 Open Access
Open Access
ARTICLE
The effect of celastrol in combination with 5-fluorouracil on proliferation and apoptosis of gastric cancer cell lines
1 Medical Plants Research Center, Basic Health Sciences Institute, Shahrekord University of Medical Sciences, Shahrekord, Iran
2 Department of Pharmaceutics, College of Pharmacy, Al-Zahraa University for Women, Karbala, Iraq
3 Cellular and Molecular Research Center, Basic Health Sciences Institute, Shahrekord University of Medical Sciences, Shahrekord, Iran
* Corresponding Author: MAJID ASADI-SAMANI. Email:
Oncology Research 2024, 32(7), 1231-1237. https://doi.org/10.32604/or.2024.047187
Received 28 October 2023; Accepted 16 April 2024; Issue published 20 June 2024
Abstract
Background: Despite the availability of chemotherapy drugs such as 5-fluorouracil (5-FU), the treatment of some cancers such as gastric cancer remains challenging due to drug resistance and side effects. This study aimed to investigate the effect of celastrol in combination with the chemotherapy drug 5-FU on proliferation and induction of apoptosis in human gastric cancer cell lines (AGS and EPG85-257). Materials and Methods: In this in vitro study, AGS and EPG85-257 cells were treated with different concentrations of celastrol, 5-FU, and their combination. Cell proliferation was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The synergistic effect of 5-FU and celastrol was studied using Compusyn software. The DNA content at different phases of the cell cycle and apoptosis rate was measured using flow cytometry. Results: Co-treatment with low concentrations (10% inhibitory concentration (IC10)) of celastrol and 5-FU significantly reduced IC50 (p < 0.05) so that 48 h after treatment, IC50 was calculated at 3.77 and 6.9 μM for celastrol, 20.7 and 11.6 μM for 5-FU, and 5.03 and 4.57 μM for their combination for AGS and EPG85-257 cells, respectively. The mean percentage of apoptosis for AGS cells treated with celastrol, 5-FU, and their combination was obtained 23.9, 41.2, and 61.9, and for EPG85-257 cells 5.65, 46.9, and 55.7, respectively. In addition, the 5-FU and celastrol-5-FU combination induced cell cycle arrest in the synthesis phase. Conclusions: Although celastrol could decrease the concentration of 5-fluorouracil that sufficed to suppress gastric cancer cells, additional studies are required to arrive at conclusive evidence on the anticancer effects of celastrol.Keywords
Gastric cancer was responsible for over one million new cancer cases and approximately 769,000 cancer deaths (the fifth leading cancer in terms of incidence and the fourth leading cancer in terms of mortality) in 2020 worldwide. The incidence rate of gastric cancer is twice in men. Gastric cancer is both the leading high level of cancer and cancer deaths among men in South Central Asian countries including Iran, Afghanistan, Turkmenistan, and Kyrgyzstan [1].
Treatments for gastric cancer include chemotherapy, radiotherapy, surgery, immunotherapy, or often a combination of two or more. Currently, chemotherapy is the standard treatment of choice although the therapeutic effects of chemotherapy drugs are not adequately satisfactory and may cause numerous side effects [2]. As a chemotherapy drug, 5-fluorouracil (5-FU) inhibits DNA synthesis and cancer cell death by inhibiting thymidylate synthase and consequently thymidine nucleotide synthesis. 5-FU is one of the most widely used drugs for the treatment of gastrointestinal cancers, yet it is not tolerated by some patients due to unpleasant side effects [2,3]. Therefore, great emphasis has recently been put on research on anticancer drugs that are compatible with the body system and produce comparably fewer side effects, or whose efficacy is increased and/or whose side effects are reduced in combination with available drugs [4,5].
Celastrol, isolated from Tripterygium wilfordii belonging to the boxwood family, is a natural active ingredient. The genus Tripterygium is widely used in traditional medicine to treat swelling, fever, chills, ulcers, and inflammation [6]. Celastrol is a pentacyclic triterpenoid from a small class of natural triterpenoids with a wide range of bioactivities [7]. Because of the in vivo anti-inflammatory effect of celastrol, the compound is considered an appropriate choice to modulate inflammation and immune responses [8]. This compound plays a substantial role in treating inflammatory and autoimmune diseases such as arthritis and lupus [9,10]. In addition, studies have confirmed the antitumor effects of celastrol in various types of cancers such as breast, prostate, and colon both in vitro and in vivo, as well as its impacts on the inhibition of cancer cell proliferation and apoptosis [11,12]. Besides that, celastrol is used to treat chronic nephritis, hepatitis, lupus erythematosus, ankylosing spondylitis, and various skin diseases [13]. Furthermore, numerous studies have demonstrated that celastrol leads to the reduction of Crohn’s disease inflammation and cancer cell differentiation in prostate cancer, and even the compound seems to decrease the tumor necrosis factor (TNF)-α and interleukin (IL)6 cytokines by influencing the nuclear factor κB (NF-κB) pathway [14–16].
Because most chemotherapy drugs cause damage to healthy cells and lead to drug resistance, the combination of herbal compounds with chemotherapy drugs has been recommended to reduce their doses, which consequently decreases their side effects [4,5]. Considering that the effectiveness of different drugs may vary across different cancer types and even different cell lines within a particular cancer, this study was conducted to investigate the effect of celastrol in combination with 5-FU in the treatment of metastatic and resistant gastric cancer cell lines, as well as the signaling pathways involved in these effects.
In this experimental in vitro study, the AGS and EPG85-257 cells were purchased from the Iran Pasteur Institute Cell Bank (Tehran, Iran) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (GIBCO, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; GIBCO, Grand Island, NY, USA), and 1% penicillin/streptomycin (GIBCO, Grand Island, NY, USA) at 37°C with 5% CO2.
Celastrol was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO; Samchun, Korea) and phosphate-buffered saline (PBS) to obtain a completely homogeneous solution. The final DMSO concentration in the cell culture did not exceed 0.02%. 5-FU (5-FU PhaRes solution for injection at 5 g/100 mL) was procured from Pharma Resources GmbH (Germany).
Investigation of cell proliferation using MTT assay
To investigate the growth-inhibiting (anti-proliferative) effects of celastrol and 5-FU on AGS and EPG85-257 cell lines, the cells were treated with different concentrations of celastrol, 5-FU, and their combination, and then cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. For determination of best combination concentration of celastrol and 5-FU, a relative low dose [10% inhibitory concentration (IC10)] of celastrol was combined with 5-FU at different concentrations.
In the MTT assay, each experiment was performed 3–5 times and the cells were poured into a 96-well culture plate. After 24 h, the cells were incubated with different concentrations of celastrol, 5-FU, and their combination for 48 h. The MTT (Sigma-Aldrich, Germany) solution was prepared at 5 mg/mL in serum-free RPMI and after incubation of the cells for an appropriate duration, the cell supernatant was removed and each well was rinsed with 100 μL of serum-free PBS. Next, the MTT solution was diluted with PBS at 1:5 ratio, and then 60 μL of the resulting solution was added to each well and incubated at 37°C for 4 h. Afterward, the contents of the wells were slowly removed and 100 µL of DMSO was added to them; 10 min later, the optical absorbance of the plates was read at 490 nm wavelength. Cell proliferation rates were compared by comparing the optical absorbance of different wells and the control well. Then, the IC50 value was determined using probit regression in the SPSS software.
The synergistic anti-proliferative effect of 5-FU and celastrol was evaluated using a freely available trial version of CompuSyn software (ComboSyn, Inc. NJ, USA). The anti-proliferative effect of 5-FU+celastrol was maintained at 0.5 IC50, 1 IC50, and 2 IC50 (10 + 2, 20 + 4 and 40 + 8 for AGS cells and 6 + 3.5, 12 + 7 and 30 + 15 μM for EPG85-257 cells). The anti-proliferative effects were investigated using the MTT assay as per the above-explained procedure.
Evaluation of apoptosis by flow cytometry
To investigate apoptosis, cells were cultured in 6-well plates for 24 h. Then, they were incubated for 48 h with the 0.5 IC50s of celastrol and 5-FU, and the 0.5 IC50s of celastrol and 5-FU combination based on the MTT assay results.
The apoptosis rate of cells under the influence of 5-FU, celastrol, and their combination was measured according to the instructions of the apoptosis detection kit (FITC Annexin V) and flow cytometry kit (Partec, Germany). For this purpose, the cells were separated from the bottom of the plates and washed with calcium buffer twice; then, 10 μL of Annexin V was mixed with 100 μL of cells and incubated in the dark on ice for 20 min; afterward, the cells were washed and 10 μL of propidium iodide stain was added. The resulting mixture was incubated in the dark on ice for 10 min, and the samples were analyzed using flow cytometry [17].
Investigation of DNA content and cell cycle
DNA content at different phases of the cell cycle was determined using BD Cycletest plus DNA reagent kit (BD Biosciences, CA, USA) and flow cytometry after treatment with 5-FU, celastrol, and their combination (at the concentrations used for investigation of apoptosis). To this end, the cells were cultured in 6-well plates for 24 h. Then, they were further incubated for 48 h with the 0.5 IC50s of celastrol and 5-FU, and the 0.5 IC50 of celastrol and 5-FU combination cultured in a 6-well plate and treated with the studied concentrations of celastrol and 5-FU. Cells were washed with cool PBS and fixated with ethanol 70% after centrifugation at 800 rpm. The cells were then left at 20°C until further investigations. To study cell cycle phases, the control and treated samples were dissolved in 20 mg propidium iodide and 20 mg RNase dissolved in 1 ml PBS; afterward, the samples were studied using flow cytometry. The cell population was calculated at the sub-G1, G0/G1, S, and G2/M phases using the device software.
The Kruskal-Wallis test in SPSS version 20 was used to investigate the relationship between the data. The probit regression in the same software was used to calculate IC50 and IC10. The curves were plotted using GraphPad Prism 5 Demo software. A p-value < 0.05 was considered significance level.
Growth-inhibiting effect and combination index value of celastrol and 5-FU
The MTT assay results on cell proliferation showed that cell viability was significantly dependent on the doses of celastrol and 5-FU (p < 0.05; Fig. 1). Co-treatment of 5-FU and IC10 of celastrol significantly reduced the IC50 of 5-FU in both cell lines. The IC50 values of the AGS cells treated with 5-FU, celastrol, and their combination were calculated at 20.7, 3.77, and 5.03 μM, and those of the EPG85-257 cells at 11.6, 6.9, and 4.57 μM, respectively (Table 1).
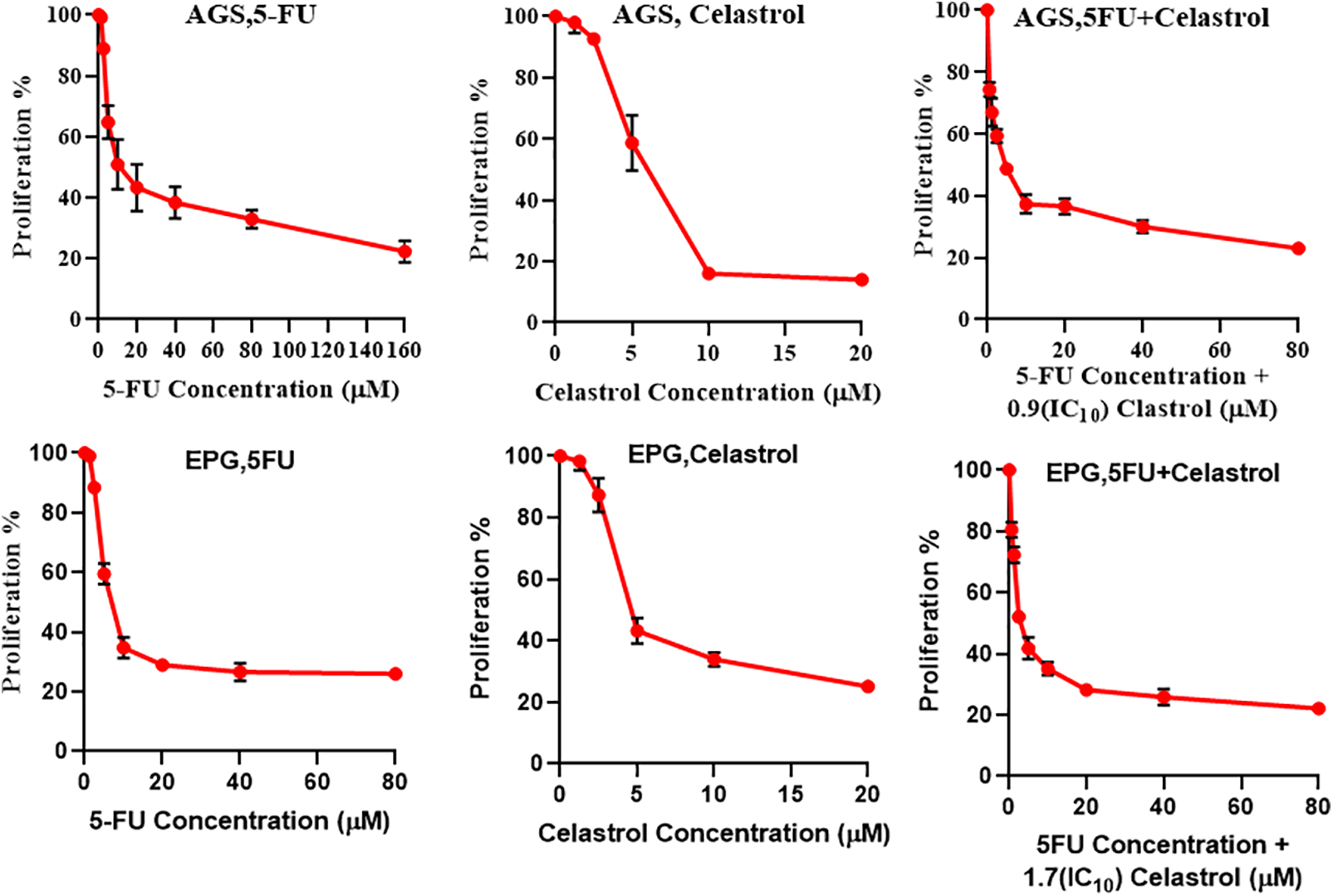
Figure 1: Cells were treated at 48 h with different concentrations of the two compounds separately and their combination, and then cell viability was measured using MTT assay. For determination of the best combination concentration of celastrol and 5-fluorouracil, 10% inhibitory concentration (IC10) of celastrol was combined with 5-FU at different concentrations.
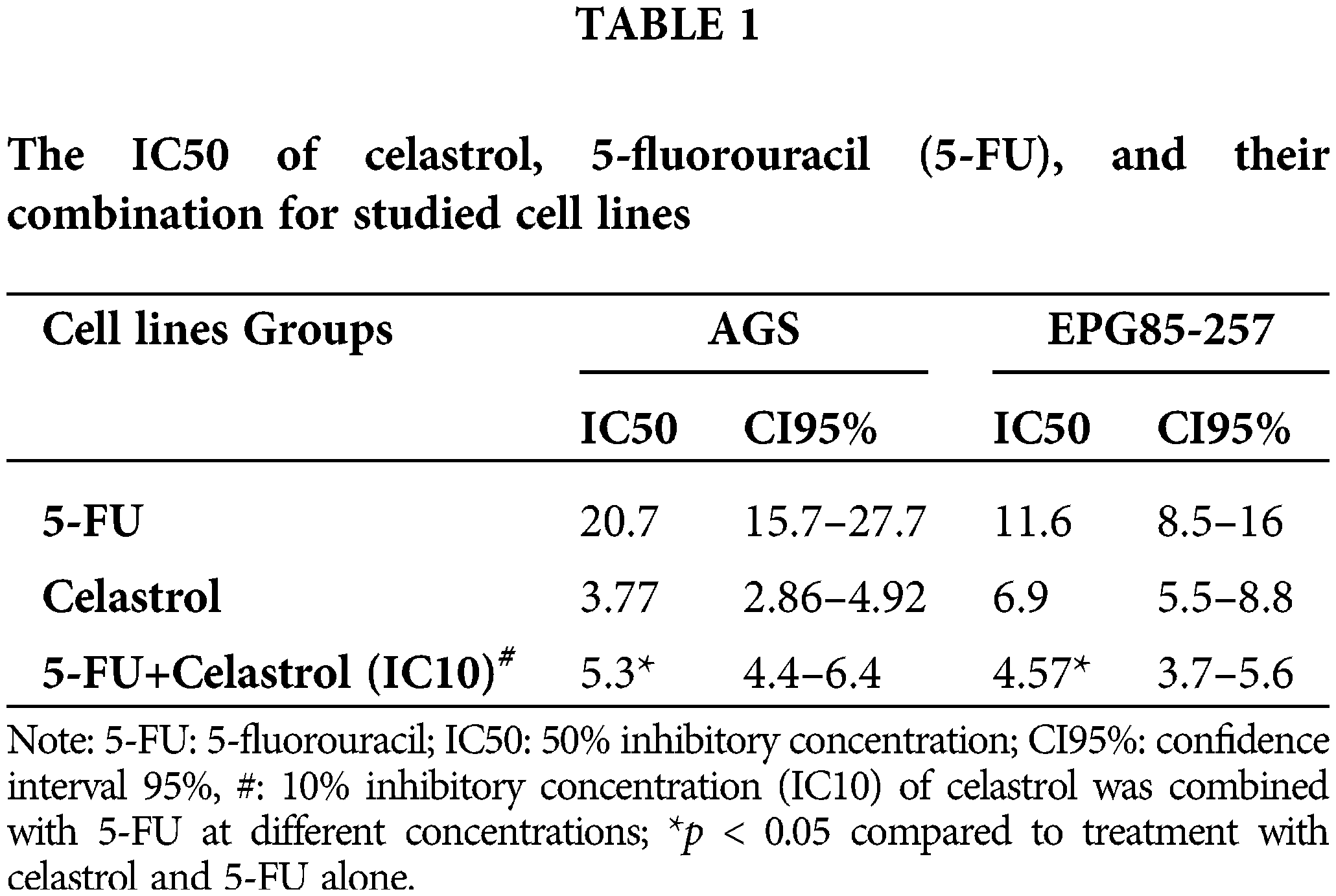
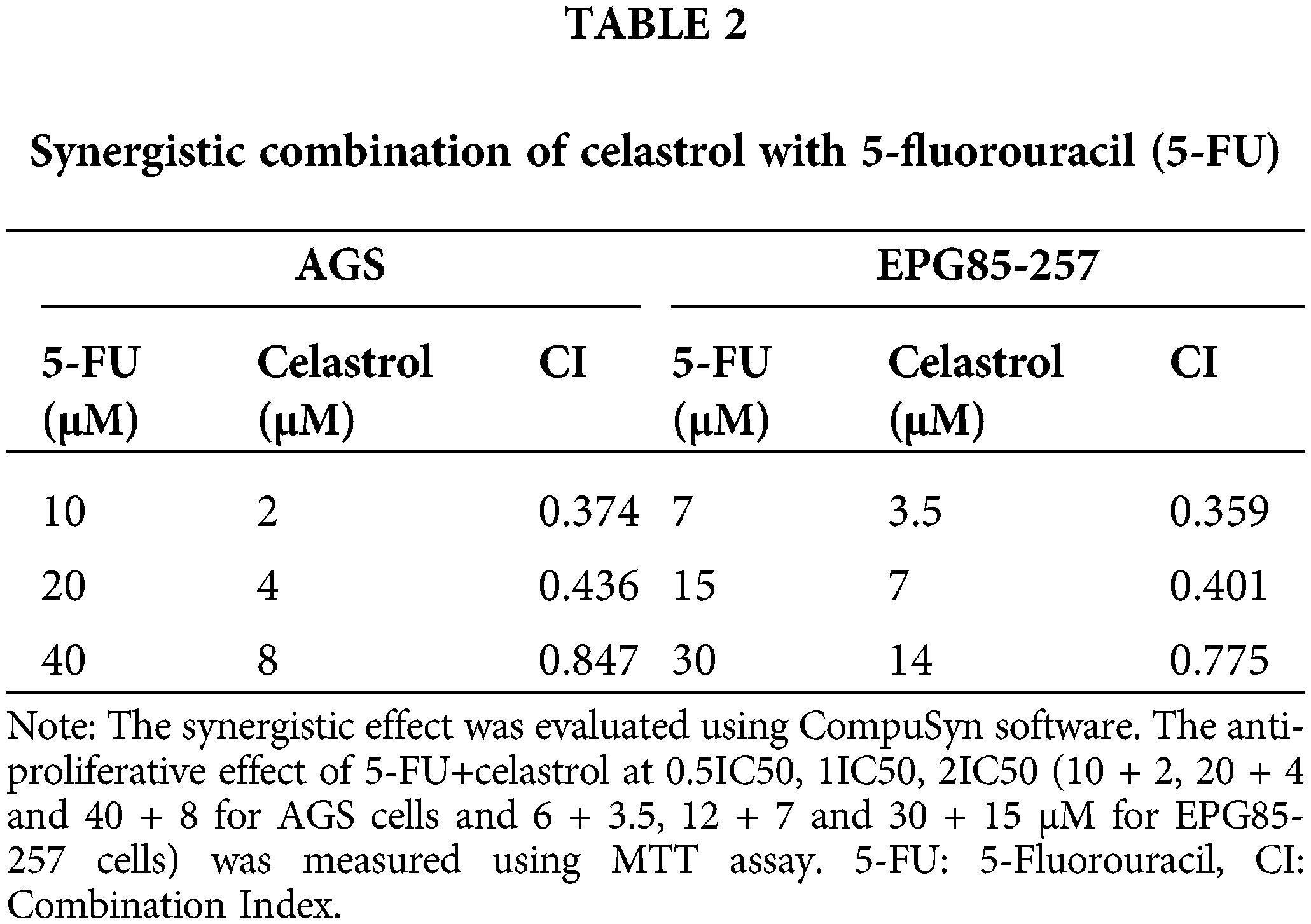
The synergistic action of celastrol and 5-FU was confirmed using CompuSyn software. Our observations showed that the combination indices of all the studied doses were <1, indicating a synergistic activity between the two agents. A lower combination index (denoting greater synergy) in both cell lines was observed for co-treatment with 0.5 IC50 celastrol and 0.5 IC50 5-FU (Table 2).
Effects of celastrol and 5-FU alone and their combination on apoptosis induction by flow cytometry
The flow cytometry results showed that celastrol, 5-Fu, and their combination induced cell death due to apoptosis in the cell lines. The mean percentage of apoptosis for AGS cells treated with celastrol, 5-FU, and their combination was calculated at 23.9, 41.2, and 61.9, and for EPG85-257 cells at 5.65, 46.9, and 55.7, respectively. Apoptosis increased in co-treated cells with celastrol and 5-FU compared with the control (p < 0.05; Fig. 2).
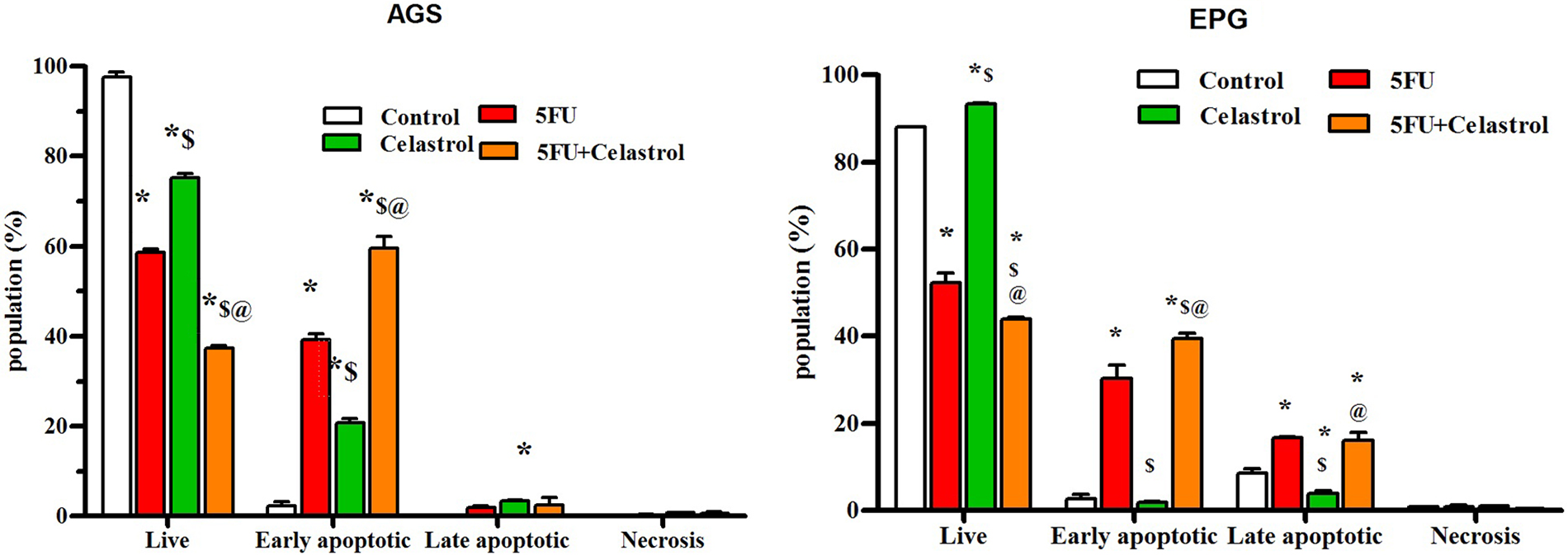
Figure 2: Cells were treated with the 0.5 IC50 of celastrol (2 μM for AGS cells and 3.5 μM for EPG cells), 5-FU (10 μM for AGS cells and 7 μM for EPG cells), and their combination for 48 h, and stained with propidium iodide (PI) and Annexin V-fluorescein isothiocyanate isomer (FITC), and then the rate of apoptosis was measured using flow cytometry; Data are presented as the mean ± SD of three independent experiments (*p < 0.05 vs. control group; $p < 0.05 vs. 5-FU group; @p < 0.05 vs. celastrol group).
Effect of celastrol, 5-FU, and their combination on cell cycle inhibition
The cells treated with celastrol, 5-FU, and their combination for 48 h showed a typical DNA pattern that represented sub-G1, G1, S, and G2/M phases of the cell cycle. The treated AGS cells with 10 μM (0.5 IC50) 5-FU increased the S population and decreased the G0/G1 and G2/M populations compared with the control group (p < 0.05). The AGS cells treated with 2 μM (0.5 IC50) celastrol increased the G2/M population and decreased the G0/G1 population compared with the control group (p < 0.05). Co-treatment of AGS cells with 10 μM 5-FU and 2 μM celastrol increased the S population compared with the control (p < 0.05) and celastrol (p < 0.05) groups. The EPG85-257 cells treated with 7 μM (0.5IC50) 5-FU increased the sub-G1, S, and G2/M population and decreased the G0/G1 population compared with the control group (p < 0.05). Co-treatment of EPG85-257 cells with 7 μM 5-FU and 3.5 μM celastrol increased the sub-G1 and S populations compared with the control group (p < 0.05) and increased the G0/G1 population and decreased the G2/M population compared with 5-FU (p < 0.05, Fig. 3).
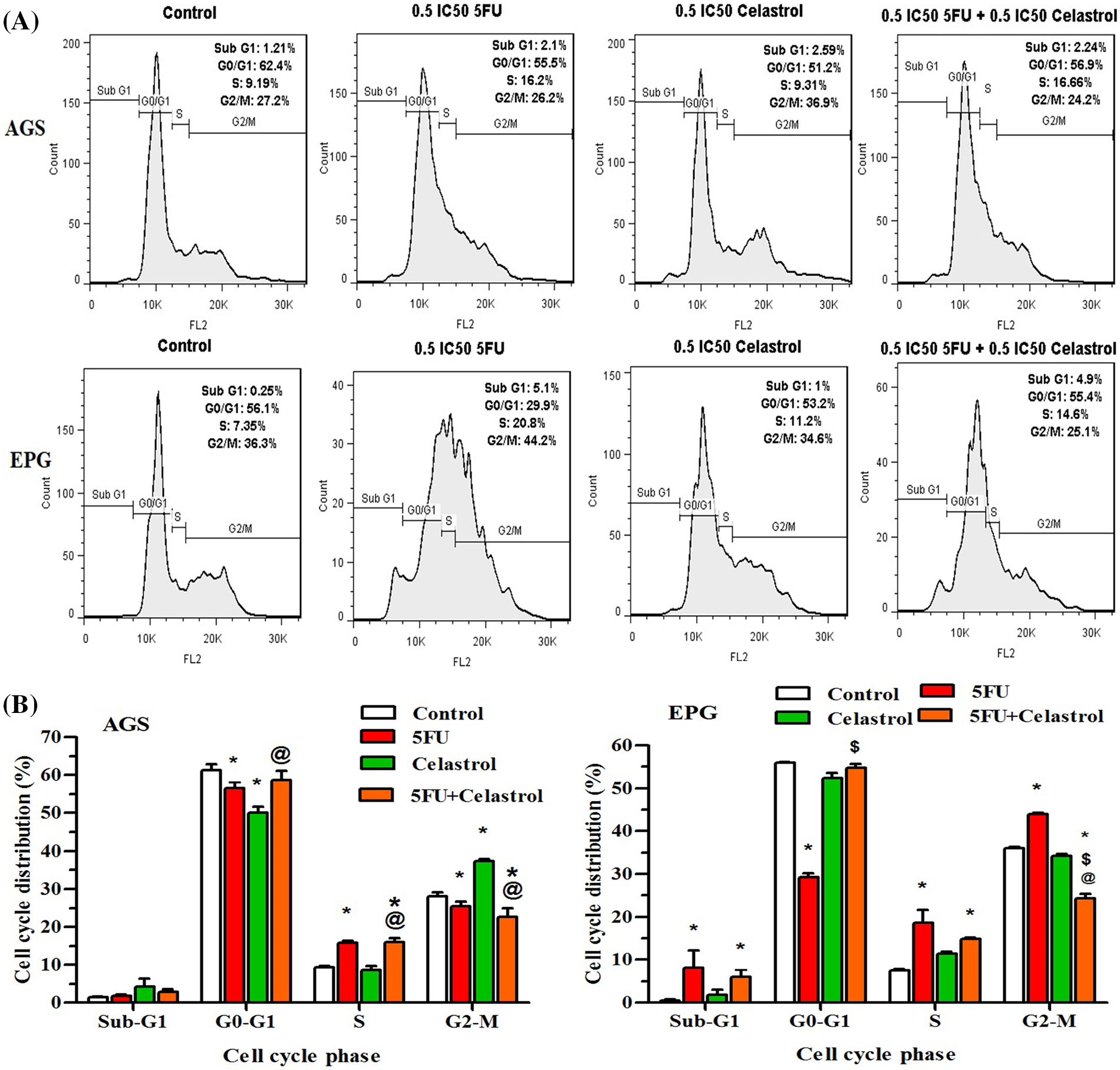
Figure 3: Cells were treated with 0.5IC 50 of celastrol (2 μM for AGS cells and 3.5 μM for EPG cells), 5-fluorouracil (10 μM for AGS cells and 7 μM for EPG cells), and their combination for 48 h and examined using BD Cycletest along with DNA reagent kit and flow cytometry; Data are presented as the mean ± SD of three independent experiments (*p < 0.05 vs. control group; $p < 0.05 vs. 5-FU group; @p < 0.05 vs. celastrol group).
The aim of this study was to investigate the effect of celastrol and 5-FU co-treatment on cell proliferation, cell cycle, and apoptosis induction in the AGS and EPG85-257 gastric cancer cell lines. The results of this study showed that co-treatment could increase the efficacy of the synthetic drug by inhibiting cell growth and inducing apoptosis along with reducing the dose of 5-FU.
In the present study, the anti-proliferative activity of celastrol on AGS and EPG85-257 cell lines was investigated. After co-treatment with celastrol and 5-FU, the cell viability significantly decreased in a dose-dependent manner. Some medicinal plants have been reported to produce anticancer effects by inducing programmed cell death [18]. The results of the present study showed that co-treatment of cells with 5-FU and celastrol could arrest the cell cycle at the synthesis phase and induce programmed cell death in early apoptosis. Celastrol is an immunomodulatory compound that produces anti-inflammatory effects by acting on the NF-κB and mitogen-activated protein kinase transcription factors [19]. The effects of celastrol have also been reported on tumor onset inhibition, tumor invasion, and metastasis in a wide range of tumor cells, as well as inhibitory impacts on cancer models in vivo [20]. In the study of Yang et al., celastrol isolated from Tripterygium inhibited the growth of breast cancer cells by inducing apoptosis [21]. Another study showed the efficacy of celastrol for inhibiting cell growth and proliferation and inducing cell death in the HL-60 cell line, prostate cancer, and glioma cells [22]. Ni et al. investigated the effect of celastrol on cell cycle arrest and apoptosis in human multiple myeloma and observed its impact on cell cycle arrest in myeloma cells [23]. Yoon et al. reported that celastrol caused the death of several cell lines of breast cancer and colon cancer by inducing apoptosis. In this study, they observed that celastrol increased mitochondrial calcium levels and dilated the endoplasmic reticulum by inhibiting proteasomes in the cell lines [24]. Celastrol can enhance the anticancer effect of gambogic acid by inhibiting NF-kB in squamous cell carcinoma cell lines [25]. As a potent proteasome inhibitor, celastrol has also been reported to induce apoptosis and cell cycle arrest in some cancer cells [26].
Raja et al. in a study on the anticancer activity of celastrol in combination with chemotherapy drugs for the treatment of human breast cancer, observed the efficacy of celastrol combined with chemotherapy drugs in vitro and in vivo [27]. These results are consistent with the results of the present study. In addition, it has been suggested that celastrol might be used as an effective anticancer agent to deal with drug resistance in lung cancer patients [28]. Celastrol has also been suggested as a promising new adjunctive therapy to treat hormone-resistant prostate cancer in vitro and in vivo [29]. Celastrol plays a role in the NF-κB pathway and produces an effect on this transcription factor through various mechanisms; the compound reduces the expression of IκB kinase and NF-κB, inhibits the NF-κB P60 subunit, and prevents its binding to DNA [23]. Also, it inhibits the enzyme by targeting cysteine 179 of the IKK kinase lope [30]. Therefore, it may decrease the expressions and concentrations of TNF-α and IL-6 in inflammatory diseases such as cancer [31,32].
Taken together, in the present study, celastrol could decrease the concentration of 5-FU required to suppress the AGS and EPG85-257 gastric cancer cell lines. As well, celastrol in combination with 5-FU induced cell cycle arrest and apoptosis. However, these observations deserve more and more research to contribute favorably to gastric cancer treatment.
Acknowledgement: The authors would like to thank the Basic Health Sciences Institute of Shahrekord University of Medical Sciences for their support, cooperation, and assistance in conducting the study. In addition, we would like to thank Prof. Mahmoud Rafieian for final editing our manuscript.
Funding Statement: This study was supported by Shahrekord University of Medical Sciences, Shahrekord, Iran (Ethics Code: IR.SKUMS.REC.1397.119, Grant No. 3696 and Ethics Code: IR.SKUMS.REC.1401.197, Grant No. 6651).
Author Contributions: MTM and MAS made substantial contributions to the conception and design of the study and/or the acquisition, analysis, and interpretation of the data. All authors contributed to collecting samples, performing experiments, and analyzing data. All authors participated in drafting, critically revising the manuscript, providing important intellectual content, revisimg the manuscript and approving the final version for submission.
Availability of Data and Materials: The data and materials used in the present study are available from the corresponding authors upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I. et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660 [Google Scholar] [PubMed] [CrossRef]
2. Ishida, K., Ito, C., Ohmori, Y., Kume, K., Sato, K. A. et al. (2017). Inhibition of PI3K suppresses propagation of drug-tolerant cancer cell subpopulations enriched by 5-fluorouracil. Scientific Reports, 7(1), 2262. https://doi.org/10.1038/s41598-017-02548-9 [Google Scholar] [PubMed] [CrossRef]
3. Park, S., Ha, S., Kwon, H. W., Kim, W. H., Kim, T. Y. et al. (2017). Prospective evaluation of changes in tumor size and tumor metabolism in patients with advanced gastric cancer undergoing chemotherapy: Association and clinical implication. Journal of Nuclear Medicine, 58(6), 899–904. https://doi.org/10.2967/jnumed.116.182675 [Google Scholar] [PubMed] [CrossRef]
4. Li, J., Wu, Y., Wang, D., Zou, L., Fu, C. et al. (2019). Oridonin synergistically enhances the anti-tumor efficacy of doxorubicin against aggressive breast cancer via pro-apoptotic and anti-angiogenic effects. Pharmacological Research, 146, 104313. https://doi.org/10.1016/j.phrs.2019.104313 [Google Scholar] [PubMed] [CrossRef]
5. Lagoa, R., Silva, J., Rodrigues, J. R., Bishayee, A. (2020). Advances in phytochemical delivery systems for improved anticancer activity. Biotechnology Advances, 38, 107382. https://doi.org/10.1016/j.biotechadv.2019.04.004 [Google Scholar] [PubMed] [CrossRef]
6. Shi, J., Li, J., Xu, Z., Chen, L., Luo, R. et al. (2020). Celastrol: A review of useful strategies overcoming its limitation in anticancer application. Frontiers in Pharmacology, 11, 558741. https://doi.org/10.3389/fphar.2020.558741 [Google Scholar] [PubMed] [CrossRef]
7. Yadav, P., Jaswal, V., Sharma, A., Kashyap, D., Tuli, H. S. et al. (2018). Celastrol as a pentacyclic triterpenoid with chemopreventive properties. Pharmaceutical Patent Analystvol, 7(4), 155–167. https://doi.org/10.4155/ppa-2017-0035 [Google Scholar] [PubMed] [CrossRef]
8. Chen, J., Xuan, J., Gu, Y. T., Shi, K. S., Xie, J. J. et al. (2017). Celastrol reduces IL-1β induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo. Biomedicine & Pharmacotherapy, 91, 208–219. https://doi.org/10.1016/j.biopha.2017.04.093 [Google Scholar] [PubMed] [CrossRef]
9. Liu, Y., Xiao, N., Du, H., Kou, M., Lin, L. et al. (2020). Celastrol ameliorates autoimmune disorders in Trex1-deficient mice. Biochemical Pharmacology, 178, 114090. https://doi.org/10.1016/j.bcp.2020.114090 [Google Scholar] [PubMed] [CrossRef]
10. Xiang, G., Shi, K., Wang, J. (2022). Celastrol alleviates murine lupus nephritis via inducting CD4+ Foxp3+ regulatory T cells. Folia Histochemica et Cytobiologica, 60(3), 237–246. https://doi.org/10.5603/FHC.a2022.0020 [Google Scholar] [PubMed] [CrossRef]
11. Lim, H. Y., Ong, P. S., Wang, L., Goel, A., Ding, L. et al. (2021). Celastrol in cancer therapy: Recent developments, challenges and prospects. Cancer Letters, 521, 252–267. https://doi.org/10.1016/j.canlet.2021.08.030 [Google Scholar] [CrossRef]
12. Zeng, X., Zhu, X., Tian, Q., Tan, X., Sun, N. et al. (2022). Celastrol-conjugated chitosan oligosaccharide for the treatment of pancreatic cancer. Drug Delivery, 29(1), 89–98. https://doi.org/10.1080/10717544.2021.2018521 [Google Scholar] [PubMed] [CrossRef]
13. Zhao, J., Zhang, F., Xiao, X., Wu, Z., Hu, Q. et al. (2021). Tripterygium hypoglaucum (Levl.) Hutch and its main bioactive components: Recent advances in pharmacological activity, pharmacokinetics and potential toxicity. Frontiers in Pharmacology, 12, 715359. https://doi.org/10.3389/fphar.2021.715359 [Google Scholar] [PubMed] [CrossRef]
14. Ng, S. W., Chan, Y., Chellappan, D. K., Madheswaran, T., Zeeshan, F. et al. (2019). Molecular modulators of celastrol as the keystones for its diverse pharmacological activities. Biomedicine & Pharmacotherapy, 109, 1785–1792. https://doi.org/10.1016/j.biopha.2018.11.051 [Google Scholar] [PubMed] [CrossRef]
15. An, L., Li, Z., Shi, L., Wang, L., Wang, Y. et al. (2020). Inflammation-targeted celastrol nanodrug attenuates collagen-induced arthritis through NF-κB and Notch1 pathways. Nano Letters, 20(10), 7728–7736. https://doi.org/10.1021/acs.nanolett.0c03279 [Google Scholar] [PubMed] [CrossRef]
16. Wu, M., Chen, W., Yu, X., Ding, D., Zhang, W. et al. (2018). Celastrol aggravates LPS-induced inflammation and injuries of liver and kidney in mice. American Journal of Translational Research, 10(7), 2078 [Google Scholar] [PubMed]
17. Kotawong, K., Chaijaroenkul, W., Muhamad, P., Na-Bangchang, K. (2018). Cytotoxic activities and effects of atractylodin and β-eudesmol on the cell cycle arrest and apoptosis on cholangiocarcinoma cell line. Journal of Pharmacological Sciences, 136(2), 51–56. https://doi.org/10.1016/j.jphs.2017.09.033 [Google Scholar] [PubMed] [CrossRef]
18. Khan, M. I., Bouyahya, A., Hachlafi, N. E., Menyiy, N. E., Akram, M. et al. (2022). Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: A review on recent investigations. Environmental Science and Pollution Research, 29, 24411–24444. https://doi.org/10.1007/s11356-021-17795-7 [Google Scholar] [PubMed] [CrossRef]
19. Pinna, G. F., Fiorucci, M., Reimund, J. M., Taquet, N., Arondel, Y. et al. (2004). Celastrol inhibits pro-inflammatory cytokine secretion in Crohn’s disease biopsies. Biochemical and Biophysical Research Communications, 322(3), 778–786. https://doi.org/10.1016/j.bbrc.2004.07.186 [Google Scholar] [PubMed] [CrossRef]
20. Kannaiyan, R., Shanmugam, M. K., Sethi, G. (2011). Molecular targets of celastrol derived from Thunder of God Vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Letters, 303(1), 9–20. https://doi.org/10.1016/j.canlet.2010.10.025 [Google Scholar] [PubMed] [CrossRef]
21. Yang, H. S., Kim, J. Y., Lee, J. H., Lee, B. W., Park, K. H. et al. (2011). Celastrol isolated from Tripterygium regelii induces apoptosis through both caspase-dependent and -independent pathways in human breast cancer cells. Food and Chemical Toxicology, 49(2), 527–532. https://doi.org/10.1016/j.fct.2010.11.044 [Google Scholar] [PubMed] [CrossRef]
22. Feng, Y., Wang, W., Zhang, Y., Fu, X., Ping, K. et al. (2022). Synthesis and biological evaluation of celastrol derivatives as potential anti-glioma agents by activating RIP1/RIP3/MLKL pathway to induce necroptosis. European Journal of Medicinal Chemistry, 229, 114070. https://doi.org/10.1016/j.ejmech.2021.114070 [Google Scholar] [PubMed] [CrossRef]
23. Ni, H., Zhao, W., Kong, X., Li, H., Ouyang, J. (2014). NF-kappa B modulation is involved in celastrol induced human multiple myeloma cell apoptosis. PLoS One, 9(4), e95846. https://doi.org/10.1371/journal.pone.0095846 [Google Scholar] [PubMed] [CrossRef]
24. Yoon, M. J., Lee, A. R., Jeong, S. A., Kim, Y. S., Kim, J. Y. et al. (2014). Release of Ca2+ from the endoplasmic reticulum and its subsequent influx into mitochondria trigger celastrol-induced paraptosis in cancer cells. Oncotarget, 5(16), 6816. https://doi.org/10.18632/oncotarget.2256 [Google Scholar] [PubMed] [CrossRef]
25. He, D., Xu, Q., Yan, M., Zhang, P., Zhou, X. et al. (2009). The NF-kappa B inhibitor, celastrol, could enhance the anti-cancer effect of gambogic acid on oral squamous cell carcinoma. BMC Cancer, 9, 343. https://doi.org/10.1186/1471-2407-9-343 [Google Scholar] [PubMed] [CrossRef]
26. Li, X., Zhu, G., Yao, X., Wang, N., Hu, R. et al. (2018). Celastrol induces ubiquitin-dependent degradation of mTOR in breast cancer cells. OncoTargets and Therapy, 11, 8977. https://doi.org/10.2147/OTT.S187315 [Google Scholar] [PubMed] [CrossRef]
27. Raja, S. M., Clubb, R. J., Ortega-Cava, C., Williams, S. H., Bailey, T. A. et al. (2011). Anticancer activity of Celastrol in combination with ErbB2-targeted therapeutics for treatment of ErbB2-overexpressing breast cancers. Cancer Biology & Therapy, 11(2), 263–276. https://doi.org/10.4161/cbt.11.2.13959 [Google Scholar] [PubMed] [CrossRef]
28. Xu, S. W., Law, B. Y., Mok, S. W., Leung, E. L., Fan, X. X. et al. (2016). Autophagic degradation of epidermal growth factor receptor in gefitinib-resistant lung cancer by celastrol. International Journal of Oncology, 49, 1576–1588. https://doi.org/10.3892/ijo.2016.3644 [Google Scholar] [PubMed] [CrossRef]
29. Dai, Y., DeSano, J. T., Meng, Y., Ji, Q., Ljungman, M. et al. (2009). Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. International Journal of Radiation Oncology*Biology*Physics, 74(4), 1217–1225. https://doi.org/10.1016/j.ijrobp.2009.03.057 [Google Scholar] [PubMed] [CrossRef]
30. Lee, J. H., Koo, T. H., Yoon, H., Jung, H. S., Jin, H. Z. et al. (2006). Inhibition of NF-κB activation through targeting IκB kinase by celastrol, a quinone methide triterpenoid. Biochemical Pharmacology, 72(10), 1311–1321. https://doi.org/10.1016/j.bcp.2006.08.014 [Google Scholar] [PubMed] [CrossRef]
31. Gao, Q., Qin, H., Zhu, L., Li, D., Hao, X. (2020). Celastrol attenuates collagen-induced arthritis via inhibiting oxidative stress in rats. International Immunopharmacology, 84, 106527. https://doi.org/10.1016/j.intimp.2020.106527 [Google Scholar] [PubMed] [CrossRef]
32. Wang, H., Ahn, K. S., Alharbi, S. A., Shair, O. H., Arfuso, F. et al. (2020). Celastrol alleviates gamma irradiation-induced damage by modulating diverse inflammatory mediators. International Journal of Molecular Sciences, 21(3), 1084. https://doi.org/10.3390/ijms21031084 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools