DOI:10.32604/phyton.2020.012985

| Phyton-International Journal of Experimental Botany DOI:10.32604/phyton.2020.012985 |  |
| Article |
1College of Food Science and Technology, Shanghai Ocean University, Shanghai, 201306, China.
2National Experimental Teaching Demonstration Center for Food Science and Engineering Shanghai Ocean University, Shanghai, 201306, China
3Shanghai Engineering Research Center of Aquatic Product Processing and Preservation, Shanghai, 201306, China
4Shanghai Professional Technology Service Platform on Cold Chain Equipment Performance and Energy Saving Evaluation, Shanghai, 201306, China
*Corresponding Authors: Jun Mei. Email: jmei@shou.edu.cn; Jing Xie. Email: jxie@shou.edu.cn
Received: 20 July 2020; Accepted: 09 September 2020
Abstract: Low-temperature storage is extensively used to optimize the postharvest life of various fresh fruits. However, red pitahaya (Hylocereus polyrhizus) fruits are sensitive to chilling injury (CI), which leads to the limitation of low-temperature storage. In this study, red pitahaya fruits were stored at 2, 4, 6, 8, and 10°C, respectively, for 27 days to determine the appropriate storage temperature. During the storage of red pitahaya fruits, storage at 8°C was more effective in suppressing decay and maintaining quality than other low temperatures. Low-temperature (2, 4, and 6°C) storage decreased weight loss (WL) and maintained higher content of titratable acidity (TA), soluble sugars (SS), and total phenolics (TP) but different degrees of CI were detected. No CI was observed at 8°C and 10°C. Red pitahay as stored at 8 and 10°C were associated with better color evaluation, lower electrolyte leakage (EL), respiration rate, and lipoxygenase (LOX) activity, and higher fruit firmness, superoxide dismutase (SOD) activity, and catalase (CAT) activity. However, higher storage temperature (10°C) resulted in higher metabolic activity leading to lower quality and antioxidant capacities compared with 8°C. Therefore, our results demonstrated that red pitahaya stored at 8°C exhibited a protective effect on fruit quality and resisted CI development during storage.
Keywords: Red pitahaya; quality; low temperature; chilling injury
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
Red pitahayas (Hylocereus polyrhizus), originating from Latin America and the West Indies [1], have attracted much attention worldwide due to attractive appearance, high nutritional value, and various biological activities [2–4]. However, the fruits are highly perishable, mainly caused by fungal diseases, degreening, and physiological disorders [5,6], which shortens its postharvest shelf life during distribution and marketing. Since the major quantum of fruits undergoes for fresh consumption [7], adequate and sound preservation technology for fruits’ quality maintenance and long-term storage are required for both the consumers and food processing markets.
Some methods preserving post-harvest red pitahaya fruits have been developed, such as Gamma irradiation [8], 1-methylcyclopropene treatment [5], and active package [9]. Nevertheless, these preservation technologies combined with low-temperature storage of fruits could have a more beneficial influence on maintaining fruit quality. Low-temperature storage can effectively delay fruit decay and prolong the storage period of red pitahayas. Obenland et al. [10] and Punitha et al. [11] reported that the fruits stored at 5°C or 6°C could maintain the quality and visual appearance better than those at higher temperatures. However, red pitahayas are sensitive to low temperatures such as 2°C resulting in CI, which limits the application of low-temperature storage [12,13].
CI is a suite of physiological defects that affects the marketability and storability of most tropical fruits [14,15]. In recent years, reports on horticultural products found that fruits responded to chilling temperatures mainly in two ways. On the one hand, the molecular level of CI affects bio-membrane conformation and structure [16], with an increase in electrolyte leakage and lipid peroxidation [17,18], causing a drastic fruit softening [19]. On the other hand, low-temperature storage may induce an uncoupling in the respiratory chain, giving rise to reactive oxygen species (ROS) [20] and the excessive accumulation of ROS can further poison cells and contribute to CI [21]. Yet, fruits can naturally protect themselves against oxidative damage by producing antioxidant enzymes, such as SOD and CAT [22].
Little information is available on the comprehensive evaluation of the physio-chemical changes concerning CI in red flesh pitahayas during cold storage. The object of this study was to investigate the physio-chemical changes in red pitahayas during cold storage at 2, 4, 6, 8, and 10°C and to determine optimum low-temperature without CI in red pitahayas.
Red pitahayas were purchased from a local supermarket. Fresh fruits that were free from disease and of uniform size (560 ± 50 g per fruit), shape, and color were randomly divided into five groups and stored at 2, 4, 6, 8, and 10°C (± 0.2°C), respectively. The relative humidity was set within the range of 70%–80%. The quality evaluation of red pitahaya samples was performed on 0, 6th, 12th, 15th, 18th, 21st, 24th, and 27th day. The samples were transferred to 23 ± 1°C with 70% R.H. for 24 h before the quality determinations.
CI evaluation was done in the sensory assessment laboratory and controlled white lighting. In each session, 6 trained panelists tested 30 codified samples (6 fruits from each group). Each panelist was asked to rate the extent of the CI based on the percentage of pitting, browning, and shriveling [23] in the peel surface as follows: 0 = no injury, 0% of the area affected; 1 = slight injury, up to 25%; 2 = moderate injury, from 26% to 50%; 3 = severe injury, from 51% to 75%; and 4 = extremely severe injury, higher than 75%. Therefore, the Chilling injury index (CII) ranged from 0 to 4. The CII was expressed as follows:
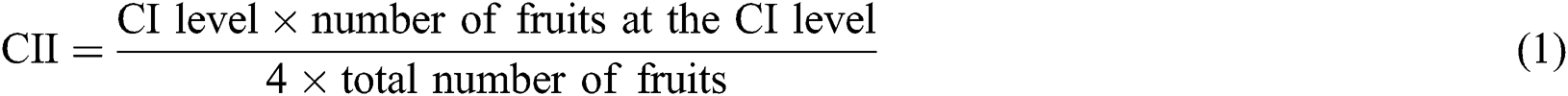
Lightness (L), chroma (C), and hue angle (H) were evaluated on four locations around the equatorial plane of the fruit peel using a colorimeter (CR-400, Konica Minolta Investment Ltd., Shanghai, China).
The WL of red pitahaya was recorded before storage and after storage. The WL was expressed as the percentage of fruit weight.
Fruit firmness was determined in the equatorial region of the fruits using a penetrometer (GY-4, Yueqing Handpi Instruments Co., Ltd., Zhejiang, China) with a 3-mm diameter tip and the results were expressed in Newton (N).
EL was measured according to Ruiling et al. [5] with some modifications. Cylindrical fruits (6 mm in diameter and 2 mm thick) were rinsed with deionized water and incubated in distilled water at 20°C for 1 h to determine the initial EL (E0). The samples were boiled in water for 30 min, cooled at room temperature, and the total conductivity (Et) was measured. The rate of EL was calculated as follows:

Respiration rate was estimated by the method of Deng et al. [24] with some modifications. One fruit was sealed in one glass container that contained 10 mL 0.4 mol/L NaOH at room temperature for 30 min. Then, 5 mL saturated BaCl2 and two drops of phenolphthalein were added, and the solution was titrated with 0.1 mol/L oxalic acid until the endpoint. The respiration rate of the samples was expressed as mg·kg−1·h−1 CO2.
TA was calculated based on malic acid as the predominant acid and was expressed as percent (w/v) of malic acid at 25°C. Measurements were performed in triplicate.
SS was determined according to the method by Zhang et al. [25] with some modifications. 1 g sample was homogenized with 10 mL distilled water. The slurry was centrifuged at 4 000 rpm for 10 min at 4°C. The supernatant was quantitative to 25 mL and used for SS analysis. Results were expressed as a percentage of glucose.
TP was measured with Folin-Ciocalteu reagent according to Ruiling et al. [5] with some modifications. Samples (2 g) were homogenized with 5 mL of pre-cooled methanol (80%, v/v) in darkness at 4°C. After centrifugation, the supernatant (1 mL) was added to the reaction solution consisted of 4 mL of 7.5% (w/v) Na2CO3 and 5 mL of Folin-Ciocalteu reagent. Then the reaction mixture was incubated in darkness at 25°C for 1 h. The absorbance at 765 nm was measured using a spectrophotometer (UV-2100, Unico (Shanghai) instrument Co., Ltd, China). The results were calculated from a standard curve of gallic acid and expressed as gallic acid equivalents (GAE) based on fresh weight basis (g·kg−1).
Frozen pulp tissue (5 g) was homogenized in various precooled extraction buffer with a tissue grinder (Crl-6010, Kinematica, Kriens-LU, Switzerland) to prepare crude extracts for assay of the following enzymes: 10 mL of 100 mmol/L sodium phosphate (pH 6.0) containing polyvinyl polypyrrolidone (PVPP) for SOD. 20 mL of 50 mmol/L sodium phosphate buffer (pH 7.0) containing PVPP for CAT. 25 mL of 1 mol/L Tris-HCl (pH 7.6) for LOX.
One unit of SOD was calculated as the amount of enzyme which leads to 50% inhibition of the reduction rate of nitro tetrazolium blue chloride (NBT) per second and the results were expressed as U·g−1 based on fresh weight. CAT activity was calculated from the change at 240 nm, and the results were expressed as U·g−1 based on fresh weight, where U = 0.01 ΔA240 nm per min. The activity of LOX was assayed according to Xu et al. [26]. LOX activity was calculated from the change at 234 nm, and the results were expressed as U·g−1 based on fresh weight, where U = 0.01 ΔA234 nm per min.
Measurements of all the indices were conducted in sextuplicate. The data of the figures were shown as the mean ± standard deviation. The experimental data was analyzed using Duncan multiple comparison test (One-way ANOVA) using SPSS version 21.0 (IBM Corp., New York, USA). The significance level among all treatments was set equal to 5% (p < 0.05). All graph preparation was performed in OriginPro version 2020 (OriginLab Corp., Northampton, MA, USA).
CI in red pitahaya has some typical symptoms including uneven brown specks of the skin, browning and shriveling, and abnormal ripening [27,28]. The red pitahayas stored at 2°C showed some pitting at the end of storage and CI did not appear in others. As shown in Fig. 1a, different degrees of CI appearance occurred on the fruits stored at room temperature for 24 h after cold storage at 2, 4, and 6°C, which was similar to those of Liu et al. [29] and Wang et al. [30]. They found that the CI did not appear until the pitahaya fruits were transferred from the low temperature to the room temperature (about 20°C). The CI of red pitahayas stored at 2, 4, and 6°C began to appear on the 12th 15th, and 21st day, respectively, and the CI index sharply increased from that time. Probably because cold storage conditions triggered metabolic adaptation, for example, degradation of reserves, fermentation, and amino acid mobilization [31]. The temperatures no more than 6°C were too low for defense mechanisms of red pitahayas to cure themselves, which made fruits different. Based on these differences, after fruits were exposed to the room temperature, various degrees of CI appeared. No visual CI symptoms were observed on red pitahaya fruits stored at 8°C and 10°C during storage. These results were in agreement with the findings of Balois-Morales et al. [32], who found the CI of pitahayas stored at 3°C was significantly higher than those of 7°C. Similarly, Nair et al. [33] pointed out that CI appeared more intensely in mango fruits stored at 0°C than those of 5°C and CI was more severe for longer storage time.
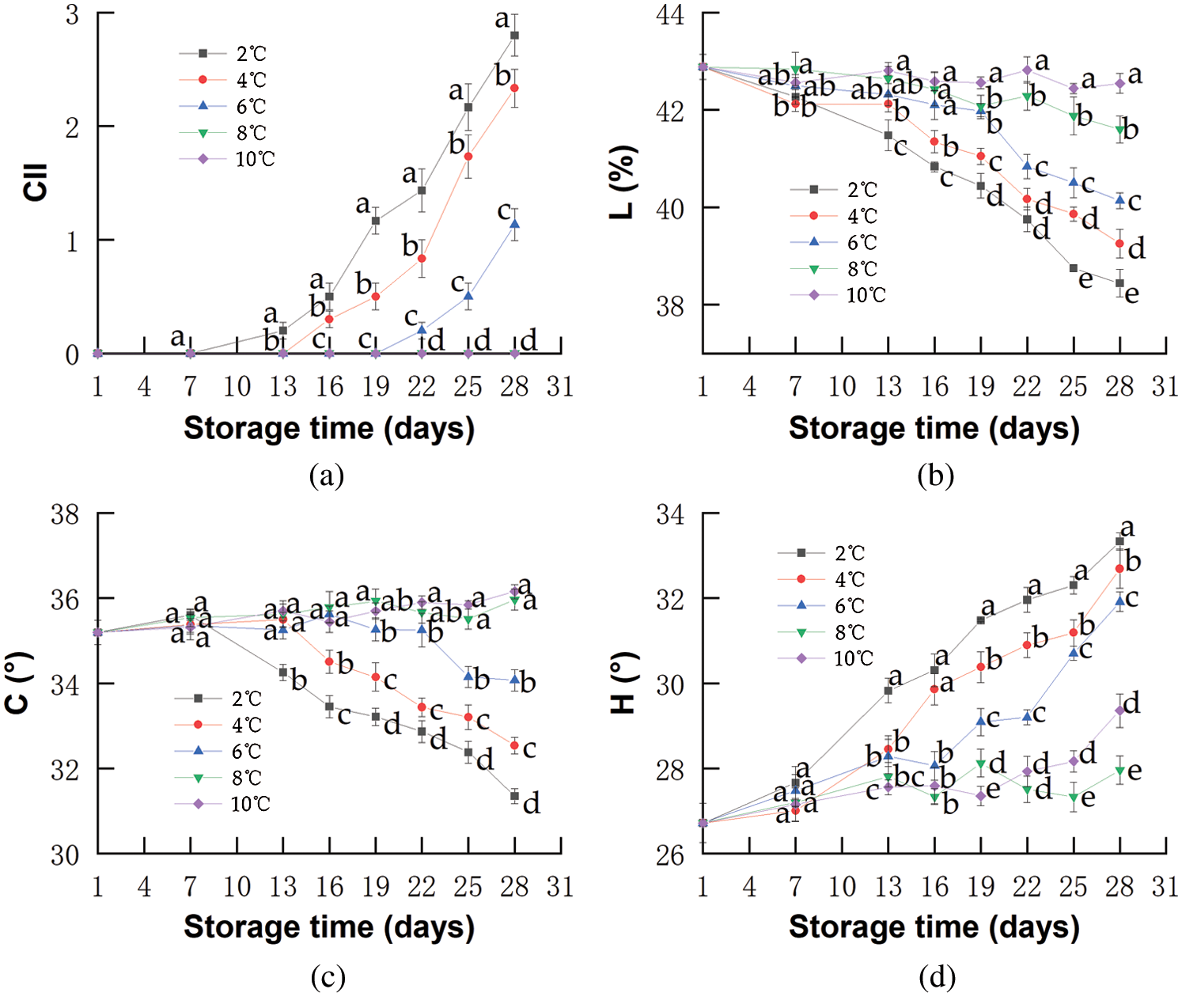
Figure 1: Changes in CII (a), Lightness (b), Chroma (c), Hue angle (d) of red pitahayas during cold storage (2°C  ; 4°C
; 4°C  ; 6°C
; 6°C  ; 8°C
; 8°C  ; 10°C
; 10°C  ) and an extra period of 1 day at 23°C. The data show means ± SD of six replicates. Different letters on the same day indicate significant differences (p < 0.05)
) and an extra period of 1 day at 23°C. The data show means ± SD of six replicates. Different letters on the same day indicate significant differences (p < 0.05)
The color attributes (L, C, and H) values of exocarp adjoining to the equator of red pitahayas were shown in Figs. 1b–1d. The L* values of red pitahayas stored at 10°C were maintained stable for 27 days. The reduction of L* value was observed in other treatments throughout storage due to the development of brown surface. This result revealed that fruits were significantly darker when stored at lower temperatures, which was in agreement with previous reports [32,34]. Furthermore, the remarkable reduction of L* values of red pitahayas stored at 2, 4, and 6°C might result from pigmentation caused by CI. The association between a reduced L* value and an increased CI during cold storage has been reported in pineapple [35] and pomegranate [36]. Low-temperature storage prevented the development of chroma. Chroma values of red pitahayas stored at 2, 4, and 6°C decreased notably (Fig. 1c) and chroma was lower in red pitahayas stored at the lower temperatures. This suggests that the red color lost purity at low temperatures (below 6°C) [37]. However, there was no significant difference (p > 0.05) of chroma between red pitahayas stored at 8 and 10°C, and chroma increased slightly. The results were in accordance with Nyanjage et al. [38] who observed a decrease in chroma value of mango fruits stored at 4°C with CI and an increasing tendency for chroma during storage at 13 and 20°C without CI. This was because the fruits with CI did not have the capacity to increase color saturation in post-cold storage, an incapacity interpreted as a manifestation of CI [39,40]. Red pitahayas stored at lower temperatures showed higher H (Fig. 1d), suggesting a tendency to orange colorations, which was due to the irregular ripening with low temperature [39]. However, the hue value of fruits stored at 10°C for 27 days was higher than those stored at 8°C for 27 days due to rot and chlorophyll degradation as the ripening process progressed [41]. Corrales Garcia et al. [42] also found the pitayas rewarming from 4°C decreased the pinkish-red color and the pitayas rewarming from 8°C were not greatly affected. In short, cold storage significantly influenced the color of red pitahaya and 8°C was an effective way to maintain good appearances such as higher L value, higher C value, and lower H value.
The WL is considered a limiting factor for marketing because it causes the skin to wrinkle and fruits to have lower visual appeal [43]. As shown in Fig. 2a, there was a continuous increase in WL in all samples during storage. The WL in red pitahayas stored at 10°C was 17.0% at the end of storage, which was higher than that in other samples. Cold storage could inhibit the evaporation from the tissue surface (a result of transpiration processes) and decrease the WL [44]. Brunini et al. [45] reported the WL in pitahayas stored at 13°C was 2.1% and higher than stored at 8°C on the 25th day. However, red pitahayas stored at 2°C had higher WL than that at 4 and 6°C and there was no significant difference among these treatments. Probably because decay caused by CI increased the loss of water so that the effect of protecting WL by low temperatures was covered. Proulx et al. [46] found that the WL of papayas stored at 0°C maintained a slightly higher level compared with the those stored at 5°C.
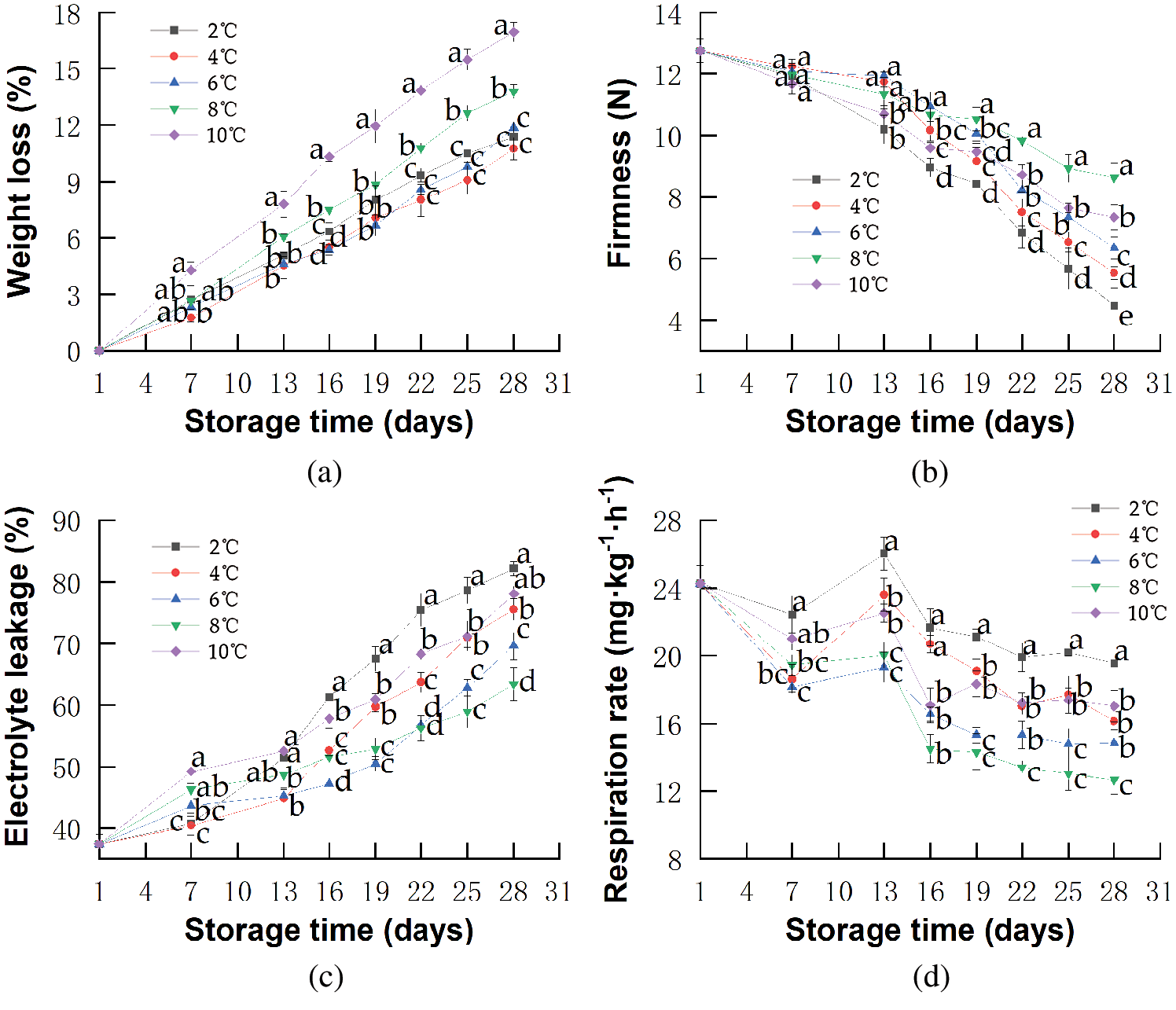
Figure 2: Changes in WL (a), Firmness (b), EL (c), Respiration rate (d) of red pitahayas during cold storage (2°C; 4°C; 6°C; 8°C 10°C) and an extra period of 1 day at 23°C. The data show means ± SD of six replicates. Different letters on the same day indicate significant differences (p < 0.05)
The fruit firmness is closely related to the fruit ripeness, and is also used to determine the suitability of fruits for critical steps in postharvest handling, including storage in bulk bins, passing across a grading line, or whether a particular line of fruits meets the requirement for export. All these processes are problematic when the fruits are too soft [47]. The firmness of all red pitahaya samples decreased slightly from the 1st day to the 13th day (Fig. 2b) presumably because of low pectinase enzyme activities controlled by cold temperature [48], but after storage at low temperatures for over 2 weeks, a quick decrease of firmness turned to be notable owing to continuous cell wall degradation induced by pectinase and oxidative damage caused by senescence and chilling disorder. After 27 days of cold storage, the firmness of red pitahayas stored at 10°C decreased rapidly from 12.8 N on the 1st day to 7.3 N at the end of storage, while the decrease was delayed by storing at 8°C, slowly from 12.8 N to 8.6 N. Kan et al. [49] reported that peaches kept at 5°C showed higher firmness than those stored at 20°C. Red pitahayas showed a higher reduction in firmness at high temperatures due to a higher activity of cell wall degrading enzymes that lead to fruit softening [50]. On the other hand, the reduction of red pitahaya firmness after 18 days at 2, 4, or 6°C was higher than that stored at 8 and 10°C. Ming et al. [51] reported that the Hami melons stored at 3°C had higher firmness than those stored at 0.5°C, which was explained by disorders as a result of exposure to near-threshold temperatures over a long period, producing irreversible consequences such as the damage of membranes, rupture of cell compartments, and EL [39].
EL usually reflects the permeability and integrity of the cell membrane, which is considered to be the primary site for the development of CI, and thus membrane penetrability might be involved in improving resistance to cold stress [52]. In this study, EL in red pitahayas increased slightly from the 1st day to the 7th day and then increased sharply (Fig. 2c). A major increase in the fruits stored at 2°C was observed on the 13th day of storage. A relatively smooth increase in the fruits stored at 8 and 10°C, suggesting that low-temperature storage (2, 4, and 6°C) could cause cellular membrane damage in red pitahayas. Probably because chilling storage led to the damage of cellular compartmentalization and increase of EL [53]. Furthermore, the rapid increase of EL of red pitahayas stored at 10°C could be attributed to the cell integrity loss caused by the senescence process and mold contamination [54]. EL was significantly (p < 0.05) lower at 8°C and storing at 8°C could inhibit the loss of membrane integrity caused by both CI and senescence process.
Fruit respiration rate is indicative of the intensity of metabolic activity and biochemical reactions taking place in fruits, which can greatly affect fruit quality [55]. As showed in Fig. 2d, low-temperature storage significantly (p < 0.05) impacted the respiration rate. The respiration rates in red pitahayas from all samples first decreased, then reached a peak on the 13th day and finally descended at a slow pace until the end of the storage. Rosas-Benítez et al. [56] showed a similar respiration pattern in pitahayas stored at 12°C. Red pitahayas stored at 8°C showed a lower respiration rate than those stored at 10°C. Raza et al. [57] found that mango stored at 10°C showed a 1-fold lower respiration rate during storage as compared to the fruits stored at 12°C. Presumably, down-regulation of the respiration at low temperatures might save internal O2 and relieve hypoxic conditions in the fruits [58]. The lowest respiration rates observed were at 8°C, not at 2, 4 or 6°C and the respiration rate values for the fruits ranged in ascending order as follows: 8°C (12.6 mg·kg−1·h−1) < 6°C (14.9 mg·kg−1·h−1) < 4°C (16.1 mg·kg−1·h−1) < 2°C (19.5 mg·kg−1·h−1). The higher respiration rate at 2°C compared to 8°C was probably due to the change of mitochondria and their electron transport pathway leading to CI development during 28-day storage [11,59]. Albornoz et al. [60] reported that the respiration rate values of cherry tomato stored at 5°C were higher than that at 12.5°C.
TA is the indicator of acidity content of fleshy fruits [61]. The TA value on the 1st day was over 0.4% and showed a significant decrease (p < 0.05) in all samples during storage (Fig. 3a). The highest value of TA was observed at 2°C at the end of storage, while the lowest level was found those in at 10°C. This indicated that lower storage temperatures could delay the loss of acidity during storage. Pal Singh et al. [62] found that the extent of decrease in TA concentration of Japanese plums was higher at 5°C than that at 0°C, which probably due to using organic acids as substrates during the metabolic reactions. Additionally, TA slightly increased in red pitahayas stored on the 7th day at 2°C compared to those on the 1st day resulting from increasing the ability to accumulate malate and decreasing leakage of solutes, such as protons or protonated forms of organic acids by low temperature [63]. Ding et al. [64] reported that mango fruits stored at 5°C for 10 days had higher TA values than those at harvest.

Figure 3: Changes in TA (a) and SS (b) of red pitahayas during cold storage (2°C; 4°C; 6°C; 8°C; 10°C) and an extra period of 1 day at 23°C. The data show means ± SD of six replicates. Different letters on the same day indicate significant differences (p < 0.05)
SS changes are easily found in fruits under cold stress conditions, which plays a crucial role in osmoregulation and cryoprotection in fruits [65]. There was an increase in the SS at the beginning and then had a decrease (Fig. 3b). The increased total SS of red pitahayas resulted from starch breakdown or hydrolysis of mucilage of the fruits as a response to low temperatures [38,66]. The decrease in total SS could be explained by metabolic processes and lacking the accumulation of carbohydrates in the fruits [10,67]. The red pitahayas stored at 6, 8, and 10°C diminished SS rapidly from the 7th day and those stored at 2 and 4°C remained significantly (p < 0.05) high during storage. Probably because higher levels of reducing-sugar played a positive role in improving chilling tolerance, which contributed to signal pathway regulation [68]. Hong et al. [69] found that higher total SS was also observed in summer pineapple fruits held at 6°C than those at 10 and 25°C due to the rapid sugar conversion rate during storage [57]. The accumulated SS acted as an important anti-freeze solute in response to low-temperature stress [52,70].
Oxidative damage is regarded as an early response to low temperatures. To get rid of oxidative stress, fruits have applied ROS scavenging strategy. SOD and CAT play essential roles in this defense [4,71]. Besides, LOX regulates fruit development at different stages, which is tightly associated with the generation and identification of stress signals [51].
SOD is a major actor in eliminating highly reactive and toxic superoxide radicals generated during metabolic processes in fruits [72]. As shown in Fig. 4a, SOD activities of red pitahayas stored at 8 and 10°C increased sharply at the beginning in response to the presence of growing toxic ROS, such as superoxide radicals, hydrogen peroxide, hydroxyl radicals, and simple oxygen [73]. On the 13th day, the level of SOD activities in red pitahayas stored at 10°C was significantly lower than that at 8°C (p < 0.05), which resulted from further senescence and water loss during storage resulting in more production of ROS and afterward the destruction of the dynamic balance of protective enzyme including SOD. Yuanzhi et al. [74] reported that higher levels of superoxide dismutase activity were observed in the rambutan fruits stored at 10°C than that at 25°C. Moreover, the faster-decreased SOD activities were noticed at lower temperatures (Fig. 4a) caused by severe chilling stress and resulted in more accumulation of superoxide anions, then leading to greater CI incidence [15,75]. Besides, we observed that low-temperature storage could delay the increase rate of SOD activities probably owing to the reduction of the reaction rate of enzyme production and catalysis by low temperature. Qian et al. [76] also demonstrated that low temperature effectively delayed the increase of SOD activities in stored blueberries.
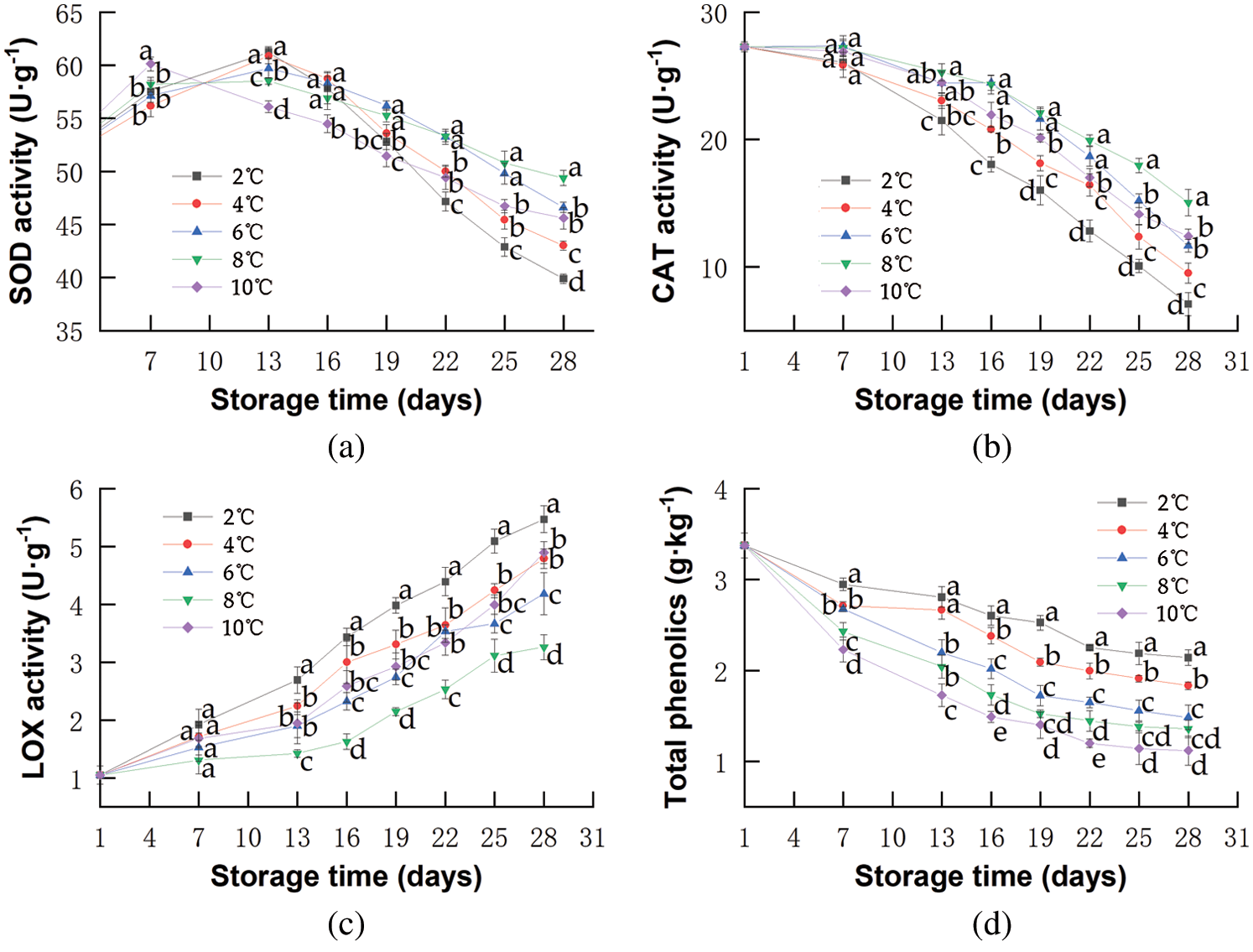
Figure 4: Changes in SOD activity (a), CAT activity (b), LOX activity (c), TP (d) of red pitahayas during cold storage (2°C; 4°C; 6°C; 8°C; 10°C) and an extra period of 1 day at 23°C. The data show means ± SD of six replicates. Different letters on the same day indicate significant differences (p < 0.05)
CAT protects cells against ROS by catalyzing the decomposition of hydrogen peroxide to oxygen and water, which prevents H2O2 from accumulating in tissues [77]. As shown in Fig. 4b, CAT activities of red pitahayas decreased in all samples during storage. At the end of storage, the CAT activities of red pitahayas stored at 2, 4, 6, and 8°C were 7.09, 9.51, 11.66, and 15.05 U·g−1, respectively. This showed that higher temperatures inhibited the decrease of CAT activities. Huang et al. [78] reported that the CAT activity in the navel orange stored at 20°C was slightly higher than that at 6°C. The high CAT activity has been proposed to impart cold tolerance [62], which could partly explain why there was no CI discovered in red pitahayas stored at 8°C. In contrast, red pitahayas stored at 10°C showed a sharper decrease in CAT activity than those at 8°C, indicating being less capable of alleviating the formation of hydroxyl radical perhaps owing to senescence [76].
LOX can introduce oxygen into unsaturated fatty acids to form unstable hydroperoxides that continually form free radicals, leading to cell damage and apoptosis [79]. LOX activity increased in all samples during storage (Fig. 4c) owing to the deoxygenation of polyunsaturated fatty acids, which resulted in toxic hydroperoxy fatty acids and membrane damage. A similar effect was also reported by Handong et al. [43]. LOX activity of red pitahayas stored at lower temperatures had higher values indicating severer lipid membrane peroxidation under cold stress. Ming et al. [51] also found Hami melons stored at 0.5°C had higher LOX activity values than those at 3°C, which reflected a more severe oxidation level of lipid membranes due to the development of CI. LOX activity of red pitahayas stored at 10°C was also higher than that at 8°C due to rotting, which was coincident with the result of EL. Xiujuan et al. [79] reported that the LOX activity of peaches stored at 25°C was much higher and underwent greater changes than that at 4°C.
As ROS usually accumulates in reaction to chilling stress, phenols are antioxidant compounds that protect fruit cells from oxidative damage attributed to ROS [80]. The TP decreased in all samples due to the oxidation of monohydric or dihydric phenols and the degradation of phenolic as a result of enzymatic activities that occurred in the fruits [81]. As shown in Fig. 4d, the TP content of red pitahayas stored at 2, 4, 6, 8, and 10°C decreased from 3.37 to 2.14, 1.83, 1.48, 1.36, and 1.12 g·kg−1, respectively, on the 28th day. The TP contents of kiwifruit and kinnow also showed temperature dependence and the degradation of phenolic occurred faster with higher temperatures [82,83]. Therefore, low-temperature storage is effective to limit the degradation of phenolic compounds.
Pearson correlation was of great significance (p < 0.01) between total phenols and antioxidant enzymes SOD, CAT, and LOX. Total phenols content was directly correlated with SOD activities (R = 0.38) and CAT activities (R = 0.43) while inversely correlated with LOX activity (R = −0.36). It demonstrated that the content of total phenols was probably related to the activities of antioxidant enzymes such as SOD, CAT, and LOX during low-temperature storage of red pitahayas. And weak correlation was maybe because the mechanism of response to CI was slightly different between phenolic compounds and antioxidant enzymes (Fig. 4).
Low-temperature storage had an impact on red pitahaya metabolism during storage. Cold storage at temperatures below 10°C could maintain fruits a good quality by reducing the key physiological and biochemical parameters studied. However, red pitahayas are susceptible to CI. Worse appearance evaluation, lower firmness, higher EL and respiration rate, and more toxic ROS indicated by TP and enzyme activities were observed during low-temperature storage (2, 4, and 6°C). Low-temperature (8 and 10°C) storage contributed to remarkably better fruit quality of red pitahayas. Compared with storage at 10°C, red pitahaya fruits stored at 8°C maintained higher TA, SS, SOD activity, and CAT activity and inhibited LOX activity, cell membranes damage, and respiratory metabolism, thereby effectively extending the shelf time. In conclusion, our current study demonstrated that the better storage temperature for red pitahaya fruits was 8°C for 27 days without CI.
Funding Statement: J. X. and J. M. gratefully acknowledge the funding from Key Project of Science and Technology Commission of Shanghai Municipality, Grant No. 19395800100; Shanghai Professional Technology Service Platform on Cold Chain Equipment Performance and Energy Saving Evaluation, Grant No. 17DZ2293400.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Wu, Y. W., Xu, J., He, Y. Z., Shi, M. Y., Han, X. M. et al. (2019). Metabolic profiling of pitaya (Hylocereus polyrhizus) during fruit development and maturation. Molecules, 24(6), 16. DOI 10.3390/molecules24061114.
2. Wahdaningsih, S., Wahyuono, S., Riyanto, S., Murwanti, R. (2020). Terpenoid-lupeol of red dragon fruit (Hylocereus polyrhizus) and its immunomodulatory activity. Pakistan Journal of Pharmaceutical Sciences, 33(2), 505–510. DOI 10.36721/pjps.2020.33.2.Reg.505-510.1. [Google Scholar] [CrossRef]
3. Fan, P. H., Huber, D. J., Su, Z. H., Hu, M. J., Gao, Z. Y. et al. (2018). Effect of postharvest spray of apple polyphenols on the quality of fresh-cut red pitaya fruit during shelf life. Food Chemistry, 243, 19–25. DOI 10.1016/j.foodchem.2017.09.103.
4. Vijayakumar, R., Abd Gani, S. S., Zaidan, U. H., Halmi, M. I. E., Karunakaran, T. et al. (2020). Exploring the potential use of Hylocereus polyrhizus peels as a source of cosmeceutical sunscreen agent for its antioxidant and photoprotective properties. Evidence-Based Complementary and Alternative Medicine, 2020, 7520736. DOI 10.1155/2020/7520736. [Google Scholar] [CrossRef]
5. Ruiling, L., Haiyan, G., Hangjun, C., Xiangjun, F., Weijie, W. (2019). Synergistic effect of 1-methylcyclopropene and carvacrol on preservation of red pitaya (Hylocereus polyrhizus). Food Chemistry, 283, 588–595. DOI 10.1016/j.foodchem.2019.01.066. [Google Scholar] [CrossRef]
6. Wu, Q., Zhou, Y., Zhang, Z., Li, T., Jiang, Y. et al. (2020). Effect of blue light on primary metabolite and volatile compound profiling in the peel of red pitaya. Postharvest Biology and Technology, 160, 111059. DOI 10.1016/j.postharvbio.2019.111059. [Google Scholar] [CrossRef]
7. Jalgaonkar, K., Mahawar, M. K., Bibwe, B., Kannaujia, P. (2020). Postharvest profile, processing and waste utilization of dragon fruit (Hylocereus Spp.A review. Food Reviews International, 27(1), 1–27. DOI 10.1080/87559129.2020.1742152. [Google Scholar] [CrossRef]
8. Uthairatanakij, A., Cholmaitri, C., Aiamla-or, S., Jitareerat, P. (2018). Gamma irradiation as phytosanitary treatment for red flesh dragon fruit. Acta Horticulturae, 56(1210), 145–150. DOI 10.17660/ActaHortic.2018.1210.20. [Google Scholar] [CrossRef]
9. Aziz, M. S. H., Manuhara, G. J., Utami, R., Khasanah, L. U. (2018). The application of active paper incorporated with oleoresin of cinnamon leaf (Cinnamomum burmanii) distillation residues on maintaining dragon fruits (Hylocereus costaricensis) quality during storage. IOP Conference Series: Materials Science and Engineering, 333, 012063. DOI 10.1088/1757-899x/333/1/012063. [Google Scholar] [CrossRef]
10. Obenland, D., Cantwell, M., Lobo, R., Collin, S., Sievert, J. et al. (2016). Impact of storage conditions and variety on quality attributes and aroma volatiles of pitahaya (Hylocereus spp.). Scientia Horticulturae, 199, 15–22. DOI 10.1016/j.scienta.2015.12.021. [Google Scholar] [CrossRef]
11. Punitha, V., Boyce, A. N., Chandran, S. (2010). Effect of storage temperatures on the physiological and biochemical properties of Hylocereus polyrhizus. Acta Horticulturae, 48(875), 137–144. DOI 10.17660/ActaHortic.2010.875.16. [Google Scholar] [CrossRef]
12. de Freitas, S. T., Mitcham, E. J. (2013). Quality of pitaya fruit (Hylocereus undatus) as influenced by storage temperature and packaging. Scientia Agricola, 70(4), 257–262. DOI 10.1590/S0103-90162013000400006. [Google Scholar] [CrossRef]
13. Phong Nguyen, N. V., Tung, T., Clark, C. J., Woolf, A. B. (2018). Effect of storage temperature and low temperature conditioning on quality and chilling injury of ‘LĐ1’ red fleshed dragon fruit. Acta Horticulturae, 56(1213), 123–128. DOI 10.17660/ActaHortic.2018.1213.16. [Google Scholar] [CrossRef]
14. Gwanpua, S. G., Jabbar, A., Zhao, M., Heyes, J. A., East, A. R. (2018). Investigating the potential of dual temperature storage as a postharvest management practice to mitigate chilling injury in kiwifruit. International Journal of Refrigeration, 86, 62–72. DOI 10.1016/j.ijrefrig.2017.12.004. [Google Scholar] [CrossRef]
15. Valenzuela, J. L., Manzano, S., Palma, F., Carvajal, F., Garrido, D. et al. (2017). Oxidative stress associated with chilling injury in immature fruit: Postharvest technological and biotechnological solutions. International Journal of Molecular Sciences, 18(7), 26. DOI 10.3390/ijms18071467. [Google Scholar] [CrossRef]
16. Phornvillay, S., Pongprasert, N., Wongs-Aree, C., Uthairatanakij, A., Srilaong, V. (2020). Physio-biochemical responses of okra (Abelmoschus esculentus) to oxidative stress under low temperature storage. Horticulture Journal, 89(1), 69–77. DOI 10.2503/hortj.UTD-105. [Google Scholar] [CrossRef]
17. Zhao, Q. X., Jin, M. J., Guo, L.Y., Pei, H. H., Rao, J. et al. (2020). Modified atmosphere packaging and 1-methylcyclopropene alleviate chilling injury of ‘Youhou’ sweet persimmon during cold storage. Food Packaging and Shelf Life, 24, 100479. DOI 10.1016/j.fpsl.2020.100479. [Google Scholar] [CrossRef]
18. Liu, J., Li, Q. X., Chen, J. J., Jiang, Y. M. (2020). Revealing further insights on chilling injury of postharvest bananas by untargeted lipidomics. Foods, 9(7), 894. DOI 10.3390/foods9070894. [Google Scholar] [CrossRef]
19. Fathi-Najafabadi, A., Salvador, A., Navarro, P., Gil, R., Besada, C. (2020). Effect of temperature during and immediately after CO2-deastringency treatment on internal flesh browning after cold storage of persimmon fruit. Scientia Horticulturae, 268, 109363. DOI 10.1016/j.scienta.2020.109363. [Google Scholar] [CrossRef]
20. Ling, L., Kitazawa, H., Rongfei, Z., Xiangyou, W., Liming, Z. et al. (2019). New insights into the chilling injury of postharvest white mushroom (Agaricus bisporus) related to mitochondria and electron transport pathway under high O2/CO2 controlled atmospheres. Postharvest Biology and Technology, 152, 45–53. DOI 10.1016/j.postharvbio.2019.02.015. [Google Scholar] [CrossRef]
21. Wanli, Z., Handong, Z., Haitao, J., Yan, X., Jiankang, C. et al. (2020). Multiple 1-MCP treatment more effectively alleviated postharvest nectarine chilling injury than conventional one-time 1-MCP treatment by regulating ROS and energy metabolism. Food Chemistry, 330, 127256. DOI 10.1016/j.foodchem.2020.127256. [Google Scholar] [CrossRef]
22. Huajun, S., Xin, Z., Qian, Z., Yingbo, Z., Ximan, K. et al. (2020). Disorder of membrane metabolism induced membrane instability plays important role in pericarp browning of refrigerated ‘Nanguo’ pears. Food Chemistry, 320, 126684. DOI 10.1016/j.foodchem.2020.126684. [Google Scholar] [CrossRef]
23. Narvaez-Cuenca, C. E., Espinal-Ruiz, M., Restrepo-Sanchez, L. P. (2011). Heat shock reduces both chilling injury and the overproduction of reactive oxygen species in yellow pitaya (Hylocereus megalanthus) fruits. Journal of Food Quality, 34(5), 327–332. DOI 10.1111/j.1745-4557.2011.00398.x. [Google Scholar] [CrossRef]
24. Deng, B., Guo, M., Liu, H., Tian, S., Zhao, X. (2019). Inhibition of autophagy by hydroxychloroquine enhances antioxidant nutrients and delays postharvest fruit senescence of Ziziphus jujuba. Food Chemistry, 296, 56–62. DOI 10.1016/j.foodchem.2019.05.189. [Google Scholar] [CrossRef]
25. Zhang, J., Lu, J., Mantri, N., Jiang, L., Ying, S. et al. (2018). An effective combination storage technology to prolong storability, preserve high nutrients and antioxidant ability of astringent persimmon. Scientia Horticulturae, 241, 304–312. DOI 10.1016/j.scienta.2018.07.017. [Google Scholar] [CrossRef]
26. Xu, F., Lu, F., Xiao, Z., Li, Z. (2020). Influence of drop shock on physiological responses and genes expression of apple fruit. Food Chemistry, 303, 125424. DOI 10.1016/j.foodchem.2019.125424. [Google Scholar] [CrossRef]
27. Dueñas, G. Y. M., Narváez, C. C. E., Restrepo, S. L. P. (2009). Heat shock improves refrigerated storage performance of yellow pitaya. Agronomía Colombiana, 27(1), 105–110. [Google Scholar]
28. Nerd, A., Gutman, F., Mizrahi, Y. (1999). Ripening and postharvest behaviour of fruits of two Hylocereus species (Cactaceae). Postharvest Biology and Technology, 17(1), 39–45. DOI 10.1016/S0925-5214(99)00035-6. [Google Scholar] [CrossRef]
29. Liu, Y., Chen, Y. R., Wang, C. Y., Chan, D. E., Kim, M. S. (2006). Development of hyperspectral imaging technique for the detection of chilling injury in cucumbers; spectral and image analysis. Applied Engineering in Agriculture, 22(1), 101–111. DOI 10.13031/2013.20176. [Google Scholar] [CrossRef]
30. Wang, J. W., Dong, S. Z., Jiang, Y. G., He, H. S., Liu, T. et al. (2020). Influence of long-term cold storage on phenylpropanoid and soluble sugar metabolisms accompanied with peel browning of Nanguo’ pears during subsequent shelf life. Scientia Horticulturae, 260, 10. DOI 10.1016/j.scienta.2019.108888. [Google Scholar] [CrossRef]
31. Gonzalez, C., Zanor, M. I., Ré, M. D., Otaiza, S., Asis, R. et al. (2019). Chilling tolerance of Micro-Tom fruit involves changes in the primary metabolite levels and in the stress response. Postharvest Biology and Technology, 148, 58–67. DOI 10.1016/j.postharvbio.2018.10.010. [Google Scholar] [CrossRef]
32. Balois-Morales, R., Pena-Valdivia, C. B., Arroyo-Pena, V. B. (2013). Symptoms and sensitivity to chilling injury of pitahaya (Hylocereus undatus (haw.) britton & rose) fruits during postharvest. Agrociencia, 47(8), 795–813. [Google Scholar]
33. Nair, S., Singh, Z., Tan, S. C. (2004). Chilling injury in relation to ethylene biosynthesis in ‘Kensington Pride’ mango fruit. Journal of Horticultural Science & Biotechnology, 79(1), 82–90. DOI 10.1080/14620316.2004.11511740. [Google Scholar] [CrossRef]
34. Zhu, D., Liang, J., Liu, H., Cao, X., Ge, Y. et al. (2018). Sweet cherry softening accompanied with moisture migration and loss during low-temperature storage. Journal of the Science of Food and Agriculture, 98(10), 3651–3658. DOI 10.1002/jsfa.8843. [Google Scholar] [CrossRef]
35. Preyanuch, S., Suriyan, S., Pannipa, Y., Chalermchai, W. A., Panida, B. (2020). Chilling injury alleviation of Queen pineapple cv. ‘Sawi’ fruit by acetyl salicylate immersion. Horticulture, Environment, and Biotechnology, 61(1), 83–92. DOI 10.1007/s13580-019-00202-z. [Google Scholar] [CrossRef]
36. Koushesh Saba, M., Lolav, Z. (2019). Preharvest methyl jasmonate’s impact on postharvest chilling sensitivity, antioxidant activity, and pomegranate fruit quality. Journal of Food Biochemistry, 43(3), e12763. DOI 10.1111/jfbc.12763. [Google Scholar] [CrossRef]
37. Resende, N. S., Goncalves, G. A. S., Reis, K. C., Tonoli, G. H. D., Boas, E. (2018). Chitosan/cellulose nanofibril nanocomposite and its effect on quality of coated strawberries. Journal of Food Quality, 13(1), 1–13. DOI 10.1155/2018/1727426. [Google Scholar] [CrossRef]
38. Nyanjage, M. O., Wainwright, H., Bishop, C. F. H. (2001). Effects of hot water treatments and storage temperatures on the ripening and the use of electrical impedance as an index for assessing post-harvest changes in mango fruits. Annals of Applied Biology, 139(1), 21–29. DOI 10.1111/j.1744-7348.2001.tb00126.x. [Google Scholar] [CrossRef]
39. Quiroz-Gonzalez, B., Corrales-Garcia, J. J. E., Colinas-Leon, M. T. B., Ybarra-Moncada, M. C. (2017). Identification of variables correlated with chilling injury in pitahaya (Hylocereus undatus haworth). Agrociencia, 51(2), 153–172. [Google Scholar]
40. Corrales-Garcia, J., Canche-Canche, E. (2008). Physical and physiological changes in low-temperature-stored pitahaya fruit (Hylocereus undatus). Journal of the Professional Association for Cactus Development, 10, 108–119. [Google Scholar]
41. Sobral, R. R. S., Santos, R. C. D., De Jesus, M. O., Alves, P. F. S., Mizobutsi, G. P. et al. (2019). Effect of ripening stages on shelf life and quality of pitaya fruits during storage. Journal of Experimental Agriculture International, 37(2), 1–12. DOI 10.9734/jeai/2019/v37i230263. [Google Scholar] [CrossRef]
42. Corrales Garcia, J., Canche-Canche, E. (2008). Physical and physiological changes in low-temperature-stored pitahaya fruit (Hylocereus undatus). Journal of the Professional Association for Cactus Development, 10, 108–119. [Google Scholar]
43. Mukama, M., Ambaw, A., Berry, T. M., Opara, U. L. (2019). Analysing the dynamics of quality loss during precooling and ambient storage of pomegranate fruit. Journal of Food Engineering, 245, 166–173. DOI 10.1016/j.jfoodeng.2018.10.020. [Google Scholar] [CrossRef]
44. Zhao, H. D., Liu, B. D., Zhang, W. L., Cao, J. K., Jiang, W. B. (2019). Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biology and Technology, 147, 113–122. DOI 10.1016/j.postharvbio.2018.09.013. [Google Scholar] [CrossRef]
45. Brunini, M. A., Cardoso, S. S., Guimaraes, J. E. R., Oliveira, A. L., Pereira, M. et al. (2017). Pitaya quality during refrigerated storage. Acta Horticulturae, 55(1178), 129–134. DOI 10.17660/ActaHortic.2017.1178.23. [Google Scholar] [CrossRef]
46. Proulx, E., Cecilia, M., do Nascimento Nunes, M. C.,Emond, J. P., Brecht, J. (2005). Quality attributes limiting papaya postharvest life at chilling and non-chilling temperatures. Proceedings of the Florida State Horticultural Society, 118, 389–395. [Google Scholar]
47. Li, H., Pidakala, P., Billing, D., Burdon, J. (2016). Kiwifruit firmness: Measurement by penetrometer and non-destructive devices. Postharvest Biology and Technology, 120, 127–137. DOI 10.1016/j.postharvbio.2016.06.007. [Google Scholar] [CrossRef]
48. Reynolds, A. G., Knox, A., Profio, F. D. (2018). Evaluation of macerating pectinase enzyme activity under various temperature, pH and ethanol regimes. Beverages, 2306-5710, 4(1), 10. DOI 10.3390/beverages4010010. [Google Scholar] [CrossRef]
49. Kan, J. A., Wang, H. M., Jin, C. H. (2011). Changes of reactive oxygen species and related enzymes in mitochondrial respiration during storage of harvested peach fruits. Agricultural Sciences in China, 10(1), 149–158. DOI 10.1016/S1671-2927(11)60317-9. [Google Scholar] [CrossRef]
50. Chen, H. J., Cao, S. F., Fang, X. J., Mu, H. L., Yang, H. L. et al. (2015). Changes in fruit firmness, cell wall composition and cell wall degrading enzymes in postharvest blueberries during storage. Scientia Horticulturae, 188, 44–48. DOI 10.1016/j.scienta.2015.03.018. [Google Scholar] [CrossRef]
51. Ning, M., Tang, F. X., Zhang, Q., Zhao, X. X., Yang, L. P. et al. (2019). The quality of Gold Queen Hami melons stored under different temperatures. Scientia Horticulturae, 243, 140–147. DOI 10.1016/j.scienta.2018.08.005. [Google Scholar] [CrossRef]
52. Zhao, H. D., Jiao, W. X., Cui, K., Fan, X. G., Shu, C. et al. (2019). Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chemistry, 289, 426–435. DOI 10.1016/j.foodchem.2019.03.088. [Google Scholar] [CrossRef]
53. Rasouli, M., Koushesh Saba, M., Ramezanian, A. (2019). Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Scientia Horticulturae, 247, 27–34. DOI 10.1016/j.scienta.2018.12.004. [Google Scholar] [CrossRef]
54. Perez-Tello, G. O., Silva-Espinoza, B. A., Vargas-Arispuro, I., Briceno-Torres, B. O., Martinez-Tellez, M. A. (2001). Effect of temperature on enzymatic and physiological factors related to chilling injury in carambola fruit (Averrhoa carambola L.). Biochemical and Biophysical Research Communications, 287(4), 846–851. DOI 10.1006/bbrc.2001.5670. [Google Scholar] [CrossRef]
55. Kingwascharapong, P., Arisa, K., Karnjanapratum, S., Tanaka, F., Tanaka, F. (2020). Effect of gelatin-based coating containing frog skin oil on the quality of persimmon and its characteristics. Scientia Horticulturae, 260, 108864. DOI 10.1016/j.scienta.2019.108864. [Google Scholar] [CrossRef]
56. Rosas-Benítez, A., Trujillo-Cárdenas, L., Valle-Guadarrama, S., Salinas-Moreno, Y., García-Cruz, L. (2016). Quality attributes of pitaya (Stenocereus pruinosus) fruit handled in postharvest with and without thorns under refrigerated storage. Revista Chapingo Serie Horticultura, 22(3), 191–207. DOI 10.5154/r.rchsh.2016.04.011. [Google Scholar] [CrossRef]
57. Raza, S. A., Khan, A. S., Malik, A. U., Amin, M., Asad, H. U. et al. (2013). Respiration rate, physico-chemical fruit quality and consumer acceptability for fajri mango under different storage temperatures. Pakistan Journal of Agricultural Sciences, 50(4), 585–590. [Google Scholar]
58. Ho, Q. T., Hertog, M. L. A. T. M., Verboven, P., Ambaw, A., Rogge, S. et al. (2018). Down-regulation of respiration in pear fruit depends on temperature. Journal of Experimental Botany, 69(8), 2049–2060. DOI 10.1093/jxb/ery031. [Google Scholar] [CrossRef]
59. Li, L., Kitazawa, H., Zhang, R., Wang, X., Zhang, L. et al. (2019). New insights into the chilling injury of postharvest white mushroom (Agaricus bisporus) related to mitochondria and electron transport pathway under high O2/CO2 controlled atmospheres. Postharvest Biology and Technology, 152, 45–53. DOI 10.1016/j.postharvbio.2019.02.015. [Google Scholar] [CrossRef]
60. Albornoz, K., Cantwell, M. I., Zhang, L., Beckles, D. M. (2019). Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Scientific Reports, 9(1), 14. DOI 10.1038/s41598-019-38877-0. [Google Scholar] [CrossRef]
61. Bose, S. K., Howlader, P., Jia, X., Wang, W., Yin, H. (2019). Alginate oligosaccharide postharvest treatment preserve fruit quality and increase storage life via Abscisic acid signaling in strawberry. Food Chemistry, 283, 665–674. DOI 10.1016/j.foodchem.2019.01.060. [Google Scholar] [CrossRef]
62. Pal Singh, S., Zora, S. (2013). Dynamics of enzymatic and non-enzymatic antioxidants in Japanese plums during storage at safe and lethal temperatures. LWT - Food Science and Technology, 50(2), 562–568. DOI 10.1016/j.lwt.2012.08.008. [Google Scholar] [CrossRef]
63. Etienne, A., Génard, M., Lobit, P., Mbeguié-A-Mbéguié, D., Bugaud, C. (2013). What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. Journal of Experimental Botany, 64(6), 1451–1469. DOI 10.1093/jxb/ert035. [Google Scholar] [CrossRef]
64. Ding, Z. S., Tian, S. P., Zheng, X. L., Zhou, Z. W., Xu, Y. (2007). Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiologia Plantarum, 130(1), 112–121. DOI 10.1111/j.1399-3054.2007.00893.x. [Google Scholar] [CrossRef]
65. Yingying, W., Feng, X., Xingfeng, S. (2017). Changes in soluble sugar metabolism in loquat fruit during different cold storage. Journal of Food Science and Technology, 54(5), 1043–1051. DOI 10.1007/s13197-017-2536-5. [Google Scholar] [CrossRef]
66. Burdon, J., Pidakala, P., Martin, P., Billing, D., Boldingh, H. (2016). Fruit maturation and the soluble solids harvest index for ‘Hayward’ kiwifruit. Scientia Horticulturae, 213, 193–198. DOI 10.1016/j.scienta.2016.10.027. [Google Scholar] [CrossRef]
67. Borsani, J., Budde, C. O., Porrini, L., Lauxmann, M. A., Lombardo, V. A. et al. (2009). Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. Journal of Experimental Botany, 60(6), 1823–1837. DOI 10.1093/jxb/erp055. [Google Scholar] [CrossRef]
68. Wang, L., Shan, T., Xie, B., Ling, C., Shao, S. et al. (2019). Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chemistry, 272, 530–538. DOI 10.1016/j.foodchem.2018.08.085. [Google Scholar] [CrossRef]
69. Hong, K. Q., Xu, H. B., Wang, J. N., Zhang, L. B., Hu, H. G. et al. (2013). Quality changes and internal browning developments of summer pineapple fruit during storage at different temperatures. Scientia Horticulturae, 151, 68–74. DOI 10.1016/j.scienta.2012.12.016. [Google Scholar] [CrossRef]
70. Palma, F., Carvajal, F., Lluch, C., Jamilena, M., Garrido, D. (2014). Changes in carbohydrate content in zucchini fruit (Cucurbita pepo L.) under low temperature stress. Plant Science, 217–218, 78–86. DOI 10.1016/j.plantsci.2013.12.004. [Google Scholar] [CrossRef]
71. Cao, S. F., Shao, J. R., Shi, L. Y., Xu, L. W., Shen, Z. M. et al. (2018). Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Scientific Reports, 8(1), 11. DOI 10.1038/s41598-017-18324-8. [Google Scholar] [CrossRef]
72. Imahori, Y., Takemura, M., Bai, J. (2008). Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage. Postharvest Biology and Technology, 49(1), 54–60. DOI 10.1016/j.postharvbio.2007.10.017. [Google Scholar] [CrossRef]
73. Trivellini, A., Cocetta, G., Francini, A., Ferrante, A. (2017). Reactive Oxygen Species Production and Detoxification During Leaf Senescence. Singapore: Springer Singapore. [Google Scholar]
74. Yuanzhi, S., Jianghui, X., Ping, C., Wen, L. (2013). Changes in some chemical components and in the physiology of rambutan fruit (Nephelium lappaceum L.) as affected by storage temperature and packing material. Fruits, 68(1), 15–24. DOI 10.1051/fruits/2012045. [Google Scholar] [CrossRef]
75. Chongchatuporn, U., Ketsa, S., van Doorn, W. G. (2013). Chilling injury in mango (Mangifera indica) fruit peel: Relationship with ascorbic acid concentrations and antioxidant enzyme activities. Postharvest Biology and Technology, 86, 409–417. DOI 10.1016/j.postharvbio.2013.07.023. [Google Scholar] [CrossRef]
76. Qian, Z., Chao, M., Shunchang, C., Baodong, W., Xiuying, L. et al. (2013). Changes in antioxidative metabolism accompanying pitting development in stored blueberry fruit. Postharvest Biology and Technology, 88, 88–95. [Google Scholar]
77. Gan, X. H., Hu, D. Y., Wang, Y. J., Yu, L., Song, B. (2017). Novel trans-ferulic acid derivatives containing a chalcone moiety as potential activator for plant resistance induction. Journal of Agricultural and Food Chemistry, 65(22), 4367–4377. DOI 10.1021/acs.jafc.7b00958. [Google Scholar] [CrossRef]
78. Huang, R. H., Liu, J. H., Lu, Y. M., Xia, R. X. (2008). Effect of salicylic acid on the antioxidant system in the pulp of ‘Cara cara’ navel orange (Citrus sinensis L. Osbeck) at different storage temperatures. Postharvest Biology and Technology, 47(2), 168–175. DOI 10.1016/j.postharvbio.2007.06.018. [Google Scholar] [CrossRef]
79. Xiujuan, A., Yin, X., Li, J., Chen, H., Zhifang, Y. (2019). Effects of postharvest temperature on apoptosis-related enzyme activity and gene expression in peach fruits (Prunus persica L. cv. Xiahui 8). Scientia Horticulturae, 245, 178–184. DOI 10.1016/j.scienta.2018.10.020. [Google Scholar] [CrossRef]
80. Kashash, Y., Holland, D., Porat, R. (2019). Molecular mechanisms involved in postharvest chilling tolerance of pomegranate fruit. Journal of the Science of Food and Agriculture, 99(13), 5617–5623. DOI 10.1002/jsfa.9933. [Google Scholar] [CrossRef]
81. Mercado Camargo, J., Taron Dunoyer, A., García-Zapateiro, L. A. (2016). The effect of storage temperature and time on total phenolics and enzymatic activity of sapodilla (Achras sapota L.). Revista Facultad Nacional de Agronomía Medellín, 69(2), 7955–7963. DOI 10.15446/rfna.v69n2.59140. [Google Scholar] [CrossRef]
82. Ah-Na, K., Hyun-Jin, K., Jiyeon, C., Ho Jin, H., Kerr, W. I. et al. (2018). Degradation kinetics of phenolic content and antioxidant activity of hardy kiwifruit (Actinidia arguta) puree at different storage temperatures. LWT-Food Science and Technology, 89, 535–541. DOI 10.1016/j.lwt.2017.11.036. [Google Scholar] [CrossRef]
83. Shah, S. W. A., Jahangir, M., Qaisar, M., Khan, S. A., Mahmood, T. et al. (2015). Storage stability of kinnow fruit (Citrus reticulata) as affected by CMC and guar gum-based silver nanoparticle coatings. Molecules, 20(12), 22645–22661. DOI 10.3390/molecules201219870. [Google Scholar] [CrossRef]