 Open Access
Open Access
ARTICLE
A Nomogram for Predicting Survival for Patients with Brain Metastatic and EGFR Mutation Advanced Non-Small Cell Lung Cancer
1 Department of Thoracic Oncology and State Key Laboratory of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, 610041, China
2 West China School of Medicine, Sichuan University, Chengdu, 610041, China
3 West China School of Medicine, Department of Postgraduate Students, Sichuan University, Chengdu, 610041, China
4 Department of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, 610041, China
* Corresponding Authors: QIN ZHANG. Email: ; HUASHAN SHI. Email:
# Contributed equally to this study
Oncology Research 2025, 33(4), 895-904. https://doi.org/10.32604/or.2024.053363
Received 29 April 2024; Accepted 05 August 2024; Issue published 19 March 2025
Abstract
Background: Non-small cell lung cancer (NSCLC) is often accompanied by brain metastasis (BM), and the prognosis of patients with BM is poor. This study assesses the prognostic impact of BM in NSCLC patients with epidermal growth factor receptor (EGFR) mutations. Methods: We retrospectively evaluated 692 advanced NSCLC patients with EGFR mutations treated with tyrosine kinase inhibitors (TKIs) at West China Hospital from 2015 to 2019. The overall survival rate (OS), progression-free survival rate (PFS), objective response rate (ORR), disease control rate (DCR), and clinical parameters of the BM and non-BM groups were compared. Univariable and multivariable regressions were performed to identify independent prognostic factors, followed by validation of a predictive nomogram using receiver operating characteristics and calibration curves. Immune infiltration in tumor tissues was assessed by immunostaining. Results: NSCLC patients with BM exhibited a higher frequency of other-site and multi-organ metastases than those without BM. The BM group demonstrated significantly worse OS (26.2 vs. 39.1 months, p < 0.001) and PFS (12.3 vs. 18.8 months, p < 0.001), although the DCR (p = 0.831) and ORR (p = 0.653) were similar in both groups. BM was identified as an independent predictor of poor prognosis. The nomogram performed well, achieving a C index of 0.73, with consistent calibration curves for predicted and actual prognoses. Additionally, fewer peripheral lymphocytes were observed in the BM group. Conclusions: BM is a significant risk factor for NSCLC patients, potentially linked to lymphocytopenia.Keywords
Lung cancer is the most prevalent malignancy worldwide and also ranks first in terms of mortality rates [1]. Non-small cell lung cancer (NSCLC) accounts for 85% of all lung tumors and is histologically classified into adenocarcinomas (LUAD), squamous cell carcinomas (LUSC), and large cell carcinomas [2]. An estimated 20%–40% of the patients in the advanced stages of cancer develop brain metastases (BM), and lung cancer alone accounts for 59% of all BM [3]. BM occurs in almost half of the lung cancer cases and significantly worsens patient prognosis [4–6]. Furthermore, epidermal growth factor receptor (EGFR) mutations occur in 44%–63% of NSCLC patients and are established driver oncogenes for NSCLC that can increase the risk of BM [7,8]. EGFR-targeting tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib, erlotinib, and afatinab, have shown promising results in treating advanced NSCLC [9]. Compared to cytotoxic drugs or whole brain radiotherapy (WBRT), EGFR-TKIs can reduce the progression risk of the central nervous system (CNS) in NSCLC patients harboring EGFR mutations. In addition, EGFR-TKIs have demonstrated significant clinical benefits in terms of progression-free survival (PFS) and objective response rate (ORR), with more tolerable side effects [10–12].
The tumor microenvironment, especially the immune landscape, is a key determinant of patient prognosis and treatment outcomes [13,14]. However, whether the intracranial lymphatic system influences the survival of cancer patients with BM is unclear. This study aimed to determine the impact of BM on the prognosis of NSCLC patients treated with EGFR-TKIs and elucidate the potential mechanisms from the perspective of the immune system.
The Hospital Information System of West China Hospital, Chengdu, China was screened for treatment-naïve patients with pathologically confirmed, EGFR-mutated, metastatic NSCLC, who received EGFR-TKI as first-line therapy. The inclusion criteria were: (1) histologically confirmed stage IV metastatic NSCLC; (2) first-line chemotherapy (EGFR-TKI) as the sole therapy; (3) Eastern Cooperative Oncology Group (ECOG) performance status of 0–3; and (4) with EGFR mutation. The Human Investigation Committee (IRB) of West China Hospital, Sichuan University approved this study (20211349). Informed written consent was obtained from all study participants. Following the criteria, 692 patients were selected and divided into the BM (n = 346) and non-BM (n = 346) groups. The median age was 58 years in the BM group and 59 years in the non-BM group. TNM staging was established according to the guidelines of the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control [15] Data on age, gender, smoking history, histological classification, progression type, therapy (including targeted therapy with or without chemotherapy, radiation, or immunotherapy), and peripheral lymphocyte counts were collected. The range of lymphocyte and cutoff values was determined based on laboratory parameters and prior reports [16–18]. The United Nations has identified 60 years as a criterion for the older population in developing countries [19,20]. PFS was defined as the time from initiating first-line therapy to confirmed disease progression or death, and overall survival (OS) was defined as the time from diagnosis to death from any cause or the date of the last follow-up. Follow-up was conducted at regular intervals until death or until December 2019 through clinical visits or telephone interviews.
The diagnostic method was selected per the judgment of the attending physician. According to the current diagnostic guidelines, possible BM was detected using enhanced computed tomography (CT) scans. Enhanced magnetic resonance imaging (MRI) or 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) imaging was performed in cases where CT scans were insufficient to determine the presence of BM. All diagnoses were verified by two or more oncologists and imaging specialists, and the diagnostic guidelines were consistent during the observation period.
Statistical analyses were conducted using SPSS version 26.0 (IBM Corp., Armonk, NY, USA) and R (R packages, version 4.2.2). The following R packages were used: survival (version 3.2-13) for survival analysis, UpSetR (version 1.4.0) for Fig. 1, survminer (version 0.4.9) for Fig. 2, MatrixModels (version 0.5-0), haven (version 2.5.1), stringi (version 1.7.6), Hmisc (version 5.1-1), lattice (version 0.20-45), Formula (version 1.2-5), ggplot2 (version 3.4.4), foreign (version 0.8-82), rms (version 6.5-0) and timeROC (version 0.4) for Fig. 3 [16,21]. According to Cochran’s rule, the Chi-square test or Fischer’s exact test was employed to compare qualitative variables. PFS and OS curves for the BM and non-BM groups were plotted using the Kaplan-Meier method, and survival rates were compared using the log-rank test. Independent predictive factors of BM were further identified through multivariate analysis using a Cox proportional hazards model. Factors demonstrating significant association in the univariate analysis (p < 0.05) were included in the multivariate analysis. The independent variables were incorporated into the Cox regression model using the enter method. The independent risk factors were included in a prognostic nomogram. Receiver operating characteristic (ROC) analysis, decision curve analysis (DCA), and calibration curves were plotted to evaluate the nomogram’s predictive accuracy, clinical applicability, and discriminative ability. p-values < 0.05 were deemed statistically significant.
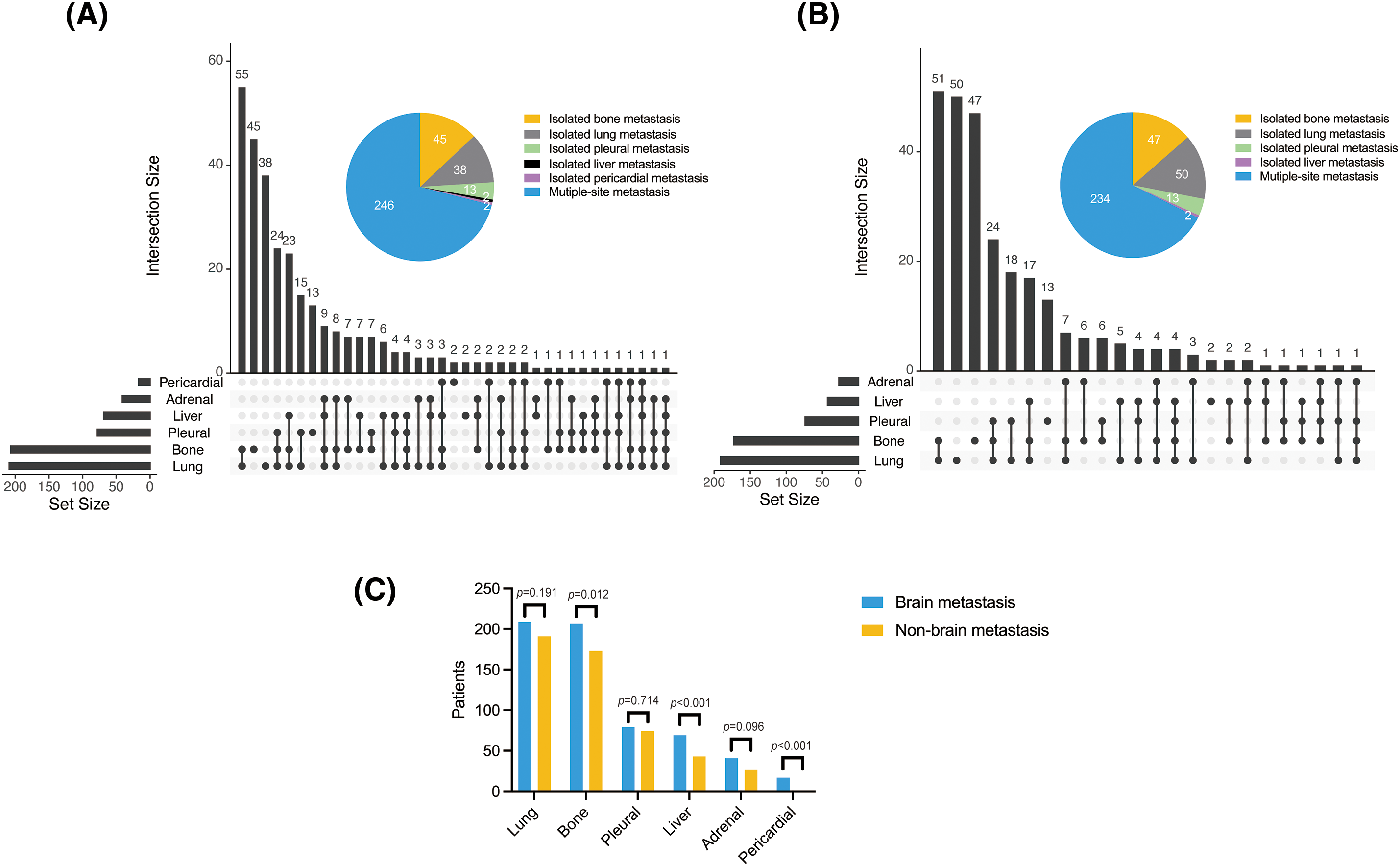
Figure 1: Organ metastases in the (A) BM and (B) non-BM groups, and (C) comparison of both groups

Figure 2: BM affects the survival and treatment outcomes for NSCLC patients. (A and B) The OS and PFS of NSCLC patients in the (A) BM and (B) non-BM groups after EGFR-TKIs therapy. PR/CR in the (C) BM and (D) non-BM groups. (E) Column chart showing ORR in the BM and non-BM groups. HR, hazard ratio; PR, partial response; SD, stable disease; PD, progressive disease; CR, complete response.
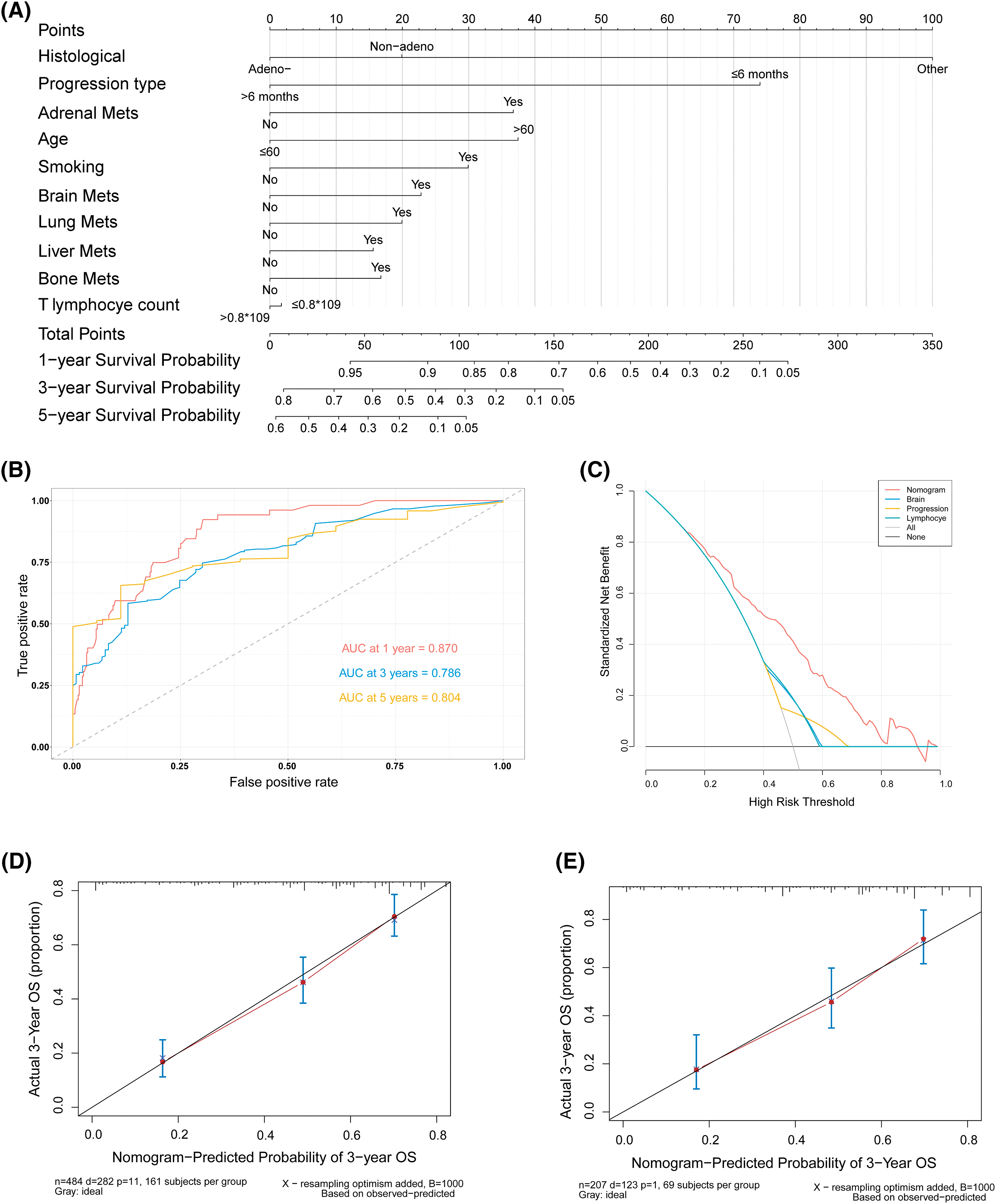
Figure 3: A nomogram and the validation for predicting the OS of patients. (A) Nomogram for predicting 1-, 3-, and 5-year OS of 484 patients (7/10 of the total number) obtained by random sampling. (B) ROC curves for 1-, 3-, and 5-year OS in the training cohort. (C) DCA for the nomogram and three other factors. (D) Calibration curves for internal validation to predict 3-year OS in the training cohort. (E) Calibration curves for external validation of 3-year OS with 3/10 of the total number of patients (n = 208) obtained by random sampling. AUC, the area under the curve; DCA, decision curve analysis; ROC, receiver operating characteristic
A total of 692 patients were included in this study, comprising 274 (39.6%) males and 418 (60.4%) females. The mean age of the participants was 58.4 years, ranging from 22 to 83 years. All patients were categorized into the BM (n = 346) and non-BM (n = 346) groups. Gender distribution, smoking history, EGFR mutation, and other clinical parameters were similar in both groups. Patients with BM tended to be younger (median age 58 years) than those in the non-BM group (median age 59 years), albeit without statistical significance. EGFR mutations included exon 19 deletion (n = 350 [50.6%]), L858 substitution (n = 297 [42.9%]), and others (n = 45 [6.5%]). Additionally, 666 (96.2%) of the patients were diagnosed with adenocarcinomas, among whom 50% had BM and the remaining 50% did not exhibit BM (non-BM group). Patients received targeted therapy alone (n = 471 [68.1%]) or in combination with chemotherapy, radiation, or immunotherapy (n = 221 [31.9%], Table 1).
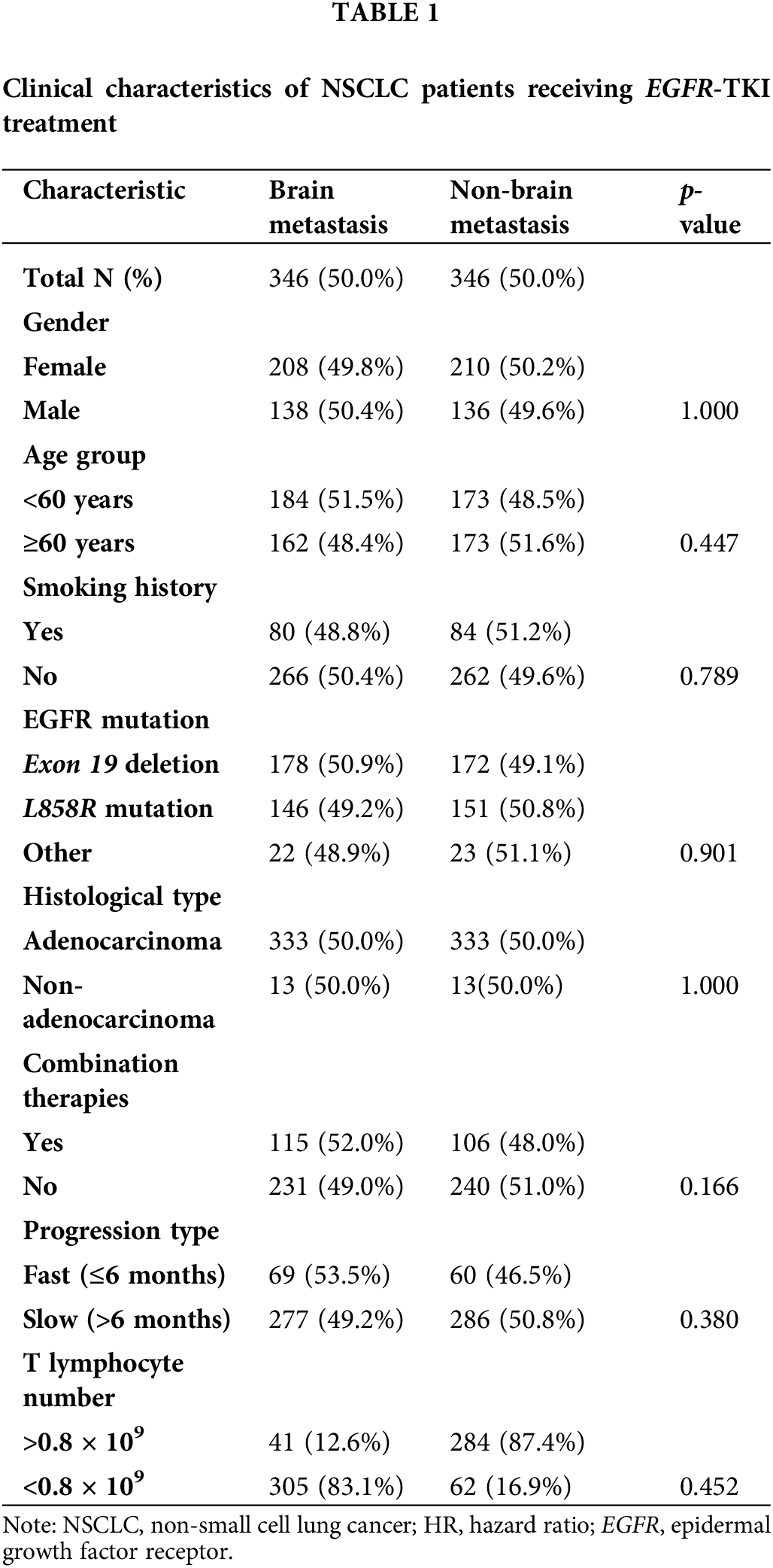
The lungs (60.4%), bone (59.8%), pleura (22.8%), liver (19.9%), adrenal glands (11.8%), and pericardium (4.9%) were most frequently colonized in the BM group. Lung metastases were defined as significantly enhanced bilateral lung lesions in contrast-enhanced CT images. And tumor lesions beyond the unilateral lung and regional node were determined as distant metastases. Thoracic radiologists independently reviewed these images to diagnose lung metastases. Notably, lung cancer can spread directly through the airways, facilitating metastasis within the lungs. In the non-BM group, metastasis was detected in the lungs (55.2%), bones (50%), pleura (21.4%), liver (12.4%), and adrenal glands (7.8%), but not in the pericardium. Bone (p = 0.012), liver (p < 0.001), and pericardial (p < 0.001) metastases were more common in the BM group, and the frequencies of adrenal (p = 0.096), lung (p = 0.191), and pleural (p = 0.714) metastases were slightly higher in the BM group compared to the non-BM group, albeit without statistical significance. In addition, the frequency of multiple organ metastasis (≥3) in the BM group was 27.7% (96/346) compared to only 19.7% (68/346) in the non-BM group (p < 0.001; Fig. 1). Thus, BM in NSCLC patients increase the likelihood of metastases to other organs.
The median follow-up times for the BM and non-BM groups were 42.1 months (95% confidence interval (CI): 37.1–47.1 months) and 40.3 months (95% CI: 38.0–42.6 months), respectively. The BM group had a significantly shorter median OS of 26.2 months (95% CI: 24.2–30.4 months) compared to 39.1 months (95% CI: 32–46 months) in the non-BM group (hazard ratio (HR) = 1.612, 95% CI: 1.322–1.965, p < 0.001). Likewise, the median PFS was only 12.3 months (95% CI: 11.5–13.6 months) in the BM group compared to 18.8 months (95% CI: 16–21 months) in the non-BM group (HR = 1.73, 95% CI: 1.420–2.108), p < 0.001; Fig. 2A,B). One patient in each BM group and the non-BM group achieved CR (Fig. 2C,D). The DCR was similar for both groups at 87.28% and 88.15%, respectively (p = 0.653), as was the ORR at 65.03% and 67.63%, respectively (p = 0.831) (Fig. 2E).
Univariate and multivariate Cox regression
Univariate analysis revealed that factors such as advanced age, gender, absence of exon deletion, L858R mutation, non-adenocarcinoma histological type, smoking, rapid progression, reduced T lymphocyte counts in the tumor, and metastases to the lung, liver, adrenal gland, bone, and brain were associated with poor OS and PFS. Variables showing significant associations with prognosis (p < 0.05) in the univariate analysis were included in the multivariate analyses. The multivariate analysis further identified age, histological type, smoking, type of progression, number of T lymphocytes, and metastases to the lung and distant sites as independent predictors of OS (Table 2). Additionally, age, histological type, type of progression, smoking, and metastases to the adrenal glands, bone, and brain were determined to be independent predictors of PFS (Table 3).
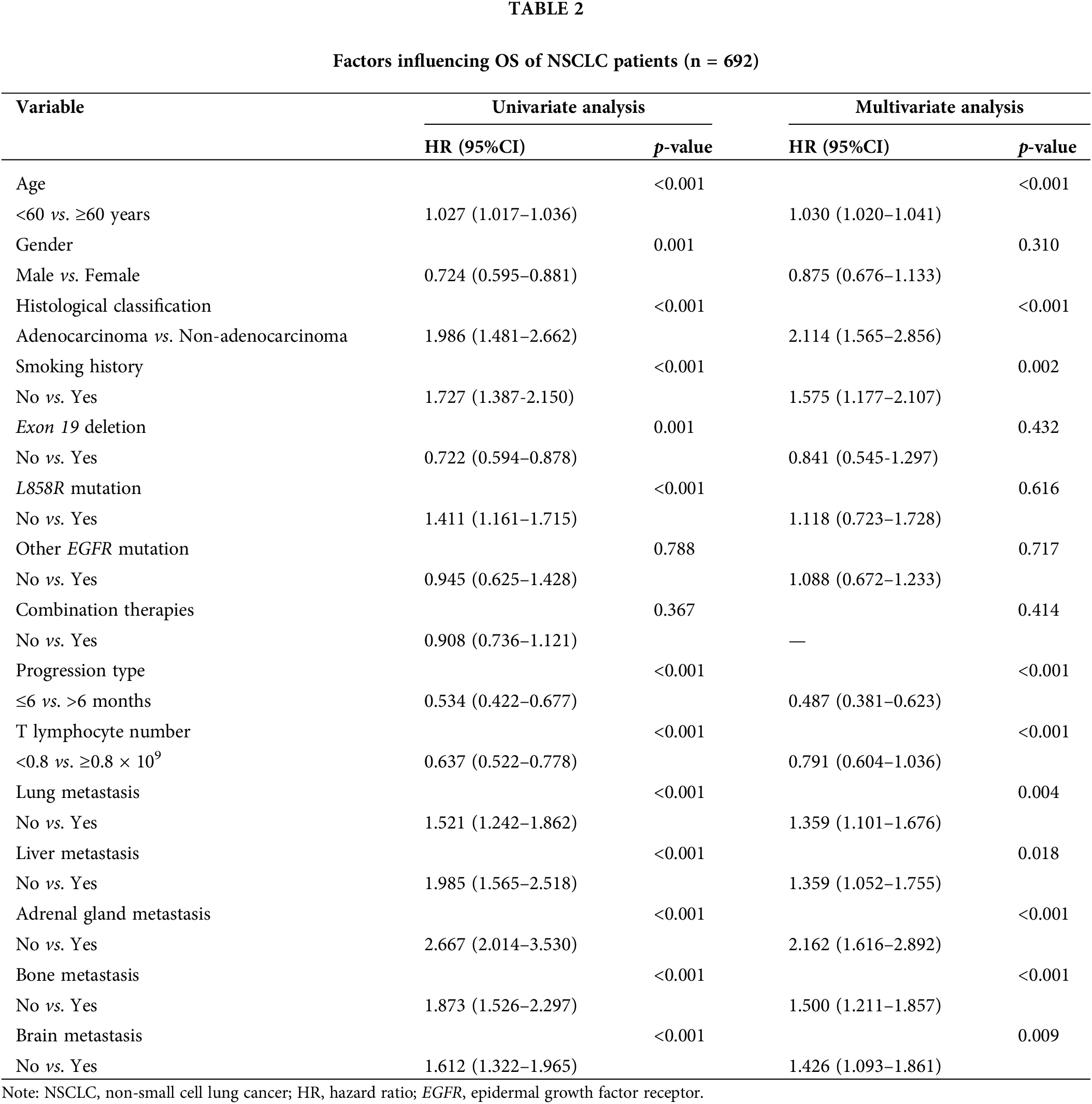
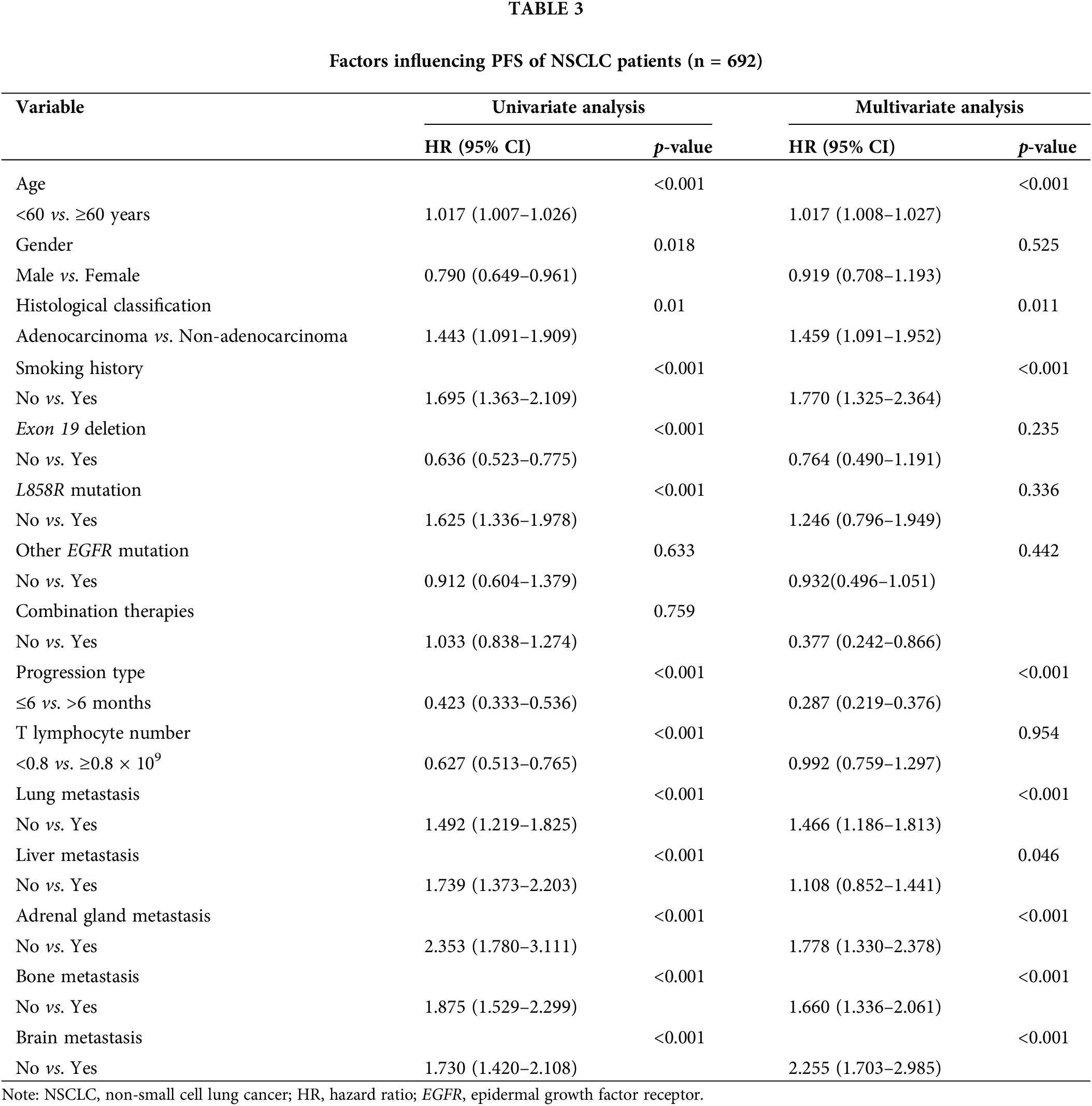
Nomogram development and validation
Based on the results above, a predictive nomogram for OS was developed using age, gender, histological classification, smoking history, progression type, T lymphocyte number, and multi-organ metastases as the independent risk factors (Fig. 3A). 484 patients were included as the training cohort, obtained by random sampling. The total scores for predicting 1-, 3-, and 5-year OS were calculated by adding that of the individual predictive factors. The nomogram exhibited good discriminatory ability, as evidenced by the area under the ROC curves and a C-index of 0.73. The area under the curve summarized the overall diagnostic accuracy of the test (Fig. 3B), while the DCA indicated its clinical practicality (Fig. 3C) [22]. Furthermore, the actual and predicted 3-year OS rates were proven to be concordant with internal validation (484 patients) and external validation (the remaining 208 patients) (Fig. 3D–E).
Over 90% of cancer-related deaths are attributed to metastasis, and this process is regulated by multiple factors related to the tumor microenvironment [23,24]. Lung cancer cells preferentially metastasize to the brain, with the highest frequency of BM observed in small cell lung cancer (SCLC) at 31%, followed by LUADs at 21%, large cell carcinomas at 21%, and LUSCs at 8% [4,25]. Several hypotheses, starting from the “seed and soil” theory proposed by Paget in 1889, have been put forth to explain the mechanisms underlying tumor metastasis, including epithelial-mesenchymal transition (EMT), cancer stem cells, and circulating tumor cells (CTCs) [26]. Several studies have shown that BM negatively impacts the prognosis of lung cancer. While EGFR-TKIs have achieved higher efficacy than other treatment modalities, the impact of BM on the prognosis of NSCLC patients following EGFR-TKI therapy remains poorly understood.
Advanced age can significantly worsen both the overall and progression-free survival of lung cancer patients, which can be attributed to the age-related decline in immune function and poor tolerance to treatment [27]. Furthermore, adenocarcinomas have a better prognosis than non-adenocarcinomas, most likely due to the biological characteristics of LUADs and recent therapeutic advances [28]. Smoking has been associated with a significant increase in the risk of lung cancer [29]. There is evidence that smoking induces the aggregation of the M2-type tumor-associated macrophages (TAMs) around NSCLC tissues and accelerates tumor development [30]. In addition, patients with multi-organ metastases had poor OS and PFS, which may be associated with higher tumor load, more complications due to invasion of multiple organs, and poor treatment effect. Rapid tumor progression generally indicates a worse prognosis due to primary drug resistance, which enhances the proliferative capacity and invasiveness of tumor cells, leading to suboptimal treatment effects. Furthermore, secondary mutations in KRAS, C-MET, and TP53 [30] contribute to tumor heterogeneity and unpredictable cancer development, increasing malignancy and adversely affecting patient survival. Increasing evidence also suggests that BM negatively impacts the outcomes of EGFR-TKI therapy in NSCLC patients [31–33]. This effect could be attributed to the distinct genetic landscapes of the primary tumor and the metastatic clones, as well as the low concentration of the drug in cerebrospinal fluid.
Interestingly, the peripheral lymphocyte counts were markedly lower in the patients with BM than in the non-BM group in this study. According to the univariate Cox regression model, peripheral lymphocyte count <0.8 × 109/L correlated with worse OS, which underscores the impact of the tumor microenvironment on treatment outcomes and prognosis. Results from other studies have also indicated that the number of peripheral blood lymphocytes is negatively correlated with the survival of cancer patients [34,35]. Ikarashi et al. [36] reported lower infiltration and abundance of various immune cell populations in the BM microenvironment, including CD4+ T cells, CD8+ T cells, and Tregs. These novel insights regarding the tumor immune microenvironment could be crucial in optimizing therapeutic strategies by modulating immune functions and improving OS for NSCLC patients.
This study has certain limitations that should be addressed. First, there were inevitable biases due to its retrospective design. In addition, there may have been selection bias since the treatment was determined at the attending physician’s discretion rather than randomization. This is relevant as the radiological imaging tests used to detect BM differ in specificities and sensitivities. Furthermore, the sample size in the immunofluorescence experiments was small, which limits the generalization of the results. Finally, this was a single-center study, and the findings must be validated using multi-center cohorts.
BM often deteriorates the quality of life and imposes a significant financial burden on patients. Consequently, developing novel therapeutic strategies for NSCLC patients with BM is urgently needed. Cancer cells sensitive to EGFR-TKIs may retain their responsiveness to the targeted drug during tumor progression. Therefore, combining EGFR-TKIs with another treatment modality may inhibit the cancer sub-clones resistant to EGFR-TKIs. The FLAURA2 study demonstrated a longer median PFS in the osimertinib-chemotherapy group compared to the osimertinib group alone (24.9 months vs. 13.8 months) [37]. An ongoing phase III study, COMPEL (NCT04765059) [38], is comparing chemotherapy (pemetrexed plus platinum) plus osimertinib against chemotherapy plus placebo. Enhanced therapeutic outcomes are anticipated in the future. Additionally, combining EGFR-TKI with radiotherapy has been shown to prolong both CNS-PFS and OS in NSCLC patients with BM [39,40]. Further ongoing studies evaluate whether radiotherapy combined with EGFR-TKI is more effective than EGFR-TKI alone [41].
Our study strongly suggests that BM is an independent predictor of poor prognosis for NSCLC patients with EGFR mutations receiving EGFR-TKIs, which may be attributed to a decline in peripheral lymphocytes. NSCLC patients with BM are more likely to experience metastases in other organs and have shorter survival times. Additionally, we identified a unique pattern of lymphatic infiltration with drainage vessels in the brain tumor tissues. These findings provide novel insights into the role of BM in the progression and prognosis of NSCLC and may inform future treatment strategies.
Acknowledgement: Some data were obtained from the clinical database of palliative care for advanced lung cancer. We want to thank the Cancer Psychology and Health Management Committee of the Sichuan Cancer Society for their guidance.
Funding Statement: This work was supported by Sichuan Province Central Government Guide Local Science and Technology Development Project (No. 2023ZYD0169).
Author Contributions: Study conception and design: Qing Zhang, Huashan Shi; data collection: Weigang Xiu, Wenjing Liao; analysis and interpretation of results: Jiyun Pang, Yueyun Chen; draft manuscript preparation: Jiyun Pang, Weigang Xiu, Yueyun Chen. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: The Human Investigation Committee (IRB) of West China Hospital, Sichuan University approved this study (20211349). Informed written consent was obtained from all study participants.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. doi:10.3322/caac.v74.3. [Google Scholar] [PubMed] [CrossRef]
2. Relli V, Trerotola M, Guerra E, Alberti S. Abandoning the notion of non-small cell lung cancer. Trends Mol Med. 2019;25(7):585–94. doi:10.1016/j.molmed.2019.04.012. [Google Scholar] [PubMed] [CrossRef]
3. Hanibuchi M, Kim SJ, Fidler IJ, Nishioka Y. The molecular biology of lung cancer brain metastasis: an overview of current comprehensions and future perspectives. J Med Invest. 2014;61(3–4):241–53. [Google Scholar] [PubMed]
4. Yousefi M, Bahrami T, Salmaninejad A, Nosrati R, Ghaffari P, Ghaffari SH. Lung cancer-associated brain metastasis: molecular mechanisms and therapeutic options. Cell Oncol. 2017;40(5):419–41. doi:10.1007/s13402-017-0345-5. [Google Scholar] [PubMed] [CrossRef]
5. Budczies J, von Winterfeld M, Klauschen F, Bockmayr M, Lennerz JK, Denkert C, et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6(1):570–83. doi:10.18632/oncotarget.v6i1. [Google Scholar] [CrossRef]
6. Sørensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6(9):1474–80. doi:10.1200/JCO.1988.6.9.1474. [Google Scholar] [PubMed] [CrossRef]
7. Maity S, Pai KSR, Nayak Y. Advances in targeting EGFR allosteric site as anti-NSCLC therapy to overcome the drug resistance. Pharmacol Rep. 2020;72(4):799–813. doi:10.1007/s43440-020-00131-0. [Google Scholar] [PubMed] [CrossRef]
8. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–11. doi:10.1016/j.lungcan.2015.01.020. [Google Scholar] [PubMed] [CrossRef]
9. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi:10.1056/NEJMoa0810699. [Google Scholar] [PubMed] [CrossRef]
10. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZresults from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–14. [Google Scholar] [PubMed]
11. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi:10.1056/NEJMoa1913662. [Google Scholar] [PubMed] [CrossRef]
12. Heon S, Yeap BY, Lindeman NI, Joshi VA, Butaney M, Britt GJ, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18(16):4406–14. doi:10.1158/1078-0432.CCR-12-0357. [Google Scholar] [PubMed] [CrossRef]
13. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221(2019):107753. doi:10.1016/j.pharmthera.2020.107753. [Google Scholar] [PubMed] [CrossRef]
14. Peng H, Wu X, Zhong R, Yu T, Cai X, Liu J, et al. Profiling tumor immune microenvironment of non-small cell lung cancer using multiplex immunofluorescence. Front Immunol. 2021;12:750046. doi:10.3389/fimmu.2021.750046. [Google Scholar] [PubMed] [CrossRef]
15. Chassagnon G, Bennani S, Revel MP. New TNM classification of non-small cell lung cancer. Rev Pneumol Clin. 2017;73(1):34–9 (In French). doi:10.1016/j.pneumo.2016.12.006. [Google Scholar] [PubMed] [CrossRef]
16. Xiu W, Zheng J, Zhou Y, Du H, Li J, Li W, et al. A nomogram for the prediction of the survival of patients with advanced non-small cell lung cancer and interstitial lung disease. Cancer Med. 2023;12(10):11375–84. doi:10.1002/cam4.v12.10. [Google Scholar] [CrossRef]
17. Cho BC, Pas TD, Kalofonos H, Wang Q, Ramlau R, Cheng Y, et al. Prognostic factors in early-stage NSCLC: analysis of the placebo group in the MAGRIT study. Anticancer Res. 2019;39(3):1403–9. doi:10.21873/anticanres.13255. [Google Scholar] [PubMed] [CrossRef]
18. Fuchs J, Früh M, Papachristofilou A, Bubendorf L, Häuptle P, Jost L, et al. Resection of isolated brain metastases in non-small cell lung cancer (NSCLC) patients-evaluation of outcome and prognostic factors: a retrospective multicenter study. PLoS One. 2021;16(6):e0253601. doi:10.1371/journal.pone.0253601. [Google Scholar] [PubMed] [CrossRef]
19. Liu J, Li C, Wang Y, Ji P, Guo S, Zhai Y, et al. Prognostic and predictive factors in elderly patients with Glioblastoma: a single-center retrospective study. Front Aging Neurosci. 2021;13:777962. [Google Scholar] [PubMed]
20. Ikawa F, Kinoshita Y, Takeda M, Saito T, Yamaguchi S, Yamasaki F, et al. Review of current evidence regarding surgery in elderly patients with Meningioma. Neurol Med Chir. 2017;57(10):521–33. doi:10.2176/nmc.ra.2017-0011. [Google Scholar] [PubMed] [CrossRef]
21. Xie D, Marks R, Zhang M, Jiang G, Jatoi A, Garces YI, et al. Nomograms predict overall survival for patients with small-cell lung cancer incorporating pretreatment peripheral blood markers. J Thorac Oncol. 2015;10(8):1213–20. doi:10.1097/JTO.0000000000000585. [Google Scholar] [PubMed] [CrossRef]
22. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–6. doi:10.1097/JTO.0b013e3181ec173d. [Google Scholar] [PubMed] [CrossRef]
23. Zhao B, Zhang W, Yu D, Xu J, Wei Y. Erlotinib in combination with bevacizumab has potential benefit in non-small cell lung cancer: a systematic review and meta-analysis of randomized clinical trials. Lung Cancer. 2018;122:10–21. doi:10.1016/j.lungcan.2018.05.011. [Google Scholar] [PubMed] [CrossRef]
24. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer, 1975–2014. Featuring Survival J Natl Cancer Inst. 2017;109(9):djx030. [Google Scholar] [PubMed]
25. Gaspar LE. Brain metastases in lung cancer. Expert Rev Anticancer Ther. 2004;4(2):259–70. doi:10.1586/14737140.4.2.259. [Google Scholar] [PubMed] [CrossRef]
26. Wang Y, Chen R, Wa Y, Ding S, Yang Y, Liao J, et al. Tumor immune microenvironment and immunotherapy in brain metastasis from non-small cell lung cancer. Front Immunol. 2022;13:829451. doi:10.3389/fimmu.2022.829451. [Google Scholar] [PubMed] [CrossRef]
27. Liu XY, Zhang X, Zhang Q, Xie HL, Ruan GT, Liu T, et al. Value of the controlling nutritional status score in predicting the prognosis of patients with lung cancer: a multicenter, retrospective study. JPEN J Parenter Enteral Nutr. 2022;46(6):1343–52. doi:10.1002/jpen.v46.6. [Google Scholar] [CrossRef]
28. Wu B, Wei S, Tian J, Song X, Hu P, Cui Y. Comparison of the survival time in the non-small cell lung cancer patients with different organ metastasis. Zhongguo Fei Ai Za Zhi. 2019;22(2):105–10 (In Chinese). [Google Scholar] [PubMed]
29. Tang A, Ahmad U, Toth AJ, Bourdakos N, Raja S, Raymond DP, et al. Non-small cell lung cancer in never- and ever-smokers: is it the same disease? J Thorac Cardiovasc Surg. 2021;161(6):1903–17.e9. doi:10.1016/j.jtcvs.2020.03.175. [Google Scholar] [PubMed] [CrossRef]
30. Cheng C, Wang P, Yang Y, Du X, Xia H, Liu J, et al. Smoking-induced M2-TAMs, via circEML4 in EVs, promote the progression of NSCLC through ALKBH5-regulated m6A modification of SOCS2 in NSCLC cells. Adv Sci. 2023;10(22):e2300953. doi:10.1002/advs.v10.22. [Google Scholar] [CrossRef]
31. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25. doi:10.1056/NEJMoa1713137. [Google Scholar] [PubMed] [CrossRef]
32. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(33):Jco2018783118. doi:10.1200/JCO.2018.78.3118. [Google Scholar] [PubMed] [CrossRef]
33. Hsu F, De Caluwe A, Anderson D, Nichol A, Toriumi T, Ho C. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer. 2016;96:101–7. doi:10.1016/j.lungcan.2016.04.004. [Google Scholar] [PubMed] [CrossRef]
34. Aldarouish M, Su X, Qiao J, Gao C, Chen Y, Dai A, et al. Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer. Int J Immunopathol Pharmacol. 2019;33(2):2058738419839592. doi:10.1177/2058738419839592. [Google Scholar] [PubMed] [CrossRef]
35. Vicente Conesa MA, Garcia-Martinez E, Gonzalez Billalabeitia E, Chaves Benito A, Garcia Garcia T, Vicente Garcia V, et al. Predictive value of peripheral blood lymphocyte count in breast cancer patients treated with primary chemotherapy. Breast. 2012;21(4):468–74. doi:10.1016/j.breast.2011.11.002. [Google Scholar] [PubMed] [CrossRef]
36. Ikarashi D, Okimoto T, Shukuya T, Onagi H, Hayashi T, Sinicropi-Yao SL, et al. Comparison of tumor microenvironments between primary tumors and brain metastases in patients with NSCLC. JTO Clin Res Rep. 2021;2(10):100230. doi:10.1016/j.jtocrr.2021.100230. [Google Scholar] [PubMed] [CrossRef]
37. Planchard D, Jänne PA, Cheng Y, Yang JC, Yanagitani N, Kim SW, et al. Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N Engl J Med. 2023;389(21):1935–48. doi:10.1056/NEJMoa2306434. [Google Scholar] [PubMed] [CrossRef]
38. Sequist LV, Peled N, Tufman A, Servidio L, Li J, Taylor R, et al. P47.11 COMPEL: chemotherapy with/without osimertinib in patients with EGFRm advanced NSCLC and progression on first-line osimertinib. J Thorac Oncol. 2021;16(10):S1101. doi:10.1016/j.jtho.2021.08.504. [Google Scholar] [CrossRef]
39. Jiang T, Min W, Li Y, Yue Z, Wu C, Zhou C. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta-analysis. Cancer Med. 2016;5(6):1055–65. doi:10.1002/cam4.2016.5.issue-6. [Google Scholar] [CrossRef]
40. Rosell R, Karachaliou N. Brain metastases in patients with EGFR-mutant non-small-cell lung cancer. Lancet Respir Med. 2017;5(9):669–71. doi:10.1016/S2213-2600(17)30265-5. [Google Scholar] [PubMed] [CrossRef]
41. Popat S, Ahn MJ, Ekman S, Leighl NB, Ramalingam SS, Reungwetwattana T, et al. Osimertinib for EGFR-mutant non-small-cell lung cancer central nervous system metastases: current evidence and future perspectives on therapeutic strategies. Target Oncol. 2023;18(1):9–24. doi:10.1007/s11523-022-00941-7. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools