 (3) and G3-0201 (3) presented frequencies of 34%, 13%, and 21%, respectively, which are classified as medium to low-polyembryony maize genotypes [3,15].
Table 3: Percentage of polyembryony of the tested genotypes
3.2 Chromosome Number in PEm and Non-PEm Plants
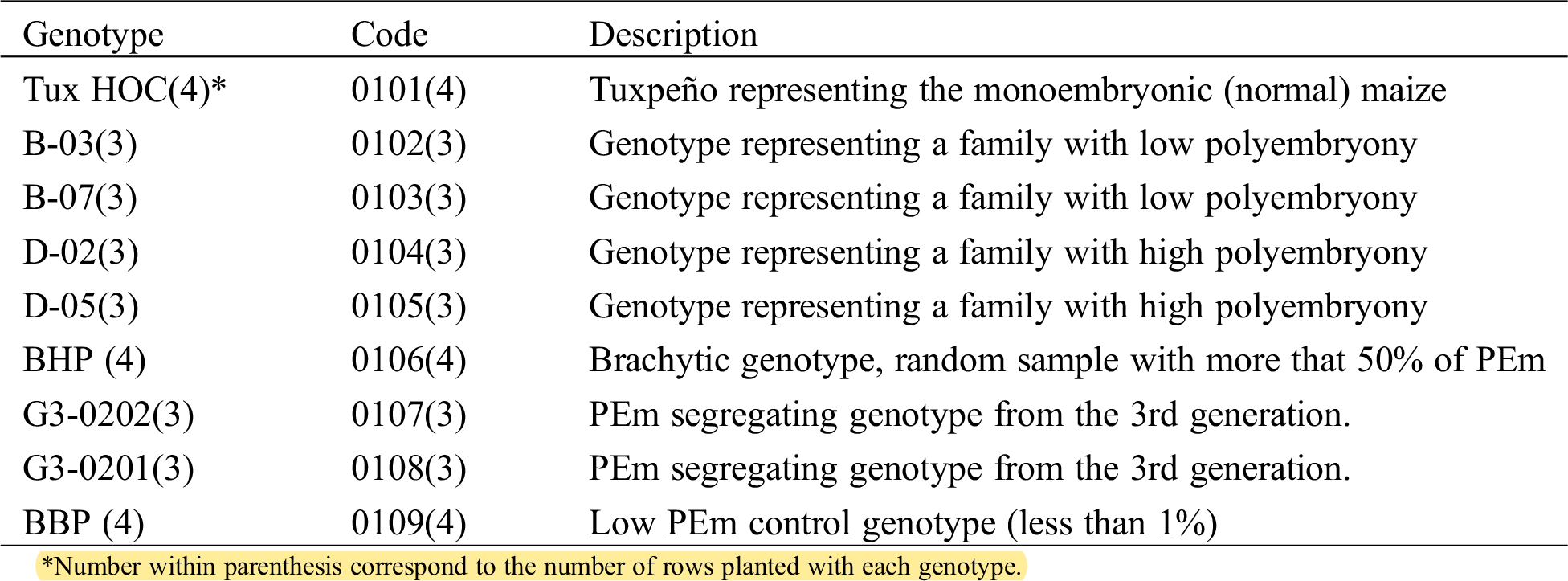
As a result of the cytogenetic study, all phases of meiosis I and II were observed in non-polyembryonic maize (Fig. 1). Prophase I (a-b), pachytene (a) phase where the nucleolus becomes visible and cross-linking is carried out; Diplotene (b) maintains the binding of homologous chromosomes by means of chiasmata; diakinesis (c) the nucleolus reduces its size, the chromosomes are more condensed, making it easier to visualize and count them; metaphase I (d) the chromosomes are aligned in the equatorial plate, the nucleolus has disappeared; anaphase I (e) phase where the spindle fibers shorten, causing migration of homologous chromosomes to opposite poles of the cell, giving rise to two groups with haploid numbers; telophase I (f) chromosomes form a compact group at each pole like two daughter nuclei, and as a result of crossing over, chromatids become genetically distinct.
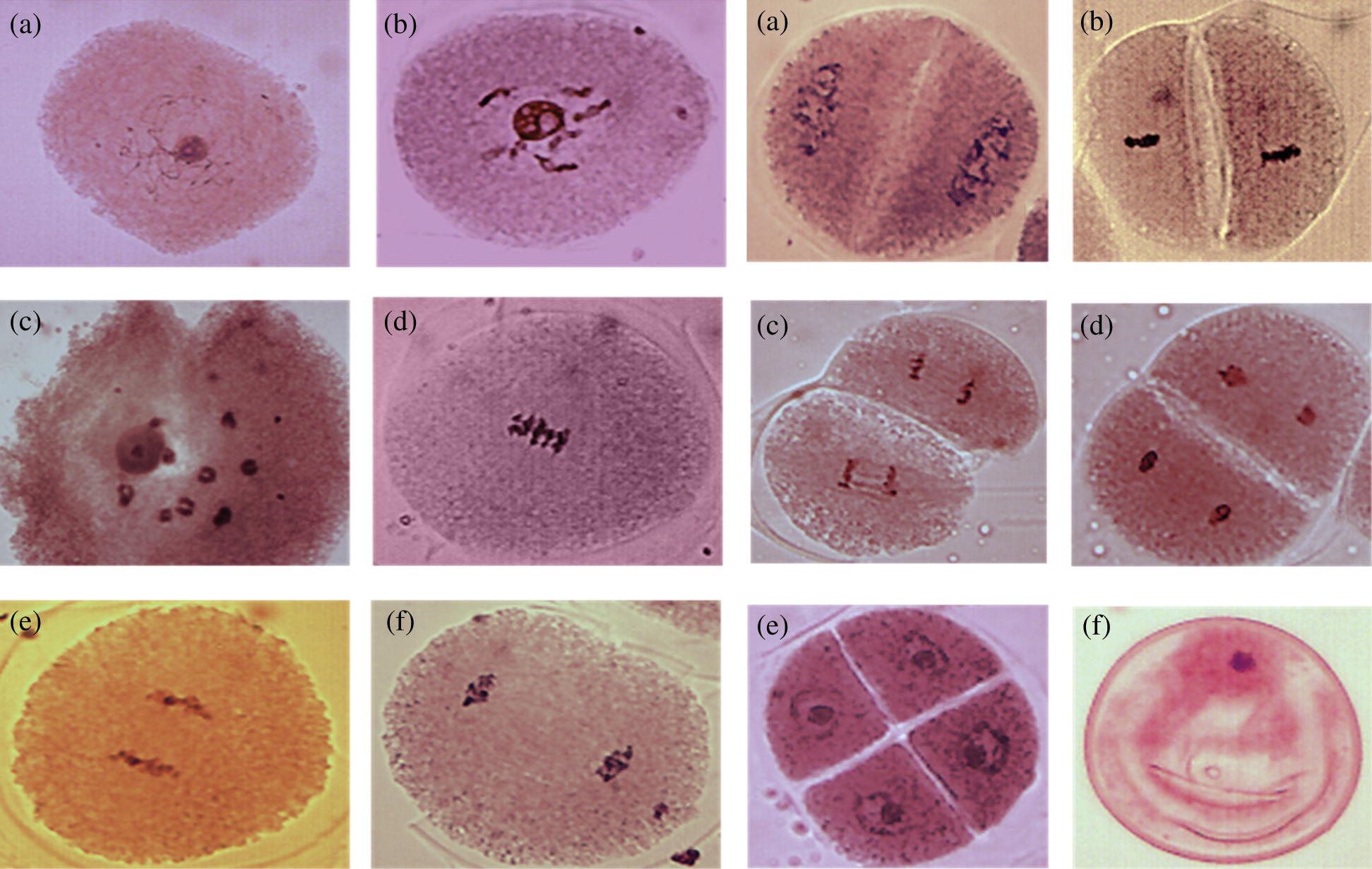
Figure 1: Left images, cells of Non- PEm maize in meiosis I. Prophase I; pachytene (a) diplotene (b); diakinesis (c); metaphase I (d); anaphase I (e); telophase I (f) and right images meiosis II. Prophase II (a); metaphase II (b); anaphase II (c); telophase II (d); tetrads (e); ripe pollen grain (f). 40 X
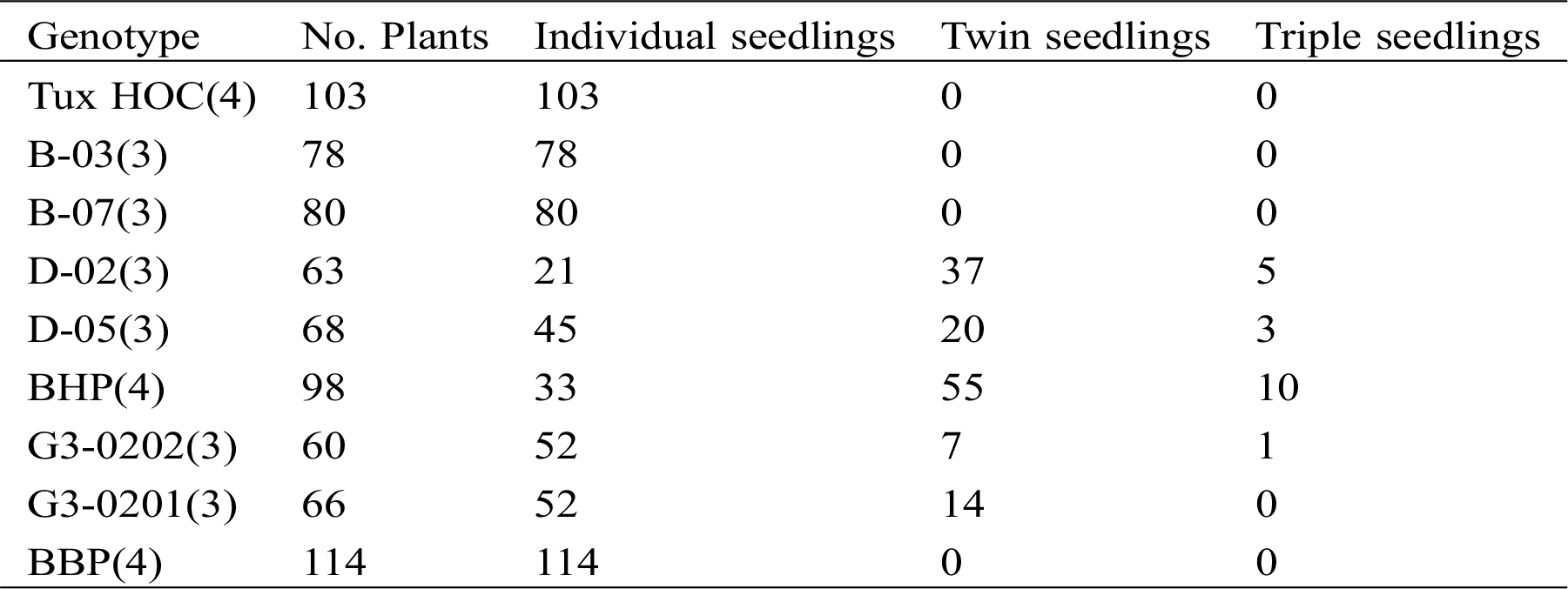
Prophase II (a), metaphase II (b), anaphase II (c) and telophase II (d) are similar to the phases of meiosis I; the tetrads (e) are a quartet of uni-nucleated cells, (f) represents the maturation of a pollen grain. The results of meiosis I and II cells from polyembryonic maize were the same as non-polyembryonic maize (Fig. 2).
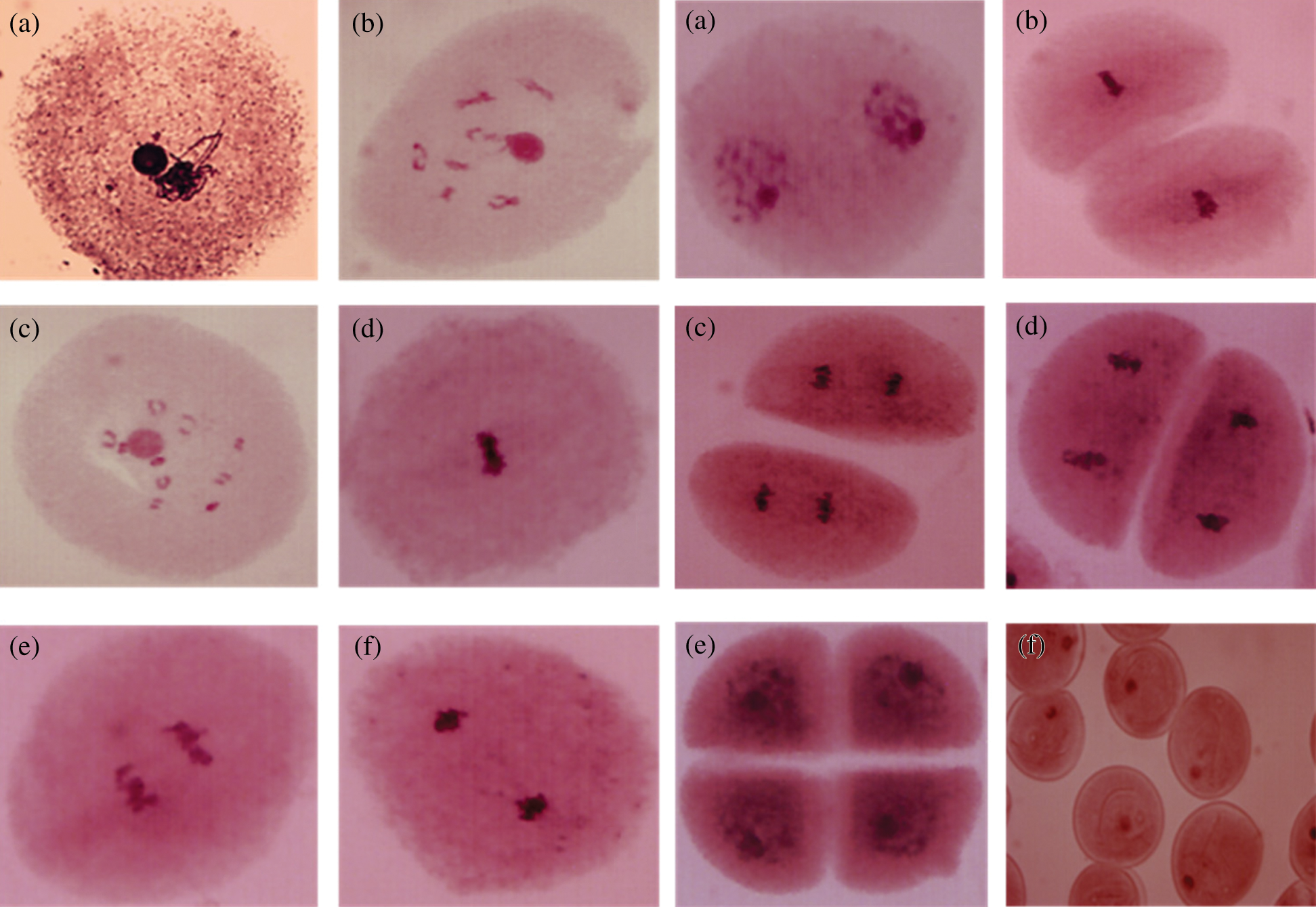
Figure 2: Left images, cells of PEm maize in meiosis I. Prophase I; pachytene (a) with chromatin agglomeration; diplotene (b); diakinesis (c); metaphase I (d); anaphase I (e); telophase I (f) and right images, meiosis II. Prophase II (a); metaphase II (b); anaphase II (c); telophase II (d); tetrads (e); ripe pollen grains (f). 40 X
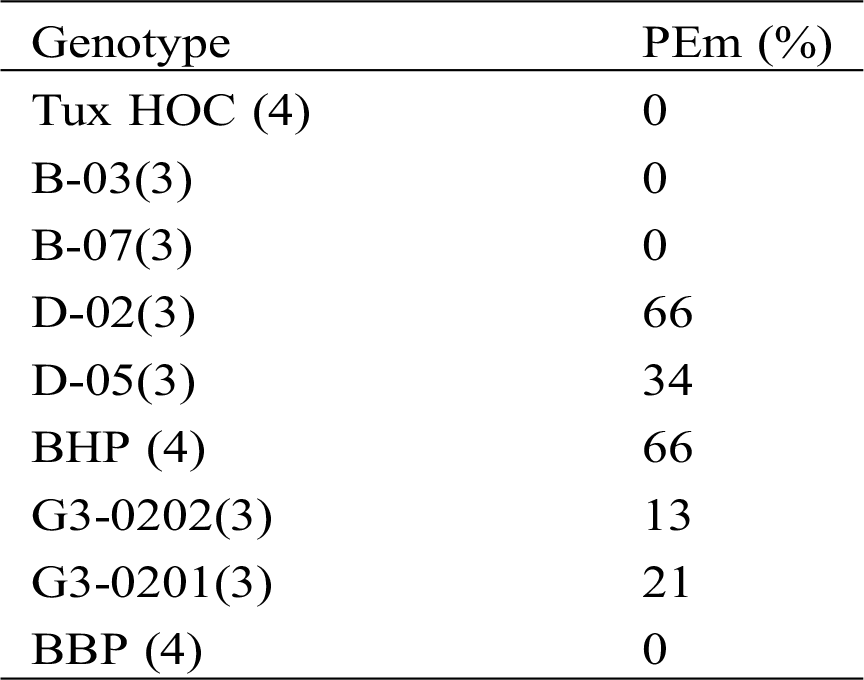
In order to identify if there is any anomality related to the number of chromosomes that is directly influencing the appearance of polyembryony (PEm) in maize, a comparison of cells in diakinesis of both non-polyembryonic (Non-PEm) (Fig. 3) and polyembryonic (PEm) maize was performed (Fig. 7). In this case, 100 cells were analyzed in prophase I sub-phase diakinesis of non-polyembryonic maize, where 100% presented 10 pairs of bivalents confirming 2n = 20, corroborating a mating and balanced segregation typical of maize without the presence of univalent or multivalent. In addition, 208 cells were analyzed in prophase I sub-phase diakinesis of polyembryonic genotypes where 100% presented 10 pairs of bivalents confirming 2n = 20 [16], corroborating a balanced mating and segregation, indicating that the sub-phase of diakinesis is the same compared to that of non-polyembryonic maize and therefore there are no cases of univalent or multivalent.
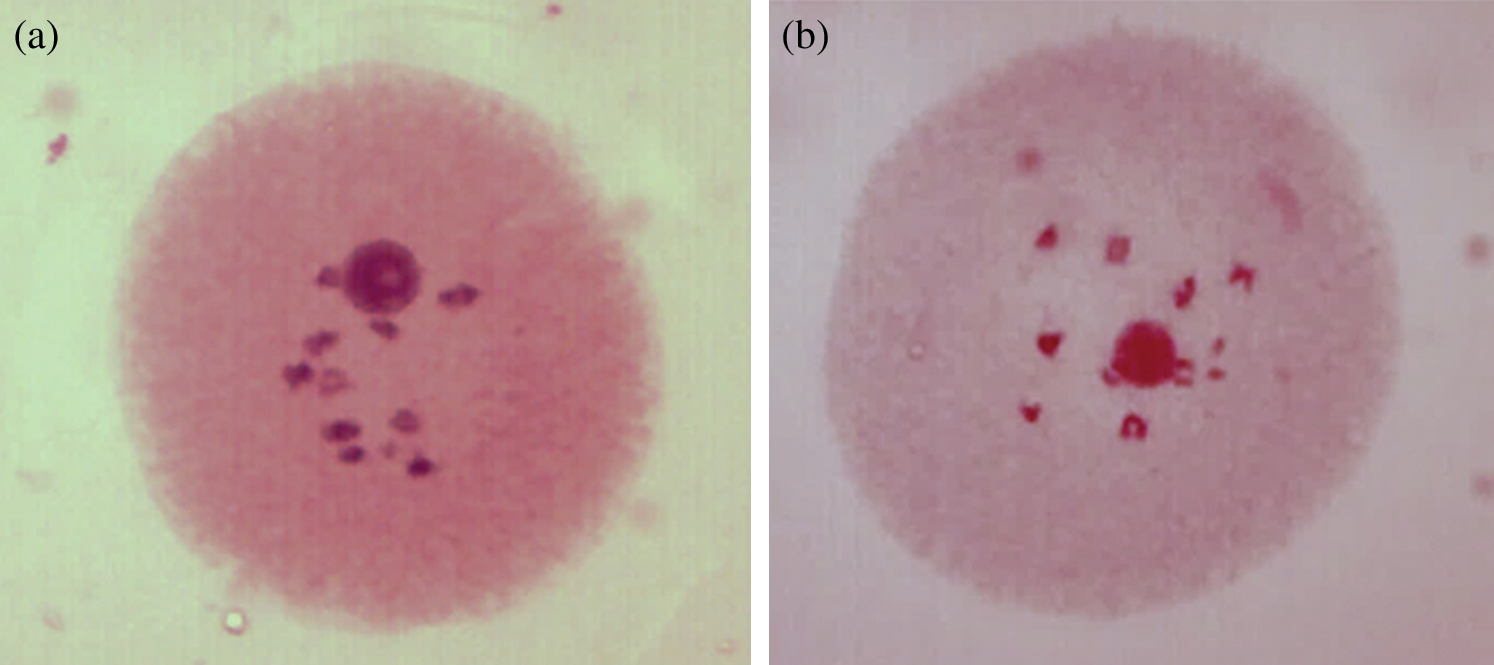
Figure 3: Cells in diakinesis of non-PEm maize (a) and PEm maize (b) with 10 pairs of bivalents
3.3 Chromosomal Meiotic Irregularities
From the 100% of the analyzed cells of polyembryonic maize in metaphase I, cases of irregular association of chromosomes in types BHP and D-02 were found in 10% as shown in Figs. 4 and 5, respectively. In addition, from 50 cases analyzed in metaphase I of polyembryonic maize cells, 5 of them from the BHP family presented irregular association of chromosomes, which may indicate the formation of microcytes present in the tetrads [17], since they do not integrate into the main nucleus, and increases the probability of formation of non-viable gametes.
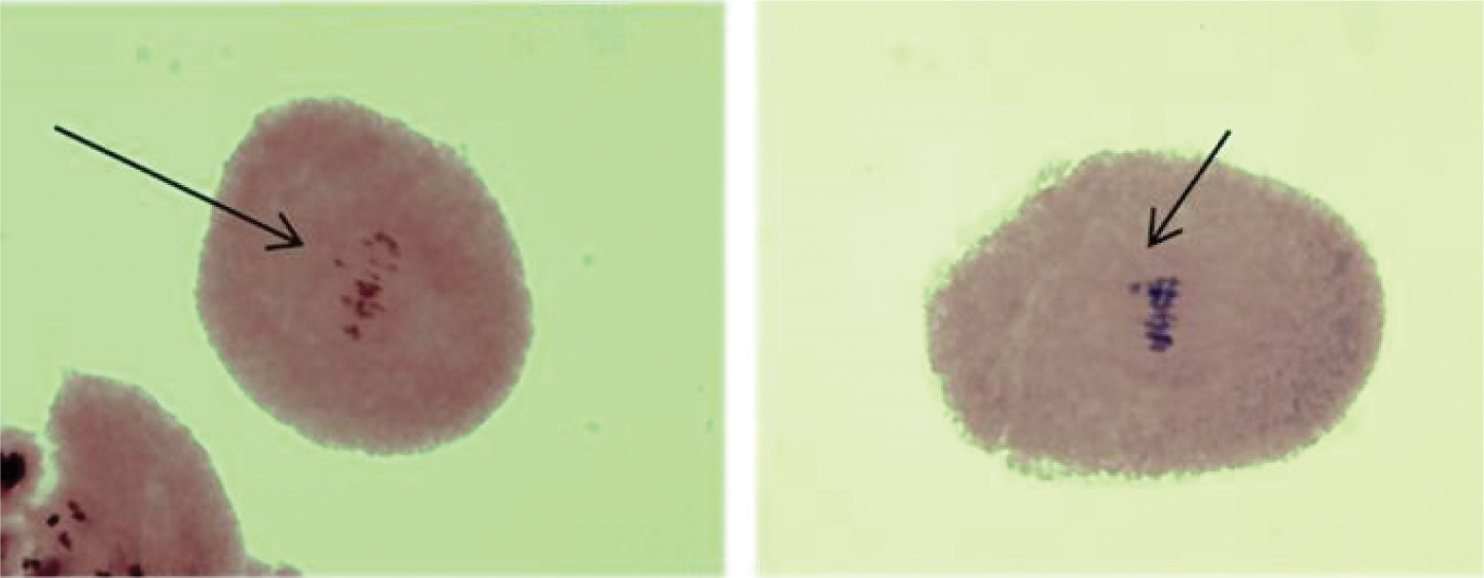
Figure 4: BHP family metaphase I cells, with irregular association of chromosomes. 40 X
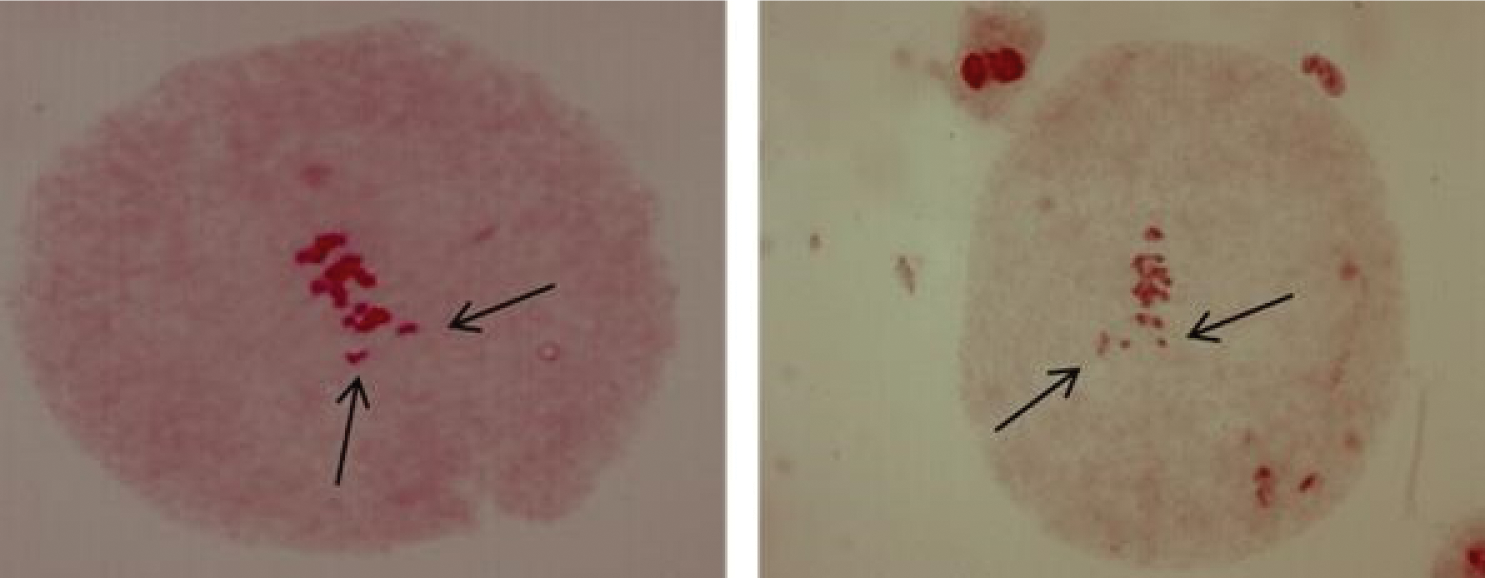
Figure 5: Cells in metaphase I of D-02 genotype, with irregular association of chromosomes. 40 X
In the case of prophase I, the pachytene sub-phase studied, in polyembryonic maize cells of the D-02 and D-05 families, 100% of these cells presented an intense agglomeration of chromatin (Fig. 6). It has been reported the st gene in maize [18], which refers to the intense agglomeration of chromosomes in different phases of both meiosis and mitosis, however, in the present study it was only found in prophase I sub -pachytene phase, which may prevent the regular segregation of chromosomes [17], and could generate non-viable gametes. Chromosomal anomalies in maize may be also induced by environmental factors. It was consignee the presence of bridges, fragments, sticky and ring chromosomes, and cells with two nuclei or with micronucleus after application of the fungicide Royal Flo [19].
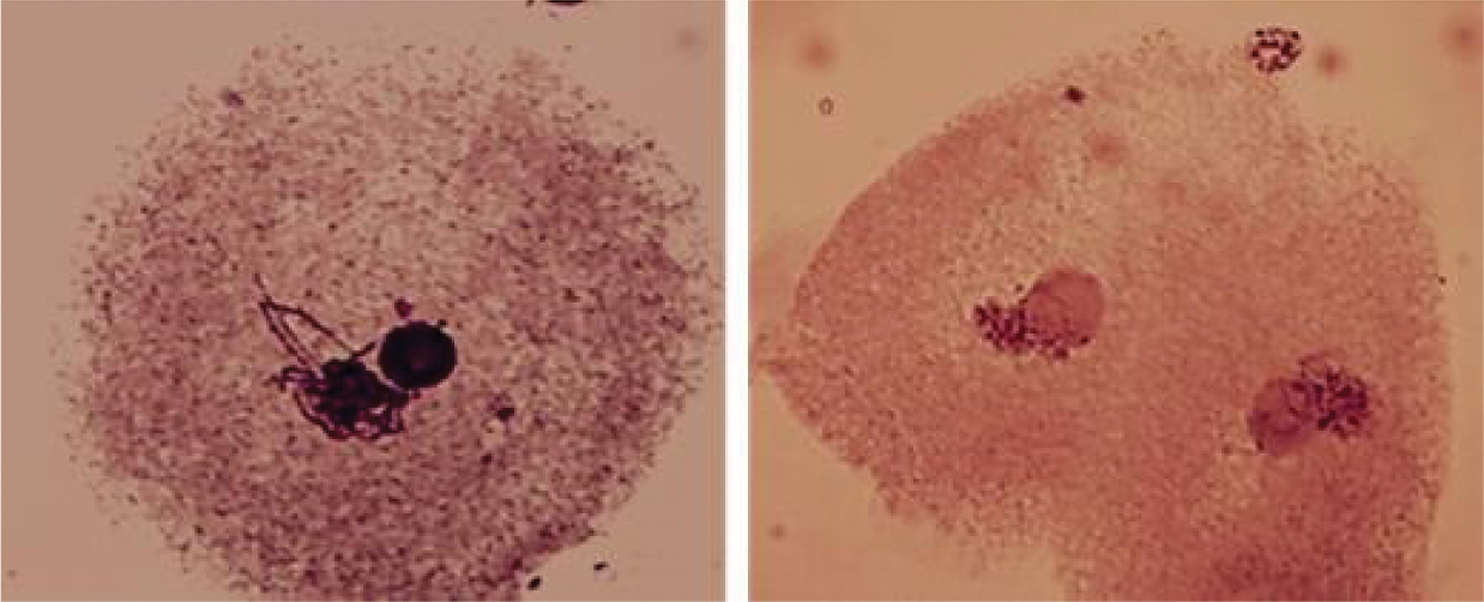
Figure 6: Cells of PEm maize from the D-02 and D-05 families in prophase I sub-phase pachytene with chromatin agglomeration. 40 X
3.4 Chromosome Number in Mitosis
From the 120 seedlings analyzed in 360 studied cases (three cytological preparations per specimen) from the 30 families of NHP half-siblings of non-polyembryonic maize, no alteration in chromosome number was found, showing all diploid constitutions (2n = 20), as can be seen in Fig. 7a. Other chromosomal measurements such as chromosome morphometry and class and knob localization may improve the identification of chromosomal abnormalities in polyembryonic maize genotypes [7]. The same condition was found in the 29 families of BHP half-siblings of polyembryonic maize; however, in a case of the family called BHP-200, the existence of cells that presented the triploid condition (2n = 30) was observed (Fig. 7b).
In this study, no chromosomal irregularities, arrangements, or numbers were strongly associated with polyembryony in maize. In other commodities, specific genes and transposable element insertions have been associated with polyembryony. The CitRWP gene is expressed at higher levels in polyembryonic citrus cultivars [20]. In addition, these authors found a miniature inverted-repeat transposable element insertion in the promoter region which co-segregated with polyembryony. While, other authors consignee that CitRKD1 gene belongs to the plant RWP-RK domain-containing protein, was transcribed in reproductive tissues of citrus varieties with the polyembryonic allele, and its transcription may be regulated by a MITE insertion in its upstream region [21].
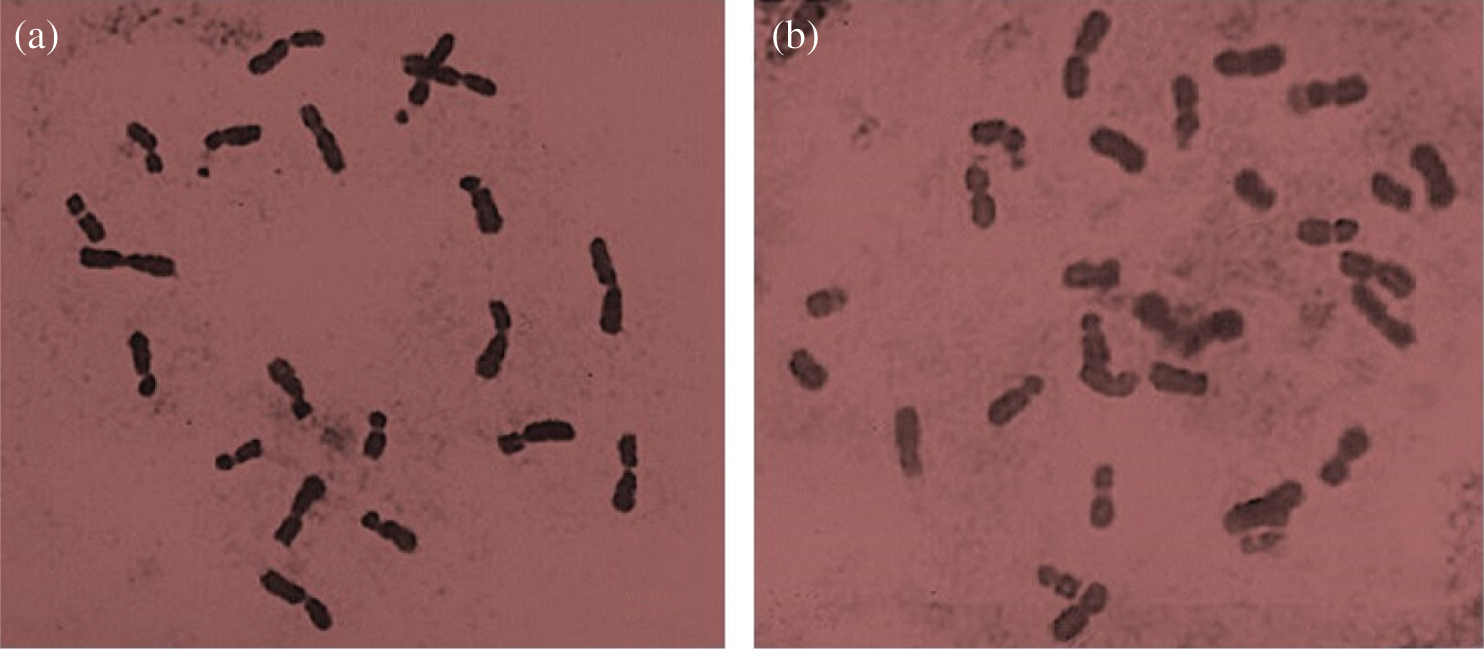
Figure 7: (a) Chromosomes in the diploid cell (2n = 20), mitotic metaphase of the NHP-89 family. (b) Chromosomes in triploid cells (2n = 30), mitotic metaphase of the BHP-200 family. 100 X
3.5 Pollen Viability in PEm Plants
Based on the observed chromosomal aberrations, it was suggested the possible formation of infeasible gametes, thus, we proceeded to analyze the viability of pollen grains in polyembryonic maize. In all the poly-embryonic maize plants, 100% pollen viability was obtained, since in none of these cases was observed an absence of nucleus or degeneration of the chromatic material (Fig. 2). Based on the pollen viability results, the idea that the found chromosomal aberrations generate unviable gametes can be discarded. There are reports that mention that chromosomal aberrations and mutations are not related in maize plants to the st gene [18], and that even when abnormalities occur during division, such as early ascension or chromatin agglutination, from this study, these may only be temporary and not transmissible to other generations. Although the existence of the st gene has not been verified in the maize populations used in this study, the results obtained can be associated with what was mentioned by [18] and with the study carried out by [17] in which chromosomal abnormalities were found in meiosis I in Lippia alba, but this one did show unfeasible pollen grains, which refers to the aberrations found during cell division. Another reason that suggests that the anomalies found in this study are not related to the formation of infeasible gametes, is the shape of the tetrads since none of these presented polyads or dyads, which may originate from failures in cytokinesis during meiosis I or II [17]. However, the tetrads found in PEm and Non-PEm maize were normal. These results may be interesting in other agricultural commodities, such as citrus cultivars which develop many nucellar embryos alongside a single zygotic embryo from a single seed [22].
It was found that polyembryony occurs at frequencies of 34 to 66% in the BHP population and from 13 to 21% in genotypes of a segregating group for polyembryony, called G3-0202 and G3-0201. Polyembryony was not observed in representative Non-PEm genotypes. In the meiotic analysis, aneuploidies cells were detected neither Pem nor Non-Pem genotypes. Exceptionally, the presence of chromosomal anomalies was observed during meiosis I, such as early segregation of chromosomes in 10% and agglomeration of chromatin in pachytene, these abnormalities were showed in genotypes with a high frequency of polyembryony, although this was not related to the formation of unviable gametes. In the chromosomal count performed in cells in mitosis, no chromosomal aberrations were found, nor the presence of monoploids. This shows that the abnormalities observed in some plants of the studied polyembryonic populations are not due to the haploid condition of their cells, nor any other change in the morphology of the chromosomes. Only in the BAP-200 family was recorded triploid in two seedlings of a total of 20 sampled cases, this ploidy condition is atypical in maize, so it is recommended to analyze a greater number of plants of this family to know better details in their reproductive behavior.
Acknowledgement: To Edilvar G. Roblero-Muñoz for his technical support during field experiments.
Funding Statement: This work was financed through the Project “Identification and sequencing of DNA regions that encode for maize polyembryony.” FON.SEC. SEP-CONACYT-BASIC SCIENCE, CB-2015-256081-Z-3061. AARC appreciates the financial support from this project for her BSc studies.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Avendaño-Sánchez, M., Espinoza-Velázquez, J., Gutiérrez-López, A., Flores-Gallegos, A. C., Rodríguez-Herrera, R. (2015). Secuencias nucleotídicas de la región ITS en familias S1 y PL de maíces poliembriónicos. Revista Mexicana de Ciencias Agrícolas, 6(3), 509–521.
2. Morgan, D. T.Jr, Rappleye, R. D. (1951). Polyembryony in maize and lily: Following X-irradiation of the pollen. Journal of Heredity, 42(2), 91–93. [Google Scholar]
3. Espinoza, J., Vega, M. C., Navarro, E., Burciaga, G. A. (1998). Poliembrionía en maíces de porte normal y enano. Agronomía Mesoamericana, 9(2), 83–88. [Google Scholar]
4. González-Vázquez, V. M., Espinoza-Velázquez, J., Mendoza-Villarreal, R., León-Castillo, D., Torres-Tapia, M. A. (2011). Caracterización de germoplasma de maíz que combina un alto contenido de aceite y poliembrionía. Universidad y Ciencia, 27(2), 157–167. [Google Scholar]
5. Espinoza-Velázquez, J., Valdés-Reyna, J., Alcalá-Rodríguez, J. M. (2012). Morfología y anatomía de radículas múltiples en plántulas de maíz derivadas de cariopsis con poliembrionía. Polibotánica, 33, 207–221. [Google Scholar]
6. Kashyap, K., Banu, S., Shrivastava, M. N., Ramchiary, N. (2018). Study of polyembryony and development of molecular markers for identification of zygotic and nucellar seedlings in Khasi mandarin (Citrus reticulata Blanco). International Journal of Environment, Agriculture and Biotechnology, 3(2), 363–372. [Google Scholar]
7. Silva, J. C., Carvalho, C. R., Clarindo, W. R. (2018). Updating the maize karyotype by chromosome DNA sizing. PLoS One, 13(1), e0190428. [Google Scholar]
8. Caetano, C. M. (2003). La aplicabilidad de la citogenética en Zea mays L.: Genes mutantes meióticos. Revista de Ciencias Agrícolas, 20(1), 27–49. [Google Scholar]
9. Mondin, M., Santos-Serejo, J. A., Bertão, M. R., Laborda, P. Pizzaia, D. et al. (2014). Karyotype variability in tropical maize sister inbred lines and hybrids compared with KYS standard line. Frontiers in Plant Science, 5(Article 544), 1–12. [Google Scholar]
10. Jiménez, L. F. (2006). Conocimientos Fundamentales de Biología. México, DF: Pearson Educación, 192. [Google Scholar]
11. Dempsey, E. (1994). Traditional analysis of maize pachytene chromosomes. In: Freeling, M. Walbot, V. (eds.The Maize Handbook. Berlin: Springer, 432-441. [Google Scholar]
12. García, V. A. (1990). Técnicas y Procedimientos de Citogenética Vegetal. Colegio de Postgraduados. Montecillo: Estado de México, 144. [Google Scholar]
13. Cardoso, M. B., Kaltchuk-Santos, E., Mundstock, E. C. D., Bodanese-Zanettini, M. H. (2004). Initial segmentation patterns of microspores and pollen viability in soybean cultured anthers: indication of chromosome doubling. Brazilian Archives of Biology and Technology, 47(5), 703–712. [Google Scholar]
14. Cardoso, M. B., Kaltchuk-Santos, E., Mundstock, E. C. D., Bodanese-Zanettini, M. H. (2004). Initial segmentation patterns of microspores and pollen viability in soybean cultured anthers: indication of chromosome doubling. Brazilian Archives of Biology and Technology, 47(5), 703–712. DOI 10.1590/S1516-89132004000500005. [Google Scholar] [CrossRef]
15. Soares, T. L., Silva, S. O., Costa, M. A. P. C., Santos-Serejo, J. A., Souza, A. D. S. et al. (2008). In vitro germination and viability of pollen grains of banana diploids. Crop Breeding and Applied Biotechnology, 8(2), 111–118. DOI 10.12702/1984-7033.v08n02a03. [Google Scholar] [CrossRef]
16. Alcalá-Rico, J. S. G. J., Espinoza-Velázquez, J., López-Benítez, A., Borrego-Escalante, F., Rodríguez-Herrera, R. et al. (2019). Agronomic performance of maize (Zea mays L.) populations segregating the polyembryony mutant. Revista Facultad de Ciencias Agrarias Universidad Del Cuyo. Argentina, 51(1), 1–18. [Google Scholar]
17. Beckett, J. B. (1991). Cytogenetic, genetic and plant breeding applications of B-A translocations in maize. In: Gupta, P. K., Tsuchiya, T. (eds.Chromosome Engineering in Plants: Genetics, Breeding, Evolution, vol. A. Amsterdam, Netherlands: Elsevier Science Publishers, 493–529. [Google Scholar]
18. Muñoz, A. M., Caetano, C. M., Vallejo, F. A., Sánchez, M. S. (2006). Comportamiento meiótico y descripción morfológica del polen de pronto alivio. Acta Agronómica, 55(2), 37–42. [Google Scholar]
19. Beadle, G. W. (1937). Chromosome aberration and gene mutation in sticky chromosome plants of Zea mays. Cytologia, 43, 56. [Google Scholar]
20. Bonea, D., Bonciu, E. (2017). Cytogenetic effects induced by the fungicide Royal Flo to maize (Zea mays L.). Caryologia, 70(3), 195–199. DOI 10.1080/00087114.2017.1318232. [Google Scholar] [CrossRef]
21. Shimada, T., Endo, T., Fujii, H. N., Nakano, M. Sugiyama, A. et al. (2018). MITE insertion-dependent expression of CitRKD1 with a RWP-RK domain regulates somatic embryogenesis in citrus nucellar tissues. BMC Plant Biology, 18, 166. [Google Scholar]
22. Woo, J., Park, Y. C., Lee, J. W., Yun, S. H. Kim, M. et al. (2019). Evaluation of polyembryony for genetic resources and efficacy of simple sequence repeat markers for the identification of nucellar and zygotic embryo-derived individuals in citrus. Applied Biological Chemistry, 62, 30. [Google Scholar]