DOI:10.32604/phyton.2020.012163

| Phyton-International Journal of Experimental Botany DOI:10.32604/phyton.2020.012163 |  |
| Review |
Salt Stress Threshold in Millets: Perspective on Cultivation on Marginal Lands for Biomass
1Department of Bioresources, School of Biological Sciences, University of Kashmir, Srinagar, 190006, India
2Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
*Corresponding Authors: Khalid Rehman Hakeem. Email: kur.hakeem@gmail.com; Reiaz Ul Rehman. Email: rreaizbiores@gmail.com
Received: 17 June 2020; Accepted: 12 August 2020
Abstract: Millets hold an immense assurance for food safety and nourishment amid ever-rising agricultural expenses and climate alterations. They are healthful, have supplementary wellbeing profit and need remarkably fewer effort overheads for crop growing. These characters draw attention to millets as a plant of preference for the humankind in the course of emergent alarm about environmental changes. Millets have the prospect to provide biomass and thus bioenergy, reduced carbon emission, carbon footprint and sustainable modern agriculture. As the rate of expansion in budding countries is increasing day by day, the scarcity of energy is a big panic and there is a mounting turn in the direction and rehearsal of waste and biomass as an energy source. Globally, at least 20% of total irrigated land has been injured by salt and 1.5 million hectares is taken away of cultivation every year. Thus, in future, we will have a requirement of efficient crops and utilisation of marginal lands for agriculture. Millet is an answer to the efficient crop. Plants are subjected to various environmental pressures (high/low temperature, heavy metal, salinity, pesticides, etc.) as well as biotic stresses (virus, bacteria, fungi, etc.) and millets are not an exception to it. Millets are categorised as glycophytes and can tolerate average salt threshold of about 6 (ECe) (dS/m) with some variation from specie to specie. Increase in the salt concentrations can lead to retarded growth and development, thus need for mitigants arise to reduce such stresses. Some mitigants to overcome the stress levels include proline, polyamine and betaines, Na2SeO3, H2S, KNO3, Mg(NO3)2, etc.
Keywords: Sustainable development; proline; salt tolerance; biomass; food security
Salt stress is the gravest factor preventing the yield of crops, having undesirable consequences on the crop, its growth rate, strength and health [1]. Saline conditions influence various irrigated areas, mostly due to the use of brackish irrigate. Internationally, greater than 45 million hectares (at least twenty per cent of total irrigated area) of irrigated land has been injured by saline conditions and 1.5 million hectares is taken away of cultivation every year [1]. The soil is assumed to be saline or influenced by salinity if it has an electrical conductivity of saturation soil extort of larger than 4 dS/m at 25°C where 4 dS m−1 ≈ 40 mM NaCl or greater [2]. Surplus salinity results in unbalanced, underdeveloped development, reduced and spotty yields. The degree of this damage is directly proportional to the quantity of salinity. Also, this type of stress tends to lessen the amount of oil in oilseed crops, in the same way, drop in yield occurs in plants that are to some extent salt-tolerant, for instance, safflower and sunflower [3,4]. Soil erosion, land degradation, biotic and abiotic factors decrease the available area for cultivation. As per United Nations Food and Agriculture Organization forecasts, in instruct to meetup worldwide food requirement, the quantity of staple cereal crops in the next thirty-two years would be greater than two times (raise by sixty to hundred percent) [5–7]. Thus in future, we will need efficient crops and utilisation of marginal lands for cultivation. Marginal and insignificant soils are fit for millets. Thus, the utilization of marginal soils and areas are desirable for bioenergy, biofuel, food, fodder, etc.
Millets are a group of variable grasses with many small seeds. These crops have a benefit of being proficient propagates in the stress conditions (scant rainfall, little soil nutrients, stress, etc.), anywhere if there is a slight possibility of growth for other crops [8]. Millets stay classified with maize and sorghum in grass sub-family panicoideae [9] whereas disagreements exist about the classification of family millet, with some evidence giving the family name Gramineae and others classifying it in family Poaceae [10,11]. About 9 millets are mostly cultivated. In a sequence of universal produce, mostly extensive cultivated millets are sorghum (Sorghum bicolor (L.) Moench), pearl millet (Pennisetum glaucum (L.) R. Br.), foxtail millet (Setaria italica (L.) P. Beauvois), proso millet (Panicum miliaceum L.) and finger millet (Eleusine coracana Gaertn.) [12]. Additional millets are little millet (Panicum sumatrense Roth. ex Roem. & Schultz), Indian barnyard millet (Echinochloa frumentacea Link), kodo millet (Paspalum scrobiculatum L.), Japanese barnyard millet (Echinochloa utilis Ohwi & Yabuno), tef (Eragrostis tef (Zucc.) Trotter) and fonio (Digitaria spp.).
In this review, we explore the benefits of millets with respect to sustainable development and effect of saline stress, salt tolerance capacity and mitigation of salt stress. Further, we will have a look on the relevance of millets to climate change, food security and livelihood.
2 Agroclimatic Conditions and Agronomical Benefits of Millets
Millets are grown frequently in developing countries [13] especially in semiarid humid areas of Asia and Africa. In these areas, millets are cultivated for both food and animal feed. Millets are significantly little granular crops which are mostly grown in marginal soils where other crops fail to propagate [14]. They are grown in severe ecological situations, particularly in insufficient wetness and in soil with a deprived nutrient that is otherwise not matched for the leading cultivated crops. Millets are rich in proteins, lipids, vitamins, minerals, dietary fibre, essential amino acids, essential fatty acids, antioxidants, polyphenols, tannin, phytic acid, phytate and oxalic acid [15]. Millets have been contributing to human health via their hypoglycaemic, anti-tumorigenic, atherosclerogenic and anti-microbial properties. The estimated protein content of millet grains is 8–15% (dry weight). Pearl millet is having the maximum protein content, i.e., 14.5% [16]. The lipid content of millets ranges from 1.43 to 6 gm/100 gm [17]. Al Juhaimi et al. [18] stated the approximate ash content of millets was 1.74% (dry weight basis). Millets are rich in vitamins that are required for normal physiological functions of the human body. Many vitamins especially vitamin-B6 and folic acid are present in millets [19]. Millets are considered to be a rich source of Vitamin E, which is an antioxidant and guards fat in membranes around cells. Millets are a good source of phenolics, flavonoids, tannins, etc. Thus help in cardiovascular diseases, cancer and diabetes. The condensed tannins have antioxidant, anti-viral, anti-inflammatory and anti-bacterial properties [20]. Moreover, being gluten-free millets are an excellent substitution to wheat. Millets are immensely consumed as snacks, bread, puddings, beverages and hold significant cultural positions. Besides providing food security to the millions, these are the means of livelihood for many especially for the people of Africa and Asia. Millets are used as feed and fodder too. Pearl millet feed is highly digestible in a vegetable state and does not contain hydrocyanic acid. Foxtail millet is used as animal feed in western countries especially for caged and wild birds. Finger millet straw is one of the most preferred feedstuffs for cattle in South Asia [21].
3 Biomass and Bioenergy from Millets
We have a continuous rise in global energy expenditure since the past few years. One of the reasons for this is a speedy expansion of population, production and industrial extension. This leads to more utilization of non-renewable fuels, such type of fuels have an obvious impact on the surrounding environment and whole ultimately. The effects of environmental change with greenhouse gases formed by the use of fossil fuels are noticeable throughout the planet [22]. So, the utilization of energy sources which can be used again and again (renewable) seems to have several returns. The only way out of this is biomass. Renewable fuels have accessibility and cause less pollution. The global production of biomass (calculated approximately) is 146 billion metric tons per annum. Biomass has the budding scope of providing a sustainable supply of energy and also meeting greenhouse gas reduction targets [23].
Millets have potential to provide biomass and thus bioenergy for sustainable development and reduced carbon emission. Panicum virgatum L. (switchgrass) and other millets turn out to be a representative species for bioethanol and biodiesel production [24–26]. The undersoil biomatter of this plant is 4–5 folds superior to corn as it can contribute 2.2 Mg C/ha/yr into soils [27,28]. Switchgrass has outstanding prospective for bioethanol production by fermentation and gas and electricity generation by gasification process. The U.S. Department of Energy selected switchgrass as the herbaceous model species for biomass energy. Characteristics like constant high biomass yield, least agricultural inputs and relatively easy to grow from seed make it a brilliant bioenergy crop [29].
Foxtail millet is cultivated widely for food and feed in Asia and Africa and has future for utilization as a C4 bioenergy crop. Zhang et al. [30] provided insight of foxtail millet (Setaria italica (L.) P. Beauvois) related to its biofuel potential and reported it as a model grass for other renewable fuel grasses, together with switchgrass and pearl millet. Genome sequence studies have shown that this plant is strongly correlated to numerous bioenergy crops at the genome level like switchgrass (Panicum virgatum L.), napiergrass (Pennisetum purpureum Schum.) and thus can serve as a potential bioenergy crop.
The proximate analysis of pearl millet biomass revealed that superior constituents are present for biofuel production in the crop. The chemical composition of pearl millet is also better to that of the other major cereals [31]. Pearl millet contains cellulose (41.6 ± 0.01%) and hemicelluloses (22.32 ± 0.65%) polymers which are necessary for biofuel production. Sweet pearl millet provides a better alternative for biofuel production than other crops as it can be used for ethanol production because it produces a high concentration of readily fermentable sugar (0.03 to 0.06 litre/kg biomass) [32].
The biofuel production capability mostly depends upon the feedstock superiority, quantity and climatic factors. For growing feedstock, which is required for biofuel manufacturing about every country is restricted by two factors, i.e., land availability and scarce water resources [33]. Developing nations use forty-seven per cent of overall energy from biomass [34]. Owing to the rapid pace of growth, the deficiency of energy is becoming an immense fear. Thus we need to explore more and more for a better future.
4 Relevance of Millets to Climate Change, Food Security, Livelihood and Future Sustainability Development
Two essential sustainability questions of the present farming are the decreasing carbon footprint and increasing energy use. Understand the suggestion of energy and carbon utilization, dept. for environment food, rural affairs [35] informed about temperature raise of 3–4°C and it is certain that agricultural production will be seriously affected, thus in future abandoned and underutilized crop resources are extremely fundamental for sustainable long-lasting agriculture [36] and millets fit into this criterion [37]. Millets are well suitable to little rainfall situations, proficiently survive extensive dry spells, improve quickly after deferred rain, root organization well-organized in soil moisture withdrawal, negligible pest and disease problems, sustainable and modest crop under little management situation in marginal land. Millets have biochemical, morpho-physiological and molecular features which contribute to enhanced tolerance to abiotic stresses than other major cereals. Principally, the quick lifecycle of millets aids in getting away from stress as they need three and a half months to finish their life cycle. The advantageous C4 photosynthetic feature is seen in millets [38]. Foxtail millet is somewhat drought tolerant crop among cereals. Compared to others it needs 257 g of H2O to manufacture 1 g of dry biomass, where 470 and 510 g is required by maize and wheat correspondingly [39,40]. The noteworthy connection among agronomic characters like panicle and grain weight, plant height, thousand-grain weight and physiologic factors with (DRI) drought-resistant index in drought circumstance is found [41]. Foxtail, as well as finger millets, are prospective crops for saline soils [42]. Millets are nutritionally very rich food, reasonably low-priced, therefore much available to poor, extended storability of yield, the extended shelf life of seeds, quality feed for animals. Thus millets are highly prized for livestock production in areas with less rainfall, have a cardinal place in local food systems, profits generating prospect for marginalized part of society as well as for women by value-adding interventions. Millets have better nutritional quality and valuable food properties, nutritionally millets are rich in protein, amino acids, vitamin A, minerals and fibre [43,44]. They can tackle the unseen appetite of the deprived people, nurture the lactating mothers, due to little glycemic index they can contribute neutraceutical profit to people having diabetes [45]. Thus millets are cardinally related to sustainable development and these characteristics of millets make-out them next-generation crops with the appeal for studies to look at the climate-resilient qualities.
All the major cereal crops like wheat, rice and maize have global warming potential of around 4 tons CO2 eq/ha, 3.4 tons CO2 eq/ha and 3.4 tons CO2 eq/ha respectively. These crops also have a high carbon equivalent emission of 1000, 956 and 935 kg C/ha for wheat, rice and maize, respectively. Even with their higher emission amounts, they are commonly cultivated as primary sources of nutrition for the global population. However, millets and sorghum have comparatively lesser carbon footprints [46]. This is other reasons for the cultivation of millets to reduce carbon footprint globally. Millets are coarse cereals and can be grown as a substitute for wheat and rice. According to FAO, the total production of millets in the world is 31019370 tonnes and the total area under cultivation is 33560087 ha [47] (Fig. 1).
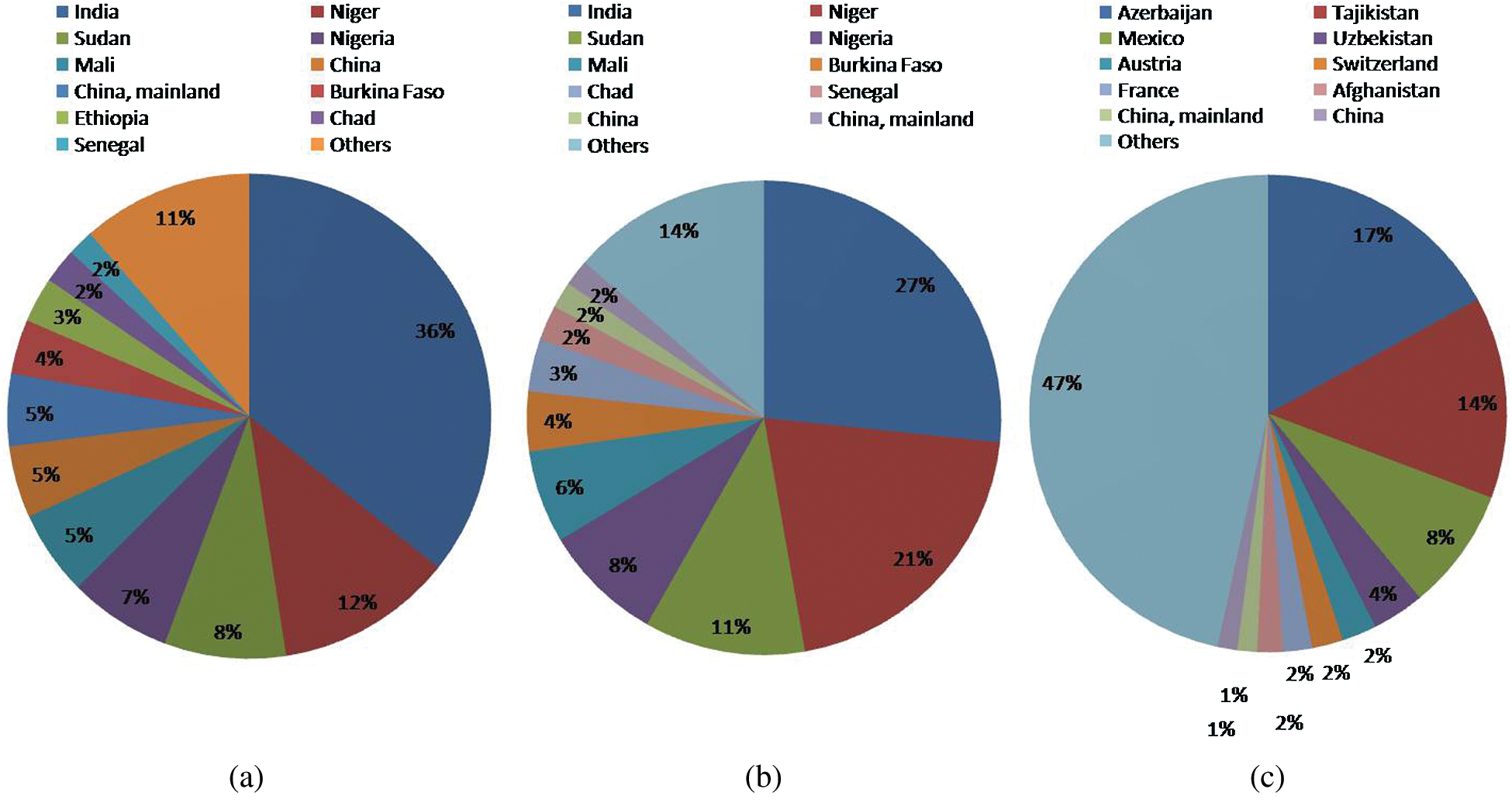
Figure 1: (A) Country-wise production (% of 31019370 tonnes in total), (B) area harvested (% of 33560087 ha in total), (C) yield (hectogram per hectare: hg/ha) of millets
5 Millets, Stress and Threshold Salt Application Required for Stress
Plants are subjected to various abiotic stresses (temperature, drought, heavy metal, salinity, pesticides, etc.) as well as biotic stresses (virus, bacteria, fungi, etc.), and millets are not an exception to it. Such conditions unpleasantly affect their progress and development and prompt a sequence of changes in morphological, physiological, biochemical and molecular aspects. Abiotic pressures can persuade varied reactions in plant life which include reformations of transportation and the metabolic route which leads to growth inhibition [48]. Ion difference and hyperosmotic pressure is the key outcome of abiotic stress and the instant effect of these crucial changes in the enhanced gathering of ROS (reactive oxygen species) that are injurious to cells at high intensities. ROS like superoxide, hydroxyl radicals, hydrogen peroxide and singlet oxygen, cause injury to cellular structures and biomolecules like DNA, proteins, carbohydrates, lipids and eventually end with cell death [49–53]. ROS are extremely reactive, deadly and can cause mutations [54]. Thus one-sidedness among ROS and antioxidants defences results in oxidative stress and when a cell is in the situation of oxidative stress, its outcomes is lethal lipid per-oxidation, DNA damage, enzyme inhibition, oxidation of proteins and inauguration of deliberate cell death or programmed (PCD) or apoptosis [55,56]. In plants chloroplasts, mitochondria and peroxisomes are cardinal sites where ROS are produced mainly. Regardless of the disparaging nature, reactive oxygen species are well-thought-out to exist as the second messengers in signalling and cellular process, provide tolerance to stress [57]. The balance between ROS and scavenging activity antioxidant decides that whether ROS work as a detrimental or signalling agent, the evenness in the activity of these two is very mandatory to retain, with the aim of battle any oxidative stress. The scavenging antioxidant security system includes non-enzymatic and enzymatic antioxidants [58]. The enzymatic scavenger resistance scheme includes catalase (CAT), guaiacol peroxidase (GPX), superoxide dismutase (SOD), glutathione reductase (GR) however glutathione (GSH), ascorbate (ASA), carotenoids, proline, tocopherols and phenolics function as probable non-enzymatic scavengers inside the cell [59]. Every stress has an unfavourable impact on plants. This injurious effect is detected on the entire plant or some parts, leading to the death of plant or drop in productivity. Further, all the cardinal processes like germination, development, pigments system, photosynthesis, water relation, nutrient imbalance and yield are affected.
The number of genes is switched on and off by transcription factors, ensuing the improved intensities of some proteins and metabolites, which are answerable for granting defence against these stresses [50,51,60] These varied pressures and stresses frequently trigger signalling paths and responses, for instance, stress proteins, upregulating of scavenging anti-oxidants and gathering of friendly solutes [61]. Such genetic produces are categorized into 3 main sets.
1. Firstly those substances that unswervingly guard cells against stresses, like heat stress proteins (HSPs) or chaperones, LEA proteins (late embryogenesis abundant proteins), osmoprotectants, antifreeze proteins, detoxification enzymes and free-radical scavengers [62,63].
2. Those that are associated in enormous signalling sequences and in controlling transcription of CDPK (calcium-dependent protein kinase) [64], MAPK (Mitogen-activated protein kinase) and SOS kinase [65], phospholipases, etc. [66].
3. Lastly, those linked in ion uptake, water uptake, transporters like aquaporins and ion transporters [67].
The minimum salt application that begins to destroy growth fluctuates noticeably within plant variety. Traditional the majority of agricultural crop species are categorised as glycophytes, having threshold resistances in a range of 1 to 10 dS/m (deci-siemens per metre), expressed as the electrical conductivity (EC) of saturated-soil extracts taken from the root zone. At EC over 10 dS/m the development of most resistant plant species, for instance, barley, cotton, sugarbeet, rye and wheat drop off with rising saline application. Several tolerate species flourish at soil salt concentration of 10 dS/m or higher are grouped in halophytes. Several halophytic plants have a budding scope as crop plants however very little or no data is on hand to guess growth as a function of soil salinity [68]. Millets are not resistant to high salt concentrations and thus are grouped into glycophytes. Similarly, salt tolerance can be expressed in mMol/L, glycophytes are cruelly repressed or destroyed by an application of 100–200 mmol/L NaCl, on the other hand, halophytes can withstand salt beyond 300 mmol/L. Halophytes stand enormous extraordinary intensities of salt amounts. For instance, in the presence of 700 mmol/L NaCl Atriplex vesicaria Heward ex Benth. can yield good yield. At the same time, Salicornia europaea L. constantly stays vigorous in 1020 mmol/L NaCl. Thus whereas glycophytes are salt sensitive and halophytes are tolerant still there are many plants which are very salt-delicate, for example, fruit trees such as avocado and citrus, these plants are restrained by few millimoles per litre concentrations of NaCl [65].
Rasool et al. [69] studied the effect of various amounts of NaCl (50–200 mM) on Setaria italica (L.) P. Beauvois and Panicum miliaceum L. and found that salt stress decreased the tolerance index (TI) of both shoots and roots, the biomass, relative water content (RWC) and photosynthetic pigments (PP) in a dose-dependent manner with respect to control. The salt treatments increased the membrane damage as evidenced by electrolyte leakage (EL) and thiobarbituric acid reactive species (TBARS). Similarly, Shah et al. [70] reported that salt stress influenced a significant modification in the level of osmolyte accumulation such as proline, glycine betaine, and antioxidant enzymes. A significant decrease of seed germination percentage, root and shoot length, photosynthetic pigments like chlorophyll a, chlorophyll b and proteins at higher concentration of NaCl added was recorded. From this, it was found that the millet crops can be sustained in optimum salinity (100 mM) condition. It was also concluded that enzymatic and non-enzymatic defence systems play a key role in generating tolerance against salt stress.
Fan et al. [71] in growth and physiological characteristics of switchgrass (Panicum virgatum L.) seedlings exposed to 0, 50, 100, 150 and 200 mmol/L of NaCl solutions. With the increasing concentration of the NaCl, the seedling growth was inhibited. The plant height decreased, leaves became smaller, photosynthetic leaf area and net photosynthetic rate reduced and dry matter accumulation decreased significantly and presenting the general traits of glycophyte, the salt tolerance threshold for P. virgatum L. was 178.6 mmol/L when taking 50% drop in biomass as the standard. El-Keblawy [72] investigated germination percentage and germination rate to salinity level in Panicum turgidum Forssk. Germination was significantly reduced and slower at the higher concentrations and was completely inhibited at 300 and 400 Mm. The results of the present study showed that the seed germination of Panicum turgidum Forssk. was greatly reduced by increasing the salt concentration and completely inhibited at 300 and 400 mM NaCl and KCl.
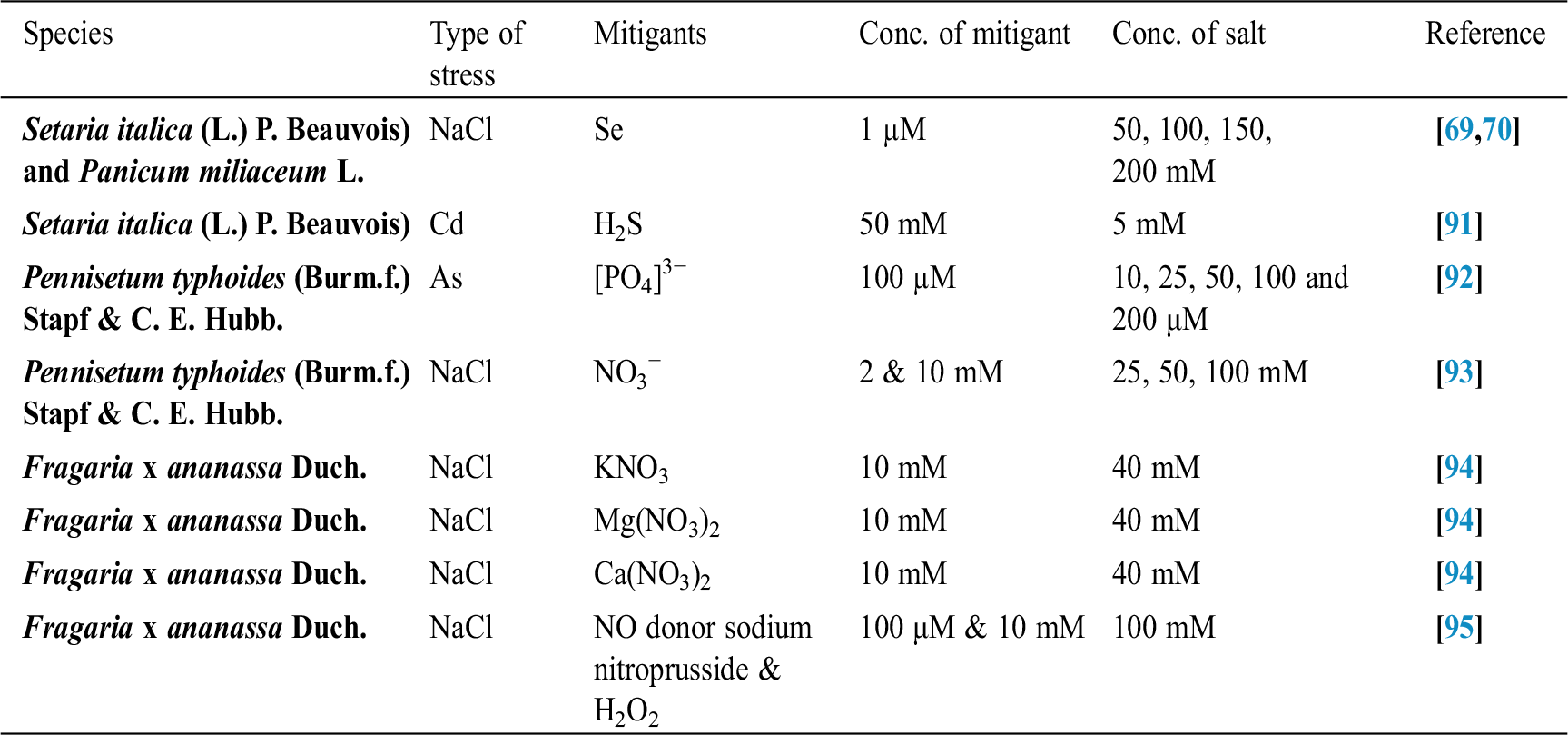
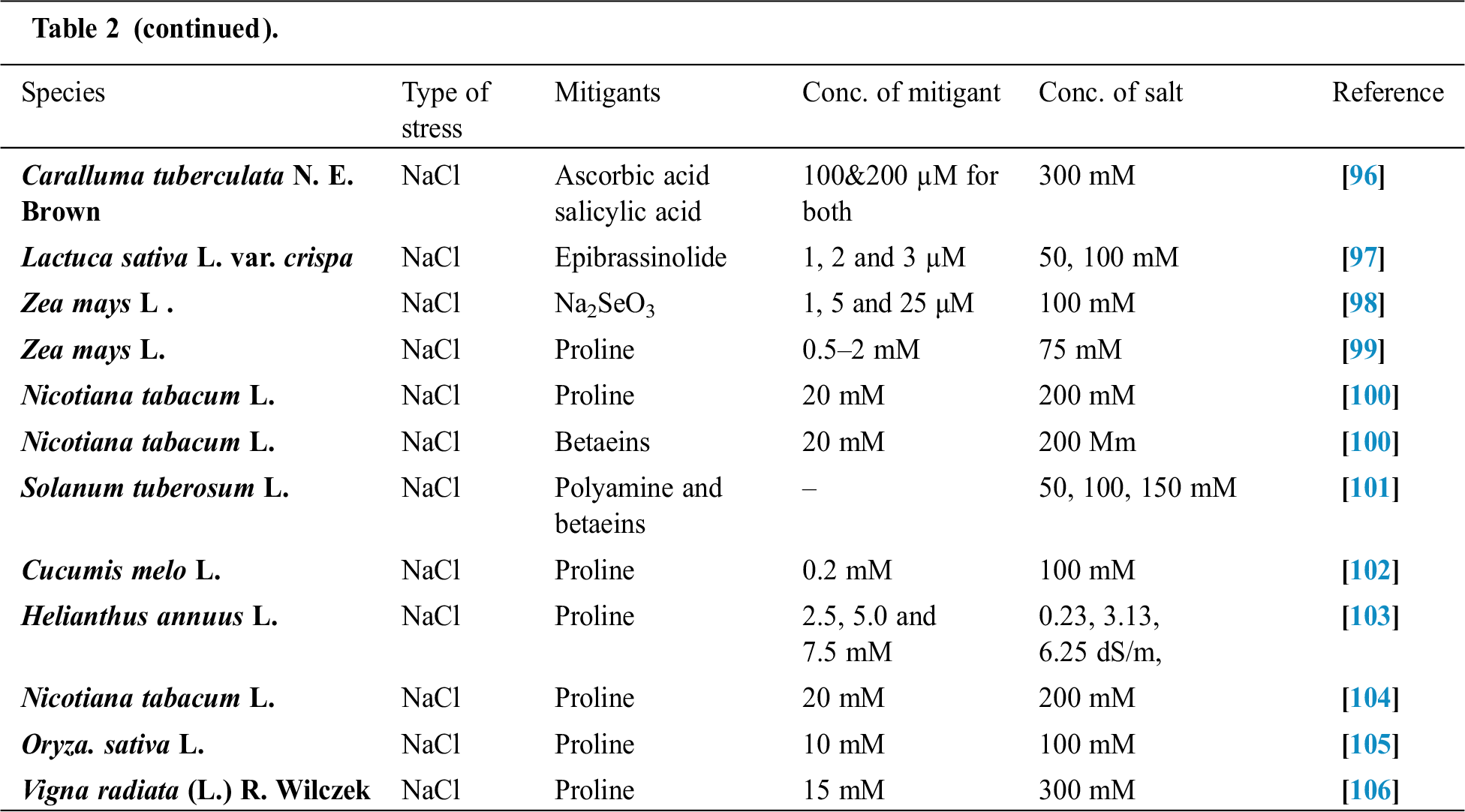
Seffino [73] evaluate the response of two cultivars (Klein Verde and Bambatsi) of Panicum coloratum L. to salinity. The salinity of 100 and 200 mmol/L NaCl delayed germination and significantly reduced germination percentages in both cultivars. Seeds that did not germinate within 16 days in saline solutions had lost viability, as very few germinated when they were transferred to water after this period. Sreenivasulu et al. [74] in differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica (L.) P. Beauvois) found that seed germination and seedling growth are normally limited by increasing concentration of NaCl. Accordingly, 5-day-old seedlings of the tolerant and the sensitive foxtail millet cultivar are grown upon high amounts of NaCl (up to 250 mM) showed differences in their growth pattern (root and shoot length). Seedlings of the tolerant cultivar were able to grow normally even at 200 mM, whereas germination of seedlings of the sensitive cultivar was strictly inhibited already at 150 mM NaCl. Kafi et al. [75] investigated relative salt tolerance of south Khorasan millets ie proso millet (Panicum miliaceum L.), foxtail millet (Setaria italica (L.) P. Beauvois) and pearl millet (Pennisetum glaucum (L.) R. Br.) and found that the yield and other yield-related parameters of millets decreased by salinity stress, this reduction was more prominent only at a high level of salinity (9.5 dS/m). Zehtabian et al. [76] studies on Panicum antidotale Retz. Salinity stress was selected in the form of four salinity treatments, including zero (authentic), 40, 120 and 200 millimolar. Salt solutions of NaCl (60%), Na2S04 (30%) and CaCl2 (15%) were used. The effect of 20 days of dry treatment and 200 millimolar salinity was observed in decreasing dry matter production more than the other treatments. Further, we can describe the salt resistance capacity of a crop by plotting its relative yield as a continuous function of soil salinity in (ECe) (dS/m). Tab. 1 shows the salt tolerance capacity of some plants whereas Tab. 2 shows some mitigants used to combat stress.
Table 1: Threshold salt tolerance of some agriculture crops
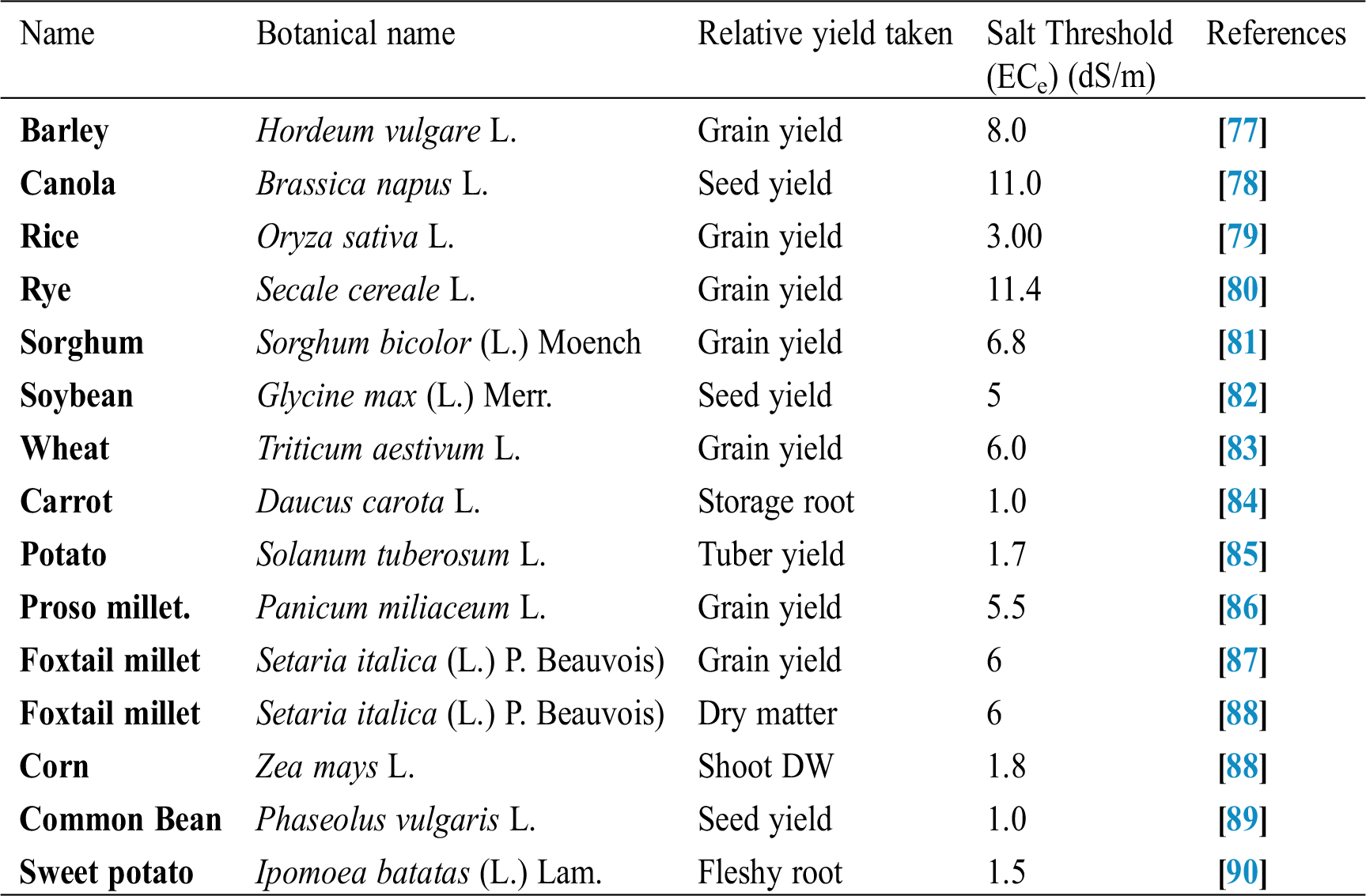
Table 2: Some mitigants used to overcome stress in plants
From the outlook of this review, we concluded that millet serves as an excellent grass for bioenergy, biomass, food Security and sustainable development. Possibly, a hale and hearty environment can be produced with food security and also our future generation will feel more safe and secure. Further millets have a scope for renewable energy generation as demanded by countries like India. Abiotic restrains like salinity is the foremost preventive factor for development and productivity of millets. Moreover, the growing worldwide population is forcing researchers to develop new and proficient plans for boosting crop production to guarantee food security in unfavourable circumstances. So far, we have various outstanding studies in millets like the significance of mitigants, threshold tolerance in millets under salt stress etc. Investigation of the stress tolerance mechanisms and genetic manipulation of millets will aid further in attaining sustainable development efforts to search out enhanced crop performance on marginal and in-significant lands. Considering the significance of mitigants in free radical scavengers at a biochemical and physiological level in salinity stress, additional search regarding role and mechanism of mitigants as protectant may perhaps add a lot to solve such adverse conditions of millets.
Acknowledgement: Thanks to the University of Kashmir (UOK) for providing fellowships to WHS and AR.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59(1), 651–681. DOI 10.1146/annurev.arplant.59.032607.092911.
2. Chinnusamy, V., Jagendorf, A., Zhu, J. K. (2005). Understanding and improving salt tolerance in plants. Crop Science, 45(2), 437–448. DOI 10.2135/cropsci2005.0437. [Google Scholar] [CrossRef]
3. Flagella, Z., Giuliani, M. M., Rotunno, T., Di Caterina, R., De Caro, A. (2004). Effect of saline water on oil yield and quality of a high oleic sunflower (Helianthus annuus L.) hybrid. European Journal of Agronomy, 21(2), 267–272. DOI 10.1016/j.eja.2003.09.001. [Google Scholar] [CrossRef]
4. Yeilaghi, H., Arzani, A., Ghaderian, M., Fotovat, R., Feizi, M. et al. (2012). Effect of salinity on seed oil content and fatty acid composition of safflower (Carthamus tinctorius L.) genotypes. Food Chemistry, 130(3), 618–625. DOI 10.1016/j.foodchem.2011.07.085. [Google Scholar] [CrossRef]
5. Ray, D. K., Mueller, N. D., West, P. C., Foley, J. A. (2013). Yield trends are insufficient to double global crop production by 2050. PLoS One, 8(6), e66428. DOI 10.1371/journal.pone.0066428. [Google Scholar] [CrossRef]
6. Wiebe, K. (2009). How to feed the world in 2050. Insights from an Expert Meeting at FAO, pp. 24–26. FAO Headquarters, Rome.
7. Spindel, J. E., McCouch, S. R. (2016). When more is better: how data sharing would accelerate genomic selection of crop plants. New Phytologist, 212(4), 814–826. DOI 10.1111/nph.14174. [Google Scholar] [CrossRef]
8. Habiyaremye, C., Matanguihan, J. B., D’Alpoim Guedes, J., Ganjyal, G. M., Whiteman, M. R. et al. (2017). Proso millet (Panicum miliaceum L.) and its potential for cultivation in the Pacific Northwest, US: a review. Frontiers in Plant Science, 7, 1961. [Google Scholar]
9. Yang, X., Wan, Z., Perry, L., Lu, H., Wang, Q. et al. (2012). Early millet use in northern China. Proceedings of the National Academy of Sciences of the United States of America, 109(10), 3726–3730. DOI 10.1073/pnas.1115430109. [Google Scholar] [CrossRef]
10. Goron, T. L., Raizada, M. N. (2015). Genetic diversity and genomic resources available for the small millet crops to accelerate a new green revolution. Frontiers in Plant Science, 6, 157. [Google Scholar]
11. Cheng, A. (2018). Review: shaping a sustainable food future by rediscovering long-forgotten ancient grains. Plant Science, 269, 136–142. DOI 10.1016/j.plantsci.2018.01.018. [Google Scholar] [CrossRef]
12. Crawford, G. W., Lee, G. A. (2003). Agricultural origins in the Korean Peninsula. Antiquity, 77(295), 87–95. DOI 10.1017/S0003598X00061378. [Google Scholar] [CrossRef]
13. McDonough, C. M., Rooney, L. W., Serna-Saldivar, S. O. (2000). The millets. Food science and technology, pp. 177–202. New York: Marcel Dekker. [Google Scholar]
14. Baker, R. D. (2003). Millet production-guide A-414. Las Cruces, New Mexico: New Mexico State University, College of Agriculture and Home Economics. [Google Scholar]
15. Gopalan, C., Rama Sastri, B. V., Balasubramanian, S. C. (1971). Nutritive value of Indian foods. Revised by Narasingha Rao BS, Deosthale YG, Pant KC. Hyderabad, India: National Institute of Nutrition and Indian Council of Medical Research. [Google Scholar]
16. Taylor, J. R. N., Taylor, J. (2017). Proteins from sorghum and millets. In Sudarshan, R. Nadathur, Janitha P. D. Wanasundara, Laurie Scanlin (eds.Sustainable protein sources, pp. 79–104. San Diego: Academic Press. [Google Scholar]
17. Kumar, A., Tomer, V., Kaur, A., Kumar, V., Gupta, K. (2018). Millets: a solution to agrarian and nutritional challenges. Agriculture & Food Security, 7(1), 31. DOI 10.1186/s40066-018-0183-3. [Google Scholar] [CrossRef]
18. Al Juhaimi, F., Şimşek, Ş., Ghafoor, K., Babiker, E. E., Özcan, M. M. et al. (2019). Effect of varieties on bioactive properties and mineral contents of some sorghum, millet and lupin seeds. Journal of Oleo Science, 68(11), 1063–1071. DOI 10.5650/jos.ess19113. [Google Scholar] [CrossRef]
19. Dayakar Rao, B., Bhaskarachary, K., Arlene Christina, G. D., Sudha Devi, G., Vilas, A. T. et al. (2017). Nutritional and health benefits of millets. Rajendranagar, Hyderabad: ICAR_Indian Institute of Millets Research, 112. [Google Scholar]
20. Xiang, J., Apea-Bah, F. B., Ndolo, V. U., Katundu, M. C., Beta, T. (2019). Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chemistry, 275, 361–368. DOI 10.1016/j.foodchem.2018.09.120. [Google Scholar] [CrossRef]
21. Hanna, W. W., Baltensperger, D. D., Seetharam, A. (2004). Pearl millet and other millets. Warm-Season (C4) Grasses, 45, 537–560. [Google Scholar]
22. Anderson, T. R., Hawkins, E., Jones, P. D. (2016). CO2, the greenhouse effect and global warming: from the pioneering work of Arrhenius and Callendar to today’s earth system models. Endeavour, 40(3), 178–187. DOI 10.1016/j.endeavour.2016.07.002. [Google Scholar] [CrossRef]
23. Balat, M., Ayar, G. (2005). Biomass energy in the world, use of biomass and potential trends. Energy Sources, 27(10), 931–940. DOI 10.1080/00908310490449045. [Google Scholar] [CrossRef]
24. Qin, X., Mohan, T., El-Halwagi, M., Cornforth, G., McCarl, B. A. (2006). Switchgrass as an alternate feedstock for power generation: an integrated environmental, energy and economic life-cycle assessment. Clean Technologies and Environmental Policy, 8(4), 233–249. DOI 10.1007/s10098-006-0065-4. [Google Scholar] [CrossRef]
25. Ohlrogge, J., Chapman, K. (2011). The seeds of green energy: expanding the contribution of plant oils as biofuels. Biochemist, 33(2), 34–38. DOI 10.1042/BIO03302034.
26. Williams, P. R., Inman, D., Aden, A., Heath, G. A. (2009). Environmental and sustainability factors associated with next-generation biofuels in the U.S.: what do we really know? Environmental Science & Technology, 43(13), 4763–4775. DOI 10.1021/es900250d. [Google Scholar] [CrossRef]
27. Zan, C., Fyles, J., Girouard, P., Samson, R., Doan, M. (1997). Carbon storage in switchgrass and short-rotation willow plantations. In Making a business from biomass in energy, environment, chemicals, fibers, and materials. In: Overend, R. P., Chornet, E. (eds.) Proceedings of the Third Biomass Conference of the Americas: Energy, Environment, Agriculture, And Industry, pp. 355–361. New York, Montréal, Québec, Canada: Pergamon. [Google Scholar]
28. Lemus, R., Lal, R. (2005). Bioenergy crops and carbon sequestration. Critical Reviews in Plant Sciences, 24(1), 1–21. DOI 10.1080/07352680590910393. [Google Scholar] [CrossRef]
29. Sanderson, M. A., Adler, P. R. (2008). Perennial forages as second generation bioenergy crops. International Journal of Molecular Sciences, 9(5), 768–788. DOI 10.3390/ijms9050768. [Google Scholar] [CrossRef]
30. Zhang, G., Liu, X., Quan, Z., Cheng, S., Xu, X. et al. (2012). Genome sequence of foxtail millet (Setaria italica L.) provides insights into grass evolution and biofuel potential. Nature Biotechnology, 30(6), 549–554. DOI 10.1038/nbt.2195. [Google Scholar] [CrossRef]
31. Packiam, M., Subburamu, K., Desikan, R., Uthandi, S., Subramanian, M. et al. (2018). Suitability of pearl millet as an alternate lignocellulosic feedstock for biofuel production in India. Journal of Applied & Environmental Microbiology, 6(2), 51–58. [Google Scholar]
32. Crepeau, M., Khelifi, M., Vanasse, A. (2010). Preliminary investigation into the pressing process of sweet pearl millet and sweet sorghum biomass for ethanol production, pp. 1–8. XVIIth World Congress of the International Commission of Agricultural and Biosystems Engineering, Quebec City Canada. [Google Scholar]
33. Singh, R., Setiawan, A. D. (2013). Biomass energy policies and strategies: harvesting potential in India and Indonesia. Renewable and Sustainable Energy Reviews, 22, 332–345. DOI 10.1016/j.rser.2013.01.043. [Google Scholar] [CrossRef]
34. Thomas, P., Soren, N., Rumjit, N. P., James, J. G., Saravanakumar, M. P. (2017). Biomass resources and potential of anaerobic digestion in Indian scenario. Renewable and Sustainable Energy Reviews, 77, 718–730. DOI 10.1016/j.rser.2017.04.053. [Google Scholar] [CrossRef]
35. Department for Environment Food and Rural Affairs (2014). Climate change agreements (2014Climate Change Explained. https://www.gov.uk/guidance/climate-change-explained. [Google Scholar]
36. Mal, B. (2007). Neglected and underutilized crop genetic resources for sustainable agriculture. Indian Journal of Plant Genetic Resources, 20(1), 1–14. [Google Scholar]
37. Dutta, M., Phogat, B. S., Dhillon, B. S. (2007). Genetic improvement and utilization of major underutilized crops in India. Breeding of neglected and underutilized crops, spices and herbs, pp. 251–298. Enfield (NHUSA: Science Publishers. [Google Scholar]
38. Aubry, S., Brown, N. J., Hibberd, J. M. (2011). The role of proteins in C3 plants prior to their recruitment into the C4 pathway. Journal of Experimental Botany, 62(9), 3049–3059. DOI 10.1093/jxb/err012. [Google Scholar] [CrossRef]
39. Bandyopadhyay, T., Muthamilarasan, M., Prasad, M. (2017). Millets for next generation climate-smart agriculture. Frontiers in Plant Science, 8, 1266. DOI 10.3389/fpls.2017.01266. [Google Scholar] [CrossRef]
40. Li, P., Brutnell, T. P. (2011). Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. Journal of Experimental Botany, 62(9), 3031–3037. DOI 10.1093/jxb/err096. [Google Scholar] [CrossRef]
41. Zhang, W. Y., Liu, B. H., Xie, J. X., Li, J. M., Gu, G. Q. et al. (2012). Screening of indexes for drought tolerance test at booting stage in foxtail millet. Journal of Plant Genetic Resources, 13(5), 765–772. [Google Scholar]
42. Krishnamurthy, L., Upadhyaya, H. D., Purushothaman, R., Gowda, C. L. L., Kashiwagi, J. et al. (2014). The extent of variation in salinity tolerance of the minicore collection of finger millet (Eleusine coracana L. Gaertn.) germplasm. Plant Science, 227, 51–59. DOI 10.1016/j.plantsci.2014.07.001. [Google Scholar] [CrossRef]
43. Saleh, A. S., Zhang, Q., Chen, J., Shen, Q. (2013). Millet grains: nutritional quality, processing, and potential health benefits. Comprehensive Reviews in Food Science and Food Safety, 12(3), 281–295. DOI 10.1111/1541-4337.12012. [Google Scholar] [CrossRef]
44. Hulse, J. H., Laing, E. M., Pearson, O. E. (1980). Sorghum and the millets: their composition and nutritive value. London: Academic Press. [Google Scholar]
45. USDA/HNIS. (1984). Composition of foods: cereal grains and pasta, pp. 8–20. Washington DC, USA: United State Department of Agriculture/Human Nutrition Information Service, Agriculture Handbook. [Google Scholar]
46. Saxena, R., Vanga, S. K., Wang, J., Orsat, V., Raghavan, V. (2018). Millets for food security in the context of climate change: a review. Sustainability, 10(7), 2228. DOI 10.3390/su10072228. [Google Scholar] [CrossRef]
47. Food and Agriculture Organisation of the United Nations, Statistics (FAOSTATS) (2020). Crop production. http://www.fao.org/faostat/en/#data/QC. [Google Scholar]
48. Jaleel, C. A., Manivannan, P., Kishorekumar, A., Sankar, B., Gopi, R. et al. (2007). Alterations in osmoregulation, antioxidant enzymes and indole alkaloid levels in Catharanthus roseus exposed to water deficit. Colloids and Surfaces B: Biointerfaces, 59(2), 150–157. DOI 10.1016/j.colsurfb.2007.05.001. [Google Scholar] [CrossRef]
49. Smirnoff, N. (1993). The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist, 125(52), 27–58. DOI 10.1111/j.1469-8137.1993.tb03863.x. [Google Scholar] [CrossRef]
50. Ahmad, P., Sarwat, M., Sharma, S. (2008). Reactive oxygen species, antioxidants and signaling in plants. Journal of Plant Biology, 51(3), 167–173. DOI 10.1007/BF03030694. [Google Scholar] [CrossRef]
51. Tuteja, N., Ahmad, P., Panda, B. B., Tuteja, R. (2009). Genotoxic stress in plants: shedding light on DNA damage, repair and DNA repair helicases. Mutation Research/Reviews in Mutation Research, 681(2–3), 134–149. DOI 10.1016/j.mrrev.2008.06.004. [Google Scholar] [CrossRef]
52. Mittler, R., Vanderauwera, S., Gollery, M., Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends in Plant Science, 9(10), 490–498. DOI 10.1016/j.tplants.2004.08.009.
53. Foyer, C. H., Noctor, G. (2005). Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment, 28(8), 1056–1071. DOI 10.1111/j.1365-3040.2005.01327.x. [Google Scholar] [CrossRef]
54. Halliwell, B. (1997). Antioxidants and human disease: a general introduction. Nutrition Reviews, 55(1), S44–S49. DOI 10.1111/j.1753-4887.1997.tb06100.x. [Google Scholar] [CrossRef]
55. Mishra, S., Jha, A. B., Dubey, R. S. (2011). Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma, 248(3), 565–577. DOI 10.1007/s00709-010-0210-0. [Google Scholar] [CrossRef]
56. Malik, B., Pirzadah, T. B., Tahir, I., Rehman, R. U., Hakeem, K. R. et al. (2014). Plant signaling: response to reactive oxygen species. In: Hakeem, K., Rehman, R., Tahir, I. (eds.Plant signaling: Understanding the molecular crosstalk, pp. 1–38. Springer, New Delhi. [Google Scholar]
57. Yan, J., Tsuichihara, N., Etoh, T., Iwai, S. (2007). Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant, Cell & Environment, 30(10), 1320–1325. DOI 10.1111/j.1365-3040.2007.01711.x. [Google Scholar] [CrossRef]
58. Sharma, P., Jha, A. B., Dubey, R. S., Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012(1), 217037. DOI 10.1155/2012/217037. [Google Scholar] [CrossRef]
59. Noctor, G., Foyer, C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology, 49(1), 249–279. DOI 10.1146/annurev.arplant.49.1.249. [Google Scholar] [CrossRef]
60. Jaleel, C. A., Riadh, K., Gopi, R., Manivannan, P., Inès, J. et al. (2009). Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiologiae Plantarum, 31(3), 427–436. DOI 10.1007/s11738-009-0275-6. [Google Scholar] [CrossRef]
61. Knight, H., Knight, M. R. (2001). Abiotic stress signalling pathways: specificity and cross-talk. Trends in Plant Science, 6(6), 262–267. DOI 10.1016/S1360-1385(01)01946-X. [Google Scholar] [CrossRef]
62. Bray, E. A., Bailey-Serres, J., Weretilnyk, E. (2000). Responses to abiotic stress. In: Gruissem, W., Jones, R. (eds.) Biochemistry & Molecular Biology of Plants, pp. 1158–1203. Rockville: American Society of Plant Physiologists. [Google Scholar]
63. Wang, W. X., Barak, T., Vinocur, B., Shoseyov, O., Altman, A. (2003). Abiotic resistance and chaperones: possible physiological role of SP1, a stable and stabilizing protein from\xA0populus. In: Vasil, I. K. (eds.Plant Biotechnology 2002 and Beyond, pp. 439–443. Springer, Dordrecht. [Google Scholar]
64. Ludwig, A. A., Romeis, T., Jones, J. D. (2004). CDPK-mediated signalling pathways: specificity and cross-talk. Journal of Experimental Botany, 55(395), 181–188. DOI 10.1093/jxb/erh008. [Google Scholar] [CrossRef]
65. Zhu, J. K. (2001). Cell signaling under salt, water and cold stresses. Current Opinion in Plant Biology, 4(5), 401–406. DOI 10.1016/S1369-5266(00)00192-8. [Google Scholar] [CrossRef]
66. Frank, W., Munnik, T., Kerkmann, K., Salamini, F., Bartels, D. (2000). Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell, 12(1), 111–123. DOI 10.1105/tpc.12.1.111. [Google Scholar] [CrossRef]
67. Blumwald, E. (2000). Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology, 12(4), 431–434. DOI 10.1016/S0955-0674(00)00112-5. [Google Scholar] [CrossRef]
68. Maas, E. V. (1993). Plant growth response to salt stress. In: Lieth, H., Al Masoom, A. A. (eds.Towards the rational use of high salinity tolerant plants. Tasks for vegetation science, vol. 27, pp. 279–291. Springer, Dordrecht. [Google Scholar]
69. Rasool, A., Shah, W. H., Tahir, I., Alharby, H. F., Hakeem, K. R. et al. (2020). Exogenous application of selenium (Se) mitigates NaCl stress in proso and foxtail millets by improving their growth, physiology and biochemical parameters. Acta Physiologiae Plantarum, 42(7), 1–13. DOI 10.1007/s11738-020-03109-w. [Google Scholar] [CrossRef]
70. Shah, W. H., Rasool, A., Tahir, I., Rehman, R. U. (2020). Exogenously applied selenium (Se) mitigates the impact of salt stress in Setaria italica L. and Panicum miliaceum L.\xA0The Nucleus, 1–13. DOI 10.1007/s13237-020-00326-z. [Google Scholar] [CrossRef]
71. Fan, X. F., Hou, X. C., Zhu, Y., Wu, J. Y. (2012). Impacts of salt stress on the growth and physiological characteristics of Panicum virgatum seedlings. Ying Yong Sheng Tai Xue Bao, 23(6), 1476–1480. [Google Scholar]
72. El-Keblawy, A. (2004). Salinity effects on seed germination of the common desert range grass, Panicum turgidum. Seed Science and Technology, 32(3), 873–878. DOI 10.15258/sst.2004.32.3.24. [Google Scholar] [CrossRef]
73. Seffino, L. G. (1998). Salinity effects on the early development stages of Panicum coloratum: cultivar differences. Grass and Forage Science, 53(3), 270–278. DOI 10.1046/j.1365-2494.1998.00139.x. [Google Scholar] [CrossRef]
74. Sreenivasulu, N., Grimm, B., Wobus, U., Weschke, W. (2000). Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiologia Plantarum, 109(4), 435–442. DOI 10.1034/j.1399-3054.2000.100410.x. [Google Scholar] [CrossRef]
75. Kafi, M., Zamani, G., Ghoraishi, G. (2009). Relative salt tolerance of south Khorasan millets.\xA0Desert,\xA014(1), 63–70. [Google Scholar]
76. Zehtabian, G. R., Azarnivand, H., Sharifi, K. M. M. (2002). Effect of drought and salinity stress on three range species: Agropyron intermedium, Avena barbata and Panicum antidotale. Iranian Journal of Natural Resources, 54(4), 409–421. [Google Scholar]
77. Ayers, A. D., Brown, J. W., Wadleigh, C. H. (1952). Salt tolerance of barley and wheat in soil plots receiving several salinization Regimes. Agronomy Journal, 44(6), 307–310. DOI 10.2134/agronj1952.00021962004400060006x.
78. Francois, L. E. (1994). Growth, seed yield, and oil content of canola grown under saline conditions. Agronomy Journal, 86(2), 233–237. DOI 10.2134/agronj1994.00021962008600020004x.
79. Venkateswarlu, J., Ramesam, M., Murali, G. V., Rao, M. (1972). Salt tolerance in rice varieties. Journal of the Indian Society of Soil Science, 20(2), 169–173.
80. Francois, L. E., Donovan, T. J., Lorenz, K., Maas, E. V. (1989). Salinity effects on rye grain yield, quality, vegetative growth, and emergence. Agronomy Journal, 81(5), 707–712. DOI 10.2134/agronj1989.00021962008100050001x.
81. Francois, L. E., Donovan, T., Maas, E. V. (1984). Salinity effects on seed yield, growth, and germination of grain sorghum. Agronomy Journal, 76(5), 741–744. DOI 10.2134/agronj1984.00021962007600050008x.
82. Bernstein, L., Ogata, G. (1966). Effects of salinity on nodulation, nitrogen fixation, and growth of soybeans and alfalfa. Agronomy Journal, 58(2), 201–203. DOI 10.2134/agronj1966.00021962005800020025x.
83. Asana, R. D., Kale, V. R. (1965). A study of salt tolerance of four varieties of wheat. Indian Journal of Plant Physiology, 8, 5–22.
84. Bernstein, L. (1953). Salt tolerance of five varieties of carrots. Proceedings of the American Society of Horticulture Sciences, 61, 360–366.
85. Bernstein, L., Ayers, A. D., Wadleigh, C. H. (1951). The salt tolerance of white rose potatoes. Proceedings of the American Society for Horticultural Science, 57, 231–236.
86. Shannon, M. C., Wheeler, E. L., Saunders, R. M. (1981). Salt tolerance of Australian channel millet. Agronomy Journal, 73(5), 830–832. DOI 10.2134/agronj1981.00021962007300050020x.
87. Kubsad, V. S., Hunshal, C. S., Vishwanath, D. P., Patil, S. L., Gowda, D. S. M. (1995). Dry matter accumulation in Setaria as influenced by saline water irrigation. Journal-Maharashtra Agricultural Universities, 20, 3–5.
88. Ravikovitch, S., Porath, A. (1967). The effect of nutrients on the salt tolerance of crops. Plant and Soil, 26(1), 49–71. DOI 10.1007/BF01978675.
89. Hoffman, G. J., Rawlins, S. L. (1970). Design and performance of sunlit climate chambers. Transactions of the ASAE, 13(5), 656–660. DOI 10.13031/2013.38687.
90. Greig, J. K., Smith, F. W. (1962). Salinity effects on sweetpotato growth. Agronomy Journal, 54(4), 309–313. DOI 10.2134/agronj1962.00021962005400040009x.
91. Tian, B., Qiao, Z., Zhang, L., Li, H., Pei, Y. (2016). Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiology and Biochemistry, 109, 293–299. DOI 10.1016/j.plaphy.2016.10.006.
92. Sharma, I., Travlos, I. S. (2012). Phosphate supply as a promoter of tolerance to arsenic in pearl millet. International Journal of Plant Production, 6(4), 443–456.
93. Albassam, B. A. (2001). Effect of nitrate nutrition on growth and nitrogen assimilation of pearl millet exposed to sodium chloride stress. Journal of Plant Nutrition, 24(9), 1325–1335. DOI 10.1081/PLN-100106984.
94. Yildirim, E., Karlidag, H., Turan, M. (2009). Mitigation of salt stress in strawberry by foliar K, Ca and Mg nutrient supply. Plant, Soil and Environment, 55(5), 213–221. DOI 10.17221/383-PSE.
95. Christou, A., Manganaris, G. A., Fotopoulos, V. (2014). Systemic mitigation of salt stress by hydrogen peroxide and sodium nitroprusside in strawberry plants via transcriptional regulation of enzymatic and non-enzymatic antioxidants. Environmental and Experimental Botany, 107, 46–54. DOI 10.1016/j.envexpbot.2014.05.009.
96. Rehman, R. U., Zia, M., Abbasi, B. H., Lu, G., Chaudhary, M. F. (2014). Ascorbic acid and salicylic acid mitigate NaCl stress in Caralluma tuberculata Calli. Applied Biochemistry and Biotechnology, 173(4), 968–979. DOI 10.1007/s12010-014-0890-6.
97. Ekinci, M., Yildirim, E., Dursun, A., Turan, M. (2012). Mitigation of salt stress in lettuce (Lactuca sativa L. var. Crispa) by seed and foliar 24-epibrassinolide treatments. HortScience, 47(5), 631–636. DOI 10.21273/HORTSCI.47.5.631.
98. Jiang, C., Zu, C., Lu, D., Zheng, Q., Shen, J. et al. (2017). Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Scientific Reports, 7(1), 42039. DOI 10.1038/srep42039.
99. Perveen, S., Nazir, M. (2018). Proline treatment induces salt stress tolerance in maize (Zea mays L. CV. Safaid Afgoi). Pakistan Journal of Botany, 50(4), 1265–1271.
100. Hoque, M. A., Okuma, E., Banu, M. N. A., Nakamura, Y., Shimoishi, Y. et al. (2007). Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. Journal of Plant Physiology, 164(5), 553–561. DOI 10.1016/j.jplph.2006.03.010.
101. Ranganayakulu, G. S., Veeranagamallaiah, G., Sudhakar, C. (2013). Effect of salt stress on osmolyte accumulation in two groundnut cultivars (Arachis hypogaea L.) with contrasting salt tolerance. African Journal of Plant Science, 7(12), 586–592. DOI 10.5897/AJPS11.063.
102. Yan, Z., Guo, S., Shu, S., Sun, J., Tezuka, T. (2011). Effects of proline on photosynthesis, root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. African Journal of Biotechnology, 10(80), 18381–18390.
103. Sadak, M. S., Mostafa, H. A. (2015). Physiological role of pre-sowing seed with proline on some growth, biochemical aspects, yield quantity and quality of two sunflower cultivars grown under seawater salinity stress. Scientia Agriculturae, 9(1), 60–69.
104. Banu, M. N. A., Hoque, M. A., Watanabe-Sugimoto, M., Matsuoka, K., Nakamura, Y. et al. (2009). Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. Journal of Plant Physiology, 166(2), 146–156. DOI 10.1016/j.jplph.2008.03.002.
105. Nounjan, N., Nghia, P. T., Theerakulpisut, P. (2012). Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. Journal of Plant Physiology, 169(6), 596–604. DOI 10.1016/j.jplph.2012.01.004.
106. Hossain, M. A., Fujita, M. (2010). Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiology and Molecular Biology of Plants, 16(1), 19–29. DOI 10.1007/s12298-010-0003-0.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |