DOI:10.32604/phyton.2020.011752

| Phyton-International Journal of Experimental Botany DOI:10.32604/phyton.2020.011752 |  |
| Article |
Effects of Barium Stress in Brassica juncea and Cakile maritima: The Indicator Role of Some Antioxidant Enzymes and Secondary Metabolites
1Laboratory of Resources, Materials and Ecosystems, Faculty of Sciences of Bizerte, University of Carthage, Jarzouna, Bizerte, 7021, Tunisia
2Centro de Recursos Naturais e Ambiente, Instituto Superior Técnico, Universidade de Lisboa, Lisbon, 1049-001, Portugal
3Instituto Português do Mare da Atmosfera, Rua Dr. Alfredo Magalhães Ramalho, Lisbon, 1495-006, Portugal
4Centro de Recursos Naturais e Ambiente, DBE, Instituto Superior Técnico, Universidade de Lisboa, Lisbon, 1049-001, Portugal
5Marine and Environmental Sciences Centre, Faculdade de Ciências, Universidade de Lisboa, Campo Grande, Lisbon, 1749-016, Portugal
*Corresponding Author: Noomene Sleimi. Email: noomene.sleimi@gmail.com
Received: 28 May 2020; Accepted: 01 September 2020
Abstract: Soil contamination by toxic trace metal elements, like barium (Ba), may stimulate various undesirable changes in the metabolic activity of plants. The plant responses are fast and with, direct or indirect, generation of reactive oxygen species (ROS). To cope with the stress imposed by the ROS production, plants developed a dual cellular system composed of enzymatic and non-enzymatic players that convert ROS, and their by-products, into stable nontoxic molecules. To assess the Ba stress response of two Brassicaceae species (Brassica juncea, a glycophyte, and Cakile maritime, a halophyte), plants were exposure to different Ba concentrations (0, 100, 200, 300 and 500 µM). The plants response was evaluated through their morphology and development, the determination of plant leaves antioxidant enzymatic activities and by the production of plants secondary metabolites. Results indicated that the two Brassicaceae species have the ability to survive in an environment containing Ba (even at 500 µM). The biomass production of C. maritima was slightly affected whereas an increase in biomass B. juncea was noticed. The stress imposed by Ba activated the antioxidant defense system in the two species, noticed by the changes in the leaves activity of catalase (CAT), ascorbate peroxidase (APX) and guaicol peroxidase (GPX), and of the secondary metabolites, through the production of total phenols and flavonoids. The enzymatic response was not similar within the two plant species: CAT and APX seem to have a more important role against the oxidative stress in C. maritima while in B. juncea is GPX. Overall, total phenols and flavonoids production was more significant in the plants aerial part than in the roots, of the both species. Although the two Brassicaceae species response was different, in both plants catalytic and non-catalytic transformation of ROS occurs, and both were able to overcome the Ba toxicity and prevent the cell damage.
Keywords: Brassica juncea; Cakile maritima; antioxidant enzymes; barium stress; oxidative stress; secondary metabolites
The increase in anthropogenic activities such as intensive agriculture, metallurgical industries, heavy traffic, atmospheric deposition, mining and dumping of waste generate an increase in the concentration of several toxic trace metal elements (TME) such as mercury, lead, cadmium, etc. beyond optimal levels in soil, water and air [1]. Pollution by TME presents a significant problem for ecological systems because they are not degraded by chemical or biological processes, which makes them difficult to eliminate from the environment [2]. The tolerance or sensitivity to abiotic stress depends on the type of stress, the duration of the stress, type of plant species as well as the stage of development of the plant [3].
Soil contamination by TME can induce many undesirable changes in the metabolic activity of plants. Barium (Ba) is one of these contaminants. Over the past 40 years, Ba has been used extensively in the industrial and medical fields, particularly in the petroleum industry, the steel industry and as an agent for radiography of the gastrointestinal tract (GI) [4]. Barium is considered the 14th most abundant element on earth’s crust. This element soil contents ranges between 265 and 835 µg.g−1 dry weight (DW), depending on the type of soil [5]. In fact, Ba has been reported to have several toxic effects on the environment [6] and can exist in the form of BaSO4, BaCO3, BaHCO3, BaNO3, BaCl2, BaCl+, BaOH+, BaF−, BaB(OH)4+, Ba2+ and Ba(CH3COO)2 [7]. Moreover, Ba is not considered essential for organisms and can be toxic for animals and plants in high concentrations [8]. The toxicity of Ba is linked to its solubility, the more soluble it is the more toxic it becomes.
It has been reported that as free ionic metal, Ba2+, Cd2+ and other metallic elements can modify plant performance from the subcellular level to the ecosystem level [9]. Similarly, the interaction of free ionic metals with cellular components can trigger a variety of metabolic responses in seconds, sometimes with direct or indirect generation of reactive oxygen species (ROS) [10].
One of the promptest consequences when plant cells are exposed to toxic concentrations of TME is the production of ROS, i.e., hydroxyl radicals (•OH) and superoxide (O•−2), as well as non-radicals, such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) [11] and are highly reactive and deleterious and could cause damage to nucleic acids, proteins and lipids [12]. However, plants develop several adaptation mechanisms in response to environmental stress. Plants have developed a dual cellular system composed of enzymatic and non-enzymatic players to recover/balance the ROS production [12]. The enzymatic mechanism comprises enzymes like superoxide dismutase (SOD), ascorbate and guaiacol peroxidase (APX, GPX), glutathione-S-transferases (GST) and catalase (CAT). The non-enzymatic mechanism is the non-enzymatic response regulating the production of polyamines, as well as low molecular weight compounds, such as reduced glutathione (GSH), vitamins (such as ascorbic acid and α-tocopherol), osmolytes (like proline) and secondary metabolites, notably flavonoids, phenolics and carotenoids [13]. Indeed, the activation of cells antioxidant system is a key element in the plants tolerance to metals [14]. The accumulation of secondary metabolites in plants generally occurs under different types of stress [15] such us TME which will be chelated by secondary metabolites [16].
Lajayer et al. [17] reported that following exposure to TME stress, the production of secondary metabolites increased. Among these secondary metabolites are phenols and flavonoids. Enhanced biosynthesis of phenolics helps protecting plants from oxidative stress after exposure to metallic stress [18–20]. Phenolics can chelate heavy metals [21]. Several studies have reported the increase in total phenols in Brassica juncea following exposure to Cu [22], Cr [18], Cd [23], Pb [24] and in Fagopyrum esculentum exposed to Al [25] as well as in Zea mays following exposure to Cu, Pb and Cd [26]. Flavonoids have been reported to enhance the process of metal chelation, which helps in reducing the levels of harmful hydroxyl radicals in plant cells [27], and it has been observed that flavonoid levels in plants have found to be enhanced by an excess of metal [20,28]. However, the flavonoid content was increased in Brassica juncea exposed to Pb [19,24] and in Withania somnifera exposed to Cd [29].
In this sense, the aim of this study was to evaluate the stress response of two Brassicaceae (Brassica juncea and Cakile maritima) after Ba exposure. The effect of the different Ba concentrations on plants morphology and development, enzymatic activity and the production of secondary metabolites have been studied.
Seeds of Brassica juncea and Cakile maritima were disinfected with calcium hypochlorite for 15 minutes. The disinfected seeds were rinsed thoroughly and soaked in distilled water for 2 h. The seeds were then placed in plastic pots containing a mixture of perlite/gravel (2:1, v/v) as a substrate and were daily irrigated with tap water. After germination, the seedlings were irrigated with a Hewitt [30] nutritive solution (pH was between 6.5 and 7) until the beginning of treatment. After 4 weeks, the uniform plants were selected for the experiments and divided into 5 groups of twelve plants for each species. The plants were exposed regularly (3 times a week) to different concentrations of Ba supplied as BaCl2 (0, 100, 200, 300 and 500 µM) added into the nutrient solution. Experiments were performed in a greenhouse under semi-controlled conditions with a natural photoperiod (14 h light), mean temperature 25 ± 5°C, and relative humidity between 60 and 90% (Fig. 1).

Figure 1: Photo of the culture at beginning of treatment (Brassica juncea (A), Cakile maritima (B)) and at end of treatment (Brassica juncea (C))
After 45 days, the plants were separated into 2 lots of 6 plants. The first lot was used for the measurement of the enzyme activities. Shoots (stem and leaves) were frozen in liquid nitrogen and stored at −80°C. For the second batch, shoots were separated from the roots. To remove traces of metal adsorbed on the root’s surfaces, these organs were dipped in a cold solution of CaCl2 for 5 min [31] and afterwards rinsed 3 times with cold distilled water. The fresh weight (FW) was immediately determined and dry weight (DW) was measured after oven drying at 60°C until constant weight.
The tolerance index (TI), which is a measure of the tolerance of the plant to TME, was determined by comparing the dry biomass of plants subjected to metal treatment with the control as described by Sleimi [32]:
Tolerance index = (Biomass of treated plants/Biomass of control plant) × 100.
Leaves (0.4 g) were ground in a mortar, in 2 mL of potassium phosphate buffer (pH 7.0), containing Na-ascorbate (5 mM) and EDTA (0.2 mM). The mixture was filtered through four layers of mir-a clothon and centrifuged at 6000 rpm for 15 min at 4°C. The resulting supernatant was used as a crude enzyme extract. The activities of CAT (EC1.11.1.6), GPX (EC1.11.1.7) and APX (EC1.11.1.11) were determined according to Aebi [33], Fielding et al. [34] and Nakano et al. [35], respectively. The details of methods were described in detail by Sleimi et al. [36].
2.4 Total Phenols and Flavonoids Level Assay
Approximately 30 mg of dry matter (shoots/roots) was added to 10 mL a methanol/water mixture (80/20; v/v). The macerate was kept overnight, centrifuged at 2500 rpm for 30 min and then filtered. For the determination of polyphenols, the method of Velioglu et al. [37] was followed. A 100 µl of the macerate was mixed with 500 µL of Folin-Ciocalteu. After 5 min of incubation in the dark, 400 µl of Na2CO3 at 7.5% was added. After 90 min incubation in the dark and at room temperature, the mixture was centrifuged at 5000 rpm for 3–4 min and the supernatant absorbance was measured at 765 nm. Gallic acid was used as standard reference for the quantitative estimation. The dosage of flavonoids was determined from the spectrometric method adapted by Quettier-Deleu et al. [38]. To 500 µl of macerate, 500 µl of freshly prepared methanoic solution of 2% AlCl3 was added and incubate for 30 min. The measurement was done at 430 nm. Rutin was used as standard reference for the quantitative estimation.
All data presented are the mean values of 6 replicates, which are presented along with the standard error (±) in bars in the figures and table. The effect of TME on the variability of the response parameters was assessed using regression analyses and One-way ANOVA. Statistical analyses were done using Statistica 8. Tukey’s HSD test was performed to define which specific mean pairs were significantly different (p < 0.05).
The Ba effects on the development of Brassica juncea and Cakile maritima were evaluated based on the production of fresh biomass and are presented in Tab. 1. Results showed an opposite effect of Ba on the production of fresh biomass in these two Brassicaceae. In fact, the used Ba concentrations promoted the growth of B. juncea, whereas for C. maritima a decrease in the production of fresh biomass was observed. For B. juncea an increase in the production of fresh biomass was noticed in the whole plant. It varied from 8677 mg in the control to 13488 mg for 100 µM of Ba and 9568 mg for the high concentration (500 µM) (Tab. 1).
Table 1: Fresh weight (shoots, roots and whole plant), shoots-roots ratio (S/R) and tolerance index (TI) of B. juncea and C. maritma in response to different concentrations of Ba. Values with different letters are significantly different at p ≤ 0.05
For the roots, the plants treated with 100 μM Ba showed the highest biomass compared to the control. An increase of 2.51 fold was noticed. Even with the highest concentrations, there was an increase in the production of root fresh biomass.
The variation of the fresh biomass of C. maritima exposed to the different concentrations of Ba showed a decrease in the shoots biomass. For the whole plant, the production decreased with increasing the concentration (100, 200, 300 µM) but it increased again with 500 µM of Ba. However, roots showed an increase in the production of fresh biomass. The highest value was 1339 mg with 500 µM and 1074 mg in the control. Nevertheless, the deviations observed compared to the control were not significant (Tab. 1).
The TI values decreased in C. maritima in the aerial parts, showing a decrease in the biomass. Whereas, B. juncea noted an increase in TI in the aerial parts and the roots.
The CAT, GPX and APX activities (expressed in Δ OD.min−1.g−1) are presented in the Figs. 2, 3 and 4 respectively.

Figure 2: Catalase (CAT) activity in the leaves of B. juncea (A) and C. maritima (B) exposed to barium (Ba). Bars marked with different letters are significantly different at p < 0.05

Figure 3: Ascorbate Peroxidase (APX) activity in the leaves of B. juncea (A) and C. maritima (B) exposed to barium (Ba). Bars marked with different letters are significantly different at p < 0.05

Figure 4: Guaiacol Peroxidase (GPX) activity in the leaves of B. juncea (A) and C. maritima (B) exposed to barium. Bars marked with different letters are significantly different at p < 0.05
The exposure of 200, 300 and 500 μM of Ba decreased the CAT activity in the leaves of B. juncea, reaching almost half in the 500 µM comparing to the control. No significant differences were found between the control plants (79 Δ OD.min−1.g−1) and those treated with 100 µM Ba (80 Δ OD.min−1.g−1) (Fig. 2A).
Unlike B. juncea, CAT activity in C. maritime leaves increased significantly from 51, in the control, to 158, 129 and 145 Δ OD.min−1.g−1 in the groups 200, 300 and 500 µM, respectively, while the group exposed to 100 µM of Ba (35 Δ OD.min−1.g−1) showed no significant differences relative to the control (Fig. 2B).
Generally, treatment of B. juncea with Ba did not affect APX activity. A slight increase in APX activity was observed in the 300 and 500 µM groups, but not statistically significant (p < 0.05) as shown in Fig. 3A. All the Ba concentrations used stimulated the APX activity compared to the control in C. maritime leaves (Fig. 3B). APX activity showed no statistically significant differences among the groups treated with Ba (p < 0.05), with mean values of 0. 09 Δ OD.min−1.g−1 for the control group and 0.18, 0.17, 0.17 and 0.18 Δ OD.min−1.g−1 for the 100, 200, 300 and 500 µM groups, respectively.
GPX activity showed an increase with all Ba concentrations in B. juncea leaves except in the 500 µM group. The highest activity was noted in the leaves of plants exposed to 100 and 300 µM, while the lowest GPX activity was observed in the 500 µM group (2.7 Δ OD.min−1.g−1) (Fig. 4A). In C. maritima, GPX activity did not show any variation following the treatment with Ba, with the exception of treatment with 100 μM. The addition of 100 μM of Ba decreased the GPX activity from 0.9 in the control to 0.46 Δ OD.min−1.g−1.
3.3 Total Phenols and Flavonoids Level
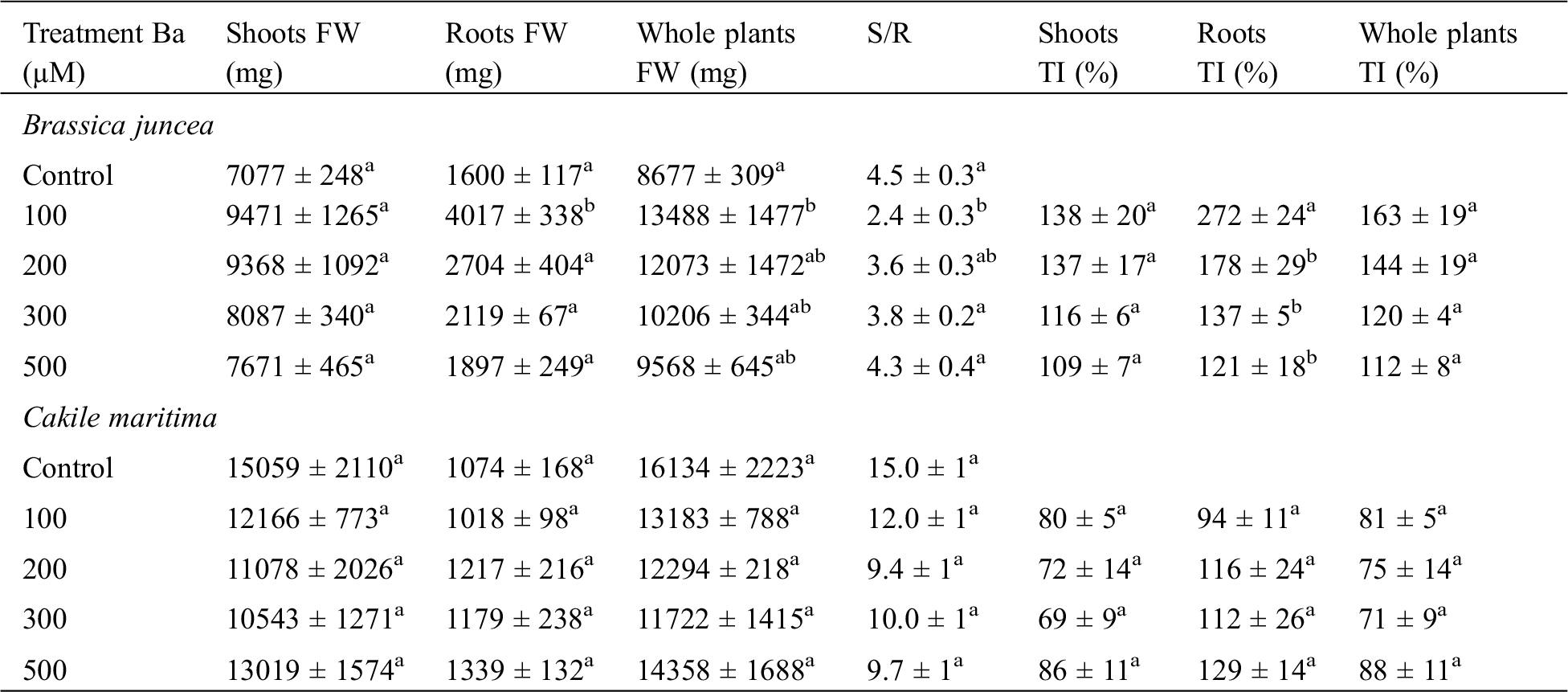
The addition of Ba to the irrigation solution induced a significant increase in the production of total phenols (TP) in the aerial parts of both plants treated (Fig. 5). The production of TP in response to stress by Ba showed a clear difference between shoots and roots. It increased from 7.5 in the control to 18 mg.g−1 (DW) with the highest concentration of Ba in B. juncea shoot. On the other hand, when compared with the control, no significant variation (p ≤ 0.05) was observed in the production of TP in B. juncea roots.
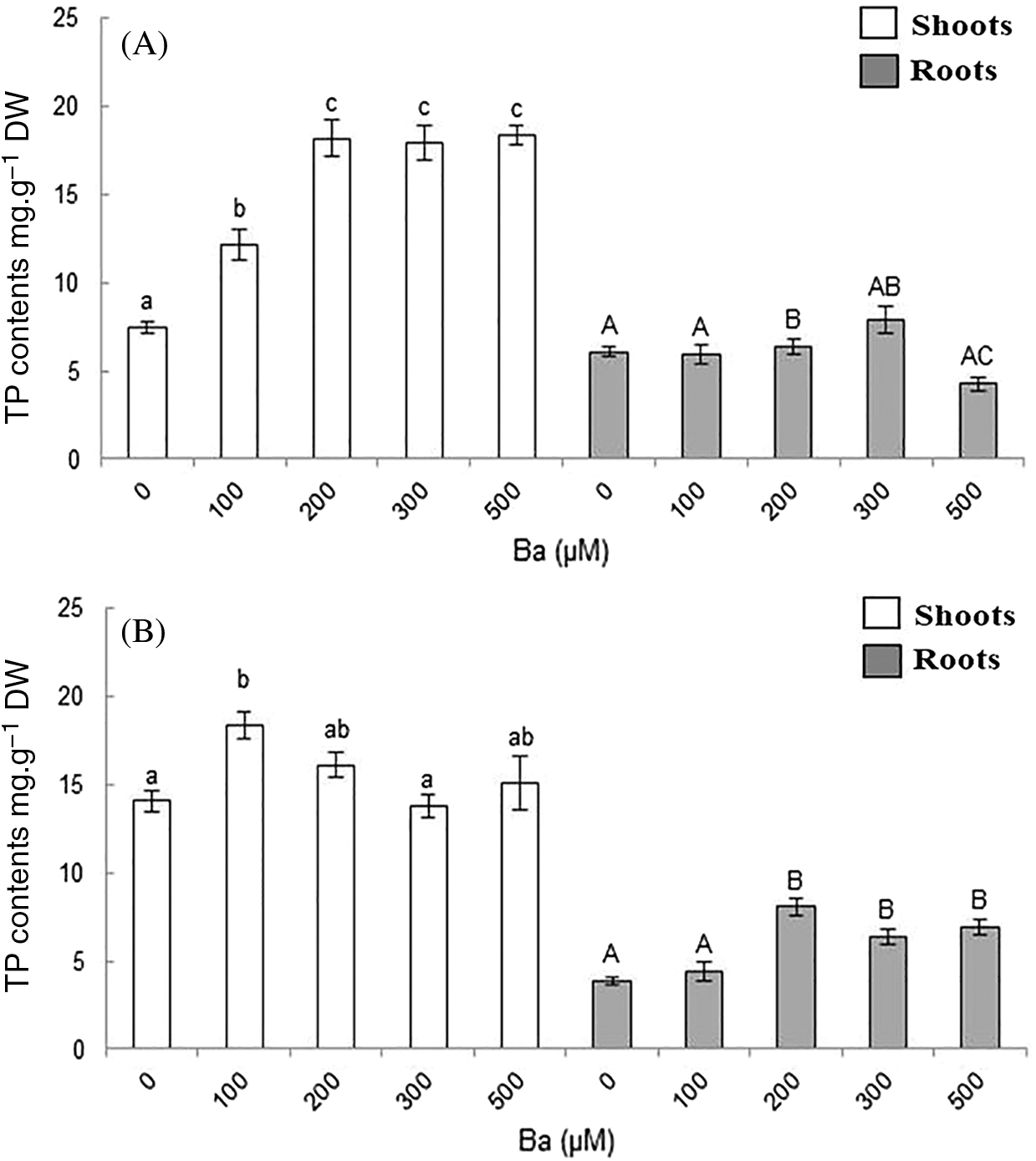
Figure 5: Total phenols (TP) content in shoots and roots in B. juncea (A) and C. maritima (B) subjected to barium (Ba) stress. Values with different letters are significantly different at p ≤ 0.05
Results showed that there was, in general, an increase in the TP content in the aerial parts of C. maritima. Except with the 300 µM concentration, which showed no variation comparing to the control. The maximum production (18 mg.g−1 DW) was noted in the plants treated with 100 µM of Ba.
For roots of C. maritima, with the exception of the 100 µM of Ba, a stimulation of TP production was observed with the increase of Ba concentration in the irrigation solution (Fig. 5B). Values observed were 3.84, 8.1, 6.36 and 6.94 mg.g−1 DW in the control, 200 µM, 300 µM and 500 µM, respectively.
The variation of flavonoids content was considered non-significant in B. juncea (Fig. 6A). In the aerial parts, the highest content was observed with 200 μM of Ba (12 mg.g−1 DW) comparing to the control (9.4 mg.g−1 DW). Flavonoids production did not show any significant variation in the roots of B. juncea compared to the control (Fig. 6A).
Figure 6: Variation of flavonoid content in the shoots and roots in B. juncea (A) and C. maritima (B) treated with different concentrations of barium (Ba). Values with different letters are significantly different at p ≤ 0.05
The treatment of C. maritima with Ba showed that the concentration 100 µM stimulated the production of flavonoids in the plant. The flavonoids content increased from 9.1 to 12 mg.g−1 DW in the aerial parts, and it decreased with the other concentrations compared to the control. In the roots, the values vary from 1.4 in the control to 1.6 mg.g−1 DW with 100 μM. The values were lower than the control for the other doses.
Information on the toxicity of Ba to plants is limited. However, many studies [39,40] suggest that Ba is toxic to plants. In the present study, we tested the effect of 100, 200, 300 and 500 µM of Ba on plant growth, some antioxidant enzymes activity response and the production of secondary metabolites on B. juncea and C. maritima. The results indicate that B. juncea tolerates Ba better than C. maritime, based on plant biomass.
Scientists consider the biomass of shoots as a determinant of the plant’s capacity to tolerate the stress of TME [41]. The fresh biomass of aerial parts of two brassicaceae studied were not affected in the same way under Ba stress. Comparing with the control, the shoots biomass of B. juncea increased even with the highest concentration of Ba (500 µM), while C. maritima has shown a decrease in its fresh biomass. But it should be mentioned that statistically, this decrease was not significant. The effect of Ba on the development of plants may vary depending on the species. It has been reported that Ba caused a reduction in the production of bean biomass [39] and soybeans [40]. On the other hand, Coscione et al. [42], with concentrations up to 300 mg.Kg−1 of Ba, found no toxic effect on plants cultivated in a rhodic hapludox.
Our results showed that all the concentrations of Ba used in the assays had a positive effect on the development of B. juncea mainly at 100 and 200 µM. Suwa et al. [40] noted that 100 µM Ba significantly inhibited growth and photosynthetic activity of soybean leaves. They explained the negative effect of Ba on reduced plant growth by reduced assimilation of CO2 caused by limited photosynthetic activity.
Both B. juncea and C. maritima were able to maintain good growth and survive under Ba stress. Our results are supported by previous studies which reported that B. juncea [43] and C. maritima [44] are capable of growing in soils contaminated with heavy metals. These authors reported that B. juncea tolerated and even grew well at high doses of zinc, while C. maritima is a cadmium tolerant plant. The tolerance of plants to TME may be linked to the development of mechanisms such as the sequestration of metals in tissues or cells compartments [45], which implies a restriction of the two upward movements in the shoots (avoidance mechanism) and translocation of excess metals into the leaves [46].
The purpose of TI is to characterize plant tolerance. Ghosh et al. [47] defined the plant’s TI by its capacity to grow well and to tolerate a large amount of concentration of metals. The TI values of B. juncea showed an increase in the biomass of the aerial parts and of the roots, thus proving tolerance to Ba, whereas in C. maritima, the TI showed a decrease, except with 200, 300 and 500 µM at the level of the roots. Despite this decrease, C. maritima survived until the end of treatment. These results suggest that B. juncea tolerates Ba better than C. maritima.
In response to abiotic stress, plants typically produce ROS [48]. ROS are rapidly detoxified by cellular enzymes which can mitigate the caused oxidative damage. In our study, CAT was clearly activated in the leaves of C. maritima since its activity showed a clear increase with the high Ba doses used. Our results are in agreement with Melo et al. [49] who observed an increase in this enzyme in Glycine max. L. stressed by Ba. It was clear that this increase in the activity of CAT has been triggered in response to a production of ROS induced by Ba stress. But we did not obtain the same response of CAT in B. juncea. Ba treatment decreased CAT activity except with the low dose (100 µM). This was probably due to the effect of high Ba concentrations. Rastgoo et al. [50] reported that CAT was not able to protect cells against ROS under high concentration of TME. The decrease in some enzyme activities at high concentrations of TME may be due to an imbalance in metabolism and the generation of ROS [51].
The APX activity showed the same reaction to metallic stress in the two Brassicaceae. Nonetheless, the APX activity increased slightly but not significantly in B. juncea, in response to all the concentrations of Ba used, except with 200 μM in B. juncea. Whereas in C. maritima, a significant increase in APX activity was noticed for all Ba concentrations. In fact, APX and GPX, powerful H2O2 scavengers, seem to maintain its levels because the uncontrolled export of this toxic ROS from organelles to the cytosol can have an undesirable effect due to the formation of hydroxyl radicals by the Haber-Weiss reaction catalyzed by a metal [52].
The accumulation of TME in plants generates an inhibition or stimulation of the activity of various antioxidant enzymes [53]. A clear decrease in GPX activity was observed with 100 µM in C. maritima. However, GPX activity increased following Ba stress, mainly with 100 and 300 µM in B. juncea. Also, Al Mahmud et al. [54] reported that GPX and APX activity had increased in B. juncea subjected to stress by Cd. Therefore, results indicate that a high activity of CAT and APX can serve as a better defense tool to resist the oxidative stress induced by Ba in C. maritime, while in B. juncea, the APX and GPX activity play the defense role against oxidative stress.
The difference in the activity of antioxidant enzymes in plants under environmental stress such as TME, depends on the species (physiological and genetic potential of the plant), the duration of treatment as well as the concentration of TME [55]. Stress caused by TME can lead an increase in ROS production, which interacts with various molecules, leading to lipid peroxidation and damage to the cell membrane system [52].
Phenols have the ability to slow down the formation of free radicals [56]. The metal chelating and ROS scavenging properties of phenols and complex forming property of flavonoids protects the plants from the effects of TME [57]. Metal ions including nickel, lanthanum, europium, silver and cadmium, and oxalate have been influenced the secondary metabolite production in various plants [58]. Our results show a significant increase in the TP content in B. juncea leaves with 500 μM of Ba. In fact, a 2.45 fold increase of the TP content in the aerial parts occurred compared to the control. In the root, TP content had a slight increase followed by a decrease with the highest concentration of Ba (500 µM). The increase in TP content under metal stress has been mentioned in other studies. Handa et al. [59] found that the content of total polyphenols was significantly increased in B. juncea under the influence of Cr (an increase of 44.9% in level of total phenols was observed in Cr treated plants compared to control).
Also our results are in agreement with earlier works conducted on Lactuca sativa and Brassica napus treated with waste water contaminated by TME where the contents of phenols were increased [60]. Lycopersicon esculentum subjected to Cu stress were also reported to have enhanced levels of phenols [61]. Under Ba stress, the aerial parts of C. maritima did not show a significant increase in TP content. This can be explained by the fact that this halophyte used other defense mechanisms against the stress caused by Ba. However, the TP content was significantly increased at roots compared to the control.
The role of secondary metabolites in counteracting ROS stress is well documented [62]. In plants, phenolic compounds have a special chemical structure in plants which protect cells against oxidative stress by chelating metals and binding to free radicals accompanied by less lipid peroxidation [57]. Ma et al. [63] reported that there is a direct correlation between TME and the expression of phenylalanine ammonia lyase (PAL), the first gene intervening in the phenylpropanoid pathway. PAL is a key enzyme in the biosynthesis of phenolic compounds such as flavonoids and anthocyanins, which enhances the increase in phenolics and flavonoids in response to certain biotic and abiotic stresses [64]. These data may explain the accumulation of phenolic acids in plant cells stressed by TME. Handa et al. [59] reported that enhanced expression of PAL could be the reason for increased accumulation of total phenols and flavonoids in B. juncea plants under Cr stress.
Flavonoids inhibit lipid peroxidation and improve the stability and fluidity of the membrane, preventing the release of ROS and the inhibition of peroxidation reactions [65]. In our study, only with 200 µM of Ba, a significant increase in the flavonoid content was observed, in relation to the control, in the aerial parts of B. juncea. Even in the root, treatment with Ba did not modify the production of flavonoids. Generally, the same behavior was observed in C. maritima stressed by the Ba. Only a significant variation in flavonoid content in the aerial parts was noted with 100 µM of Ba, compared to the control, while there is a significant decrease with 300 and 500 μM in the roots. These results are in agreement with the studies carried out by Babula et al. [66]. In their work they observed a decrease in flavonoids in the shoots and roots of Hyperium perforatum in response to the stress of lanthanum and cadmium. Berni et al. [67] explained the non-intervention of flavonoids in the defense against oxidative stress in these studies, by the fact that plants can rather invest first in the pathway to produce phenolic acids (hydroxycinnamic acids) and do not activate genes involved in the following steps (which lead to the formation of flavonoids and anthocyanins) to save energy, while being able to counter stress via phenolic compounds.
Generally, the application of stress by the Ba on the two plants has helped to activate the anti-oxidant defense system: Enzymatic system (increase in the activity of enzymes like CAT, APX and GPX) and non-enzymatic system (production of TP and flavonoids). The production of antioxidant enzymes and certain secondary metabolites may present a defense mechanism for these two brassicaceae against metal stress.
C. maritima and B. juncea, can cope and survive in an environment containing Ba. Although biomass production of C. maritima was slightly affected by Ba, B. juncea showed an increase in biomass. It seems that B. juncea tolerates Ba better than C. maritima. The ability of two brassicaceae to survive in an environment contaminated with this element may be linked to the development of some defense mechanisms against stress induced by TME, such as, the stimulation of the activity of some antioxidant enzymes as well as the production of secondary metabolites. However, the study of other tolerance parameters such as lipid peroxidation and the measurement of toxicity parameters (e.g., endogenous H2O2 and Hydroxyl radicals) may help to better understand the mechanism of tolerance and/or adaptation of the two brassicaceae species to Ba stress.
Funding Statement: This work has been supported by LISBOA-01-0145-FERDER-031863 project, co-funded by FEDER through POR Lisboa 2020 (Programa Operacional de Lisboa) from Portugal 2020 and Fundação para a Ciência e a Tecnologia (PTDC/CTA-AMB/31863/2017). The authors also gratefully acknowledge the support of CERENA (strategic project FCT-UIDB/04028/2020) and MARE (strategic project FCT-UIDB/04292/2020).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Mahajan, M., Kuiry, R., Pal, P. K. (2020). Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. Journal of Applied Research on Medicinal and Aromatic Plants, 18, 100255 (In press).
2. Huo, Y., Pyo Kang, J., Chan Ahn, J., Ju Kim, Y., Piao, C. H. et al. (2020). Siderophore-producing rhizobacteria reduce heavy metal-induced oxidative stress in Panax ginseng Meyer. Journal of Ginseng Research (In press). DOI 10.1016/j.jgr.2019.12.008. [Google Scholar] [CrossRef]
3. Mahawar, L., Shekhawat, G. S. (2019). EsHO 1 mediated mitigation of NaCl induced oxidative stress and correlation between ROS, antioxidants and HO 1 in seedlings of Eruca sativa: Underutilized oil yielding crop of arid region. Physiology and Molecular Biology of Plants, 25(4), 895–904. DOI 10.1007/s12298-019-00663-7. [Google Scholar] [CrossRef]
4. Kravchenko, J., Darrah, T. H., Miller, R. K., Lyerly, H. K., Vengosh, A. (2014). A review of the health impacts of barium from natural and anthropogenic exposure. Environmental Geochemistry and Health, 36(4), 797–814. DOI 10.1007/s10653-014-9622-7. [Google Scholar] [CrossRef]
5. Lide, D. R. (2005). CRC handbook of chemistry and physics, pp. 4–51. New York: CRC Press. [Google Scholar]
6. Lu, Q., Xua, X., Liang, L., Xua, Z., Shang, L. et al. (2019). Barium concentration, phytoavailability, and risk assessment in soil-rice systems from an active barium mining region. Applied Geochemistry, 106, 142–148. DOI 10.1016/j.apgeochem.2019.05.010. [Google Scholar] [CrossRef]
7. McGrath, M., Davison, W., Hamilton-Taylor, J. (1989). Biogeochemistry of barium and strontium in a softwater lake. Science of the Total Environment, 87–88(1), 287–295. DOI 10.1016/0048-9697(89)90242-8. [Google Scholar] [CrossRef]
8. Lamb, D. T., Matanitobua, V. P., Palanisami, T., Megharaj, M., Naidu, R. (2013). Bioavailability of barium to plants and invertebrates in soils contaminated by barite. Environmental Science & Technology, 47(9), 4670–4676. DOI 10.1021/es302053d. [Google Scholar] [CrossRef]
9. Ernst, W. H. O., Verkleij, J. A. C., Schat, H. (1992). Metal tolerance in plants. Acta Botanica Neerlandica, 41(3), 229–248. DOI 10.1111/j.1438-8677.1992.tb01332.x. [Google Scholar] [CrossRef]
10. Babu, T. S., Marder, J. B., Tripuranthakam, S., Dixon, D. G., Greenberg, B. M. (2001). Synergistic effects of a photooxidized polycyclic aromatic hydrocarbon and copper on photosynthesis and plant growth: Evidence that in vivo formation of reactive oxygen species is a mechanism of copper toxicity. Environmental Toxicology and Chemistry, 20(6), 1351–1358. DOI 10.1002/etc.5620200626. [Google Scholar] [CrossRef]
11. Tamás, L., Mistrík, I., Zelinová, V. (2017). Heavy metal-induced reactive oxygen species and cell death in barley root tip. Environmental and Experimental Botany, 140, 34–40. DOI 10.1016/j.envexpbot.2017.05.016. [Google Scholar] [CrossRef]
12. Gill, S. S., Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930. DOI 10.1016/j.plaphy.2010.08.016. [Google Scholar] [CrossRef]
13. Akram, N. A., Shafiq, F., Ashraf, M. (2017). Ascorbic acid-A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Frontiers in Plant Science, 8, 613. DOI 10.3389/fpls.2017.00613. [Google Scholar] [CrossRef]
14. Mahawar, L., Kumar, R., Shekhawat, G. S. (2018). Evaluation of heme oxygenase 1 (HO 1) in Cd and Ni induced cytotoxicity and crosstalk with ROS quenching enzymes in two to four leaf stage seedling of Vigna radiate. Protoplasma, 255(2), 527–545. DOI 10.1007/s00709-017-1166-0. [Google Scholar] [CrossRef]
15. Hatami, M., Ghorbanpour, M. (2016). Changes in phytochemicals in response to rhizospheric microorganism infection. In: Choudhary, D. K., Varma, A., (eds.) Microbial Mediated Induced Systemic Resistance in Plants, pp. 1–14. Springer, Singapore. DOI 10.1007/978-981-10-0388-2_1. [Google Scholar] [CrossRef]
16. Eghbaliferiz, S., Iranshahi, M. (2016). Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: updated review of mechanisms and catalyzing metals. Phytotherapy Research, 30(9), 1379–1391. DOI 10.1002/ptr.5643. [Google Scholar] [CrossRef]
17. Lajayer, H. A., Savaghebi, G., Hadian, J., Hatami, M., Pezhmanmehr, M. (2017). Comparison of copper and zinc effects on growth, micro-and macronutrients status and essential oil constituents in pennyroyal (Mentha pulegium L.). Brazilian Journal of Botany, 40(2), 379–388. DOI 10.1007/s40415-016-0353-0. [Google Scholar] [CrossRef]
18. Handa, N., Kohli, S. K., Sharma, A., Thukral, A. K., Bhardwaj, R. et al. (2019). Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. Environmental and Experimental Botany, 161, 180–192. DOI 10.1016/j.envexpbot.2018.11.009. [Google Scholar] [CrossRef]
19. Kohli, S. K., Handa, N., Sharma, A., Gautam, V., Arora, S. et al. (2018). Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environmental Sciences and Pollution Research, 25(15), 15159–15173. DOI 10.1007/s11356-018-1742-7. [Google Scholar] [CrossRef]
20. Kaur, R., Yadav, P., Sharma, A., Kumar Thukral, A., Kumar, V. et al. (2017). Castasterone and citric acid treatment restores photosynthetics attributes in Brassica juncea L. under Cd (II) toxicity. Ecotoxixology and Environmental Safety, 145, 466–475. DOI 10.1016/j.ecoenv.2017.07.067. [Google Scholar] [CrossRef]
21. Sharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M. et al. (2019). Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules, 24(13), 2452. DOI 10.3390/molecules24132452. [Google Scholar] [CrossRef]
22. Poonam, R. K., Bhardwaj, R., Sirhindi, G. (2015). Castasterone regulated polyphenolic metabolism and photosynthetic system in Brassica juncea plants under copper stress. Journal of Pharmacognosy and Phytochemistry, 4, 282–289. [Google Scholar]
23. Kaur, P., Bali, S., Sharma, A., Vig, A. P., Bhardwaj, R. (2018). Role of earthworms in phytoremediation of cadmium (Cd) by modulating the antioxidative potential of Brassica juncea L. Applied Soil Ecology, 124, 306–316. DOI 10.1016/j.apsoil.2017.11.017. [Google Scholar] [CrossRef]
24. Kohli, S. K., Handa, N., Sharma, A., Kumar, V., Kaur, P. et al. (2017). Synergistic effect of 24-epibrassinolide and salicylic acid on photosynthetic efficiency and gene expression in Brassica juncea L. under Pb stress. Turkish Journal of Biology, 41, 943–953. DOI 10.3906/biy-1707-15. [Google Scholar] [CrossRef]
25. Smirnov, O. E., Kosyan, A. M., Kosyk, O. I., Taran, N. Y. (2015). Response of phenolic metabolism induced by aluminium toxicity in Fagopyrum esculentum moench. plants. Ukrainian Biochemical Journal, 87(6), 129–135. DOI 10.15407/ubj87.06.129. [Google Scholar] [CrossRef]
26. Kısa, D., Elmastaş, M., Öztürk, L., Kayır, Ö. (2016). Responses of the phenolic compounds of Zea mays under heavy metal stress. Applied Biological Chemistry, 59(6), 813–820. DOI 10.1007/s13765-016-0229-9. [Google Scholar] [CrossRef]
27. Williams, R. J., Spencer, J. P., Rice-Evans, C. (2004). Flavonoids: Antioxidants or signalling molecules? Free Radical Biology and Medicine, 36(7), 838–849. DOI 10.1016/j.freeradbiomed.2004.01.001. [Google Scholar] [CrossRef]
28. Handa, N., Kohli, S. K., Sharma, A., Thukral, A. K., Bhardwaj, R. et al. (2018). Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. South African Journal of Botany, 119, 1–10. DOI 10.1016/j.sajb.2018.08.003. [Google Scholar] [CrossRef]
29. Mishra, B., Sangwan, N. S. (2019). Amelioration of cadmium stress in Withania somnifera by ROS management: Active participation of primary and secondary metabolism. Plant Growth Regulation, 87(3), 403–412. DOI 10.1007/s10725-019-00480-8. [Google Scholar] [CrossRef]
30. Hewitt, E. J. (1966). Sand and water culture methods used in study of plant nutrition. Common Wealth Bureau of Horticulture Technical Communication, 22, 547. [Google Scholar]
31. Stolt, J. P., Sneller, F. E. C., Brynelsson, T., Lundborg, T., Schat, H. (2003). Phytochelatin and cadmium accumulation in wheat. Environmental and Experimental Botany, 49(1), 21–28. DOI 10.1016/S0098-8472(02)00045-X. [Google Scholar] [CrossRef]
32. Sleimi, N., Abdelly, C. (2003). Salt tolerance strategy of two fodder halophytes species: Spartina alterniflora and Suaeda fruticosa. In: Lieth, H., Mochtchenko, M., (eds.) Cash Crop Halophytes, pp. 79–85. Netherland: Kluwer Academic Publishers. [Google Scholar]
33. Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126. [Google Scholar]
34. Fielding, J. L., Hall, J. L. (1978). A biochemical and cytochemical study of peroxidase activity in roots of Pisum sativum. Journal of Experimental Botany, 29(4), 969–981. DOI 10.1093/jxb/29.4.969. [Google Scholar] [CrossRef]
35. Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880. [Google Scholar]
36. Sleimi, N., Guerfali, S., Bankaji, I. (2015). Biochemical indicators of salt stress in Plantago maritima: Implications for environmental stress assessment. Ecological Indicators, 48, 570–577. DOI 10.1016/j.ecolind.2014.08.035. [Google Scholar] [CrossRef]
37. Velioglu, Y. S., Mazza, G., Gao, L., Oomah, B. D. (1998). Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of Agricultural and Food Chemistry, 46(10), 4113–4117. DOI 10.1021/jf9801973. [Google Scholar] [CrossRef]
38. Quettier-Deleu, C., Gressier, B., Vasseur, J., Dine, T., Brunet, C. et al. (2000). Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and four. Journal of Ethnopharmacology, 72(1–2), 35–42. DOI 10.1016/S0378-8741(00)00196-3. [Google Scholar] [CrossRef]
39. Lugany, M., Poschenrieder, C., Barceló, J. (2000). Assessment of barium toxicity in bush beans. Archives of Environmental Contamination and Toxicology, 39(4), 440–444. DOI 10.1007/s002440010125. [Google Scholar] [CrossRef]
40. Suwa, R., Jayachandran, K., Nguyen, N. T., Boulenouar, A., Fujita, K. et al. (2008). Barium toxicity effects in soybean plants. Archives of Environmental Contamination and Toxicology, 55(3), 397–403. DOI 10.1007/s00244-008-9132-7. [Google Scholar] [CrossRef]
41. Gurajala, H. K., Cao, X., Tang, L., Ramesh, T. M., Lu, M. et al. (2019). Comparative assessment of Indian mustard (Brassica juncea L.) genotypes for phytoremediation of Cd and Pb contaminated soils. Environmental Pollution, 254, 113085. DOI 10.1016/j.envpol.2019.113085. [Google Scholar] [CrossRef]
42. Coscione, A. R., Berton, R. S. (2009). Barium extraction potential by mustard, sunflower and castor bean. Scientia Agricola, 66(1), 59–63. DOI 10.1590/S0103-90162009000100008. [Google Scholar] [CrossRef]
43. Chaudhry, H., Nisar, N., Mehmood, S., Iqbal, M., Arif Nazir, A. et al. (2020). Indian Mustard Brassica juncea efficiency for the accumulation, tolerance and translocation of zinc from metal contaminated soil. Biocatalysis and Agricultural Biotechnology, 23, 101489. DOI 10.1016/j.bcab.2019.101489. [Google Scholar] [CrossRef]
44. Taamalli, M., Ghabrichea, R., Amari, T., Mnasri, M., Zolla, L. et al. (2014). Comparative study of Cd tolerance and accumulation potential between Cakile maritima L. (halophyte) and Brassica juncea L. Ecological Engineering, 71, 623–627. [Google Scholar]
45. Weis, J. S., Weis, P. (2004). Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environment International, 30(5), 685–700. DOI 10.1016/j.envint.2003.11.002. [Google Scholar] [CrossRef]
46. Verkleij, J. A., Schat, H. (1990). Mechanism of metal tolerance in higher plants. In: Show, J. (ed.Evolutionary Aspects of heavy metal tolerance in plants, pp. 179–193. CRC Press, Boca Raton, FL. [Google Scholar]
47. Ghosh, M., Singh, S. P. (2005). A comparative study of cadmium phytoextraction by accumulator and weed species. Environmental Pollution, 133(2), 365–371. DOI 10.1016/j.envpol.2004.05.015. [Google Scholar] [CrossRef]
48. Jaspers, P., Kangasjärvi, J. (2010). Reactive oxygen species in abiotic stress signaling. Physiologia Plantarum, 138(4), 405–413. DOI 10.1111/j.1399-3054.2009.01321.x. [Google Scholar] [CrossRef]
49. Melo, L. C. A., Alleoni, L. R. F., Carvalho, G., Azevedo, R. A. (2011). Cadmium- and barium-toxicity effects on growth and antioxidant capacity of soybean (Glycine max L.) plants, grown in two soil types with different physicochemical properties. Journal of Plant Nutrition and Soil Science, 174(5), 847–859. DOI 10.1002/jpln.201000250. [Google Scholar] [CrossRef]
50. Rastgoo, L., Alemzadeh, A. (2011). Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Australian Journal of Crop Science, 5, 375–383. [Google Scholar]
51. Rai, V., Vajpayee, P., Singh, S. N., Mehrotra, S. (2004). Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Science, 167(5), 1159–1169. DOI 10.1016/j.plantsci.2004.06.016. [Google Scholar] [CrossRef]
52. Singh, S., Eapen, S., D’Souza, S. F. (2005). Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere, 62(2), 233–246. DOI 10.1016/j.chemosphere.2005.05.017. [Google Scholar] [CrossRef]
53. Zheng, G., Ly, H. P., Gao, S., Wang, S. R. (2010). Effects of cadmium on growth and antioxidant responses in Glycyrrhiza uralensis seedlings. Plant, Soil and Environment, 56(11), 508–515. DOI 10.17221/30/2010-PSE. [Google Scholar] [CrossRef]
54. Al Mahmuda, J., Hasanuzzamanc, M., Nahard, K., Bhuyana, B., Fujita, M. (2018). Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicology and Environmental Safety, 147, 990–1001. DOI 10.1016/j.ecoenv.2017.09.045. [Google Scholar] [CrossRef]
55. Tamàs, L., Dudíková, J., Ďurčeková, K., Huttová, J., Mistrík, I. et al. (2008). The impact of heavy metals on the activity of some enzymes along the barley root. Environmental and Experimental Botany, 62(1), 86–91. DOI 10.1016/j.envexpbot.2007.07.009. [Google Scholar] [CrossRef]
56. Foti, M. C. (2007). Antioxidant properties of phenols. Journal of Pharmacy and Pharmacology, 59(12), 1673–1685. DOI 10.1211/jpp.59.12.0010. [Google Scholar] [CrossRef]
57. Michalak, A. (2006). Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish Journal of Environmental Studies, 15(4), 523–530. [Google Scholar]
58. Zhao, J., Zhu, W. H., Hu, Q. (2001). Selection of fungal elicitors to increase indole alkaloid accumulation in Catharanthus roseus suspension cell culture. Enzyme and Microbial Technology, 28(7–8), 666–672. DOI 10.1016/S0141-0229(01)00309-X. [Google Scholar] [CrossRef]
59. Handa, N., Kohli, S. K., Sharma, A., Thukral, A. K., Bhardwaj, R. et al. (2019). Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environmental and Experimental Botany, 161, 180–192. DOI 10.1016/j.envexpbot.2018.11.009. [Google Scholar] [CrossRef]
60. Hassanein, R. A., Hashem, H. A., El-Deep, M. H., Shouman, A. (2013). Soil contamination with heavy metals and its effect on growth, yield and physiological responses of vegetable crop plants (Turnip and Lettuce). Journal of Stress Physiology & Biochemistry, 9(4), 145–162. [Google Scholar]
61. Chakraborty, N., Chandra, S., Acharya, K. (2015). Sublethal heavy metal stress stimulates innate immunity in tomato. Scientific World Journal, 2015(5), 1–7. DOI 10.1155/2015/208649. [Google Scholar] [CrossRef]
62. Bartwal, A., Mall, R., Lohani, P., Guru, S. K., Arora, S. (2013). Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. Journal of Plant Growth Regulation, 32(1), 216–232. DOI 10.1007/s00344-012-9272-x. [Google Scholar] [CrossRef]
63. Ma, C., Liu, H., Guo, H., Musante, C., Coskun, S. H. et al. (2016). Defense mechanisms and nutrient displacement in Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environmental Science: Nano, 3(6), 1369–1379. DOI 10.1039/C6EN00189K. [Google Scholar] [CrossRef]
64. Şen, A. (2012). Oxidative stress studies in plant tissue culture, antioxidant enzyme. In: El-Missiry, M. A. (ed.Biochemistry, genetics and molecular biology “Antioxidant Enzyme”, pp. 59–88. Technology & Medicine Open Access Book Publisher. [Google Scholar]
65. Kadioglu, A., Saruhan, N., Sağlam, A., Terzi, R., Acet, T. (2011). Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regulation, 64(1), 27–37. DOI 10.1007/s10725-010-9532-3. [Google Scholar] [CrossRef]
66. Babula, P., Klejdus, B., Kovacik, J., Hedbavny, J., Hlavna, M. (2015). Lanthanum rather than cadmium induces oxidative stress and metabolite changes in Hypericum perforatum. Journal of Hazardous Materials, 286, 334–342. DOI 10.1016/j.jhazmat.2014.12.060. [Google Scholar] [CrossRef]
67. Berni, R., Luyckxc, M., Xud, X., Legayd, S., Sergeantd, K. et al. (2019). Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environmental and Experimental Botany, 161, 98–106. DOI 10.1016/j.envexpbot.2018.10.017. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |