DOI:10.32604/phyton.2020.010597

| Phyton-International Journal of Experimental Botany DOI:10.32604/phyton.2020.010597 |  |
| Review |
Changes in Phyto-Chemical Status upon Viral Infections in Plant: A Critical Review
1Institute of Agricultural Sciences, University of the Punjab, Lahore, 54590, Pakistan
2Department of Forestry, Range and Wildlife Management, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Bahawalpur, 63100, Pakistan
*Corresponding Author: Tehmina Bahar. Email: tehminabahar21@gmail.com
Received: 12 March 2020; Accepted: 01 July 2020
Abstract: Most damaging plant diseases have been caused by viruses in the entire world. In tropical and subtropical areas, the damage caused by plant virus leads to great economic and agricultural losses. Single stranded DNA viruses (geminiviruses) are the most perilous pathogens which are responsible for major diseases in agronomic and horticultural crops. Significantly begomoviruses and mastreviruses are the biggest genus of plant infecting viruses, transmitted though Bemisia tabaci and members of Cicadellidae respectively. Plants possesses some naturally existing chemicals term as phyto-chemicals which perform important functions in the plant. Some antioxidant enzymes are used by plants for self-defense upon foreign invasion of infection. This review explains the present perceptive of influence of viral infections on phyto-chemicals, oxidative enzymes and biochemical changes occurring in the plant. Viral infection mediated phyto-chemical changes in plants mainly includes: up and down regulation of photosynthetic pigment, increase in the concentration of phenolic compounds, elevation of starch content in the leaf and up & down regulation of anti-oxidative enzymes including (GPX) guaiacol peroxidase, (PPO) polyphenol oxidase, (APX) ascorbate peroxidase, (SOD) superoxide dismutase and (CTA) catalase. These changes lead to initiation of hypersensitive response, by thicken of the leaf lamina, lignification under the leaf surface, blocking to stomatal openings, systematic cell death, generation of reactive oxidative species (ROS), activation of pathogen mediated resistance pathways i.e., production of salicylic acid and jasmonic acid. Collectively all the physiological changes in the plant due to viral infection supports the activation of defense mechanism of the plant to combat against viral infection by limiting virus in specific area, followed with the production of barriers for pathogen, accumulation of starch in the leaf and excess production of (ROS). These strategies used by the plant to prevent the spread of virus in whole plant and to minimize the risk of severe yield loss.
Keywords: Anti-oxidative enzymes; defense mechanism; phyto-chemicals; viral infection
Plant infecting Gemini viruses are present almost everywhere in the world. Therefore, they became a center of scientific considerations related to plant diseases [1]. Among all single stranded DNA viruses ‘geminiviruses’ are responsible for most of plant destructive diseases. Specifically, the group begomoviruses and masteviruses are biggest genus of plant infecting viruses, transmitted by the whitefly and leaf hopper [2]. Plants possess some naturally existing chemicals known as phyto-chemicals which are of two types such as primary metabolites and secondary metabolites [3]. Phyto-chemical analysis of the plant leaf extract, revealed the presence of secondary metabolites i.e., flavonoids, phenols, polyphenols, some antioxidant enzymes, i.e., POX, PPO, APX, CAT and major biomolecules i.e., chlorophyll, carbohydrates, etc. These metabolites are responsible for various important functions in the plants specifically task related to activation of defense mechanism in plants [4]. Any biotic or abiotic stress in plants becomes the cause of change in chemical configuration of primary metabolites and alters the number of phyto-chemicals [5]. Literature confirmed that viral infection affects a lot of physiological and biochemical changes in plants [6]. In 1970 it was investigated that many viruses in different host plants were responsible to change the metabolic activities within the plants [7]. In the past few years it has widely been reported that viruses were not only involved in altering the concentration of phyto-chemicals but also found responsible to stimulate some compounds in plant which activated its defense mechanism against the biotic stress [8]. Interactions between virus and plant have been considered to affect the crop production. Plant virus not only disturbs the secondary metabolites production but also increases their numbers as well. It affects the enzymatic activity of antioxidant enzymes in the plants [9]. PCR results confirmed that begomoviruses also changed phyto-chemicals concentration resulting in activation of defense mechanism [8]. It has been reported that virus altered the secondary metabolites present in the fruit as well. Plant virus involved in increasing the tolerances in plants towards them by activating the antioxidant defense mechanism through enhancing positive biochemical changes in plants [10]. Viral infection enhanced the concentration of phenol, proline and activity of the antioxidant enzymes i.e., CAT, PPO, SOD and POX was also increased due to the viral infection [11] (Fig. 1).
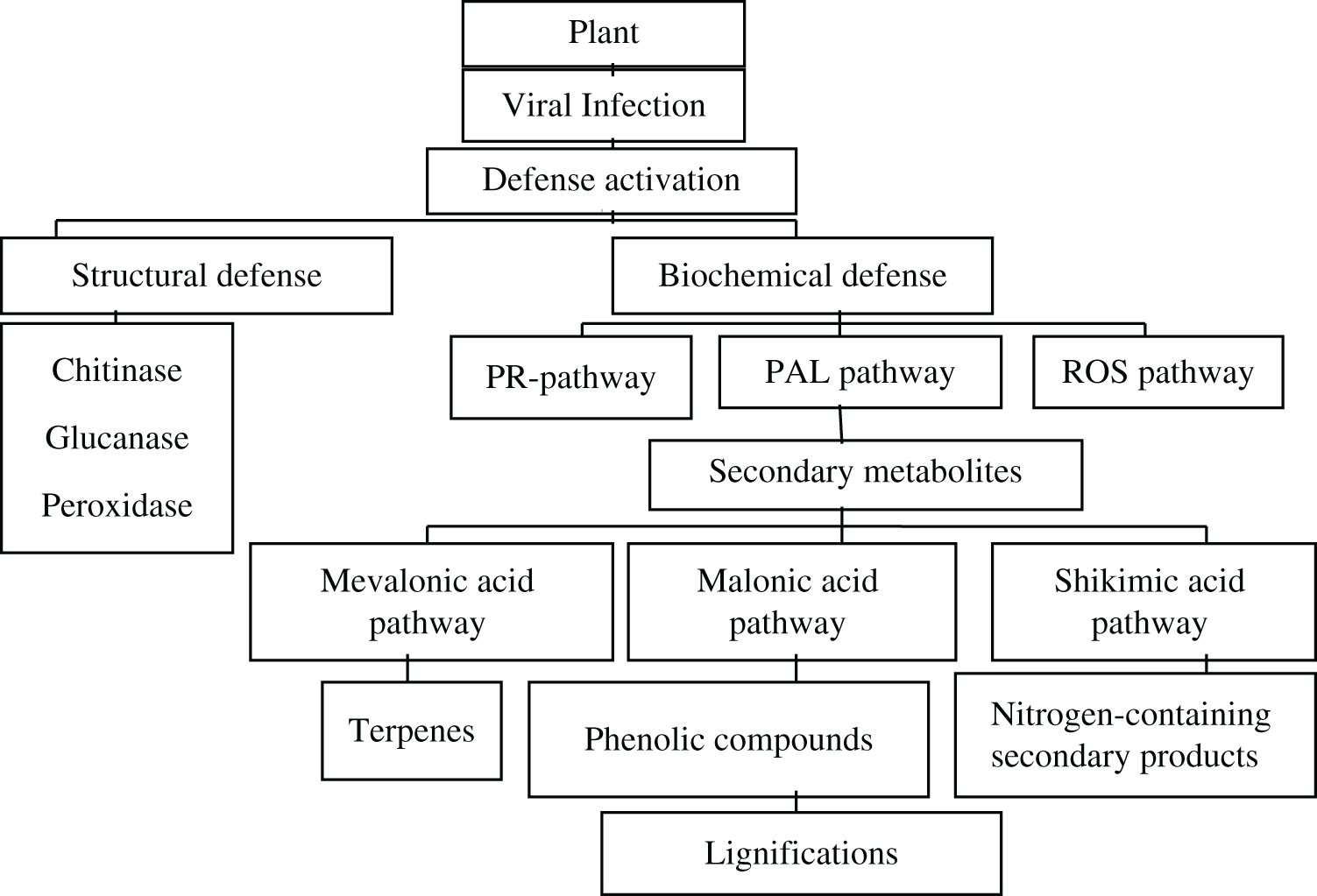
Figure 1: Virus mediated activation of defense pathways in plant [4,5,10]
Objective of this review study was:
1. To elaborate the role of viral pathogens in activation of plant defense mechanism.
2. To understand the phyto-chemicals changes occurring in the plants due to viral invasion.
3. Role of antioxidant enzymes in plant defense activation.
This study will help to further investigate the activation of secondary metabolites in plant upon infection and how they are associated with the activation of plant defense mechanism.
2 Impact of Viral Infection on Phyto-Chemicals
On pumpkin plant, infection of Tomato leaf curl palampur virus (ToLCPMV) affected the chlorophyll content of leaf, this virus affected both chlorophyll a and b, by reducing their amount in the plant [8]. According to Sinha et al. [12]. Banana bunchy top virus (BBTV) upon infection reduced the concentration of chlorophyll a and b, maximum reduction was observed in chlorophyll b than chlorophyll a. Reported reason behind this reduction was the deposition of carbohydrates in the leaf of plant [13,14]. Aucuba mosaic virus in tomato upon infection did not affect the chlorophyll in older leaves; younger leaves have shown no change. Cucumber mosaic disease was supported by the formation of chlorosis strips on the leaf which confirmed the change in chlorophyll concentration in the leaf [15]. It is reported that in tomato, infection of TLCV altered chlorophyll concentration in the plant, decline in chlorophyll concentration was observed in infected leaf as compared to the healthy leaf, increased concentration of chlorophyll was measured at high temperature while low temperature reduced the concentration of chlorophyll [5]. In okra, viral infection decreased the concentration of chlorophyll inside the plant leaf [16]. According to various researchers the Yellow vein clearing mosaic virus (YVCMV) which was responsible for vein clearing mosaic disease in okra caused drastic reduction in the amount of chlorophyll in leaf of okra [17]. It was found that in Egypt (TYLCV) causing infection in tomato reduced the chlorophyll content leading to the reduction of tomato production causing great economic loss to the farmer [11]. It was also reported that Moroccan watermelon mosaic virus (MWMV) infection in Cucurbita moschata caused reduction in both chlorophyll a and b. Their estimation showed that quantity of chlorophyll a was affected more than chlorophyll b [18]. The quantity of photosynthetic pigment should reduce in diseased plants compared to the healthy plants. In eggplant, infection of begomovirus TYLCV which is transmitted by whitefly changed the phyto-chemical and biochemical compounds in tomato, leaf analysis revealed that mild change in chlorophyll concentration was identified [19]. In previous studies, TYLCV was responsible for the reduction of Mg++ which is a main component of chlorophyll [20]. This reduction also led to the decrease of chlorophyll in tomato plant while, high temperature supported the reduction. On TYLCV infection, tomato plant showed chlorosis at high temperature, causing reduction in chlorophyll concentration in the plant [21]. Infection of Bean yellow mosaic virus (BYMV) caused gradual reduction of chlorophyll in Viciafaba infected leaf [22].
Significant impact of pathogenic viruses on the carbohydrate metabolism of infected plant has been reported. Different viruses caused varied infections in plants such as some completely altered carbohydrate synthesis, and translocation process while others have mild effect [23]. Carbohydrates play a significant role in the plant body but accumulation of excessive starch in the leaf produced viral symptoms [24] Watson [13] reported that sucrose contents increased in infected plant on the incidence of viral infection. Carbohydrates have major role in the production of antioxidant enzymes [25]. Strong link between the activation of defense mechanism with carbohydrate concentration has been confirmed by scientific reports [26,27]. Results of Fryer et al. [28] showed that BBTV infection increased the sugar content by interfering with the photo inhibitory processes in the banana plant and symptoms appeared on infected areas. On the incidence of banana bunchy top infection amount of ROS increased in the plants. ROS sucrose produced within infected plant resulted in chlorosis [29]. In tomato plant decrease in insoluble and soluble sugars in stem and leaf was observed due to infection caused by TLCV in infected plant [30]. Changes in carbohydrates concentration due to viral infection also affect the leaf color, no change was observed in color of healthy leaf while infected leaf showed dark gray color [21].
Polyphenols are secondary metabolites, which play significant function during host-pathogen interaction, disease development and activation of defense mechanism in diseased plants. Defense mechanism got strengthen by the formation of lignin under the leaf surface that acts as a physical barrier to resist the multiplication of pathogen. This might be due to increased concentration of phenolic compounds upon viral infection in the plants [20]. Enhanced amount of phenol due to the viral infection in plant was measured by Rai et al. [31] stating that increased amount of phenol resulted in the elevation of plant defense mechanism [32]. Increased levels of phenols also suggested an acceleration of phenol synthesizing pathway following pathogen infection. Anuradha et al. [5] results showed that the level of total phenols was elevated in P. edulis fruit due to infection of TeMV. Similarly Jaiswal et al. [8] and Jabeen et al. [33] have reported that Yellow vein mosaic virus (YVMV) infection in pumpkin plant resulted in increase of phenolic compounds which boasted the defense mechanism in the plant, these compounds were elevated unto 73% in the infected plant leaf while 300% in the infected fruit as compared to the healthy plant. Infection of CMV in tomato increased the amount of phenols which helped in lignifications of cell wall and played role in plant defense by boosting the immunity [34]. The research studies conducted by Jaiswal et al. [8], Song et al. [29], Khalil et al. [30], and El-Dougdoug et al. [35] also supported the fact that TYLCV enhanced the amounts of phenol in the plants. Huston et al. [36] reported that infected tomato varieties have more phenolic compounds that activate the resistance phenomena against viruses in plant and also boost the antioxidants in plant for further fight against viral infection. Although, viral infections changed the number of phenolic compounds in plant, but infection of CTV did not show any change in the phenolic concentration [37]. Secondary metabolites specially polyphenols and flavonoid compounds were affected due to viral infection in members of Passifloraceae, results suggested that viral infection enhanced their amounts, in P. edulis fruit by 58.3% while in leaf this increase observed was 43.1% [9].
3 Effect of Viral Infection of Oxidative Enzymes
First enzyme that is reported to show quick defense against viral infection is peroxidase [37]. Lignification, polymerization, suberification, cell wall elongation, resistance and wound healing are all the processes which are due to POX enzyme [38]. Banana bunchy top infection caused high peroxidase activity in bananas cultivar, while healthy crop showed less POX action in the plant, similar results were observed upon the infection of (TMV) in tobacco plant [39]. Likewise beans infected with (BYMV) [40], Potato’s infected with potato virus Y [41], TMV and Tomato mosaic virus (ToMV) causing infection in tomato and bell pepper [42], TMV infecting tobacco plants [43], In tomato plants infection of TYLCV [44], in banana plants infected with BBTV [45], Capsicum annum infection of Gemini virus [46] and Cotton leaf curl burewala virus (CLCuBuV) infection in cotton [47] supported these results. Polyphenol oxidase is involved in AOS process. The accumulation of AOX at the infection site damaged the membrane and destroyed the chlorophyll in the plant which leads to POX during senescence, in green leaves there was a correlation which was observed at the time of increase in POX and decrease in chlorophyll [41]. Peroxidases perform their role in plant defense by the production of pathogenesis-related proteins. POX helped to remove the hydrogen peroxide from the cell. Peroxidase was also found involved in formation of a wall of lignin around the cell which limits pathogens to cross from the place of penetration [48].
3.2 Effect on Polyphenol Oxidase (PPO)
Plant defense was boosted in the presence of polyphenol oxides, when plant membrane gets damaged on pathogenic invasion, phenols in the plant produced chlorogenic acid, which create an unfavorable environment for the pathogen to spread, polyphenol increased the phenol production that leads to restrict the spread of pathogen [47]. Banana bunchy top infection caused the elevation in PPO activity in banana cultivars. PPO after combining with phenols shows activation of defense in the plant towards pathogen [49].
Catalase is an enzyme, which can hold oxygen to protect the cell from peroxides, as peroxides cause toxic effect on plant health by development of H2O2 from substrate [50,51] BBTV supported the activity of CAT in banana leaf, likewise in peanuts the infection of Arachis hypogeal increased the activity of catalase [52] similarly elevation in catalase was observed when cotton was inoculated with CLCuBuV [47]. The amount of CAT was greater in non-inoculated susceptible cotton variety (CIM-496), in comparison with the non-inoculated resistant variety (NIAB-11) after with the inoculation of elevation of CAT activity reported to be maximum. High activity was observed in genotype Ravi, which was resistant to the CLCuBuV, CAT activity was upto 40%. NIAB-11 showed 34.4% CAT activity, CIM-496 showed 22.22% activity of CAT [47]. In viral infected P. edulis plant the amount of catalase increased upto 52.8% hence the tolerance capacity of plant toward virus also increased said [9].
3.4 Effect on Ascorbate Peroxidase (APX)
Main role of APX is to hold ROS in plants, activity of ascorbate peroxide was high during BBTV infection in banana, same results were obtained by infection of begomoviruses in Hibiscus cannbinus [53], Nicotiana benthamiana plant have high APX activity due to infection of Pepper mild mottle virus (PMMV) [54], Sunflower chlorotic mottle virus (SCMV) infection in sunflower [55]. Over production of ascorbate peroxide boosted the production of peroxidase, led to grip more reactive oxygen species, in past reports it has been stated that the compatible relation between host and pathogen increased the amount of APX in epidermal cells as well as in mesophyll cells, cell itself have no mechanism to restrict the pathogen spread, increased amount of APX allowed cell to remain viable [56]. This mechanism has been studied in Plum pox virus (PPV) infection in apricot [57]. Another report showed that APX activity increased up to 44.6% due to infection of CMV in cucumber plant Jaiswal et al. [8] and Lan et al. [9] reported that viral infection increased the APX activity upto 100% due to viral infection of ToLCPMV in pumpkin. In viral infected P. edulis plant the amount of GR increased up to 31.8% hence the tolerance capacity of plant toward virus also increased said [9].
3.5 Effect on Superoxide Dismutase
SOD reported to be another scavenging enzyme which increases the rate of dis-mutation of SO radical into active oxygen species. Viral infection increased SOD activity in the plant [50], above statement is supported by the findings of Hernández et al. [57]. It is reported that decrease of SOD in peaches infected with Plum pox virus, Phaseolus vulgaris L. infected with White clover mosaic virus (WClMV) [58] black gram infected with Urdbean leaf crikle virus (ULCV) [59] and Soybean mosaic virus (SMV) infection in resistant variety of soybean [60]. Plants enhance the SOD quantity for defense against viral infection [61]. It is reported that Mycosphaerella fragariae infection in strawberry increased the amount of SOD in the infected leave [61]. In resistant variety of Ravi cultivar showed more SOD in infectious plant parts [62]. CLCuBuV infection elevated the quantity of SOD in cotton genotype [47]. Viral infected P. edulis plant the amount of SOD increased up to 66.7% hence the tolerance capacity of plant toward virus also increased said Lan et al. [9]. The behavior of anti-oxidative enzymes and their isoforms were checked in both non-inoculated healthy and infected plants one month later from viral infection. In infected leaf samples there was a substantial increase in the activities of SOD about 273%, in comparison with the leaves of uninfected seedlings [8].
4 Effect of Viral Infection on Activation of Defense Mediated Pathways
Infection of CMV induced HR response in the plant, salicylic acid production increased in the plant which showed resistance towards pathogen, no increase was noticed in susceptible varieties [63]. Cultivar of tobacco, which was resistant towards TMV produced hypersensitive response, upon infection the level of salicylic acid in infected plant became several fold more than non-infected plant [64]. Infection of Turnip crinkle virus (TCV) on Arabidopsis triggered the hypersensitive response in the plant, but HR response was depended upon the SA but not or JA/ethylene [65]. Infection of cauliflower mosaic virus (CaMV) in cauliflower plant was investigated by setting three maker genes, for identification of salicylic acid signaling pathway, reactive oxygen and jasmonic acid pathways respectively, results suggested that salicylic acid signaling was very low, but RO and JC acid pathways were visible in virus infecting plant (Fig. 2). PR-1 expression, salicylic signaling was less until 8th post inoculation but then elevated sharply as the viral infection increased. On the other hand, GST1- and PDF1.2 markers which are for observing reactive oxygen species and jasmonic acid signaling showed their elevation upon viral infection after 2 h of post inoculation [66]. It is studied that in tomato, infection of TWV activated plants defense mechanism by stimulating the production of plant hormones i.e., Jasmonate and salicylate [67] Upon Infection of TuMV in Arabidopsis, levels of SA and ET were more than doubled than the healthy plant, on the contrary JA concentration became four fold in infected plant and continue to increase in infected plants [68] Infection of citrus exocortis virus CEVd and Tomato mosaic virus (ToMV) also elevated the jasmonic acid production up to 150 folds [69] According to past researches protein C2 that encodes for Beat beet severe curly top virus (BSCTV) caused the rise of salicylic defense pathway [70] Likewise in peas infection of Clover yellow vein virus (CIYVV) was responsible for activation of hypersensitive response in diseased plants ultimately leading towards cell death. Wild strain of CIYVV was responsible for cell death in peas. Furthermore, the aggressiveness of CIYVV strain in susceptible peas increased due to SA production [71]. Pathogen identification with the help of R gene was studied; results concluded that upon infection plant showed hypersensitive response, boost the production of reactive oxygen species and up regulate salicylic acid which results in activation of local as well as systemic gene involved in defense. In tobacco activation of N-gene due to infection of TMV produced pathogen related proteins and establishment of SAR on plant pathogen interaction [72] Citrus leprosis virus C which is responsible for Leprosis disease in citrus, triggered the production of reactive oxygen species (ROS) upon interaction with mites which were non viruliferous, plants locally produced ROS and activates the production of the salicylic acid (SA) and jasmonate pathways. On the other hand, JA pathway activating genes were suppressed upon viral infection. Viral infection intensified the ROS burst and cell death and enhanced the expression of genes involved in the RNA silencing mechanism and SA pathway [73].
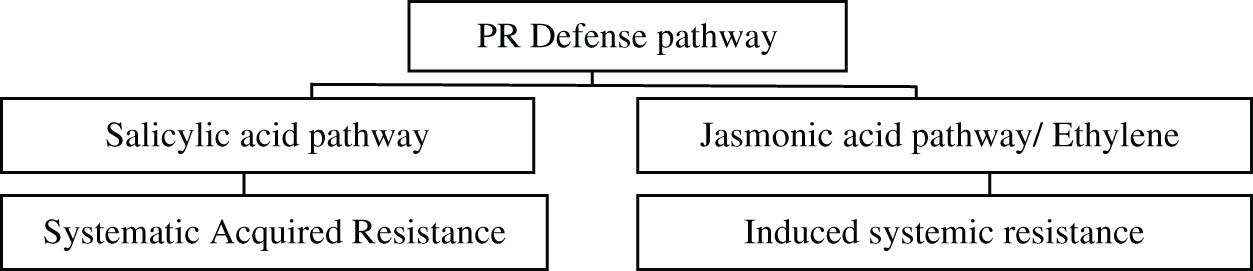
Figure 2: PR-Defense pathway in plant [66,69,73]
From present review it is summarized that upon viral infections in the plants due to the activation of secondary metabolism pathway based on ecological influence and adaptability of genetic code of the plant cell, some naturally existing primary metabolites synthesized into secondary metabolites or phyto-chemicals [3]. Some important secondary metabolites are flavonoids, phenols, polyphenols, some antioxidant enzymes i.e., POX, PPO, APX, CAT and major biomolecules, i.e., chlorophyll, carbohydrates etc. These metabolites perform task related to activation of defense mechanism in plants. Upon viral infection plant activates its defense mechanism to deal with foreign invasion. Generally, two types of defense responses are shown by the plant towards infection, i.e., structural defense and biochemical defense [10,34]. Certain enzymes like chitinase, peroxidases help to give structural defense by controlling the multiplication of virus, furthermore polymerization, suberification, cell wall elongation, resistance and wound healing are all the processes which provide structural defense and are triggered by POX enzyme. Viral infection in many plants increased the activity of superoxide dismutase and polyphenol oxidase, resulting in enhancement of reactive oxygen species that ultimately activate plant defense mechanism [59]. Along with these enzymes, phenols have association with plant defense and the enzymes play a role as a biomarker for plant-viral interaction study [5,47,74]. In biochemical defense mechanism specific pathways are activated which include PR-pathways, PAL pathway, ROS pathways etc., and boost the plant defense by producing secondary metabolites. Concentration of phenolic compounds increased in infected plant resulting in lignifications under the infected leaf surface that act as barrier to pathogenic viruses to multiply in the host cell. Infection of virus induces HR response in the plant cells. Salicylic acid production increased in the plant having resistanance towards pathogen [63]. Cultivars which have resistance against viral pathogen produce hypersensitive response, upon infection the level of salicylic acid became several folds high in infected plants as compare to the non-infected plants [64,68–70] causing disturbance in primary metabolites and antioxidant enzymes activity within the plant. Viral infection enhances the enzymatic activity of the antioxidant’s enzymes, i.e., CAT, PPO, SOD and POX was also increased due to the viral infection [11]. Host plant infected with pathogenic viruses show severe reduction in photosynthetic pigment and chlorophyll content due to accumulation of carbohydrates in leaves tissue. Some specific viruses cause decrease in chlorophyll a and some has effects on chlorophyll b. Due to reduction in chlorophyll, plant undergo chlorosis. Reactive oxygen species production enhanced at the site of necrosis, showing hypersensitive response against viral infection. This review suggests that virus induce resistance in plant by altering the primary phyto-chemicals and antioxidant enzymes to initiate defense mechanism of the host plant. So that plant may thus combat viral invasion as other type of stresses mostly due to presence of natural antioxidants [75].
This study will help to further investigate that what is the genetic difference among cultivars which shows more resistance against viral strain as compared to the susceptible cultivars. Is there any gene involved which is controlling the defense mechanism and making them more resistant? Is there any gene which is controlling the production of secondary metabolites? Moreover, as secondary metabolites are boosting defense mechanism in plant, is there any other way to trigger the production of secondary metabolites other than viral infections? These are the questions which can be addressed with further research.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Jeske, H. (2009). Geminiviruses. In: TT viruses, pp. 185–226. Berlin, Heidelberg: Springer.
2. Inoue-Nagata, A. K., Lima, M. F., Gilbertson, R. L. (2016). A review of geminivirus diseases in vegetables and other crops in Brazil: current status and approaches for management. Horticultura Brasileira, 34(1), 8–18. DOI 10.1590/S0102-053620160000100002. [Google Scholar] [CrossRef]
3. Banu, K. S., Cathrine, L. (2015). General techniques involved in phytochemical analysis. International Journal of Advanced Research in Chemical Science, 2(4), 25–32. [Google Scholar]
4. Kala, S. C., Mallikarjuna, K., Aruna, P. (2012). Qualitative phyto chemical analysis of seed and leaf callus extracts of canthium parviflorum lam. Guntur district, Andhra Pradesh. International Journal of Pharma and Bio Sciences, 3(4), 177–182. [Google Scholar]
5. Anuradha, C., Selvarajan, R., Vasantha, S., Suresha, G. S. (2015). Biochemical characterization of compatible plant virus interaction: a case study with bunchy top virus-banana host-pathosystem. Plant Pathology Journal, 14(4), 212–222. DOI 10.3923/ppj.2015.212.222. [Google Scholar] [CrossRef]
6. Radwan, D. E. M., Fayez, K. A., Mahmoud, S. Y., Hamad, A., Lu, G. (2007). Physiological and metabolic changes of Cucurbita pepo leaves in response to zucchini yellow mosaic virus (ZYMV) infection and salicylic acid treatments. Plant Physiology and Biochemistry, 45(6–7), 480–489. DOI 10.1016/j.plaphy.2007.03.002. [Google Scholar] [CrossRef]
7. Miteva, E., Hristova, D., Nenova, V., Maneva, S. (2005). Arsenic as a factor affecting virus infection in tomato plants: changes in plant growth, peroxidase activity and chloroplast pigments. Scientia Horticulturae, 105(3), 343–358. DOI 10.1016/j.scienta.2005.01.026. [Google Scholar] [CrossRef]
8. Jaiswal, N., Singh, M., Dubey, R. S., Venkataramanappa, V., Datta, D. (2013). Phytochemicals and antioxidative enzymes defence mechanism on occurrence of yellow vein mosaic disease of pumpkin (Cucurbita moschata). 3 Biotech, 3(4), 287–295. DOI 10.1007/s13205-012-0100-6. [Google Scholar] [CrossRef]
9. Lan, H., Lai, B., Zhao, P., Dong, X., Wei, W. et al. (2020). Cucumber mosaic virus infection modulated the phytochemical contents of Passiflora edulis. Microbial Pathogenesis, 138, 103828. DOI 10.1016/j.micpath.2019.103828. [Google Scholar] [CrossRef]
10. Chen, S., Yu, N., Yang, S., Zhong, B., Lan, H. (2018). Identification of Telosma mosaic virus infection in Passiflora edulis and its impact on phytochemical contents. Virology Journal, 15(1), 168. DOI 10.1186/s12985-018-1084-6. [Google Scholar] [CrossRef]
11. Sofy, A. R., El-Dougdoug, K. A., Mousa, A. A., Refaey, E. E. (2017). Impact of two TYLCV Egyptian isolates on metabolic and antioxidant activities in some tomato cultivars. International Journal of Advanced Research in Biological Sciences, 4(2), 110–133. [Google Scholar]
12. Sinha, A., Srivastava, M. (2010). Biochemical changes in mungbean plants infected by mungbean yellow mosaic virus. International Journal of Virology, 6(3), 150–157. DOI 10.3923/ijv.2010.150.157. [Google Scholar] [CrossRef]
13. Watson, M. A. (1955). The effect of sucrose spraying on symptoms caused by beet yellows virus in sugar beet. Annals of Applied Biology, 43(4), 672–685. DOI 10.1111/j.1744-7348.1955.tb02511.x. [Google Scholar] [CrossRef]
14. Endo, T., Okuda, T., Tamura, M., Yasuoka, Y. (2001). Estimation of net photosynthetic rate based on in-situ hyperspectral data. Journal of Optical Microsystems, 4151, 214–221. [Google Scholar]
15. Sheffield, F. M. L. (1933). The development of assimilatory tissue in Solanaceous hosts infected with aucuba mosaic of tomato. Annals of Applied Biology, 20(1), 57–69. DOI 10.1111/j.1744-7348.1933.tb07427.x. [Google Scholar] [CrossRef]
16. Arooj, S., Iftekhar, Y., Mubeen, M., Ullah, M. I., Sajid, A. et al. (2019). Effect of environmental factors on biochemical properties of tomato leaf curl virus infected leaves of tomato. Pakistan Journal of Phytopathology, 31(1), 105–111. DOI 10.33866/phytopathol.031.01.0467. [Google Scholar] [CrossRef]
17. Sayed, M. K. A., Hossain, M. B., Shahriar, S. A. (2018). Performance of selected phytochemicals and botanical nutrient on physiological features of okra varieties against yellow vein clearing mosaic virus. Asian Journal of Agricultural and Horticultural Research, 1(3), 1–8. DOI 10.9734/AJAHR/2018/41527. [Google Scholar] [CrossRef]
18. Mofunanya, A. A. J., Edu, E. A. (2015). Physiological and biochemical changes in Cucurbita moschata Duch. Ex. Poir inoculated with a Nigerian strain of Moroccan Watermelon Mosaic Virus (MWMVLagenariabreviflora isolate. International Journal of Plant Pathology, 6(2), 36–47. DOI 10.3923/ijpp.2015.36.47. [Google Scholar] [CrossRef]
19. Khan, M. M., Khan, M. Y., Muhammad, R., Ullah, K., Yasir, M. et al. (2018). Morphological and biochemical characters of eggplant (Solanum melongena) conferring resistance against whitefly (Bemisia tabaci). Journal of Enomology and Zoology Studies, 6(5), 915–920. [Google Scholar]
20. Singh, H. P., Kaur, S., Batish, D. R., Kohli, R. K. (2014). Ferulic acid impairs rhizogenesis and root growth, and alters associated biochemical changes in mung bean (Vigna radiata) hypocotyls. Journal of Plant Interactions, 9(1), 267–274. DOI 10.1080/17429145.2013.820360. [Google Scholar] [CrossRef]
21. Arooj, S., Iftekhar, Y., Mubeen, M., Ullah, M. I., Sajid, A. et al. (2019). Effect of environmental factors on biochemical properties of tomato leaf curl virus infected leaves of tomato. Pakistan Journal of Phytopathology, 31(1), 105–111. DOI 10.33866/phytopathol.031.01.0467. [Google Scholar] [CrossRef]
22. Hemida, S. K. (2005). Effect of bean yellow mosaic virus on physiological parameters of Vicia faba and Phaseolus vulgaris. International Journal of Agriculture and Biology, 7(2), 154–157. [Google Scholar]
23. Gaddam, S. A., Kotakadi, V. S., Reddy, M. N., Saigopal, D. V. R. (2012). Antigenic relationships of citrus yellow mosaic virus by immunological methods. Asian Journal of Plant Science and Research, 2, 566–569. [Google Scholar]
24. Holmes, F. O. (1929). Local lesions in tobacco mosaic. Botanical Gazette, 87(1), 39–55. DOI 10.1086/333923. [Google Scholar] [CrossRef]
25. Couée, I., Sulmon, C., Gouesbet, G., El Amrani, A. (2006). Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany, 57(3), 449–459. DOI 10.1093/jxb/erj027. [Google Scholar] [CrossRef]
26. Sulmon, C., Gouesbet, G., Couée, I., El Amrani, A. (2004). Sugar-induced tolerance to atrazine in Arabidopsis seedlings: interacting effects of atrazine and soluble sugars on psbA mRNA and D1 protein levels. Plant Science, 167(4), 913–923. DOI 10.1016/j.plantsci.2004.05.036. [Google Scholar] [CrossRef]
27. Loreti, E., Poggi, A., Novi, G., Alpi, A., Perata, P. (2005). A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiology, 137(3), 1130–1138. DOI 10.1104/pp.104.057299. [Google Scholar] [CrossRef]
28. Fryer, M. J., Oxborough, K., Mullineaux, P. M., Baker, N. R. (2002). Imaging of photo-oxidative stress responses in leaves. Journal of Experimental Botany, 53(372), 1249–1254. [Google Scholar]
29. Song, X. S., Wang, Y. J., Mao, W. H., Shi, K., Zhou, Y. H. et al. (2009). Effects of cucumber mosaic virus infection on electron transport and antioxidant system in chloroplasts and mitochondria of cucumber and tomato leaves. Physiologia Plantarum, 135(3), 246–257. DOI 10.1111/j.1399-3054.2008.01189.x. [Google Scholar] [CrossRef]
30. Khalil, R. R., Bassiouny, F. M., El-Dougdoug, K. A., Abo-Elmaty, S., Yousef, M. S. (2014). A dramatic physiological and anatomical changes of tomato plants infecting with tomato yellow leaf curl germinivirus. International Journal of Agricultural Sustainability, 10, 1213–1229. [Google Scholar]
31. Rai, V. P., Jaiswal, N., Kumar, S., Singh, S. P., Kumar, R. et al. (2010). Response of total phenols and peroxidase activity in Chilli exposed to pepper leaf curl virus disease. Vegetable Science, 37(1), 78–80. [Google Scholar]
32. Siddique, Z., Akhtar, K. P., Hameed, A., Sarwar, N., Imran-Ul-Haq, K. S. A. (2014). Biochemical alterations in leaves of resistant and susceptible cotton genotypes infected systemically by cotton leaf curl Burewala virus. Journal of Plant Interactions, 9(1), 702–711. DOI 10.1080/17429145.2014.905800. [Google Scholar] [CrossRef]
33. Jabeen, N., Ahmed, N., Ghani, M. Y., Sofi, P. A. (2009). Role of phenolic compounds in resistance to chilli wilt. Communications in Biometry and Crop Science, 4(2), 52–61. [Google Scholar]
34. Sudhakar, N., Nagendra-Prasad, D., Mohan, N., Murugesan, K. (2007). Induction of systemic resistance in Lycopersicon esculentum cv. PKM1 (tomato) against Cucumber mosaic virus by using ozone. Journal of Virological Methods, 139(1), 71–77. DOI 10.1016/j.jviromet.2006.09.013. [Google Scholar] [CrossRef]
35. El-Dougdoug, K. A., Sofy, A. R., Mousa, A. A., Refaey, E. E. (2014). Monitoring variability responses of cultivated potato varieties infected with Potato virus Y pepper isolate. Egyptian Journal of Virology, 11(2), 82–101. DOI 10.1186/1743-422X-11-82. [Google Scholar] [CrossRef]
36. Hutson, R. A., Smith, I. M. (1980). Phytoalexins and tyloses in tomato cultivars infected with Fusarium oxysporum f.sp. lycopersici or Verticillium albo-atrum. Physiological Plant Pathology, 17(3), 245–257. DOI 10.1016/S0048-4059(80)80018-X. [Google Scholar] [CrossRef]
37. Iftikhar, Y., Mughal, S. M., Khan, M. M., Khan, M. A., Batool, A. et al. (2011). Some biochemical changes in tristeza infected citrus trees in Pakistan. International Journal of Science and Nature, 2, 621–624. [Google Scholar]
38. Maksimov, I., Troshina, N., Surina, O., Cherepanova, E. (2014). Salicylic acid increases the defense reaction against bunt and smut pathogens in wheat calli. Journal of Plant Interactions, 9(1), 306–314. DOI 10.1080/17429145.2013.832424. [Google Scholar] [CrossRef]
39. Lagrimini, L. M., Rothstein, S. (1987). Tissue specificity of tobacco peroxidase isozymes and their induction by wounding and tobacco mosaic virus infection. Plant Physiology, 84(2), 438–442. DOI 10.1104/pp.84.2.438. [Google Scholar] [CrossRef]
40. Radwan, D. E. M., Fayez, K. A., Mahmoud, S. Y., Lu, G. (2010). Modifications of antioxidant activity and protein composition of bean leaf due to Bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiologiae Plantarum, 32(5), 891–904. DOI 10.1007/s11738-010-0477-y. [Google Scholar] [CrossRef]
41. Milavec, M., Ravnikar, M., Kovač, M. (2001). Peroxidases and photosynthetic pigments in susceptible potato infected with potato virus YNTN. Plant Physiology and Biochemistry, 39(10), 891–898. DOI 10.1016/S0981-9428(01)01303-1. [Google Scholar] [CrossRef]
42. Madhusudhan, K. N., Srikanta, B. M., Shylaja, M. D., Prakash, H. S., Shetty, H. S. (2009). Changes in antioxidant enzymes, hydrogen peroxide, salicylic acid and oxidative stress incompatible and incompatible host-tobamovirus interaction. Journal of Plant Interactions, 4(3), 157–166. DOI 10.1080/17429140802419516. [Google Scholar] [CrossRef]
43. Király, Z., Barna, B., Kecskés, A., Fodor, J. (2009). Down-regulation of antioxidative capacity in a transgenic tobacco which fails to develop acquired resistance to necrotization caused by TMV. Free Radical Research, 36(9), 981–991. DOI 10.1080/1071576021000006581. [Google Scholar] [CrossRef]
44. Dieng, H., Satho, T., Hassan, A. A., Aziz, A. T., Morales, R. E. et al. (2011). Peroxidase activity after viral infection and whitefly infestation in juvenile and mature leaves of Solanum lycopersicum. Journal of Phytopathology, 159(11–12), 707–712. DOI 10.1111/j.1439-0434.2011.01830.x. [Google Scholar] [CrossRef]
45. Devanathan, M., Ramaiah, M., Sundar, A. R., Murugan, M. (2005). Changes of peroxidase and polyphenol oxidase in bunchy top nana virus infected and healthy cultivars of banana. Annals of Plant Physiology, 19(1), 114. [Google Scholar]
46. Meena, R. K., Patni, V., Arora, D. K. (2008). Study on phenolics and their oxidative enzyme in Capsicum annuum L. infected with geminivirus. Asian Journal of Experimental Sciences, 22(3), 307–310. [Google Scholar]
47. Siddique, Z., Akhtar, K. P., Hameed, A., Sarwar, N., Imran-Ul-Haq, K. S. A. (2014). Biochemical alterations in leaves of resistant and susceptible cotton genotypes infected systemically by cotton leaf curl Burewala virus. Journal of Plant Interactions, 9(1), 702–711. DOI 10.1080/17429145.2014.905800. [Google Scholar] [CrossRef]
48. Almagro, L., Gómez Ros, L. V., Belchi-Navarro, S., Bru, R., Ros Barceló, A. et al. (2008). Class III peroxidases in plant defence reactions. Journal of Experimental Botany, 60(2), 377–390. DOI 10.1093/jxb/ern277. [Google Scholar] [CrossRef]
49. Ngadze, E., Icishahayo, D., Coutinho, T. A., Van der Waals, J. E. (2012). Role of polyphenol oxidase, peroxidase, phenylalanine ammonia lyase, chlorogenic acid, and total soluble phenols in resistance of potatoes to soft rot. Plant Diseases, 96(2), 186–192. DOI 10.1094/PDIS-02-11-0149. [Google Scholar] [CrossRef]
50. Hameed, A., Iqbal, N. (2014). Chemo-priming with mannose, mannitol and H2O2 mitigate drought stress in wheat. Cereal Research Communications, 42(3), 450–462. DOI 10.1556/CRC.2013.0066. [Google Scholar] [CrossRef]
51. Patel, S. J., Subramanian, R. B., Jha, Y. S. (2011). Biochemical and molecular studies of early blight disease in tomato. Phytoparasitica, 39(3), 269–283. DOI 10.1007/s12600-011-0156-6. [Google Scholar] [CrossRef]
52. Kobeasy, M. I., El-Beltagi, H. S., El-Shazly, M. A., Khattab, E. A. (2011). Induction of resistance in Arachis hypogaea L. against Peanut mottle virus by nitric oxide and salicylic acid. Physiological and Molecular Plant Pathology, 76(2), 112–118. DOI 10.1016/j.pmpp.2011.07.005. [Google Scholar] [CrossRef]
53. Sarkar, T. S., Majumdar, U., Roy, A., Maiti, D., Goswamy, A. M. et al. (2010). Production of nitric oxide in host-virus interaction: a case study with a compatible begomovirus-kenaf host-pathosystem. Plant Signaling & Behavior, 5(6), 668–676. DOI 10.4161/psb.5.6.11282. [Google Scholar] [CrossRef]
54. Hakmaoui, A., Pérez-Bueno, M. L., García-Fontana, B., Camejo, D., Jiménez, A. et al. (2012). Analysis of the antioxidant response of Nicotiana benthamiana to infection with two strains of Pepper mild mottle virus. Journal of Experimental Botany, 63(15), 5487–5496. DOI 10.1093/jxb/ers212. [Google Scholar] [CrossRef]
55. Rodríguez, M., Taleisnik, E., Lenardon, S., Lascano, R. (2010). Are sunflower chlorotic mottle virus infection symptoms modulated by early increases in leaf sugar concentration. Journal of Plant Physiology, 167(14), 1137–1144. DOI 10.1016/j.jplph.2010.03.004. [Google Scholar] [CrossRef]
56. Burhenne, K., Gregersen, P. L. (2000). Up-regulation of the ascorbate-dependent antioxidative system in barley leaves during powdery mildew infection. Molecular Plant Pathology, 1(5), 303–314. DOI 10.1046/j.1364-3703.2000.00034.x. [Google Scholar] [CrossRef]
57. Hernández, J. A., Talavera, J. M., Martínez-Gómez, P., Dicenta, F., Sevilla, F. (2001). Response of antioxidative enzymes to plum pox virus in two apricot cultivars. Physiologia Plantarum, 111(3), 313–321. DOI 10.1034/j.1399-3054.2001.1110308.x. [Google Scholar] [CrossRef]
58. Clarke, S. F., Guy, P. L., Burritt, D. J., Jameson, P. E. (2002). Changes in the activities of antioxidant enzymes in response to virus infection and hormone treatment. Physiologia Plantarum, 114(2), 157–164. DOI 10.1034/j.1399-3054.2002.1140201.x. [Google Scholar] [CrossRef]
59. Ashfaq, M., Khan, M. A., Javed, N., Mughal, S. M., Shahid, M. et al. (2010). Effect of urdbean leaf crinkle virus infection on total soluble protein and antioxidant enzymes in blackgram plants. Pakistan Journal of Botany, 42(1), 447–454. [Google Scholar]
60. Zhuang, B. C., Xu, B., Liao, L. (1993). Change of superoxide dismutase, peroxidase and storage protein in soyabean leaves after inoculation with soyabean mosaic virus. Acta Phytopathologica Sinica, 23(3), 261–265. [Google Scholar]
61. Ehsani-Moghaddam, B., Charles, M. T., Carisse, O., Khanizadeh, S. (2006). Superoxide dismutase responses of strawberry cultivars to infection by Mycosphaerella fragariae. Journal of Plant Physiology, 163(2), 147–153. DOI 10.1016/j.jplph.2005.04.025. [Google Scholar] [CrossRef]
62. Govrin, E. M., Levine, A. (2000). The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Current Biology, 10(13), 751–757. DOI 10.1016/S0960-9822(00)00560-1. [Google Scholar] [CrossRef]
63. Whitham, S. A., Yang, C., Goodin, M. M. (2006). Global impact: elucidating plant responses to viral infection. Molecular Plant-Microbe Interactions, 19(11), 1207–1215. DOI 10.1094/MPMI-19-1207. [Google Scholar] [CrossRef]
64. Malamy, J., Carr, J. P., Klessig, D. F., Raskin, I. (1990). Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science, 250(4983), 1002–1004. DOI 10.1126/science.250.4983.1002. [Google Scholar] [CrossRef]
65. Kachroo, P., Yoshioka, K., Shah, J., Dooner, H. K., Klessig, D. F. (2000). Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell, 12(5), 677–690. DOI 10.1105/tpc.12.5.677. [Google Scholar] [CrossRef]
66. Love, A. J., Yun, B. W., Laval, V., Loake, G. J., Milner, J. J. (2005). Cauliflower mosaic virus, a compatible pathogen of Arabidopsis, engages three distinct defense-signaling pathways and activates rapid systemic generation of reactive oxygen species. Plant Physiology, 139(2), 935–948. DOI 10.1104/pp.105.066803. [Google Scholar] [CrossRef]
67. Thaler, J. S., Karban, R., Ullman, D. E., Boege, K., Bostock, R. M. (2002). Crosstalk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia, 131(2), 227–235. DOI 10.1007/s00442-002-0885-9. [Google Scholar] [CrossRef]
68. Casteel, C. L., De Alwis, M., Bak, A., Dong, H., Whitham, S. A. et al. (2015). Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiology, 169(1), 209–218. DOI 10.1104/pp.15.00332. [Google Scholar] [CrossRef]
69. Bellés, J. M., Garro, R., Fayos, J., Navarro, P., Primo, J. et al. (1999). Gentisic acid as a pathogen-inducible signal, additional to salicylic acid for activation of plant defenses in tomato. Molecular Plant-Microbe Interactions, 12(3), 227–235. DOI 10.1094/MPMI.1999.12.3.227. [Google Scholar] [CrossRef]
70. Yang, L. P., Fang, Y. Y., An, C. P., Dong, L., Zhang, Z. H. et al. (2013). C 2-mediated decrease in DNA methylation, accumulation of si RNA s, and increase in expression for genes involved in defense pathways in plants infected with beet severe curly top virus. Plant Journal, 73(6), 910–917. DOI 10.1111/tpj.12081. [Google Scholar] [CrossRef]
71. Atsumi, G., Kagaya, U., Kitazawa, H., Nakahara, K. S., Uyeda, I. (2009). Activation of the salicylic acid signaling pathway enhances Clover yellow vein virus virulence in susceptible pea cultivars. Molecular Plant-Microbe Interactions, 22(2), 166–175. DOI 10.1094/MPMI-22-2-0166. [Google Scholar] [CrossRef]
72. van Verk, M. C.,Pappaioannou, D., Neeleman, L., Bol, J. F., Linthorst, H. J. (2008). A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiology, 146(4), 1983–1995. DOI 10.1104/pp.107.112789. [Google Scholar] [CrossRef]
73. Arena, G. D., Ramos-González, P. L., Nunes, M. A., Ribeiro-Alves, M., Camargo, L. E. et al. (2016). Citrus leprosis virus C infection results in hypersensitive-like response, suppression of the JA/ET plant defense pathway and promotion of the colonization of its mite vector. Frontiers in Plant Science, 7, 1757. DOI 10.3389/fpls.2016.01757. [Google Scholar] [CrossRef]
74. Deng, B., Fang, S., Shang, X., Fu, X., Yang, W. (2019). Influence of genotypes and environmental factors on leaf triterpenoid content and growth of Cyclocarya paliurus. Journal of Forestry Research, 30(3), 789–798. DOI 10.1007/s11676-018-0680-z. [Google Scholar] [CrossRef]
75. Theapparat, Y., Khongthong, S., Rodjan, P., Lertwittayanon, K., Faroongsarng, D. (2019). Physicochemical properties and in vitro antioxidant activities of pyroligneous acid prepared from brushwood biomass waste of Mangosteen, Durian, Rambutan, and Langsat. Journal of Forestry Research, 30(3), 1139–1148. DOI 10.1007/s11676-018-0675-9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |