 Open Access
Open Access
ARTICLE
The interplay mechanism between IDH mutation, MGMT-promoter methylation, and PRMT5 activity in the progression of grade 4 astrocytoma: unraveling the complex triad theory
1 Department of Pathology, Faculty of Medicine, King Abdulaziz University, Rabigh, Saudi Arabia
2 Department of Pathology, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
3 Department of Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
4 Department of Surgery, Faculty of Medicine, University of Tabuk, Tabuk, Saudi Arabia
5 Section of Neurosurgery, Department of Surgery, King Abdulaziz Medical City, Jeddah, Saudi Arabia
6 Department of Pediatrics, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
7 Department of Clinical Biochemistry, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
8 Department of Neurosciences, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
9 Department of Surgery, King Abdulaziz Specialist Hospital, Taif, Saudi Arabia
* Corresponding Author: MAHER KURDI. Email:
Oncology Research 2024, 32(6), 1037-1045. https://doi.org/10.32604/or.2024.051112
Received 28 February 2024; Accepted 22 March 2024; Issue published 23 May 2024
Abstract
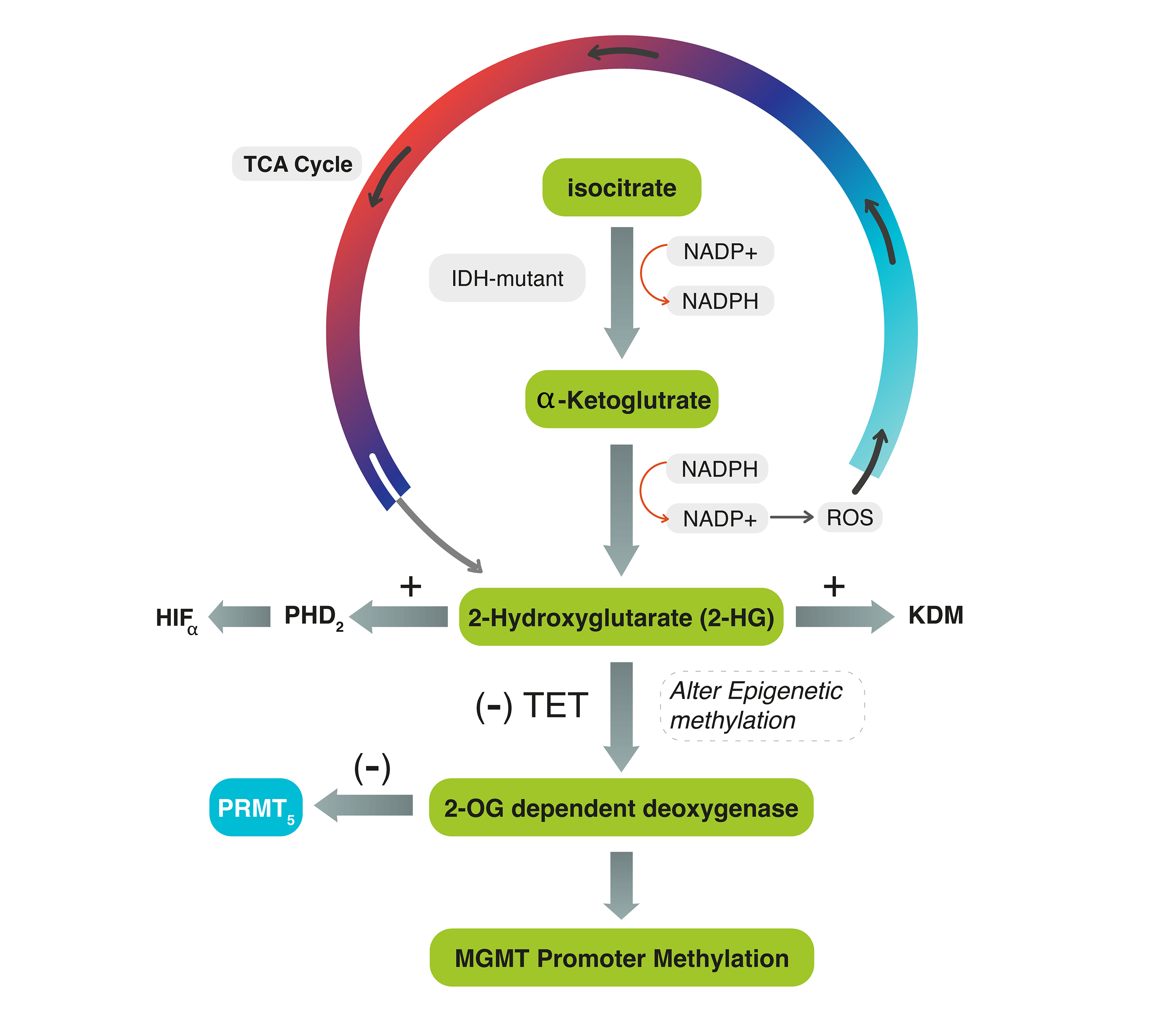
Background: The dysregulation of Isocitrate dehydrogenase (IDH) and the subsequent production of 2-Hydroxyglutrate (2HG) may alter the expression of epigenetic proteins in Grade 4 astrocytoma. The interplay mechanism between IDH, O-6-methylguanine-DNA methyltransferase (MGMT)-promoter methylation, and protein methyltransferase proteins-5 (PRMT5) activity, with tumor progression has never been described. Methods: A retrospective cohort of 34 patients with G4 astrocytoma is classified into IDH-mutant and IDH-wildtype tumors. Both groups were tested for MGMT-promoter methylation and PRMT5 through methylation-specific and gene expression PCR analysis. Inter-cohort statistical significance was evaluated. Results: Both IDH-mutant WHO grade 4 astrocytomas (n = 22, 64.7%) and IDH-wildtype glioblastomas (n = 12, 35.3%) had upregulated PRMT5 gene expression except in one case. Out of the 22 IDH-mutant tumors, 10 (45.5%) tumors showed MGMT-promoter methylation and 12 (54.5%) tumors had unmethylated MGMT. All IDH-wildtype tumors had unmethylated MGMT. There was a statistically significant relationship between MGMT-promoter methylation and IDH in G4 astrocytoma (p-value = 0.006). Statistically significant differences in progression-free survival (PFS) were also observed among all G4 astrocytomas that expressed PRMT5 and received either temozolomide (TMZ) or TMZ plus other chemotherapies, regardless of their IDH or MGMT-methylation status (p-value=0.0014). Specifically, IDH-mutant tumors that had upregulated PRMT5 activity and MGMT-promoter methylation, who received only TMZ, have exhibited longer PFS. Conclusions: The relationship between PRMT5, MGMT-promoter, and IDH is not tri-directional. However, accumulation of D2-hydroxyglutarate (2-HG), which partially activates 2-OG-dependent deoxygenase, may not affect their activities. In IDH-wildtype glioblastomas, the 2HG-2OG pathway is typically inactive, leading to PRMT5 upregulation. TMZ alone, compared to TMZ-plus, can increase PFS in upregulated PRMT5 tumors. Thus, using a PRMT5 inhibitor in G4 astrocytomas may help in tumor regression.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools