DOI:10.32604/phyton.2020.013388

| Phyton-International Journal of Experimental Botany DOI:10.32604/phyton.2020.013388 |  |
| Review |
Biological and Functional Properties of Wedelolactone in Human Chronic Diseases
1Department of Biotechnology, Institute of Biotechnology, College of Life and Applied Science, Yeungnam University, Gyeongsan, Gyeongbuk, 38541, Korea
2Department of Forestry, North Eastern Regional Institute of Science and Technology, Nirjuli, 791109, India
3Stemforce, 313 Institute of Industrial Technology, Yeungnam University, Gyeongsan, Gyeongbuk, 38541, Korea
4Food Science and Technology Program, Beijing Normal University-Hong Kong Baptist University, United International College, Zhuhai, 519000, China
5Department of Genetic Engineering and Biotechnology, University of Rajshahi, Rajshahi, 6205, Bangladesh
*Corresponding Authors: Muhammad Nurul Matin. Email: nmatin@ru.ac.bd; Sang Gu Kang. Email: kangsg@ynu.ac.kr
Received: 05 August 2020; Accepted: 28 September 2020
Abstract: Medicinal herbs are well known and studied over the past millennia in most of the developing countries as a rational means of treatment against various diseases and disorders. Wedelolactone (WDL), a major bioactive compound in Eclipta prostrata L (Eclipta alba L), has been reported with potential benefits in human health against chronic diseases. However, a comprehensive study on WDL pharmacological benefits in various ailments, to the best of our knowledge, is not yet reported. Thereof, the present review provides the recent therapeutic applications in reference to biological and functional activities against major human chronic diseases, including cardiovascular, cancer, diabetes mellitus, liver disease, Alzheimer’s disease, and androgenetic alopecia. In this study, we collected all the relevant experimental information on WDL from Scientific databases such as PubMed, Web of Science, Science Direct, and Google Scholar. Conclusively, WDL is recognized as a key anti-oxidant with both specific regulator and inhibitor of major drug targetable proteins in human chronic diseases and disorders. Hence, WDL as a novel therapeutic bioactive molecule is advised to explore further for relevant pharmacological activities.
Keywords: Wedelolactone; Eclipta alba L; Ecliptaprostrata L; bioactivity; bioavailability
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
The natural sources of plant food products, such as whole grains, berries, fruits, vegetables, and bioactive non-nutrient plant compounds called phytochemicals are intensively researched for their possible use in drug discovery and improvement. The use of these natural compounds have gained popularity in nutraceutical and pharmacological purposes due to their potential benefits and safety [1–4]. Currently, medicinal plants are widely used as traditional and preventive medicine, especially in the treatment of metabolic disorders [5,6].
Polyphenols are secondary plant metabolites that act to defend the plants against ultraviolet radiation, oxidants, and pathogens [7,8]. These natural phytochemicals are abundantly found in vegetables, fruits, cereals, whole grains, legumes, coffee, and cocoa [7]. Structurally, polyphenols are classified into numerous classes based on the number and arrangement of phenolic rings. Among them, flavonoids are the major abundant class of polyphenols in the human diet and more than 4,000 types of polyphenols have been identified [9,10]. In general, these plant polyphenols promote health by their powerful antioxidant properties.
The main structural feature of phytochemicals in plants is the presence of hydroxyl groups attached to one or more aromatic rings. Plant polyphenols are classified into two major groups: Flavonoids and non-flavonoids. Flavonoids have a common flavone core consisting of 15 carbon atoms, which is further divided into flavonols, flavone-3-ols, anthocyanidins, flavones, and chalcones. Non-flavonoids have an aromatic ring with one or more hydroxyl groups. These included stilbenes, phenolic acids, saponin and other polyphenols like tannin and curcumin. It has been estimated that dietary intake of polyphenols is approximately 1.2 g/day [11]. Among the various interesting biological properties exhibited by plant polyphenols, their potential against various diseases have attracted much attention.
Among many phytochemicals, coumestan, known as 6H-benzofouro[3,2-c][1]benzopyran-6-one, represents the basic ring system for a number of naturally occurring wedelolactone (WDL) and many other compounds with antibacterial, antifungal, antimyotoxic, phytoestrogen, and phytoalexin activities [12]. Many of their biological activities can be attributed to their action as phytoestrogens. Further, polyphenols have shown beneficial effects in the experimental studies as edible health-promoting supplements [13].
In this review article, we describe the therapeutic potential and biological activities of WDL with underlying molecular mechanisms by citing available scientific reports. We also discuss the limitations and possibilities of WDL in pharmaceutical and clinical development. In addition, the structural changes, physico-chemical, pharmacokinetics, drug delivery and evaluation characteristics of WDL have been discussed.
For relevant literature an internet search was conducted using PubMed, Web of Science, Science Direct and Google scholar typing the keywords “Eclipta prostrata L” OR “Eclipta alba L” AND “Wedelolactone-antioxidant, antidiabetic, anticancer, antimicrobial, cardiovascular, hepatoprotective, osteoporosis, anti-hair loss and neurotoxicity” in title/abstract/keywords, without date restriction to retrieve the majority of the research articles (in vitro, in vivo and clinical).
Wedelolactone and dimethyl wedelolactone (DWL) are the coumestans present in Eclipta prostrata L (Fig. 1A) (synonym: Eclipta alba L. “Bhringaraj”). The plant belonging to the family Asteraceae is a small, branched annual herb inhabiting at tropical and subtropical regions of the world with white flower heads. E. prostrata is one of the oriental herbs known as ‘Hanryoncho’ in Korea and China. The plant grows abundantly in cool, humid locations throughout India and Northern Asia [14]. E. prostrata, a traditional herbal medicinal plant, has been a long used in Asia and South America for the treatment of various human diseases [15].
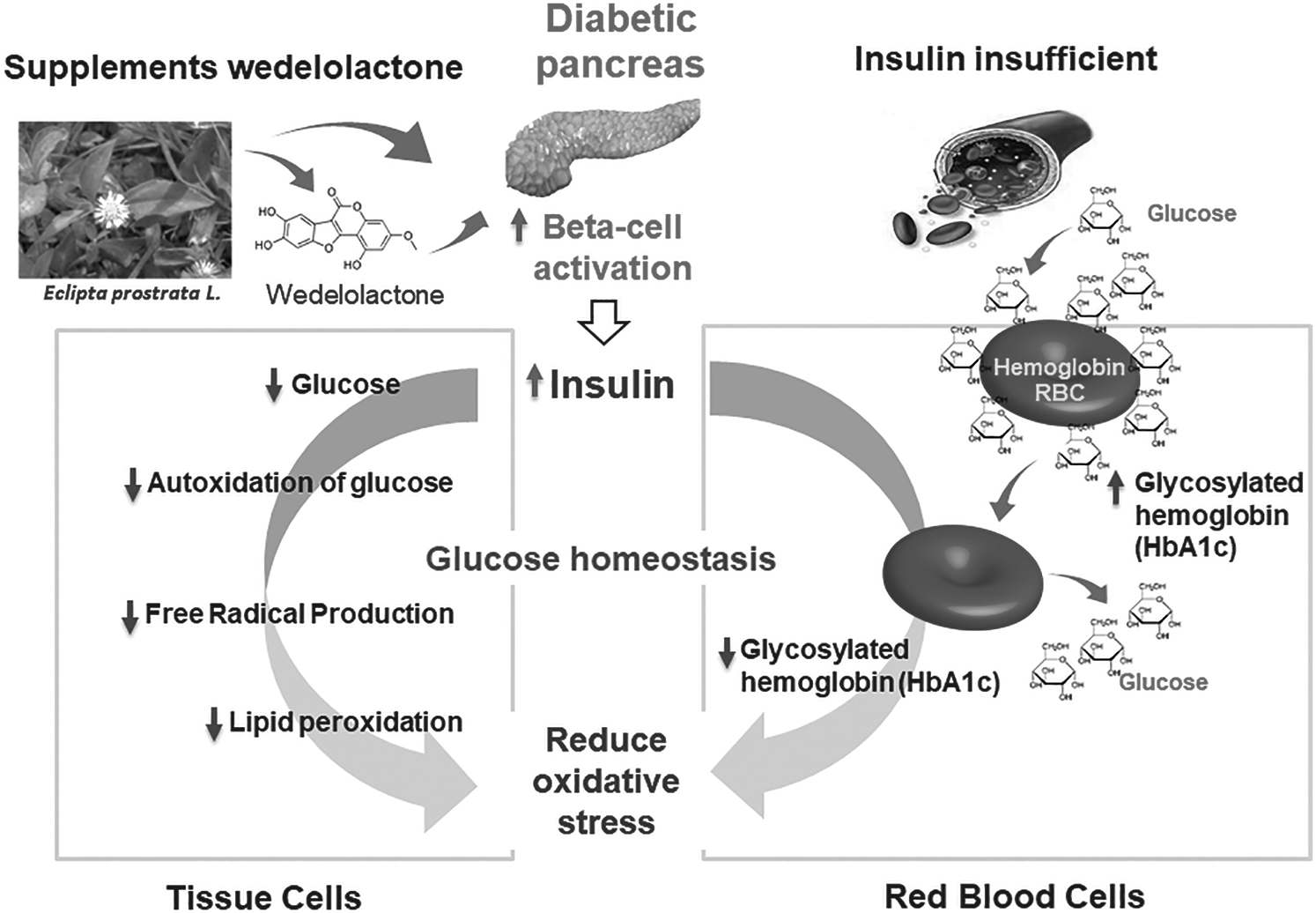
Figure 1: Eclipta alba L. (A), and structure of wedelolactone (B)
Wedelolactone (Fig. 1B), a natural coumestan was first obtained from the Wedeliacalandulacea extract in 1956 and later isolated from Bhringaraj (Eclipta alba L.) which is an acrid, bitter ethno-medicine used widely for dermatological and trichological health as well as in the treatment of cirrhosis, infective hepatitis, and hepatic enlargement.
Chemical name: 7-methoxy 5,11,12-trihydroxy-coumestan
Chemical formula: C16H10O7
Molar mass: 314.249 g/Mol
Boiling point: 498.4°C at 760 mmHg
Density: 1.655 g/cm3
Pubmed CID: 5281813
3.3 Extraction Methods of the WDL
3.3.1 Solvent or Soxhlet Extraction Method
Shanshol et al. [16] used a Soxhlet apparatus to extract WDL from aerial parts of E. alba. The shade dried plant material powder was used to extract WDL. Various solvents such as water, hexane, absolute methanol, and absolute ethanol were used at 50°C for 36 h to extract the material. The extracted material was filtered and concentrated using a rotary evaporator and the dried material was kept at 4°C until further use. The extracted sample was also evaluated for different phytochemical constituents. Patil et al. [17] also used this method for extracting WDL from W. calendulacea using methanol as a solvent for 24 h and dried using a rotary vacuum evaporator.
3.3.2 Ultrasound-Assisted Extraction
In this method, asonochemical reactor consists of an ultrasonic probe was employed to extract WDL from E. prostrata. Response surface methodology and central composite design were used to optimize the process parameters [18]. The important parameters for optimizing the process were type of solvent, temperature, extraction time, solvent to material ratio, ultrasonic power and extraction time. In the process, the powdered material (screened from #80-100 standard filter) was soaked in the solvent for about an hour in a vessel and ultrasonication was performed with 50% duty cycle at 5 s ON and 5 s OFF cycle by dipping 1.0 cm probe into the solvent. Savita et al. [19] used this method to extract WDL from E. alba with 1:5 ratio of methanol, herb powder, and found 0.36% yield of WDL.
3.3.3 Maceration Followed by Percolation
In this method, air-dried and coarse powder of whole plant of E. alba was used and macerated for 24 h in methanol and then percolated in a vessel until a colorless percolate was obtained [19]. The percolated extract was dried using a vacuum rotary evaporator at 40°C and obtained a green color sticky mass. The isolation of WDL was done through column chromatography using 60–120 mesh silica gel and toluene as an eluting solvent. The isolated material was further purified by preparative high-performance thin layer chromatography and found 0.38% yield. Finally, it was characterized and confirmed by spectroscopic techniques.
3.3.4 Supercritical CO2 Extraction
Patil et al. [17] extracted WDL from W. calendulacea using supercritical CO2 method. In this method, the extraction column was filled with sample powder and fixed in a column oven. A chiller unit was used to pass CO2 to achieve the desired working pressure. A solvent pump was employed to introduce methanol. The whole system was thermostatically maintained and then six port valves were opened to pass the supercritical CO2 to start the extraction cycle. The first cycle was static to achieve complete contact with sample, while the second cycle was dynamic, in which steady flow of CO2 to sample was achieved. The extract was collected in a vessel and analyzed to confirm WDL. They found that the concentration of WDL decreased when the pressure and temperature were increased from 25.0 to 35 MPa and 40 to 80°C, respectively. Similarly, when the extraction time was increased from 30 to 90 mins, the concentration of WDL was found to increase [17]. Also, Savita et al. [19] extracted 0.002–0.013% WDL using supercritical fluid extraction at different pressure (4000–6000 psi) and temperature (40–50°C) with a CO2 flow rate of 23.98 mL/min from E. alba.
3.3.5 Microwave-Assisted Extraction
Dang et al. [20,21] used a reflux system equipped with round bottom flask to extract WDL from E. alba. In their procedure, 90% ethanol was used as an extraction solvent with 200W microwave radiation for 30 mins. Then, extracted mixture was filtered and the process was repeated with the same sample three times. Afterward, the extract was dried using a rotary vacuum evaporator at 50°C, the dried extract was dissolved in hot water and filtered. The aqueous solution was extracted by ethyl acetate for three times, dehydrated over anhydrous sodium sulfate and finally 80%–90% solvent of the solvent was evaporated at 50°C under vacuum. Then, a silica gel column was used to purify WDL with dichloromethane, methanol and small amount of acetic acid as mobile phase. Final purification was performed using dilution crystallization with single factor analysis and response surface methodology. This resulted in 77.66% yield and 99.46% purity of WDL [21].
3.3.6 Ultra-High Pressure-Assisted Extraction
In this method, high-speed counter-current chromatography was employed with ultra-high-pressure extraction (UHPE) to extract and purify isodemethyl-WDL and WDL from E. alba [22]. The crude powdered herb was sieved using 60–80 mesh, filled into a polyethylene bag with the extraction solvent, sealed and placed in an ultra-high-pressure vessel for extraction. Then, the extract was centrifuged for 5 mins at 6000 rpm and filtered through 0.45 micron membrane filter. The WDL content was determined using high performance liquid chromatography. Afterward, two phase solvent system, i.e., petroleum ether-ethyl acetate-methanol-water in the ratio of 3:7:5:5 was used for high speed counter current chromatographic separation. By this method, 300 mg of crude extract yielded 23.5 mg WDL, 6.8 mg isodemethyl-WDL and 5.5 mg luteolin with more than 95% purity in a one-step separation. Various UHPE parameters such as time, solvent, pressure and solid-liquid ratio were optimized using orthogonal array. The optimum conditions were found to be 80% aqueous methanol, 3 mins extraction time, 1:20 solid-liquid ratio and 200 MPa pressure for good yield.
3.3.7 Aqueous Two-Phase System (ATPS) Extraction Method
In this method, ATPS was prepared from various mol. wt. of polyethylene glycol (PEG) and sodium citrate salt [23]. A fixed amount of E. alba dried powder was taken in a conical flask at a specified pH. Then, the mixture was agitated at a temperature of 30 ± 2°C and 600 rpm for 2 h using a magnetic stirrer. Afterward, the mixture was centrifuged at 8000 g rpm for 10 mins, the resultant mixture was collected using a separating funnel and analyzed by high performance liquid chromatography. It was observed that as the concentration of sodium citrate, mol. wt. of PEG, concentration of PEG and pH were increased from 14% to 16%, 4000 to 6000, 12% to 18% w/v and 5 to 7, respectively, the yield was found to increase, whereas, further increase in the range of parameters resulted in a decreased yield [23]. The optimized parameters for ATPS system were PEG 6000, PEG concentration 18% (w/v), salt concentration 17.96% (w/v), and pH 7 to get an extraction yield of 6.73 mg/g [23].
Pharmacokinetic studies involving albino rats indicated that WDL administration with paracetamol did not alter the bioavailability of paracetamol in plasma. The UV λmax for WDL was found to be 351, 248, and 208 nm in the spectrometric assessment. The following are the physical and chemical properties observed by WDL. Melting point: 310°C, retention time (HPLC):11 mins. There was no change in Cmax between paracetamol and WDL, but the Tmax of paracetamol was 2 h in control group rats, while WDL oral treatment with paracetamol resulted in a shift of Tmax to 2 to 3 h without substantially affecting the area under the curve [24]. A validation study showed that inter- and intra-day accuracy of quality control WDL specimens was within the acceptance threshold (85%–115 %). Mean recovery was found to be within the acceptable limit of >95% (98.8% WDL) for marker quality control samples. It was found that WDL was stable at ambient temperature for 6 hours and below 8°C for 15 days [25]. In a quantification study of WDL in mice plasma, an apparent distribution of 53.5 L/kg and a clearance of 6.39 L/h/kg were observed. In addition, the area under the conc-time curves AUC/0-24 for WDL was 27.5 ng/h/mL. The concentration of plasma above the lower limit of quantitation was noted up to 24 h showed 10.54 h of Mean Residence Time (MRT0-inf) for WDL, which may be the reason for its sustained pharmacological activity [26]. Chen et al. [27] studied the pharmacokinetic of WDL in rats and found that 5.00 mg/kg WDL was rapidly absorbed through gastrointestinal tract. This study provided a theoretical basis for pharmacological research on pre-clinical medicine.
4 Therapeutic Application of WDL
4.1 Free Radical Scavenging Activity
Free radicals are extremely reactive molecular species with an unpaired electron in the exterior valence orbitals that causes them to capture electrons from other substances to neutralize themselves. It has been known that the overproduction of free radicals, including reactive oxygen and nitrogen species (RONS) play a key role in the development of many chronic diseases. It has been experimentally demonstrated that WDL extracted from E. alba has antioxidant potency to scavenge nitric oxide radical/superoxide [28]. The mechanistic investigations showed single electron transfer to be the primary pathway and Fe2+ chelation is a secondary pathway. Both of these pathways can be assigned to the catechol moiety of WDL rather than the cumestane skeleton [29].
4.2 WDL as an Effective Antimicrobial Agent
In recent years, phytochemicals with antimicrobial properties have gained overwhelming attention pharmaceutical applications. Staphylococcus epidermidis, Staphylococcus aureus and Salmonella typhimurium are the most vulnerable species to the antimicrobial activity of WDL. The most resistant strain of bacteria was Shigella flexneri. These finding indicated that WDL is a promising antimicrobial agent [30]. It has been experimentally demonstrated that crude extract of E. alba has antimicrobial, antibacterial and antiviral activities [31–33]. More recently, it has been demonstrated that WDL showed excellent activity against Salmonella typhimurium and Staphylococcus epidermidis. The minimum inhibitory concentration (MIC) and zone of inhibition of Staphylococcus epidermidis and S. Typhimurium were 15 µg/ml; 10.3 mm and 25 µg/ml; 9.2 mm, respectively. E. coli was a highly resistant bacteria strain [34].
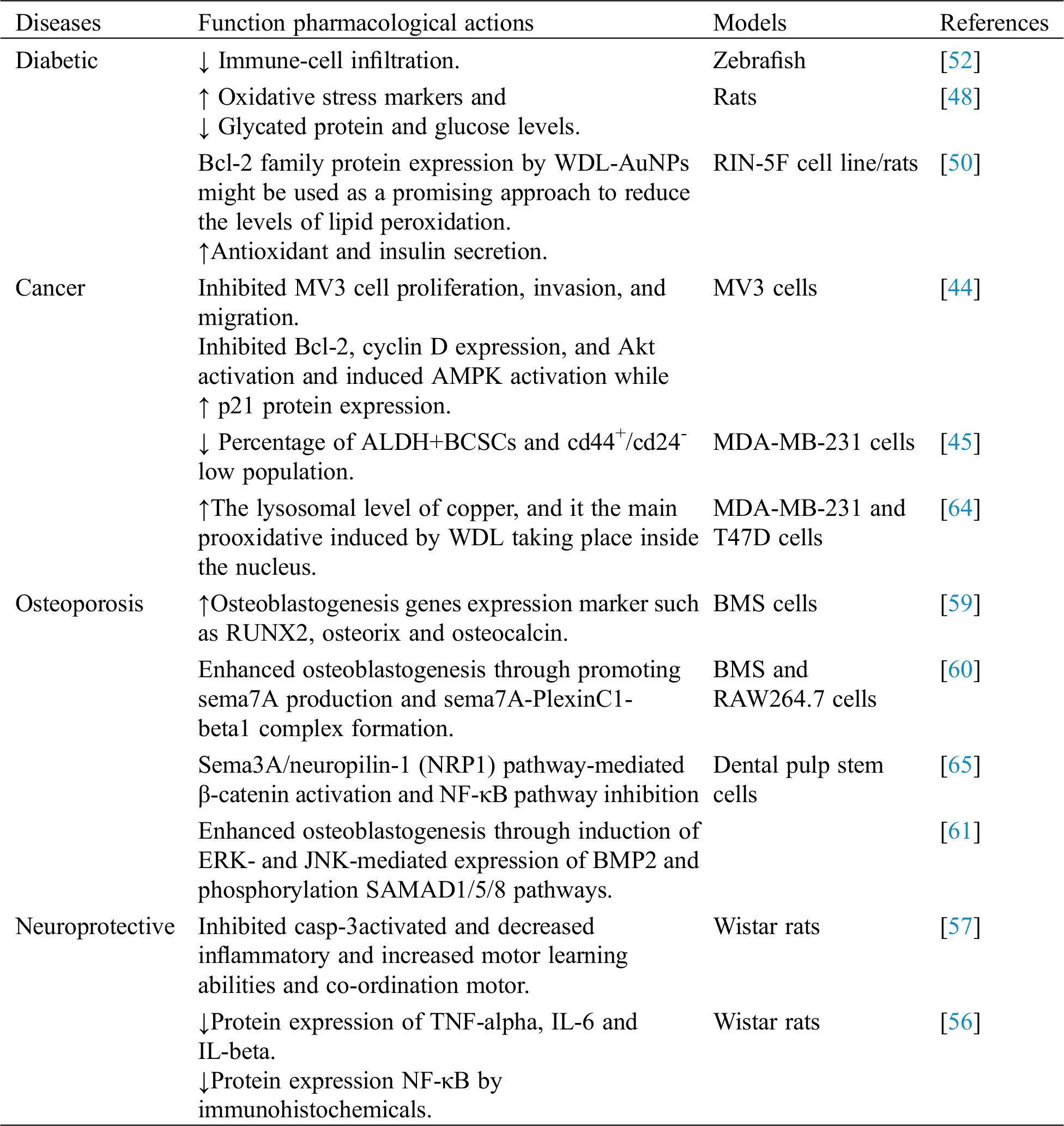
Cancer is one of the world's main health issues and is the second leading cause of death following heart disease [35]. The latest report released by the world health organization shows that 8.8 million individuals died in 2015 as a result of cancer, which accounts for around 16% of the world’s total fatalities. WDL has been shown to exhibit significant decreases in the protein values, nuclear accumulation, DNA binding and transcriptional activities of c-Myc. It can efficiently down-regulate the oncogenic role of cancer cells in c-Myc, that can be a new route to develop a therapeutic approach for Myc-driven prostate cancer [36]. In addition, WDL can specifically inhibit the estrogen receptor (ER) signaling and block the 17β-estradiol (E2) activated cell proliferation in the estrogen cancers [37]. The results of the study found that WDL specifically enhanced interferons (IFN-α)-induced signal transducers and activators of transcription 1 (STAT1) phosphatase by inhibition of STAT1 phosphatase T-cell protein tyrosine phosphatase (TCPTP) and possibly interacted with the c-terminal auto-inhibitory domain of TCPTP, to treat apoptosis of cancer cells [38]. The release quantity of liposomal indocyanine green (Lip-ICG) WDL reached upto 96.7% at 8 h, the release of drug on site was achieved under NIR irradiation. In addition, Lip-ICG WDL under near infrared (NIR) clearly inhibited the development of HepG2 cells, and early stage apoptosis in Hep G2 cell lines of rats were 33.7 percent. In addition, the tumor mice treated with the medication were inhibited to 81% [39]. It has been evaluated that WDL can destroy breast cancer cells, mediated by proteasomal protein targets (p21,27,53 and Bax) mortification pathway [40]. The results also suggested that WDL triggers selectivity induced caspase-3 protein apoptosis cells in prostate cancer via molecular mechanism involving the downregulation of protein kinase [41]. A recent study evaluated the in vivo effect of WDL (30 μg/ml) played an important role for regulation of zymosan induced inflammation signals in bone marrow-derived macrophages (BMs), mediated by zymosan secretion of tumor necrosis factor-α (TNF-α), interleukin-1, interleukin-6, interleukin-12 but not interleukin-10. Furthermore, decreased levels of superoxide generation, NADPH oxidase, phosphorylation of p47phox in BMDMs by pre-treatment of WDL have been demonstrated [42]. Another interesting study has found that WDL had a promisingaction in suppressing early tumor promotion events triggered by ultraviolet (UV) B radiation exposure as shown by ornithine decarboxylase, vimentin and vascular-endothelial growth factor expression [43]. Peng et al. [44] investigated the effect of WDL suppression in the proliferationof cell nuclear antigen and melanoma cell MV3 cell cycle proteins through AMP-activated protein kinase and Akt signal pathway. It has also been reported that WDL-coated poly(lactic-co-glycolic acid)-nanoparticles (PLGA-NPs) restricted the transition of epithelial cells to mesenchymal cells thereby preventing the cells from migrating and invading and reduced the growth of breast cancer stem cells in MDA-MB-231 cells [45]. Recently, Zhang et al. [46] showed that a combinational therapeutic approach (chemo photothermal) using WDL liposome coated gold nano-shells inhibits the tumor cells upon NIR irradiation andpromotes targeted drug release. The hyperthermia effect of gold nanoshells enhanced the release of drug onto the targeted site inhibited the 143B tumor cells up to 95.73%. In another study, WDL was shown to inhibit the protein cyclin D and increase p21 expression protein. Also, WDL inhibited activation of Akt but triggered activation of AMPK [47].
4.4 Antidiabetic Effect of WDL
Diabetes is generally characterized by hyperglycemia. In the past decade, it has been proved that WDL has good antioxidant and antiglycation activities. The significantly decreased levels of glycosylated haemoglobin (HbA1c) and glycated serum proteins, indicated the low generation of advanced glycation products (AGEs) and decreased pancreatic beta cells. Shahab et al. [48] reported that WDL has anti-glycating and anti-oxidation properties. A recent study has demonstrated that WDL has apparent lipid-lowering impacts and its underlying mechanism is mediated by peroxisome proliferator-activated receptor-α (PPARα)/lipoprotein lipase (LPL) and low-density lipoprotein receptor (LDLR) up-regulated expression and AMPK activation. Furthermore, WDL has been shown to cure hepatic steatosis. By directly scavenging reactive oxygen species (ROS) and raising the activity of several antioxidant enzymes, WDL shielded the liver from ROS attack [49]. It has been recently reported that regulation of Bcl-2 family protein expression by WDL-gold nanoparticles might be used as a promising approach to reduce the levels of lipid peroxidation, improve antioxidant, increase insulin secretion and activate the PI3K/Akt pathway in RIN-5F cells/rat [50] (Fig. 2). Kumar et al. [51] has studied the hypoglycemic effect of WDL using α-amylase and α-glucosidase properties [51]. It was noted that the inhibitory effect on α-glucosidase of WDL was as same as to the standard drug acarbose, i.e., 80.65% and α-amylase activity showed much higher potential than acarbose, which is 93.83% while acarbose showed only 42.23%. Further, molecular docking of WDL with glibenclamide has also been studied. WDL showed anti-diabetic activity in the zebrafish model [52].

Figure 2: Scheme indicates the mechanism of oxidative stress reduction of WDL. Diabetes causes destruction of beta-cells, results in decreased insulin level that leads to significant increase in blood glucose level. Increased glucose binds to protein molecules and as result increases glycosylated haemoglobin (HbA1c). WDL treatment protects beta-cells from diabetic condition free radicals via its antioxidant activity thereby improve insulin levels and decrease glucose in blood leading to reduced HbA1c. WDL treatment significantly decreases the glucose auto-oxidation, free radical levels, lipid peroxidation, and improves antioxidants
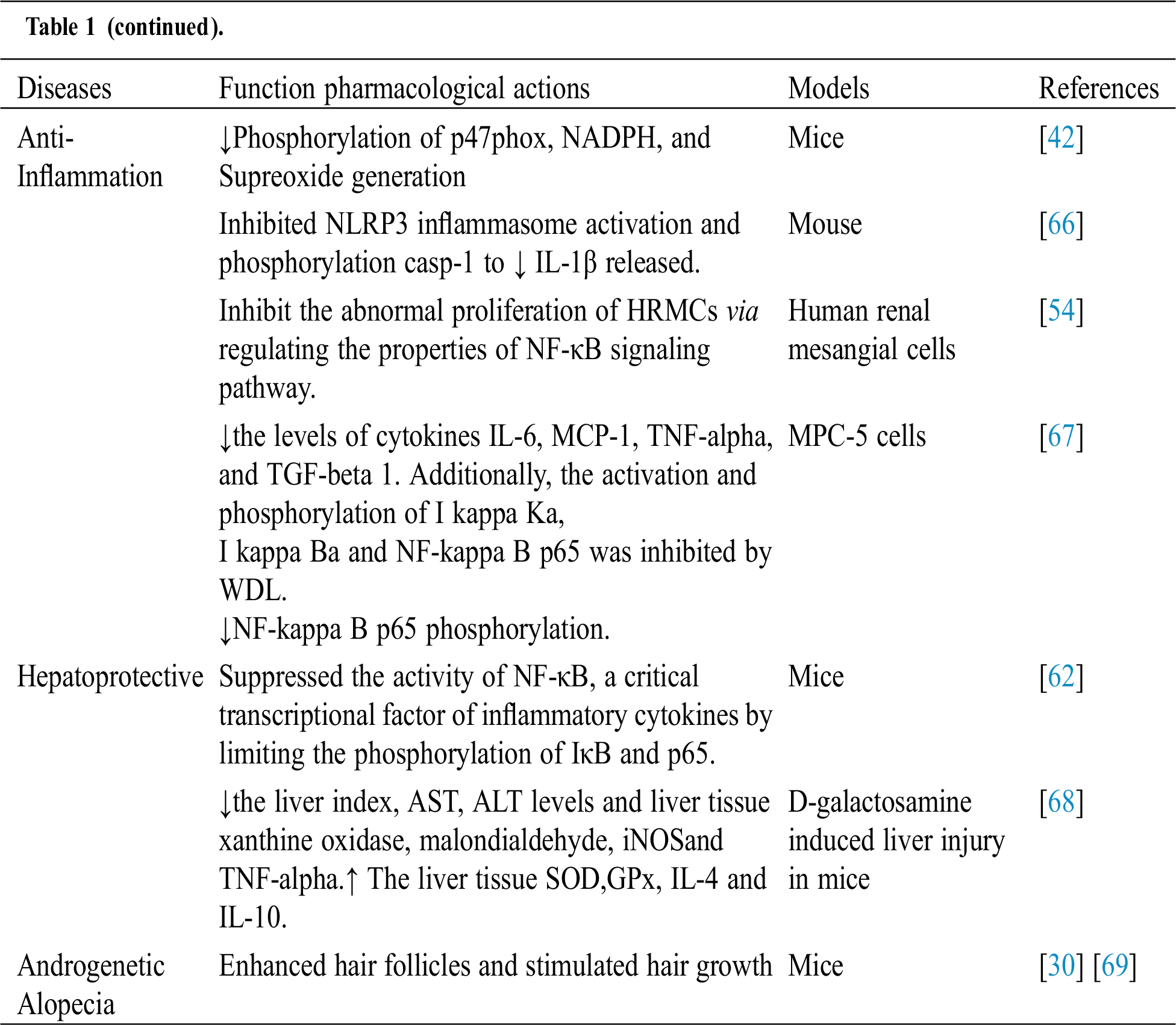
Many studies showed that WDL and its active constituents possessed anti-inflammatory properties. WDL, a natural compound has been shown to inhibit the activation of nuclear factor-kappa B (NF-κB) pathway. It possesses anti-inflammatory activity to inhibit cytokines including IL-1b and IKK in disease condition [53]. The results showed that WDL from E. alba could be a novel drug for the therapy of kidney damage caused by the inflammation response of human renal mesangial cells through influencing the NF-κB signaling pathway [54]. Furthermore, the treatment of WDL (30 µg/ml) significantly decreased phosphorylation of p47phox, superoxide generation, and nicotinamide adenine dinucleotide phosphate oxidase [42]. A study by Yuan et al. [55] found that WDL (10µM) treatment inhibited the inducible nitric oxides synthase (iNOS) and cyclooxygenase 2(Cox2) protein expression in lipopolysaccharide-stimulated cells.
Some individuals, especially elderly people, have a high risk for degenerative nerve diseases such as Parkinson’s and Alzheimer diseases. Recent investigations revealed that WDL could protect motor neurons from Al-caused toxicity by enhancing antioxidant status, brain-derived neurotrophic factor, and prevent excitotoxicity of glutamate. In brain, excitotoxicity can cause neuron damage by the over-activations and excessive stimulation of receptors for the neurotransmitter glutamate, NMDA receptor for N-methyl-D-aspartic acid (NMDA), and AMPA receptor for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA). Moreover, WDL inhibits the activation of caspase-3 and reduces inflammatory cytokines [56]. In addition, WDL therapy reduced lactate dehydrogenase (LDH), caspase-3 and m-calpain operations. The reports highly supported the usefulness of both compounds in preventing quinolinic acid-induced glutamate excitotoxicity. After WDL treatment, vascular endothelial growth factor (VEGF), insulin-like growth factor type 1 (IGF-1) and N-acetylasparatate (NAA) levels were improved in the brain [57].
Cardiovascular diseases (CVD) are heart and blood vessel disorders such as coronary heart disease, hypertensive heart diseases, stroke, rheumatic heart disease and other conditions. Four of five deaths from CVD are caused by heart attacks and strokes. WDL has exhibited cardiovascular protective effects by dyslipidemia, such as in the reduced triglycerides (TG), very low-density lipoprotein cholesterol (VLDL-C), total cholesterol (TC), upregulation of PPAR-α and activation of AMPK [49]. Another report shows that WDL may have therapeutic effect by activating AMPK and significantly decreasing protein expression TGFβ/Raf-MAPK [58]. The administration of WDL suppressed Akt and neointima to and inhibited cyclin D1 and cyclin-dependent kinase inhibitor p21 in balloon-injured common carotid arteries (CCAs) when compared with untreated CCAs. Based on these results, it can be stated that WDL exhibits potential therapeutic efficacy in treating cardiovascular ailments in rats [44].
Osteoporosis, a chronic metabolic bone disease, is a major health problem. Studies have found that WDL exerted a promising beneficial effect on osteoclast differentiation and osteoblastogenesis protein markers such as osteocalcin, runx2 and osteorix, which can encumber osteoclastic bone resorption. It has also been shown that WDL inhibits migration of MV3 cells. WDL caused the development of Bax pro-apoptotic protein but inhibited the development of apoptotic Bcl-2 protein. WDL inhibited cycline D expression while significantly improving p21 protein expression. WDL has been shown to inhibit the activation of Akt but cause activation of AMPK [59]. Studies have also demonstrated that WDL can efficiently protect mesenchymal stem cells against hydroxyl radical-induced oxidative damage. This protective effect may provide application of WDL in mesenchymal stem cells transplantation (osteoporosis); radical scavengers via an electron transfer- radical adduct formation reaction RAF pathway [29]. It has been evaluated that WDL semaphoring 4D (SEMA4D) inhibits the formation of SEMA4D/PLEXIN-B1. The cell culture of bone marrow stromal cells with RAW264.7 cell, addition, WDL at the dose of tartrate-resistant acid phosphatase properties. The study demonstrated that WDL reduced the progression of osteoclastogenesis but stimulated osteoblastogenesis [60]. Moreover, the gene expression bone morphogenetic protein-2 and phosphorylation levels of SMAD-1/5/8 were significantly decreased after WDL treatment [61].
Hepatitis is an inflammation of the liver that can cause a range of health problems. The liver is one of the body's main organs and plays a basic role in regulating various functions, including metabolism, secretion, storage, and detoxification of endogenous and exogenous substances. WDL improved hepatic lipid metabolism and improves hepatic steatosis mediated by AMPK activation [49]. Luo et al. [62] showed that WDL could suppress NF-κB activity, a significant transcription factor for inflammatory cytokines by restricting IκB and p65 phosphorylation. The authors of the studies concluded that WDL shows hepatoprotective activity by inhibiting topoisomerase activity [63].
Androgenetic alopecia (AGA) is commonly related with aging type of hair loss symptoms in men [70]. AGA affects up to 80% men and 50% women in their lifetime [71]. It is the result of the effects of dehydrotestosterone (DHT) converted from testosterone by enzymatic functions of 5-alpha reductase. High dose of DHT affect androgen-sensitive hair follicles and cause progressing hair loss [72]. Another type of hair loss, Alopecia areata is caused by autoimmune disorder in hair follicles that results in hair loss resulting in spot baldness [73]. Currently, inhibitors for a type 25-α reductase, such as finasteride, dutasteride and minoxidil, are main drugs in treating AGA and promoting hair regrowth [74,75]. In order to reduce side effects, AGA hair loss has been treated with natural products even in a traditional way without using drugs such as finasteride and dutasteride. Formulation of herbal extracts and natural compounds is mostly applied to reduce progression of hair loss [75]. E. alba showed hair growth promotion effects in traditional medicine in Korea and China. Evidential examples have been applied to prevent hair loss using E. alba extracts which increased the hair follicles and improved hair growth in mice [30,69] (Fig. 3 and Tab. 1).
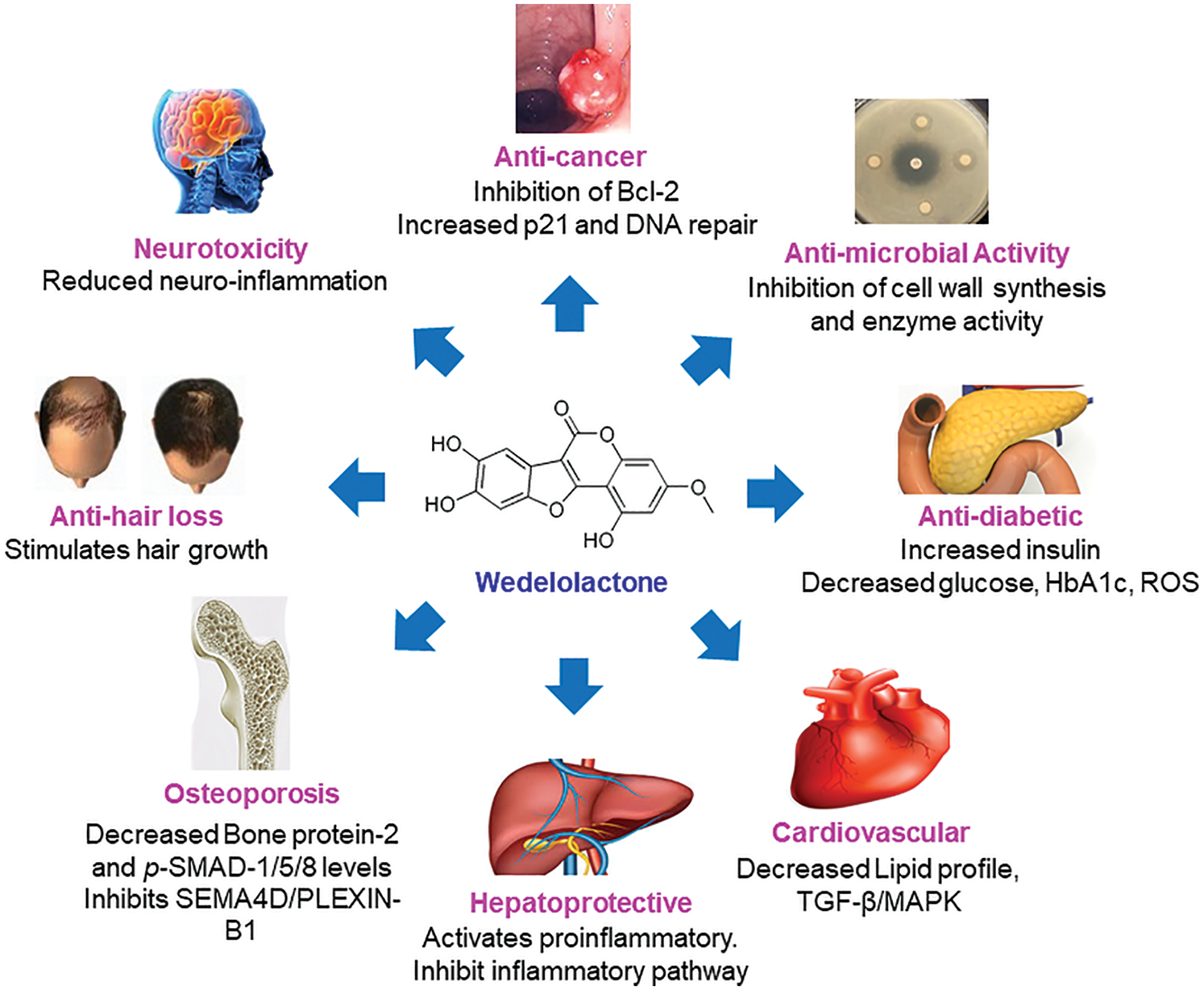
Figure 3: Pharmacological mechanisms of WDL
Table 1: Biological activities of WDL
Natural fresh plant sources of high phytochemicals and antioxidants have the potential to be useful supplements in the discipline of integrative medicine. This review presents comprehensive information derived from recently published scientific works that have documented the biological activity of WDL in therapeutic applications. The antibacterial, analgesic, antioxidant, cytotoxic, antidiabetic, anti-inflammatory, neuroprotective, cardioprotective and hepatoprotective activities of WDL and the mechanisms of action have also been discussed in this review.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Pan, S. Y., Zhou, S. F., Gao, S. H., Yu, Z. L., Zhang, S. F. et al. (2013). New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evidence-Based Complementary and Alternative Medicine, 2013(1), 1–25. DOI 10.1155/2013/627375.
2. NagoorMeeran, M. F., Goyal, S. N., Suchal, K., Sharma, C., Patil, C. R. et al. (2018). Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: A pentacyclic triterpenoid of therapeutic promise. Frontiers in Pharmacology, 9, 892. DOI 10.3389/fphar.2018.00892.
3. Rubab, S., Rizwani, G. H., Bahadur, S., Shah, M., Alsamadany, H. et al. (2020). Neuropharmacological potential of various morphological parts of Camellia sinensis L. Saudi Journal of Biological Sciences, 27(1), 567–573. DOI 10.1016/j.sjbs.2019.11.025.
4. Ishtiaq, S., Hanif, U., Shaheen, S., Bahadur, S., Liaqat, I. et al. (2020). Antioxidant potential and chemical characterization of bioactive compounds from a medicinal plant Colebrokea oppositifolia Sm. Anais da Academia Brasileira de Ciências, 92(2), e20190387. DOI 10.1590/0001-3765202020190387. [Google Scholar] [CrossRef]
5. Bahadur, S., Khan, M. S., Shah, M., Shuaib, M., Ahmad, M. et al. (2020). Traditional usage of medicinal plants among the local communities of Peshawar valley. Pakistan Acta EcologicaSinica, 40(1), 1–29. DOI 10.1016/j.chnaes.2018.12.006. [Google Scholar] [CrossRef]
6. Ashfaq, S., Ahmad, M., Zafar, M., Sultana, S., Bahadur, S. et al. (2019). Medicinal plant biodiversity used among the rural communities of arid regions of northern Punjab, Pakistan. Indian Journal of Traditional Knowledge, 81(2), 226–241. [Google Scholar]
7. Pandey, K. B., Rizvi, S. I. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity, 2(5), 270–278. DOI 10.4161/oxim.2.5.9498. [Google Scholar] [CrossRef]
8. Vinayagam, R., Jayachandran, M., Xu, B. (2016). Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytotherapy Research, 30(2), 184–199. DOI 10.1002/ptr.5528. [Google Scholar] [CrossRef]
9. Vinayagam, R., Xu, B. (2015). Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutrition & Metabolism, 12(1), 60. DOI 10.1186/s12986-015-0057-7. [Google Scholar] [CrossRef]
10. Harborne, J. B., Williams, C. A. (2000). Advances in flavonoid research since 1992. Phytochemistry, 55(6), 481–504. DOI 10.1016/S0031-9422(00)00235-1. [Google Scholar] [CrossRef]
11. Habauzit, V., Morand, C. (2012). Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Therapeutic Advances in Chronic Disease, 3(2), 87–106. DOI 10.1177/2040622311430006. [Google Scholar] [CrossRef]
12. Lenza, V. A., Morkel, L. J. F., Coppede, J. S., Fernandes, V. C., Rossi, N. M. et al. (2009). Antimicrobial activities of ethanol extract and coumestans from Eclipta alba (L.) Hassk.(Asteraceae). Latin American Journal of Pharmacy, 28, 863–868. [Google Scholar]
13. Cory, H., Passarelli, S., Szeto, J., Tamez, M., Mattei, J. (2018). The role of polyphenols in human health and food systems: A mini-review. Frontiers in Nutrition, 5, 87. DOI 10.3389/fnut.2018.00087. [Google Scholar] [CrossRef]
14. Chopra, R. N., Nayar, S. L., Chopra, I. C., Asolkar, L., Kakkar, K. (1956). Glossary of Indian medicinal plants, New Delhi. Council of Scientific & Industrial Research, 55, 1–329. [Google Scholar]
15. Feng, L., Zhai, Y. Y., Xu, J., Yao, W. F., Cao, Y. D. et al. (2019). A review on traditional uses, phytochemistry and pharmacology of Eclipta prostrata (L.) L. Journal of Ethnopharmacology, 245, 112109. DOI 10.1016/j.jep.2019.112109. [Google Scholar] [CrossRef]
16. Shanshol, Z. A., Alaubydi, M. A., Sadik, A. (2013). The extraction and partial purification of wedelolactone from local Eclipta alba plant. Iraqi Journal of Science, 54(5), 1084–1089. [Google Scholar]
17. Patil, A. A., Sachin, B. S., Wakte, P. S., Shinde, D. B. (2014). Optimization of supercritical fluid extraction and HPLC identification of wedelolactone from Wedelia calendulacea by orthogonal array design. Journal of Advanced Research, 5(6), 629–635. DOI 10.1016/j.jare.2013.09.002. [Google Scholar] [CrossRef]
18. Fang, X., Wang, J., Wang, Y., Li, X., Zhou, H. et al. (2014). Optimization of ultrasonic-assisted extraction of wedelolactone and antioxidant polyphenols from Eclipta prostrate L. using response surface methodology. Separation and Purification Technology, 138, 55–64. DOI 10.1016/j.seppur.2014.10.007. [Google Scholar] [CrossRef]
19. Savita, K., Prakashchandra, K. (2011). Optimization of extraction conditions and development of a sensitive HPTLC method for estimation of wedelolactone in different extracts of Eclipta alba. International Journal of Pharmaceutical Sciences and Drug Research, 3(1), 56–61. [Google Scholar]
20. Shi, D., Ding, H., Xu, S. (2014). Optimization of microwave-assisted extraction of wedelolactone from Eclipta alba using response surface methodology. Frontiers of Chemical Science and Engineering, 8(1), 34–42. DOI 10.1007/s11705-014-1401-6. [Google Scholar] [CrossRef]
21. Ding, H., Wang, Y., Gao, Y., Han, X., Liu, S. et al. (2017). Purification of wedelolactone from Eclipta alba and evaluation of antioxidant activity. Separation Science and Technology, 52(17), 2732–2741. DOI 10.1080/01496395.2017.1374973. [Google Scholar] [CrossRef]
22. Zhao, H., Cheng, S., Zhang, L., Dong, H., Zhang, Y. et al. (2019). Ultra-high-pressure-assisted extraction of wedelolactone and isodemethylwedelolactone from Ecliptae Herba and purification by high-speed counter-current chromatography. Biomedical Chromatography, 33(6), e4497. DOI 10.1002/bmc.4497. [Google Scholar] [CrossRef]
23. Gharat, N. N., Rathod, V. K. (2020). Response surface methodology for the extraction of wedelolactone from Eclipta alba using aqueous two-phase extraction. Preparative Biochemistry & Biotechnology, 50(8), 827–833. DOI 10.1080/10826068.2020.1753071. [Google Scholar] [CrossRef]
24. Sagar, B. P. S., Panwar, R., Goswami, A., Kadian, K., Tyagi, K. et al. (2006). Pharmacokinetic interactions of antihepatotoxic wedelolactone with paracetamol in wistar albino rats. Pharmaceutical Biology, 44(7), 554–561. DOI 10.1080/13880200600885242. [Google Scholar] [CrossRef]
25. Shailajan, S., Menon, S., Singh, D., Swar, G. (2016). Validated analytical RP-HPLC method for quantitation of wedelolactone from Eclipta alba and marketed Ayurvedic formulations. Pharmacognosy Journal, 8(2), 132–139. DOI 10.5530/pj.2016.2.6. [Google Scholar] [CrossRef]
26. Cheruvu, H. S., Yadav, N. K., Valicherla, G. R., Arya, R. K., Hussain, Z. et al. (2018). LC-MS/MS method for the simultaneous quantification of luteolin, wedelolactone and apigenin in mice plasma using hansen solubility parameters for liquid-liquid extraction: Application to pharmacokinetics of Eclipta alba chloroform fraction. Journal of Chromatography B, 1081, 76–86. DOI 10.1016/j.jchromb.2018.01.035. [Google Scholar] [CrossRef]
27. Chen, Q., Wu, X., Gao, X., Song, H., Zhu, X. (2019). Development and validation of an ultra-performance liquid chromatography method for the determination of wedelolactone in rat plasma and its application in a pharmacokinetic study. Molecules, 24(4), 762. DOI 10.3390/molecules24040762. [Google Scholar] [CrossRef]
28. Unnikrishnan, K. P., Fathima, A., Hashim, K. M., Balachandran, I. (2007). Antioxidant studies and determination of wedelolactone in Eclipta alba. Journal of Plant Sciences, 2(4), 459–464. DOI 10.3923/jps.2007.459.464. [Google Scholar] [CrossRef]
29. Li, X., Wang, T., Liu, J., Liu, Y., Zhang, J. et al. (2020). Effect and mechanism of wedelolactone as antioxidant-coumestan on OH-treated mesenchymal stem cells. Arabian Journal of Chemistry, 13(1), 184–192. DOI 10.1016/j.arabjc.2017.03.008. [Google Scholar] [CrossRef]
30. Dalal, S., Kataria, S. K., Sastry, K. V., Rana, S. V. S. (2010). Phytochemical screening of methanolic extract and antibacterial activity of active principles of hepatoprotective herb, Eclipta alba. Ethnobotanical Leaflets, 14, 248–258. [Google Scholar]
31. Venkatesan, S., Ravi, R. (2004). Antifungal activity of Eclipta alba. Indian Journal of Pharmaceutical Sciences, 66(1), 97–98. [Google Scholar]
32. Ma-Ma, K., Nyunt, N., Tin, K. M. (1978). The protective effect of Eclipta alba on carbon tetrachloride-induced acute liver damage. Toxicology and Applied Pharmacology, 45(3), 723–728. DOI 10.1016/0041-008X(78)90165-5.
33. Karthikumar, S., Vigneswari, K., Jegatheesan, K. (2007). Screening of antibacterial and antioxidant activities of leaves of Eclipta prostrata (L). Scientific Research and Essays, 2(4), 101–104. [Google Scholar]
34. Dalal, S., Rana, S., Sastry, K., Kataria, S. (2009). Wedelolactone as an antibacterial agent extracted from Eclipta alba. Internet Journal of Microbiology, 7, 1–11. [Google Scholar]
35. Daher, I. N., Daigle, T. R., Bhatia, N., Durand, J. B. (2012). The prevention of cardiovascular disease in cancer survivors. Texas Heart Institute Journal, 39(2), 190. [Google Scholar]
36. Sarveswaran, S., Ghosh, R., Parikh, R., Ghosh, J. (2016). Wedelolactone, an anti-inflammatory botanical, interrupts c-Myc oncogenic signaling and synergizes with enzalutamide to induce apoptosis in prostate cancer cells. Molecular Cancer Therapeutics, 15(11), 2791–2801. DOI 10.1158/1535-7163.MCT-15-0861. [Google Scholar] [CrossRef]
37. Xu, D., Lin, T. H., Yeh, C. R., Cheng, M. A., Chen, L. M. et al. (2014). The wedelolactone derivative inhibits estrogen receptor-mediated breast, endometrial, and ovarian cancer cells growth. BioMed Research International, 2014(17), 1–11. DOI 10.1155/2014/713263. [Google Scholar] [CrossRef]
38. Chen, Z., Sun, X., Shen, S., Zhang, H., Ma, X. et al. (2013). Wedelolactone, a naturally occurring coumestan, enhances interferon-γ signaling through inhibiting STAT1 protein dephosphorylation. Journal of Biological Chemistry, 288(20), 14417–14427. DOI. DOI 10.1074/jbc.M112.442970. [Google Scholar] [CrossRef]
39. Zhang, X., Li, N., Liu, Y., Ji, B., Wang, Q. et al. (2016). On-demand drug release of ICG-liposomal wedelolactone combined photothermal therapy for tumor. Nanomedicine: Nanotechnology, Biology and Medicine, 12(7), 2019–2029. DOI 10.1016/j.nano.2016.05.013. [Google Scholar] [CrossRef]
40. Nehybová, T., Šmarda, J., Daniel, L., Stiborek, M., Kanický, V. et al. (2017). Wedelolactone acts as proteasome inhibitor in breast cancer cells. International Journal of Molecular Sciences, 18(4), 729. DOI 10.3390/ijms18040729. [Google Scholar] [CrossRef]
41. Sarveswaran, S., Gautam, S. C., Ghosh, J. (2012). Wedelolactone, a medicinal plant-derived coumestan, induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCε without inhibiting Akt. International Journal of Oncology, 41(6), 2191–2199. DOI 10.3892/ijo.2012.1664. [Google Scholar] [CrossRef]
42. Cuong, T. T., Diem, G. H., Doan, T. T., Huy, N. Q., Phuong, N. et al. (2018). Wedelolactone from Vietnamese Ecliptaprostrata (L) L. protected Zymosan-induced shock in Mice. Iranian Journal of Pharmaceutical Research: IJPR, 17(2), 653. [Google Scholar]
43. Ali, F., Khan, B. A., Sultana, S. (2016). Wedelolactone mitigates UVB induced oxidative stress, inflammation and early tumor promotion events in murine skin: Plausible role of NF-kB pathway. European Journal of Pharmacology, 786, 253–264. DOI 10.1016/j.ejphar.2016.05.008. [Google Scholar] [CrossRef]
44. Peng, L., Huang, X., Jin, X., Jing, Z., Yang, L. et al. (2017). Wedelolactone, a plant coumarin, prevents vascular smooth muscle cell proliferation and injury-induced neointimal hyperplasia through Akt and AMPK signaling. Experimental Gerontology, 96, 73–81. DOI 10.1016/j.exger.2017.06.011. [Google Scholar] [CrossRef]
45. Das, S., Mukherjee, P., Chatterjee, R., Jamal, Z., Chatterji, U. (2019). Enhancing chemosensitivity of breast cancer stem cells by downregulating SOX2 and ABCG2 using wedelolactone-encapsulated nanoparticles. Molecular Cancer Therapeutics, 18(3), 680–692. DOI 10.1158/1535-7163.MCT-18-0409. [Google Scholar] [CrossRef]
46. Zhang, X., Liu, Y., Luo, L., Li, L., Xing, S. et al. (2019). A chemo-photothermal synergetic antitumor drug delivery system: Gold nanoshell coated wedelolactone liposome. Materials Science and Engineering C, 101, 505–512. DOI 10.1016/j.msec.2019.04.006. [Google Scholar] [CrossRef]
47. Peng, Y. G., Zhang, L. (2019). Wedelolactone suppresses cell proliferation and migration through AKT and AMPK signaling in melanoma. Journal of Dermatological Treatment, 30(4), 389–395. DOI 10.1080/09546634.2018.1527996. [Google Scholar] [CrossRef]
48. Shahab, U., Faisal, M., Alatar, A. A., Ahmad, S. (2018). Impact of wedelolactone in the anti-glycation and anti-diabetic activity in experimental diabetic animals. IUBMB Life, 70(6), 547–552. DOI 10.1002/iub.1744. [Google Scholar] [CrossRef]
49. Zhao, Y., Peng, L., Yang, L. C., Xu, X. D., Li, W. J. et al. (2015). Wedelolactone regulates lipid metabolism and improves hepatic steatosis partly by AMPK activation and up-regulation of expression of PPARα/LPL and LDLR. PLoS One, 10(7), e0132720. DOI 10.1371/journal.pone.0132720. [Google Scholar] [CrossRef]
50. Ramachandran, V., Arokia Vijaya Anand, M., David, E., Venkatachalam, K., Vijayakumar, S. et al. (2020). Antidiabetic activity of gold nanoparticles synthesized using wedelolactone in RIN-5F cell line. Antioxidants, 9(1), 8. DOI 10.3390/antiox9010008. [Google Scholar] [CrossRef]
51. Kumar, V., Sharma, K., Ahmed, B., Al-Abbasi, F. A., Anwar, F. et al. (2018). Deconvoluting the dual hypoglycemic effect of wedelolactone isolated from Wedelia calendulacea: Investigation via experimental validation and molecular docking. RSC Advances, 8(32), 18180–18196. DOI 10.1039/C7RA12568B. [Google Scholar] [CrossRef]
52. Delgadillo-Silva, L. F., Tsakmaki, A., Akhtar, N., Franklin, Z. J., Konantz, J. et al. (2019). Modelling pancreatic β-cell inflammation in zebrafish identifies the natural product wedelolactone for human islet protection. Disease Models & Mechanisms, 12(1), dmm036004. DOI 10.1242/dmm.036004. [Google Scholar] [CrossRef]
53. Kobori, M., Yang, Z., Gong, D., Heissmeyer, V., Zhu, H. et al. (2004). Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death & Differentiation, 11(1), 123–130. DOI 10.1038/sj.cdd.4401325. [Google Scholar] [CrossRef]
54. Shen, P., Yang, X., Jiang, J., Wang, X., Liang, T. (2017). Wedelolactone from Eclipta alba inhibits lipopolysaccharide-enhanced cell proliferation of human renal mesangial cells via NF-κB signaling pathway. American Journal of Translational Research, 9(5), 2132. [Google Scholar]
55. Yuan, F., Chen, J., Sun, P. P., Guan, S., Xu, J. (2013). Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. Journal of Biomedical Science, 20(1), 84. DOI 10.1186/1423-0127-20-84. [Google Scholar] [CrossRef]
56. Maya, S., Prakash, T., Goli, D. (2018). Evaluation of neuroprotective effects of wedelolactone and gallic acid on aluminium-induced neurodegeneration: Relevance to sporadic amyotrophic lateral sclerosis. European Journal of Pharmacology, 835, 41–51. DOI 10.1016/j.ejphar.2018.07.058. [Google Scholar] [CrossRef]
57. Maya, S., Prakash T., Goli D. (2018). Effect of wedelolactone and gallic acid on quinolinic acid-induced neurotoxicity and impaired motor function: Significance to sporadic amyotrophic lateral sclerosis. Neurotoxicology, 68, 1–12. DOI 10.1016/j.neuro.2018.06.015. [Google Scholar] [CrossRef]
58. Yang, J. Y., Tao, L. J., Liu, B., You, X. Y., Zhang, C. F. et al. (2019). Wedelolactone attenuates pulmonary fibrosis partly through activating AMPK and regulating Raf-MAPKs signaling pathway. Frontiers in Pharmacology, 10, 151. DOI 10.3389/fphar.2019.00151. [Google Scholar] [CrossRef]
59. Dong, P., Zhu, D., Deng, X., Zhang, Y., Ma, J. et al. (2019). Effect of hydroxyapatite nanoparticles and wedelolactone on osteoblastogenesis from bone marrow mesenchymal stem cells. Journal of Biomedical Materials Research Part A, 107(1), 145–153. DOI 10.1002/jbm.a.36541. [Google Scholar] [CrossRef]
60. Deng, X., Liang, L. N., Zhu, D., Zheng, L. P., Yu, J. H. et al. (2018). Wedelolactone inhibits osteoclastogenesis but enhances osteoblastogenesis through altering different semaphorins production. International Immunopharmacology, 60, 41–49. DOI 10.1016/j.intimp.2018.04.037. [Google Scholar] [CrossRef]
61. Zhu, D., Deng, X., Han, X. F., Sun, X. X., Pan, T. W. et al. (2018). Wedelolactone enhances osteoblastogenesis through ERK-and JNK-mediated BMP2 expression and Smad/1/5/8 phosphorylation. Molecules, 23(3), 561. DOI 10.3390/molecules23030561. [Google Scholar] [CrossRef]
62. Luo, Q., Ding, J., Zhu, L., Chen, F., Xu, L. et al. (2018). Hepatoprotective effect of wedelolactone against concanavalin A-induced liver injury in mice. American Journal of Chinese Medicine, 46(04), 819–833. DOI 10.1142/S0192415X1850043X. [Google Scholar] [CrossRef]
63. Benes, P., Knopfova, L., Trcka, F., Nemajerova, A., Pinheiro, D. et al. (2011). Inhibition of topoisomerase IIα: Novel function of wedelolactone. Cancer Letters, 303(1), 29–38. DOI 10.1016/j.canlet.2011.01.002. [Google Scholar] [CrossRef]
64. Kučírková, T., Stiborek, M., Dúcka, M., Navrátilová, J., Pristov, J. B. (2018). Anti-cancer effects of wedelolactone: interactions with copper and subcellular localization. Metallomics, 10(10), 1524–1531. DOI 10.1039/C8MT00191J.
65. Wang, C., Song, Y., Gu, Z., Lian, M., Huang, D. et al. (2018). Wedelolactone enhances odontoblast differentiation by promoting Wnt/β-catenin signaling pathway and suppressing NF-κB signaling pathway. Cellular Reprogramming, 20(4), 236–244. DOI 10.1089/cell.2018.0004.
66. Wei, W., Ding, M., Zhou, K., Xie, H., Zhang, M. et al. (2017). Protective effects of wedelolactone on dextran sodium sulfate induced murine colitis partly through inhibiting the NLRP3 inflammasome activation via AMPK signaling. Biomedicine & Pharmacotherapy, 94, 27–36. DOI 10.1016/j.biopha.2017.06.071.
67. Zhu, M. M., Wang, L., Yang, D., Li, C., Pang, S. T. et al. (2019). Wedelolactone alleviates doxorubicin-induced inflammation and oxidative stress damage of podocytes by IκK/IκB/NF-κB pathway. Biomedicine & Pharmacotherapy, 117, 109088. DOI 10.1016/j.biopha.2019.109088.
68. Wang, W., Tian, C., Lv, Y., Wang, G. (2020). Protective effect of Wedelolactone on acute D-Galactosamine-induced acute liver injury in mice. LATIN American Journal of Pharmacy, 39(6), 1122–1127.
69. Lee, K. H., Choi, D., Jeong, S. I., Kim, S. J., Lee, C. H. et al. (2019). Ecliptaprostrata promotes the induction of anagen, sustains the anagen phase through regulation of FGF-7 and FGF-5. Pharmaceutical Biology, 57(1), 105–111. DOI 10.1080/13880209.2018.1561729. [Google Scholar] [CrossRef]
70. Gupta, M., Mysore, V. (2016). Classifications of patterned hair loss: A review. Journal of Cutaneous and Aesthetic Surgery, 9(1), 3–12. DOI 10.4103/0974-2077.178536. [Google Scholar] [CrossRef]
71. Piraccini, B. M., Alessandrini, A. (2014). Androgenetic alopecia. Giornaleitaliano di dermatologia e venereologia: Organoufficiale, Societaitaliana di dermatologia e sifilografia, 149(1), 15. [Google Scholar]
72. Roy, R. K., Thakur, M., Dixit, V. K. (2008). Hair growth promoting activity of Eclipta alba in male albino rats. Archives of Dermatological Research, 300(7), 357–364. DOI 10.1007/s00403-008-0860-3. [Google Scholar] [CrossRef]
73. Trüeb, R. M., Dias, M. F. R. G. (2018). Alopecia areata: A comprehensive review of pathogenesis and management. Clinical Reviews in Allergy & Immunology, 54(1), 68–87. DOI 10.1007/s12016-017-8620-9. [Google Scholar] [CrossRef]
74. Yim, E., Nole, K. L. B., Tosti, A. (2014). 5α-Reductase inhibitors in androgenetic alopecia. Current Opinion in Endocrinology, Diabetes and Obesity, 21(6), 493–498. DOI 10.1097/MED.0000000000000112. [Google Scholar] [CrossRef]
75. Adil, A., Godwin, M. (2017). The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. Journal of the American Academy of Dermatology, 77(1), 136–141. DOI 10.1016/j.jaad.2017.02.054. [Google Scholar] [CrossRef]