 Open Access
Open Access
ARTICLE
Comparative analysis of breast and lung cancer survival rates and clinical trial enrollments among rural and urban patients in Georgia
Winship Cancer Institute, Emory University School of Medicine, Emory University, Atlanta, 30322, USA
* Corresponding Author: TATIANA KURILO. Email:
Oncology Research 2024, 32(9), 1401-1406. https://doi.org/10.32604/or.2024.050266
Received 01 February 2024; Accepted 16 April 2024; Issue published 23 August 2024
Abstract
Objectives: Rural patients have poor cancer outcomes and clinical trial (CT) enrollment compared to urban patients due to attitudinal, awareness, and healthcare access differential. Knowledge of cancer survival disparities and CT enrollment is important for designing interventions and innovative approaches to address the stated barriers. The study explores the potential disparities in cancer survival rates and clinical trial enrollments in rural and urban breast and lung cancer patients. Our hypotheses are that for both cancer types, urban cancer patients will have longer 5-year survival rates and higher enrollment rates in clinical trials than those in rural counties. Methods: We compared breast and lung cancer patients’ survival rates and enrollment ratios in clinical trials between rural (RUCC 4-9) and urban counties in Georgia at a Comprehensive Cancer Center (CCC). To assess these differences, we carried out a series of independent samples t-tests and Chi-Square tests. Results: The outcomes indicate comparable 5-year survival rates across rural and urban counties for breast and lung cancer patients, failing to substantiate our hypothesis. While clinical trial enrollment rates demonstrated a significant difference between breast and lung cancer patients at CCC, no significant variation was observed based on rural or urban classification. Conclusion: These findings underscore the need for further research into the representation of rural patients with diverse cancer types at CCC and other cancer centers. Further, the findings have considerable implications for the initiation of positive social change to improve CT participation and reduce cancer survival disparities.Keywords
The rural populations have poor cancer survival outcomes compared to their urban counterparts [1]. The survival disparities have been attributed to the longer cancer pathway for rural populations due to attitudinal and awareness issues as well as the inherent inequalities in access to health and transport infrastructure between the rural and urban regions [2,3]. Further, rural-residing cancer patients have substantially lower CT participation rates due to several barriers, including poor transport system, limited health literacy and awareness, and longer travel time [4]. Research and knowledge on the extent of cancer survival disparities and CT enrollment differential are important as they offer useful insight to initiate relevant interventions to reduce inequalities in health access.
Medical science has experienced remarkable strides in cancer management, with clinical trials being pivotal for advancing efficacious therapies. However, patient engagement in clinical trials remains a challenge, with only 3% of adult cancer patients enrolling in such trials [5]. Addressing some of the barriers impeding patient participation is of paramount significance [6]. There is a paucity of studies focusing on clinical trial participation among rural cancer patients who grapple with inferior outcomes due to their restricted access to medical care, diagnostic delays, and a prevalence of emergency-based diagnoses [7]. The present study addresses this gap by assessing whether there is a difference in cancer survival rates among urban vs. rural Georgian patients with lung and breast cancer and whether there are different rates of participation in clinical trials conducted at a Comprehensive Cancer Center (CCC). The study also aims to assess whether enrolling in a CCC clinical trial impacts survival.
Our study’s three hypotheses are specified as:
HA1: Urban cancer patients will have a longer 5-year survival rate than rural cancer patients.
HA2: Urban cancer patients will participate in CCC clinical trials more frequently than rural patients.
HA3: Clinical trials participants will have longer survival rates than non-participants.
The study was conducted in Georgia, encompassing urban and rural locations [8]. The ages of the population studied were limited to 45–65 years because this study focused on the middle-aged cancer population, given the rising incidence rates of cancer in this age group [9,10]. Participants of all sexes, including male, female, and non-binary individuals, were included in this study. There was no randomization in this research. Power analysis was performed to determine the ideal sample size. The sample consisted of one-thousand, three-hundred and sixty-seven lung cancer patients (n = 1367) and two-thousand, two-hundred and sixty-four breast cancer patients (n = 2264). The sample was randomly selected in terms of rural-urban residence. For lung cancer patients, 1147 participants were urban patients while 221 were rural patients. Among the breast cancer patients, 2258 were from urban areas while only six were from rural areas.
Data to determine the 5-year survival rates for each rural-urban classification scheme (RUCC) level was taken from Georgia Cancer Registries for lung and breast cancer from 2010 to 2016. RUCC has nine categories, with 1–3 representing metropolitan areas of differing population sizes and 4–9 representing nonmetropolitan regions characterized by population size and proximity to metropolitan areas [11].
We compared the average 5-year survival rates for breast and lung cancer patients in rural and urban Georgia counties using independent sample t-tests. Before conducting the t-test analysis, we ensured the data met certain assumptions. First, the homogeneity of variance using Levene’s test was assessed, which returned non-significant results for both breast cancer (F = 4.91, p = 0.06) and lung cancer (F = 2.44, p = 0.16) tests. These findings validate that the assumption of variance homogeneity was met [12].
Additionally, we examined the normality of the data distribution using Shapiro-Wilk’s normality tests. The tests indicated non-significance for both breast cancer (W = 0.88, p = 0.16) and lung cancer (W = 0.92, p = 0.42) tests, suggesting that the survival rates approximated a normal distribution. With these assumptions confirmed, we proceeded to conduct independent sample t-tests.
We compared the frequency of rural patients in clinical trials to urban patients participating in clinical trials at the CCC in Georgia. The comparison was done between Georgia rural and urban patients with lung and breast cancer enrolled in CCC clinical trials, and Georgia rural patients with lung and breast cancer who were patients at the CCC but not enrolled in CCC clinical trials through a quantitative, non-experimental comparative application.
A series of Chi-Square tests of independence were conducted. The proportion of the population engaged in clinical trials was the dependent variable, while urban/rural designation and breast/lung cancer diagnosis constituted the independent factors.
Five-year relative cancer-related data were collected from the SEER Tumor Registry, CCC Tumor Registry (CCC), and OnCore subject accrual data (CCC). The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI) is an authoritative source of information on cancer incidence and survival in the United States. SEER currently collects and publishes cancer incidence and survival data from population-based cancer registries covering approximately 35% of the U.S. population [13]. Since this registry contains data from 1975 to 2017, the information utilized was from 2010 to 2016 (6 years). The OnCore Enterprise Research system, or OnCore, is a vendor-supported Clinical Trials Management System (CTMS).
OnCore allows research teams to track subject details for specific trials. OnCore was used in the study to access data on rural and urban CCC patients with lung and breast cancer enrolled in clinical trials [14]. The procedures followed were in accordance with the ethical standards of the Helsinki Declaration. The approval of the Emory University Institutional Review Board (IRB) Committee was obtained before data collection and statistical analysis [15]. The IRB approval number is STUDY00004364. The IRB has granted a complete waiver of HIPAA authorization and informed consent. Protected Health Information of which use or access has been determined to be necessary by the IRB: data related to cancer type and survival outcomes from OnCore, Winship Tumor Registry, Georgia Cancer Registry, Rural Georgia SEER Registry (zip codes are only HIPAA identifier).
Rural vs. urban area Georgian patients’ cancer survival rates
Breast Cancer: Of the patients with breast cancer living in urban areas, 89.13% were surviving at the 5-year mark. In contrast, 85.40% of the patients with breast cancer living in rural areas were surviving at the 5-year mark (Table 1). The t-test analysis was conducted to determine if the observed survival rates in urban and rural areas were significantly different. The t-test outcomes (Table 2) indicated that the disparity in breast cancer survival rates was not statistically significant (t(7) = 2.23, p = 0.06).
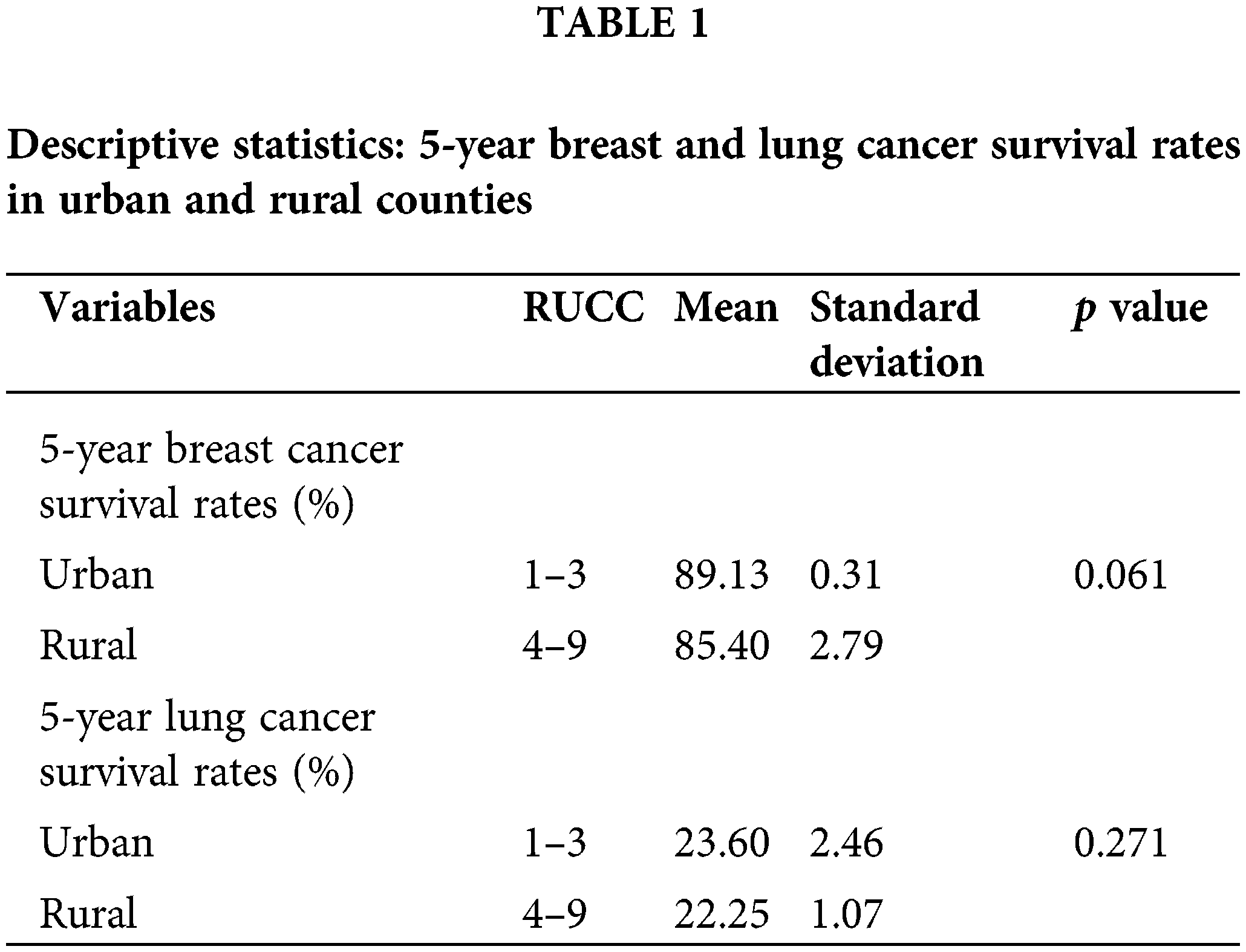
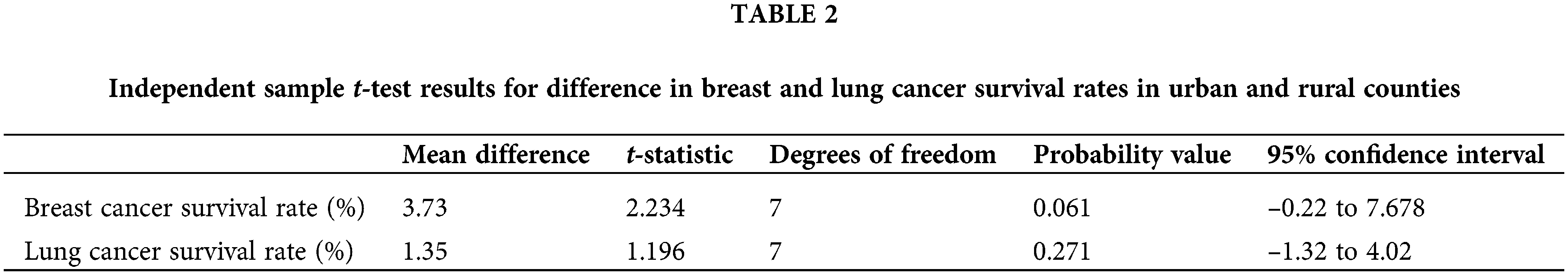
Lung Cancer: Comparatively, lung cancer survival rates were notably lower than breast cancer in both rural and urban counties in Georgia. Of the lung cancer patients in urban counties, only 23.60% were surviving at 5 years while 22.25% of lung cancer patients in rural counties were surviving at the 5-year mark.
The estimated mean difference did not exhibit statistical significance at the 5% level (t(7) = 1.20, p = 0.27). The results meant that there was no evidence to validate the hypothesis that the lung cancer survival rates were significantly higher among urban patients compared to rural patients.
Rural vs. urban area Georgian cancer patients’ clinical trials enrollment
Lung Cancer: There were 1,367 lung cancer and 2,264 breast cancer patients who were treated at the CCC during the period of interest, 2010 to 2016. When comparing the clinical trial enrollment rate by region, 8.2% of lung cancer patients from urban areas were enrolled in clinical trials (CT) compared to 5.5% of patients from rural areas (Table 3). The chi-square results are summarized in Table 4. The test was not significant (χ(1) = 1.94, p = 0.16) indicating the absence of differential CT enrollment rate between urban and rural lung cancer patients.
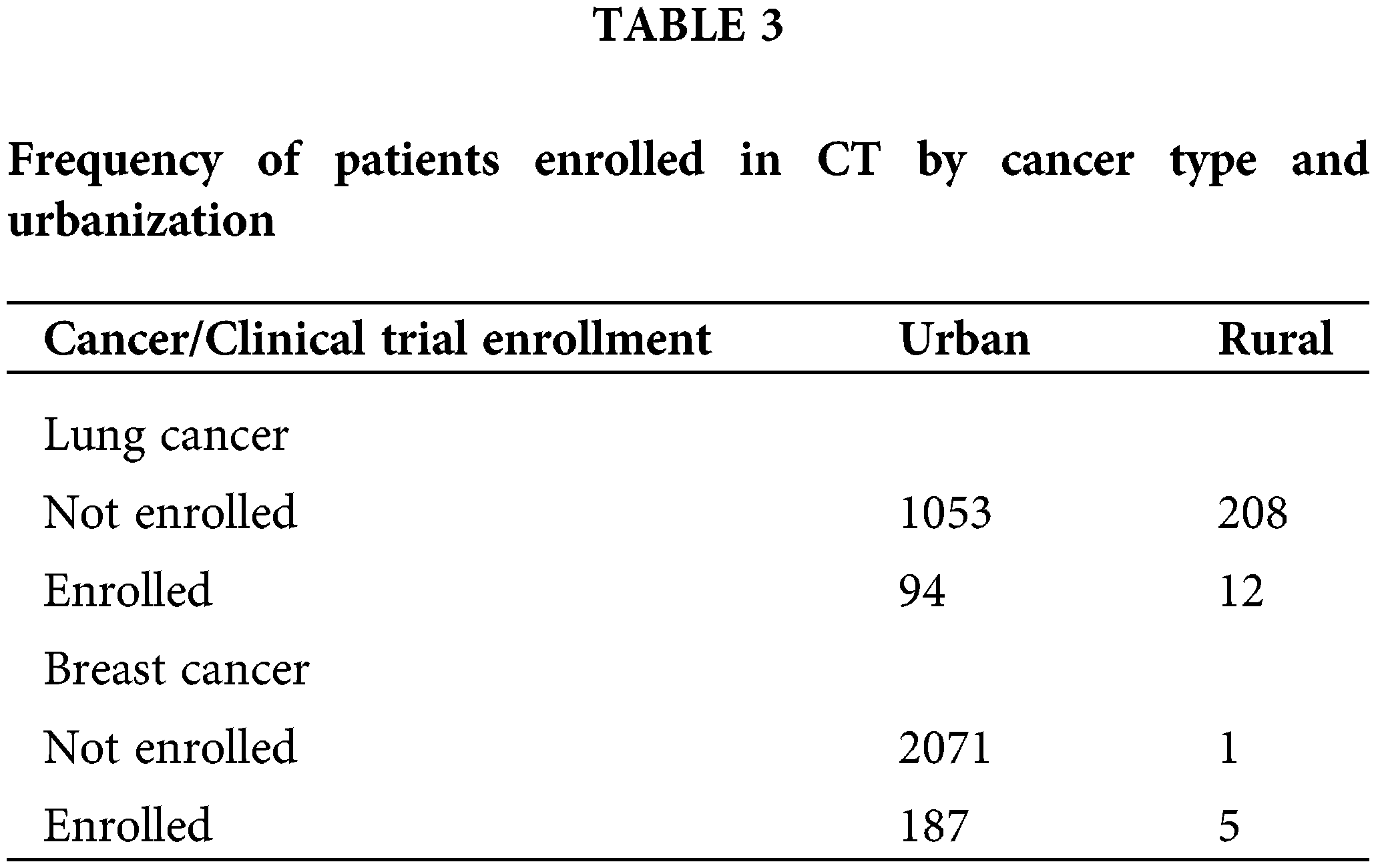
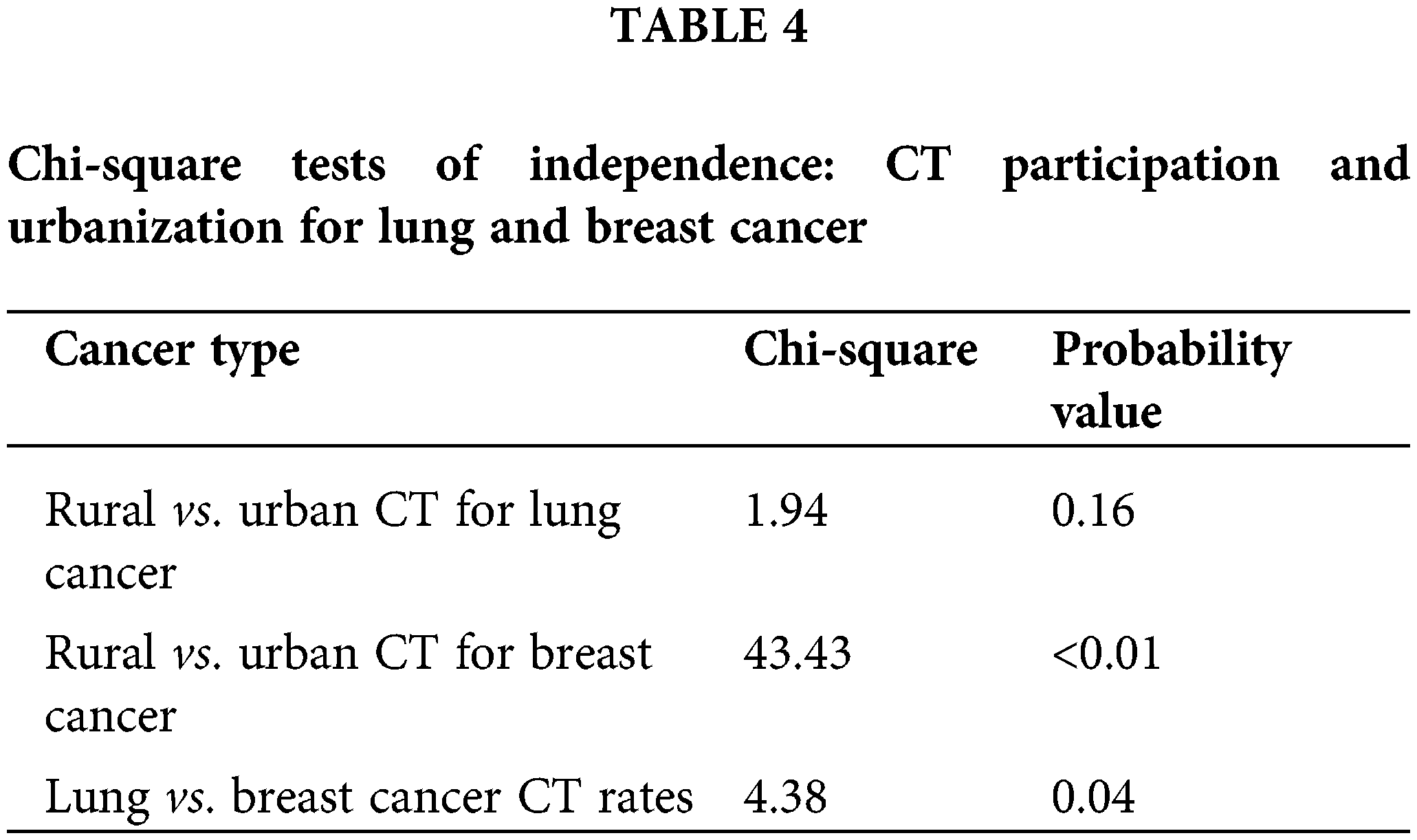
Breast Cancer: The analysis was repeated for breast cancer patients, among whom, 83.3% of rural patients were involved in clinical trials compared to 8% of urban patients. Even though chi-square analysis indicates a significantly different CT enrollment rate (χ(1) = 43.43, p < 0.01), the analysis was not reliable as one of the cells had an expected count of less than 5, violating a critical chi-square test assumption.
When considering the difference in the clinical trial enrollment rate of lung and breast cancer patients, breast cancer patients were shown to have a significantly higher CT enrollment rate compared to lung cancer patients (χ(1) = 4.38, p = 0.04). Overall, there was no evidence of significantly different CT enrollment rate of lung cancer patients in rural vs. urban areas while the sample of rural area breast cancer patients was too small to draw conclusions about the significance of the difference in CT participation rates. However, breast cancer patients participated in clinical trials at a higher rate than lung cancer patients.
Rural vs. urban area Georgian CCC CT participation cancer survival rates
A comparison of the 5-year survival rates among Georgia’s rural and urban patients enrolled in clinical trials, showed no significant difference based on t-test analysis (see Tables 5 and 6). Individual-level data were examined to assess this within a Comprehensive Cancer Center (CCC) in metropolitan Georgia. The results for the breast cancer patients indicate that on average, CCC clinical trial participants had higher cancer survival rates (M = 7.92, SD = 3.06) compared to non-participants (M = 7.46, SD = 3.89). The outcome of independent samples t-test confirmed that the difference in breast cancer survival rates by CCC clinical trial participation for both rural and urban patients was not statistically significant at 5% (t(249.58) = −1.93, p = 0.054).
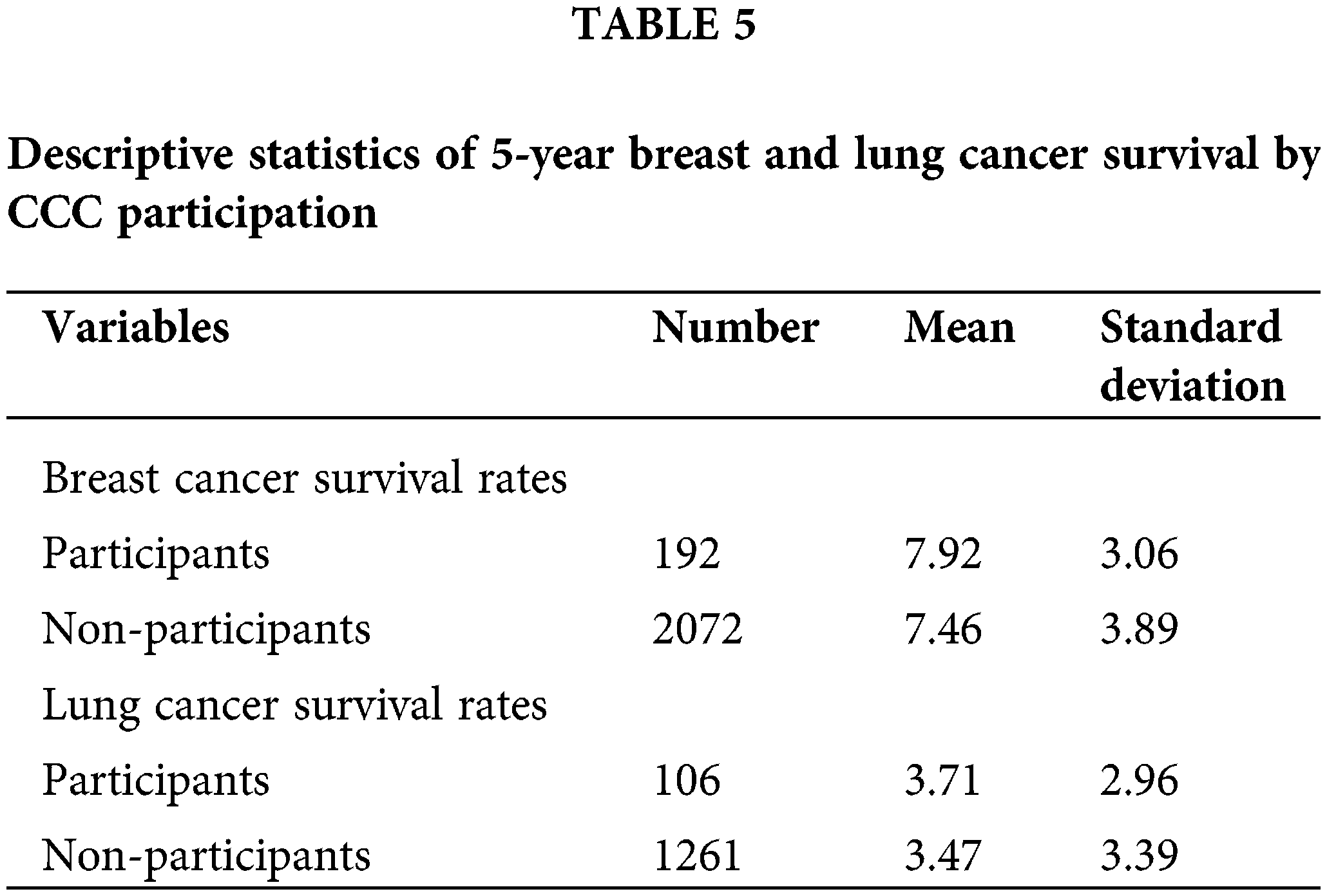
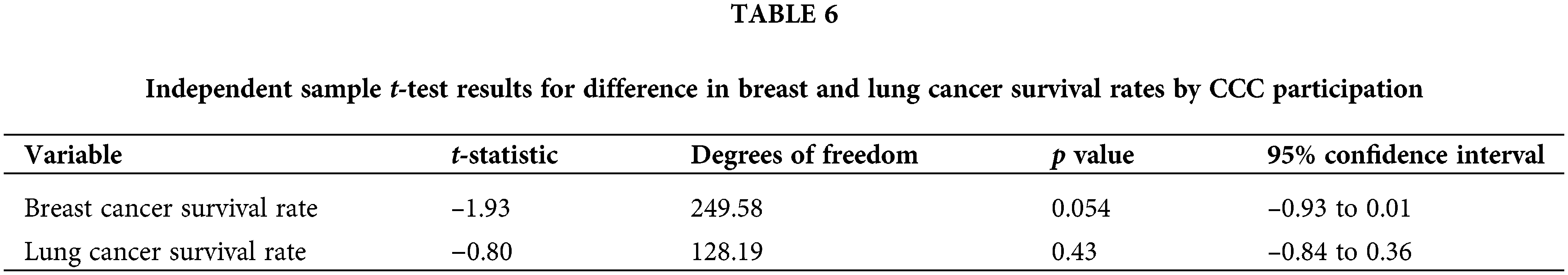
Similarly, for the lung cancer patients, on average, the CCC clinical trial participants had higher cancer survival chances (M = 3.71, SD = 2.96) compared to non-participants (M = 3.47, SD = 3.39). The findings based on the independent samples t-test analysis indicated that there was no significant difference in lung cancer survival rates for rural and urban patients by CCC clinical trial participation (t(128.19) = −0.80, p = 0.43).
The analysis results did not support the hypotheses of higher breast and lung cancer survival rates for urban patients compared to rural patients. There was no evidence to support HA1 indicating that in Georgia, there are comparable cancer survival rates among urban and rural patients. However, these findings contrast the insight based on earlier evidence [16], which showed that cancer mortality is higher in rural compared to urban areas of the USA.
Further, there was a significant disparity in the clinical trial enrollment rate of breast cancer and lung cancer patients. For lung cancer, urban areas had a higher mean enrollment percentage than rural areas. However, this difference was not statistically significant. In contrast, for breast cancer, rural areas had a much higher mean enrollment percentage than urban areas. Even though the difference was statistically significant, the small sample size resulted in the violation of a key chi-square assumption making the finding unreliable. The results are inconsistent with the outcome of previous studies [17,18], which showed that breast cancer patients from rural areas had higher total intervals and longer diagnostic intervals compared to their urban counterparts. Health literacy and attitudinal factors were the most important factors that contributed to the lower CT enrollment rate among rural patients.
There was no substantial evidence to support HA2 based on the findings of the independent samples t-test. For breast and lung cancer patients in rural and urban Georgia, there were comparable cancer survival rates between CCC clinical trial participants and non-participants. The results indicate that participation in CCC clinical trial did not have a significant effect on breast and lung cancer survival outcomes. The results are inconsistent with the findings of previous studies [19] where it has been noted that participation in clinical trials offers between cancer survival chances due to access to state-of-the-art cancer care that exceeds the standard care guidelines.
The study aimed to close the literature gap by comparing the survival rates and the enrollment ratios of breast and lung cancer patients from rural counties to those from urban counties. The presented data and statistical analysis provide valuable insights into the differences in cancer survival rates and clinical trial enrollment between rural and urban areas in Georgia. While it is commonly assumed that cancer patients in urban areas fare better than cancer patients in rural areas, the lack of significant differences in the 5-year survival rates and the clinical trial enrollment rate of breast and lung cancer patients indicates that for this sample the stated assumption was not substantiated. The lack of significant differences in 5-year survival rates between rural and urban patients could be due to a variety of factors.
Within the CCC setting in Georgia, it is possible that the quality of care and access to treatment are relatively uniform across rural and urban patients, leveling the survival rates. The CCC has established three network sites throughout Georgia giving direct access to CCC specialty physicians to community physicians affiliated with the three sites. Two sites are in RUCC 3 counties and one in RUCC 4 county, whereas the CCC is in RUCC 1 county. Having dispersed network sites could help community physicians improve quality of care [20].
Several factors could contribute to higher breast cancer clinical trial enrollment. One possibility is the difference in the level of public awareness and support for different types of cancer. Breast cancer awareness has received significant attention and support due to the active advocacy of national breast cancer organizations, such as groups like the Susan G. Komen Foundation [21]. This has led to increased public awareness, fundraising, and early detection efforts for breast cancer. Lung cancer awareness, while increasing, faces challenges related to stigma and limited early detection options [22]. Future research should explore these potential factors in more detail to provide a comprehensive understanding of the observed results.
Additionally, within the CCC dataset, a distinctive disparity in patient distribution was noted. Among lung cancer patients, 19% originated from rural counties, while 81% were from metropolitan areas. For breast cancer patients, a mere 0.3% hailed from rural locales, with the overwhelming majority (99.7%) originating from metropolitan regions. The underrepresentation of rural patients with breast and lung cancer in the CCC compared to the state-level dataset highlights lack of geographic coverage, and differences in ability to access the CCC facility, which is located in a metropolitan area of Georgia. The biased sample composition could help explain the present research problem where breast and lung cancer patients from rural areas tend to have low CT enrollment rates and have longer diagnostic intervals.
This difference suggests rural individuals diagnosed with cancer may face unique challenges accessing Comprehensive Cancer Centers (CCCs), which are commonly located in urban areas. The necessity of traveling long distances to reach these urban centers may influence their healthcare decisions. Factors such as limited access to transportation, financial constraints, and the physical toll of travel may deter rural patients from seeking care at CCCs, even if these centers offer advanced treatments and clinical trials.
The insights gleaned from this research hold the potential to inform decision-making within the CCCs, guide patient education initiatives, facilitate networking and outreach efforts, secure grants, and drive future studies and developments. With a bias on lung and breast cancer, the results and findings depict the prevalence and survival rates in urban and rural areas. Future studies could delve into the specific challenges rural patients face when seeking care at urban CCCs, quantify the impact of travel distance on healthcare decisions, and evaluate the effectiveness of interventions aimed at mitigating this barrier.
This study encountered limitations such as the lack of more recent Rural-Urban Continuum Codes (RUCC), though the available dataset remains pertinent as of its last update on December 10, 2020. Additionally, reliance on secondary data precluded comprehensive exploration of all factors impacting rural populations. The study’s temporal scope of five years might not capture longitudinal changes, and the focus on Georgian CCC trials restricts generalizability to broader populations. Notably, this analysis omitted consideration of urban and rural Georgian populations’ racial and socioeconomic statuses. These factors are key confounding factors as they influence cancer survival outcomes [23,24]. However, given the secondary dataset limitation and the need to safeguard participants’ privacy, it was not possible to obtain detailed information on participants’ age, gender, racial profile, and socioeconomic statuses.
The study findings provide insights into cancer survival rates and clinical trial enrollment trends among rural and urban patients in Georgia. Despite the widely believed assumption that rural patients receive subpar cancer care compared to their urban counterparts, the results of the present study indicated that urban patients did not have a superior survival rate and clinical trial participation rate than rural patients. While limitations were present, the study contributes to understanding the nuances of cancer care in different geographic settings.
The study findings also contribute to highlighting the extent of health inequity specifically with respect to breast and lung cancer care across the rural and urban population. Further, the findings have considerable implications in informing the need for a positive social change as a proactive intervention to improve cancer survival outcomes and CT participation/engagement for both rural and urban cancer patients. There is a need for future research encompassing diverse populations and comprehensive variables to enhance our understanding of the complex dynamics between cancer survival outcomes, CT participation, and urbanicity.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Kurilo, Pentz; data collection: Kurilo, Pentz; analysis and interpretation of results: Kurilo, Pentz; draft manuscript preparation: Kurilo, Pentz. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data underlying this article are available in NIH SEER Research Database SEER Incidence Data, 1975–2020 (cancer.gov). Additional data underlying this article were provided by Winship Cancer Institute of Emory University under permission. Data will be shared on request to the corresponding author with permission of Winship Cancer Institute.
Ethics Approval: This study was a retrospective data analysis and did not contain any in vivo experiments. The approval of the Emory University Institutional Review Board Committee was obtained before data collection and statistical analysis. The IRB approval number is STUDY00004364. The IRB has granted a complete waiver of HIPAA authorization and informed consent. Protected Health Information of which use or access has been determined to be necessary by the IRB: data related to cancer type and survival outcomes from OnCore, Winship Tumor Registry, Georgia Cancer Registry, Rural Georgia SEER Registry (zip codes are only HIPAA identifier).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Yabroff, K. R., Han, X., Zhao, J., Nogueira, L., Jemal, A. (2020). Rural cancer disparities in the United States: A multilevel framework to improve access to care and patient outcomes. JCO Oncology Practice, 16(10), 1–6. [Google Scholar]
2. Bergin, R. J., Emery, J., Bollard, R. C., Falborg, A. Z., Jensen, H. et al. (2018). Rural-urban disparities in time to diagnosis and treatment for colorectal and breast cancer. Cancer Epidemiology and Prevention Biomarkers, 27(9), 1–33. [Google Scholar]
3. Greiner, B., Lee, M., Nelson, B., Hartwell, M. (2021). The pink elephant in the room: Declining public interest in breast cancer and the impact of marketing efforts. Journal of Cancer Policy, 28, 100287. https://doi.org/10.1016/j.jcpo.2021.100287. [Google Scholar] [PubMed] [CrossRef]
4. Pathak, S., George, N., Monti, D., Robinson, K., Politi, M. C. (2019). Evaluating adaptation of a cancer clinical trial decision aid for rural cancer patients: A mixed-methods approach. Journal of Cancer Education, 34(4), 803–809. https://doi.org/10.1007/s13187-018-1377-x. [Google Scholar] [PubMed] [CrossRef]
5. Maciejewski, M. L. (2020). Quasi-experimental design. Biostatistics Epidemiology, 4(1), 38–47. https://doi.org/10.1080/24709360.2018.1477468 [Google Scholar] [CrossRef]
6. Unger, J. M., Barlow, W. E., Martin, D. P., Ramsey, S. D., LeBlanc, M. et al. (2016). Comparison of survival outcomes among cancer patients in and out of clinical trials. Journal of the National Cancer Institute, 106(3), 1–8. [Google Scholar]
7. Whelan, C. J., Gatenby, R. A. (2020). Special collection on ecological and evolutionary approaches to cancer control: Cancer finds a conceptual home. Cancer Research, 80(14), 1–4. [Google Scholar]
8. Parisi, A., Porzio, G., Ficorella, C. (2020). Multimodality treatment in metastatic gastric cancer: From past to next future. Cancers, 12(9), 1–24. [Google Scholar]
9. National Institutes of Health (NIH). (2021). Age and cancer risk, vol. 2, no. 36, pp. 45–50. https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed on 15/04/2024). [Google Scholar]
10. Ugai, T., Sasamoto, N., Lee, H. Y., Ando, M., Song, M. et al. (2022). Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nature Reviews Clinical Oncology, 19, 656–673. https://doi.org/10.1038/s41571-022-00672-8. [Google Scholar] [PubMed] [CrossRef]
11. Kurilo, T. (2023). Survival rates and clinical trial participation among rural cancer patients (Ph.D. Thesis). Walden University, USA. https://scholarworks.waldenu.edu/dissertations/11750 (accessed on 15/04/2024). [Google Scholar]
12. Blee, S. M., Facdol, J., Dixon, M. D., Master, V., Switchenko, J. M. et al. (2022). Dissemination of validated health literacy videos: A tailored approach. Cancer Medicine, 11(7), 1678–1687. https://doi.org/10.1002/cam4.v11.7 [Google Scholar] [CrossRef]
13. Freedman, R. A., Dockter, T. J., Lafky, J. M., Hurria, A., Muss, H. J. et al. (2018). Promoting accrual of older patients with cancer to clinical trials: An alliance for clinical trials in oncology member survey (A171602). The Oncologist, 23(9), 1016–1023. https://doi.org/10.1634/theoncologist.2018-0033. [Google Scholar] [PubMed] [CrossRef]
14. Galewitz, P., Miller, A. (2021). Record number of Americans sign up for ACA health insurance. Kaiser Family Foundation, 6(1), 12. [Google Scholar]
15. Giffin, R. B., Lebovitz, Y., English, R. A. (2010). Transforming clinical research in the United States: Challenges and opportunities: Workshop summary. 1st edition. Washington DC: National Academies Press. [Google Scholar]
16. Ocana, A., Tannock, I. F. (2011). When are “positive” clinical trials in oncology truly positive? Journal of the National Cancer Institute, 103(1), 16–20. https://doi.org/10.1093/jnci/djq463. [Google Scholar] [PubMed] [CrossRef]
17. National Cancer Institute (NCI). (2020). Overview of the SEER program. Washington DC: USA Department of Health and Human Services. https://seer.cancer.gov/about/overview.html (accessed on 15/04/2024). [Google Scholar]
18. Huang, G. D., Bull, J., McKee, K. J., Mahon, E., Harper, B. et al. (2018). Clinical trials recruitment planning: A proposed framework from the clinical trials transformation initiative. Contemporary Clinical Trials, 66(16), 74–79. [Google Scholar] [PubMed]
19. Lin, Y., Wimberly, M. C., da Rosa, P., Hoover, J., Athas, W. F. (2018). Geographic access to radiation therapy facilities and disparities of early-stage breast cancer treatment. Geospatial Health, 13(1), 93–101. [Google Scholar]
20. Iskandar, K., Molinier, L., Hallit, S., Sartelli, M., Hardcastle, T. C. et al. (2021). Surveillance of antimicrobial resistance in low-and middle-income countries: A scattered picture. Antimicrobial Resistance & Infection Control, 10, 1–19. [Google Scholar]
21. Unger, J. M., Hershman, D. L., Till, C., Minasian, L. M., Osarogiagbon, R. U. et al. (2021). “When offered to participate”: A systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. Journal of the National Cancer Institute, 113(3), 244–257. https://doi.org/10.1093/jnci/djaa155. [Google Scholar] [PubMed] [CrossRef]
22. Bu, X., Li, S., Cheng, A. S., Ng, P. H., Xu, X. et al. (2022). Breast cancer stigma scale: A reliable and valid stigma measure for patients with breast cancer. Frontiers in Psychology, 13, 1–11. [Google Scholar]
23. Blevins Primeau, A. S. (2019). Barriers to cancer clinical trial enrollment. Cancer Therapy Advisor, 19(6), 26–28. [Google Scholar]
24. Miller, C. J., Smith, S. N., Pugatch, M. (2020). Experimental and quasi-experimental designs in implementation research. Psychiatry Research, 283, 1–7. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools